Expression Improvement of Recombinant Plasmids of the Interleukin-7 Gene in Chitosan-Derived Nanoparticles and Their Elevation of Mice Immunity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pig Lymphoblasts In Vitro
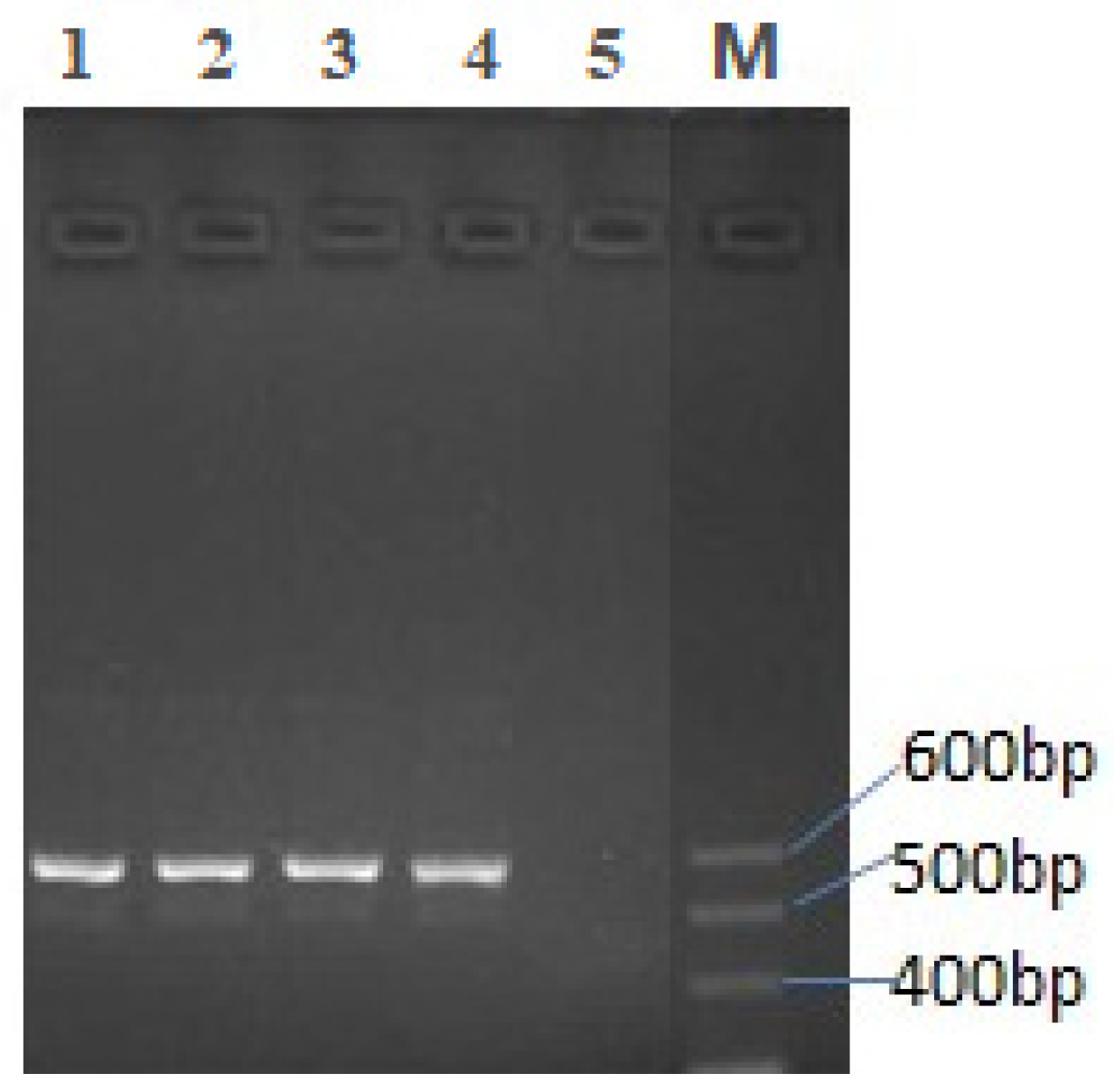
2.2. Cloning & Sequencing of TPIL-7 Gene
2.3. Construction of VTPIL-7 & Preparation of Plasmid Nanoparticles
2.4. Transfection, Expression Identification & Bioactivity Analysis of TPIL-7 Gene
2.5. Mice Inoculation
2.6. Bioactivity Analysis of TPIL-7 In Vivo
2.6.1. Quantity of Blood Cells
2.6.2. Analysis of Immunocompetent T Cells by Flow Cytometry (FCM)
2.6.3. Assay of Mouse IgG1, IgG2a and IgG
2.6.4. Transcription Analysis of Cytokines & TLRs Gene
2.7. Effect of TPIL-7 on Rabies Vaccination
2.7.1. Animal and Vaccination
2.7.2. ELISA Assay of Specific IgG for RABV
2.7.3. Virus Neutralizing Antibody (VNA) Titers Measurement
2.8. Statistical Analysis
3. Results
3.1. Cloning and Sequencing TPIL-7 Gene
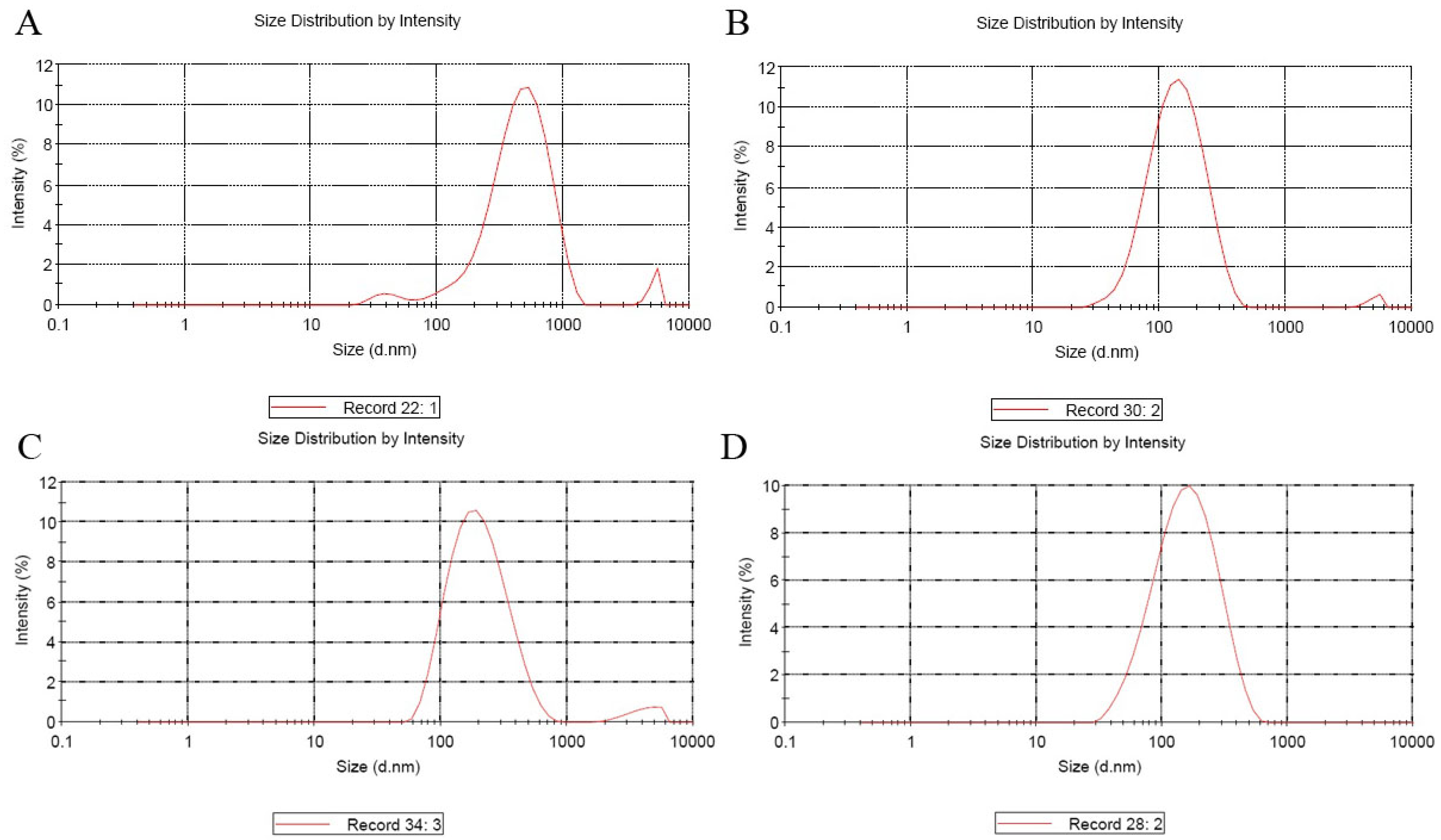
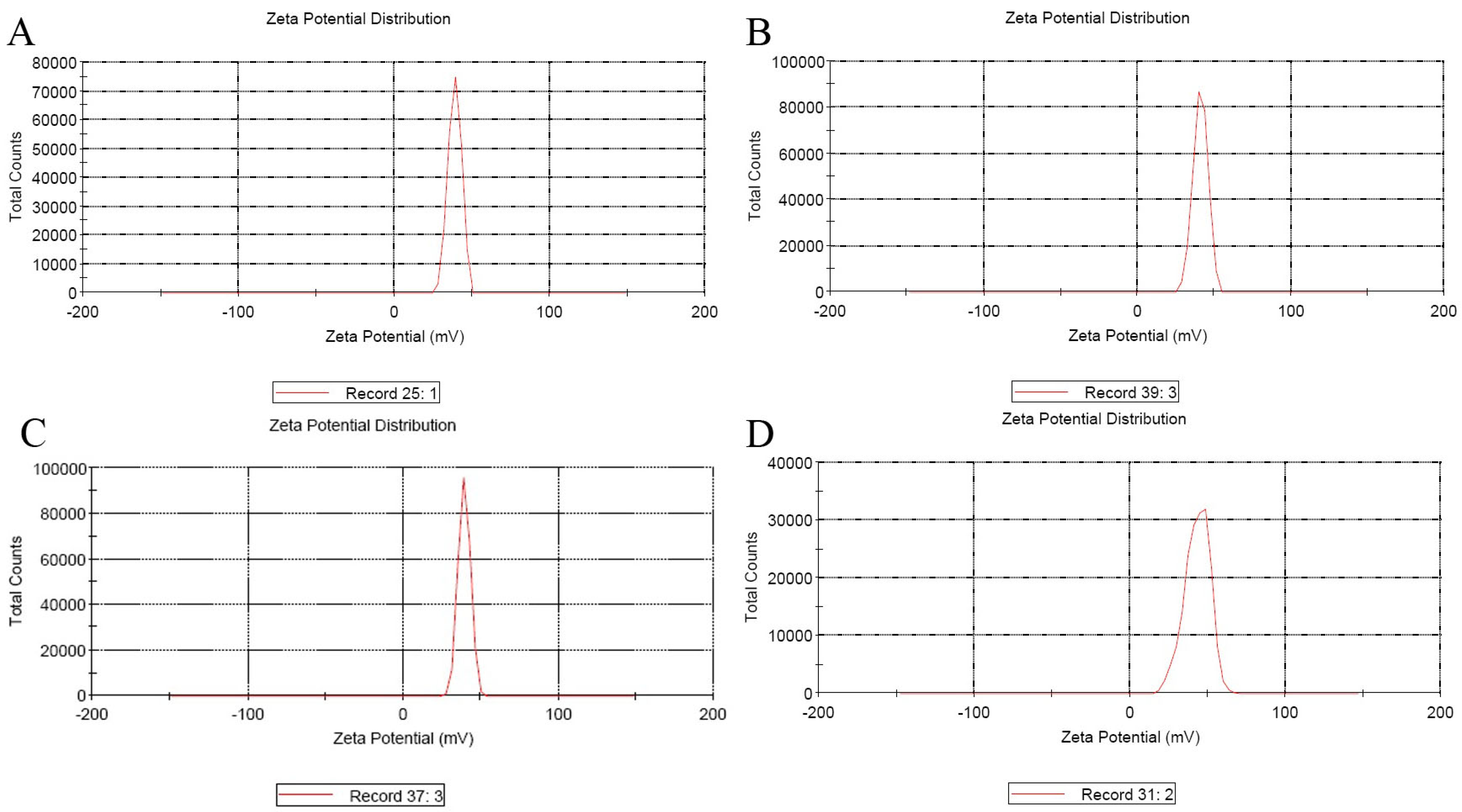
3.2. Nanoparticles Sizes and Zeta Potential
3.3. Transcription Expression and In Vitro Proliferation of Pig Lymphoblasts
3.4. Elevation of Mice Immunity
3.4.1. Immune Changes in the Blood
3.4.2. Change of CD4+ and CD8+ T Lymphocytes
3.4.3. Change of IgG1, IgG2a and IgG
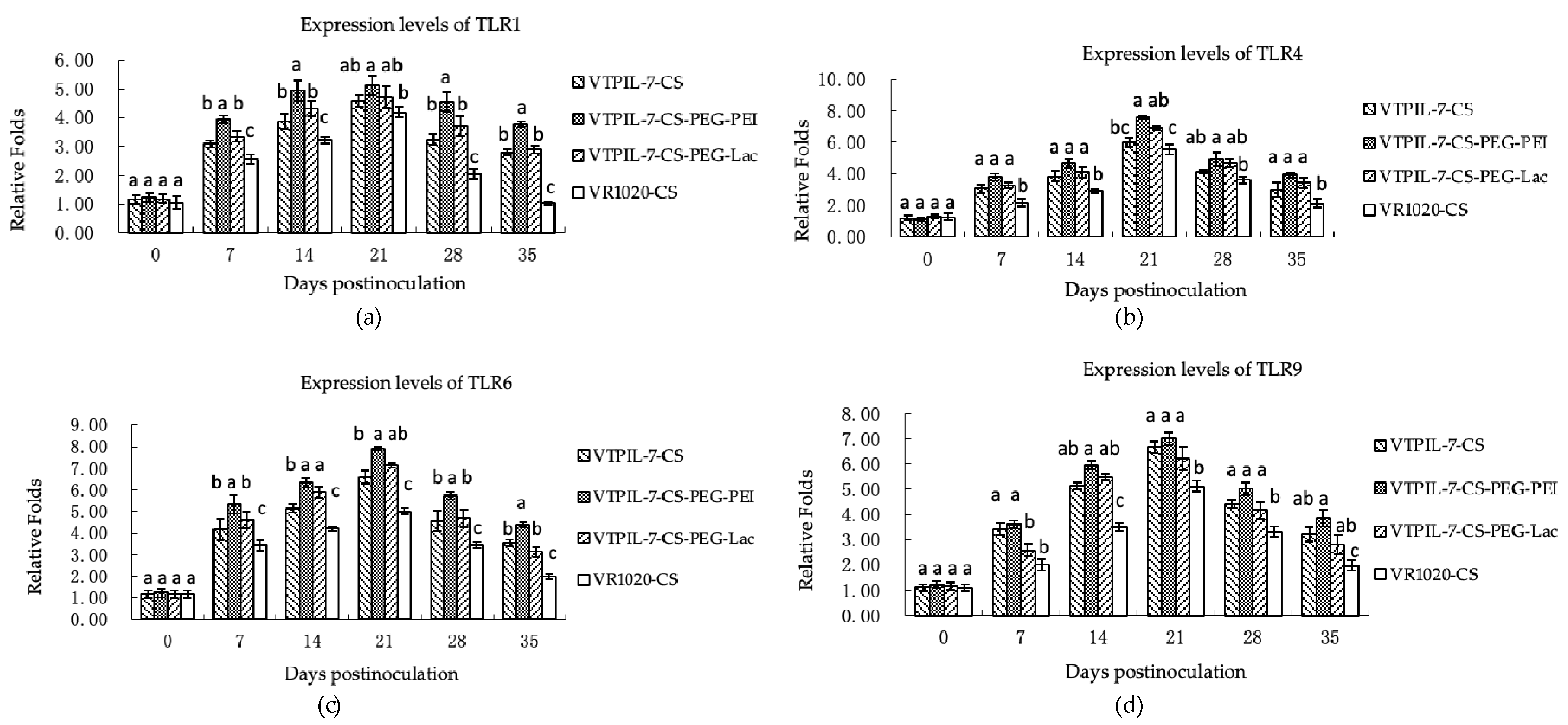
3.4.4. Change of TLRs Gene Expression
