Structure-Function of the Human WAC Protein in GABAergic Neurons: Towards an Understanding of Autosomal Dominant DeSanto–Shinawi Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics
2.2. Animals
2.3. DNA Vector Generation
2.4. Immuno-Fluorescence Labeling and Imaging
2.5. MGE Primary Cultures
2.6. Western Blotting
2.7. Statistics and Cell Assessments
3. Results
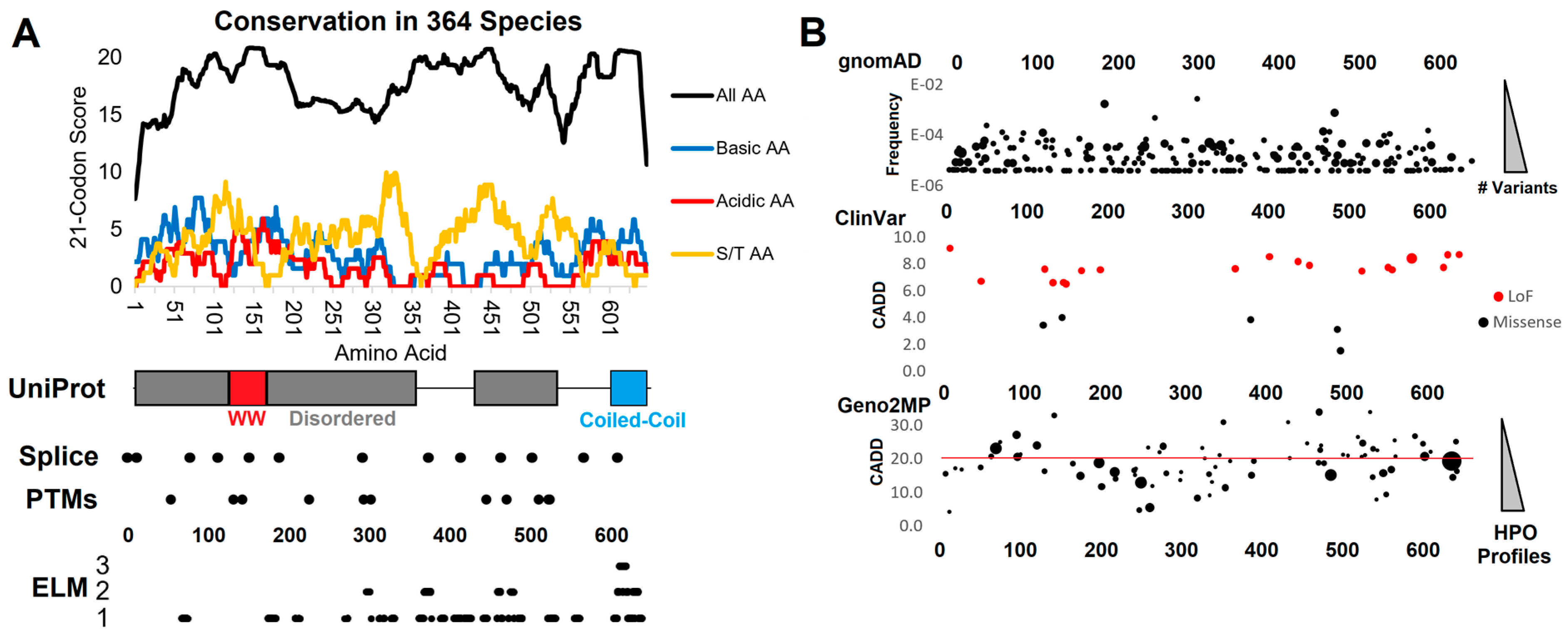
3.1. Functional Domain and Motif Annotations of WAC
3.2. Human Clinical Variants Annotated to Functional Motifs of WAC
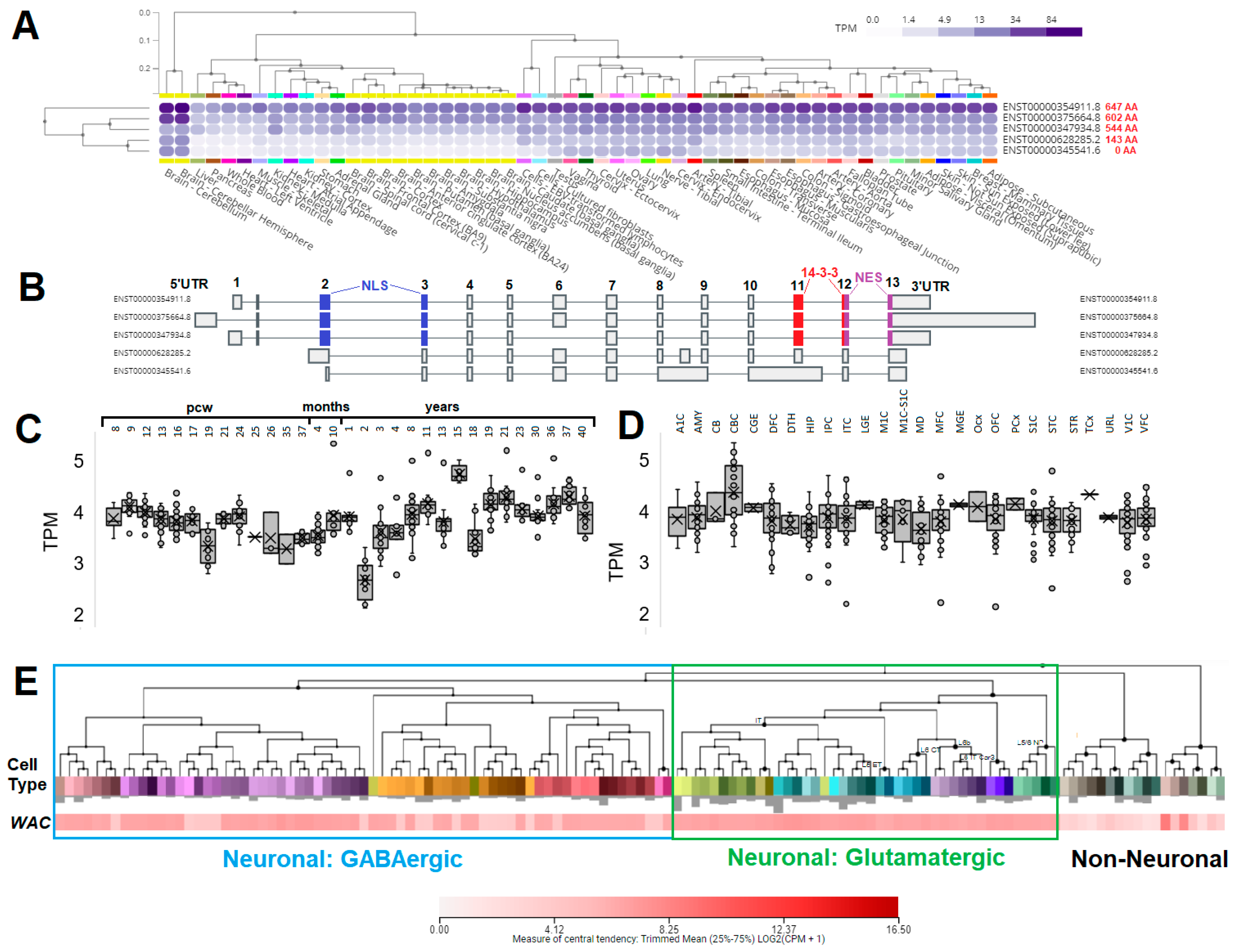
3.3. Expression of WAC within Human Brain and Neural Cell Types
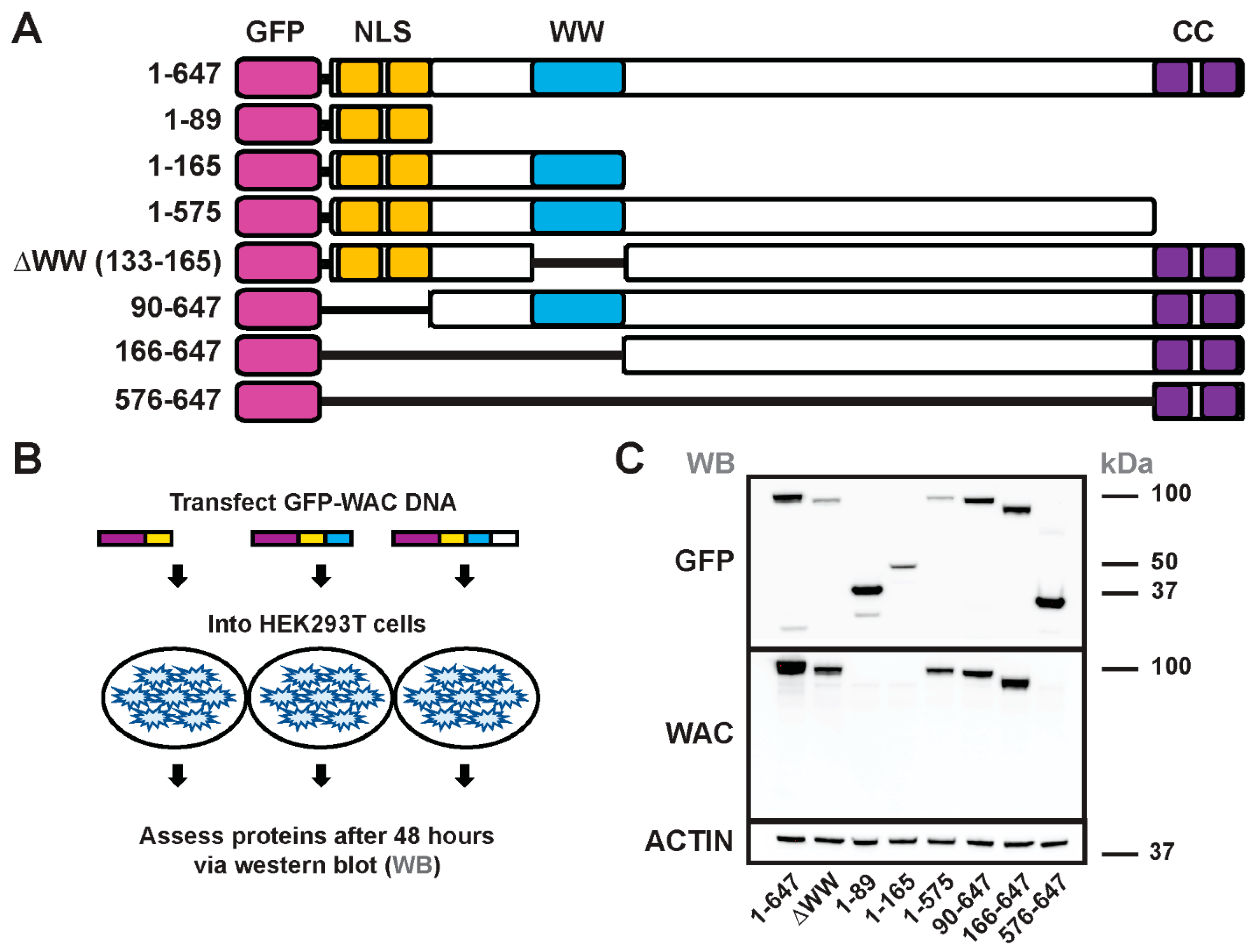
3.4. Generation and Structure/Function of GFP-WAC Mutant Fusion Proteins
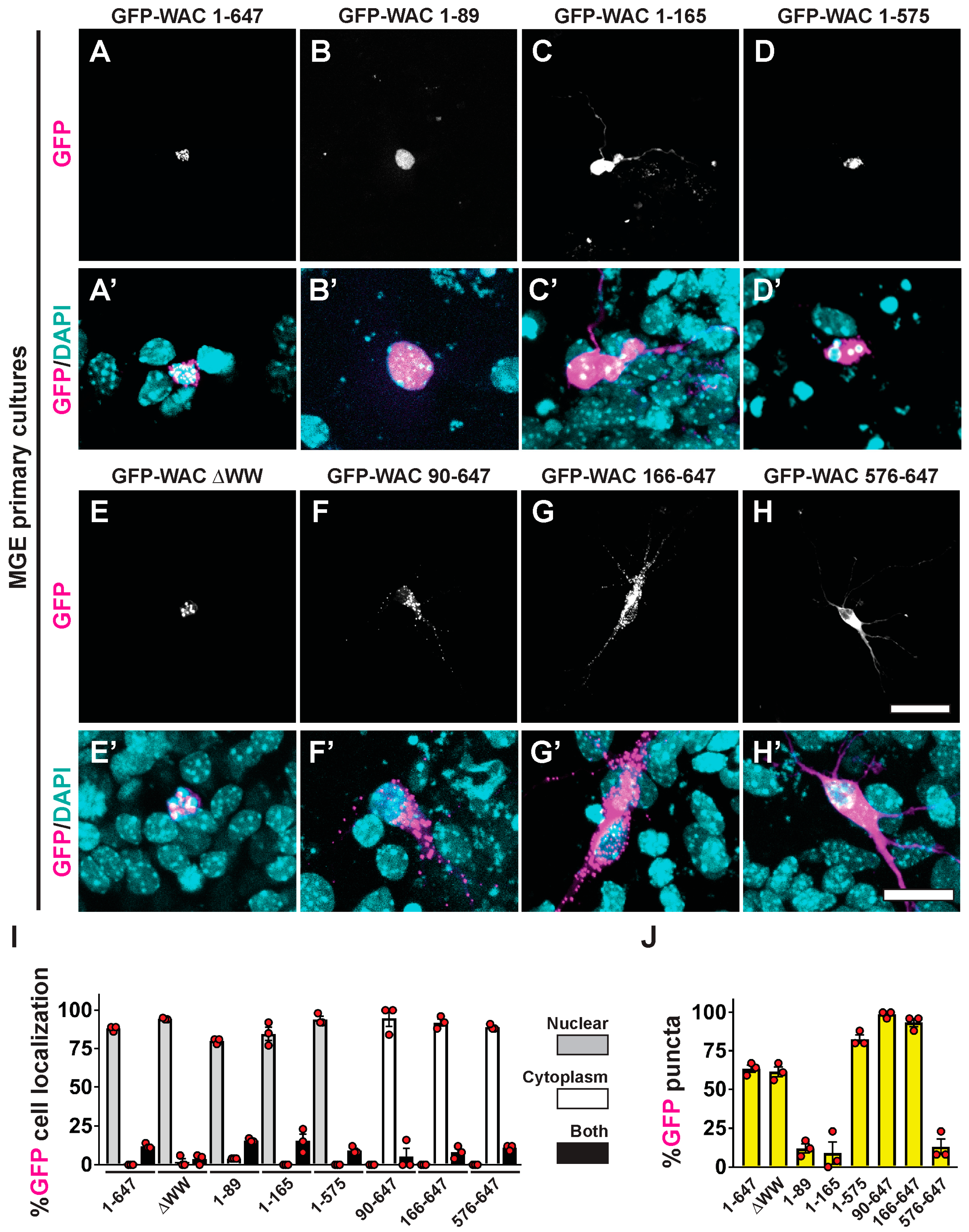
3.5. WAC Deletion Mutant Proteins Exhibit Differential Targeting and Aggregation in Neurons
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, K.J. The Genetics of Neurodevelopmental Disease. Curr. Opin. Neurobiol. 2011, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gilman, S.R.; Chiang, A.H.; Sanders, S.J.; Vitkup, D. Genotype to Phenotype Relationships in Autism Spectrum Disorders. Nat. Neurosci. 2015, 18, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Bupp, C.P.; English, B.K.; Rajasekaran, S.; Prokop, J.W. Introduction to Personalized Medicine in Pediatrics. Pediatr. Ann. 2022, 51, e381–e386. [Google Scholar] [CrossRef] [PubMed]
