Evaluation of BAFF, APRIL and CD40L in Ocrelizumab-Treated pwMS and Infectious Risk
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sample Collection
2.3. Measurement of Plasma B-Cell-Activating Factors and Immunoglobulin Levels
2.4. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics of Study Population
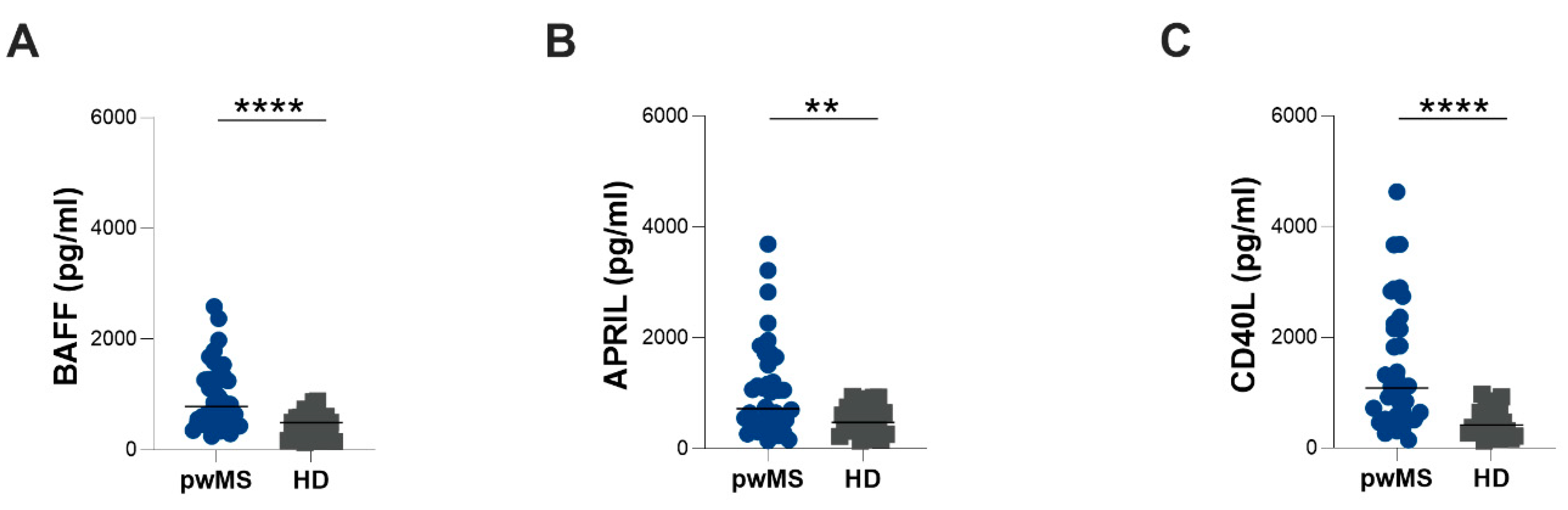
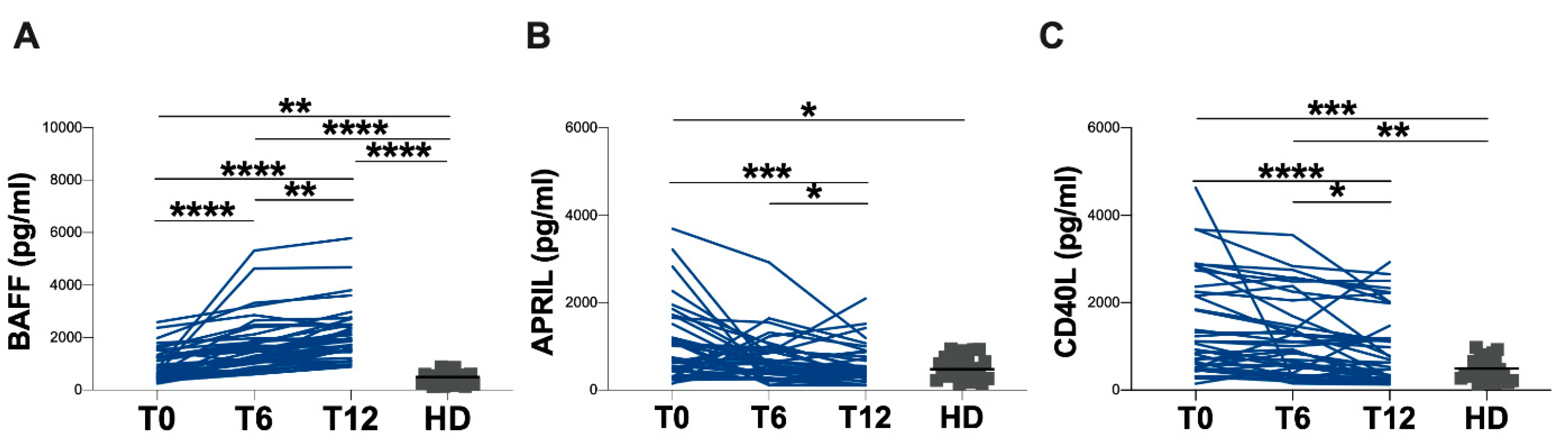
3.2. Evaluation of Plasma BAFF, APRIL and CD40L Levels
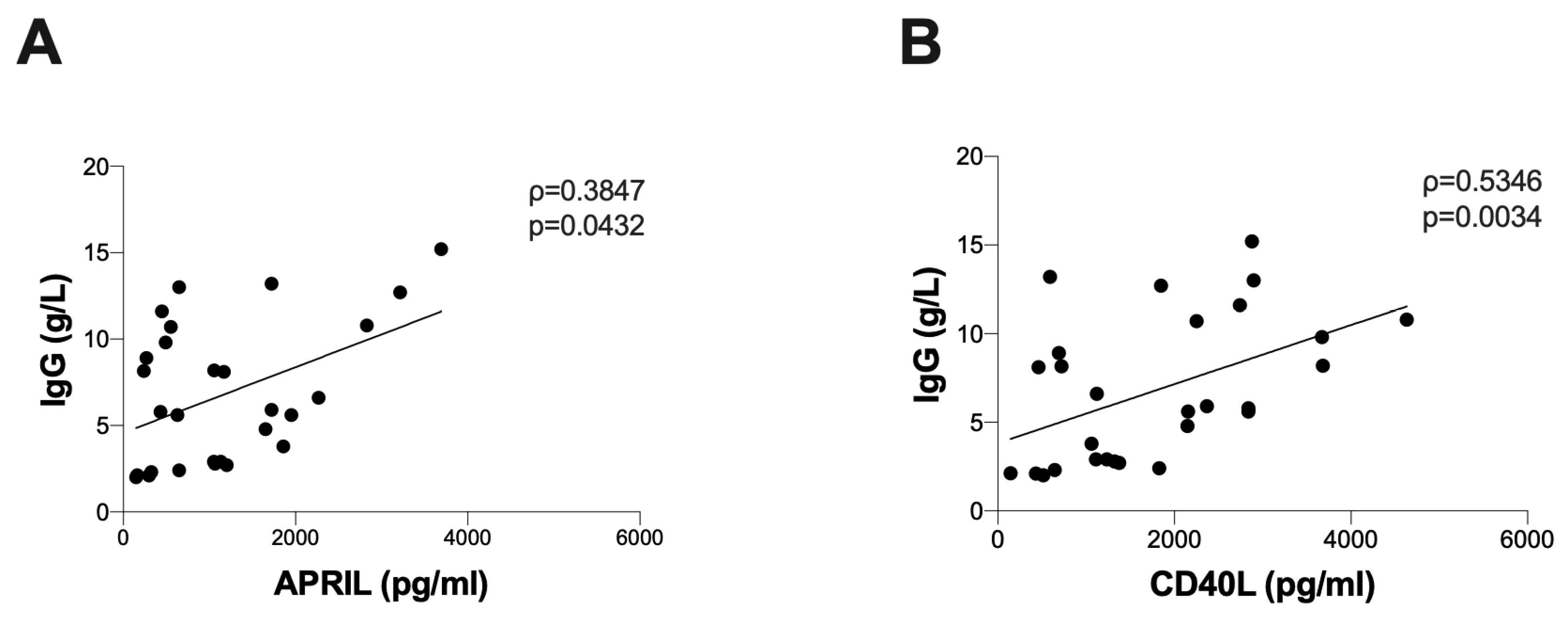
3.3. Correlation between B Lymphocyte Activation Markers and Immunoglobulin Levels
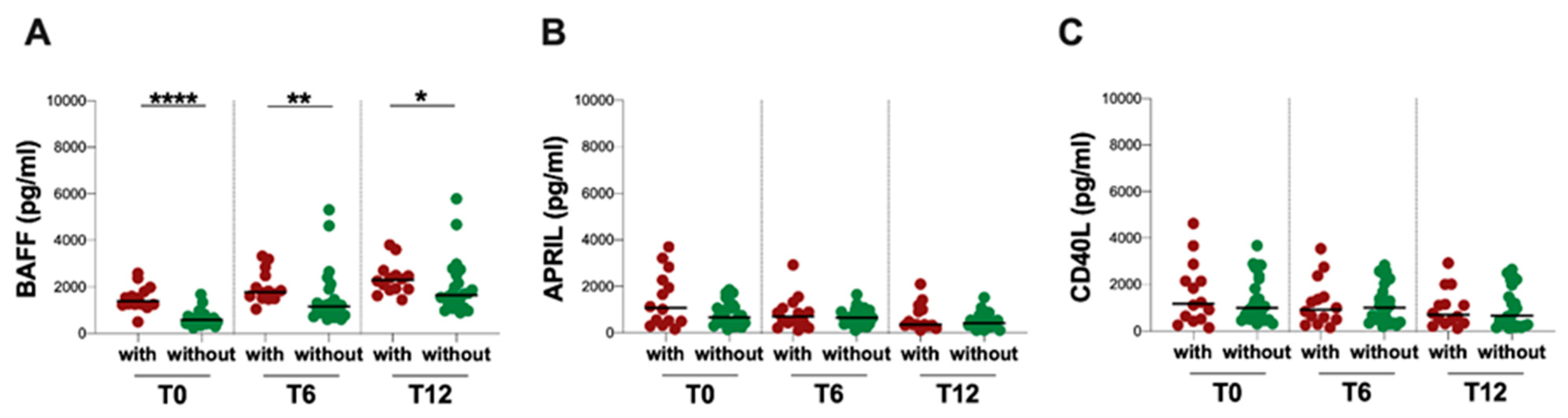
3.4. Infectious Events and Plasma BAFF, APRIL and CD40L Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet Lond. Engl. 2008, 372, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, M.; Poretto, V.; Favaretto, A.; Alessio, S.; Bernardi, V.; Romualdi, C.; Rinaldi, F.; Perini, P.; Gallo, P. Cortical Lesion Load Associates with Progression of Disability in Multiple Sclerosis. Brain J. Neurol. 2012, 135, 2952–2961. [Google Scholar] [CrossRef]
- Scalfari, A.; Romualdi, C.; Nicholas, R.S.; Mattoscio, M.; Magliozzi, R.; Morra, A.; Monaco, S.; Muraro, P.A.; Calabrese, M. The Cortical Damage, Early Relapses, and Onset of the Progressive Phase in Multiple Sclerosis. Neurology 2018, 90, e2107–e2118. [Google Scholar] [CrossRef] [PubMed]
- Howell, O.W.; Reeves, C.A.; Nicholas, R.; Carassiti, D.; Radotra, B.; Gentleman, S.M.; Serafini, B.; Aloisi, F.; Roncaroli, F.; Magliozzi, R.; et al. Meningeal Inflammation Is Widespread and Linked to Cortical Pathology in Multiple Sclerosis. Brain J. Neurol. 2011, 134, 2755–2771. [Google Scholar] [CrossRef]
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The Compartmentalized Inflammatory Response in the Multiple Sclerosis Brain Is Composed of Tissue-Resident CD8+ T Lymphocytes and B Cells. Brain J. Neurol. 2018, 141, 2066–2082. [Google Scholar] [CrossRef] [PubMed]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological Mechanisms in Progressive Multiple Sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Vidal-Jordana, A.; Sastre-Garriga, J. Treatment of Multiple Sclerosis—Success from Bench to Bedside. Nat. Rev. Neurol. 2019, 15, 53–58. [Google Scholar] [CrossRef]
