Impacts of Deoxygenation and Hypoxia on Shark Embryos Anti-Predator Behavior and Oxidative Stress
Abstract
Simple Summary
Abstract
1. Introduction
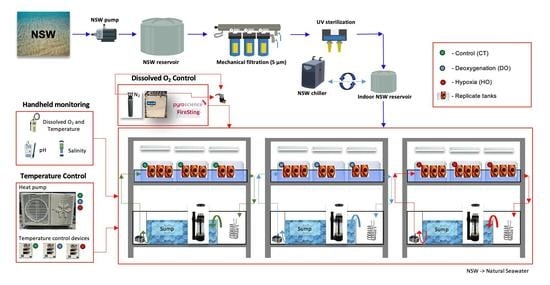
2. Materials and Methods
2.1. Collection and Incubation
2.2. Biological Response
2.3. Biochemical Analysis
2.3.1. Tissue Processing
2.3.2. Total Protein Content
2.3.3. Antioxidant Enzymes
Superoxide Dismutase Activity (SOD)
Catalase Activity (CAT)
Glutathione Peroxidase Activity (GPx)
Glutathione-S-Transferase (GST)
2.4. Protein Repair and Elimination
2.4.1. Heat Shock Response (HSP70)
2.4.2. Ubiquitin Content (Ub)
2.4.3. Lipid Peroxidation
2.5. Statistical Analysis
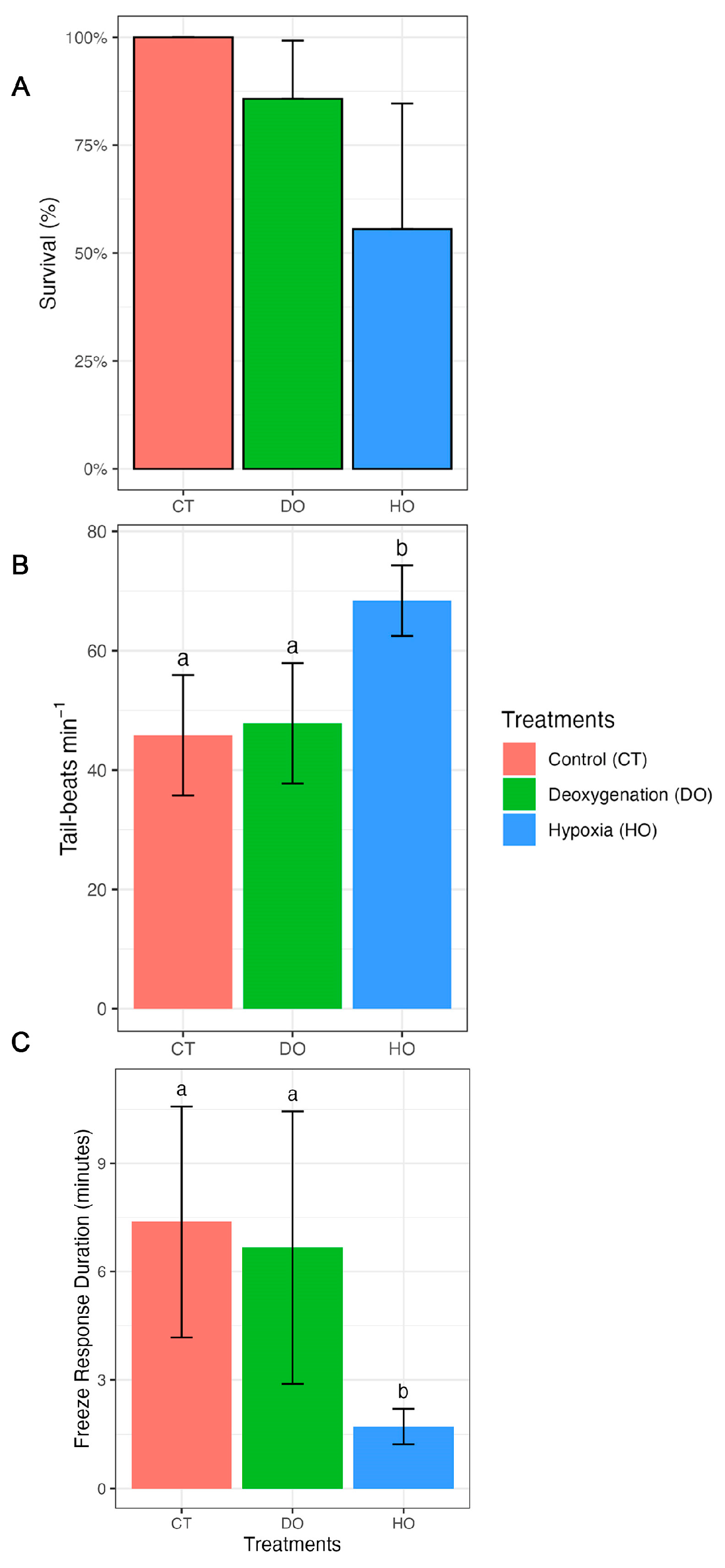
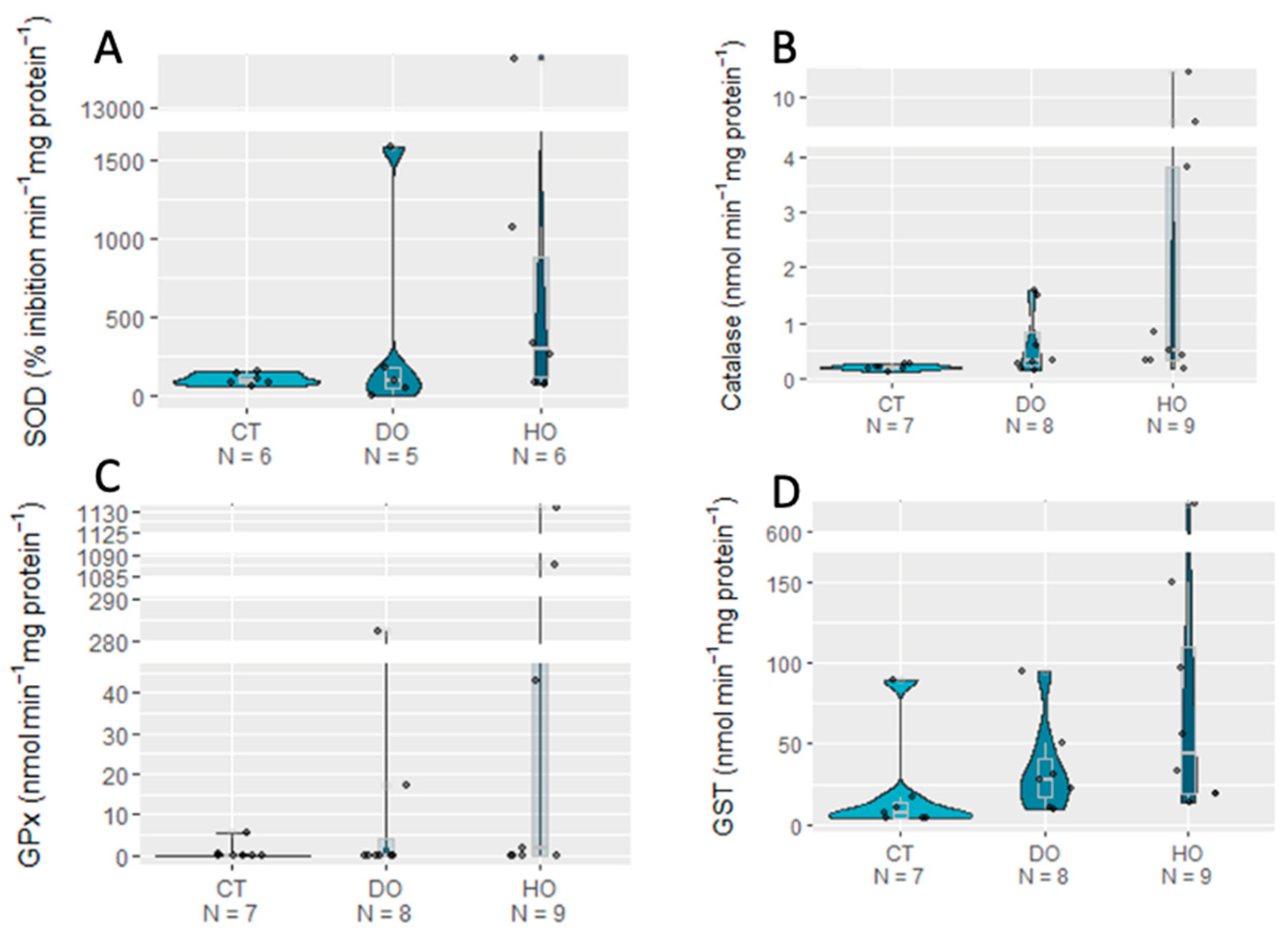
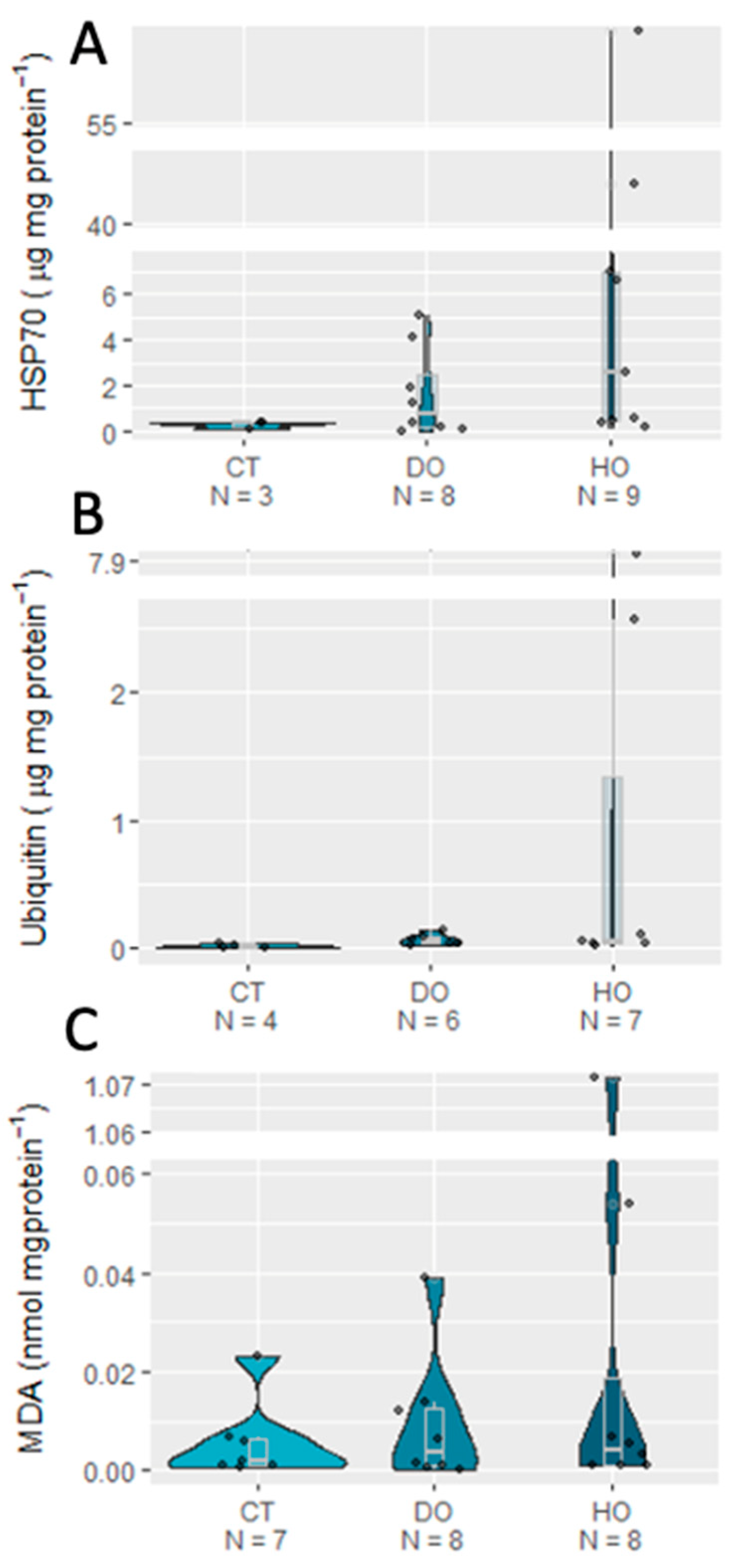
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, M.C.; Deutsch, C.; Ito, T. Finding Forced Trends in Oceanic Oxygen. Glob. Biogeochem. Cycles 2016, 30, 381–397. [Google Scholar] [CrossRef]
- Stramma, L.; Johnson, G.C.; Sprintall, J.; Mohrholz, V. Expanding Oxygen-Minimum Zones in the Tropical Oceans. Science 2008, 320, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.O.; Sampaio, E.; Santos, C.P.; Rosa, R. Impacts of Low Oxygen on Marine Life: Neglected, but a Crucial Priority for Research. Biol. Bull. 2022, 243, 104–119. [Google Scholar] [CrossRef]
- Santos, C.P.; Sampaio, E.; Pereira, B.P.; Pegado, M.R.; Borges, F.O.; Wheeler, C.R.; Bouyoucos, I.A.; Rummer, J.L.; Frazão Santos, C.; Rosa, R. Elasmobranch Responses to Experimental Warming, Acidification, and Oxygen Loss—A Meta-Analysis. Front. Mar. Sci. 2021, 8, 735377. [Google Scholar] [CrossRef]
