Muscle Activity during Passive and Active Movements in Preterm and Full-Term Infants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
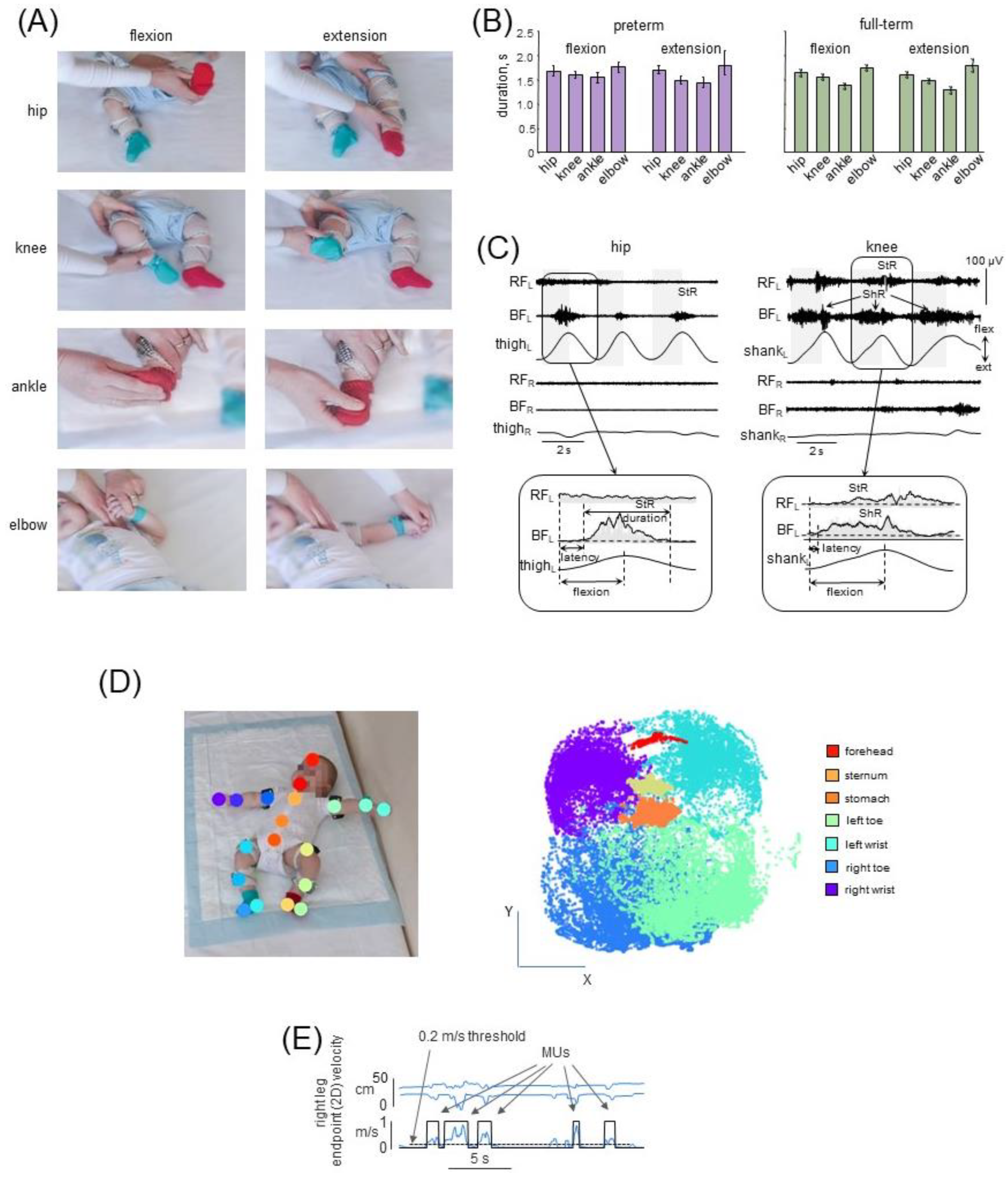
2.2.1. Muscle Responses to Passive Joint Movements (PM)
2.2.2. Spontaneous Movements (SM)
2.3. Data Recording
2.4. Data Analysis
2.4.1. Muscle Responses to Passive Joint Movements
2.4.2. Spontaneous Movements
2.5. Statistics
3. Results
3.1. Passive Movements
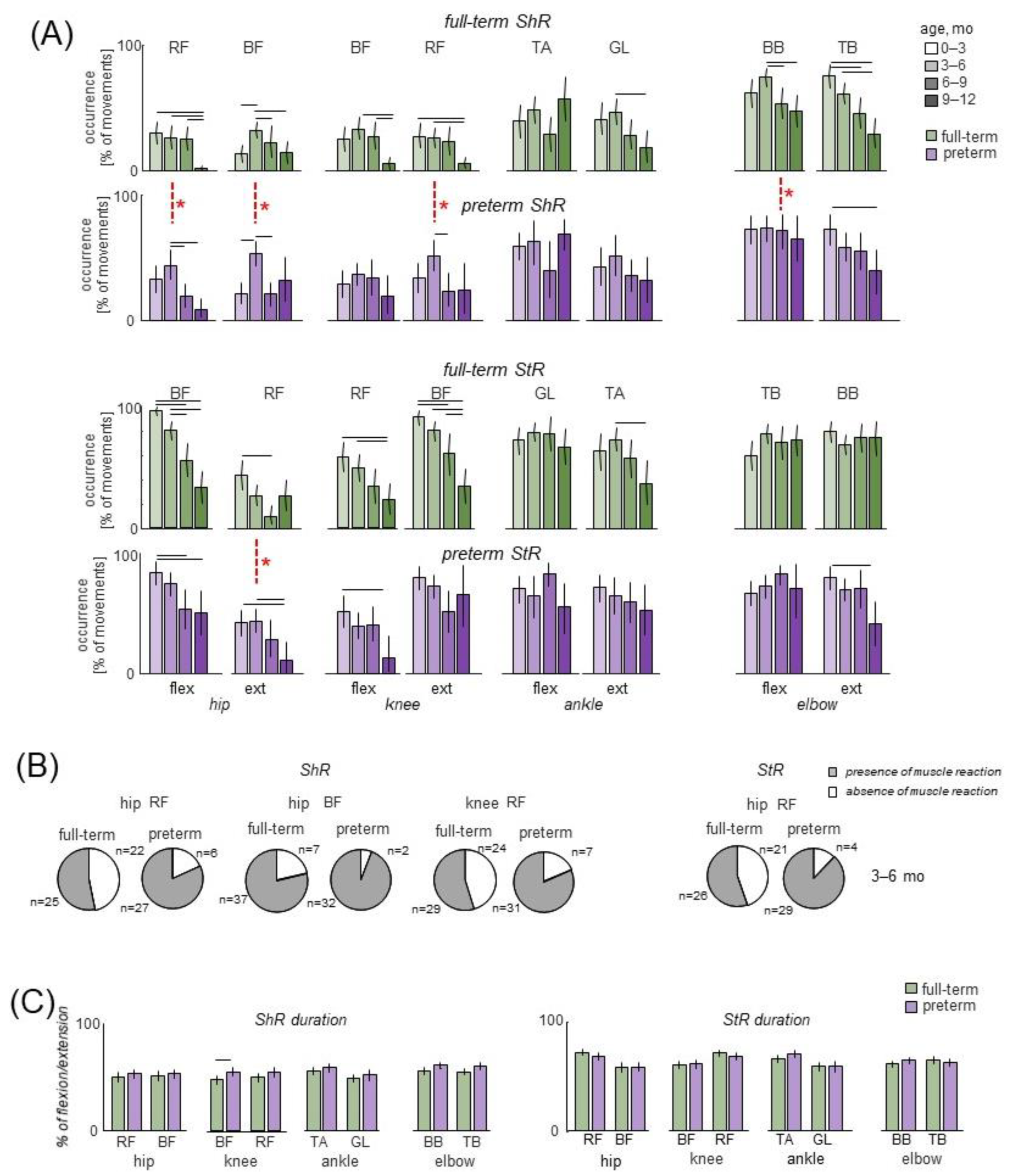
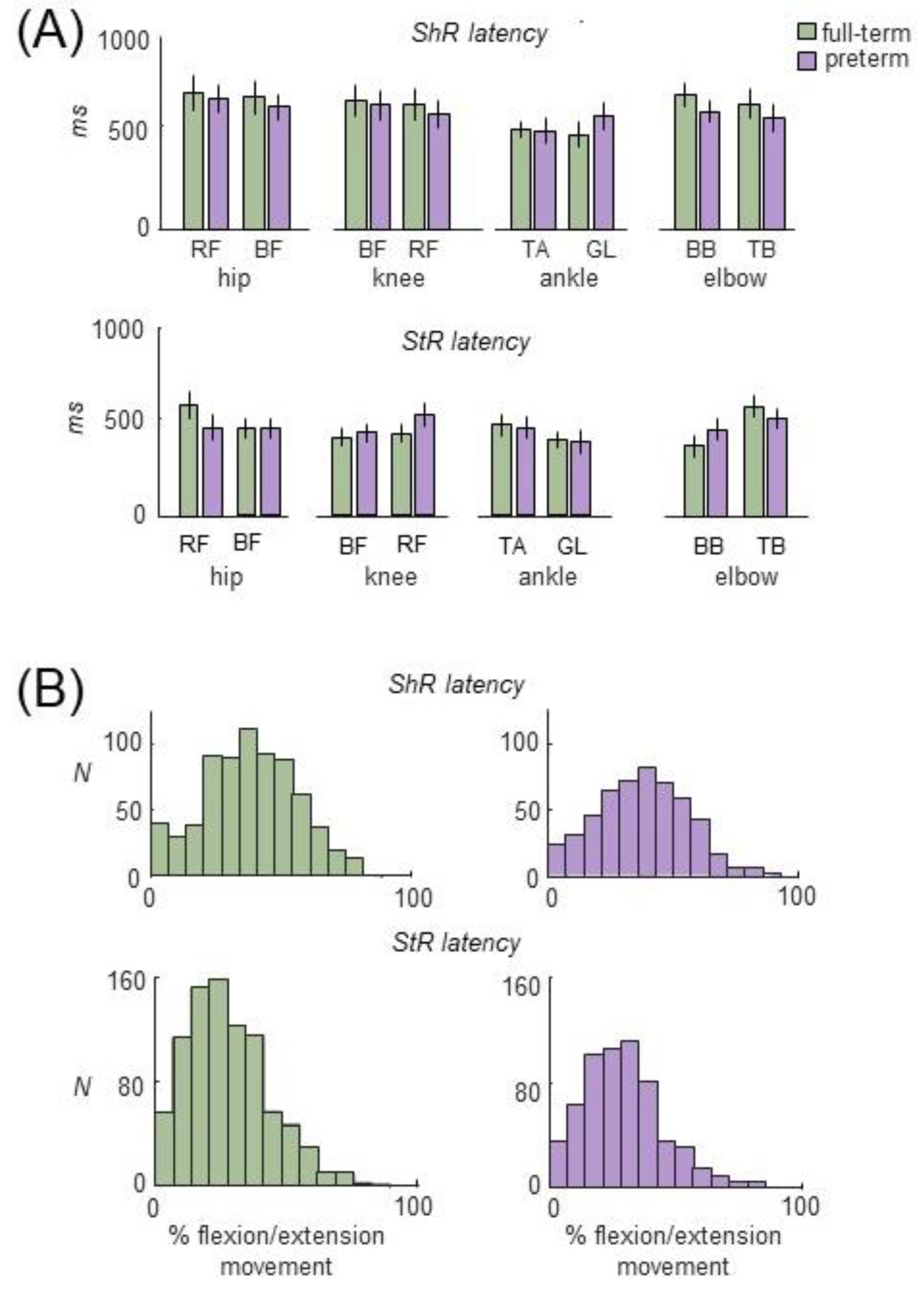
3.2. Muscle Responses to Passive Movements in Preterm and Full-Term Infants
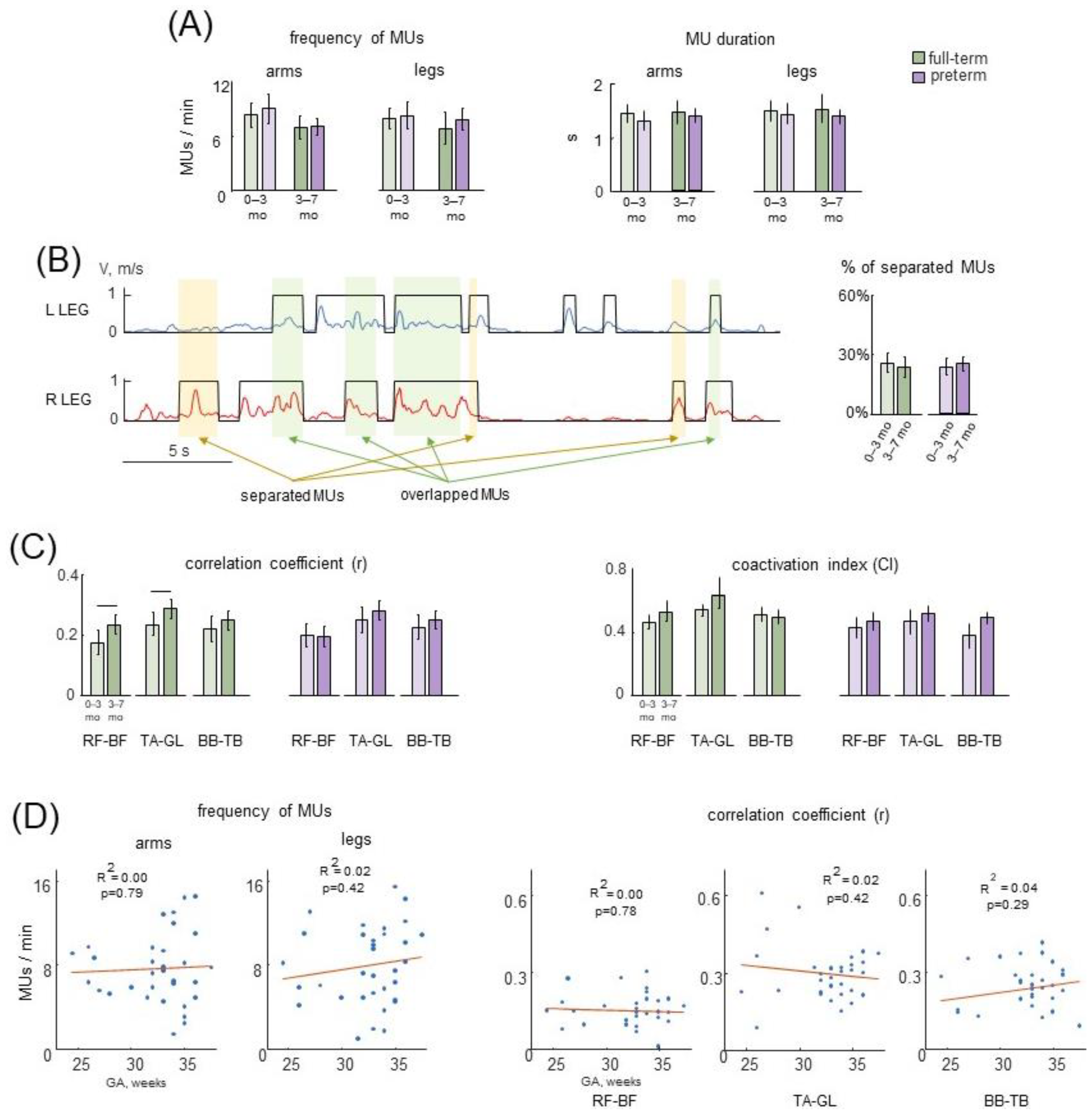
3.3. Selected Movement Episodes (MUs) of Spontaneous Activity
3.4. Characteristics of MUs in Preterm and Full-Term Infants
4. Discussion
4.1. Limitations of the Study
4.2. Comparison of Muscle Activities during Passive and Active (Spontaneous) Movements
4.3. Muscle Responses to Passive Movements
4.4. StR vs. ShR
4.5. Muscle Activities during Spontaneous Movements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Personius, K.E.; Balice-Gordon, R.J. Loss of Correlated Motor Neuron Activity during Synaptic Competition at Developing Neuromuscular Synapses. Neuron 2001, 31, 395–408. [Google Scholar] [CrossRef]
- Vecchio, A.D.; Sylos-Labini, F.; Mondì, V.; Paolillo, P.; Ivanenko, Y.; Lacquaniti, F.; Farina, D. Spinal Motoneurons of the Human Newborn Are Highly Synchronized during Leg Movements. Sci. Adv. 2020, 6, eabc3916. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, L.; Fabrizi, L.; Patten, D.; Worley, A.; Meek, J.; Boyd, S.; Slater, R.; Fitzgerald, M. Postnatal Temporal, Spatial and Modality Tuning of Nociceptive Cutaneous Flexion Reflexes in Human Infants. PLoS ONE 2013, 8, e76470. [Google Scholar] [CrossRef]
- Payne, V.G.; Isaacs, L.D. Human Motor Development: A Lifespan Approach, 9th ed.; Routledge: New York, NY, USA, 2017; ISBN 978-1-315-21304-0. [Google Scholar]
- Egmose, I.; Varni, G.; Cordes, K.; Smith-Nielsen, J.; Væver, M.S.; Køppe, S.; Cohen, D.; Chetouani, M. Relations between Automatically Extracted Motion Features and the Quality of Mother-Infant Interactions at 4 and 13 Months. Front. Psychol. 2017, 8, 2178. [Google Scholar] [CrossRef]
- Inácio, A.R.; Nasretdinov, A.; Lebedeva, J.; Khazipov, R. Sensory Feedback Synchronizes Motor and Sensory Neuronal Networks in the Neonatal Rat Spinal Cord. Nat. Commun. 2016, 7, 13060. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J.; Sinning, A.; Yang, J.-W.; Reyes-Puerta, V.; Stüttgen, M.C.; Kirischuk, S.; Kilb, W. Spontaneous Neuronal Activity in Developing Neocortical Networks: From Single Cells to Large-Scale Interactions. Front. Neural Circuits 2016, 10, 40. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Neural Substrate and Clinical Significance of General Movements: An Update. Dev. Med. Child Neurol. 2018, 60, 39–46. [Google Scholar] [CrossRef]