- Liu, R.; Du, S.; Zhao, L.; Jain, S.; Sahay, K.; Rizvanov, A.; Lezhnyova, V.; Khaibullin, T.; Martynova, E.; Khaiboullina, S.; et al. Autoreactive Lymphocytes in Multiple Sclerosis: Pathogenesis and Treatment Target. Front. Immunol. 2022, 13, 996469. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Kappos, L.; Li, D.; Calabresi, P.A.; O’Connor, P.; Bar-Or, A.; Barkhof, F.; Yin, M.; Leppert, D.; Glanzman, R.; Tinbergen, J.; et al. Ocrelizumab in Relapsing-Remitting Multiple Sclerosis: A Phase 2, Randomised, Placebo-Controlled, Multicentre Trial. Lancet 2011, 378, 1779–1787. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Buonomo, A.R.; Viceconte, G.; Calabrese, M.; De Luca, G.; Tomassini, V.; Cavalla, P.; Maniscalco, G.T.; Ferraro, D.; Nociti, V.; Radaelli, M.; et al. Management of Hepatitis B Virus Prophylaxis in Patients Treated with Disease-Modifying Therapies for Multiple Sclerosis: A Multicentric Italian Retrospective Study. J. Neurol. 2022, 269, 3301–3307. [Google Scholar] [CrossRef]
- Barkhof, F.; Kappos, L.; Wolinsky, J.S.; Li, D.K.B.; Bar-Or, A.; Hartung, H.-P.; Belachew, S.; Han, J.; Julian, L.; Sauter, A.; et al. Onset of Clinical and MRI Efficacy of Ocrelizumab in Relapsing Multiple Sclerosis. Neurology 2019, 93, e1778–e1786. [Google Scholar] [CrossRef] [PubMed]
- Margoni, M.; Preziosa, P.; Filippi, M.; Rocca, M.A. Anti-CD20 Therapies for Multiple Sclerosis: Current Status and Future Perspectives. J. Neurol. 2022, 269, 1316–1334. [Google Scholar] [CrossRef] [PubMed]
- Forsthuber, T.G.; Cimbora, D.M.; Ratchford, J.N.; Katz, E.; Stüve, O. B Cell-Based Therapies in CNS Autoimmunity: Differentiating CD19 and CD20 as Therapeutic Targets. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418761697. [Google Scholar] [CrossRef]
- Avery, D.T.; Kalled, S.L.; Ellyard, J.I.; Ambrose, C.; Bixler, S.A.; Thien, M.; Brink, R.; Mackay, F.; Hodgkin, P.D.; Tangye, S.G. BAFF Selectively Enhances the Survival of Plasmablasts Generated from Human Memory B Cells. J. Clin. Investig. 2003, 112, 286–297. [Google Scholar] [CrossRef]
- Kannel, K.; Alnek, K.; Vahter, L.; Gross-Paju, K.; Uibo, R.; Kisand, K.V. Changes in Blood B Cell-Activating Factor (BAFF) Levels in Multiple Sclerosis: A Sign of Treatment Outcome. PLoS ONE 2015, 10, e0143393. [Google Scholar] [CrossRef]
- Samy, E.; Wax, S.; Huard, B.; Hess, H.; Schneider, P. Targeting BAFF and APRIL in Systemic Lupus Erythematosus and Other Antibody-Associated Diseases. Int. Rev. Immunol. 2017, 36, 3–19. [Google Scholar] [CrossRef]
- Karnell, J.L.; Rieder, S.A.; Ettinger, R.; Kolbeck, R. Targeting the CD40-CD40L Pathway in Autoimmune Diseases: Humoral Immunity and Beyond. Adv. Drug Deliv. Rev. 2019, 141, 92–103. [Google Scholar] [CrossRef]
- Zahednasab, H.; Siroos, B.; Balood, M.; Aleagha, M.S.E.; Harirchian, M.H. Soluble CD40 Ligand Derived from Serum Is Not Correlated with Early MS. Mult. Scler. Relat. Disord. 2017, 14, 29–31. [Google Scholar] [CrossRef]
