Simple Summary
Scientists are exploring the use of new genetic tools to engineer crop varieties which can result in desirable traits such as improved yield, disease resistance, and enhanced nutritional value. By manipulating the plants’ genetic makeup, they are able to develop transgenic plants capable of producing valuable biotechnological compounds. The development of plant-based biomanufacturing could offer a more sustainable and cost-effective method to produce useful products compared to traditional manufacturing process.
Abstract
The development of recombinant DNA technology during the past thirty years has enabled scientists to isolate, characterize, and manipulate a myriad of different animal, bacterial, and plant genes. This has, in turn, led to the commercialization of hundreds of useful products that have significantly improved human health and well-being. Commercially, these products have been mostly produced in bacterial, fungal, or animal cells grown in culture. More recently, scientists have begun to develop a wide range of transgenic plants that produce numerous useful compounds. The perceived advantage of producing foreign compounds in plants is that compared to other methods of producing these compounds, plants seemingly provide a much less expensive means of production. A few plant-produced compounds are already commercially available; however, many more are in the production pipeline.
1. Introduction
Plant biotechnology has two main objectives: (i) the use of plants as a means of producing a wide range of products that have been previously synthesized using recombinant bacteria, fungi, or animal cells in culture, and (ii) the creation of new, unique, and better-performing varieties of crop plants. Some of the new and unique varieties of crop plants include plants resistant to insects, viruses, and herbicides; plants with increased nutritional content; plants that are resistant to a range of abiotic environmental stresses such as high levels of soil salinity and drought; plants with modified fruits and flowers; plants that are resistant to a wide range of fungal and bacterial pathogens; and plants with a significant increase in yield. In addition, the most prominent products that are either currently produced in plants or are being developed for eventual production in plants include antibodies and antibody fragments; human and animal vaccines; and a wide range of animal and human therapeutic agents (Figure 1).
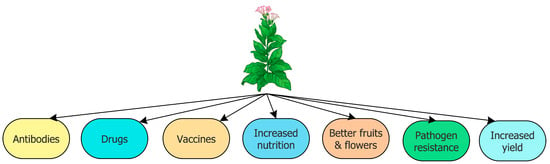
Figure 1.
Schematic representation of some transgenic plants and the products that they produce.
2. Early Engineered Plants
Plants that are resistant to insect predation, damage from plant viruses, and various herbicides (used to prevent the growth of weeds) were genetically engineered beginning ~30–40 years ago, and a number of these engineered plants have been commercially available for many years. Thus, these plants are only mentioned briefly in this review, where the focus is on the development of some of the more recent genetically modified plants.
2.1. Insect Resistance
Beginning with the 1940s through the 1970s, several powerful chemical insecticides were developed and employed globally on a massive scale. The use of these insecticides had a dramatic effect in reducing the damage to crop plants that resulted from insect predation. The most effective and most widely use of these chemical insecticides was the compound dichlorodiphenyltrichloroethane or DDT [1]. Unfortunately, it was eventually discovered that most of these chemical insecticides, and DDT in particular, had negative effects on animals, ecosystems, and humans [2]. Moreover, many of these chemicals, notwithstanding their apparent effectiveness as insecticides, persisted for many years in the environment and accumulated in increasing concentrations through food chains. With the widespread development of transgenic plants in the early 1980s, scientists began developing plants that were resistant to insect predation [3,4,5,6,7,8,9,10]. For the most part, this involved isolating genes encoding bacterial insect-toxigenic proteins from various strains of the bacterium Bacillus thuringiensis and expressing those genes in transgenic plants; this approach has turned out to be highly effective and without significant negative consequences. In the past 25 years, the availability and commercial use of transgenic plants expressing one or more of several different B. thuringiensis insecticidal toxins has increased dramatically throughout the US and many other countries of the world [11]. At the present time, 44 countries worldwide (with the notable exception of countries belonging to the European Union) have given regulatory approval to 40 different genetically engineered crops with insect and herbicide-resistant plants being the most commonly introduced traits [12]. Currently, regulatory approved B. thuringiensis-engineered plants include cotton, cowpea, eggplant, maize, poplar, potato, rice, soybean, sugarcane, and tomato.
2.2. Virus Resistance
It has been estimated that there are nearly 2000 known different plant viruses [13], the great majority of which have a small single-stranded RNA genome (often less than 10 kilobases). These viruses can cause significant damage to crop plants, thereby dramatically reducing plant yields. The viral coat protein is generally the most abundant protein in small single-stranded RNA viruses. Consequently, many transgenic plants have been engineered to express a small single-stranded RNA viral coat protein gene and, as a result are often protected against the systematic spread and the subsequent deleterious effects of that virus [14,15,16,17,18,19,20]. It is believed that the cloned and expressed viral coat protein gene inhibits the expression of the small single-stranded RNA virus through the mechanism of RNA interference (RNAi). In addition, given the similarity in structure and mechanisms of infectivity of many small plant viruses, the abovementioned viral coat protein gene approach has been found to be highly effective for many different plants, including alfalfa, corn, tobacco, tomato, sugar beet, cucumber, papaya, potato, rice, zucchini, soybean, grapevine, squash, pumpkin, plum, and muskmelon, against several different viruses. Moreover, numerous transgenic plants expressing a small single-stranded RNA viral coat protein gene have been approved by regulatory authorities and subsequently commercialized. Unfortunately, the viral coat protein strategy is only effective when transgenic plants are challenged by closely related viruses. Therefore, scientists have sought to develop transgenic plants that are resistant to a broad spectrum of plant viruses. While several different approaches have been attempted to achieve this end, this remains a work in progress.
2.3. Herbicide Resistance
Every year a large portion of worldwide crop production is lost through weed infestation. This loss of productivity notwithstanding, an estimated ~USD 35–40 billion is spent annually by farmers on >100 different chemical herbicides [21]. Since most herbicides do not discriminate between weeds and crop plants, scientists have developed several different herbicide-resistant crop plants, including plants resistant to triazines, sulfonylureas, imidazolinones, aryloxphenoxypropionates, cyclohexanediones, bromoxynil, phenoxycarboxylic acids, glufosinate, cyanamide, and dalapon. This approach has been so successful that currently ~60% of the transgenic crops that are planted globally have been modified to be herbicide-resistant. However, herbicide-resistant crops do not have a higher yield than non-herbicide-resistant crops [22]. On the other hand, herbicide-resistant crops are preferred for “improved and simplified weed control, less labour and fuel cost, no-till planting/planting flexibility, yield increase, extended time window for spraying, and in some cases decreased pesticide input” [23].
Worldwide, the most widely used chemical herbicide is glyphosate (commercially sold as Roundup®(Bayer, USA), which is claimed by its producer (the Monsanto Corporation, now a division of Bayer) to be safe, cheap, effective, and environmentally friendly [24]. Transgenic plants that have been shown to be resistant to this herbicide include soybean, corn, canola, tobacco, soybean, petunia, tomato, potato, alfalfa, sorghum, sugar beet, Indian mustard, and cotton. Treatment of plants in the field with glyphosate should kill all (or most) of the weeds while the engineered crop plants are not affected. With the widespread acceptance of this herbicide there is, however, some concern that (i) there is too much dependence upon the use of a single herbicide and, as a result, there are numerous instances of weeds acquiring naturally occurring resistance [25,26]; and (ii) some reports have suggested that long term exposure to this herbicide may be toxic or carcinogenic to humans [27]. Thus, there are some recent literature reports of plants that have been engineered to be resistant to other herbicides that researchers hope will provide an effective alternative to the widespread use of glyphosate [28,29,30]. However, glyphosate is likely to continue to be the dominant herbicide worldwide until some of these newer approaches can be shown to safe and efficacious in the field with a large number of different transgenic plants [31].
3. Antibody Production
Currently, therapeutic monoclonal antibodies are mostly produced from transgenic Chinese hamster ovary (CHO) cells grown in culture, typically in stirred tank bioreactors with volumes as large as 25,000 L. Unfortunately, this process, which requires highly specialized fermentation equipment and media, and highly controlled conditions, yields only a modest level of monoclonal antibody protein per run (which takes around two weeks’ time). Producing monoclonal antibodies from transgenic CHO cells grown in culture results in an average monoclonal antibody treatment retailing for nearly USD 100,000 per annum [32] with production cost being estimated to be from USD 100 to 200 per gram. This results in the average treatment with a monoclonal antibody costing between USD 15,000 and USD 200,000 per year. On the other hand, with current technology, functional monoclonal antibodies can be produced in transgenic plants at only 1–10% of the cost of using CHO cells, notwithstanding the fact that it takes longer to grow transgenic plants than it does to produce monoclonal antibodies from CHO cells in culture. As an alternative to transgenic plants, transient gene expression can produce a high protein yield in a much shorter period of time [33]. In addition, unlike the requirement to grow CHO cells in very expensive large fermenters, plants can easily be grown on a relatively large scale in semi-contained and less expensive greenhouse facilities.
The majority of human proteins, including human antibodies, are glycosylated, with sugar molecules covalently attached to either a hydroxyl group of an antibody molecule serine or threonine residue, or to an amide moiety of an asparagine residue [34,35,36,37]. There are significant differences in the patterns of protein glycosylation produced by humans versus plants. That is, humans and plants add different carbohydrate molecules to the surface-facing amino acids of antibody molecules. While differences in surface carbohydrate molecules do not alter the activity of the antibody molecules, “other properties, such as protein folding, stability, solubility, susceptibility to proteases, blood clearance rate, and antigenicity, can be affected profoundly” [38]. To overcome any potential problems that might occur as a consequence of using incorrectly glycosylated plant-produced monoclonal antibodies, scientists have preemptively genetically engineered several plant hosts to glycosylate foreign proteins produced in those plants so that the glycosylation pattern of plant-produced proteins is nearly identical to the glycosylation pattern of the same proteins produced in human cells [36,39]. According to Castilho and Steinkellner [36], “Plants have demonstrated a high degree of tolerance to changes in the N-glycosylation pathway, allowing recombinant proteins to be modified into human-like structures in a specific and controlled manner.” Thus, in the correct genetic background, plant-produced antibodies do not show any significant differences in their ability to bind to their target antigens.
In early experiments, scientists synthesized cDNAs (separately encoding light and heavy antibody chains) that were previously isolated from mouse hybridoma mRNAs and expressed these cDNAs in transgenic tobacco plants [40]. Plants that expressed single (light or heavy) chains from the same antibody molecule were then genetically crossed with one another to produce progeny in which both light and heavy chains were produced in the same plant, with the hope that these chains would correctly associate with one another to form functional complete antibody molecules. Notwithstanding this long and somewhat cumbersome process, some transgenic tobacco plants producing functional antibodies were obtained. Since that time, several more efficient systems have been developed for the synthesis of monoclonal antibodies in plants.
Table 1 summarizes some of the full-size monoclonal antibodies that have been produced in plants [39,41,42,43,44,45,46,47,48]. In addition, this table does not include the numerous plant-synthesized antibody fragments or single chain antibodies that have been produced. Since scientists can produce full-size active monoclonal antibodies with glycosylation patterns similar or identical to those found in human cells, in the future more monoclonal antibodies are likely to be synthesized in plants rather than in CHO cells. This should allow monoclonal antibodies to be produced in larger amounts and much less expensively than is currently the case.

Table 1.
Some full-size monoclonal antibodies that have been produced in plants.
4. Pharmaceutical Production
Given the fact that many human therapeutic agents are commercially produced in animal cells in culture, the resultant proteins end up being relatively expensive. This is because animal cells in culture grow slowly, do not produce a large biomass, and require specialized fermentation media and equipment. As an alternative to animal cells, scientists have begun to produce a number of therapeutic proteins in genetically engineered plants (in a process that is euphemistically called pharming). A few of these plant-synthesized pharmaceuticals have been approved for human use while several others are currently being tested in clinical trials (Table 2). For example, one group of scientists showed that transgenic rice could simultaneously produce three functional proteins which together could neutralize HIV-1 [57]. These proteins included the monoclonal antibody 2G12, and the lectins griffithsin and cyanovirin-N. This protein mixture enhanced both the activity and the binding of the antibody to the HIV protein gp120, resulting in the virus’s neutralization. Thus, this procedure not only decreased the cost of producing this anti-HIV cocktail, but it also increased the potency of the individual components.

Table 2.
Some plant-synthesized pharmaceuticals.