- Sokoloff, G.; Hickerson, M.M.; Wen, R.Y.; Tobias, M.E.; McMurray, B.; Blumberg, M.S. Spatiotemporal Organization of Myoclonic Twitching in Sleeping Human Infants. Dev. Psychobiol. 2020, 62, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Prechtl, H.F.; Einspieler, C.; Cioni, G.; Bos, A.F.; Ferrari, F.; Sontheimer, D. An Early Marker for Neurological Deficits after Perinatal Brain Lesions. Lancet 1997, 349, 1361–1363. [Google Scholar] [CrossRef]
- Karch, D.; Kang, K.-S.; Wochner, K.; Philippi, H.; Hadders-Algra, M.; Pietz, J.; Dickhaus, H. Kinematic Assessment of Stereotypy in Spontaneous Movements in Infants. Gait Posture 2012, 36, 307–311. [Google Scholar] [CrossRef]
- Smith, C.C.; Paton, J.F.R.; Chakrabarty, S.; Ichiyama, R.M. Descending Systems Direct Development of Key Spinal Motor Circuits. J. Neurosci. 2017, 37, 6372–6387. [Google Scholar] [CrossRef]
- Friel, K.M.; Williams, P.T.J.A.; Serradj, N.; Chakrabarty, S.; Martin, J.H. Activity-Based Therapies for Repair of the Corticospinal System Injured during Development. Front. Neurol. 2014, 5, 229. [Google Scholar] [CrossRef]
- de Vries, J.I.; Visser, G.H.; Prechtl, H.F. The Emergence of Fetal Behaviour. I. Qualitative Aspects. Early Hum. Dev. 1982, 7, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.P.; Carman, R. Developmental Profiles of Spontaneous Movements in Infants. Early Hum. Dev. 1994, 39, 109–126. [Google Scholar] [CrossRef]
- Zuzarte, I.; Indic, P.; Sternad, D.; Paydarfar, D. Quantifying Movement in Preterm Infants Using Photoplethysmography. Ann. Biomed. Eng. 2019, 47, 646–658. [Google Scholar] [CrossRef]
- Gima, H.; Ohgi, S.; Morita, S.; Karasuno, H.; Fujiwara, T.; Abe, K. A Dynamical System Analysis of the Development of Spontaneous Lower Extremity Movements in Newborn and Young Infants. J. Physiol. Anthr. 2011, 30, 179–186. [Google Scholar] [CrossRef]
- Kanemaru, N.; Watanabe, H.; Taga, G. Increasing Selectivity of Interlimb Coordination during Spontaneous Movements in 2- to 4-Month-Old Infants. Exp. Brain Res. 2012, 218, 49–61. [Google Scholar] [CrossRef]
- Ohmura, Y.; Gima, H.; Watanabe, H.; Taga, G.; Kuniyoshi, Y. Developmental Changes in Intralimb Coordination during Spontaneous Movements of Human Infants from 2 to 3 Months of Age. Exp. Brain Res. 2016, 234, 2179–2188. [Google Scholar] [CrossRef]