- Gorbacheva, V.; Ayasoufi, K.; Fan, R.; Baldwin, W.M.; Valujskikh, A. B Cell Activating Factor (BAFF) and a Proliferation Inducing Ligand (APRIL) Mediate CD40-Independent Help by Memory CD4 T Cells. Am. J. Transplant. 2015, 15, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Von Büdingen, H.-C.; Bar-Or, A.; Zamvil, S.S. B Cells in Multiple Sclerosis: Connecting the Dots. Curr. Opin. Immunol. 2011, 23, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Trivino, T.; Braithwaite, D.; Bacchetti, P.; Waubant, E. Rituximab in Relapsing and Progressive Forms of Multiple Sclerosis: A Systematic Review. PLoS ONE 2013, 8, e66308. [Google Scholar] [CrossRef]
- Kappos, L.; Hartung, H.-P.; Freedman, M.S.; Boyko, A.; Radü, E.W.; Mikol, D.D.; Lamarine, M.; Hyvert, Y.; Freudensprung, U.; Plitz, T.; et al. Atacicept in Multiple Sclerosis (ATAMS): A Randomised, Placebo-Controlled, Double-Blind, Phase 2 Trial. Lancet Neurol. 2014, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.S.; Buttmann, M.; Meuth, S.G.; Dirks, P.; Muros-Le Rouzic, E.; Eggebrecht, J.C.; Hieke-Schulz, S.; Leemhuis, J.; Ziemssen, T. Safety, Adherence and Persistence in a Real-World Cohort of German MS Patients Newly Treated With Ocrelizumab: First Insights From the CONFIDENCE Study. Front. Neurol. 2022, 13, 863105. [Google Scholar] [CrossRef]
- Ciardi, M.R.; Iannetta, M.; Zingaropoli, M.A.; Salpini, R.; Aragri, M.; Annecca, R.; Pontecorvo, S.; Altieri, M.; Russo, G.; Svicher, V.; et al. Reactivation of Hepatitis B Virus With Immune-Escape Mutations After Ocrelizumab Treatment for Multiple Sclerosis. Open Forum Infect. Dis. 2018, 6, ofy356. [Google Scholar] [CrossRef]
- Epstein, D.J.; Dunn, J.; Deresinski, S. Infectious Complications of Multiple Sclerosis Therapies: Implications for Screening, Prophylaxis, and Management. Open Forum Infect. Dis. 2018, 5, ofy174. [Google Scholar] [CrossRef]
- Moiola, L.; Barcella, V.; Benatti, S.; Capobianco, M.; Capra, R.; Cinque, P.; Comi, G.; Fasolo, M.M.; Franzetti, F.; Galli, M.; et al. The Risk of Infection in Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies: A Delphi Consensus Statement. Mult. Scler. Houndmills Basingstoke Engl. 2021, 27, 331–346. [Google Scholar] [CrossRef]
- Dudek, M.I.R.; Thies, K.; Kammenhuber, S.; Bösel, J.; Rösche, J. HSV-2-Encephalitis in a Patient with Multiple Sclerosis Treated with Ocrelizumab. J. Neurol. 2019, 266, 2322–2323. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S.; Bzowej, N.H.; Wong, J.B. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Pasculli, P.; Iannetta, M.; Perri, V.; Tartaglia, M.; Crisafulli, S.G.; Merluzzo, C.; Baione, V.; Mazzochi, L.; Taglietti, A.; et al. Infectious Risk in Multiple Sclerosis Patients Treated with Disease-Modifying Therapies: A Three-Year Observational Cohort Study. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 20552173211065732. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, M.R.; Zingaropoli, M.A.; Iannetta, M.; Prezioso, C.; Perri, V.; Pasculli, P.; Lichtner, M.; d’Ettorre, G.; Altieri, M.; Conte, A.; et al. JCPyV NCCR Analysis in PML Patients with Different Risk Factors: Exploring Common Rearrangements as Essential Changes for Neuropathogenesis. Virol. J. 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, C.; Ciotti, M.; Brazzini, G.; Piacentini, F.; Passerini, S.; Grimaldi, A.; Landi, D.; Nicoletti, C.G.; Zingaropoli, M.A.; Iannetta, M.; et al. Diagnostic Value of JC Polyomavirus Viruria, Viremia, Serostatus and MicroRNA Expression in Multiple Sclerosis Patients Undergoing Immunosuppressive Treatment. J. Clin. Med. 2022, 11, 347. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Imaging and Laboratory Technology Use of Anticoagulants in Diagnostic Laboratory Investigations. 2002. Available online: https://apps.who.int/iris/handle/10665/65957 (accessed on 4 April 2023).
- Winkelmann, A.; Loebermann, M.; Barnett, M.; Hartung, H.-P.; Zettl, U.K. Vaccination and Immunotherapies in Neuroimmunological Diseases. Nat. Rev. Neurol. 2022, 18, 289–306. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liao, Q.; Wen, H.; Zhang, Y. Disease Modifying Therapies in Relapsing-Remitting Multiple Sclerosis: A Systematic Review and Network Meta-Analysis. Autoimmun. Rev. 2021, 20, 102826. [Google Scholar] [CrossRef]
- Kurtzke, J.F. A Reassessment of the Distribution of Multiple Sclerosis. Acta Neurol. Scand. 1975, 51, 137–157. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Multiple Sclerosis in Time and Space—Geographic Clues to Cause. J. Neurovirol. 2000, 6 (Suppl. 2), S134–S140. [Google Scholar]
- Brück, W.; Gold, R.; Lund, B.T.; Oreja-Guevara, C.; Prat, A.; Spencer, C.M.; Steinman, L.; Tintoré, M.; Vollmer, T.L.; Weber, M.S.; et al. Therapeutic Decisions in Multiple Sclerosis: Moving beyond Efficacy. JAMA Neurol. 2013, 70, 1315–1324. [Google Scholar] [CrossRef]
- Grebenciucova, E.; Pruitt, A. Infections in Patients Receiving Multiple Sclerosis Disease-Modifying Therapies. Curr. Neurol. Neurosci. Rep. 2017, 17, 88. [Google Scholar] [CrossRef]