- DeSanto, C.; D’Aco, K.; Araujo, G.C.; Shannon, N.; Study, D.D.; Vernon, H.; Rahrig, A.; Monaghan, K.G.; Niu, Z.; Vitazka, P.; et al. WAC Loss-of-Function Mutations Cause a Recognisable Syndrome Characterised by Dysmorphic Features, Developmental Delay and Hypotonia and Recapitulate 10p11.23 Microdeletion Syndrome. J. Med. Genet. 2015, 52, 754–761. [Google Scholar] [CrossRef]
- Lugtenberg, D.; Reijnders, M.R.F.; Fenckova, M.; Bijlsma, E.K.; Bernier, R.; van Bon, B.W.M.; Smeets, E.; Vulto-van Silfhout, A.T.; Bosch, D.; Eichler, E.E.; et al. De Novo Loss-of-Function Mutations in WAC Cause a Recognizable Intellectual Disability Syndrome and Learning Deficits in Drosophila. Eur. J. Hum. Genet. EJHG 2016, 24, 1145–1153. [Google Scholar] [CrossRef]
- Leonardi, E.; Bellini, M.; Aspromonte, M.C.; Polli, R.; Mercante, A.; Ciaccio, C.; Granocchio, E.; Bettella, E.; Donati, I.; Cainelli, E.; et al. A Novel WAC Loss of Function Mutation in an Individual Presenting with Encephalopathy Related to Status Epilepticus during Sleep (ESES). Genes 2020, 11, 344. [Google Scholar] [CrossRef]
- Alawadhi, A.; Morgan, A.T.; Mucha, B.E.; Scheffer, I.E.; Myers, K.A. Self-Limited Focal Epilepsy and Childhood Apraxia of Speech with WAC Pathogenic Variants. Eur. J. Paediatr. Neurol. 2021, 30, 25–28. [Google Scholar] [CrossRef]