3.4.5. Expression Increase of Cytokine Genes
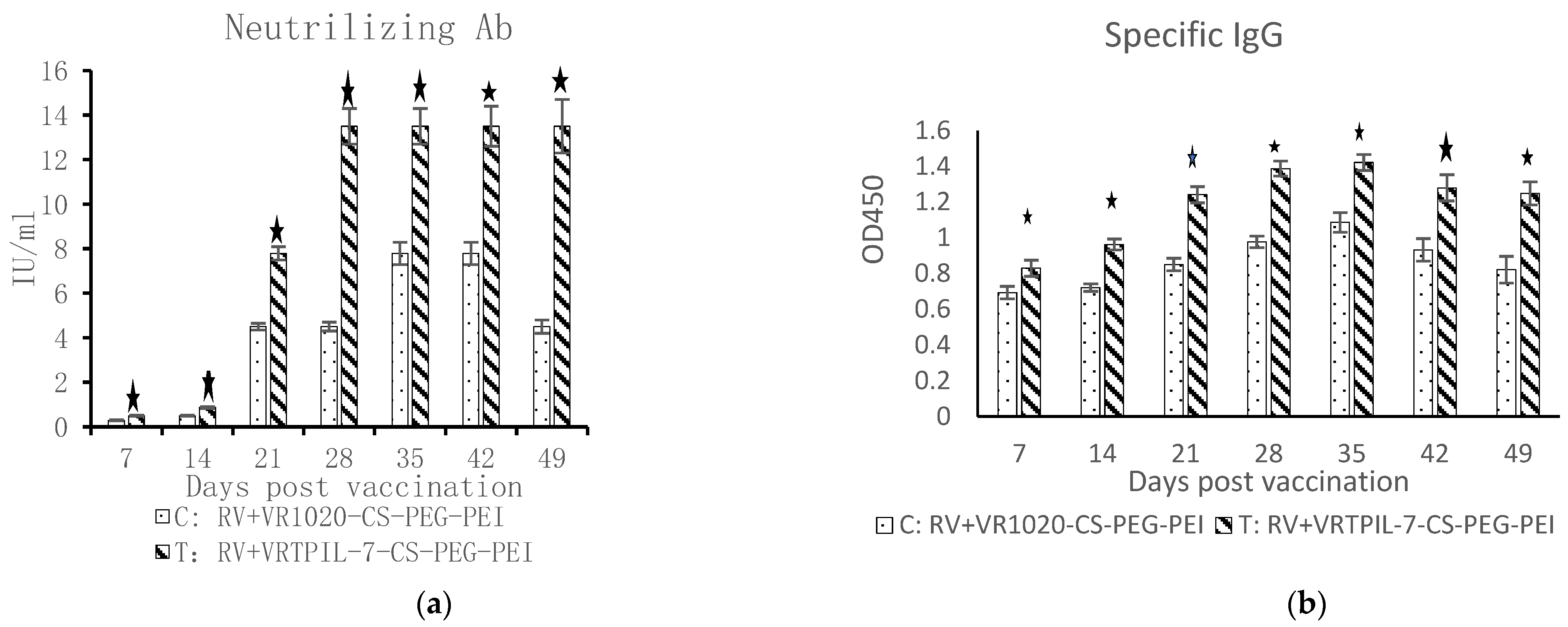
3.5. Regulation on Specific Humoral Immunity to Rabies Vaccination
4. Discussion
4.1. Nanoparticles for Gene Delivery
4.2. The Role of Chitosan and Its Derivatives in Promoting Immunity
4.3. Enhancement of Systemic and Adaptive Immunity by IL-7 Gene Delivery
4.4. Safety and Growth Performance of Animals Treated with Recombinant IL-7 Gene
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmeister, R.; Khaled, A.R.; Benbernou, N.; Rajnavolgyi, E.; Muegge, K.; Durum, S.K. Interleukin-7: Physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999, 10, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Kittipatarin, C.; Khaled, A.R. Interlinking interleukin-7. Cytokine 2007, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Teague, T.K.; Tan, C.; Marino, J.H.; Davis, B.K.; Taylor, A.A.; Huey, R.W.; Van De Wiele, C.J. CD28 expression redefines thymocyte development during the pre-T to DP transition. Int. Immunol. 2010, 22, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Namen, A.E.; Lupton, S.; Hjerrild, K.; Wignall, J.; Mochizuki, D.Y.; Schmierer, A.; Goodwin, R.G. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature 1988, 333, 571–573. [Google Scholar] [CrossRef]
- Corfe, S.A.; Paige, C.J. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2012; Volume 24, pp. 198–208. [Google Scholar]