- Sampaio, E.; Santos, C.; Rosa, I.C.; Ferreira, V.; Pörtner, H.-O.; Duarte, C.M.; Levin, L.A.; Rosa, R. Impacts of Hypoxic Events Surpass Those of Future Ocean Warming and Acidification. Nat. Ecol. Evol. 2021, 5, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Keeling, R.F.; Körtzinger, A.; Gruber, N. Ocean Deoxygenation in a Warming World. Annu. Rev. Mar. Sci. 2010, 2, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, S.; Stramma, L.; Visbeck, M. Decline in Global Oceanic Oxygen Content during the Past Five Decades. Nature 2017, 542, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining Oxygen in the Global Ocean and Coastal Waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [PubMed]
- Mattiasen, E.G.; Kashef, N.S.; Stafford, D.M.; Logan, C.A.; Sogard, S.M.; Bjorkstedt, E.P.; Hamilton, S.L. Effects of Hypoxia on the Behavior and Physiology of Kelp Forest Fishes. Glob. Change Biol. 2020, 26, 3498–3511. [Google Scholar] [CrossRef]
- Dowling, D.K.; Simmons, L.W. Reactive Oxygen Species as Universal Constraints in Life-History Evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 1737–1745. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871747-8. [Google Scholar]
- Lesser, M.P. Oxidative Stress in Marine Environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as Potential Antioxidants: A Systematic Review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Fundamental Aspects of Reactive Oxygen Species, or What’s the Matter with Oxygen? Ann. N. Y. Acad. Sci. 1999, 893, 13–18. [Google Scholar] [CrossRef]
- Wang, L.; Groves, M.J.; Hepburn, M.D.; Bowen, D.T. Glutathione S-Transferase Enzyme Expression in Hematopoietic Cell Lines Implies a Differential Protective Role for T1 and A1 Isoenzymes in Erythroid and for M1 in Lymphoid Lineages. Haematologica 2000, 85, 7. [Google Scholar]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of Plant Heat-Shock Proteins and Molecular Chaperones in the Abiotic Stress Response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Hanna, J.; Meides, A.; Zhang, D.P.; Finley, D. A Ubiquitin Stress Response Induces Altered Proteasome Composition. Cell 2007, 129, 747–759. [Google Scholar] [CrossRef]