- Morales, J.A.; Valenzuela, I.; Cuscó, I.; Cogné, B.; Isidor, B.; Matalon, D.R.; Gomez-Ospina, N. Clinical and Molecular Characterization of Five New Individuals with WAC-Related Intellectual Disability: Evidence of Pathogenicity for a Novel Splicing Variant. Am. J. Med. Genet. A 2022, 188, 1396–1406. [Google Scholar] [CrossRef]
- Sanders, S.J.; He, X.; Willsey, A.J.; Ercan-Sencicek, A.G.; Samocha, K.E.; Cicek, A.E.; Murtha, M.T.; Bal, V.H.; Bishop, S.L.; Dong, S.; et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron 2015, 87, 1215–1233. [Google Scholar] [CrossRef]
- Fu, J.M.; Satterstrom, F.K.; Peng, M.; Brand, H.; Collins, R.L.; Dong, S.; Wamsley, B.; Klei, L.; Wang, L.; Hao, S.P.; et al. Rare Coding Variation Provides Insight into the Genetic Architecture and Phenotypic Context of Autism. Nat. Genet. 2022, 54, 1320–1331. [Google Scholar] [CrossRef]
- Xu, G.M.; Arnaout, M.A. WAC, a Novel WW Domain-Containing Adapter with a Coiled-Coil Region, Is Colocalized with Splicing Factor SC35. Genomics 2002, 79, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yu, X. WAC, a Functional Partner of RNF20/40, Regulates Histone H2B Ubiquitination and Gene Transcription. Mol. Cell 2011, 41, 384–397. [Google Scholar] [CrossRef] [PubMed]
- McKnight, N.C.; Jefferies, H.B.J.; Alemu, E.A.; Saunders, R.E.; Howell, M.; Johansen, T.; Tooze, S.A. Genome-Wide SiRNA Screen Reveals Amino Acid Starvation-Induced Autophagy Requires SCOC and WAC. EMBO J. 2012, 31, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- David-Morrison, G.; Xu, Z.; Rui, Y.-N.; Charng, W.