PI3K/AKT/mTOR Signaling Pathway in HPV-Driven Head and Neck Carcinogenesis: Therapeutic Implications
Abstract
Simple Summary
Abstract
1. Introduction
2. PI3K/Akt/mTOR Activation
2.1. PI3K/Akt/mTOR Activation under Physiological Conditions
2.2. PI3K/Akt/mTOR Activation in Cancer
3. Human Papillomavirus in Head and Neck Carcinogenesis
3.1. HPV Structure and Tropism
3.2. HPV’s Role in Oropharyngeal Cancers
4. PI3K/Akt/mTOR Pathway Activation in HPV-Positive HNCs
4.1. PI3K/Akt/mTOR Activation: Epidemiological Aspects
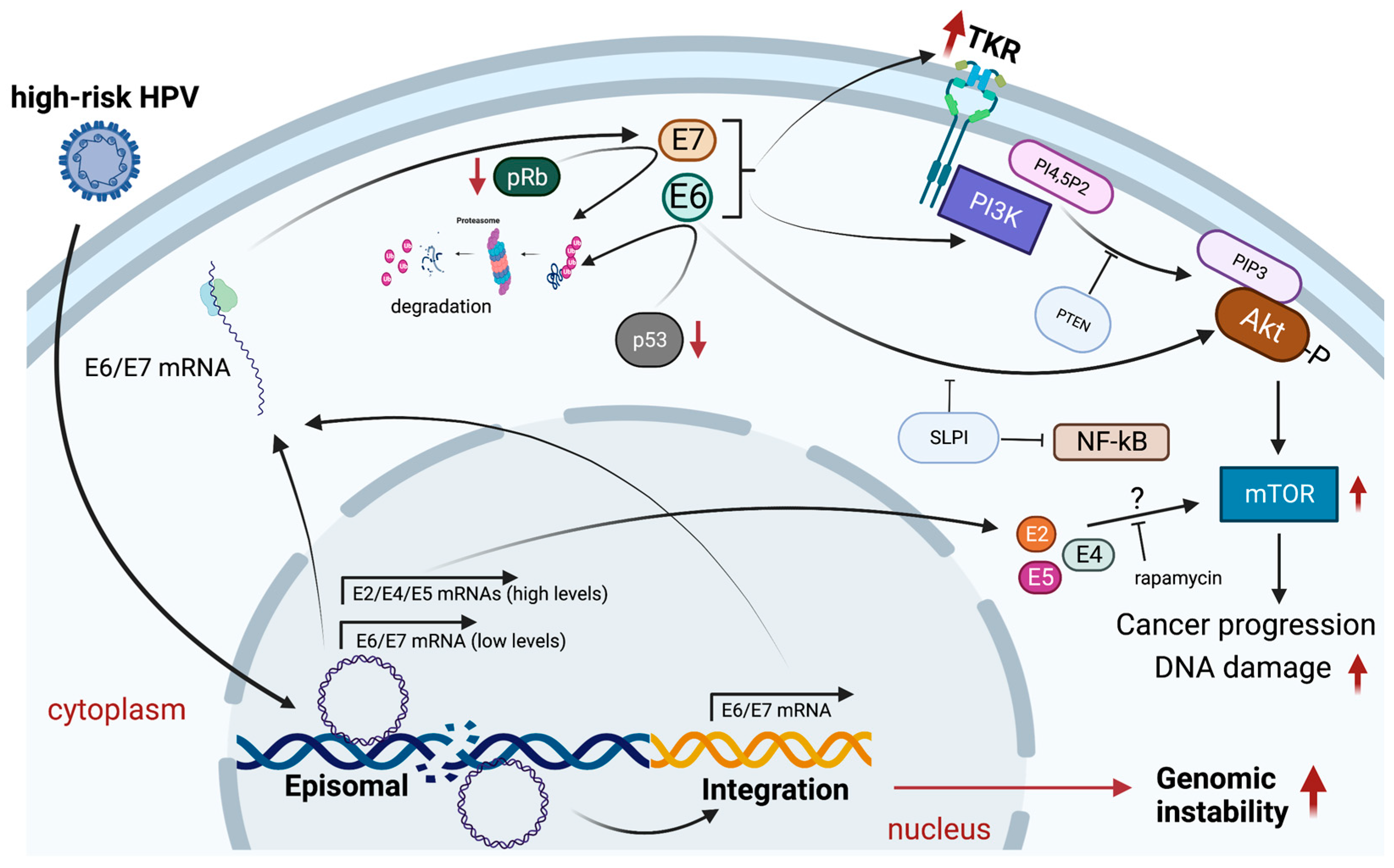
4.2. Mechanisms of PI3K/Akt/mTOR Activation by HPV
5. PI3K/AKT/mTOR Pathway Targeting in HPV-Positive HNSCCs
5.1. HPV Infection in HNC Treatment
5.2. Chemotherapy in HPV-Positive HNCs
6. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, M.S.; Siddiqui, S.A.; Perween, R. Epidemiological profile of head and neck cancer patients in Western Uttar Pradesh and analysis of distributions of risk factors in relation to site of tumor. J. Cancer Res. Ther. 2017, 13, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160270. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, F.; Yin, Y.; Liu, S.; Li, P.; Zhang, X.; Chen, D.; Liu, Y.; Wang, J.; Wang, K.; et al. Prevalence of Human Papillomavirus Type-16 in Head and Neck Cancer Among the Chinese Population: A Meta-Analysis. Front. Oncol. 2018, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Hanker, A.B.; Kaklamani, V.; Arteaga, C.L. Challenges for the Clinical Development of PI3K Inhibitors: Strategies to Improve Their Impact in Solid Tumors. Cancer Discov. 2019, 9, 482–491. [Google Scholar] [CrossRef]
- Marquard, F.E.; Jücker, M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharm. 2020, 172, 113729. [Google Scholar] [CrossRef]
- Isaacsson Velho, P.H.; Castro, G.; Chung, C.H. Targeting the PI3K Pathway in Head and Neck Squamous Cell Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Janecka-Widła, A.; Majchrzyk, K.; Mucha-Małecka, A.; Biesaga, B. EGFR/PI3K/Akt/mTOR pathway in head and neck squamous cell carcinoma patients with different HPV status. Pol. J. Pathol. 2021, 72, 296–314. [Google Scholar] [CrossRef]
- Altomare, D.A.; Khaled, A.R. Homeostasis and the importance for a balance between AKT/mTOR activity and intracellular signaling. Curr. Med. Chem. 2012, 19, 3748–3762. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Costa, C.; Ciraolo, E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J. Endocrinol. 2007, 194, 243–256. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ouyang, G.; Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef]
- Gibbons, J.J.; Abraham, R.T.; Yu, K. Mammalian target of rapamycin: Discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin. Oncol. 2009, 36 (Suppl. S3), S3–S17. [Google Scholar] [CrossRef]
- Bidkhori, G.; Benfeitas, R.; Klevstig, M.; Zhang, C.; Nielsen, J.; Uhlen, M.; Boren, J.; Mardinoglu, A. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc. Natl. Acad. Sci. USA 2018, 115, E11874–E11883. [Google Scholar] [CrossRef]
- Narayanankutty, A. Phytochemicals as PI3K/Akt/mTOR Inhibitors and Their Role in Breast Cancer Treatment. Recent Pat. Anticancer Drug Discov. 2020, 15, 188–199. [Google Scholar] [CrossRef]
- Aziz, A.U.R.; Farid, S.; Qin, K.; Wang, H.; Liu, B. PIM Kinases and Their Relevance to the PI3K/AKT/mTOR Pathway in the Regulation of Ovarian Cancer. Biomolecules 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Khazaei, M.; Hasanzadeh, M.; ShahidSales, S.; Joudi Mashhad, M.; Farazestanian, M.; Sadeghnia, H.R.; Rezayi, M.; Maftouh, M.; Hassanian, S.M.; et al. Therapeutic Potential of Targeting PI3K/AKT Pathway in Treatment of Colorectal Cancer: Rational and Progress. J. Cell. Biochem. 2018, 119, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Quinn, D.I. Differentiating mTOR inhibitors in renal cell carcinoma. Cancer Treat. Rev. 2013, 39, 709–719. [Google Scholar] [CrossRef]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted therapy for head and neck cancer: Signaling pathways and clinical studies. Signal Transduct. Target. 2023, 8, 31. [Google Scholar] [CrossRef]
- Kourea, H.P.; Nikolaou, M.; Tzelepi, V.; Adonakis, G.; Kardamakis, D.; Tsapanos, V.; Scopa, C.D.; Kalofonos, C.; Decavalas, G. Expression of phosphorylated Akt, mTOR and MAPK in type I endometrial carcinoma: Clinical significance. Anticancer Res. 2015, 35, 2321–2331. [Google Scholar] [PubMed]
- Antonuzzo, L.; Del Re, M.; Barucca, V.; Spada, F.; Meoni, G.; Restante, G.; Danesi, R.; Di Costanzo, F.; Fazio, N. Critical focus on mechanisms of resistance and toxicity of m-TOR inhibitors in pancreatic neuroendocrine tumors. Cancer Treat. Rev. 2017, 57, 28–35. [Google Scholar] [CrossRef]