- Bradley, L.M.; Haynes, L.; Swain, S.L. IL-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005, 26, 172–176. [Google Scholar] [CrossRef]
- Ceredig, R.; Rolink, A.G. The key role of IL-7 in lymphopoiesis. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2012; Volume 24, pp. 159–164. [Google Scholar]
- Dooms, H. Interleukin-7: Fuel for the autoimmune attack. J. Autoimmun. 2013, 45, 40–48. [Google Scholar] [CrossRef]
- Lundström, W.; Fewkes, N.M.; Mackall, C.L. IL-7 in human health and disease. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2012; Volume 24, pp. 218–224. [Google Scholar]
- Mazzucchelli, R.I.; Riva, A.; Durum, S.K. The human IL-7 receptor gene: Deletions, polymorphisms and mutations. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2012; Volume 24, pp. 225–230. [Google Scholar]
- Vonarbourg, C.; Diefenbach, A. Multifaceted roles of interleukin-7 signaling for the development and function of innate lymphoid cells. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2012; Volume 24, pp. 165–174. [Google Scholar]
- Zhang, N.Z.; Zhou, D.H.; Shi, X.C.; Nisbet, A.J.; Huang, S.Y.; Ciren, D.; Wu, S.-W.; Zhu, X.-Q. First report of Chlamydiaceae seroprevalence in Tibetan pigs in Tibet, China. Vector-Borne Zoonotic Dis. 2013, 13, 196–199. [Google Scholar] [CrossRef]