- Prezioso, C.; Zingaropoli, M.A.; Iannetta, M.; Rodio, D.M.; Altieri, M.; Conte, A.; Vullo, V.; Ciardi, M.R.; Palamara, A.T.; Pietropaolo, V. Which Is the Best PML Risk Stratification Strategy in Natalizumab-Treated Patients Affected by Multiple Sclerosis? Mult. Scler. Relat. Disord. 2020, 41, 102008. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Patterson, K.R.; Bar-Or, A. Reassessing B Cell Contributions in Multiple Sclerosis. Nat. Immunol. 2018, 19, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Pröbstel, A.-K.; Sanderson, N.S.R.; Derfuss, T. B Cells and Autoantibodies in Multiple Sclerosis. Int. J. Mol. Sci. 2015, 16, 16576–16592. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, M.; Meinl, E. B Cells in MS and NMO: Pathogenesis and Therapy. Semin. Immunopathol. 2014, 36, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Lisak, R.P.; Nedelkoska, L.; Benjamins, J.A.; Schalk, D.; Bealmear, B.; Touil, H.; Li, R.; Muirhead, G.; Bar-Or, A. B Cells from Patients with Multiple Sclerosis Induce Cell Death via Apoptosis in Neurons in Vitro. J. Neuroimmunol. 2017, 309, 88–99. [Google Scholar] [CrossRef]
- Montalban, X.; Belachew, S.; Wolinsky, J.S. Ocrelizumab in Primary Progressive and Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 1694. [Google Scholar] [CrossRef]
- Lam, J.H.; Smith, F.L.; Baumgarth, N. B Cell Activation and Response Regulation During Viral Infections. Viral Immunol. 2020, 33, 294–306. [Google Scholar] [CrossRef]
- Elsner, R.A.; Shlomchik, M.J. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity 2020, 53, 1136–1150. [Google Scholar] [CrossRef]
- Iannetta, M.; Landi, D.; Cola, G.; Campogiani, L.; Malagnino, V.; Teti, E.; Coppola, L.; Di Lorenzo, A.; Fraboni, D.; Buccisano, F.; et al. B- and T-Cell Responses After SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Receiving Disease Modifying Therapies: Immunological Patterns and Clinical Implications. Front. Immunol. 2021, 12, 796482. [Google Scholar] [CrossRef]
- Dominelli, F.; Zingaropoli, M.A.; Tartaglia, M.; Tortellini, E.; Guardiani, M.; Perri, V.; Pasculli, P.; Ciccone, F.; Malimpensa, L.; Baione, V.; et al. Multiple Sclerosis-Disease Modifying Therapies Affect Humoral and T-Cell Response to MRNA COVID-19 Vaccine. Front. Immunol. 2022, 13, 1050183. [Google Scholar] [CrossRef]
- Krumbholz, M.; Theil, D.; Derfuss, T.; Rosenwald, A.; Schrader, F.; Monoranu, C.-M.; Kalled, S.L.; Hess, D.M.; Serafini, B.; Aloisi, F.; et al. BAFF Is Produced by Astrocytes and Up-Regulated in Multiple Sclerosis Lesions and Primary Central Nervous System Lymphoma. J. Exp. Med. 2005, 201, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ospina, F.E.; Betancur, J.F.; Suso, J.P.; Muñoz-Buitron, E.; Cañas, C.A.; Tobón, G.J. Role of the Cytokine BAFF in Autoimmune Diseases: Physiopathology and Therapeutic Targets. Rev. Colomb. Reumatol. Engl. Ed. 2016, 23, 177–194. [Google Scholar] [CrossRef]