- Kanazawa, H.; Kawai, M.; Kinai, T.; Iwanaga, K.; Mima, T.; Heike, T. Cortical Muscle Control of Spontaneous Movements in Human Neonates. Eur. J. Neurosci. 2014, 40, 2548–2553. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Yamada, Y.; Tanaka, K.; Kawai, M.; Niwa, F.; Iwanaga, K.; Kuniyoshi, Y. Open-Ended Movements Structure Sensorimotor Information in Early Human Development. Proc. Natl. Acad. Sci. USA 2023, 120, e2209953120. [Google Scholar] [CrossRef]
- Ritterband-Rosenbaum, A.; Herskind, A.; Li, X.; Willerslev-Olsen, M.; Olsen, M.D.; Farmer, S.F.; Nielsen, J.B. A Critical Period of Corticomuscular and EMG–EMG Coherence Detection in Healthy Infants Aged 9–25 Weeks. J. Physiol. 2017, 595, 2699–2713. [Google Scholar] [CrossRef]
- Sylos-Labini, F.; La Scaleia, V.; Cappellini, G.; Fabiano, A.; Picone, S.; Keshishian, E.S.; Zhvansky, D.S.; Paolillo, P.; Solopova, I.A.; d’Avella, A.; et al. Distinct Locomotor Precursors in Newborn Babies. Proc. Natl. Acad. Sci. USA 2020, 117, 9604–9612. [Google Scholar] [CrossRef]
- Hautala, S.; Tokariev, A.; Roienko, O.; Häyrinen, T.; Ilen, E.; Haataja, L.; Vanhatalo, S. Recording Activity in Proximal Muscle Networks with Surface EMG in Assessing Infant Motor Development. Clin. Neurophysiol. 2021, 132, 2840–2850. [Google Scholar] [CrossRef]
- Solopova, I.A.; Zhvansky, D.S.; Dolinskaya, I.Y.; Keshishian, E.S.; Selionov, V.A.; Sylos-Labini, F.; Lacquaniti, F.; Ivanenko, Y. Muscle Responses to Passive Joint Movements in Infants During the First Year of Life. Front. Physiol. 2019, 10, 1158. [Google Scholar] [CrossRef]
- Myklebust, B.M.; Gottlieb, G.L. Development of the Stretch Reflex in the Newborn: Reciprocal Excitation and Reflex Irradiation. Child Dev. 1993, 64, 1036–1045. [Google Scholar] [CrossRef]
- Teulier, C.; Ulrich, B.D.; Martin, B. Functioning of Peripheral Ia Pathways in Infants with Typical Development: Responses in Antagonist Muscle Pairs. Exp. Brain Res. 2011, 208, 581–593. [Google Scholar] [CrossRef]
- Thelen, E.; Fisher, D.M. The Organization of Spontaneous Leg Movements in Newborn Infants. J. Mot. Behav. 1983, 15, 353–377. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Van Eykern, L.A.; Klip-Van den Nieuwendijk, A.W.; Prechtl, H.F. Developmental Course of General Movements in Early Infancy. II. EMG Correlates. Early Hum. Dev. 1992, 28, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Sylos-Labini, F.; La Scaleia, V.; Cappellini, G.; Dewolf, A.; Fabiano, A.; Solopova, I.A.; Mondì, V.; Ivanenko, Y.; Lacquaniti, F. Complexity of Modular Neuromuscular Control Increases and Variability Decreases during Human Locomotor Development. Commun. Biol. 2022, 5, 1256. [Google Scholar] [CrossRef]
- Safronov, V.A. Regulation of muscle tone. Biofizika 1970, 15, 1103–1111. [Google Scholar] [PubMed]
