Assessment of the Relationship between the Total Occlusal Area of the Human Permanent Upper First and Second Molars and the Robusticity of the Facial Skeleton in Sex-Different Cranial Samples of Homo Sapiens: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. The Total Occlusal Area
2.3. Traits of the Facial Skeleton
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Relative TOCA of Upper Molars and the Relative General Facial Robusticity
4.2. TOCA of the Upper Molars and Robusticity of Examined Facial Regions
4.3. The Potential Influence of Diet and Non-Masticatory Activities on the Values of TOCA
4.3.1. Meaning of Diet
4.3.2. Meaning of Non-Masticatory Activities
4.4. Meaning of the Results and the Direction of Further Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demes, B.; Creel, N. Bite Force, Diet, and Cranial Morphology of Fossil Hominids. J. Hum. Evol. 1988, 17, 657–670. [Google Scholar] [CrossRef]
- Eng, C.M.; Lieberman, D.E.; Zink, K.D.; Peters, M.A. Bite Force and Occlusal Stress Production in Hominin Evolution. Am. J. Phys. Anthropol. 2013, 151, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Lahr, M.M. The Evolution of Modern Human Diversity: A Study in Cranial Variation; Cambridge University Press: Cambridge, UK, 1996; ISBN 0 521 47393 4. [Google Scholar]
- Lahr, M.M.; Wright, R.V.S. The Question of Robusticity and the Relationship between Cranial Size and Shape in Homo sapiens. J. Hum. Evol. 1996, 31, 157–191. [Google Scholar] [CrossRef]
- Bernal, V.; Perez, S.I.; González, P.N. Variation and Causal Factors of Craniofacial Robusticity in Patagonian Hunter-Gatherers from Late Holocene. Am. J. Hum. Biol. 2006, 18, 748–765. [Google Scholar] [CrossRef]
- Brachetta-Aporta, N.; Toro-Ibacache, V. Differences in Masticatory Loads Impact Facial None Surface Remodeling in an Archaeological Sample of South American Individuals. J. Archaeol. Sci. Rep. 2021, 38, 103034. [Google Scholar] [CrossRef]
- Gross, M.D.; Arbel, G.; Hershkovitz, I. Three-dimensional Finite Element Analysis of the Facial Skeleton on Simulated Occlusal Loading. J. Oral Rehabil. 2001, 28, 684–694. [Google Scholar] [CrossRef]
- Hilloowala, R.A.; Trent, R.B. Supraorbital Ridge and Masticatory Apparatus II: Humans (Eskimos). Hum. Evol. 1988, 3, 351–356. [Google Scholar] [CrossRef]
- Janovic, A.; Saveljic, I.; Vukicevic, A.; Nikolic, D.; Rakocevic, Z.; Jovicic, G.; Filipovic, N.; Djuric, M. Occlusal Load Distribution Through the Cortical and Trabecular Bone of the Human Mid-facial Skeleton in Natural Dentition: A Three-dimensional Finite Element Study. Ann. Anat. 2015, 197, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ledogar, J.A.; Dechow, P.C.; Wang, Q.; Gharpure, P.H.; Gordon, A.D.; Baab, K.L.; Smith, A.L.; Weber, G.W.; Grosse, I.R.; Ross, C.F.; et al. Human Feeding Biomechanics: Performance, Variation, and Functional Constraints. PeerJ 2016, 4, e2242. [Google Scholar] [CrossRef]
- Lieberman, D.E. The Evolution of the Human Head; Harvard University Press: Cambridge, MA, USA, 2011; ISBN 978-0-674-04636-8. [Google Scholar]
- Paschetta, C.; de Azevedo, S.; Castillo, L.; Martínez-Abadías, N.; Hernández, M.; Lieberman, D.E.; González-José, R. The Influence of Masticatory Loading on Craniofacial Morphology: A Test Case Across Technological Transitions in the Ohio Valley. Am. J. Phys. Anthropol. 2010, 141, 297–314. [Google Scholar] [CrossRef]
- Pinhasi, R.; Eshed, V.; Shaw, P. Evolutionary Changes in the Masticatory Complex Following the Transition to Farming in the Southern Levant. Am. J. Phys. Anthropol. 2008, 135, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Prado, F.B.; Noritomi, P.Y.; Freire, A.R.; Rossi, A.C.; Neto, F.H.; Caria, P.H.F. Stress Distribution in Human Zygomatic Pillar Using Three-Dimensional Finite Element Analysis. Int. J. Morphol. 2013, 31, 1386–1392. [Google Scholar] [CrossRef]
