Escape Survival and Scale Damage Assessment of Red Mullet (Mullus barbatus Linnaeus, 1758) during Bottom Trawling in the Central Mediterranean Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
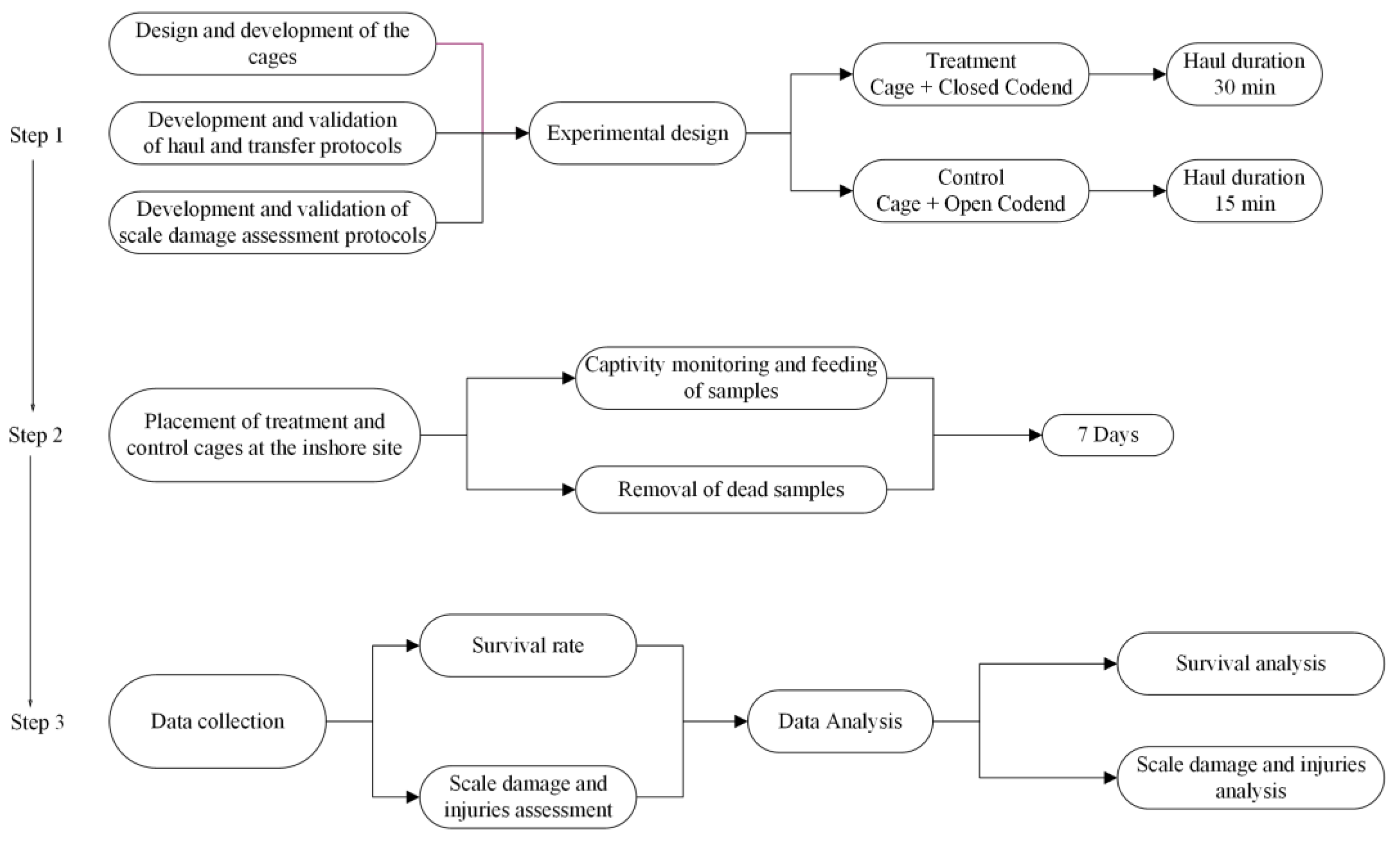
2.2. Overview of the Scientific Approach
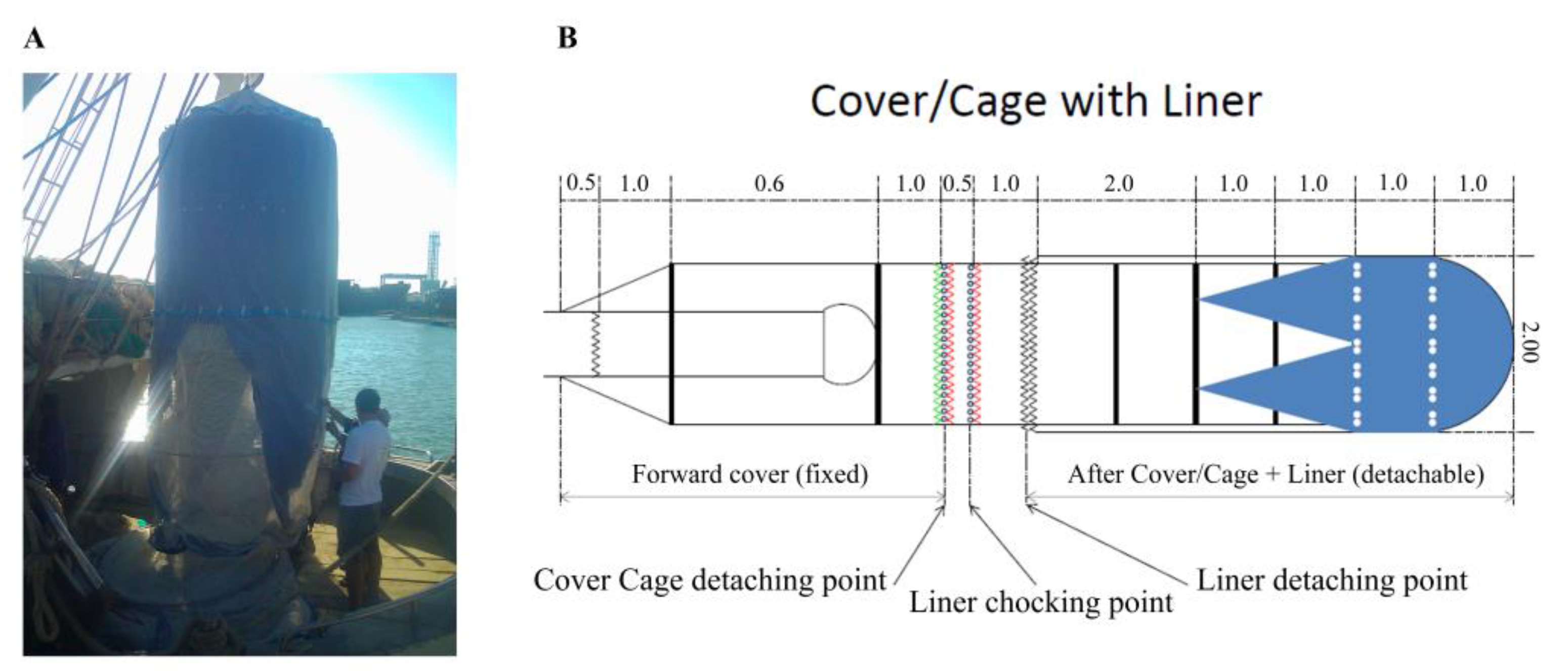
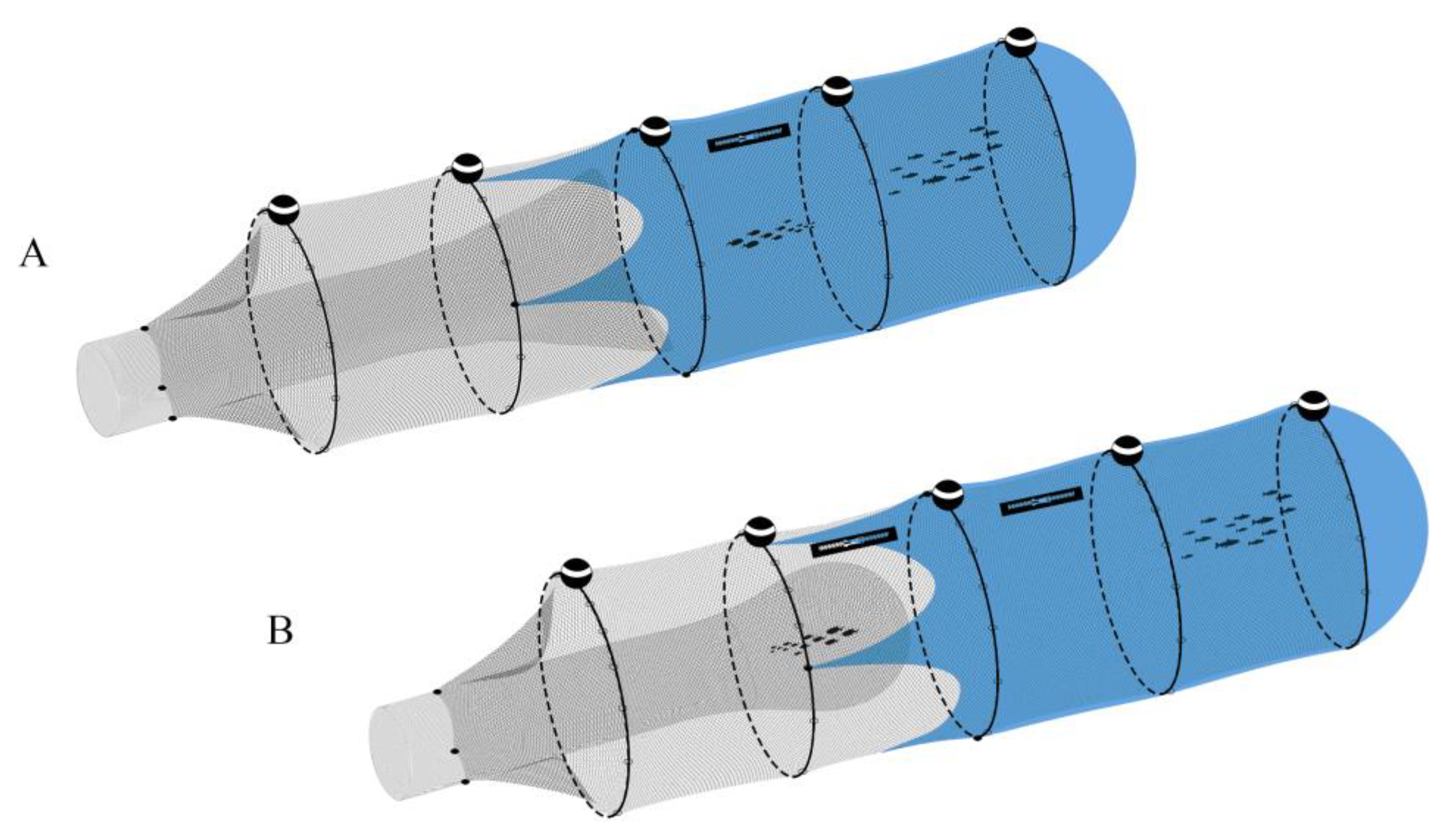
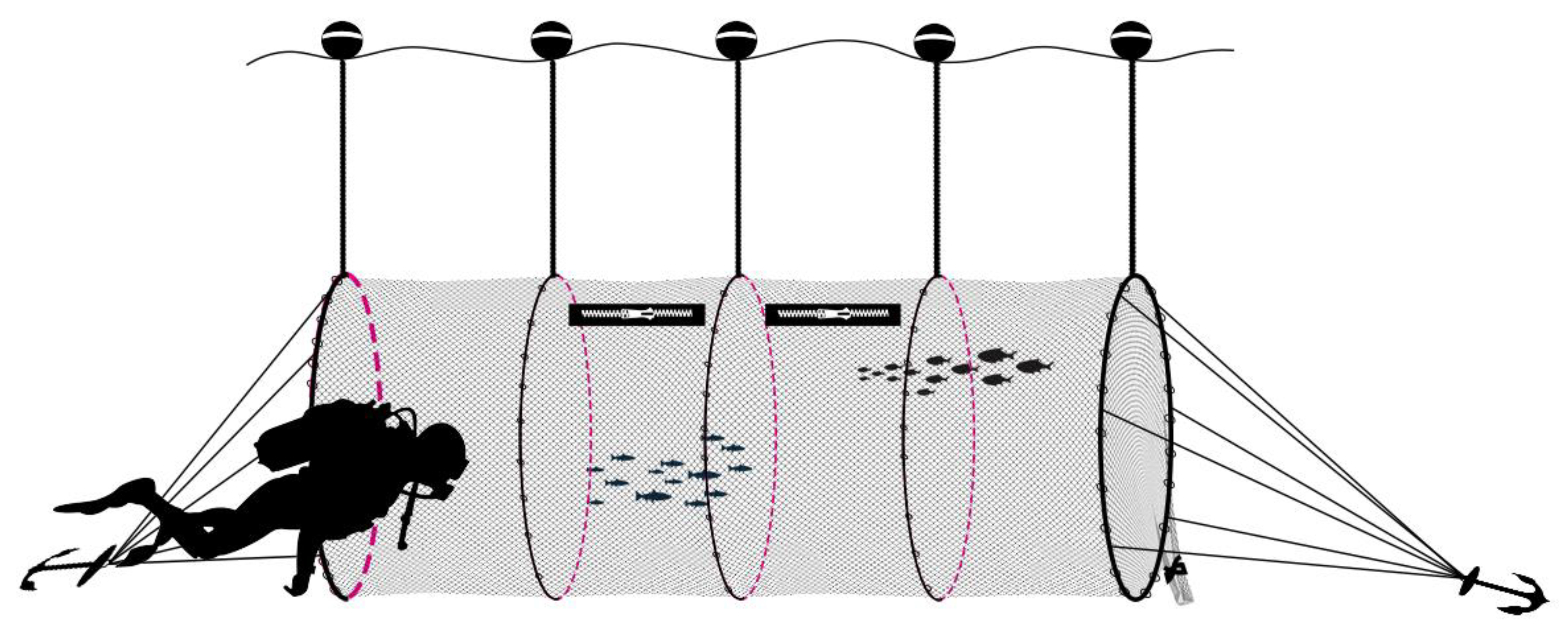
2.3. Cage Description
2.4. Sampling at Sea
2.5. Monitoring of Fish Survival
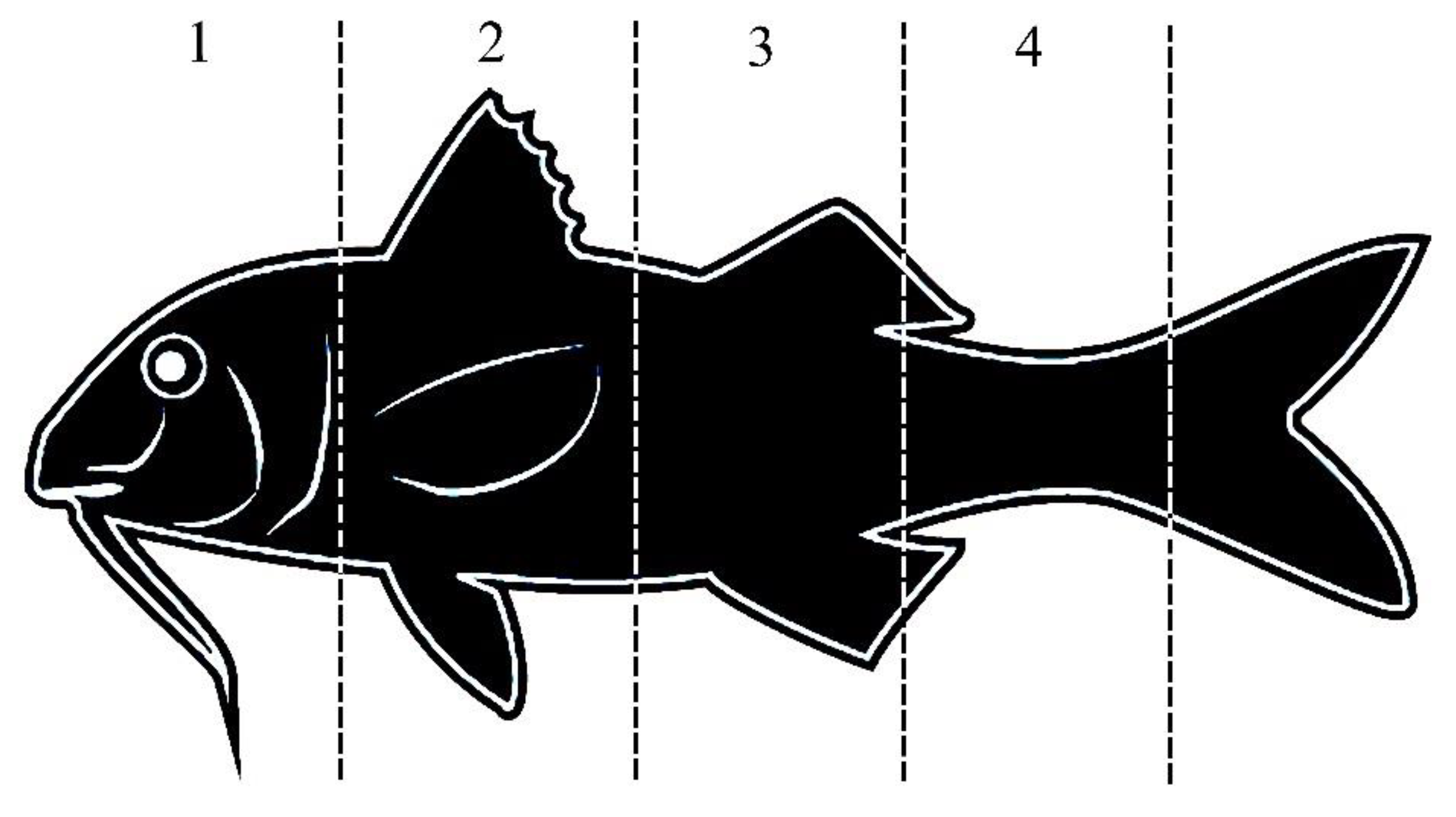
2.6. Scale Damage and Injury Analysis
2.7. Survival Analysis
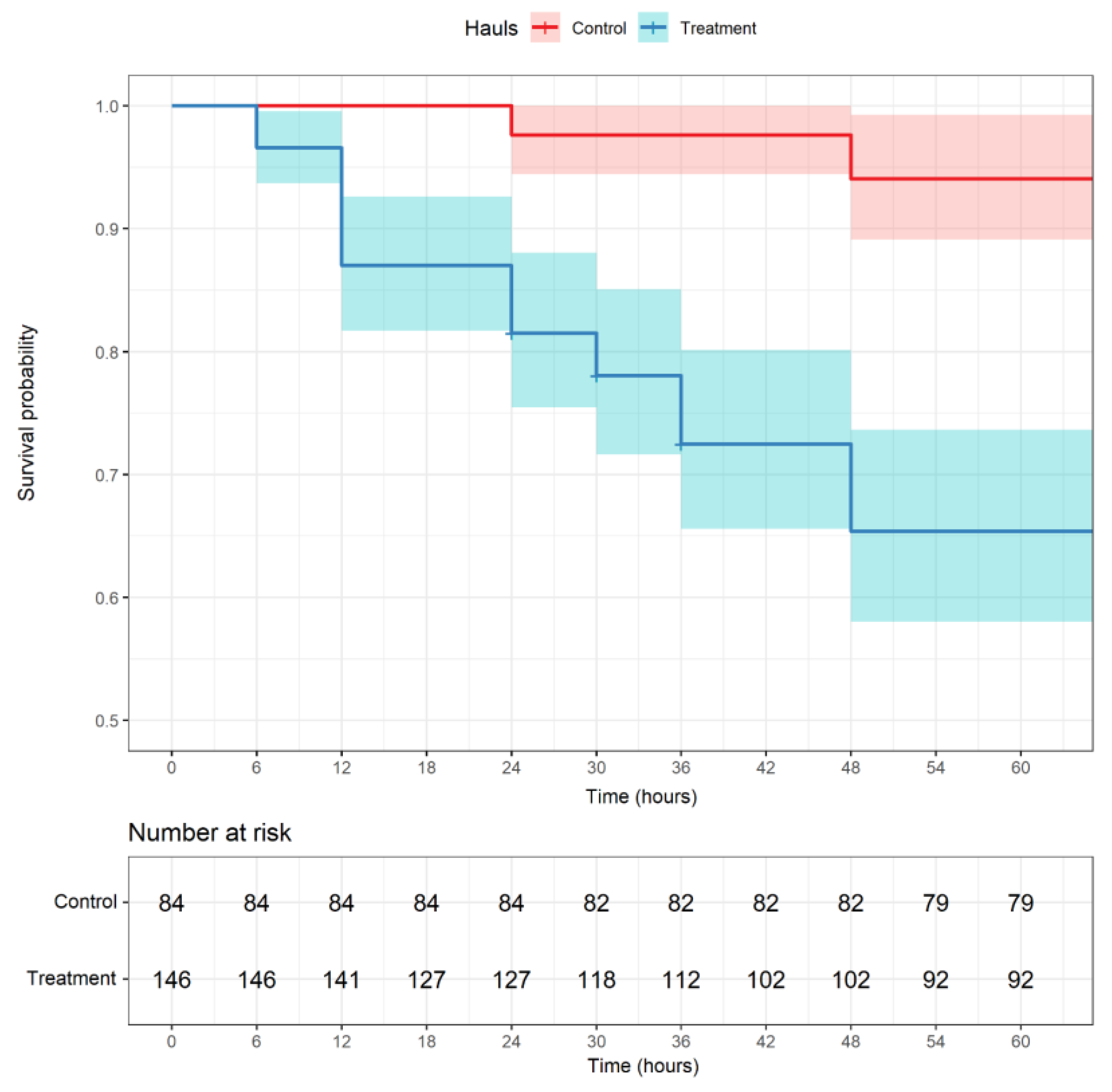
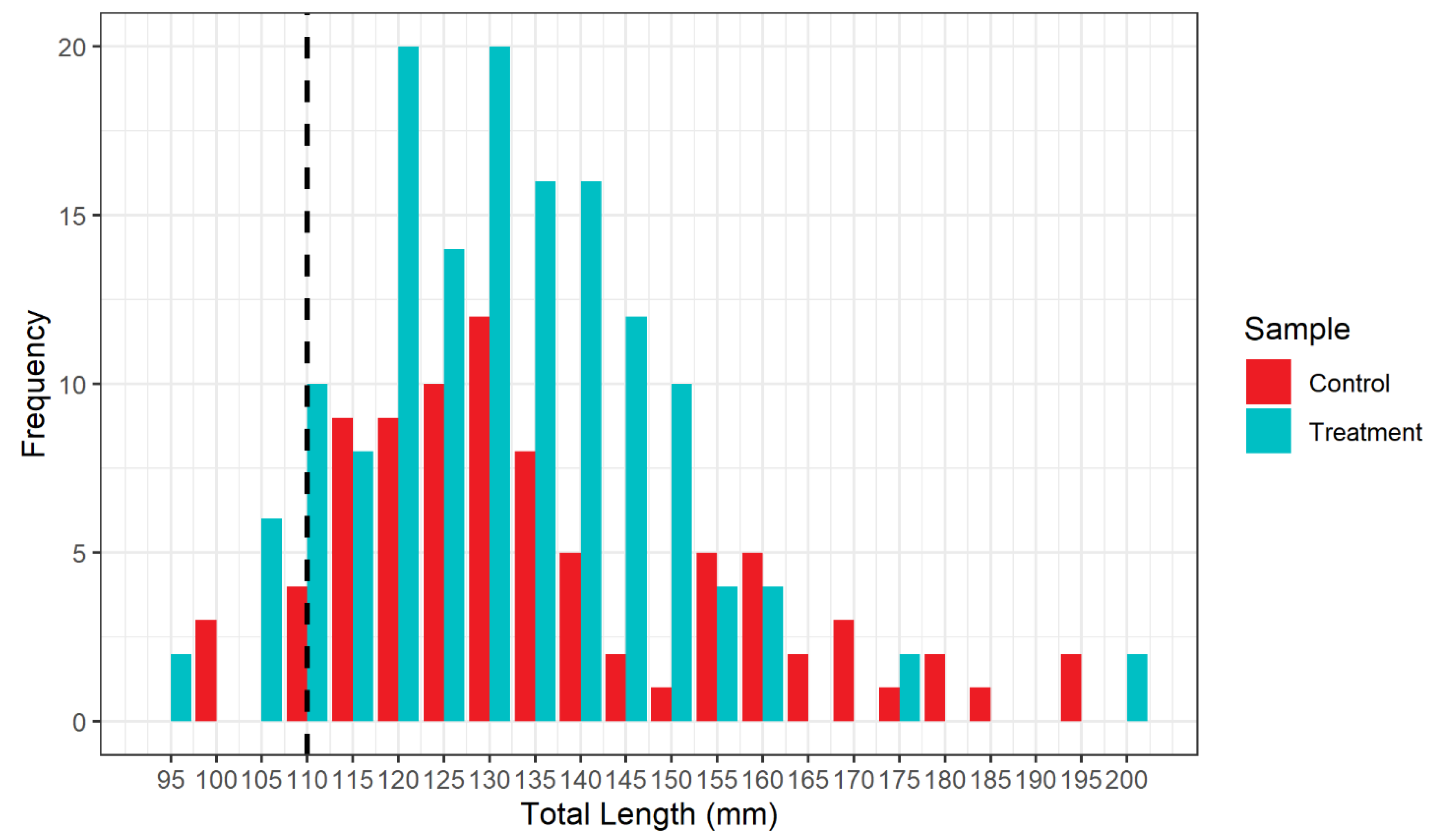
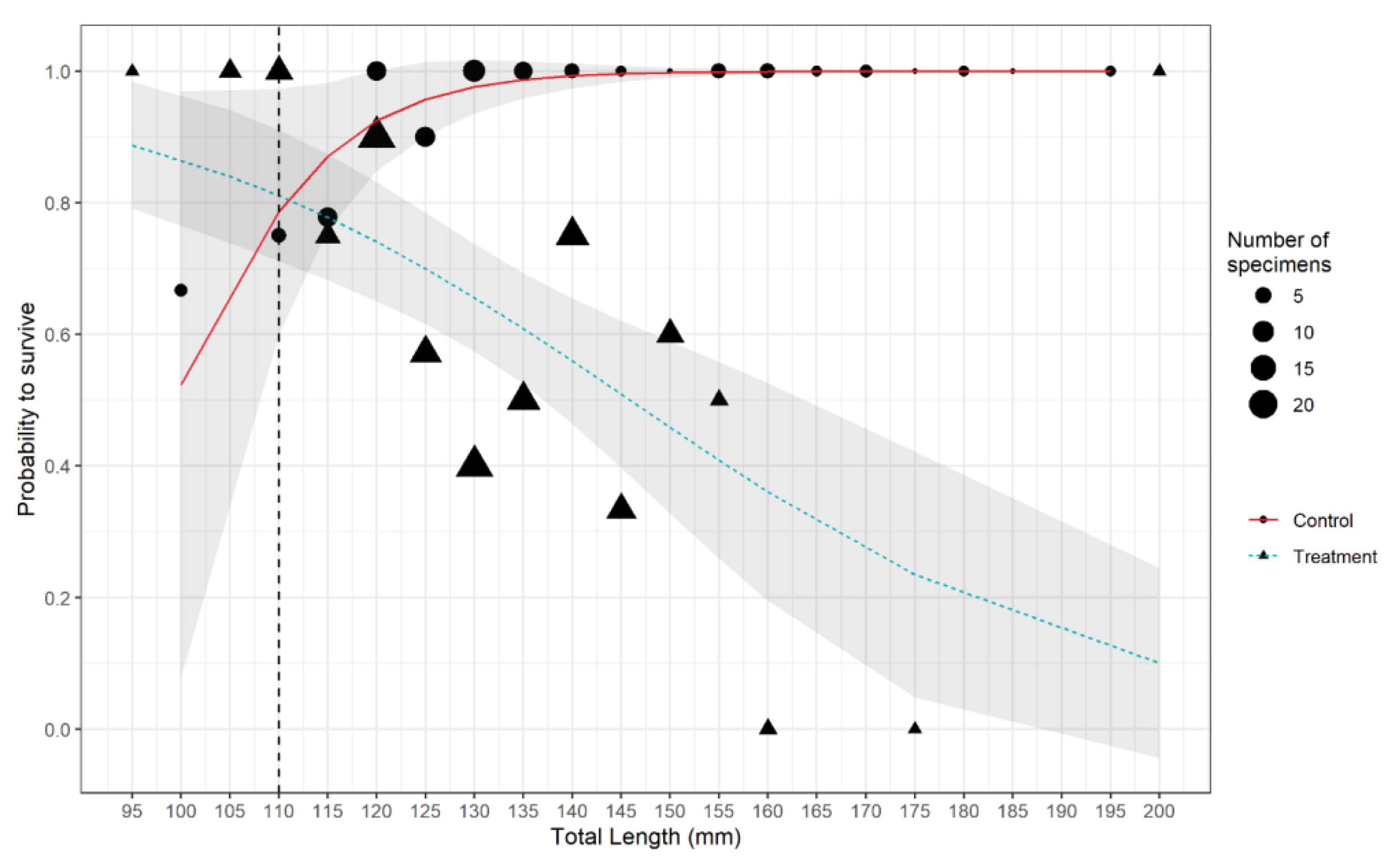
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennings, S.; Kaiser, M.J. The effects of fishing on marine ecosystems. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 1998; Volume 34, pp. 201–352. [Google Scholar]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Crowder, L.B.; Murawski, S.A. Fisheries bycatch: Implications for management. Fisheries 1998, 23, 8–17. [Google Scholar] [CrossRef]