-L.; Jaiswal, M.; Yamamoto, S.; Xiong, B.; Zhang, K.; Sandoval, H.; Duraine, L.; et al. WAC Regulates MTOR Activity by Acting as an Adaptor for the TTT and Pontin/Reptin Complexes. Dev. Cell 2016, 36, 139–151. [Google Scholar] [CrossRef]
- Qi, F.; Chen, Q.; Chen, H.; Yan, H.; Chen, B.; Xiang, X.; Liang, C.; Yi, Q.; Zhang, M.; Cheng, H.; et al. WAC Promotes Polo-like Kinase 1 Activation for Timely Mitotic Entry. Cell Rep. 2018, 24, 546–556. [Google Scholar] [CrossRef]
- Stafford, A.M.; Pacheco-Vergara, M.; Uhl, K.L.; Jager, T.E.; Li, X.; Jeong, J.; Vogt, D. A Murine Wac Model Exhibits Phenotypes Relevant to DeSanto–Shinawi Syndrome. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wonders, C.P.; Anderson, S.A. The Origin and Specification of Cortical Interneurons. Nat. Rev. Neurosci. 2006, 7, 687–696. [Google Scholar] [CrossRef]
- Prokop, J.W.; Lazar, J.; Crapitto, G.; Smith, D.C.; Worthey, E.A.; Jacob, H.J. Molecular Modeling in the Age of Clinical Genomics, the Enterprise of the next Generation. J. Mol. Model. 2017, 23, 75. [Google Scholar] [CrossRef]
- Prokop, J.W.; Jdanov, V.; Savage, L.; Morris, M.; Lamb, N.; VanSickle, E.; Stenger, C.L.; Rajasekaran, S.; Bupp, C.P. Computational and Experimental Analysis of Genetic Variants. Compr. Physiol. 2022, 12, 3303–3336. [Google Scholar] [CrossRef]
- Maglott, D.; Ostell, J.; Pruitt, K.D.; Tatusova, T. Entrez Gene: Gene-Centered Information at NCBI. Nucleic Acids Res. 2005, 33, D54–D58. [Google Scholar] [CrossRef]