Within the next 15–20 years, many more pharmaceutical proteins are likely to be produced in transgenic plants. Tobacco plants are presently the most popular host for the synthesis of pharmaceutical proteins because of (i) the ease of genetically transforming these plants, (ii) tobacco’s rapid growth, and (ii) the large size of the tobacco leaf, which yields large amounts of the target protein [70]. However, in an effort to determine the optimum plant host for foreign proteins, scientists are currently expressing pharmaceutical proteins in a range of different plants. Other plants that have been used to produce foreign proteins include maize, wheat, tomato, potato, mustard, banana, rice, and soybean. For example, some pharmaceutical proteins have been synthesized in rice kernels, which are subsequently ground into a powder and put into easy-to-deliver and consume gelatin capsules. It is important to keep in mind that the purification of plant-produced proteins may present unique challenges and is certainly expected to be different from purifying the same protein from animal cells in culture.
Using traditional techniques (i.e., Agrobacterium tumefaciens’ transformation of plants) to produce pharmaceutical proteins may be effective; however, it is a relatively time-consuming and labor-intensive process [71]. On the other hand, this approach is currently being replaced by transient expression systems as well as the use of plant cell cultures [71] wherein the time to produce a foreign protein may be reduced by 10-fold or more. Unfortunately, these approaches are currently significantly more expensive than relying on A. tumefaciens’ transformation followed by plant growth.
As a consequence of concerns among both the public and the scientific community that transgenic plants producing pharmaceuticals could threaten traditional agriculture, the environment and/or human health, it is currently necessary to grow these plants under contained conditions [72]. However, using transient expression systems as well as plant cell cultures should enable scientists to safely produce large amounts of pharmaceuticals in plants.
5. Vaccine Production
Antigens that are the basis for conventional vaccines may be produced (similar to various protein pharmaceuticals) in transgenic plants under contained conditions or using either transient expression systems or plant cell cultures. In many countries, conventional vaccines are often too expensive to allow for widespread dissemination, even with the decreased cost of producing the vaccine antigen(s) in transgenic plants. With COVID-19 vaccines, despite donations from wealthy nations, by mid-2021, fewer than “1% of people in low-income countries are fully vaccinated, and just 10% are in lower-middle-income countries, compared with more than half in high-income countries” [73]. For COVID-19, as well as many other diseases, it would therefore be advantageous if vaccines could be delivered directly and inexpensively to individuals in countries that do not have a sufficient infrastructure of modern roads and refrigeration. One way to do this is to produce vaccines in plants that are edible (by humans or animals) and do not require specialized production facilities, sterilization, packaging, or refrigeration. Moreover, unlike conventional vaccines, edible vaccines do not have to be purified prior to their use.
Most current vaccines are delivered by injection. However, since the majority of pathogens enter the body through mucosal sites [74,75], using mucosal vaccination (including through the use of oral vaccines) instead of vaccination by injection, it is possible to create increased levels of protective immunity. Unfortunately, only a small number of mucosal vaccines have been approved for human use; this is a consequence of the lack of effective mucosal delivery systems. On the other hand, with the rapid development of more plant-based edible vaccines, the dearth of mucosal vaccines will soon be a thing of the past. Following the ingestion of an edible vaccine and its subsequent transit into the small intestine, the vaccine antigen is taken up by M cells that line the intestine. Then the vaccine antigen is transported to other cells in the mucosal immune system including macrophages and B cells. The macrophages bind the vaccine antigen and then display it on the macrophage cell surface to helper T cells. The now “activated” helper T cells secrete small molecules that in turn activate B cells that have bound vaccine antigens to surface-localized antibodies. Once the B cells are activated, they synthesize and release antigen-directed antibodies that can eventually neutralize the antigen [76].
Many of the earliest edible vaccines were produced in potatoes, a plant that is relatively easy to genetically manipulate. However, cooking potatoes to make them more palatable inactivates many of the protein antigens that comprise the vaccine. Researchers have therefore tested a number of other plants as delivery vehicles for edible vaccines. These include bananas, tomatoes, lettuce, carrots, peanuts, corn, and rice (Table 3) [77,78,79,80,81,82,83,84]. It is expected that, within the next few years, some of the edible vaccines that have been successfully tested in small animals will subsequently be tested in humans. Thus, the era of large-scale commercialization of edible vaccines will likely become a reality within the next 10–15 years.

Table 3.
Some plant-based oral vaccines and oral booster vaccines.
6. Increasing Nutritional Content
Humans depend on food not only as a source of energy but also for essential nutrients and minerals that maintain the immune system and a healthy lifestyle. However, it is estimated that micronutrient and mineral deficiencies affect almost 800 million people globally, and the majority are people residing in developing countries [103]. In addition to this, poverty and limited access to nutritional food can lead to several disorders, especially among the female population [104].
The availability of a diverse genetic pool for nutritional improvement has enabled the use of genetic modification techniques to develop crop varieties with increased amounts of essential vitamins and minerals. Of particular note is the development of ‘golden’ crops targeted to improve the diets of populations in developing countries that mostly rely on cereals and starchy roots and tubers. For example, ‘golden’ rice, ‘golden’ potato, and ‘golden’ banana have been engineered to accumulate high levels of provitamin A. In golden rice (the first one of these crops to be developed), manipulations in the endosperm-specific carotenoid pathways led to enhanced production of β-carotene, a precursor to vitamin A in rice. By introduction of genes from two different sources, the phytoene desaturase gene (ctrl) from the bacterium Erwinia uredovora, the phytoene synthase (psy) gene and lycopene β-cyclase (lyc) gene from daffodil (Narcissus pseudonarcissus), the β-carotene accumulation in rice endosperm was improved following Agrobacterium-mediated transformation [105]. Subsequently, by replacing the daffodil gene with a maize gene (under the control of the glutelin promoter) scientists created a new version of golden rice, i.e., golden rice2 (GR2) that produced up to 23 times more beta carotene compared to the original golden rice, with a total carotenoid level of up to 37 mg/g dry weight in the endosperm, of which 31 mg/g dry weight was β-carotene [106]. In a separate study by Diretto et al. [107], a combination of various approaches boosted the potato carotenoid content. The highest carotenoid and beta-carotene content was reported in the Desiree cultivar by using a ‘mini pathway’ of bacterial origin for carotenoid biosynthesis, i.e., by transforming the Desiree cultivar with three genes encoding phytoene synthase (CrtB), phytoene desaturase (CrtI), and lycopene beta-cyclase (lyc) from Erwinia under a tuber-specific promoter. The resulting ‘golden’ tubers had 20-fold more carotenoids (114 mg/g dry weight) and >3000-fold more β-carotene (47 mg/g dry weight) compared to the wild-type plant. According to a recent study, “a 150 g serving of boiled golden potatoes has the potential to contribute 42 and 23% of the daily requirement of retinol activity equivalents (RAE), as well as 34 and 17% of the daily vitamin E requirement for children and women of reproductive age, respectively” [108]. A transgenic Cavendish desert banana, displaying a golden-orange phenotype, was developed in Australia and showed an increase in pro-vitamin A when ZmPsy1 and MtPsy2a phytoene synthase genes were overexpressed in fruit pulp. Subsequent field trials in Australia and Uganda showed that the transgene was stable through three successive generations [109]. Clinical trials on some of the ‘golden crops’ are supportive and can prove to be useful in research programs to address malnutrition [110].
There has been considerable progress in elucidating biosynthetic pathways and identifying human-health-related metabolites and facilitating stacking of human health traits. For instance, maize that was genetically engineered by simultaneously targeting three different biosynthesis pathways contained 169-fold more β-carotene, 6-fold more ascorbate, and double the amount of folate compared to the wild-type controls [111]. In another study, a transgenic rice line expressing AtNAS1, PvFerritin, CRT1, and ZmPSY genes showed enhanced iron, zinc, and b-carotene levels in the rice endosperm [112]. Such studies have opened the door to introduce multi-nutrient traits in key crops such as rice, banana, cassava, and sorghum [113]. In China, the goal of this research is to use gene technologies to produce rice with enhanced vitamin E, iron, zinc, and folate combined with the golden rice trait, as well as improved grain protein content [114].
Upregulating a biosynthetic pathway by modifying transcription factor (TF) expression is another effective strategy to increase amounts of desirable vitamins. For instance, overexpression of NAC TFs that are specific to plants and are involved in many developmental processes have been linked to increases in micronutrients such as zinc and iron. In wheat, NAC-like TF NAM-B1, which might be linked to early onset of senescence, increased the concentration of zinc in grains [115]. Desired traits can also be obtained by the use of engineered TFs to control the expression of a target gene, and thus zinc finger TFs were designed to increase the amount of tocopherol by modifying the expression of the endogenous γ-tocopherol methyl transferase gene, resulting in a 20-fold increase in alpha-tocopherol in transgenic Arabidopsis compared to the control seeds [116].
There has recently been considerable progress on genetic modification of plants for improved protein production in crops. Genome editing technologies aimed at modifying the amino acid quality and quantity have been developed and sold to consumers. The best known examples of this are the CalynoTM (Calyxt, USA), a high-oleic soybean oil, NutriterraTM (Nuseed, USA) a canola product with high levels of docosahexaenoic acid (DHA) and Sicilian RougeTM (Sanatech Seed, Tokyo, Japan) a GABA (γ-aminobutyric acid)-enriched tomato variety [117,118,119].
Some notable achievements in altering carbohydrates that impact gut health by modifying the amylose content of starch include metabolically genetically engineered potato (75% more amylose), and wheat with >70% amylose [120,121]. More recently, high-amylose rice plants have been produced through CRISPR/CaS9 targeted mutagenesis of SBE11b and SB1 genes. Furthermore, knocking out the SBE11b gene led to longer amylopectin chains, subsequently improving nutritional properties of starch in the rice grain [122]. CRISPR/CaS 9 gene editing has also been used to improve the digestibility of RFOs (raffinose family oligosaccharides) in soybean. By targeting the GmGOLS1A and GmGOLS1B genes, researchers were able to realize a 33% decrease in the total RFO content in soybean seeds. Interestingly, in this case there were increases in protein and fat content in some of the mutant lines. Furthermore, the authors observed no differences in the agronomic traits between the mutant and wild-type plants [123].
Recently, anthocyanins have attracted interest due to their potential role in preventing obesity and diabetes [124]. Initially, the mode of action of anthocyanins was attributed directly to their antioxidant properties; however, new reports are now emerging that address their cell-molecular interactions, including modulation of gene expression, signaling, and DNA methylation [125]. Many of the genes involved in anthocyanin biosynthesis are available and this has enabled scientists to enhance plant anthocyanin content. For instance, a high anthocyanin content was achieved in the purple tomato variety by engineering the ROS1 and Del regulatory genes [126]. However, incorporating this trait in rice has been unsuccessful despite being tried by numerous researchers, thus illustrating challenges associated with engineering complex biosynthetic pathways in plant [127]. Nevertheless, one group developed purple endosperm rice by using a multi-gene assembly system called TGSII (TransGene Stacking II) for precise effects in rice using CRISPR/Cas-9 genome editing [128]. This experiment suggests extensive gene modification may be required to confer anthocyanin biosynthesis in target species, and that the difficulties in assembling multiple genes into single transformation vectors may require development of a more effective vector system.
7. Modifying Fruits and Flowers
Fruits contribute a large portion of vitamins, minerals, antioxidants, phytochemicals, and fiber to our diet and are integral to a healthy lifestyle. Although fruits are mainly grown for consumption, improving aesthetic qualities such as flavor and color could have a significant impact on nutrition if it shifted eating preferences toward more healthy food choices. In the past few decades, consumers have experienced a significant decline in fruit quality, with flavor being one of their major complaints [129]. Developing flavor and taste improvement traits are therefore important quality and health attributes from a consumer’s perspective; however, growers have put most of their efforts into breeding varieties for high yield, disease resistance, size, postharvest shelf life, and large-scale production [130].
Sugar, organic acids, and volatile chemicals have played a large role in shaping fruit flavor. For example, around 200 volatile chemicals have been identified from various cultivars of musk melon (Cucumus melo) that contribute to flavor [131]. Genetic manipulation of flavor-inducing genes has allowed researchers to generate various desired mutations enabling development of fruit varieties with superior flavor. For example, modifying the geraniol synthase gene in tomato (increased terpenoids) and FaFAD1 gene in strawberry (increased γ-decalactone) subsequently produced tastier fruits [132,133]. More recently, gene silencing has been applied to improve taste and fruit flavor. For example, this approach was shown to be effective in strawberry, where the suppression of ADP-glucose phosphorylase and aldose-6-phosphate reductase genes, respectively, by RNAi increased the sugar content in the flesh of the fruit [134].