- Baab, K.L.; Freidline, S.E.; Wang, S.L.; Hanson, T. Relationship of Cranial Robusticity to Cranial Form, Geography and Climate in Homo sapiens. Am. J. Phys. Anthropol. 2010, 141, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Lalueza, C.; Garcıa-Moro, C. Fueguian Cranial Morphology: The Adaptation to a Cold, Harsh Environment. Am. J. Phys. Anthropol. 1997, 103, 103–117. [Google Scholar] [CrossRef]
- Larsen, C.S. Biological Changes in Human Populations with Agriculture. Annu. Rev. Anthropol. 1995, 24, 185–213. [Google Scholar] [CrossRef]
- Larsen, C.S. Bioarchaeology: Interpreting Behavior from the Human Skeleton; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Sardi, M.L.; Novellino, P.S.; Pucciarelli, H.M. Craniofacial Morphology in the Argentine Center-West: Consequences of the Transition to Food Production. Am. J. Phys. Anthropol. 2006, 130, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Von Cramon-Taubadel, N. Evolutionary Insights into Global Patterns of Human Cranial Diversity: Population History, Climatic and Dietary Effects. J. Anthropol. Sci. 2013, 91, 1–36. [Google Scholar] [CrossRef]
- Clement, A.F.; Hillson, S.W. Intrapopulation Variation in Macro Tooth Wear Patterns: A Case Study from Igloolik, Canada. Am. J. Phys. Anthropol. 2012, 149, 517–524. [Google Scholar] [CrossRef]
- Edmonds, H.M.; Glowacka, H. The Ontogeny of Maximum Bite Force in Humans. J. Anat. 2020, 237, 529–542. [Google Scholar] [CrossRef]
- Hlusko, L.J.; Mahaney, M. Quantitative Genetics, Pleiotropy, and Morphological Integration in the Dentition of Papio hamadryas. Evol. Biol. 2009, 36, 5–18. [Google Scholar] [CrossRef]
- Hlusko, L.J.; Richard, D.S.; Mahaney, M.C. Modularity in the Mammalian Dentition: Mice and Monkeys Share a Common Dental Genetic Architecture. J. Exp. Zool. B Mol. Dev. Evol. 2011, 316, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Robles, A.; Polly, P.D. Morphological Integration in the Hominin Dentition: Evolutionary, Developmental, and Functional Factors. Evolution 2012, 66, 1024–1043. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Hughes, T.E.; James, H.; Townsend, G.C. Strong Genetic Influence on Hypocone Expression of Permanent Maxillary Molars in South Australian Twins. Dental Anthropol. 2009, 22, 1–7. [Google Scholar]
- Townsend, G.; Bockmann, M.; Hughes, T.; Brook, A. Genetic, Environmental and Epigenetic Influences on Variation in Human Tooth Number, Size and Shape. Odontology 2012, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.; Townsend, G. Genetic and Environmental Contributions to Variation in Human Tooth Size. Heredity 2001, 86, 685–693. [Google Scholar] [CrossRef]
- Cheverud, J.M. Phenotypic, Genetic, and Environmental Morphological Integration in the Cranium. Evolution 1982, 36, 499–516. [Google Scholar] [CrossRef]
- Enlow, D.H.; Moyers, R.E.; Hunter, W.S.; McNamara, J.A., Jr. A Procedure for the Analysis of Intrinsic Facial form and Growth. Am. J. Orthod. 1969, 56, 6–23. [Google Scholar] [CrossRef]
- Enlow, D.H.; Hans, M.G. Essentials of Facial Growth; W.B. Saunders Co.: Philadelphia, PA, USA, 1996. [Google Scholar]
- Bastir, M.; Rosas, A. Hierarchical Nature of Morphological Integration and Modularity in the Human Posterior Face. Am. J. Phys. Anthropol. 2005, 128, 26–34. [Google Scholar] [CrossRef]
- Noback, M.L.; Harvati, K. The Contribution of Subsistence to Global Human Cranial Variation. J. Hum. Evol. 2015, 80, 34–50. [Google Scholar] [CrossRef]
- Reyes-Centeno, H.; Ghirotto, S.; Harvati, K. Genomic Validation of the Differential Preservation of Population History in the Human Cranium. Am. J. Phys. Anthropol. 2017, 162, 170–179. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Stringer, C.B.; Kimbel, W.H.; Wood, B.; Harvati, K.; O’Higgins, P.; Bromage, T.G.; Juan-Luis, A. The Evolutionary History of the Human Face. Nat. Ecol. Evol. 2019, 3, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Weidenreich, F. The Torus Occipitalis and Related Structures and their Transformations in the Course of Human Evolution. Bull. Geol. Soc. China 1940, 19, 479–544. [Google Scholar] [CrossRef]