- Santiago, J.L.; Ballesteros, M.A.; Chapela, R.; Silva, C.; Nielsen, K.N.; Rangel, M.; Erzini, K.; Wise, L.; Campos, A.; Borges, M.F.; et al. Is Europe ready for a results-based approach to fisheries management? The voice of stakeholders. Mar. Pol. 2015, 56, 86–97. [Google Scholar] [CrossRef]
- Pravin, P.; Gibinkumar, T.R.; Sabu, S.; Boopendranath, M. Hard bycatch reduction devices for bottom trawls: A review. Fish. Technol. 2011, 48, 107–118. [Google Scholar]
- Gorelli, G.; Sardà, F.; Company, J.B. Fishing effort increase and resource status of the deep-sea red shrimp Aristeus antennatus (Risso 1816) in the Northwest Mediterranean Sea since the 1950s. Rev. Fish. Sci. Aquac. 2016, 24, 192–202. [Google Scholar] [CrossRef]
- Geraci, M.L.; Ragonese, S.; Scannella, D.; Falsone, F.; Gancitano, V.; Mifsud, J.; Gambin, M.; Said, A.; Vitale, S. Batoid Abundances, Spatial Distribution, and Life History Traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a Knowledge Gap through Three Decades of Survey. Animals 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- EU. Regulation (EU) 2019/1241 of the European Parliament and of the Council of 20 June 2019 on the conservation of fisheries resources and the protection of marine ecosystems through technical measures. Off. J. Eur. Union 2019, 198, 97. [Google Scholar]