- Gujrati, H.; Ha, S.; Waseem, M.; Wang, B.D. Downregulation of miR-99b-5p and Upregulation of Nuclear mTOR Cooperatively Promotes the Tumor Aggressiveness and Drug Resistance in African American Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 9643. [Google Scholar] [CrossRef]
- Verdugo, E.; Puerto, I.; Medina, M. An update on the molecular biology of glioblastoma, with clinical implications and progress in its treatment. Cancer Commun. 2022, 42, 1083–1111. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Razi, S.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Transl. Oncol. 2022, 18, 101364. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Kumar, S. PI3K/AKT/mTOR pathway in multiple myeloma: From basic biology to clinical promise. Leuk. Lymphoma 2018, 59, 2524–2534. [Google Scholar] [CrossRef]
- Smith, S.M. Targeting mTOR in mantle cell lymphoma: Current and future directions. Best Pract. Res. Clin. Haematol. 2012, 25, 175–183. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Human papillomavirus & cervical cancer. Indian J. Med. Res. 2009, 130, 209. [Google Scholar]
- Ribeiro, A.L.; Caodaglio, A.S.; Sichero, L. Regulation of HPV transcription. Clinics 2018, 73, e486s. [Google Scholar] [CrossRef]
- Sichero, L.; Sobrinho, J.S.; Villa, L.L. Identification of novel cellular transcription factors that regulate early promoters of human papillomavirus types 18 and 16. J. Infect. Dis. 2012, 206, 867–874. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Ouda, A.M.; Elsabagh, A.A.; Elmakaty, I.M.; Gupta, I.; Vranic, S.; Al-Thawadi, H.; Al Moustafa, A.E. HPV and Recurrent Respiratory Papillomatosis: A Brief Review. Life 2021, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Mahal, B.A.; Catalano, P.J.; Haddad, R.I.; Hanna, G.J.; Kass, J.I.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.I.; Alawi, F. Human Papillomavirus and Oropharyngeal Cancer. Dent. Clin. N. Am. 2018, 62, 111–120. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Berman, T.A.; Schiller, J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Newcombe, R.; Fiander, A.; Powell, J.; Rolles, M.; Thavaraj, S.; Robinson, M.; Powell, N. Human Papillomavirus-associated oropharyngeal cancer: An observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer 2013, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Poeta, M.L.; Manola, J.; Goldwasser, M.A.; Forastiere, A.; Benoit, N.; Califano, J.A.; Ridge, J.A.; Goodwin, J.; Kenady, D.; Saunders, J.; et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2007, 357, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Sano, D.; Oridate, N. The molecular mechanism of human papillomavirus-induced carcinogenesis in head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2016, 21, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Mallen-St Clair, J.; Alani, M.; Wang, M.B.; Srivatsan, E.S. Human papillomavirus in oropharyngeal cancer: The changing face of a disease. Biochim. Biophys. Acta 2016, 1866, 141–150. [Google Scholar] [CrossRef]
- Mittal, S.; Banks, L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat. Res. Rev. Mutat. Res. 2017, 772, 23–35. [Google Scholar] [CrossRef]
- Paver, E.C.; Currie, A.M.; Gupta, R.; Dahlstrom, J.E. Human papilloma virus related squamous cell carcinomas of the head and neck: Diagnosis, clinical implications and detection of HPV. Pathology 2020, 52, 179–191. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Chaturvedi, A.K. HPV-associated Oropharyngeal Cancers—Are They Preventable? Cancer Prev. Res. 2011, 4, 1346–1349. [Google Scholar] [CrossRef]
- Gao, G.; Johnson, S.H.; Kasperbauer, J.L.; Eckloff, B.W.; Tombers, N.M.; Vasmatzis, G.; Smith, D.I. Mate pair sequencing of oropharyngeal squamous cell carcinomas reveals that HPV integration occurs much less frequently than in cervical cancer. J. Clin. Virol. 2014, 59, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Olthof, N.C.; Speel, E.J.; Kolligs, J.; Haesevoets, A.; Henfling, M.; Ramaekers, F.C.; Preuss, S.F.; Drebber, U.; Wieland, U.; Silling, S.; et al. Comprehensive analysis of HPV16 integration in OSCC reveals no significant impact of physical status on viral oncogene and virally disrupted human gene expression. PLoS ONE 2014, 9, e88718. [Google Scholar] [CrossRef]
- Morgan, I.M.; DiNardo, L.J.; Windle, B. Integration of Human Papillomavirus Genomes in Head and Neck Cancer: Is It Time to Consider a Paradigm Shift? Viruses 2017, 9, 208. [Google Scholar] [CrossRef]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, M.; Pedamallu, C.S.; Gehlenborg, N.; Freeman, S.S.; Danilova, L.; Bristow, C.A.; Lee, S.; Hadjipanayis, A.G.; Ivanova, E.V.; Wilkerson, M.D.; et al. Characterization of HPV and host genome interactions in primary head and neck cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15544–15549. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, L.J.; Rocha-Zavaleta, L.; Lizano, M.; Ramírez-Alcántara, K.M.; Madrid-Marina, V.; Manzo-Merino, J. Alteration of the IFN-Pathway by Human Papillomavirus Proteins: Antiviral Immune Response Evasion Mechanism. Biomedicines 2022, 10, 2965. [Google Scholar] [CrossRef]