Improving quality traits to make some fruit more appealing, with a longer shelf life, has been a key objective of both governments and the private sector. The Flavr SavrTM tomato developed by Calgene (California, USA) was the first genetically engineered fruit to be approved for cultivation and human consumption and it helped pave the way for the development of other genetically modified crops that are now widely available [135]. Pink-flesh pineapples (Pink GlowTMDelMonte (California, USA)) were produced by expressing a tangerine phytoene synthase (Psy) gene and suppressing endogenous lycopene β-cyclase (β-Lyc) and lycopene ε-cyclase (ε-Lyc) gene expression using RNAi [136]. Additionally, it was possible to alter endogenous ethylene biosynthesis by suppressing meristem-specific ACC synthase gene expression by using RNAi to reduce precocious flowering in Pink GlowTM [136]. Similarly, by using RNAi to mediate the silencing of the β-carotene hydroxylase gene, it was possible to produce a sweet orange variety that was deep yellow in color. Moreover, the β-carotene content in the pulp of transgenic fruits increased 26-fold, thereby significantly improving the health benefits of the fruit [137] Researchers have also manipulated genes involved in the regulation of the anthocyanin pathway that can influence fruit coloration. This has been described in several species including grape berries, strawberries, and apple, where MYB regulatory genes were shown to undergo anthocyanin synthesis to increase anthocyanin production, allowing further enhancement of fruit colors [138,139,140]. As more mechanisms are emerging, increasing anthocyanin pigmentation to further improve fruit color traits seems plausible.
Postharvest losses are a continuing threat in the production chain and result in decreasing returns and lower profits [141]. The main determinant for the postharvest damage of fruits is the rate of softening, which limits fruit shelf life and increases wastage. Therefore, engineering fruits for traits with better resistance to postharvest losses offers potential value. Increased shelf life can be achieved by engineering resistance to ethylene or by the inhibition of ethylene biosynthesis genes. By silencing the key enzymes of ethylene biosynthesis in apple, a significant improvement in fruit shelf life was observed. Notably, the ethylene-suppressed fruits were firmer than the wild-type controls, suggesting the additional role of ethylene in fruit development [142]. Similarly in pear, kiwifruit, and papaya, mutation of ethylene biosynthesis genes resulted in non-ripening phenotypes [143,144,145]. Other traits that have been successfully modified to improve apples include reducing browning by inactivating polyphenol oxidases (PPOs) and other fruit quality traits. For example, the ArcticTM Apple(Okanagan Specialty Fruits, British Columbia, Canada), was developed by silencing the PPOs and currently there are three commercial varieties available: ArcticTM Golden Delicious, ArcticTM Fuji, and ArcticTM Granny Smith [146,147]. In tomato, traditional breeding for traits to slow ripening has detrimental effects on tomato fruit flavor, color, firmness, and nutrition. However, when scientists used an antisense approach targeting the pectate lyase gene to control ripening in tomato, the resultant transgenic tomato lines showed no difference in fruit firmness or color compared to the control non-transgenic lines. Additionally, the genetically engineered lines also had the same yield of fruit as the control lines, which may also benefit the farmer [148]. In another study, repression of MADS box gene expression (MaMADS1 MaMADS2) in banana plants extended plant shelf life, and delayed color development and softening [141]. Thus, by using genetic solutions researchers were able to control ripening in banana, which is an important carbohydrate and nutrient source for millions of people.
The use of newer techniques such as CRISPR/Cas9 genome editing provides innovation in fruit crops as these approaches have the potential in some jurisdictions to circumvent the regulatory constraints generally associated with genetically modified organisms (GMOs) [149]. In fruit crops, researchers have used this technique to introduce precise changes at chosen genomic sites to improve flavor and nutrient content, modified flowering, and ripening times. For example, a tomato plant with benefits for both the consumer and the farmer was generated by genome editing. The CRISPR/Cas9-edited variety had uniform growth and produced larger (both size and number), tasty, and sweeter fruit. Notably, the researchers were able to increase the nutritional value of the tomato by lycopene enhancement (~500-fold) and greater vitamin C content, both linked to increased health benefits [150]. Many fruits and vegetables are relatively low in vitamin C, which has led to efforts to increase the vitamin C content of these foods through genetic engineering. One approach to increasing the vitamin C content of crops involves the use of transgenes that encode enzymes involved in vitamin C biosynthesis. For example, Bulley et al. [151] have shown in tomato, strawberry, and potato that overexpression of the GDP-L-galactose phosphorylase (GGP or VTC2) gene from kiwi fruit leads to increased accumulation of vitamin C.
Early flowering generally enables plants to have more flowers and fruits. For instance, accelerated flowering and early fruit yield were achieved in cultivated tomato plants modified by CRISPR/CaS9-mediated SELFPRUNING5G(SP5G) gene editing [152]. These tools are available for development of cultivars adapted to specific geographical locations.
Recently, scientists have focused on improvement and enhancement of quality attributes in ornamental plant species. Plant characteristics such as flower color and postharvest life can be addressed through genetic engineering. In early years, white-colored flowers generated by antisense RNA or RNAi targeting of structural genes, e.g., the chalcone synthase (CHS) gene, were produced in several plant species such as petunia, tobacco, and the garden plant Torenia hybrida [153,154,155]. In 1991, the Calgene Pacific company developed a rose variety called Blue Moon that was genetically modified to synthesize delphenedin, a pigment found in most blue flowers [156]. Today, work is ongoing to produce GM flowers with a broad color range and other features. Simultaneous targeting of two genes for flower color modification from white to yellow was attempted in African violet, a commercially valuable ornamental plant. Overexpression of chalcone 4-O-glucosyltransferase (4CGT) and aureusidin synthase (AS1) genes in African violet led to a color change of the flower petals. The flowers of the yellow-colored cultivar accumulated aureusidin 6-o-glucoside (AOG), a yellow-pigmented compound. In this case, both genes are required to induce expression of AOG to change the color of African violet flowers from white to yellow [157].
In a more recent study, mutations in the dihydroflavonol-4-reductase-B (DFR-B) gene resulted in white flowers in CRISPR/Cas9-edited Ipomea nil (Pharbitis) plants [158]. Furthermore, modification of a carotenoid cleavage dioxygenase (CCD) gene in Ipomea nil using the same approach caused white petals to turn pale yellow [159]. Ornamentals like petunia and torenia can represent model plants for studying floral color modifications produced by genetic manipulation. Transformation of torenia plants with a CRISPR/Cas9 construct targeting flavanone-3-hydroxylase (F3H), a flavonoid biosynthetic gene, resulted in pale blue flowers [160]. Genes responsible for other flower traits, such as enhancing flower longevity, have also been edited. For instance, editing the ethylene biosynthesis gene to decrease the production of ethylene in petunia improved flower longevity in the mutants compared to the wild-type counterparts [161]. Additionally, the transmission of the edited gene was also confirmed in the T1 generation with similar results to those of the T0 mutants. Such studies may accelerate the development and commercialization of genome-edited ornamentals.
The delivery of preassembled Cas9 protein-gRNA ribonucleoproteins (RNPs) into plant cells can also result in genome editing without the integration of the transgene. Thus, scientists were able to deliver RNPs across the protoplast membrane and subsequently generated petunias with modified pale purple-pink color from an engineered protoplast [162]. However, the overall efficiency of this transient system was very low (only 1 out of 67 transformed lines exhibited the desired color). The low efficiency of this system notwithstanding, these studies offer a new mode of gene editing; however, it remains to be seen whether sufficiently high editing efficiencies can be achieved to make this a popular editing process.
8. Fungal and Bacterial Resistance
Plant pathogens reduce crop yields by adversely affecting plant growth and development. It has been estimated that diseases globally reduce crop yields by 20–40% [163]. In the past decade, there has been a surge in the use of genome editing technologies in generating crops that are resistant to a wide range of pathogens. Several studies of targeted mutagenesis in crop plants, including deletions, insertions, and replacement of DNA of various sizes at targeted sites, have proven to be a promising approach to improve plant resistance to pathogens.
Developing plant resistance by modifying host S genes such as those belonging to the mlo (Mildew Resistant Locus O) gene family has been effective in apple, tomato, barley, and wheat [164]. However, a common disadvantage of S gene mutation is the concomitant negative impact on plant growth and productivity [165,166]. Therefore, a CRISPR/Cas9-induced targeted deletion in the MLO-B1 locus resulted in a mutant wheat variety (Tamlo-R32) that thrived better and maintained growth and yields while conserving resistance to powdery mildew [167]. In another study conducted by Peng et al. [168] on citrus plants, CRISPR/Cas9-targeted modification of the S gene CsLOB1 was performed to enhance resistance to citrus canker. Some recent experiments on rice reported the use of CRISPR/Cas9 technology to induce mutagenesis in the promoter region of bacterial blight S genes, OsSWEET14 and OsSWEET11 [169]. Antony et al. [170] developed blight-resistant rice plants with an OsSWEET14 TDNA insertion mutant but when compared to wild-type plants the mutants had smaller seeds. In contrast, resistance against rice blight with no growth defects was detected in the TALE-edited OsSWEET14 gene in super basmati rice [171]. These results suggest that engineering S genes through genome editing technology is a potential strategy to enhance rice resistance to blight caused by Xanthomonas oryzae pv. Oryzae. Thus, with rice production threatened by bacterial blight causing major crop losses, rapid and durable methods are desperately needed.
In addition to the knockout of host genes to improve disease resistance, endophyte-derived genes can serve as additional routes for improvement of wheat traits. In a recent study by Wang et al. [172], the authors identified a Fusarium resistance gene (Fhb7), which was shown to be horizontally transferred from an endophytic fungus and conferred resistance to Fusarium head blight (FHB), a significant fungal disease of wheat. The researchers demonstrated that introgression of Fhb7 into the genome of many commercial wheat cultivars conferred tolerance to FHB without negatively affecting growth yields, suggesting that Fhb7 is a potential candidate for engineering blight resistance in elite wheat varieties. Similarly, in tomato plants, CRISPR/Cas 9-mediated knockout of the DMR6 gene enhanced resistance to bacterial pathogens including P. syringae, P. capsici, and Xanthomonas spp., with no adverse effects on plant development and growth. As more targets are identified, it is expected that there will be many more successful studies on durable and broad-spectrum disease resistance using the CRISPR/Cas9 approach in a wide range of crops.
The effect of overexpression of plant proteins such as ribosome-inactivating proteins (RIPs) on performance of fungal and bacterial pathogens has been investigated [173]. Some interesting examples, such as overexpression of the PhRIP gene in transgenic potato, protected the plants against damage from Botrytis cinerea and Rhizoctonia solani, whereas expressing the RIP alpha-MMC gene improved resistance to rice blast fungus in rice [174,175]. Other proteins, including PFLP (plant ferredoxin-like protein) and HRAP (hypersensitive response-assisting proteins), are effective against multiple bacterial pathogens when they are overexpressed in rice, banana, and other species [176,177]. Recently, overexpression of PFLP and HRAP genes in greenhouse and field-grown bananas indicated that both genes are effective against bacterial wilt caused by Xanthomonas spp. [178]. In an earlier study, a combination of both genes did not provide any additional benefits in banana, yet the authors speculate that bananas expressing both genes may be more durable [179]. Furthermore, the disease-resistance trait was passed on to the next generation of transgenic lines. Additionally, the authors noted no difference in the agronomic traits, including yield and flowering of field-grown symptom-free bananas. These examples are helpful in revealing the biological function of plant proteins that can be beneficial when engineering crop tolerance to pathogen attacks.
Harpins may act in extracellular spaces in plant tissue, facilitating recognition by the plant [180]. Harpins are effective against multiple pathogens when overexpressed in tobacco, rice, canola, and cotton [181,182,183,184,185]. The effect of overexpression of harpinxooc encoding the hrf2 gene on the performance of an oomycete pathogen was investigated by Niu et al. [186]. The results demonstrate that transgenic soybeans expressing the hrf2 gene showed enhanced resistance to Phytophthora sojae. This study provides a valuable insight toward the functional role of the hrf2 gene in plant defense against P. sojae, opening new avenues for understanding other important pathogens as well as subsequently engineering broad-spectrum disease resistance in soybean.
The ability of plants to utilize diverse classes of immune receptors to perceive the presence of pathogenic microbes makes possible the transferring and engineering of these receptors to improve recognition capacities [187]. Recently, Ercoli et al. [188] conducted studies to show how the XA21 receptor in rice recognizes and resists infection caused by Xanthomonas oryzae. Other immune receptors have been targeted for modification in potato, apple, and rice. A late blight-resistant potato with a NOD-like receptor (NLR) introduced from a wild relative is currently on the market in the UK [12]. In apples, modification of the HcrVf2 gene encoding such a receptor conferred resistance against the devastating fungal scab Venturia inaequalis [189]. Xu and colleagues [190] showed that rice plants constantly expressing an immune regulator gene, NPR1 (non-expressor of pathogenesis-related genes 1), conferred resistance to bacterial blight but displayed growth defects. However, when the authors controlled NPR1 expression, it resulted in increased accumulation of NPR1 upon pathogen infection, enhancing resistance to bacterial blight without negatively affecting plant growth and grain yield. Although this method enables researchers to obtain plants with strong immunity, durability can be challenging because pathogens are evolving rapidly.