- Andrews, C.J.; Burke, D.; Lance, J.W. The Response to Muscle Stretch and Shortening in Parkinsonian Rigidity. Brain 1972, 95, 795–812. [Google Scholar] [CrossRef]
- Walsh, E.G. Shortening Reactions in the Human Forearm. J. Physiol. 1976, 256, 116. [Google Scholar]
- Katz, R.; Rondot, P. Muscle Reaction to Passive Shortening in Normal Man. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 90–99. [Google Scholar] [CrossRef]
- Landau, W.M.; Struppler, A.; Mehls, O. A Comparative Electromyographic Study of the Reactions to Passive Movement in Parkinsonism and in Normal Subjects. Neurology 1966, 16, 34–48. [Google Scholar] [CrossRef]
- Gurfinkel, V.; Cacciatore, T.W.; Cordo, P.; Horak, F.; Nutt, J.; Skoss, R. Postural Muscle Tone in the Body Axis of Healthy Humans. J. Neurophysiol. 2006, 96, 2678–2687. [Google Scholar] [CrossRef]
- Esslen, E. Stretch Reflex and Shortening Reaction. A Quantitative Analysis of Phasic and Tonic Stretch Reflex in Clinical States of Spasticity and Rigidity. Electroencephalogr. Clin. Neurophysiol. 1969, 27, 719. [Google Scholar]
- Gurfinkel, V.S.; Ivanenko, I.P. Low-threshold reactions of human muscles at rest. Hum. Physiol. 1987, 13, 317–323. [Google Scholar]
- Miscio, G.; Pisano, F.; Del Conte, C.; Pianca, D.; Colombo, R.; Schieppati, M. The Shortening Reaction of Forearm Muscles: The Influence of Central Set. Clin. Neurophysiol. 2001, 112, 884–894. [Google Scholar] [CrossRef]
- Angel, R.W. Shortening Reaction in Patients with Cerebellar Ataxia. Ann. Neurol. 1982, 11, 272–278. [Google Scholar] [CrossRef]
- Andrews, C.J.; Neilson, P.; Knowles, L. Electromyographic Study of the Rigidospasticity of Athetosis. J. Neurol. Neurosurg. Psychiatry 1973, 36, 94–103. [Google Scholar] [CrossRef]
- Wright, W.G.; Gurfinkel, V.S.; Nutt, J.; Horak, F.B.; Cordo, P.J. Axial Hypertonicity in Parkinson’s Disease: Direct Measurements of Trunk and Hip Torque. Exp. Neurol. 2007, 208, 38–46. [Google Scholar] [CrossRef]
- Andrews, K.; Fitzgerald, M. Cutaneous Flexion Reflex in Human Neonates: A Quantitative Study of Threshold and Stimulus-Response Characteristics after Single and Repeated Stimuli. Dev. Med. Child Neurol. 1999, 41, 696–703. [Google Scholar] [CrossRef]
- Shadmehr, R. Distinct Neural Circuits for Control of Movement vs. Holding Still. J. Neurophysiol. 2017, 117, 1431–1460. [Google Scholar] [CrossRef]
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Bernstein, N.A. Studies of the Biodynamics of Walking, Running and Jumping. Moscow, Researches of the Central Scientific Institute of Physical Culture. In Human Motor Actions. Bernstein Reassessed; Whiting, H.T.A., Ed.; Publisher: Amsterdam, The Netherlands, 1940; pp. 171–222, (In Russian). [Google Scholar] [CrossRef]