- Lo Cigno, I.; Calati, F.; Albertini, S.; Gariglio, M. Subversion of Host Innate Immunity by Human Papillomavirus Oncoproteins. Pathogens 2020, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A. Regulation of the Innate Immune Response during the Human Papillomavirus Life Cycle. Viruses 2022, 14, 1797. [Google Scholar] [CrossRef]
- Um, S.J.; Rhyu, J.W.; Kim, E.J.; Jeon, K.C.; Hwang, E.S.; Park, J.S. Abrogation of IRF-1 response by high-risk HPV E7 protein in vivo. Cancer Lett. 2002, 179, 205–212. [Google Scholar] [CrossRef]
- Won, H.S.; Jung, C.K.; Chun, S.H.; Kang, J.H.; Kim, Y.S.; Sun, D.I.; Kim, M.S. Difference in expression of EGFR, pAkt, and PTEN between oropharyngeal and oral cavity squamous cell carcinoma. Oral Oncol. 2012, 48, 985–990. [Google Scholar] [CrossRef]
- Yarbrough, W.G.; Whigham, A.; Brown, B.; Roach, M.; Slebos, R. Phosphoinositide kinase-3 status associated with presence or absence of human papillomavirus in head and neck squamous cell carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, S98–S101. [Google Scholar] [CrossRef]
- Martin, D.; Abba, M.C.; Molinolo, A.A.; Vitale-Cross, L.; Wang, Z.; Zaida, M.; Delic, N.C.; Samuels, Y.; Lyons, J.G.; Gutkind, J.S. The head and neck cancer cell oncogenome: A platform for the development of precision molecular therapies. Oncotarget 2014, 5, 8906–8923. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Guthrie, V.B.; Masica, D.L.; Tokheim, C.; Kang, H.; Richmon, J.; Agrawal, N.; Fakhry, C.; Quon, H.; Subramaniam, R.M.; et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann. Oncol. 2015, 26, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.H.; Jung, C.K.; Won, H.S.; Kang, J.H.; Kim, Y.S.; Kim, M.S. Divergence of P53, PTEN, PI3K, Akt and mTOR expression in tonsillar cancer. Head Neck 2015, 37, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Dubot, C.; Bernard, V.; Sablin, M.P.; Vacher, S.; Chemlali, W.; Schnitzler, A.; Pierron, G.; Ait Rais, K.; Bessoltane, N.; Jeannot, E.; et al. Comprehensive genomic profiling of head and neck squamous cell carcinoma reveals FGFR1 amplifications and tumour genomic alterations burden as prognostic biomarkers of survival. Eur. J. Cancer 2018, 91, 47–55. [Google Scholar] [CrossRef]
- Kiessling, S.Y.; Broglie, M.A.; Soltermann, A.; Huber, G.F.; Stoeckli, S.J. Comparison of PI3K Pathway in HPV-Associated Oropharyngeal Cancer with and without Tobacco Exposure. Laryngoscope Investig. Otolaryngol. 2018, 3, 283–289. [Google Scholar] [CrossRef]
- Khanna, S.; Palackdharry, S.; Roof, L.; Wicker, C.A.; Mark, J.; Zhu, Z.; Jandorav, R.; Molinolo, A.; Takiar, V.; Wise-Draper, T.M. Determining the molecular landscape and impact on prognosis in HPV-associated head and neck cancer. Cancers Head Neck 2020, 5, 11. [Google Scholar] [CrossRef]
- Moura, A.C.; Assad, D.X.; Amorim Dos Santos, J.; Porto de Toledo, I.; Barra, G.B.; Castilho, R.M.; Squarize, C.H.; Guerra, E.N.S. Worldwide prevalence of PI3K-AKT-mTOR pathway mutations in head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 160, 103284. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Zuo, Z.; Keck, M.K.; Khattri, A.; Pedamallu, C.S.; Stricker, T.; Brown, C.; Pugh, T.J.; Stojanov, P.; Cho, J.; et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 2015, 21, 632–641. [Google Scholar] [CrossRef]
- Nichols, A.C.; Palma, D.A.; Chow, W.; Tan, S.; Rajakumar, C.; Rizzo, G.; Fung, K.; Kwan, K.; Wehrli, B.; Winquist, E.; et al. High frequency of activating PIK3CA mutations in human papillomavirus-positive oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 617–622. [Google Scholar] [CrossRef]
- Lechner, M.; Frampton, G.M.; Fenton, T.; Feber, A.; Palmer, G.; Jay, A.; Pillay, N.; Forster, M.; Cronin, M.T.; Lipson, D.; et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV− tumors. Genome Med. 2013, 5, 49. [Google Scholar] [CrossRef]
- Lui, V.W.; Hedberg, M.L.; Li, H.; Vangara, B.S.; Pendleton, K.; Zeng, Y.; Lu, Y.; Zhang, Q.; Du, Y.; Gilbert, B.R.; et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013, 3, 761–769. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.; Borsu, L.; Ghossein, R.; Katabi, N.; Ganly, I.; Dogan, S. A proportion of primary squamous cell carcinomas of the parotid gland harbour high-risk human papillomavirus. Histopathology 2016, 69, 921–929. [Google Scholar] [CrossRef]
- Hanna, G.J.; Kacew, A.; Chau, N.G.; Shivdasani, P.; Lorch, J.H.; Uppaluri, R.; Haddad, R.I.; MacConaill, L.E. Improved outcomes in PI3K-pathway-altered metastatic HPV oropharyngeal cancer. JCI Insight 2018, 3, e122799. [Google Scholar] [CrossRef]