An important strategy in the fight against Verticillium wilt (VW), caused by fungi belonging to the genus Verticillium, is to silence genes essential for spore production, hyphal development, and pathogenicity. RNAi-mediated silencing of the VdRGS1 gene has been achieved in cotton, resulting in transgenic plants with enhanced resistances to VW. With the help of RNAi technology, Govindrajulu et al. [191] showed that the transgenic lettuce containing a modified construct of highly abundant message #34 (HAM34) and cellulose synthase (CES1) genes showed resistance against a biotrophic pathogen that causes downy mildew of lettuce. Similarly, a PsFUZ7 RNAi construct expressed in transgenic wheat conferred strong resistance to wheat stripe rust. Additionally, knocking down the transcription factor gene OsERF922 (ethylene responsive factor) using RNAi results in increased resistance against the pathogen Magnoporthe oryzae [192]. The improvement of rice blast resistance via CRISPR/Cas9, which targeted knockdown of the OsERF922 gene in a japonica rice variety cultivated in northern China, was reported by Wang et al. [193]. This modification led to no detrimental effects on rice growth and development. Overall, RNAi appears to be a promising approach to control the detrimental effects of many fungi and oomycetes.
9. Increasing Plant Yield
One of the main objectives of growing plants, whether the plants are transgenic or non-transgenic, is to increase the yield of the target plant. Moreover, plant yield is important whether the plant is intended to be used as a food or as the source of a cloned protein. In addition, increasing crop yields can decrease the amount of land that is required for agricultural production [194].
In recent years, scientists have developed several unique schemes intended to increase the yield of various plants. For example, one group of scientists developed an approach that they called ‘speed breeding’ that is applicable to essentially all crops [195]. Speed breeding entails extending the plant’s photoperiod, controlling the plant’s growth temperature, and selection of fast-growing seeds. Of course, to more rapidly grow plants, controlling their growth environment requires the extensive use of greenhouses. In this environment, the lighting may be supplemented with artificial electric lamps [196], the photoperiod may be extended [197], and the wavelength of the lighting may be altered [198]. Following these protocols, researchers have reduced the growth cycle of many different plants to an average of half of what it was previously, thereby enabling the controlled growth of additional generations of the same plant [195].
Scientists have developed several different schemes in an effort to increase plant crop yields. For example, traditional varieties of wheat and rice allocate a significant fraction of their resources to producing vegetative tissues rather than grain or reproductive tissues [199]. However, semi-dwarf varieties previously developed by conventional breeding during the so-called green revolution [200] allocate a greater portion of their resources to grain rather than to vegetative (leaf) tissues. By genetic modulation of the levels of the phytohormones gibberellin and brassinosteroid, it should be possible to produce dwarf plant strains that allocate even more resources to grain instead of vegetative tissues, and thereby further increase the grain yield.
In another study, researchers observed that they were able to genetically modify tomato plants to increase their harvest index, which is the ratio of fruit yield to total plant biomass [201]. This was accomplished by introducing a chloroplast-targeted cyanobacterial flavodoxin gene into tomato plants. In this case, the transgenic plants were generally smaller than the wild-type plants with a higher number of tomato fruits per plant. Moreover, the overall yield of tomato fruit could be augmented by increasing the density of plants in the field (i.e., by planting the transgenic plants close together). In a separate study, scientists increased the expression of the maize (corn) MADS-box transcription factor gene zmm28 by placing this gene under the control of a moderate-level constitutive maize promoter [202]. In field trials, the transgenic plants with increased expression of the zmm28 gene showed an increase in carbon assimilation, nitrogen utilization, and plant growth, all leading to an increase in grain yield relative to the cultivar of wild-type maize used in this study. Methylation of the N6 position of adenosine residues in RNA molecules is common in plants (as well as in other higher eukaryotes), and this modification is believed to regulate RNA processing and metabolism [203]. In a recent experiment, scientists introduced and expressed the human demethylase FTO gene into rice and potato plants [204]. Expression of this transgene resulted in an exceptionally large increase in rice grain yields in plants that were grown in the greenhouse and a smaller, although still highly significant, increase in rice and potato yield and biomass when plants were grown in the field. In this experiment, expression of the transgene caused an increase of root meristem proliferation and tiller bud formation as well as overall plant photosynthetic efficiency and drought tolerance, suggesting that modulating RNA methylation is an effective way to improve plant yield. Scientists who generated transgenic rice by overexpressing the rice gene encoding a plasma membrane H+-ATPase 1 gene (i.e., OSA1) found that the resultant transgenic plants significantly improved their utilization of nitrogen and carbon resources compared to wild-type [205]. As a result of this manipulation, the transgenic plants showed a large increase in grain yield.
In addition to introducing exogenous genes to increase plant yield, some researchers have used the CRISPR/Cas9 system to modify some of the existing plant genes (i.e., this technique enables scientists to directly alter specific plant genes) to achieve the same ends as genetic transformation [206]. In one instance, in the T2 generation following the genomic modification, three of the four rice plant mutants that were created yielded an increased number of rice grains and had a larger grain size. Also using the CRISPR/Cas9 system, the alteration of multiple genes in a single tomato cultivar worked together to affect the yield of rice. In this case, the CRISPR/Cas9 system was used to edit six independent loci that were important for controlling the yield and productivity in a wild tomato (Solanum pimpinellifolium) crop line [150]. When the engineered tomato plant was compared to the wild-type plant, it was observed to have a three-fold increase in fruit size and a ten-fold increase in fruit number. In addition to these examples, researchers have used the CRISPR/Cas9 system to modify an already shortened version of canola (by conventional breeding) so that the plant has more branches, resulting in the formation of more flowers and pods. This was achieved by knocking out genes for receptors that perceive the hormone strigolactone [207]. Another group of researchers used the CRISPR/Cas9 system to knock out the rice OsPDCD5 gene, which is involved in programmed cell death [208]. Mutating this gene decreased auxin synthesis by the modified plant in addition to lowering gibberellin and cytokinin synthesis and signaling pathways. Moreover, rice that contained mutations in the OsPDCD5 gene had an increased yield of rice grains.
From the above cursory exploration of some strategies that have been employed to increase plant fruit or grain yield, it is clear that plant yield is controlled by a relatively large number of different genes. The expression of some of these genes may be increased while the expression of others is decreased with the same ultimate result, i.e., the yield is increased. Moreover, in some instances, the addition of foreign genes may also result in an increase in yield. While most of these genetic manipulations have yet to be tested and proven in the field, there is every reason to expect that the approaches described here will eventually lead to plants with much greater fruit, seed, and grain yield than is currently available from wild-type plants, including plants that have been manipulated by traditional breeding techniques.
10. Conclusions
Given the current ease and rapidity with which higher plants can be genetically engineered to yield both highly improved plants and plants inexpensively yielding compounds designed to improve human health and life, it is likely that within the next 10–20 years plants will replace most other means of producing these compounds, i.e., the use of bacterial, fungal, and animal cells in culture. In this regard, following extensive testing, the proven safety and efficaciousness of purified compounds will negate the issue of how these compounds are produced. It will, however, become necessary to develop new and different means of growing transgenic plants for the large-scale production of compounds such as pharmaceuticals. It is therefore possible that plants producing specific useful compounds will be grown hydroponically as a means of protecting valuable agricultural land [209].
Author Contributions
Writing—original draft preparation, Z.N. and B.R.G.; writing—review and editing, Z.N. and B.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data were reported in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DDT—A Brief History and Status. 2023. Available online: https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status (accessed on 11 January 2023).
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Schnepf, H.E.; Whiteley, H.R. Cloning and Expression of the Bacillus Thuringiensis Crystal Protein Gene in Escherichia Coli. Proc. Natl. Acad. Sci. USA 1981, 78, 2893–2897. [Google Scholar] [CrossRef] [PubMed]
- Ely, S. The Engineering of Plants to Express Bacillus Thuringiensis δ-Endotoxins. In Bacillus Thuringiensis, an Environmental Biopesticide: Theory and Practice; Entwistle, P.F., Cory, J.S., Bailey, M.J., Higgs, S., Eds.; John Wiley & Sons: Chichester, UK, 1993; pp. 105–124. [Google Scholar]
- Aronson, A.I.; Shai, Y. Why Bacillus Thuringiensis Insecticidal Toxins Are so Effective: Unique Features of Their Mode of Action. FEMS Microbiol. Lett. 2001, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cerda, H.; Paoletti, M.G. Genetic Engineering with Bacillus Thuringiensis and Conventional Approaches for Insect Resistance in Crops. Crit. Rev. Plant Sci. 2004, 23, 317–323. [Google Scholar] [CrossRef]
- Halpin, C. Gene stacking in transgenic plants—The challenge for 21st century plant biotechnology. Plant Biotechnol. J. 2005, 3, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Strategies for Enhanced Crop Resistance to Insect Pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef]
- Rauf, I.; Javaid, S.; Naqvi, R.Z.; Mustafa, T.; Amin, I.; Muktar, Z.; Jander, G.; Mansoor, S. In-Planta Expression of Insecticidal Proteins Provides Protection against Lepidopteran Insects. Sci. Rep. 2019, 9, 6745. [Google Scholar] [CrossRef] [PubMed]
- Montagu, M. The Future of Plant Biotechnology in a Globalized and Environmentally Endangered World. Genet. Mol. Biol. 2020, 43, e20190040. [Google Scholar] [CrossRef] [PubMed]
- Bel, Y.; Ferré, J.; Hernández-Martínez, P. Bacillus Thuringiensis Toxins: Functional Characterization and Mechanism of Action. Toxins 2020, 12, 785. [Google Scholar] [CrossRef]
- ISAAA Inc. Available online: http://www.isaaa.org/gmapprovaldatabase/ (accessed on 11 January 2023).
- Gaur, R.K.; Ali, A.; Cheng, X.; Mäkinen, K.; Agindotan, B.; Wang, X. Editorial: Plant Viruses, Volume II: Molecular Plant Virus Epidemiology and Its Management. Front. Microbiol. 2021, 12, 756807. [Google Scholar] [CrossRef]
- Beachy, R.N.; Loesch-Fries, S.; Tumer, N.E. Coat Protein-Mediated Resistance against Virus Infection. Annu. Rev. Phytopathol. 1990, 28, 451–474. [Google Scholar] [CrossRef]
- Fitchen, J.H.; Beachy, R.N. Genetically Engineering Protection against Viruses in Transgenic Plants. Annu. Rev. Microbiol. 1993, 47, 739–763. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Gonsalves, D. Resistance of Transgenic Hybrid Squash ZW-20 Expressing the Coat Protein Genes of Zucchini Yellow Mosaic Virus and Watermelon Mosaic Virus 2 to Mixed Infections by Both Potyviruses. Bio/Technology 1995, 13, 1466–1473. [Google Scholar] [CrossRef]
- Tricoli, D.M.; Carney, K.J.; Russell, P.F.; McMaster, J.R.; Groff, D.W.; Hadden, K.C.; Himmel, P.T.; Hubbard, J.P.; Boeshore, M.L.; Quemada, H.D. Field Evaluation of Transgenic Squash Containing Single or Multiple Virus Coat Protein Gene Constructs for Resistance to Cucumber Mosaic Virus, Watermelon Mosaic Virus 2, and Zucchini Yellow Mosaic Virus. Bio/Technology 1995, 13, 1458–1465. [Google Scholar] [CrossRef]
- Gonsalves, D. Control of papaya ringspot virus in papaya: A case study. Annu. Rev. Phytopathol. 1998, 36, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Lindbro, J.A.; Falk, B.W. The Impact of “coat Protein-Mediated Virus Resistance in Applied Plant Pathology and Basic Research. Phytopathology 2017, 107, 624–634. [Google Scholar] [CrossRef]
- Niraula, P.M.; Fondong, V.N. Development and Adoption of Genetically Engineered Plants for Virus Resistance: Advances, Opportunities and Challenges. Plants 2021, 10, 2339. [Google Scholar] [CrossRef]
- Report Linker. Available online: https://www.reportlinker.com/report-summary/Pesticide/20359/North-American-Pesticide-Industry.html (accessed on 6 April 2023).
- Schütte, G.; Eckerstorfer, M.; Rastelli, V.; Reichenbecher, W.; Restrepo-Vassalli, S.; Ruohonen-Lehto, M.; Saucy, A.-G.W.; Mertens, M. Herbicide resistance and biodiversity: Agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ. Sci. Eur. 2017, 29, 5. [Google Scholar] [CrossRef]
- UCBiotech. Available online: http://ucbiotech.org/biotech_info/PDFs/Sankula_2005_BiotechnologyDerivedCropsPlantedin2004.pdf (accessed on 11 January 2023).