- Cuellar-Tamez, R.X.; Villarreal-Calderon, J.R.; Rubio-Infante, N.; Castillo, E.C.; García-Garza, M.; Elizondo-Montemayor, L.; García-Rivas, G. Bariatric Surgery-Induced Weight Loss Reduces B Cell Activating Cytokines and IgG Immunoglobulins Related to Autoimmunity. Surg. Endosc. 2021, 35, 5147–5158. [Google Scholar] [CrossRef] [PubMed]
- Mackay, F.; Schneider, P. Cracking the BAFF Code. Nat. Rev. Immunol. 2009, 9, 491–502. [Google Scholar] [CrossRef]
- Ng, L.G.; Mackay, C.R.; Mackay, F. The BAFF/APRIL System: Life beyond B Lymphocytes. Mol. Immunol. 2005, 42, 763–772. [Google Scholar] [CrossRef]
- Ho, S.; Oswald, E.; Wong, H.K.; Vural, A.; Yilmaz, V.; Tüzün, E.; Türkoğlu, R.; Straub, T.; Meinl, I.; Thaler, F.; et al. Ocrelizumab Treatment Modulates B-Cell Regulating Factors in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200083. [Google Scholar] [CrossRef] [PubMed]
- Craxton, A.; Draves, K.E.; Gruppi, A.; Clark, E.A. BAFF Regulates B Cell Survival by Downregulating the BH3-Only Family Member Bim via the ERK Pathway. J. Exp. Med. 2005, 202, 1363–1374. [Google Scholar] [CrossRef]
- Dörner, T.; Radbruch, A.; Burmester, G.R. B-Cell-Directed Therapies for Autoimmune Disease. Nat. Rev. Rheumatol. 2009, 5, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Sordet, C.; Guillevin, L.; Hachulla, E.; Masson, C.; Ittah, M.; Candon, S.; Guern, V.L.; Aouba, A.; Sibilia, J.; et al. Tolerance and Efficacy of Rituximab and Changes in Serum B Cell Biomarkers in Patients with Systemic Complications of Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2007, 66, 351–357. [Google Scholar] [CrossRef]
- Lavie, F.; Miceli-Richard, C.; Ittah, M.; Sellam, J.; Gottenberg, J.; Mariette, X. Increase of B Cell-activating Factor of the TNF Family (BAFF) after Rituximab Treatment: Insights into a New Regulating System of BAFF Production. Ann. Rheum. Dis. 2007, 66, 700–703. [Google Scholar] [CrossRef]
- Vallerskog, T.; Heimbürger, M.; Gunnarsson, I.; Zhou, W.; Wahren-Herlenius, M.; Trollmo, C.; Malmström, V. Differential Effects on BAFF and APRIL Levels in Rituximab-Treated Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis. Arthritis Res. Ther. 2006, 8, R167. [Google Scholar] [CrossRef]