- Chiosea, S.I.; Grandis, J.R.; Lui, V.W.; Diergaarde, B.; Maxwell, J.H.; Ferris, R.L.; Kim, S.W.; Luvison, A.; Miller, M.; Nikiforova, M.N. PIK3CA, HRAS and PTEN in human papillomavirus positive oropharyngeal squamous cell carcinoma. BMC Cancer 2013, 13, 602. [Google Scholar] [CrossRef]
- Beaty, B.T.; Moon, D.H.; Shen, C.J.; Amdur, R.J.; Weiss, J.; Grilley-Olson, J.; Patel, S.; Zanation, A.; Hackman, T.G.; Thorp, B.; et al. PIK3CA Mutation in HPV-Associated OPSCC Patients Receiving Deintensified Chemoradiation. J. Natl. Cancer Inst. 2020, 112, 855–858. [Google Scholar] [CrossRef]
- Islam, M.R.; Ellis, I.R.; Macluskey, M.; Cochrane, L.; Jones, S.J. Activation of Akt at T308 and S473 in alcohol, tobacco and HPV-induced HNSCC: Is there evidence to support a prognostic or diagnostic role? Exp. Hematol. Oncol. 2014, 3, 25. [Google Scholar] [CrossRef]
- Brizel, D.M.; Sibley, G.S.; Prosnitz, L.R.; Scher, R.L.; Dewhirst, M.W. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 285–289. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Busk, M.; Olthof, N.; Speel, E.J.; Horsman, M.R.; Alsner, J.; Overgaard, J. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother. Oncol. 2013, 108, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G.; Hanna, A.; Recknagel, J.; Pruetz, B.L.; Baschnagel, A.M.; Wilson, G.D. Prognostic significance of MTOR expression in HPV positive and negative head and neck cancers treated by chemoradiation. Head Neck 2020, 42, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Charette, S.T.; McCance, D.J. The E7 protein from human papillomavirus type 16 enhances keratinocyte migration in an Akt-dependent manner. Oncogene 2007, 26, 7386–7390. [Google Scholar] [CrossRef]
- Menges, C.W.; Baglia, L.A.; Lapoint, R.; McCance, D.J. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res. 2006, 66, 5555–5559. [Google Scholar] [CrossRef]
- Spangle, J.M.; Münger, K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J. Virol. 2010, 84, 9398–9407. [Google Scholar] [CrossRef]
- Molinolo, A.A.; Marsh, C.; El Dinali, M.; Gangane, N.; Jennison, K.; Hewitt, S.; Patel, V.; Seiwert, T.Y.; Gutkind, J.S. mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin. Cancer Res. 2012, 18, 2558–2568. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Valera, J.L.; Iglesias-Bartolome, R.; Amornphimoltham, P.; Palacios-Garcia, J.; Martin, D.; Califano, J.A.; Molinolo, A.A.; Gutkind, J.S. mTOR inhibition prevents rapid-onset of carcinogen-induced malignancies in a novel inducible HPV-16 E6/E7 mouse model. Carcinogenesis 2016, 37, 1014–1025. [Google Scholar] [CrossRef]
- Pollock, N.I.; Wang, L.; Wallweber, G.; Gooding, W.E.; Huang, W.; Chenna, A.; Winslow, J.; Sen, M.; DeGrave, K.A.; Li, H.; et al. Increased Expression of HER2, HER3, and HER2:HER3 Heterodimers in HPV-Positive HNSCC Using a Novel Proximity-Based Assay: Implications for Targeted Therapies. Clin. Cancer Res. 2015, 21, 4597–4606. [Google Scholar] [CrossRef]
- Brand, T.M.; Hartmann, S.; Bhola, N.E.; Peyser, N.D.; Li, H.; Zeng, Y.; Isaacson Wechsler, E.; Ranall, M.V.; Bandyopadhyay, S.; Duvvuri, U.; et al. Human Papillomavirus Regulates HER3 Expression in Head and Neck Cancer: Implications for Targeted HER3 Therapy in HPV. Clin. Cancer Res. 2017, 23, 3072–3083. [Google Scholar] [CrossRef]
- Brand, T.M.; Hartmann, S.; Bhola, N.E.; Li, H.; Zeng, Y.; O’Keefe, R.A.; Ranall, M.V.; Bandyopadhyay, S.; Soucheray, M.; Krogan, N.J.; et al. Cross-talk Signaling between HER3 and HPV16 E6 and E7 Mediates Resistance to PI3K Inhibitors in Head and Neck Cancer. Cancer Res. 2018, 78, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Bossler, F.; Kuhn, B.J.; Günther, T.; Kraemer, S.J.; Khalkar, P.; Adrian, S.; Lohrey, C.; Holzer, A.; Shimobayashi, M.; Dürst, M.; et al. Repression of Human Papillomavirus Oncogene Expression under Hypoxia Is Mediated by PI3K/mTORC2/AKT Signaling. mBio 2019, 10, e02323-18. [Google Scholar] [CrossRef] [PubMed]
- Stegeman, H.; Kaanders, J.H.; Wheeler, D.L.; van der Kogel, A.J.; Verheijen, M.M.; Waaijer, S.J.; Iida, M.; Grénman, R.; Span, P.N.; Bussink, J. Activation of AKT by hypoxia: A potential target for hypoxic tumors of the head and neck. BMC Cancer 2012, 12, 463. [Google Scholar] [CrossRef]
- Göttgens, E.L.; Bussink, J.; Ansems, M.; Hammond, E.M.; Span, P.N. AKT inhibition as a strategy for targeting hypoxic HPV-positive HNSCC. Radiother. Oncol. 2020, 149, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, Y.; Wang, X.; Yang, Y. Secretory leukocyte protease inhibitor suppresses HPV E6-expressing HNSCC progression by mediating NF-κB and Akt pathways. Cancer Cell Int. 2019, 19, 220. [Google Scholar] [CrossRef]
- Quabius, E.S.; Görögh, T.; Fischer, G.S.; Hoffmann, A.S.; Gebhard, M.; Evert, M.; Beule, A.; Maune, S.; Knecht, R.; Óvári, A.; et al. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett. 2015, 357, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Gaykalova, D.A.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Tamayo, P.; Callejas-Valera, J.L.; Allevato, M.; Gilardi, M.; Santos, J.; et al. HPV E2, E4, E5 drive alternative carcinogenic pathways in HPV positive cancers. Oncogene 2020, 39, 6327–6339. [Google Scholar] [CrossRef]