- Funke, T.; Han, H.; Healy-Fried, M.L.; Schönbrunn, E. Molecular Basis for the Herbicide Resistance of Roundup Ready Crops. Proc. Natl. Acad. Sci. USA 2006, 103, 13010–13015. [Google Scholar] [CrossRef]
- Cruz, R.; Domínguez-Martínez, P.A.; Silveira, H.; Cruz-Hipólito, H.E.; Palma-Bautista, C.; Vázquez-García, J.G.; Domínguez-Valenzuela, J.A.; Prado, R. Management of Glyphosate-Resistant Weeds in Mexican Citrus Groves: Chemical Alternatives and Economic Viability. Plants 2019, 8, 325. [Google Scholar] [CrossRef]
- Kniss, A.R. Genetically Engineered Herbicide—Resistant Crops and Herbicide—Resistant Weed Evolution in the United States. Weed Sci. 2018, 66, 260–273. [Google Scholar] [CrossRef]
- Tarazona, J.V.; Court-Marques, D.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate Toxicity and Carcinogenicity: A Review of the Scientific Basis of the European Union Assessment and Its Differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef]
- Li, Y.J.Z.; Wu, H.; Liu, C.; Huang, C.; Lan, J.; Zhao, Y.; Xie, C. Precise Base Editing of Non-Allelic Acetolactate Synthase Genes Confers Sulfonylurea Herbicide Resistance in Maize. Crop J. 2020, 8, 449–456. [Google Scholar] [CrossRef]
- Fartyal, D.; Agarwal, A.; James, D.; Borphukan, B.; Ram, B.; Sheri, V.; Agarwal, P.K.; Achary, V.M.M.; Reddy, M.K. Developing Dual Herbicide Tolerant Transgenic Rice Plants for Sustainable Weed Management. Sci. Rep. 2018, 8, 11598. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Huang, Y.; Wang, K. The Development of Herbicide Resistance Crop Plants Using CRISPR/Cas9-Mediated Gene Editing. Genes 2021, 12, 912. [Google Scholar] [CrossRef]
- Duke, S.O. Perspectives on Transgenic, Herbicide-Resistant Crops in the United States Almost 20 Years after Introduction. Pest Manag. Sci. 2015, 71, 652–657. [Google Scholar] [CrossRef]
- Hernandez, I.; Bott, S.W.; Patel, A.S.; Wolf, C.G.; Hospodar, A.R.; Sampathkumar, S.; Shrank, W.H. Pricing of Monoclonal Antibody Therapies: Higher If Used for Cancer? A. J. Manag. Care 2018, 24, 109–112. [Google Scholar]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel PEff Vector Based on the Genome of Potato Virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef]
- Schähs, M.; Strasser, R.; Stadlmann, J.; Kunert, R.; Rademacher, T.W.; Steinkellner, H. Production of a Monoclonal Antibody in Plants with a Humanized N-Glycosylation Pattern. Plant Biotechnol. J. 2007, 5, 657–663. [Google Scholar] [CrossRef]
- Casthilho, A.; Gattinger, P.; Grass, J.; Jez, J.; Pabst, M.; Altmann, F.; Gorfer, M.; Strasser, R.; Steinkellner, H. N-Glycosylation Engineering of Plants for the Biosynthesis of Glycoproteins with Bisected and Branched Complex N-Glycans. Glycobiology. 2011, 21, 813–823. [Google Scholar] [CrossRef]
- Castilho, A.; Steinkellner, H. Glyco-Engineering in Plants to Produce Human-like N-Glycan Structures. Biotechnol. J. 2012, 7, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Rozov, S.M.; Permyakova, N.V.; Deineko, E.V. Main Strategies of Plant Expression System Glycoengineering for Producing Humanized Recombinant Pharmaceutical Proteins. Biochemistry 2018, 83, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Patten, C.L. Molecular Biotechnology: Principles and Applications of Recombinant DNA Technology, 6th ed.; American Society for Microbiology Press: Washington, DC, USA, 2022. [Google Scholar]
- Yusibov, V.; Kushnit, N.; Streatfield, S.J. Antibody Production in Plants and Green Algae. Annu. Rev. Plant Biol. 2016, 67, 669–701. [Google Scholar] [CrossRef]
- Hiatt, A.; Cafferkey, R.; Bowdish, K. Production of Antibodies in Transgenic Plants. Nature 1986, 342, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, L.; Olmsted, S.S.; Moench, T.R.; Co, M.S.; Martinell, B.J.; Paradkar, V.M.; Russell, D.R.; Queen, C.; Cone, R.A.; Whaley, K.J. A Humanized Monoclonal Antibody Produced in Transgenic Plants for Immunoprotection of the Vagina against Genital Herpes. Nat. Biotechnol. 1998, 16, 1361–1364. [Google Scholar] [CrossRef]
- Lai, H.; Engle, M.; Fuchs, A.; Chen, Q. Monoclonal Antibody Produced in Plants Efficiently Treats West Nile Virus Infection in Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 2419–2424. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, D.Y.; Lee, K.-J.; Kim, Y.-K.; So, Y.-K.; Ryu, J.-S.; Oh, S.-H.; Han, Y.-S.; Ko, K.; Choo, Y.-K.; et al. Intracellular Programming of Expression, Glycosylation, and Function of a Plant-Derived Antiviral Therapeutic Monoclonal Antibody. PLoS ONE 2013, 8, e68772. [Google Scholar]
- Ma, J.K.-C.; Drossard, J.; Lewis, D.; Altmann, F.; Boyle, J.; Christou, P.; Cole, T.; Dale, P.; van Dolleweerd, C.J.; Isitt, V.; et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 2015, 13, 1106–1120. [Google Scholar] [CrossRef]
- Donini, M.; Marusic, C. Current State-of-the-Art in Plant-Based Antibody Production Systems. Biotechnol. Lett. 2019, 41, 335–346. [Google Scholar] [CrossRef]
- Nessa, M.U.; Rahman, M.A.; Kabir, Y. Plant-Produced Monoclonal Antibody as Immunotherapy for Cancer. BioMed. Res. Internat 2020, 2020, 3038564. [Google Scholar] [CrossRef]
- Singh, A.A.; Pooe, O.; Kwezi, L.; Lotter-Stark, T.; Stoychev, S.H.; Alexandra, K.; Gerber, I.; Bhiman, J.N.; Vorster, J.; Pauly, M.; et al. Plant-Based Production of Highly Potent Anti-HIV Antibodies with Engineered Posttranslational Modifications. Sci. Rep. 2020, 10, 6201. [Google Scholar] [CrossRef] [PubMed]
- Jugler, C.; Sun, H.; Grill, F.; Kibler, K.; Esqueda, A.; Lai, H.; Li, Y.; Lake, D.; Chen, Q. Potential for a plant-made SARS-CoV-2 neutralizing monoclonal antibody as a synergistic cocktail component. Vaccines 2022, 10, 772. [Google Scholar] [CrossRef]
- Rituximab Biosimilar Successfully Produced in Plants. Available online: https://www.gabionline.net/biosimilars/news/Rituximab-biosimilar-successfully-produced-in-plants (accessed on 6 April 2023).
- Zeitlin, L.; Bohorov, O.; Bohorova, N.; Hiatt, A.; Kim, D.H.; Pauly, M.H.; Velasco, J.; Whaley, K.J.; Barnard, D.L.; Bates, J.T.; et al. Prophylactic and Therapeutic Testing of Nicotiana-Derived RSV-Neutralizing Human Monoclonal Antibodies in the Cotton Rat Model. MAbs 2013, 5, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, J. Tobacco against Ebola Virus Disease. Przegl. Lek. 2015, 72, 567–571. [Google Scholar]
- Almaraz-Delgado, A.L.; Flores-Uribe, J.; Pérez-España, V.H.; Salgado-Manjarrez, E.; Badillo-Corona, J.A. Production of Therapeutic Proteins in the Chloroplast of Chlamydomonas Reinhardtii. AMB Express 2014, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Larrick, J.W.; Yu, L.; Chen, J.; Jaiswal, S.; Wycoff, K. Production of Antibodies in Transgenic Plants. Res. Immunol. 1998, 149, 603–608. [Google Scholar] [CrossRef]
- O’Hara, J.M.; Whaley, K.; Pauly, M.; Zeitlin, L.; Mantis, N.J. Plant-Based Expression of a Partially Humanized Neutralizing Monoclonal IgG Directed against an Immunodominant Epitope on the Ricin Toxin A Subunit. Vaccines 2012, 30, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Wycoff, K.; Maclean, J.; Belle, A.; Yu, L.; Tran, Y.; Roy, C.; Hayden, F. Anti-Infective Immunoadhesins from Plants. Plant Biotechnol. J. 2015, 13, 1078–1093. [Google Scholar] [CrossRef]
- Brodzik, R.; Spitsin, S.; Golovkin, M.; Bandurska, K.; Portocarrero, C.; Okulicz, M.; Steplewski, Z.; Koprowski, H. Plant-Derived EpCAM Antigen Induces Protective Anti-Cancer Response. Cancer Immunol. Immunother. 2008, 57, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Farré, G.; Molinos-Albert, L.M.; Evans, A.; Canela-Xandri, A.; Twyman, R.M.; Carrillo, J.; Ordóñez, R.A.; Shattock, R.J.; O’Keefe, B.R.; et al. Unexpected synergistic HIV neutralization by a triple microbicide produced in rice endosperm. Proc. Natl. Acad. Sci. USA 2018, 115, E7854–E7862. [Google Scholar] [CrossRef]
- Politch, J.A.; Cu-Uvin, S.; Moench, T.R.; Tashima, K.T.; Marathe, J.G.; Guthrie, K.M.; Cabral, H.; Nyhuis, T.; Brennan, M.; Zeitlin, L.; et al. Safety, Acceptability, and Pharmacokinetics of a Monoclonal Antibody-Based Vaginal Multipurpose Prevention Film (MB66): A Phase I Randomized Trial. PLoS Med. 2021, 18, e1003495. [Google Scholar] [CrossRef]
- Ventria. Available online: https://ventria.com/ (accessed on 6 April 2023).
- Invitria. Available online: https://invitria.com/ (accessed on 6 April 2023).
- Tacket, C.O. Plant-Based Vaccines against Diarrheal Diseases. Trans. Am. Clin. Climatol. Assoc. 2007, 118, 79–87. [Google Scholar]
- Landry, N.; Pillet, S.; Favre, D.; Poulin, J.-F.; Trépanier, S.; Yassine-Diab, B.; Ward, B.J. Influenza Virus-like Particle Vaccines Made in Nicotiana Benthamiana Elicit Durable, Poly-Functional and Cross-Reactive T Cell Responses to Influenza HA Antigens. Clin. Immunol. 2014, 154, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Li, J.-Y.; Tien, N.-Q.-D.; Yang, M.-S. Expression and Assembly of Cholera Toxin B Subunit and Domain III of Dengue Virus 2 Envelope Fusion Protein in Transgenic Potatoes. Protein Expr. Purif. 2017, 139, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pfizer and Protalix BioTherapeutics Announce FDA Approval of EELELYSOTM (Taliglucerase Alfa) for the Treatment of Gaucher Disease. Available online: https://www.pfizer.com/news/press-release/ (accessed on 6 April 2023).
- Mason, H.S.; Haq, T.A.; Clements, J.D.; Arntzen, C.J. Edible Vaccine Protects Mice against Escherichia Coli Heat-Labile Enterotoxin (LT): Potatoes Expressing a Synthetic LT-B Gene. Vaccines 1998, 16, 1336–1343. [Google Scholar] [CrossRef]
- Azzoni, A.R.; Kusnadi, A.R.; Miranda, E.A.; Nikolov, Z.L. Recombinant Aprotinin Produced in Transgenic Corn Seed: Extraction and Purification Studies. Biotechnol. Bioeng. 2002, 80, 268–276. [Google Scholar] [CrossRef] [PubMed]
- SemBioSys. Available online: https://www.reuters.com/article/sembiosysgenetics-idUKN3034433020090730 (accessed on 6 April 2023).
- Zhang, X.; Buehner, N.A.; Hutson, A.M.; Estes, M.K.; Mason, H.S. Tomato Is a Highly Effective Vehicle for Expression and Oral Immunization with Norwalk Virus Capsid Protein. Plant Biotechnol. J. 2006, 4, 419–432. [Google Scholar] [CrossRef]
- Orfgenetics. Available online: https://www.orfgenetics.com/ (accessed on 6 April 2023).
- Medical Life Sciences News. Available online: https://www.news-medical.net/health/Tobacco-Plants-and-Drug-Development.aspx (accessed on 11 January 2023).