- Qi, S.; Chen, J.; Guo, R.; Yu, B.; Chen, D. β-defensins gene expression in tissues of the crossbred and Tibetan pigs. Livest. Sci. 2009, 123, 161–168. [Google Scholar] [CrossRef]
- Campian, J.L.; Ghosh, S.; Kapoor, V.; Yan, R.; Thotala, S.; Jash, A.; Thotala, D. Long-acting recombinant human interleukin-7, NT-I7, increases cytotoxic CD8+ T cells and enhances survival in mouse glioma models. Clin. Cancer Res. 2022, 28, 1229–1239. [Google Scholar] [CrossRef]
- Fistonich, C.; Zehentmeier, S.; Bednarski, J.J.; Miao, R.; Schjerven, H.; Sleckman, B.P.; Pereira, J.P. Cell circuits between B cell progenitors and IL-7+ mesenchymal progenitor cells control B cell development. J. Exp. Med. 2018, 215, 2586–2599. [Google Scholar] [CrossRef]
- Yu, M.; Chen, Y.; Zeng, H.; Zheng, Y.; Fu, G.; Zhu, W.; Wang, D. PLCγ-dependent mTOR signalling controls IL-7-mediated early B cell development. Nat. Commun. 2017, 8, 1457. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Spasova, D.S.; Frimpong-Boateng, K.; Kim, H.O.; Lee, M.; Kim, K.S.; Surh, C.D. Interleukin-7 availability is maintained by a hematopoietic cytokine sink comprising innate lymphoid cells and T cells. Immunity 2017, 47, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Michelchen, S.; Micheel, B.; Hanack, K. In vitro immunization approach to generate specific murine monoclonal IgG antibodies. J. Immunol. Methods 2021, 499, 113149. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Malik, A.; Gupta, M.; Gupta, V.; Gogoi, H.; Bhatnagar, R. Novel application of trimethyl chitosan as an adjuvant in vaccine delivery. Int. J. Nanomed. 2018, 13, 7959. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Wang, Z.; Cai, L.; Wang, G.; Yang, X.; Wan, X.P.; Xu, X.H.; Li, Y.; Gao, R. Synthesis and characterization of methoxy poly (ethylene glycol)-O-chitosan-polyethylenimine for gene delivery. Carbohydr. Polym. 2010, 81, 269–274. [Google Scholar] [CrossRef]
- Beals, N.; Kasibhatla, N.; Basu, S. Efficient delivery of plasmid DNA using incorporated nucleotides for precise conjugation of targeted nanoparticles. ACS Appl. Bio Mater. 2019, 2, 717–727. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Xu, X.; Guo, T.; Xie, D.; Zhu, R.; He, L. RGD/TAT-functionalized chitosan-graft-PEI-PEG gene nanovector for sustained delivery of NT-3 for potential application in neural regeneration. Acta Biomater. 2018, 72, 266–277. [Google Scholar] [CrossRef]
- Asiri, S.M.; Khan, F.A.; Bozkurt, A. Synthesis of chitosan nanoparticles, chitosan-bulk, chitosan nanoparticles conjugated with glutaraldehyde with strong anti-cancer proliferative capabilities. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S1152–S1161. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Luo, Z.; Zhang, Y.; Cui, M.; Chen, H.; Zhao, L. Overexpression of interleukin-7 extends the humoral immune response induced by rabies vaccination. J. Virol. 