- Cambridge, G.; Stohl, W.; Leandro, M.J.; Migone, T.-S.; Hilbert, D.M.; Edwards, J.C.W. Circulating Levels of B Lymphocyte Stimulator in Patients with Rheumatoid Arthritis Following Rituximab Treatment: Relationships with B Cell Depletion, Circulating Antibodies, and Clinical Relapse. Arthritis Rheum. 2006, 54, 723–732. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Zhang, Y.; Zeng, W.; Wang, S.; Ji, P.; Pan, M.; Zhu, C.; Wang, Y. Distinct Roles of ICOS and CD40L in Human T-B Cell Adhesion and Antibody Production. Cell. Immunol. 2021, 368, 104420. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.C.; Thoman, M.; Linton, P.-J.; Deisseroth, A. Use of CD40L Immunoconjugates to Overcome the Defective Immune Response to Vaccines for Infections and Cancer in the Aged. Cancer Immunol. Immunother. 2009, 58, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Sakai, J.; Akkoyunlu, M. The Role of BAFF System Molecules in Host Response to Pathogens. Clin. Microbiol. Rev. 2017, 30, 991–1014. [Google Scholar] [CrossRef]
- Datta, S.K.; Kalled, S.L. CD40-CD40 Ligand Interaction in Autoimmune Disease. Arthritis Rheum. 1997, 40, 1735–1745. [Google Scholar] [CrossRef]
- Aarts, S.A.B.M.; Seijkens, T.T.P.; van Dorst, K.J.F.; Dijkstra, C.D.; Kooij, G.; Lutgens, E. The CD40-CD40L Dyad in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Front. Immunol. 2017, 8, 1791. [Google Scholar] [CrossRef]
- Bornacelly, A.; Mercado, D.; Acevedo, N.; Caraballo, L. The Strength of the Antibody Response to the Nematode Ascaris Lumbricoides Inversely Correlates with Levels of B-Cell Activating Factor (BAFF). BMC Immunol. 2014, 15, 22. [Google Scholar] [CrossRef]
- Gasperi, C.; Stüve, O.; Hemmer, B. B Cell-Directed Therapies in Multiple Sclerosis. Neurodegener. Dis. Manag. 2016, 6, 37–47. [Google Scholar] [CrossRef]
- Vaknin-Dembinsky, A.; Brill, L.; Orpaz, N.; Abramsky, O.; Karussis, D. Preferential Increase of B-Cell Activating Factor in the Cerebrospinal Fluid of Neuromyelitis Optica in a White Population. Mult. Scler. Houndmills Basingstoke Engl. 2010, 16, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Marco, V.D.; Petta, S.; Almasio, P.L.; Barbaria, F.; Licata, A.; Bosco, G.L.; Tripodo, C.; Stefano, R.D.; Craxì, A. Serum BLyS/BAFF Predicts the Outcome of Acute Hepatitis C Virus Infection. J. Viral Hepat. 2009, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Toubi, E.; Gordon, S.; Kessel, A.; Rosner, I.; Rozenbaum, M.; Shoenfeld, Y.; Zuckerman, E. Elevated Serum B-Lymphocyte Activating Factor (BAFF) in Chronic Hepatitis C Virus Infection: Association with Autoimmunity. J. Autoimmun. 2006, 27, 134–139. [Google Scholar] [CrossRef] [PubMed]

| pwMS (n = 38) | |
|---|---|
| Female/Male | 14/24 |
| Age, median (IQR) | 54 (47–61) |
| Years of disease, median (IQR) | 11 (6–19) |
| EDSS, median (IQR) | 5.5 (4.0–7.0) |
| Prior treatment | |
| alemtuzumab | 1 |
| azathioprine | 2 |