- Lomonossoff, G.P.; D’Aoust, M.-A. Plant-Produced Biopharmaceuticals: A Case of Technical Developments Driving Clinical Deployment. Science 2016, 353, 1237–1240. [Google Scholar] [CrossRef]
- Clark, M.; Maselko, M. Transgene biocontainment strategies for molecular farming. Front. Plant Sci. 2020, 11, 210. [Google Scholar] [CrossRef]
- Maxmen, A. The Fight to Manufacture COVID Vaccines in Lower-Income Countries. Nature 2021, 597, 455–457. [Google Scholar] [CrossRef]
- Miquel-Clopés, A.; Bentley, E.G.; Stewart, J.P.; Carding, S.R. Mucosal Vaccines and Technology. Clin. Exp. Immunol. 2019, 196, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Yuki, Y.; Nakahashi-Ouchida, R.; Fujhashi, K. Mucosal Vaccines: Wisdom from Now and Then. Internat. Immunol. 2021, 33, 767–774. [Google Scholar] [CrossRef]
- Shah, V.V.; Prajapati, R.A.; Shah, S.P.; Patel, S.R.; Patel, H.P. A Comprehensive Review on Edible Vaccine. J. Drug. Deiv. Therap. 2022, 12, 192–201. [Google Scholar] [CrossRef]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in Humans of an Edible Vaccine for Hepatitis B. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Ljunggren, M.K.; Jedlina, L.; Basalaj, K.; Legocki, A.; Wedrychowicz, H.; Kesik-Brodacka, M. A preliminary study of a lettuce-based edible vaccine expressing the cysteine proteinase of Fasciola hepatica for fasciolosis control in livestock. Front. Immunol. 2018, 9, 2592. [Google Scholar] [CrossRef]
- Bertini, E.; Merlin, M.; Gecchele, E.; Puggia, A.; Brozzetti, A.; Commisso, M.; Falorni, A.; Bini, V.; Klymyuk, V.; Pezzotti, M.; et al. Design of a type-1 diabetes vaccine candidate using edible plants expressing a major autoantigen. Front. Plant Sci. 2018, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Mandal, A.K.; Dwivedi, K.; Kumar, V. A Cross Talk between the Immunization and Edible Vaccine: Current Challenges and Future Prospects. Life Sci. 2020, 261, 118343. [Google Scholar] [CrossRef]
- Gunasekaran, B.; Gothandam, K.M. A Review on Edible Vaccine and Their Prospects. Brazil. J. Med. Biol. Res. 2020, 53, e8749. [Google Scholar] [CrossRef]
- Nakarhira, Y.; Mizuno, K.; Yamashita, H.; Tsuchikura, M.; Takeuchi, K.; Shiina, T.; Kawakami, H. Mass Production of Virus-like Particles Using Chloroplast Genetic Engineering for Highly Immunogenic Oral Vaccine against Fish Disease. Front. Plant Sci. 2021, 12, 717952. [Google Scholar] [CrossRef]
- Khalid, F.; Tahi, R.; Ellahi, M.; Amir, N.; Rizvi, S.F.A.; Hasnain, A. Emerging Trends of Edible Vaccine Therapy for Combating Human Diseases Especially COVID-19: Pros, Cons, and Future Challenges. Phytother. Res. 2022, 36, 2746–2766. [Google Scholar] [CrossRef]
- Patel, P.; Patel, R.; Patel, S.; Patel, Y.; Patel, M.; Trivedi, R. Edible vaccines: A nutritional substitute for traditional immunization. Pharmac. Rev. 2022, 16, 62–69. [Google Scholar] [CrossRef]
- Bolaños-Martínez, O.C.; Govea-Alonso, D.O.; Cervantes-Torres, J.; Hernández, M.; Fragoso, G.; Sciutto-Conde, E.; Rosales-Mendoza, S. Expression of Immunogenic Poliovirus Sabin Type 1 VP Proteins in Transgenic Tobacco. J. Biotechnol. 2020, 322, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.W.; Sherman, A.; Kwon, K.-C.; Chang, W.-J.; Daniell, H. Lettuce Plants Expressing High Levels of Factor VIII Antigen in the Chloroplast for Oral Tolerance in Hemophilia a. Blood 2017, 130, 362. [Google Scholar] [CrossRef]
- Orlova, N.A.; Kovnir, S.V.; Vorobiev, I.I.; Gabibov, A.G. Coagulation Factor IX for Hemophilia B Therapy. Acta Nat. 2012, 4, 62–73. [Google Scholar] [CrossRef]
- Martiniuk, F.; Reggi, S.; Tchou-Wong, K.-M.; Rom, W.N.; Busconi, M.; Fogher, C. Production of a Functional Human Acid Maltase in Tobacco Seeds: Biochemical Analysis, Uptake by Human GSDII Cells, and in Vivo Studies in GAA Knockout Mice. Appl. Biochem. Biotechnol. 2013, 171, 916–926. [Google Scholar] [CrossRef]
- Su, J.; Zhu, L.; Sherman, A.; Wang, X.; Lin, S.; Kamesh, A.; Norikane, J.H.; Streatfield, S.J.; Herzog, R.W.; Daniell, H. Low Cost Industrial Production of Coagulation Factor IX Bioencapsulated in Lettuce Cells for Oral Tolerance Induction in Hemophilia B. Biomaterials 2015, 70, 84–93. [Google Scholar] [CrossRef]
- Marsian, J.; Hurdiss, D.L.; Ranson, N.A.; Ritala, A.; Paley, R.; Cano, I.; Lomonossoff, G.P. Plant-Made Nervous Necrosis Virus-Like Particles Protect Fish Against Disease. Front. Plant Sci. 2019, 10, 880. [Google Scholar] [CrossRef] [PubMed]
- Detert, K.; Schmidt, H. Survival of Enterohemorrhagic Escherichia Coli O104:H4 Strain C227/11Φcu in Agricultural Soils Depends on RpoS and Environmental Factors. Pathogens 2021, 10, 1443. [Google Scholar] [CrossRef]
- Mason, H.S.; Ball, J.M.; Shi, J.J.; Jiang, X.; Estes, M.K.; Arntzen, C.J. Expression of Norwalk Virus Capsid Protein in Transgenic Tobacco and Potato and Its Oral Immunogenicity in Mice. Proc. Natl. Acad. Sci. USA 1996, 93, 5335–5340. [Google Scholar] [CrossRef]
- Mason, H.S.; Lam, D.M.; Arntzen, C.J. Expression of Hepatitis B Surface Antigen in Transgenic Plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef]
- Phan, H.T.; Pham, V.T.; Ho, T.T.; Pham, N.B.; Chu, H.H.; Vu, T.H.; Abdelwhab, E.M.; Scheibner, D.; Mettenleiter, T.C.; Hanh, T.X.; et al. Immunization with Plant-Derived Multimeric H5 Hemagglutinins Protect Chicken against Highly Pathogenic Avian Influenza Virus H5N1. Vaccines 2020, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Koya, V.; Leppla, S.H.; Daniell, H. Expression of Bacillus Anthracis Protective Antigen in Transgenic Chloroplasts of Tobacco, a Non-Food/Feed Crop. Vaccines 2004, 22, 4374–4384. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, P.B.; Hammond, J.; Dienelt, M.M.; Hooper, D.C.; Fu, Z.F.; Dietzschold, B.; Koprowski, H.; Michaels, F.H. Expression of the Rabies Virus Glycoprotein in Transgenic Tomatoes. Bio/Technology 1995, 13, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Ponndorf, D.; Meshcheriakova, Y.; Thuenemann, E.C.; Dobon Alonso, A.; Overman, R.; Holton, N.; Dowall, S.; Kennedy, E.; Stocks, M.; Lomonossoff, G.P.; et al. Plant-Made Dengue Virus-like Particles Produced by Co-Expression of Structural and Non-Structural Proteins Induce a Humoral Immune Response in Mice. Plant Biotechnol. J. 2021, 19, 745–756. [Google Scholar] [CrossRef]
- Webster, D.E.; Cooney, M.L.; Huang, Z.; Drew, D.R.; Ramshaw, I.A.; Dry, I.B.; Strugnell, R.A.; Martin, J.L.; Wesselingh, S.L. Successful Boosting of a DNA Measles Immunization with an Oral Plant-Derived Measles Virus Vaccine. J. Virol. 2002, 76, 7910–7912. [Google Scholar] [CrossRef]
- Porceddu, A.; Falorni, A.; Ferradini, N.; Cosentino, A.; Calcinaro, F.; Faleri, C.; Cresti, M.; Lorenzetti, F.; Brunetti, P.; Pezzotti, M. Transgenic Plants Expressing Human Glutamic Acid Decarboxylase (GAD65), a Major Autoantigen in Insulin-Dependent Diabetes Mellitus. Mol. Breed. 1999, 5, 553–560. [Google Scholar] [CrossRef]
- Di Bonito, P.; Grasso, F.; Mangino, G.; Massa, S.; Illiano, E.; Franconi, R.; Fanales-Belasio, E.; Falchi, M.; Affabris, E.; Giorgi, C. Immunomodulatory Activity of a Plant Extract Containing Human Papillomavirus 16-E7 Protein in Human Monocyte-Derived Dendritic Cells. Int. J. Immunopathol. Pharm. 2009, 22, 967–978. [Google Scholar] [CrossRef]
- Fay, P.C.; Attoui, H.; Batten, C.; Mohd Jaafar, F.; Lomonossoff, G.P.; Daly, J.M.; Mertens, P.P.C. Bluetongue Virus Outer-Capsid Protein VP2 Expressed in Nicotiana Benthamiana Raises Neutralising Antibodies and a Protective Immune Response in IFNAR (-/-) Mice. Vaccine X 2019, 2, 100026. [Google Scholar] [CrossRef]
- Arakawa, T.; Chong, D.K.; Langridge, W.H. Efficacy of a Food Plant-Based Oral Cholera Toxin B Subunit Vaccine. Nat. Biotechnol. 1998, 16, 292–297. [Google Scholar] [CrossRef]
- Sinha, P.; Davis, J.; Saag, L.; Wanke, C.; Salgame, P.; Mesick, J.; Horsburgh, C.R.; Hochberg, N.S. Undernutrition and Tuberculosis: Public Health Implications. J. Infect. Dis. 2019, 219, 1356–1363. [Google Scholar] [CrossRef]
- Farre, G.; Twyman, R.M.; Zhu, C.; Capell, T.; Christou, P. Nutritionally Enhanced Crops and Food Security: Scientific Achievements versus Political Expediency. Curr. Opin. Biotechnol. 2011, 22, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Al-Babili, S.; Klöti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the Provitamin A (β-Carotene) Biosynthetic Pathway into (Carotenoid-Free) Rice Endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the Nutritional Value of Golden Rice through Increased Pro-Vitamin A Content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; Al-Babili, S.; Tavazza, R.; Papacchioli, V.; Beyer, P.; Giuliano, G. Metabolic Engineering of Potato Carotenoid Content through Tuber-Specific Overexpression of a Bacterial Mini-Pathway. PLoS ONE 2007, 2, e350. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Diretto, G.; Parisi, B.; Giuliano, G.; Failla, M.L. Potential of Golden Potatoes to Improve Vitamin A and Vitamin E Status in Developing Countries. PLoS ONE 2017, 12, e0187102. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.-Y.; Khanna, H.; Kleidon, J.; Hoang, P.; Geijskes, J.; Daniells, J.; Zaplin, E.; Rosenberg, Y.; James, A.; Mlalazi, B.; et al. Golden Bananas in the Field: Elevated Fruit pro-Vitamin A from the Expression of a Single Banana Transgene. Plant Biotechnol. J. 2017, 15, 520–532. [Google Scholar] [CrossRef]
- De Steur, H.; Mehta, S.; Gellynck, X.; Finkelstein, J.L. GM Biofortified Crops: Potential Effects on Targeting the Micronutrient Intake Gap in Human Populations. Curr. Opin. Biotechnol. 2017, 44, 181–188. [Google Scholar] [CrossRef]
- Naqvi, S.; Zhu, C.; Farre, G.; Ramessar, K.; Bassie, L.; Breitenbach, J.; Perez Conesa, D.; Ros, G.; Sandmann, G.; Capell, T.; et al. Transgenic Multivitamin Corn through Biofortification of Endosperm with Three Vitamins Representing Three Distinct Metabolic Pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 7762–7767. [Google Scholar] [CrossRef]
- Singh, S.P.; Gruissem, W.; Bhullar, N.K. Single Genetic Locus Improvement of Iron, Zinc and β-Carotene Content in Rice Grains. Sci. Rep. 2017, 7, 6883. [Google Scholar] [CrossRef]
- Grand Challenges. Available online: https://www.gcgh.org (accessed on 20 February 2023).