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-Based Alignment Tool for Multiple Protein Sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: A Hub for Protein Information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The Mutational Constraint Spectrum Quantified from Variation in 141,456 Humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Hoover, J.; et al. ClinVar: Public Archive of Interpretations of Clinically Relevant Variants. Nucleic Acids Res. 2016, 44, D862–D868. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Kumar, M.; Michael, S.; Alvarado-Valverde, J.; Mészáros, B.; Sámano-Sánchez, H.; Zeke, A.; Dobson, L.; Lazar, T.; Örd, M.; Nagpal, A.; et al. The Eukaryotic Linear Motif Resource: 2022 Release. Nucleic Acids Res. 2022, 50, D497–D508. [Google Scholar] [CrossRef]
- Li, M.; Santpere, G.; Imamura Kawasawa, Y.; Evgrafov, O.V.; Gulden, F.O.; Pochareddy, S.; Sunkin, S.M.; Li, Z.; Shin, Y.; Zhu, Y.; et al. Integrative Functional Genomic Analysis of Human Brain Development and Neuropsychiatric Risks. Science 2018, 362, eaat7615. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Overly, C.C.; Sunkin, S.M. The Allen Brain Atlas: 5 Years and Beyond. Nat. Rev. Neurosci. 2009, 10, 821–828. [Google Scholar] [CrossRef]