- Gardiol, D.; Kühne, C.; Glaunsinger, B.; Lee, S.S.; Javier, R.; Banks, L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 1999, 18, 5487–5496. [Google Scholar] [CrossRef]
- Contreras-Paredes, A.; De la Cruz-Hernández, E.; Martínez-Ramírez, I.; Dueñas-González, A.; Lizano, M. E6 variants of human papillomavirus 18 differentially modulate the protein kinase B/phosphatidylinositol 3-kinase (akt/PI3K) signaling pathway. Virology 2009, 383, 78–85. [Google Scholar] [CrossRef]
- Wu, D.M.; Deng, S.H.; Zhou, J.; Han, R.; Liu, T.; Zhang, T.; Li, J.; Chen, J.P.; Xu, Y. PLEK2 mediates metastasis and vascular invasion via the ubiquitin-dependent degradation of SHIP2 in non-small cell lung cancer. Int. J. Cancer 2020, 146, 2563–2575. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Tian, Q.; Huang, R.; Wang, H.; Wang, X.; Han, F. Expression and prognostic potential of PLEK2 in head and neck squamous cell carcinoma based on bioinformatics analysis. Cancer Med. 2021, 10, 6515–6533. [Google Scholar] [CrossRef]
- Hamaguchi, N.; Ihara, S.; Ohdaira, T.; Nagano, H.; Iwamatsu, A.; Tachikawa, H.; Fukui, Y. Pleckstrin-2 selectively interacts with phosphatidylinositol 3-kinase lipid products and regu.u.ulates actin organization and cell spreading. Biochem. Biophys. Res. Commun. 2007, 361, 270–275. [Google Scholar] [CrossRef]
- Bach, T.L.; Kerr, W.T.; Wang, Y.; Bauman, E.M.; Kine, P.; Whiteman, E.L.; Morgan, R.S.; Williamson, E.K.; Ostap, E.M.; Burkhardt, J.K.; et al. PI3K regulates pleckstrin-2 in T-cell cytoskeletal reorganization. Blood 2007, 109, 1147–1155. [Google Scholar] [CrossRef]
- Guo, T.; Sakai, A.; Afsari, B.; Considine, M.; Danilova, L.; Favorov, A.V.; Yegnasubramanian, S.; Kelley, D.Z.; Flam, E.; Ha, P.K.; et al. A Novel Functional Splice Variant of. Cancer Res. 2017, 77, 5248–5258. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, C.; Denaro, N.; Merlotti, A.; Merlano, M. Head and neck cancer: Improving outcomes with a multidisciplinary approach. Cancer Manag. Res. 2017, 9, 363–371. [Google Scholar] [CrossRef]
- Dok, R.; Bamps, M.; Glorieux, M.; Zhao, P.; Sablina, A.; Nuyts, S. Radiosensitization approaches for HPV-positive and HPV-negative head and neck squamous carcinomas. Int. J. Cancer 2020, 146, 1075–1085. [Google Scholar] [CrossRef]
- Özcan-Wahlbrink, M.; Schifflers, C.; Riemer, A.B. Enhanced Radiation Sensitivity of Human Papillomavirus-Driven Head and Neck Cancer: Focus on Immunological Aspects. Front. Immunol. 2019, 10, 2831. [Google Scholar] [CrossRef]
- Perri, F.; Longo, F.; Caponigro, F.; Sandomenico, F.; Guida, A.; Della Vittoria Scarpati, G.; Ottaiano, A.; Muto, P.; Ionna, F. Management of HPV-Related Squamous Cell Carcinoma of the Head and Neck: Pitfalls and Caveat. Cancers 2020, 12, 975. [Google Scholar] [CrossRef]
- Spiotto, M.T.; Taniguchi, C.M.; Klopp, A.H.; Colbert, L.E.; Lin, S.H.; Wang, L.; Frederick, M.J.; Osman, A.A.; Pickering, C.R.; Frank, S.J. Biology of the Radio- and Chemo-Responsiveness in HPV Malignancies. Semin. Radiat. Oncol. 2021, 31, 274–285. [Google Scholar] [CrossRef]
- Kimple, R.J.; Smith, M.A.; Blitzer, G.C.; Torres, A.D.; Martin, J.A.; Yang, R.Z.; Peet, C.R.; Lorenz, L.D.; Nickel, K.P.; Klingelhutz, A.J.; et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013, 73, 4791–4800. [Google Scholar] [CrossRef]
- Tinhofer, I.; Budach, V.; Saki, M.; Konschak, R.; Niehr, F.; Jöhrens, K.; Weichert, W.; Linge, A.; Lohaus, F.; Krause, M.; et al. Targeted next-generation sequencing of locally advanced squamous cell carcinomas of the head and neck reveals druggable targets for improving adjuvant chemoradiation. Eur. J. Cancer 2016, 57, 78–86. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Q.; Zhang, Y.; Zhang, Q.; Zheng, Z.; Liu, S.; Liu, Z.; Meng, L.; Xin, Y.; Jiang, X. Immunotherapy Advances in Locally Advanced and Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma and Its Relationship with Human Papillomavirus. Front. Immunol. 2021, 12, 652054. [Google Scholar] [CrossRef]
- Galvis, M.M.; Borges, G.A.; Oliveira, T.B.; Toledo, I.P.; Castilho, R.M.; Guerra, E.N.S.; Kowalski, L.P.; Squarize, C.H. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 150, 102966. [Google Scholar] [CrossRef]