- EU. Regulation (EU) 1380/2013 of 11 December 2013 on the Common Fisheries Policy, amending Council Regulations (EC) No 1954/2003 and (EC) No 1224/2009 and repealing Council Regulations (EC) No 2371/2002 and (EC) No 639/2004 and Council Decision 2004/585/EC. Off. J. Eur. Union 2013, 22–61. [Google Scholar]
- Vitale, S.; Milisenda, G.; Gristina, M.; Baiata, P.; Bonanomi, S.; Colloca, F.; Gancitano, V.; Scannella, D.; Fiorentino, F.; Sala, A. Towards more selective Mediterranean trawl fisheries: Are juveniles and trash excluder devices effective tools for reducing undersized catches? Sci. Mar. 2018, 82, 215–223. [Google Scholar] [CrossRef]
- Vitale, S.; Enea, M.; Milisenda, G.; Gancitano, V.; Geraci, M.L.; Falsone, F.; Bono, G.; Fiorentino, F.; Colloca, F. Modelling the effects of more selective trawl nets on the productivity of European hake (Merluccius merluccius) and deep-water rose shrimp (Parapenaeus longirostris) stocks in the Strait of Sicily. Sci. Mar. 2018, 82, 199–208. [Google Scholar] [CrossRef]
- Sardo, G.; Vecchioni, L.; Milisenda, G.; Falsone, F.; Geraci, M.L.; Massi, D.; Rizzo, P.; Scannella, D.; Vitale, S. Guarding net effects on landings and discards in Mediterranean trammel net fishery: Case analysis of Egadi Islands Marine Protected Area (Central Mediterranean Sea, Italy). Front. Mar. Sci. 2023, 10, 1011630. [Google Scholar] [CrossRef]
- Sbrana, M.; De Carlo, F.; Ligas, A.; Massaro, A.; Musumeci, C.; Rossetti, I.; Sartini, M.; Vasapollo, C.; Viva, C.; Sartor, P.; et al. Testing experimental devices in the extension piece to increase the selectivity of bottom trawl in the Nw Mediterranean. Front. Mar. Sci. 2022, 9, 1017766. [Google Scholar] [CrossRef]
- Petetta, A.; Herrmann, B.; Virgili, M.; Li Veli, D.; Brinkhof, J.; Lucchetti, A. Effect of extension piece design on catch patterns in a Mediterranean bottom trawl fishery. Front. Mar. Sci. 2022, 9, 876569. [Google Scholar] [CrossRef]
- Maynou, F.; García-de-Vinuesa, A.G.; Martínez-Baños, P.; Sánchez, P.; Demestre, M. Relative Catch Performance of Two Gear Modifications Used to Reduce Bycatch of Undersized Fish and Shrimp in Mediterranean Bottom Trawl Fisheries. Mar. Coast. Fish. 2021, 13, 518–533. [Google Scholar] [CrossRef]