2017, 91, e02324-16. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, M.; Baron, B. The role of toll-like receptors in autoimmune diseases through failure of the self-recognition mechanism. Int. J. Inflam. 2017, 2017, 8391230. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, E.A.; Rifkin, I.R.; Hohlbaum, A.M.; Beaudette, B.C.; Shlomchik, M.J.; Marshak-Rothstein, A. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002, 416, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Green, N.M.; Marshak-Rothstein, A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2011; Volume 23, pp. 106–112. [Google Scholar]
- Kirtland, M.E.; Tsitoura, D.C.; Durham, S.R.; Shamji, M.H. Toll-like receptor agonists as adjuvants for allergen immunotherapy. Front. Immunol. 2020, 11, 2951. [Google Scholar] [CrossRef] [PubMed]
- Marton, C.; Patricia, M.L.; Galaine, J.; Godet, Y. An unmet need: Harmonization of IL-7 and IL-15 combination for the ex vivo generation of minimally differentiated T cells. Cell. Immunol. 2021, 363, 104314. [Google Scholar] [CrossRef]
- Park, J.Y.; Won, H.Y.; DiPalma, D.T.; Kim, H.K.; Kim, T.H.; Li, C.; Sato, N.; Hong, C.; Abraham, N.; Gress, R.E.; et al. In vivo availability of the cytokine IL-7 constrains the survival and homeostasis of peripheral iNKT cells. Cell Rep. 2022, 38, 110219. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Kronenberg, M. Going both ways: Immune regulation via CD1d-dependent NKT cells. J. Clin. Investig. 2004, 114, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]






| Genes | Primers (5′−3′) |
|---|---|
| β-actin-F | TACGCCAACACGGTGCTGTC |
| β-actin-R | GTACTCCTGCTTGCTGATCCACAT |
| TLR-1-F | GGACCTACCCTTGCAAACAA |
| TLR-1-R | GGTGGCACAAGATCACCTTT |
| TLR4-F | ACCTGGCTGGTTTACACGTC |
| TLR-4-R | CTGCCAGAGACATTGCAGAA |
| TLR-6-F | CCAAGAACAAAAGCCCTGAG |
| TLR-6-R | TGTTTTGCAACCGATTGTGT |
| TLR9-F | ACTGAGCACCCCTGCTTCTA |
| TLR9-R | AGATTAGTCAGCGGCAGGAA |
| TGF-β-F | GGGAGTAGACAAGGTACAAC |
| TGF-β-R | ACACACAGCCTCAGTT |
| IL-1b-F | TGCTGTCGGACCCAT |
| IL-1b-R | TGTGCCGTCTTTCATTAC |
| IL-2F | AAGCACAGCAGCAGCAGCAG |
| IL-2R | GCCGCAGAGGTCCAAGTTCATC |
| IL-4F | GCCATATCCACGGATGCGACAA |
| IL-4R | GGTGTTCTTCGTTGCTGTGAGGA |
| IL-6F | TCTTGGGACTGATGCTGGTGACA |
| IL-6R | AGCCTCCGACTTGTGAAGTGGTAT |
| IL-7F | TCCCGCAGACCATGTTCCATGTTTC |
| IL-7R | TTCAACTTGCGAGCAGCACGA |
| IL-23-F | TGCTGGATTGCAGAGCAGTAA |
| IL-23-R | GCATGCAGAGATTCCGAGAGA |
| Sample | T (°C) | w/w | Size (d.nm) | Zeta Potential (mV) | ||
|---|---|---|---|---|---|---|
| Average | SD (±) | Average | SD (±) | |||
| CS/DNA | 25 | 30:1 | 522.2 | 5.15 | +40.57 | 1.6 |
| PEI/DNA | 25 | 30:1 | 341 | 5.79 | +45.5 | 2.04 |