- Rosa, R.; Baptista, M.; Lopes, V.M.; Pegado, M.R.; Ricardo Paula, J.; Trübenbach, K.; Leal, M.C.; Calado, R.; Repolho, T. Early-Life Exposure to Climate Change Impairs Tropical Shark Survival. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141738. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction Risk and Conservation of the World’s Sharks and Rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a Century of Global Decline in Oceanic Sharks and Rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Pistevos, J.C.A.; Nagelkerken, I.; Rossi, T.; Olmos, M.; Connell, S.D. Ocean Acidification and Global Warming Impair Shark Hunting Behaviour and Growth. Sci. Rep. 2015, 5, 16293. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Rummer, J.L.; Munday, P.L. Biological Responses of Sharks to Ocean Acidification. Biol. Lett. 2017, 13, 20160796. [Google Scholar] [CrossRef] [PubMed]
- Kempster, R.M.; Hart, N.S.; Collin, S.P. Survival of the Stillest: Predator Avoidance in Shark Embryos. PLoS ONE 2013, 8, e52551. [Google Scholar] [CrossRef] [PubMed]
- Musa, S.M.; Czachur, M.V.; Shiels, H.A. Oviparous Elasmobranch Development inside the Egg Case in 7 Key Stages. PLoS ONE 2018, 13, e0206984. [Google Scholar] [CrossRef]
- Ripley, D.M.; De Giorgio, S.; Gaffney, K.; Thomas, L.; Shiels, H.A. Ocean Warming Impairs the Predator Avoidance Behaviour of Elasmobranch Embryos. Conserv. Physiol. 2021, 9, coab045. [Google Scholar] [CrossRef]
- Ellis, J.R.; Shackley, S.E. The Reproductive Biology of Scyliorhinus Canicula in the Bristol Channel, U.K. J. Fish Biol. 1997, 51, 361–372. [Google Scholar] [CrossRef]