- Geraci, M.L.; Sardo, G.; Scannella, D.; Falsone, F.; Di Maio, F.; Gancitano, V.; Fiorentino, F.; Chirco, P.; Massi, D.; Vitale, S. Exploring the feasibility of technological transfers of two by-catch reduction devices in the crustacean bottom trawling of the central Mediterranean. Front. Mar. Sci. 2023, 10, 1011605. [Google Scholar]
- Breen, M.; Cook, R. Inclusion of Discard and Escape Mortality Estimates in Stock Assessment Models and Its Likely Impact on Fisheries Management, ICES CM 2002/V. 2002; Volume 27, 15p.
- Suuronen, P. Mortality of Fish Escaping Trawl Gears; FAO Fisheries Technical Paper No. 478; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; 72p.
- Chopin, F.S.M.; Arimoto, T. The condition of fish escaping from fishing gears—A review. Fish. Res. 1995, 21, 315–327. [Google Scholar] [CrossRef]
- ICES. Report of the FTFB Topic Group on Unaccounted Mortality in Fisheries. In Proceedings of the ICES FTFB Working Group, The Netherlands, 10–11 April 2000. [Google Scholar]
- Broadhurst, M.K.; Suuronen, P.; Hulme, A. Estimating collateral mortality from towed fishing gear. Fish Fish. 2006, 7, 180–218. [Google Scholar] [CrossRef]
- Sala, A.; Priour, D.; Herrmann, B. Experimental and theoretical study of red mullet (Mullus barbatus) selection in codends of Mediterranean bottom trawls. Aquat. Living Resour. 2006, 19, 317–327. [Google Scholar] [CrossRef]