- Forster, O. Schlaffe Und Spastische Lähmung. In Handbuch der Normale und Pathologischen Physiologie; Bethe, A., Bergman, G., von Embden, G., Ellinger, A., Eds.; Springer: Berlin, Germany, 1927; Volume 10, pp. 893–972. [Google Scholar]
- de Groot, L.; vd Hoek, A.M.; Hopkins, B.; Touwen, B.C. Development of the Relationship between Active and Passive Muscle Power in Preterms after Term Age. Neuropediatrics 1992, 23, 298–305. [Google Scholar] [CrossRef]
- Cioni, G.; Prechtl, H.F. Preterm and Early Postterm Motor Behaviour in Low-Risk Premature Infants. Early Hum. Dev. 1990, 23, 159–191. [Google Scholar] [CrossRef]
- Wilson-Costello, D.E.; Payne, A.H. Early childhood neurodevelopmental outcomes of high-risk neonates. In Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant; Elsevier/Saunders: Philadelphia, PA, USA, 2015; pp. 1018–1031. [Google Scholar]
- Dolinskaya, I.Y.; Solopova, I.A.; Zhvansky, D.S.; Keshishian, E.S.; Ivanenko, Y. Increasing Muscle Activity Correlations during Spontaneous Movements in the First Six Months of Life. Neurosci. Lett. 2021, 756, 135957. [Google Scholar] [CrossRef]
- de Graaf, M.T.; Samsom, J.F.; Pettersen, E.M.; Schaaf, V.A.M.; van Schie, P.E.M.; de Groot, L. Vestibulospinal Component of Postural Control (Vestibular Function) in Very Preterm Infants (25 to 27 Weeks) at 3, 6, and 12 Months Corrected Age. J. Child Neurol. 2004, 19, 614–618. [Google Scholar] [CrossRef]
- Jensen, J.L.; Thelen, E.; Ulrich, B.D.; Schneider, K.; Zernicke, R.F. Adaptive Dynamics of the Leg Movement Patterns of Human Infants: III. Age-Related Differences in Limb Control. J. Mot. Behav. 1995, 27, 366–374. [Google Scholar] [CrossRef]
- Merletti, R.; Parker, P.J. Electromyography: Physiology, Engineering, and Non-Invasive Applications; IEEE Press Series on Biomedical Engineering; Wiley: Hoboken, NY, USA, 2004. [Google Scholar]
- Mathis, A.; Mamidanna, P.; Cury, K.M.; Abe, T.; Murthy, V.N.; Mathis, M.W.; Bethge, M. DeepLabCut: Markerless Pose Estimation of User-Defined Body Parts with Deep Learning. Nat. Neurosci. 2018, 21, 1281–1289. [Google Scholar] [CrossRef]
- Rudolph, K.S.; Axe, M.J.; Snyder-Mackler, L. Dynamic Stability after ACL Injury: Who Can Hop? Knee Surg. Sports Traumatol. Arthrosc. 2000, 8, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Mari, S.; Serrao, M.; Casali, C.; Conte, C.; Martino, G.; Ranavolo, A.; Coppola, G.; Draicchio, F.; Padua, L.; Sandrini, G.; et al. Lower Limb Antagonist Muscle Co-Activation and Its Relationship with Gait Parameters in Cerebellar Ataxia. Cerebellum 2014, 13, 226–236. [Google Scholar] [CrossRef]
- Martino, G.; Ivanenko, Y.P.; Serrao, M.; Ranavolo, A.; d’Avella, A.; Draicchio, F.; Conte, C.; Casali, C.; Lacquaniti, F. Locomotor Patterns in Cerebellar Ataxia. J. Neurophysiol. 2014, 112, 2810–2821. [Google Scholar] [CrossRef]