- De Steur, H.; Gellynck, X.; Blancquaert, D.; Lambert, W.; Van Der Straeten, D.; Qaim, M. Potential Impact and Cost-Effectiveness of Multi-Biofortified Rice in China. New Biotechnol. 2012, 29, 432–442. [Google Scholar] [CrossRef]
- Connorton, J.M.; Jones, E.R.; Rodríguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat Vacuolar Iron Transporter TaVIT2 Transports Fe and Mn and Is Effective for Biofortification. Plant Physiol. 2017, 174, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Van Eenennaam, A.; Li, G.; Venkatramesh, M.; Levering, C.; Gong, X.; Jamieson, A.; Rebar, E.; Shewmaker, C.; Case, C. Elevation of Seed α-Tocopherol Levels Using Plant-Based Transcription Factors Targeted to an Endogenous Locus. Metab. Eng. 2004, 6, 101–108. [Google Scholar] [CrossRef] [PubMed]
- GMO News. Available online: https://non-gmoreport.com/gene-edited-soybean-failing-due-to-slow-adoption-by-farmers-low-crop-yields/ (accessed on 20 February 2023).
- MacIntosh, S.; Shaw, M.; Connelly, M.; Yao, Z. Food and Feed Safety of NS-B5ØØ27-4 Omega-3 Canola (Brassica Napus): A New Source of Long-Chain Omega-3 Fatty Acids. Front. Nutr. 2021, 8, 716659. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. GABA-Enriched Tomato Is First CRISPR-Edited Food to Enter Market. Nat. Biotechnol. 2022, 40, 9–11. [Google Scholar] [CrossRef]
- Schwall, G.P.; Safford, R.; Westcott, R.J.; Jeffcoat, R.; Tayal, A.; Shi, Y.-C.; Gidley, M.J.; Jobling, S.A. Production of Very-High-Amylose Potato Starch by Inhibition of SBE A and B. Nat. Biotechnol. 2000, 18, 551–554. [Google Scholar] [CrossRef]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-Amylose Wheat Generated by RNA Interference Improves Indices of Large-Bowel Health in Rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiao, G.; Liu, Z.; Zhang, X.; Li, J.; Guo, X.; Du, W.; Du, J.; Francis, F.; Zhao, Y.; et al. Generation of High-Amylose Rice through CRISPR/Cas9-Mediated Targeted Mutagenesis of Starch Branching Enzymes. Front. Plant Sci. 2017, 8, 298. [Google Scholar] [CrossRef]
- Le, H.; Nguyen, N.H.; Ta, D.T.; Le, T.N.T.; Bui, T.P.; Le, N.T.; Nguyen, C.X.; Rolletschek, H.; Stacey, G.; Stacey, M.G.; et al. CRISPR/Cas9-Mediated Knockout of Galactinol Synthase-Encoding Genes Reduces Raffinose Family Oligosaccharide Levels in Soybean Seeds. Front. Plant Sci. 2020, 11, 612942. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Kortesniemi, M.; Pariyani, R.; Zhang, Y.; Yang, B. Effects of Acylated and Nonacylated Anthocyanins Extracts on Gut Metabolites and Microbiota in Diabetic Zucker Rats: A Metabolomic and Metagenomic Study. Food Res. Int. 2022, 153, 110978. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.-P.; Matros, A.; Peterek, S.; Schijlen, E.G.W.M.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of Tomato Fruit with Health-Promoting Anthocyanins by Expression of Select Transcription Factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef]
- Ogo, Y.; Ozawa, K.; Ishimaru, T.; Murayama, T.; Takaiwa, F. Transgenic Rice Seed Synthesizing Diverse Flavonoids at High Levels: A New Platform for Flavonoid Production with Associated Health Benefits. Plant Biotechnol. J. 2013, 11, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of “Purple Endosperm Rice” by Engineering Anthocyanin Biosynthesis in the Endosperm with a High-Efficiency Transgene Stacking System. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J. Improving the Flavor of Fresh Fruits: Genomics, Biochemistry, and Biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Gruber, K. Agrobiodiversity: The Living Library. Nature 2017, 544, S8–S10. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.C.; Grimm, C.C. Identification of Volatile Compounds in Cantaloupe at Various Developmental Stages Using Solid Phase Microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Davidovich-Rikanati, R.; Sitrit, Y.; Tadmor, Y.; Iijima, Y.; Bilenko, N.; Bar, E.; Carmona, B.; Fallik, E.; Dudai, N.; Simon, J.E.; et al. Enrichment of Tomato Flavor by Diversion of the Early Plastidial Terpenoid Pathway. Nat. Biotechnol. 2007, 25, 899–901. [Google Scholar] [CrossRef]
- Chambers, A.H.; Pillet, J.; Plotto, A.; Bai, J.; Whitaker, V.M.; Folta, K.M. Identification of a Strawberry Flavor Gene Candidate Using an Integrated Genetic-Genomic-Analytical Chemistry Approach. BMC Genom. 2014, 15, 217. [Google Scholar] [CrossRef]
- Park, J.-I.; Lee, Y.-K.; Chung, W.-I.; Lee, I.-H.; Choi, J.-H.; Lee, W.-M.; Ezura, H.; Lee, S.-P.; Kim, I.-J. Modification of Sugar Composition in Strawberry Fruit by Antisense Suppression of an ADP-Glucose Pyrophosphorylase. Mol. Breed. 2006, 17, 269–279. [Google Scholar] [CrossRef]
- Bruening, G.; Lyons, J.M. The Case of the FLAVR SAVR Tomato. Calif. Agr. 2000, 54, 6–7. [Google Scholar] [CrossRef]
- Firoozbady, E.; Young, T.R. Pineapple Plant Named “Rose”. U.S. Patent PP25,763 P3, 4 August 2015. [Google Scholar]
- Pons, E.; Alquézar, B.; Rodríguez, A.; Martorell, P.; Genovés, S.; Ramón, D.; Rodrigo, M.J.; Zacarías, L.; Peña, L. Metabolic Engineering of β-Carotene in Orange Fruit Increases Its in Vivo Antioxidant Properties. Plant Biotechnol. J. 2014, 12, 17–27. [Google Scholar] [CrossRef]
- Cutanda-Perez, M.-C.; Ageorges, A.; Gomez, C.; Vialet, S.; Terrier, N.; Romieu, C.; Torregrosa, L. Ectopic Expression of VlmybA1 in Grapevine Activates a Narrow Set of Genes Involved in Anthocyanin Synthesis and Transport. Plant Mol. Biol. 2009, 69, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Lin-Wang, K.; Bolitho, K.; Grafton, K.; Kortstee, A.; Karunairetnam, S.; McGhie, T.K.; Espley, R.V.; Hellens, R.P.; Allan, A.C. An R2R3 MYB Transcription Factor Associated with Regulation of the Anthocyanin Biosynthetic Pathway in Rosaceae. BMC Plant Biol. 2010, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red Colouration in Apple Fruit Is Due to the Activity of the MYB Transcription Factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Elitzur, T.; Yakir, E.; Quansah, L.; Zhangjun, F.; Vrebalov, J.; Khayat, E.; Giovannoni, J.J.; Friedman, H. Banana MaMADS Transcription Factors Are Necessary for Fruit Ripening and Molecular Tools to Promote Shelf-Life and Food Security. Plant Physiol. 2016, 171, 380–391. [Google Scholar] [CrossRef]
- Dandekar, A.M.; Teo, G.; Defilippi, B.G.; Uratsu, S.L.; Passey, A.J.; Kader, A.A.; Stow, J.R.; Colgan, R.J.; James, D.J. Effect of Down-Regulation of Ethylene Biosynthesis on Fruit Flavor Complex in Apple Fruit. Transgenic Res. 2004, 13, 373–384. [Google Scholar] [CrossRef]
- Gao, M.; Matsuta, N.; Murayama, H.; Toyomasu, T.; Mitsuhashi, W.; Dandekar, A.M.; Tao, R.; Nishimura, K. Gene Expression and Ethylene Production in Transgenic Pear (Pyrus Communis Cv. ‘La France’) with Sense or Antisense CDNA Encoding ACC Oxidase. Plant Sci. 2007, 173, 32–42. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Gunaseelan, K.; Wang, M.Y.; Luo, L.; Wang, T.; Norling, C.L.; Johnston, S.L.; Maddumage, R.; Schröder, R.; Schaffer, R.J. Dissecting the Role of Climacteric Ethylene in Kiwifruit (Actinidia Chinensis) Ripening Using a 1-Aminocyclopropane-1-Carboxylic Acid Oxidase Knockdown Line. J. Exp. Bot. 2011, 62, 3821–3835. [Google Scholar] [CrossRef]
- López-Gómez, R.; Cabrera-Ponce, J.L.; Saucedo-Arias, L.J.; Carreto-Montoya, L.; Villanueva-Arce, R.; Díaz-Perez, J.C.; Gómez-Lim, M.A.; Herrera-Estrella, L. Ripening in Papaya Fruit Is Altered by ACC Oxidase Cosuppression. Transgen. Res. 2009, 18, 89–97. [Google Scholar] [CrossRef]
- Stowe, E.; Dhingra, A. Development of the Arctic® Apple. Plant Breed. Rev. 2021, 44, 273–296. [Google Scholar] [CrossRef]
- APHIS. Interpretative Rule on Okanagan Specialty Fruits. Petition for Determination of Nonregulated Status: Arctic™ Apple (Malus × Domestica), Events GD743 and GS784, Docket No. APHIS-2012-0025. 2012. [Google Scholar]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, R.B.; Besong, T.M.D.; Sheldon, J.; Stiegelmeyer, S.; Perez, L.; et al. Genetic Improvement of Tomato by Targeted Control of Fruit Softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef]
- .Waltz, E. CRISPR-Edited Crops Free to Enter Market, Skip Regulation. Nat. Biotechnol. 2016, 34, 582. [Google Scholar] [CrossRef] [PubMed]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De Novo Domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Bulley, S.; Wright, M.; Rommens, C.; Yan, H.; Rassam, M.; Lin-Wang, K.; Andre, C.; Brewster, D.; Karunairetnam, S.; Allan, A.C.; et al. Enhancing Ascorbate in Fruits and Tubers through Over-Expression of the l-Galactose Pathway Gene GDP-l-Galactose Phosphorylase. Plant Biotechnol. J. 2012, 10, 390–397. [Google Scholar] [CrossRef]
- Soyk, S.; Müller, N.A.; Park, S.J.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; Van Eck, J.; Jiménez-Gómez, J.M.; Lippman, Z.B. Variation in the Flowering Gene SELF PRUNING 5G Promotes Day-Neutrality and Early Yield in Tomato. Nat. Genet. 2017, 49, 162–168. [Google Scholar] [CrossRef]
- van der Krol, A.R.; Lenting, P.E.; Veenstra, J.; van der Meer, I.M.; Koes, R.E.; Gerats, A.G.M.; Mol, J.N.M.; Stuitje, A.R. An Anti-Sense Chalcone Synthase Gene in Transgenic Plants Inhibits Flower Pigmentation. Nature 1988, 333, 866–869. [Google Scholar] [CrossRef]
- Nishihara, M.; Nakatsuka, T.; Yamamura, S. Flavonoid Components and Flower Color Change in Transgenic Tobacco Plants by Suppression of Chalcone Isomerase Gene. FEBS Lett. 2005, 579, 6074–6078. [Google Scholar] [CrossRef] [PubMed]
- The Blue Rose Project. Available online: https://www.suntory.com/sic/research/s_bluerose/story/ (accessed on 6 April 2023).