| daclizumab | 1 |
| dimethyl fumarate | 4 |
| fingolimod | 6 |
| glatiramer acetate | 2 |
| IFN-β | 3 |
| natalizumab | 3 |
| rituximab | 1 |
| teriflunomide | 2 |
| none | 13 |
| Plasma Ig levels | |
| IgG (g/L) | 5.8 (2.8–10.5) |
| IgA (g/L) | 2.0 (1.6–2.7) |
| IgM (g/L) | 0.9 (0.8–1.2) |
| pwMS | HD | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T6 | T12 | p § | p † | p †† | p ††† | ||
| BAFF (pg/mL) | 781 (495–1288) | 1462 (1060–2011) | 1891 (1506–2497) | <0.0001 | 494 (211–611) | 0.0073 | <0.0001 | <0.0001 |
| APRIL (pg/mL) | 724 (443–1548) | 642 (407–919) | 383 (319–791) | 0.0004 | 477 (303–751) | 0.0223 | ns | ns |
| CD40L (pg/mL) | 1087 (519–2281) | 965 (474–1781) | 665.50 (298–1599) | <0.0001 | 417 (298–724) | 0.0002 | 0.0035 | ns |
| pwMS (n = 38) | ||
|---|---|---|
| With Infectious Event (n = 14) | Without Infectious Event (n = 24) | |
| Female/Male | 3/11 | 11/13 |
| Age, median years [IQR] | 60 (50–65) | 51 (47–56) |
| Years of disease, median (IQR) | 17 (11–23) | 9 (5–14) |
| EDSS, median (IQR) | 6.5 (5.5–7) | 5 (3–6) |
| Infectious event: | ||
| HSV-1 reactivation | 2 | - |
| UTIs | 9 | - |
| RTIs | 5 | - |
| Antiviral/antibiotic treatment | 14 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingaropoli, M.A.; Pasculli, P.; Tartaglia, M.; Dominelli, F.; Ciccone, F.; Taglietti, A.; Perri, V.; Malimpensa, L.; Ferrazzano, G.; Iannetta, M.; et al. Evaluation of BAFF, APRIL and CD40L in Ocrelizumab-Treated pwMS and Infectious Risk. Biology 2023, 12, 587. https://doi.org/10.3390/biology12040587
Zingaropoli MA, Pasculli P, Tartaglia M, Dominelli F, Ciccone F, Taglietti A, Perri V, Malimpensa L, Ferrazzano G, Iannetta M, et al. Evaluation of BAFF, APRIL and CD40L in Ocrelizumab-Treated pwMS and Infectious Risk. Biology. 2023; 12(4):587. https://doi.org/10.3390/biology12040587
Chicago/Turabian StyleZingaropoli, Maria Antonella, Patrizia Pasculli, Matteo Tartaglia, Federica Dominelli, Federica Ciccone, Ambra Taglietti, Valentina Perri, Leonardo Malimpensa, Gina Ferrazzano, Marco Iannetta, and et al. 2023. "Evaluation of BAFF, APRIL and CD40L in Ocrelizumab-Treated pwMS and Infectious Risk" Biology 12, no. 4: 587. https://doi.org/10.3390/biology12040587
APA StyleZingaropoli, M. A., Pasculli, P., Tartaglia, M., Dominelli, F., Ciccone, F., Taglietti, A., Perri, V., Malimpensa, L., Ferrazzano, G., Iannetta, M., Del Borgo, C., Lichtner, M., Mastroianni, C. M., Conte, A., & Ciardi, M. R. (2023). Evaluation of BAFF, APRIL and CD40L in Ocrelizumab-Treated pwMS and Infectious Risk. Biology, 12(4), 587. https://doi.org/10.3390/biology12040587