- Myklebust, B.M. A Review of Myotatic Reflexes and the Development of Motor Control and Gait in Infants and Children: A Special Communication. Phys. Ther. 1990, 70, 188–203. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Prechtl, H.F. Developmental Course of General Movements in Early Infancy. I. Descriptive Analysis of Change in Form. Early Hum. Dev. 1992, 28, 201–213. [Google Scholar] [CrossRef]
- Hadders-Algra, M.; Klip-Van den Nieuwendijk, A.; Martijn, A.; van Eykern, L.A. Assessment of General Movements: Towards a Better Understanding of a Sensitive Method to Evaluate Brain Function in Young Infants. Dev. Med. Child Neurol. 1997, 39, 88–98. [Google Scholar] [CrossRef]
- Prechtl, H.F.; Hopkins, B. Developmental Transformations of Spontaneous Movements in Early Infancy. Early Hum. Dev. 1986, 14, 233–238. [Google Scholar] [CrossRef]
- Hopkins, B.; Prechtl, H.F.R. A Qualitative Approach to the Development of Movements during Early Infancy. In Continuity of Neural Functions from Prenatal to Postnatal Life; Prechtl, H.F.R., Ed.; Blackwell: Oxford, UK, 1984; pp. 179–197. [Google Scholar]
- Marinelli, L.; Trompetto, C.; Mori, L.; Vigo, G.; Traverso, E.; Colombano, F.; Abbruzzese, G. Manual Linear Movements to Assess Spasticity in a Clinical Setting. PLoS ONE 2013, 8, e53627. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Morgan, C.; Walker, K.; Novak, I. Cerebral Palsy—Don’t Delay. Dev. Disabil. Res. Rev. 2011, 17, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Vecchierini-Blineau, M.F.; Guiheneuc, P. Excitability of the Monosynaptic Reflex Pathway in the Child from Birth to Four Years of Age. J. Neurol. Neurosurg. Psychiatry 1981, 44, 309–314. [Google Scholar] [CrossRef]
- Hakamada, S.; Hayakawa, F.; Kuno, K.; Tanaka, R. Development of the Monosynaptic Reflex Pathway in the Human Spinal Cord. Brain Res. 1988, 470, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Solopova, I.A.; Selionov, V.A.; Blinov, E.O.; Dolinskaya, I.Y.; Zhvansky, D.S.; Lacquaniti, F.; Ivanenko, Y. Higher Responsiveness of Pattern Generation Circuitry to Sensory Stimulation in Healthy Humans Is Associated with a Larger Hoffmann Reflex. Biology 2022, 11, 707. [Google Scholar] [CrossRef]
- Gurfinkel, V.S.; Cacciatore, T.W.; Cordo, P.J.; Horak, F.B. Method to Measure Tone of Axial and Proximal Muscle. J. Vis. Exp. 2011, 58, e3677. [Google Scholar] [CrossRef]
- Safronov, V.A. Some Properties of the Response of Muscle to Passive Shortening. Hum. Physiol. 1984, 10, 294–300. [Google Scholar]