- Fukusaki, E.; Kawasaki, K.; Kajiyama, S.; An, C.-I.; Suzuki, K.; Tanaka, Y.; Kobayashi, A. Flower Color Modulations of Torenia Hybrida by Downregulation of Chalcone Synthase Genes with RNA Interference. J. Biotechnol. 2004, 111, 229–240. [Google Scholar] [CrossRef]
- Rajabi, A.; Fahmideh, L.; Keykhasaber, M.; Omran, V.G. Genetic Engineering of Novel Yellow Color African Violet (Saintpaulia Ionantha) Produced by Accumulation of Aureusidin 6-O-Glucoside. Biol. Proced. Online 2022, 24, 3. [Google Scholar] [CrossRef]
- Watanabe, K.; Kobayashi, A.; Endo, M.; Sage-Ono, K.; Toki, S.; Ono, M. CRISPR/Cas9-Mediated Mutagenesis of the Dihydroflavonol-4-Reductase-B (DFR-B) Locus in the Japanese Morning Glory Ipomoea (Pharbitis) Nil. Sci. Rep. 2017, 7, 10028. [Google Scholar] [CrossRef]
- Watanabe, K.; Oda-Yamamizo, C.; Sage-Ono, K.; Ohmiya, A.; Ono, M. Alteration of Flower Colour in Ipomoea Nil through CRISPR/Cas9-Mediated Mutagenesis of Carotenoid Cleavage Dioxygenase 4. Transgen. Res. 2018, 27, 25–38. [Google Scholar] [CrossRef]
- Nishihara, M.; Higuchi, A.; Watanabe, A.; Tasaki, K. Application of the CRISPR/Cas9 System for Modification of Flower Color in Torenia Fournieri. BMC Plant Biol. 2018, 18, 331. [Google Scholar] [CrossRef]
- Xu, J.; Kang, B.-C.; Naing, A.H.; Bae, S.-J.; Kim, J.-S.; Kim, H.; Kim, C.K. CRISPR/Cas9-Mediated Editing of 1-Aminocyclopropane-1-Carboxylate Oxidase1 Enhances Petunia Flower Longevity. Plant Biotechnol. J. 2020, 18, 287–297. [Google Scholar] [CrossRef]
- Yu, J.; Tu, L.; Subburaj, S.; Bae, S.; Lee, G.-J. Simultaneous Targeting of Duplicated Genes in Petunia Protoplasts for Flower Color Modification via CRISPR-Cas9 Ribonucleoproteins. Plant Cell Rep. 2021, 40, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Kusch, S.; Panstruga, R. Mlo-Based Resistance: An Apparently Universal “Weapon” to Defeat Powdery Mildew Disease. MPMI 2017, 30, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Büschges, R.; Hollricher, K.; Panstruga, R.; Simons, G.; Wolter, M.; Frijters, A.; van Daelen, R.; van der Lee, T.; Diergaarde, P.; Groenendijk, J.; et al. The Barley Mlo Gene: A Novel Control Element of Plant Pathogen Resistance. Cell 1997, 88, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Lefert, P.; Vogel, J. Closing the Ranks to Attack by Powdery Mildew. Trends Plant Sci. 2000, 5, 343–348. [Google Scholar] [CrossRef]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-Edited Powdery Mildew Resistance in Wheat without Growth Penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering Canker-Resistant Plants through CRISPR/Cas9-Targeted Editing of the Susceptibility Gene CsLOB1 Promoter in Citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/SgRNA-Mediated Targeted Gene Modification in Arabidopsis, Tobacco, Sorghum and Rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef] [PubMed]
- Antony, G.; Zhou, J.; Huang, S.; Li, T.; Liu, B.; White, F.; Yang, B. Rice Xa13 Recessive Resistance to Bacterial Blight Is Defeated by Induction of the Disease Susceptibility Gene Os-11N3. Plant Cell 2010, 22, 3864–3876. [Google Scholar] [CrossRef] [PubMed]
- Zafar, K.; Khan, M.Z.; Amin, I.; Mukhtar, Z.; Yasmin, S.; Arif, M.; Ejaz, K.; Mansoor, S. Precise CRISPR-Cas9 Mediated Genome Editing in Super Basmati Rice for Resistance Against Bacterial Blight by Targeting the Major Susceptibility Gene. Front. Plant Sci. 2020, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal Gene Transfer of Fhb7 from Fungus Underlies Fusarium Head Blight Resistance in Wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Zhu, F.; Zhou, Y.-K.; Ji, Z.-L.; Chen, X.-R. The Plant Ribosome-Inactivating Proteins Play Important Roles in Defense against Pathogens and Insect Pest Attacks. Front. Plant Sci. 2018, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Huang, L.; Yi, R.; Wang, S.; Ding, Y. Enhanced Resistance to Blast Fungus in Rice (Oryza sativa L.) by Expressing the Ribosome-Inactivating Protein Alpha-Momorcharin. Plant Sci. 2014, 217–218, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Salazar, R.; Cecere, B.; Ruocco, M.; Rao, R.; Corrado, G. A Comparison between Constitutive and Inducible Transgenic Expression of the PhRIP I Gene for Broad-Spectrum Resistance against Phytopathogens in Potato. Biotechnol. Lett. 2017, 39, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-E.; Ger, M.-J.; Yip, M.-K.; Chen, C.-Y.; Pandey, A.-K.; Feng, T.-Y. A Hypersensitive Response Was Induced by Virulent Bacteria in Transgenic Tobacco Plants Overexpressing a Plant Ferredoxin-like Protein (PFLP). Physiol. Mol. Plant Pathol. 2004, 64, 103–110. [Google Scholar] [CrossRef]
- Tripathi, L.; Tripathi, J.N.; Kiggundu, A.; Korie, S.; Shotkoski, F.; Tushemereirwe, W.K. Field Trial of Xanthomonas Wilt Disease-Resistant Bananas in East Africa. Nat. Biotechnol. 2014, 32, 868–870. [Google Scholar] [CrossRef]
- Tripathi, L.; Atkinson, H.; Roderick, H.; Kubiriba, J.; Tripathi, J.N. Genetically Engineered Bananas Resistant to Xanthomonas Wilt Disease and Nematodes. Food Energy Secur. 2017, 6, 37–47. [Google Scholar] [CrossRef]
- Muwonge, A.; Tripathi, J.; Kunert, K.; Tripathi, L. Expressing Stacked HRAP and PFLP Genes in Transgenic Banana Has No Synergistic Effect on Resistance to Xanthomonas Wilt Disease. S. Afr. J. Bot. 2016, 104, 125–133. [Google Scholar] [CrossRef]
- Choi, M.-S.; Kim, W.; Lee, C.; Oh, C.-S. Harpins, Multifunctional Proteins Secreted by Gram-Negative Plant-Pathogenic Bacteria. MPMI 2013, 26, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xu, M.; Zhou, T.; Wang, D.; Tian, S.; Han, L.; Dong, H.; Zhang, C. Transgenic Expression of a Functional Fragment of Harpin Protein Hpa1 in Wheat Induces the Phloem-Based Defence against English Grain Aphid. J. Exp. Bot. 2014, 65, 1439–1453. [Google Scholar] [CrossRef]
- Li, P.; Lu, X.; Shao, M.; Long, J.; Wang, J. Genetic Diversity of Harpins from Xanthomonas oryzae and Their Activity to Induce Hypersensitive Response and Disease Resistance in Tobacco. Sci. China C Life Sci. 2004, 47, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Wang, X.; Li, M.; Song, C.; Wang, Y.; Hu, D.; Wang, J. Genetic Transformation of Cotton with a Harpin-Encoding Gene HpaXooconfers an Enhanced Defense Response against Different Pathogens through a Priming Mechanism. BMC Plant Biol. 2010, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Wang, Y.; Ma, L.-L.; Qiao, J.-Q.; Shao, M.; Gao, X.-W. Assessment of Inheritance Pattern and Agronomic Performance of Transgenic Rapeseed Having Harpin Xooc-Encoding Hrf2 Gene. Transgen. Res. 2010, 19, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Collmer, A.; Beer, S.V. Harpin, Elicitor of the Hypersensitive Response Produced by the Plant Pathogen Erwinia Amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Yang, J.; Zhang, J.; He, H.; Xing, G.; Zhao, Q.; Guo, D.; Sui, L.; Zhong, X.; Yang, X. Introduction of the HarpinXooc-Encoding Gene Hrf2 in Soybean Enhances Resistance against the Oomycete Pathogen Phytophthora Sojae. Transgen. Res. 2019, 28, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Moreno, L.; Song, Y.; Thomma, B.P. Transfer and Engineering of Immune Receptors to Improve Recognition Capacities in Crops. Curr. Opin. Plant Biol. 2017, 38, 42–49. [Google Scholar] [CrossRef]
- Ercoli, M.F.; Luu, D.D.; Rim, E.Y.; Shigenaga, A.; Teixeira de Araujo, A.; Chern, M.; Jain, R.; Ruan, R.; Joe, A.; Stewart, V.; et al. Plant Immunity: Rice XA21-Mediated Resistance to Bacterial Infection. Proc. Natl. Acad. Sci. USA 2022, 119, e2121568119. [Google Scholar] [CrossRef]
- Szankowski, I.; Waidmann, S.; Degenhardt, J.; Patocchi, A.; Paris, R.; Silfverberg-Dilworth, E.; Broggini, G.; Gessler, C. Highly Scab-Resistant Transgenic Apple Lines Achieved by Introgression of HcrVf2 Controlled by Different Native Promoter Lengths. Tree Genet. Genomes 2009, 5, 349–358. [Google Scholar] [CrossRef]
- Xu, G.; Greene, G.H.; Yoo, H.; Liu, L.; Marqués, J.; Motley, J.; Dong, X. Global Translational Reprogramming Is a Fundamental Layer of Immune Regulation in Plants. Nature 2017, 545, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Epstein, L.; Wroblewski, T.; Michelmore, R.W. Host-Induced Gene Silencing Inhibits the Biotrophic Pathogen Causing Downy Mildew of Lettuce. Plant Biotechnol. J. 2015, 13, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, X.; Liu, J.; Ye, J.; Guo, Z. The Rice ERF Transcription Factor OsERF922 Negatively Regulates Resistance to Magnaporthe Oryzae and Salt Tolerance. J. Exp. Bot. 2012, 63, 3899–3911. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.-G.; Zhao, K. Enhanced Rice Blast Resistance by CRISPR/Cas9-Targeted Mutagenesis of the ERF Transcription Factor Gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Kovac, E.; Blaustein-Rejto, D.; Qaim, M. Genetically Modified Crops Support Climate Change Mitigation. Clim. Change Sustain. 2022, 27, 627–629. [Google Scholar] [CrossRef]
- Samantara, K.; Bohra, A.; Mohapatra, S.R.; Prihatini, R.; Asibe, F.; Singh, L.; Reyes, V.P.; Tiwari, A.; Maurya, A.K.; Croser, J.S.; et al. Breeding More Crops in Less Time: A Perspective on Speed Breeding. Biology 2022, 11, 275. [Google Scholar] [CrossRef]
- Wheeler, R.M. A Historical Background of Plant Lighting: An Introduction to the Workshop. Hortic. Sci. 2008, 43, 1942–1943. [Google Scholar] [CrossRef]
- Sysoeva, M.I.; Markovskaya, E.F.; Shibaeva, T.G. Plants under Continuous Light: A Review. Plant Stress 2010, 4, 5–17. [Google Scholar]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for Energy Efficient Greenhouse Lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect Leaves Caused by Brassinosteroid Deficiency Increase Biomass Production and Grain Yield in Rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar] [CrossRef]
- Ashikari, M.; Sasaki, A.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Datta, S.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S. Loss-of-Function of a Rice Gibberellin Biosynthetic Gene, GA20 Oxidase (GA20ox-2), Led to the Rice “green Revolution. Breed Sci. 2002, 52, 143–150. [Google Scholar] [CrossRef]
- Mayta, M.L.; Arce, R.C.; Zurbriggen, M.D.; Valle, E.M.; Hajirezaei, M.-R.; Zanor, M.; Carrillo, N. Expression of a Chloroplast-Targeted Cyanobacterial Flavodoxin in Tomato Plants Increases Harvest Index by Altering Plant Size and Productivity. Front. Plant Sci. 2019, 10, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lawit, S.J.; Weers, B.; Sun, J.; Mongar, N.; Hemert, J.; Melo, R.; Meng, X.; Rupe, M.; Clapp, J.; et al. Overexpression of Zmm28 Increases Maize Grain Yield in the Field. Proc. Natl. Acad. Sci. USA 2019, 116, 23850–23858. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive Analysis of MRNA Methylation Reveals Enrichment in 3′UTRs and near Stop Codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, S.; Yu, L.; Xiao, Y.; Zhang, S.; Wang, X.; Xu, Y.; Yu, H.; Li, Y.; Yang, J.; et al. RNA Demethylation Increases the Yield and Biomass of Rice and Potato Plants in Field Trials. Nat. Biotechnol. 2021, 39, 1581–1588. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Chen, X.; Xu, F.; Ding, M.; Ye, W.; Kawai, Y.; Toda, Y.; Hayashi, Y.; Suzuki, T.; et al. Plasma membrane H+-ATPase expression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis. Nat. Commun. 2021, 12, 735. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the Four Yield-Related Genes Gn1a, DEP1, GS3, and IPA1 in Rice Using a CRISPR/Cas9 System. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef]
- AlbertaFarmer Express. Available online: https://www.albertafarmexpress.ca/crops/canola/gene-editing-produces-breakthrough-canola-variety/ (accessed on 29 October 2002).
- Dong, S.; Dong, X.; Han, X.; Zhang, F.; Zhu, Y.; Xin, X.; Wang, Y.; Hu, Y.; Yuan, D.; Wang, J.; et al. OsPDCD5 Negatively Regulates Plant Architecture and Grain Yield in Rice. Proc. Natl. Acad. Sci USA 2021, 118, e2018799118. [Google Scholar] [CrossRef]
- Stegelmeir, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The use of PGPB to promote plant hydroponic growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).