- Vogt, D.; Hunt, R.F.; Mandal, S.; Sandberg, M.; Silberberg, S.N.; Nagasawa, T.; Yang, Z.; Baraban, S.C.; Rubenstein, J.L.R. Lhx6 Directly Regulates Arx and CXCR7 to Determine Cortical Interneuron Fate and Laminar Position. Neuron 2014, 82, 350–364. [Google Scholar] [CrossRef]
- Angara, K.; Pai, E.L.-L.; Bilinovich, S.M.; Stafford, A.M.; Nguyen, J.T.; Li, K.X.; Paul, A.; Rubenstein, J.L.; Vogt, D. Nf1 Deletion Results in Depletion of the Lhx6 Transcription Factor and a Specific Loss of Parvalbumin+ Cortical Interneurons. Proc. Natl. Acad. Sci. USA 2020, 117, 6189–6195. [Google Scholar] [CrossRef]
- Wundrach, D.; Martinetti, L.E.; Stafford, A.M.; Bilinovich, S.M.; Angara, K.; Prokop, J.W.; Crandall, S.R.; Vogt, D. A Human TSC1 Variant Screening Platform in Gabaergic Cortical Interneurons for Genotype to Phenotype Assessments. Front. Mol. Neurosci. 2020, 13, 573409. [Google Scholar] [CrossRef] [PubMed]
- Sudol, M.; Hunter, T. NeW Wrinkles for an Old Domain. Cell 2000, 103, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Truebestein, L.; Leonard, T.A. Coiled-coils: The Long and Short of It. Bioessays 2016, 38, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Snijders Blok, L.; Hiatt, S.M.; Bowling, K.M.; Prokop, J.W.; Engel, K.L.; Cochran, J.N.; Bebin, E.M.; Bijlsma, E.K.; Ruivenkamp, C.A.L.; Terhal, P.; et al. De Novo Mutations in MED13, a Component of the Mediator Complex, Are Associated with a Novel Neurodevelopmental Disorder. Hum. Genet. 2018, 137, 375–388. [Google Scholar] [CrossRef]