- Mortimer, J.A.; Webster, D.D. Relationships between Quantitative Measures of Rigidity and Tremor and the Electromyographic Responses to Load Perturbations in Unselected Normal Subjects and Parkinson Patients. In Cerebral Motor Control in Man Long Loop Mechanisms. Progress in Clinical Neurophysiology; Desmedt, J.E., Ed.; Karger: Basel, Switzerland, 1978; Volume 4, pp. 342–360. [Google Scholar]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L. Muscle Spindle Activity in Man during Shortening and Lengthening Contractions. J. Physiol. 1978, 277, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, M.S.; Adolph, K.E. Protracted Development of Motor Cortex Constrains Rich Interpretations of Infant Cognition. Trends Cogn. Sci. 2023, 27, 233–245. [Google Scholar] [CrossRef]
- Martin, J.H. The Corticospinal System: From Development to Motor Control. Neuroscientist 2005, 11, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L. The Control and Perception of Antagonist Muscle Action. Exp. Brain Res. 2023, 241, 1–12. [Google Scholar] [CrossRef]
- Sherrington, C. The Integrative Action of the Nervous System; Charles Scribner’s Sons: New York, NY, USA, 1906. [Google Scholar]
- Hadders-Algra, M. Early Human Motor Development: From Variation to the Ability to Vary and Adapt. Neurosci. Biobehav. Rev. 2018, 90, 411–427. [Google Scholar] [CrossRef]
- Piek, J.P.; Gasson, N.; Barrett, N.; Case, I. Limb and Gender Differences in the Development of Coordination in Early Infancy. Hum. Mov. Sci. 2002, 21, 621–639. [Google Scholar] [CrossRef]


| PM_Group 1 (0.5–3 mo) | PM_Group 2 (3–6 mo) | PM_Group 3 (6–9 mo) | PM_Group 4 (9–12 mo) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N Infants | N Movements | N Infants | N Movements | N Infants | N Movements | N Infants | N Movements | ||
| Hip flexion | Full-term | 19 16 | 203 (5.5 ± 1.7) 131 (4.2 ± 1.3) | 25 18 | 241 (5.2 ± 1.2) 175 (5.1 ± 1.3) | 13 12 | 116 (4.5 ± 1.1) 109 (4.9 ± 1.9) | 11 4 | 110 (5.1 ± 1.3) 40 (5.0 ± 1.3) |
| Preterm | |||||||||
| Hip extension | Full-term | 19 16 | 201 (5.5 ± 1.6) 126 (4.1 ± 1.3) | 25 18 | 243 (5.3 ± 1.1) 186 (5.5 ± 1.5) | 13 12 | 114 (4.5 ± 1.3) 107 (4.9 ± 1.7) | 11 4 | 103 (4.9 ± 1.3) 42 (5.2 ± 1.0) |
| Preterm | |||||||||
| Knee flexion | Full-term | 19 16 | 198 (5.0 ± 1.7) 136 (4.3 ± 1.5) | 27 19 | 239 (4.6 ± 1.3) 181 (4.7 ± 1.3) | 13 12 | 100 (4.1 ± 1.2) 95 (4.3 ± 1.5) | 11 4 | 97 (5.0 ± 1.2) 41 (5.1 ± 1.4) |
| Preterm | |||||||||
| Knee extension | Full-term | 19 16 | 195 (5.3 ± 1.8) 138 (4.3 ± 1.5) | 27 19 | 240 (4.8 ± 1.2) 190 (5.0 ± 1.6) | 13 12 | 100 (4.1 ± 1.3) 99 (4.5 ± 1.2) | 11 4 | 92 (4.3 ± 1.2) 41 (5.1 ± 1.3) |
| Preterm | |||||||||