- Musa, S.M.; Ripley, D.M.; Moritz, T.; Shiels, H.A. Ocean warming and hypoxia affect embryonic growth, fitness and survival of small-spotted catsharks, Scyliorhinus canicula. J. Fish Biol. 2020, 97, 257–264. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Oxidative Stress in Fish: A Review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Janse, M.; Firchau, B.; Mohan, P. Elasmobranch Nutrition, Food Handling, and Feeding Techniques. In The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and Their Relatives; Ohio Biological Survey, Inc.: Columbus, OH, USA, 2004; ISBN 978-0-86727-152-2. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase. J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Njemini, R.; Lambert, M.; Demanet, C.; Mets, T. Heat Shock Protein 32 in Human Peripheral Blood Mononuclear Cells: Effect of Aging and Inflammation. J. Clin. Immunol. 2005, 25, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.; Sampaio, E.; Santos, C.; Couto, A.; Pegado, M.R.; Diniz, M.; Munday, P.L.; Rummer, J.L.; Rosa, R. Absence of Cellular Damage in Tropical Newly Hatched Sharks (Chiloscyllium Plagiosum) under Ocean Acidification Conditions. Cell Stress Chaperones 2018, 23, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Mihara, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.L. Dissolved Oxygen and Fish Behavior. Environ. Biol. Fishes 1987, 18, 81–92. [Google Scholar] [CrossRef]
- Diez, J.M.; Davenport, J. Energy Exchange between the Yolk and Embryo of Dogfish (Scyliorhinus Canicula L.) Eggs Held under Normoxic, Hypoxic and Transient Anoxic Conditions. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 96, 825–830. [Google Scholar] [CrossRef]
- Di Santo, V.; Tran, A.H.; Svendsen, J.C. Progressive Hypoxia Decouples Activity and Aerobic Performance of Skate Embryos. Conserv. Physiol. 2016, 4, cov067. [Google Scholar] [CrossRef]
- Gervais, C.R.; Brown, C. Impact of Conspecific Necromones on the Oxygen Uptake Rates of a Benthic Elasmobranch. Anim. Behav. 2021, 174, 1–8. [Google Scholar] [CrossRef]