- Sala, A.; Herrmann, B.; De Carlo, F.; Lucchetti, A.; Brčić, J. Effect of codend circumference on the size selection of square-mesh codends in trawl fisheries. PLoS ONE 2016, 11, e0160354. [Google Scholar] [CrossRef]
- Brčić, J.; Herrmann, B.; Sala, A. Can a square-mesh panel inserted in front of the codend improve size and species selectivity in Mediterranean trawl fisheries? Can. J. Fish. Aquat. 2018, 75, 704–713. [Google Scholar] [CrossRef]
- Soldal, A.V.; Isaksen, B.; Marteinsson, J.E.; Engås, A. Scale Damage and Survival of Cod and Haddock Escaping from a Demersal Trawl; ICES CM 1991/B:44; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1991; 9p. [Google Scholar]
- Main, J.; Sangster, G.I. A Report on an Investigation to Assess the Scale Damage and Survival of Young Fish Escaping from a Demersal Trawl, Working Paper No. 3/88. Scott. Fish. Res. Rep. 1988; 3.
- Main, J.; Sangster, G.I. An Assessment of the Scale Damage to and Survival Rates of Young Gadoid Fish Escaping from the Cod-End of a Demersal Trawl. Scott. Fish. Res. Rep. 1990, 46, 28. [Google Scholar]
- Main, J.; Sangster, G.I. Do Fish Escaping from Codends Survive? Working Paper No. 18/91; D.A.F.S. 1991; 18.