- Shimada, T.; Fournier, A.E.; Yamagata, K. Neuroprotective Function of 14-3-3 Proteins in Neurodegeneration. Bio. Med. Res. Int. 2013, 2013, e564534. [Google Scholar] [CrossRef]

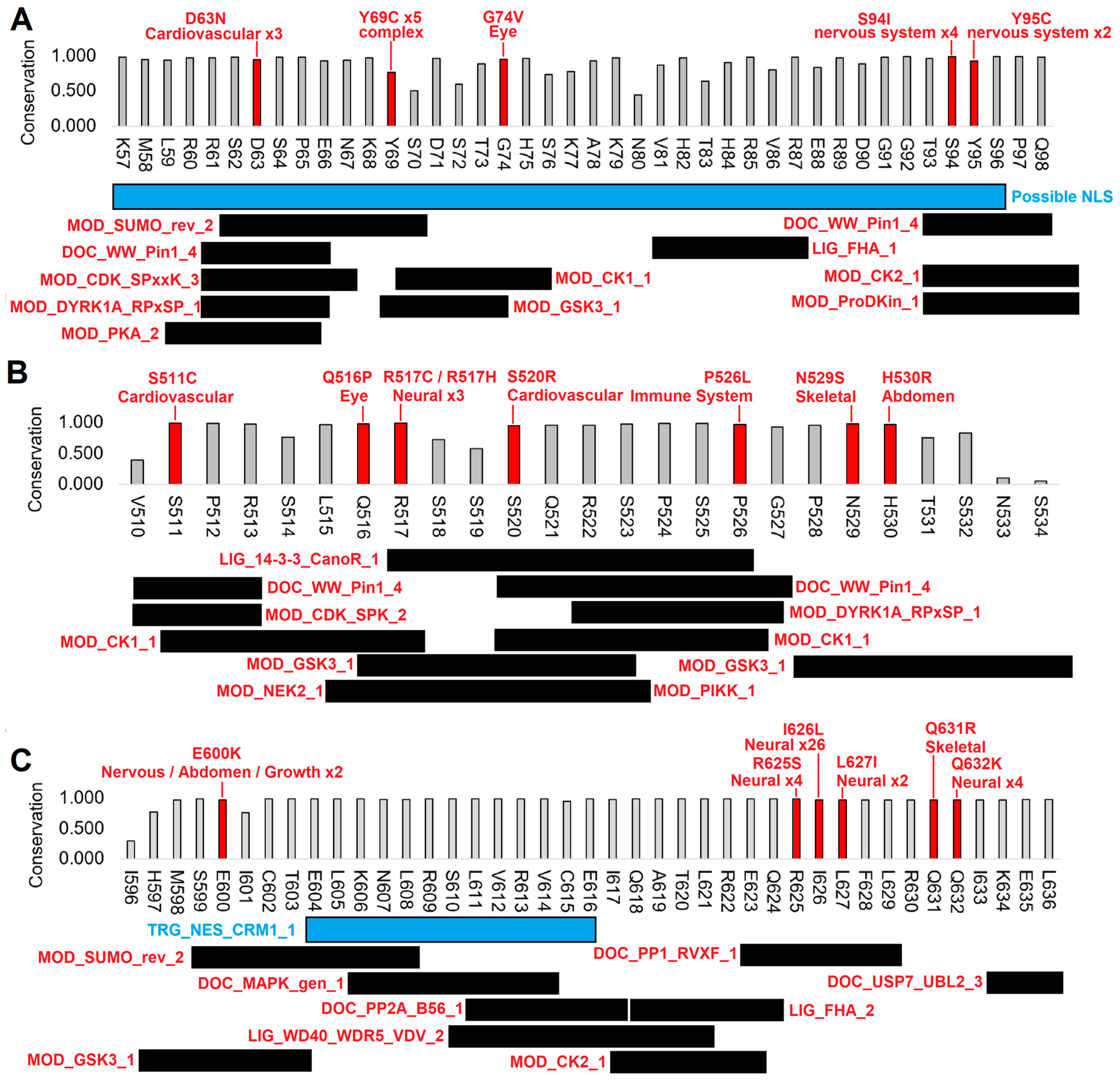
| WAC Change | Conservation Score | CADD Score | PolyPhen2 Damaging | HPO Profiles | Homozygous Count | Abnormality Noted in HPO |
|---|---|---|---|---|---|---|
| S94I | 98.87% | 27.2 | probably | 4 | 3 | nervous system |
| L426F | 98.30% | 31 | probably | 1 | 0 | nervous system |
| R517H | 98.58% | 24.6 | probably | 1 | 0 | nervous system |
| K466R | 98.87% | 24.1 | probably | 1 | 0 | nervous system |
| P557A | 97.45% | 20.8 | probably | 1 | 0 | nervous system |
| E600K | 97.17% | 22.1 | probably | 1 | 0 | nervous system, abdomen |
| Y95C | 92.63% | 20.6 | probably | 4 | 0 | nervous system, limbs, eyes |
| P464L | 93.20% | 34 | probably | 3 | 0 | nervous system, genitourinary system, skeletal system |
| H530R | 96.88% | 23 | probably | 2 | 0 | abdomen |
| K581R | 98.02% | 26.8 | probably | 2 | 0 | cardiovascular system |
| S511C | 98.58% | 26.6 | probably | 1 | 0 | cardiovascular system |
| S520R | 95.47% | 21.2 | probably | 1 | 0 | cardiovascular system |
| G74V | 95.47% | 25.1 | probably | 1 | 0 | eye |
| S449P | 99.15% | 24.7 | probably | 1 | 0 | eye |
| Q516P | 98.02% | 20.6 | probably | 1 | 0 | eye |
| T556M | 97.45% | 25.5 | probably | 1 | 0 | genitourinary system |
| P526L | 97.17% | 34 | probably | 1 | 0 | immune system, abdomen |
| S140I | 99.43% | 33 | probably | 2 | 0 | limbs |