- Sevcikova, M.; Modra, H.; Slaninova, A.; Svobodova, Z. Metals as a Cause of Oxidative Stress in Fish: A Review. Veterinární Med. 2011, 56, 537–546. [Google Scholar] [CrossRef]
- Bartosz, G. Total Antioxidant Capacity. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2003; Volume 37, pp. 219–292. ISBN 978-0-12-010337-9. [Google Scholar]
- Pegado, M.R.; Santos, C.P.; Pimentel, M.; Cyrne, R.; Sampaio, E.; Temporão, A.; Röckner, J.; Diniz, M.; Rosa, R. Lack of Oxidative Damage on Temperate Juvenile Catsharks after a Long-Term Ocean Acidification Exposure. Mar. Biol. 2020, 167, 165. [Google Scholar] [CrossRef]
- Rudneva, I.I. Blood Antioxidant System of Black Sea Elasmobranch and Teleosts. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1997, 118, 255–260. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V. Hypoxia Induces Oxidative Stress in Tissues of a Goby, the Rotan Perccottus Glenii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hassell, K.L.; Coutin, P.C.; Nugegoda, D. Hypoxia Impairs Embryo Development and Survival in Black Bream (Acanthopagrus Butcheri). Mar. Pollut. Bull. 2008, 57, 302–306. [Google Scholar] [CrossRef] [PubMed]
| Control | Deoxygenation | Hypoxia | |
|---|---|---|---|
| Temperature (°C) | 16.3 ± 0.2 | 16.1 ± 0.2 | 16.3 ± 0.1 |
| pH (pH units) | 8.07 ± 0.02 | 8.07 ± 0.02 | 8.16 ± 0.02 |
| Salinity (g/L) | 33.0 ± 0.2 | 33.0 ± 0.2 | 33.0 ± 0.2 |
| Oxygen (%) | 99.56 ± 0.69 | 92.83 ± 0.93 | 28.26 ± 3.25 |
| Estimate | SE | Df | z-Ratio | p-Value | |
|---|---|---|---|---|---|
| Survival | |||||
| CT–DO | 23.13 | 55,398.03 | NA | 0.000 | 1.0000 |
| CT–HO | 24.70 | 55,398.03 | NA | 0.000 | 1.0000 |
| DO–HO | 1.57 | 1.27 | NA | 1.234 | 0.6520 |
| Estimate | SE | Df | t-Ratio | p-Value | |
| Tail beats | |||||
| CT–DO | −2.0 | 2.86 | 54 | −0.698 | 0.4879 |
| CT–HO | −22.6 | 3.02 | 54 | −7.465 | <0.0001 |
| DO–HO | −20.6 | 3.21 | 54 | −6.405 | <0.0001 |
| Freeze response duration | |||||