- Robinson, W.E.; Carr, H.A.; Harris, J. Assessment of Juvenile Bycatch and Cod-End Survivability in the Northeast Fishing Industry; A Report of the New England Aquarium to the National Oceanic and Atmospheric Administration Pursuant to NOAA Award No. NA26FD 0039-01; 1933. [Google Scholar]
- Metin, C.; Tokaç, A.; Ulaş, A.; Duzbastilar, O.F.; Lok, A.; Ozbilgin, H.; Metin, G.; Tosunoglu, Z.; Kaykac, H.; Aydin, C. Survival of red mullet (Mullus barbatus L., 1758) after escape from a trawl codend in the Aegean Sea. Fish. Res. 2004, 70, 49–53. [Google Scholar] [CrossRef]
- Düzbastılar, F.O.; Özgül, A.; Aydın, İ.; Gul, B.; Soykan, O. A preliminary study on the survival of brown comber, Serranus hepatus (Actinopterygii, Perciformes, Serranidae), escaping from the codend of a bottom trawl. Acta Ichthyol. Piscat. 2010, 40, 27–36. [Google Scholar] [CrossRef]
- Düzbastılar, F.O.; Özbilgin, H.; Aydın, C.; Metin, G.; Ulas, A.; Lok, A.; Metin, C. Mortalities of fish escaping from square and diamond mesh codends in the Aegean Sea. Fish. Res. 2010, 106, 386–392. [Google Scholar] [CrossRef]
- Düzbastılar, F.O.; Aydın, C.; Metin, G.; Lok, A.; Ulas, A.; Ozgul, A.; Gul, B.; Metin, C.; Ozbilgin, H.; Sensurat, T.; et al. Survival of fish after escape from a 40 mm streched diamond mesh trawl codend in the Aegean Sea. Sci. Mar. 2010, 74, 755–761. [Google Scholar] [CrossRef]
- Düzbastılar, F.O. Determining escape mortality of red bandfish (Cepola macrophthalma) escaping from bottom trawl. Ege J. Fish. Aqua. Sci. 2014, 31, 61–68. [Google Scholar] [CrossRef]
- Düzbastılar, F.O.; Laleli, T.; Özgül, A.; Metin, G. Determining the severity of skin injuries of red mullet, Mullus barbatus (Actinopterygii: Perciformes: Mullidae), inflicted during escape from trawl codend. Acta Ichthyol. Piscat. 2015, 45, 75–83. [Google Scholar] [CrossRef]
- Düzbastılar, F.O.; Aydın, C.; Gül, B. Mortality of non-target flatfishes escaping from demersal trawl codends. J. Appl. Ichthyol. 2016, 32, 1194–1204. [Google Scholar] [CrossRef]
- Düzbastilar, F.O.; Breen, M.; Aydin, C.; Özbilgin, H.; Özgül, A.; Ulaş, A.; Metin, G.; Gül, B.; Lök, A. Seasonal variation in mortality of red mullet (Mullus barbatus) escaping from codends of three different sizes in the Aegean Sea. Sci. Mar. 2017, 81, 339–349. [Google Scholar] [CrossRef]
- Düzbastılar, O.; Lök, A.; Ulaş, A. Mortality of Common pandora (Pagellus erythrinus Linnaeus, 1758) escaping from demersal trawl codends: Water temperature effect. Ege J. Fish. Aqua. Sci. 2018, 35, 181–188. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Sinerchia, M.; Colloca, F. The north sector of the Strait of Sicily: A priority area for conservation in the Mediterranean Sea. Hydrobiologia 2018, 821, 235–253. [Google Scholar] [CrossRef]
- Jarboui, O.; Ceriola, L.; Fiorentino, F. Current fisheries management in the Strait of Sicily and progress towards an ecosystem approach. In Transition towards an Ecosystem Approach to Fisheries in the Mediterranean Sea—Lessons Learned through Selected Case Studies; Vasconcellos, M., Ünal, V., Eds.; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2022; Volume 681, pp. 147–162. [Google Scholar]
- Milisenda, G.; Vitale, S.; Massi, D.; Enea, M.; Gancitano, V.; Giusto, G.B.; Badalucco, C.; Gristina, M.; Garofalo, G.; Fiorentino, F. Spatio-temporal composition of discard associated with the deep water rose shrimp fisheries (Parapenaeus longirostris, Lucas 1846) in the south-central Mediterranean Sea. Med. Mar. Sci. 2017, 18, 53–63. [Google Scholar] [CrossRef]
- Geraci, M.L.; Colloca, F.; Di Maio, F.; Falsone, F.; Fiorentino, F.; Sardo, G.; Scannella, D.; Gancitano, V.; Vitale, S. How is artificial lighting affecting the catches in deep water rose shrimp trawl fishery of the Central Mediterranean Sea? Ocean Coast. Manag. 2021, 215, 105970. [Google Scholar] [CrossRef]
- Pinello, D.; Gee, J.; Accadia, P.; Sabatella, E.C.; Vitale, S.; Polymeros, K.; Fiorentino, F. Efficiency of shallow- and deep-watertrawling in the Mediterranean and its implications for discard reduction. Sci. Mar. 2018, 82, 97. [Google Scholar] [CrossRef]
- Falsone, F.; Gancitano, V.; Geraci, M.L.; Sardo, G.; Scannella, D.; Serena, F.; Vitale, S.; Fiorentino, F. Assessing the stock dynamics of Elasmobranchii off the southern coast of Sicily by using trawl survey data. Fishes 2022, 7, 136. [Google Scholar] [CrossRef]
- Di Maio, F.; Geraci, M.L.; Scannella, D.; Russo, T.; Fiorentino, F. Evaluation of the Economic Performance of Coastal Trawling off the Southern Coast of Sicily (Central Mediterranean Sea). Sustainability 2022, 14, 4743. [Google Scholar] [CrossRef]
- Breen, M.; Huse, I.; Ingolfsson, I.; Madsen, N.; Soldal, A.V. SURVIVAL: An Assessment of Mortality in Fish Escaping from Trawl Codends and Its Use in Fisheries Management, EU Final Report. [s.n.]. 2007; 300.
- Sardo, G.; Okpala, C.O.R.; Geraci, M.L.; Vitale, S. The effects of different artificial light wavelengths on some behavioural features of juvenile pelagic Atlantic horse mackerel, Trachurus trachurus (Actinopterygii: Perciformes: Carangidae). Acta Ichthyol. Piscat. 2020, 50, 85–92. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 20 August 2022).
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Dorai-Raj, S. Binomial Confidence Intervals for Several Parameterizations. 2014. Available online: https://CRAN.R-project.org/package=binom (accessed on 20 August 2022).
- Breen, M.; Catchpole, T. ICES Guidelines for Estimating Discard Survival, Breen, M., Catchpole, T., Eds.; ICES Cooperative Research Reports. 2021; 351, 219. [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Scheipl, F. Drawing Survival Curves Using ggplot2. 2021. Available online: https://rpkgs.datanovia.com/survminer/index.html (accessed on 20 August 2022).
- Therneau, T.; A Package for Survival Analysis in R. R Package Version 3.4-0. 2022. Available online: https://cran.r-project.org/package=survival (accessed on 20 August 2022).
- McCullagh, P.; Nelder, J. Generalized Linear Models, 2nd ed.; Chapman and Hall/CRC: London, UK, 1999. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Smithson, M.; Verkuilen, J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 2006, 11, 54–71. [Google Scholar] [CrossRef]
- Hartig, F.; Lohse, L. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, 2022.
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics, 2016; Volume 2, 1–189.