- Julian, R.; Savani, M.; Bauman, J.E. Immunotherapy Approaches in HPV-Associated Head and Neck Cancer. Cancers 2021, 13, 5889. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.W.; Jiang, J.; Pang, X.; Huang, M.C.; Tang, Y.J.; Liang, X.H.; Tang, Y.L. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Carper, M.B.; Troutman, S.; Wagner, B.L.; Byrd, K.M.; Selitsky, S.R.; Parag-Sharma, K.; Henry, E.C.; Li, W.; Parker, J.S.; Montgomery, S.A.; et al. An Immunocompetent Mouse Model of HPV16(+) Head and Neck Squamous Cell Carcinoma. Cell Rep. 2019, 29, 1660–1674.e1667. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Chapman, C.M.; Sun, X.; Roschewski, M.; Aue, G.; Farooqui, M.; Stennett, L.; Gibellini, F.; Arthur, D.; Pérez-Galán, P.; Wiestner, A. ON 01910.Na is selectively cytotoxic for chronic lymphocytic leukemia cells through a dual mechanism of action involving PI3K/AKT inhibition and induction of oxidative stress. Clin. Cancer Res. 2012, 18, 1979–1991. [Google Scholar] [CrossRef]
- Anderson, R.T.; Keysar, S.B.; Bowles, D.W.; Glogowska, M.J.; Astling, D.P.; Morton, J.J.; Le, P.; Umpierrez, A.; Eagles-Soukup, J.; Gan, G.N.; et al. The dual pathway inhibitor rigosertib is effective in direct patient tumor xenografts of head and neck squamous cell carcinomas. Mol. Cancer 2013, 12, 1994–2005. [Google Scholar] [CrossRef]
- Prasad, A.; Khudaynazar, N.; Tantravahi, R.V.; Gillum, A.M.; Hoffman, B.S. ON 01910.Na (rigosertib) inhibits PI3K/Akt pathway and activates oxidative stress signals in head and neck cancer cell lines. Oncotarget 2016, 7, 79388–79400. [Google Scholar] [CrossRef]
- Glorieux, M.; Dok, R.; Nuyts, S. The influence of PI3K inhibition on the radiotherapy response of head and neck cancer cells. Sci. Rep. 2020, 10, 16208. [Google Scholar] [CrossRef]
- Maira, S.M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chène, P.; De Pover, A.; Schoemaker, K.; et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Fokas, E.; Yoshimura, M.; Prevo, R.; Higgins, G.; Hackl, W.; Maira, S.M.; Bernhard, E.J.; McKenna, W.G.; Muschel, R.J. NVP-BEZ235 and NVP-BGT226, dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitors, enhance tumor and endothelial cell radiosensitivity. Radiat. Oncol. 2012, 7, 48. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wei, M.F.; Wang, C.W.; Lee, H.W.; Pan, S.L.; Gao, M.; Kuo, S.H.; Cheng, A.L.; Teng, C.M. Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor is an effective radiosensitizer for colorectal cancer. Cancer Lett. 2015, 357, 582–590. [Google Scholar] [CrossRef]
- Gil del Alcazar, C.R.; Hardebeck, M.C.; Mukherjee, B.; Tomimatsu, N.; Gao, X.; Yan, J.; Xie, X.J.; Bachoo, R.; Li, L.; Habib, A.A.; et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014, 20, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Schötz, U.; Balzer, V.; Brandt, F.W.; Ziemann, F.; Subtil, F.S.B.; Rieckmann, T.; Köcher, S.; Engenhart-Cabillic, R.; Dikomey, E.; Wittig, A.; et al. Dual PI3K/mTOR Inhibitor NVP-BEZ235 Enhances Radiosensitivity of Head and Neck Squamous Cell Carcinoma (HNSCC) Cell Lines Due to Suppressed Double-Strand Break (DSB) Repair by Non-Homologous End Joining. Cancers 2020, 12, 467. [Google Scholar] [CrossRef]
- Holzhauser, S.; Kostopoulou, O.N.; Ohmayer, A.; Lange, B.K.A.; Ramqvist, T.; Andonova, T.; Bersani, C.; Wickström, M.; Dalianis, T. Antitumor effects of FGFR and PI3K inhibitors on human papillomavirus positive and negative tonsillar and base of tongue cancer cell lines. Oncol Lett. 2019, 18, 6249–6260. [Google Scholar] [CrossRef]
- Chong, C.R.; Jänne, P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013, 19, 1389–1400. [Google Scholar] [CrossRef]
- Maiti, G.P.; Mondal, P.; Mukherjee, N.; Ghosh, A.; Ghosh, S.; Dey, S.; Chakrabarty, J.; Roy, A.; Biswas, J.; Roychoudhury, S.; et al. Overexpression of EGFR in head and neck squamous cell carcinoma is associated with inactivation of SH3GL2 and CDC25A genes. PLoS ONE 2013, 8, e63440. [Google Scholar] [CrossRef]
- Ma, W.; Concha-Benavente, F.; Santegoets, S.J.A.M.; Welters, M.J.P.; Ehsan, I.; Ferris, R.L.; van der Burg, S.H. EGFR signaling suppresses type 1 cytokine-induced T-cell attracting chemokine secretion in head and neck cancer. PLoS ONE 2018, 13, e0203402. [Google Scholar] [CrossRef]
- Guix, M.; Faber, A.C.; Wang, S.E.; Olivares, M.G.; Song, Y.; Qu, S.; Rinehart, C.; Seidel, B.; Yee, D.; Arteaga, C.L.; et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J. Clin. Investig. 2008, 118, 2609–2619. [Google Scholar] [CrossRef]
- Keysar, S.B.; Astling, D.P.; Anderson, R.T.; Vogler, B.W.; Bowles, D.W.; Morton, J.J.; Paylor, J.J.; Glogowska, M.J.; Le, P.N.; Eagles-Soukup, J.R.; et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol. Oncol. 2013, 7, 776–790. [Google Scholar] [CrossRef]
- Ihle, N.T.; Paine-Murrieta, G.; Berggren, M.I.; Baker, A.; Tate, W.R.; Wipf, P.; Abraham, R.T.; Kirkpatrick, D.L.; Powis, G. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol. Cancer 2005, 4, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, A.; Shirai, K.; Choi, M.; Laskin, J.; Kochenderfer, M.; Spira, A.; Cline-Burkhardt, V.; Winquist, E.; Hausman, D.; Walker, L.; et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann. Oncol. 2015, 26, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Rowley, B.R.; Bull, C.O.; Schneider, C.; Haegebarth, A.; Schatz, C.A.; Fracasso, P.R.; Wilkie, D.P.; Hentemann, M.; Wilhelm, S.M.; et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol. Cancer 2013, 12, 2319–2330. [Google Scholar] [CrossRef]