| CT–DO | 0.708 | 1.53 | 18 | 0.463 | 0.6491 |
| CT–HO | 5.661 | 1.47 | 18 | 3.858 | 0.0035 |
| DO–HO | 4.952 | 1.58 | 18 | 3.140 | 0.0113 |
| Chi-Squared | Df | p-Value | |
|---|---|---|---|
| Survival | 6.4566 | 2 | 0.03963 |
| Sum of Squares | Df | p-Value | |
| Tail beats | 5250.3 | 2 | 1.024 × 10−9 |
| Freeze response duration | 135.65 | 2 | 0.002595 |
| Chi-Squared | Df | p-Value | |
|---|---|---|---|
| SOD | 2.0314 | 2 | 0.3621 |
| CAT | 5.3528 | 2 | 0.06881 |
| GPx | 3.5299 | 2 | 0.1712 |
| GST | 3.1165 | 2 | 0.2105 |
| HSP70 | 3.1764 | 2 | 0.2043 |
| Ub | 2.4077 | 2 | 0.3 |
| MDA | 1.9324 | 2 | 0.3805 |
| DNA Damage | 0.68601 | 2 | 0.7096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varela, J.; Martins, S.; Court, M.; Santos, C.P.; Paula, J.R.; Ferreira, I.J.; Diniz, M.; Repolho, T.; Rosa, R. Impacts of Deoxygenation and Hypoxia on Shark Embryos Anti-Predator Behavior and Oxidative Stress. Biology 2023, 12, 577. https://doi.org/10.3390/biology12040577
Varela J, Martins S, Court M, Santos CP, Paula JR, Ferreira IJ, Diniz M, Repolho T, Rosa R. Impacts of Deoxygenation and Hypoxia on Shark Embryos Anti-Predator Behavior and Oxidative Stress. Biology. 2023; 12(4):577. https://doi.org/10.3390/biology12040577
Chicago/Turabian StyleVarela, Jaquelino, Sandra Martins, Melanie Court, Catarina Pereira Santos, José Ricardo Paula, Inês João Ferreira, Mário Diniz, Tiago Repolho, and Rui Rosa. 2023. "Impacts of Deoxygenation and Hypoxia on Shark Embryos Anti-Predator Behavior and Oxidative Stress" Biology 12, no. 4: 577. https://doi.org/10.3390/biology12040577
APA StyleVarela, J., Martins, S., Court, M., Santos, C. P., Paula, J. R., Ferreira, I. J., Diniz, M., Repolho, T., & Rosa, R. (2023). Impacts of Deoxygenation and Hypoxia on Shark Embryos Anti-Predator Behavior and Oxidative Stress. Biology, 12(4), 577. https://doi.org/10.3390/biology12040577