| Dorsiflexion | Full-term | 15 12 | 121 (3.9 ± 1.8) 95 (3.9 ± 1.4) | 22 9 | 223 (5.3 ± 1.9) 68 (4.0 ± 1.3) | 11 7 | 95 (4.3 ± 1.9) 63 (4.5 ± 1.3) | 10 4 | 89 (4.2 ± 1.3) 39 (4.9 ± 0.8) |
| Preterm | |||||||||
| Plantarflexion | Full-term | 15 12 | 126 (4.2 ± 1.6) 105 (4.3 ± 1.4) | 22 9 | 221 (5.2 ± 2.0) 69 (4.1 ± 1.4) | 11 7 | 95 (4.4 ± 2.0) 65 (4.6 ± 1.2) | 10 4 | 91 (4.3 ± 1.2) 43 (5.4 ± 1.2) |
| Preterm | |||||||||
| Elbow flexion | Full-term | 19 15 | 164 (4.4 ± 1.3) 128 (4.3 ± 1.7) | 27 18 | 237 (4.6 ± 1.2) 161 (4.7 ± 1.7) | 13 10 | 113 (4.2 ± 1.2) 91 (4.6 ± 1.4) | 11 4 | 103 (4.7 ± 1.2) 44 (5.5 ± 2.4) |
| Preterm | |||||||||
| Elbow extension | Full-term | 19 15 | 157 (4.1 ± 1.2) 118 (4.1 ± 1.5) | 27 18 | 216 (4.4 ± 1.3) 151 (4.4 ± 1.7) | 13 10 | 108 (4.1 ± 1.0) 81 (4.3 ± 1.3) | 11 4 | 95 (4.3 ± 1.2) 44 (5.5 ± 2.3) |
| Preterm | |||||||||
| SM_Group 1 (0–3 mo) | SM_Group 2 (3–7 mo) | ||
|---|---|---|---|
| N infants | Full-term | 17 | 13 |
| Preterm | 15 | 18 |
| PM_Group 1 (0.5–3 mo) | PM_Group 2 (3–6 mo) | PM_Group 3 (6–9 mo) | PM_Group 4 (9–12 mo) | ||
|---|---|---|---|---|---|
| Hip joint movements—activity in TA, GL | Full-term | 15/19 (79%) | 20/25 (80%) | 6/13 (46%) | 4/11 (36%) |
| Preterm | 14/16 (87%) | 15/18 (83%) | 6/11 (54%) | 1/4 (25%) | |
| Knee joint movements—activity in TA | Full-term | 14/19 (73%) | 19/27 (70%) | 5/13 (38%) | 5/11 (45%) |
| Preterm | 14/15 (93%) | 13/19 (68%) | 6/12 (50%) | 3/4 (75%) | |
| Ankle joint movements—activity in RF, BF | Full-term | 12/15 (80%) | 18/22 (81%) | 3/11 (27%) | 4/10 (40%) |
| Preterm | 12/12 (100%) | 8/9 (89%) | 2/6 (33%) | 1/4 (25%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolinskaya, I.Y.; Solopova, I.A.; Zhvansky, D.S.; Rubeca, D.; Sylos-Labini, F.; Lacquaniti, F.; Ivanenko, Y. Muscle Activity during Passive and Active Movements in Preterm and Full-Term Infants. Biology 2023, 12, 724. https://doi.org/10.3390/biology12050724
Dolinskaya IY, Solopova IA, Zhvansky DS, Rubeca D, Sylos-Labini F, Lacquaniti F, Ivanenko Y. Muscle Activity during Passive and Active Movements in Preterm and Full-Term Infants. Biology. 2023; 12(5):724. https://doi.org/10.3390/biology12050724
Chicago/Turabian StyleDolinskaya, Irina Y., Irina A. Solopova, Dmitry S. Zhvansky, Damiana Rubeca, Francesca Sylos-Labini, Francesco Lacquaniti, and Yury Ivanenko. 2023. "Muscle Activity during Passive and Active Movements in Preterm and Full-Term Infants" Biology 12, no. 5: 724. https://doi.org/10.3390/biology12050724
APA StyleDolinskaya, I. Y., Solopova, I. A., Zhvansky, D. S., Rubeca, D., Sylos-Labini, F., Lacquaniti, F., & Ivanenko, Y. (2023). Muscle Activity during Passive and Active Movements in Preterm and Full-Term Infants. Biology, 12(5), 724. https://doi.org/10.3390/biology12050724