- Dreyling, M.; Santoro, A.; Mollica, L.; Leppä, S.; Follows, G.; Lenz, G.; Kim, W.S.; Nagler, A.; Dimou, M.; Demeter, J.; et al. Long-term safety and efficacy of the PI3K inhibitor copanlisib in patients with relapsed or refractory indolent lymphoma: 2-year follow-up of the CHRONOS-1 study. Am. J. Hematol. 2020, 95, 362–371. [Google Scholar] [CrossRef]
- Klinghammer, K.; Politz, O.; Eder, T.; Otto, R.; Raguse, J.D.; Albers, A.; Kaufmann, A.; Tinhofer, I.; Hoffmann, J.; Keller, U.; et al. Combination of copanlisib with cetuximab improves tumor response in cetuximab-resistant patient-derived xenografts of head and neck cancer. Oncotarget 2020, 11, 3688–3697. [Google Scholar] [CrossRef]
- Furet, P.; Guagnano, V.; Fairhurst, R.A.; Imbach-Weese, P.; Bruce, I.; Knapp, M.; Fritsch, C.; Blasco, F.; Blanz, J.; Aichholz, R.; et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg. Med. Chem. Lett. 2013, 23, 3741–3748. [Google Scholar] [CrossRef]
- Elkabets, M.; Pazarentzos, E.; Juric, D.; Sheng, Q.; Pelossof, R.A.; Brook, S.; Benzaken, A.O.; Rodon, J.; Morse, N.; Yan, J.J.; et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 2015, 27, 533–546. [Google Scholar] [CrossRef]
- Graham, D.K.; DeRyckere, D.; Davies, K.D.; Earp, H.S. The TAM family: Phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 2014, 14, 769–785. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, J.; Schumaker, L.; Carter-Cooper, B.; Lapidus, R.G.; Fan, X.; Gaykalova, D.A.; Mehra, R.; Cullen, K.J.; Dan, H. Simultaneously targeting ErbB family kinases and PI3K in HPV-positive head and neck squamous cell carcinoma. Oral Oncol. 2022, 131, 105939. [Google Scholar] [CrossRef]
- Madera, D.; Vitale-Cross, L.; Martin, D.; Schneider, A.; Molinolo, A.A.; Gangane, N.; Carey, T.E.; McHugh, J.B.; Komarck, C.M.; Walline, H.M.; et al. Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3. Cancer Prev. Res. 2015, 8, 197–207. [Google Scholar] [CrossRef]
- Dunn, L.A.; Riaz, N.; Fury, M.G.; McBride, S.M.; Michel, L.; Lee, N.Y.; Sherman, E.J.; Baxi, S.S.; Haque, S.S.; Katabi, N.; et al. A Phase 1b Study of Cetuximab and BYL719 (Alpelisib) Concurrent with Intensity Modulated Radiation Therapy in Stage III-IVB Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Holzhauser, S.; Wild, N.; Zupancic, M.; Ursu, R.G.; Bersani, C.; Näsman, A.; Kostopoulou, O.N.; Dalianis, T. Targeted Therapy with PI3K and FGFR Inhibitors on Human Papillomavirus Positive and Negative Tonsillar and Base of Tongue Cancer Lines with and without Corresponding Mutations. Front. Oncol. 2021, 11, 640490. [Google Scholar] [CrossRef]
- Janku, F.; Wheler, J.J.; Naing, A.; Falchook, G.S.; Hong, D.S.; Stepanek, V.M.; Fu, S.; Piha-Paul, S.A.; Lee, J.J.; Luthra, R.; et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res. 2013, 73, 276–284. [Google Scholar] [CrossRef]
- Huang, J.; Manning, B.D. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem. Soc. Trans. 2009, 37, 217–222. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Morse, N.; Krigsfeld, G.; Gupta, G.; Higginson, D.S.; Lee, N.Y.; Morris, L.; Ganly, I.; Shiao, S.L.; Powell, S.N.; et al. Taselisib (GDC-0032), a Potent β-Sparing Small Molecule Inhibitor of PI3K, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating PIK3CA Alterations. Clin. Cancer Res. 2016, 22, 2009–2019. [Google Scholar] [CrossRef]

| Clinical Trial Number | Project Name | Drug | Target |
|---|---|---|---|
| NCT04252248 | Decitabine Treatment in HPV-Induced Anogenital and Head and Neck Cancer Patients After Radiotherapy or as Novel Late Salvage | Dacogen | DNA-demethylating agents |
| NCT03162224 | Safety and Efficacy of MEDI0457 and Durvalumab in Participants with Human Papilloma Virus (HPV) Associated Recurrent/Metastatic Head and Neck Cancer | Durvalumab | Blocking the action of PD-L1 |
| NCT05122221 | CRTE7A2-01 TCR-T Cell for HPV-16 Positive Advanced Cervical, Anal, or Head and Neck Cancers | Fludarabine + Cyclophosphamide; interleukin-2 | Inhibition of DNA synthesis + Alkylating agents; pro-inflammatory cytokine |
| NCT05108870 | TheraT® Vectors (Vaccines) Combined with Chemotherapy to Treat HPV16 Head and Neck Cancers | Carboplatin; paclitaxel | Binds to EGFR; targets microtubules |
| NCT04534205 | A Clinical Trial Investigating the Safety, Tolerability, and Therapeutic Effects of BNT113 in Combination with Pembrolizumab Versus Pembrolizumab Alone for Patients with a Form of Head and Neck Cancer Positive for Human Papilloma Virus 16 and Expressing the Protein PD-L1 | Pembrolizumab | Blocks a protein called PD-1 |