| CS-PEG-PEI/DNA | 25 | 10:1 | 169.73 | 4.072 | +43.9 | 1.113 |
| CS-PEG-LAC/DNA | 25 | 10:1 | 247.07 | 18.78 | +40.23 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, W.; Zhang, L.; Chen, J.; Gu, Y.; Lv, X.; Zhang, X.; Li, J.; Liu, H.; Gao, R. Expression Improvement of Recombinant Plasmids of the Interleukin-7 Gene in Chitosan-Derived Nanoparticles and Their Elevation of Mice Immunity. Biology 2023, 12, 667. https://doi.org/10.3390/biology12050667
Hou W, Zhang L, Chen J, Gu Y, Lv X, Zhang X, Li J, Liu H, Gao R. Expression Improvement of Recombinant Plasmids of the Interleukin-7 Gene in Chitosan-Derived Nanoparticles and Their Elevation of Mice Immunity. Biology. 2023; 12(5):667. https://doi.org/10.3390/biology12050667
Chicago/Turabian StyleHou, Wenli, Linhan Zhang, Jianlin Chen, Yiren Gu, Xuebin Lv, Xiuyue Zhang, Jiangling Li, Hui Liu, and Rong Gao. 2023. "Expression Improvement of Recombinant Plasmids of the Interleukin-7 Gene in Chitosan-Derived Nanoparticles and Their Elevation of Mice Immunity" Biology 12, no. 5: 667. https://doi.org/10.3390/biology12050667
APA StyleHou, W., Zhang, L., Chen, J., Gu, Y., Lv, X., Zhang, X., Li, J., Liu, H., & Gao, R. (2023). Expression Improvement of Recombinant Plasmids of the Interleukin-7 Gene in Chitosan-Derived Nanoparticles and Their Elevation of Mice Immunity. Biology, 12(5), 667. https://doi.org/10.3390/biology12050667