| H591R | 98.02% | 24.6 | probably | 2 | 0 | limbs |
| P347S | 98.58% | 31 | probably | 2 | 0 | skeletal system |
| Q631R | 97.17% | 25.2 | probably | 2 | 0 | skeletal system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolph, H.C.; Stafford, A.M.; Hwang, H.-E.; Kim, C.-H.; Prokop, J.W.; Vogt, D. Structure-Function of the Human WAC Protein in GABAergic Neurons: Towards an Understanding of Autosomal Dominant DeSanto–Shinawi Syndrome. Biology 2023, 12, 589. https://doi.org/10.3390/biology12040589
Rudolph HC, Stafford AM, Hwang H-E, Kim C-H, Prokop JW, Vogt D. Structure-Function of the Human WAC Protein in GABAergic Neurons: Towards an Understanding of Autosomal Dominant DeSanto–Shinawi Syndrome. Biology. 2023; 12(4):589. https://doi.org/10.3390/biology12040589
Chicago/Turabian StyleRudolph, Hannah C., April M. Stafford, Hye-Eun Hwang, Cheol-Hee Kim, Jeremy W. Prokop, and Daniel Vogt. 2023. "Structure-Function of the Human WAC Protein in GABAergic Neurons: Towards an Understanding of Autosomal Dominant DeSanto–Shinawi Syndrome" Biology 12, no. 4: 589. https://doi.org/10.3390/biology12040589
APA StyleRudolph, H. C., Stafford, A. M., Hwang, H.-E., Kim, C.-H., Prokop, J. W., & Vogt, D. (2023). Structure-Function of the Human WAC Protein in GABAergic Neurons: Towards an Understanding of Autosomal Dominant DeSanto–Shinawi Syndrome. Biology, 12(4), 589. https://doi.org/10.3390/biology12040589