| NCT05286060 | Trial of the Combination of GX-188E Vaccination, GX-I7 and Pembrolizumab in Patients with Advanced, Resectable HPV Type 16 and/or 18 Positive Head and Neck Cancer | Pembrolizumab | Blocks a protein called PD-1 |
| NCT05280457 | HPV 16-positive and/or HPV 18-positive Recurrent and/or For Patients with Metastatic Head and Neck Cancer to Evaluate GX-188E DNA Vaccination, GX-I7 and Nivolumab Combination Therapy | Nivolumab | Anti-PD1 receptor |
| NCT05678348 | Pyrimethamine as an Inhibitor of NRF2 in HPV-negative Locally Advanced Head and Neck Squamous Cell Carcinoma | Pyrimethamine | Inhibits STAT3 transcriptional activity |
| NCT01721525 | Induction Chemotherapy with Afatinib, Ribavirin, and Weekly Carboplatin/Paclitaxel for Stage IVA/IVB HPV Associated Oropharynx Squamous Cell Cancer (OPSCC) | Afatinib, ribavirin, and carboplatin/paclitaxel | Tyrosine kinase inhibitor; antiviral activity against DNA and RNA viruses; binds to EGFR; targets microtubules |
| NCT02291055 | A Study of ADXS11-001 or MEDI4736 Alone or Combination in Cervical or Human Papillomavirus (HPV)+ Head & Neck Cancer | MEDI4736 | Target PD-L1 |
| NCT01084083 | Induction Chemotherapy Followed by Cetuximab and Radiation in HPV-Associated Resectable Stage III/IV Oropharynx Cancer | Cetuximab, paclitaxel, cisplatin | Binds to EGFR; targets microtubules; binds to the N7 reactive center on purine residues |
| NCT03978689 | A Phase 1 Study in Patients with HPV16+ Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma | Keytruda | Blocks its interaction with PD-L1 and PD-L2 |
| NCT04260126 | Study of PDS0101 and Pembrolizumab Combination I/O in Subjects with HPV16+ Recurrent and/or Metastatic HNSCC | Pembrolizumab | Inhibits PD-1 |
| NCT05541016 | Blood-Based Biomarkers to Inform Treatment and Radiation Therapy Decisions for HPV Associated Oropharyngeal Squamous Cell Head and Neck Cancers-DART 2.0 | Cisplatin | Binds to the N7 reactive center on purine residues |
| NCT05582590 | Autologous T Cells Targeting HPV16 HPV18 & Survivin in Patients With R/R HPV-related Oropharyngeal Cancers | Fludarabine, cyclophosphamide | Inhibition of DNA synthesis, alkylating agents |
| NCT03795610 | Window of Opportunity Study of IPI-549 in Patients with Locally Advanced HPV+ and HPV− Head and Neck Squamous Cell Carcinoma | IPI-549 | Potent inhibitor of PI3K-γ |
| NCT05357898 | Study of SQZ-eAPC-HPV in Patients with HPV16+ Recurrent, Locally Advanced or Metastatic Solid Tumors | Pembrolizumab | Inhibits PD-1 |
| Reference | Project Name | Drug | Target |
|---|---|---|---|
| 35667295 | Simultaneously targeting ErbB family kinases and PI3K in HPV-positive head and neck squamous cell carcinoma | Afatinib; copanlisib | ErbB kinase inhibitor; FDA-approved PI3K inhibitor |
| 32085396 | Dual PI3K/mTOR Inhibitor NVP-BEZ235 Enhances Radiosensitivity of Head and Neck Squamous Cell Carcinoma (HNSCC) Cell Lines Due to Suppressed Double-Strand Break (DSB) Repair by Non-Homologous End Joining | BEZ235 | PI3K/Akt/mTOR inhibitor |
| 31292160 | Metformin Inhibits Progression of Head and Neck Squamous Cell Carcinoma by Acting Directly on Carcinoma-Initiating Cells | Metformin | Activate AMP-activated protein kinase, and inhibited mTOR signaling both in vitro and in vivo |
| 23730210 | Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition | Rapamycin | mTOR inhibitor |
| 32620624 | Tyrosine Kinase Inhibitors and Everolimus Reduce IGF1R Expression in HPV16-positive and -negative Squamous Cell Carcinoma | Everolimus | mTOR inhibitor |
| 35790279 | Targeted Treatment of HPV16-positive and -negative SCC Cells With Small Molecule Tyrosine Kinase Inhibitors and Everolimus Affects MMP2 and MMP14 Expression | Everolimus | mTOR inhibitor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguayo, F.; Perez-Dominguez, F.; Osorio, J.C.; Oliva, C.; Calaf, G.M. PI3K/AKT/mTOR Signaling Pathway in HPV-Driven Head and Neck Carcinogenesis: Therapeutic Implications. Biology 2023, 12, 672. https://doi.org/10.3390/biology12050672
Aguayo F, Perez-Dominguez F, Osorio JC, Oliva C, Calaf GM. PI3K/AKT/mTOR Signaling Pathway in HPV-Driven Head and Neck Carcinogenesis: Therapeutic Implications. Biology. 2023; 12(5):672. https://doi.org/10.3390/biology12050672
Chicago/Turabian StyleAguayo, Francisco, Francisco Perez-Dominguez, Julio C. Osorio, Carolina Oliva, and Gloria M. Calaf. 2023. "PI3K/AKT/mTOR Signaling Pathway in HPV-Driven Head and Neck Carcinogenesis: Therapeutic Implications" Biology 12, no. 5: 672. https://doi.org/10.3390/biology12050672
APA StyleAguayo, F., Perez-Dominguez, F., Osorio, J. C., Oliva, C., & Calaf, G. M. (2023). PI3K/AKT/mTOR Signaling Pathway in HPV-Driven Head and Neck Carcinogenesis: Therapeutic Implications. Biology, 12(5), 672. https://doi.org/10.3390/biology12050672