- Wang, W.; Yan, J. Shape-Restricted Regression Splines with R Package splines2. J. Data Sci. 2021, 19, 498–517. [Google Scholar] [CrossRef]
- Sangster, G.I.; Lehmann, K.; Breen, M. Commercial fishing experiments to assess the survival of haddock and whiting after escape from four sizes of diamond mesh cod-ends. Fish. Res. 1996, 25, 323–345. [Google Scholar] [CrossRef]
- Wileman, D.A.; Sangster, G.I.; Breen, M.; Ulmestrand, M.; Soldal, A.V.; Harris, R.R. Roundfish and Nephrops Survival after Escape from Commercial Fishing Gear; EU Contract Final Report. EC Contract No: FAIR-CT95-0753; European Commission: Brussels, Belgium, 1999. [Google Scholar]
- Papaconstantinou, C.; Farrugio, H. Fisheries in the Mediterranean. Med. Mar. Sci. 2000, 1, 5–18. [Google Scholar] [CrossRef]
- Tserpes, G.; Massuti, E.; Fiorentino, F.; Facchini, M.T.; Viva, C.; Jadaud, A.; Joksimovic, A.; Pesci, P.; Piccinetti, C.; Sion, L.; et al. Distribution and spatio-temporal biomass trends of red mullets across the Mediterranean. Sci. Mar. 2019, 83S1, 43–55. [Google Scholar] [CrossRef]
- ICES. Report of the Study Group on Unaccounted Fishing Mortality; ICES CM 2004/B:09; ICES: Copenhagen, Denmark, 2004. [Google Scholar]
- ICES. Joint Report of the Study Group on Unaccounted Fishing Mortality (SGUFM) and the Workshop on Unaccounted Fishing Mortality (WKUFM); ICES CM 2005/B:08; ICES: Copenhagen, Denmark, 2005. [Google Scholar]
- Soldal, A.V.; Engås, A. Survival of young gadoids excluded from a shrimp trawl by a rigid deflecting grid. ICES J. Mar. Sci. 1997, 54, 117–124. [Google Scholar] [CrossRef]
- Ingólfsson, Ó.A.; Soldal, A.V.; Huse, I.; Breen, M. Escape mortality of cod, saithe, and haddock in a Barents Sea trawl fishery. ICES J. Mar. Sci. 2007, 64, 1836–1844. [Google Scholar] [CrossRef]
- Breen, M.; Sangster, G.I.; Soldal, A.V. Evidence of Cover Induced Mortality in Fish Survival Experiments—A Cautionary Note; ICES CM 1998/Open: 2; ICES: Copenhagen, Denmark, 1998. [Google Scholar]
- Breen, M.; Sangster, G.; O’Neill, B.; Kynoch, R.; Jones, E.; Soldal, A.V. Evidence of Sampling Induced Biases in Mortality Estimates from Experiments Investigating Mortality in Fish Escaping from Towed Fishing Gears; ICES CM 2002/V:25; ICES: Copenhagen, Denmark, 2002. [Google Scholar]
- Breen, M. Investigating the Mortality of Fish Escaping from Towed Fishing Gears—A Critical Analysis. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 2004; 313p. [Google Scholar]


| Control | Treatment | |||||
|---|---|---|---|---|---|---|
| Species | Dead | Survivors | Total | Dead | Survivors | Total |
| Arnoglossus thori | // | // | // | 3 | 0 | 3 |
| Bothus podas | 1 | 0 | 1 | // | // | // |
| Dactilopterus volitans | // | // | // | 1 | 0 | 1 |
| Microchirus variegatus | // | // | // | 1 | 0 | 1 |
| Mullus barbatus | 5 | 79 | 84 | 54 | 92 | 146 |
| Pagellus acarne | // | // | // | 0 | 3 | 3 |
| Pagellus erythrinus | // | // | // | 0 | 5 | 5 |
| Serranus cabrilla | // | // | // | 1 | 2 | 3 |
| Serranus hepatus | // | // | // | 0 | 4 | 4 |
| Spicara flexuosa | // | // | // | 0 | 1 | 1 |
| Synodus saurus | // | // | // | 4 | 0 | 4 |
| Coefficients | Estimate | Std. error | z Value | p-Value | Resid. df | Resid. dev | p-Value |
|---|---|---|---|---|---|---|---|
| Intercept | −11.975 | 5.789 | −2.068 | 0.039 | |||
| TL | 0.121 | 0.050 | 2.410 | 0.016 | 31 | 63.450 | 0.025 |
| Haul_type Treatment | 17.892 | 6.007 | 2.978 | 0.003 | 31 | 93.010 | <0.001 |
| TL: haul_type Treatment | −0.161 | 0.051 | −3.132 | 0.002 | 30 | 58.405 | <0.001 |
| Coefficients | Estimate | Std. Error | z Value | p-Value | Df | LogLik | Deviance | Chisq | Chi df | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | −0.860 | 0.107 | −8.001 | 1.23−15 | ||||||
| Zone Abdominal | −2.056 | 0.159 | −12.893 | <2−16 | 11 | 587.07 | −1174.2 | 0 | 0 | <0.001 |
| Zone Anal | −2.353 | 0.168 | −13.991 | <2−16 | ||||||
| Zone Caudal | −2.212 | 0.166 | −13.328 | <2−16 | ||||||
| Haul_type Control | −4.182 | 0.636 | −6.578 | 4.76−11 | 11 | 587.07 | −1174.2 | 0 | 0 | <0.001 |
| Zone Abdominal: haul_typeControl | 2.587 | 0.636 | 4.068 | 4.74−05 | 11 | 587.07 | −1174.2 | 17.878 | 3 | <0.001 |
| Zone Anal: haul_type Control | 1.675 | 0.670 | 2.503 | 0.012 | ||||||
| Zone Caudal: haul_type Control | 1.534 | 0.669 | 2.293 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraci, M.L.; Sardo, G.; Falsone, F.; Scannella, D.; Breen, M.; Fiorentino, F.; Sala, A.; Vitale, S. Escape Survival and Scale Damage Assessment of Red Mullet (Mullus barbatus Linnaeus, 1758) during Bottom Trawling in the Central Mediterranean Sea. Biology 2023, 12, 649. https://doi.org/10.3390/biology12050649
Geraci ML, Sardo G, Falsone F, Scannella D, Breen M, Fiorentino F, Sala A, Vitale S. Escape Survival and Scale Damage Assessment of Red Mullet (Mullus barbatus Linnaeus, 1758) during Bottom Trawling in the Central Mediterranean Sea. Biology. 2023; 12(5):649. https://doi.org/10.3390/biology12050649
Chicago/Turabian StyleGeraci, Michele Luca, Giacomo Sardo, Fabio Falsone, Danilo Scannella, Michael Breen, Fabio Fiorentino, Antonello Sala, and Sergio Vitale. 2023. "Escape Survival and Scale Damage Assessment of Red Mullet (Mullus barbatus Linnaeus, 1758) during Bottom Trawling in the Central Mediterranean Sea" Biology 12, no. 5: 649. https://doi.org/10.3390/biology12050649
APA StyleGeraci, M. L., Sardo, G., Falsone, F., Scannella, D., Breen, M., Fiorentino, F., Sala, A., & Vitale, S. (2023). Escape Survival and Scale Damage Assessment of Red Mullet (Mullus barbatus Linnaeus, 1758) during Bottom Trawling in the Central Mediterranean Sea. Biology, 12(5), 649. https://doi.org/10.3390/biology12050649