- Lieberman, D.E.; McBratney, B.M.; Krovitz, G. The Evolution and Development of Cranial form in Homo sapiens. Proc. Natl. Acad. Sci. USA 2002, 99, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Nowaczewska, W.; Kubicka, A.M.; Piontek, J.; Biecek, P. The Meaning of the Shape of the Frontal Bone, Facial Retraction and Prognathism for the Degree of Gracilisation of the Supraorbital Region in Homo sapiens. Anthropol. Anz. 2022, 79, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Nowaczewska, W.; Kuźmiński, Ł.; Biecek, P. Morphological Relationship between the Cranial and Supraorbital Regions in Homo sapiens. Am. J. Phys. Anthropol. 2015, 156, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Nowaczewska, W.; Łapicka, U.; Cieślik, A.; Biecek, P. The Relationship of Cranial, Orbital and Nasal Cavity Size with the Morphology of the Supraorbital Region in Modern Homo sapiens. Anthropol. Anz. 2017, 74, 241–246. [Google Scholar] [CrossRef]
- Fiscella, G.N.; Smith, F.H. Ontogenetic Study of the Supraorbital Region in Modern Humans: A Longitudinal Test of the Spatial Model. Anthropol. Anz. 2006, 64, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.L.; Young, R.W. A Functional Approach to Craniology. Am. J. Phys. Anthropol. 1960, 18, 281–292. [Google Scholar] [CrossRef]
- Shea, B.T.; Russell, M.D. On Skull form and the Supraorbital Torus in Primates. Curr. Anthropol. 1986, 27, 257–260. [Google Scholar] [CrossRef]
- Vinyard, C.J.; Smith, F.H. Morphometric Relationships between the Supraorbital Region and Frontal Sinus in Melanesian Crania. Homo 1997, 48, 1–21. [Google Scholar]
- Vinyard, C.J.; Smith, F.H. Morphometric Testing of Structural Hypotheses of the Supraorbital Region in Modern Humans. Z. Morphol. Anthropol. 2001, 83, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Endo, B. Analysis of Stress Around the Orbit Due to Masseter and Temporalis Muscles Respectively. J. Anthropol. Soc. Jpn. 1970, 78, 251–266. [Google Scholar] [CrossRef]
- Russell, M.D. The Supraobital Torus: A Most Remarkable Peculiarity. Curr. Anthropol. 1985, 27, 337–350. [Google Scholar] [CrossRef]
- Górny, S. Crania Africana, Uganda. Mater. Prace Antropolog. 1957, 14, 1–400. [Google Scholar]
- Kruczkiewicz, E. Ossa Australica. Polska Akademia Nauk, Zakład Antropologii. Mater. Prace Antropolog. 1962, 58, 1–93. [Google Scholar]
- Loth, E. Wyprawa antropologiczna do Ugandy (Ruwenzori). Przegląd Antropolog. 1938, 12, 690–692. [Google Scholar]
- Milicerowa, H. Crania Australica. Mater. Prace Antropolog. 1955, 6, 1–268. [Google Scholar]
- Buikstra, J.E.; Ubelaker, D.H. Standards for Data Collection from Human Skeletal Remains; Arkansas Archeological Survey Research Series, No 44; Arkansas Archeological Survey: Fayetteville, NC, USA, 1994. [Google Scholar]
- White, T.D.; Folkens, P.A. Human Osteology; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Ferembach, D.; Schwindezky, I.; Stoukal, M. Recommendations for Age and Sex Diagnoses of Skeletons. J. Hum. Evol. 1980, 9, 517–549. [Google Scholar]
- Górka, K.; Alejandro Romero, A.; Pérez-Péreza, A. First Molar Size and Wear within and among Modern Hunter-Gatherers and Agricultural Populations. Homo 2015, 66, 299–315. [Google Scholar] [CrossRef]
- Górka, K.; Alejandro Romero, A.; Pérez-Péreza, A. Dental-macrowear and Diet of Tigara Foragers from Point Hope, Northern Alaska. Anthropol. Anz. 2016, 73, 257–264. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Harris, E.F.; Smith, R.N. Accounting for Measurement Error: A Critical but often Overlooked Process. Arch. Oral Biol. 2009, 54, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.M.; Scott, N.M.; Neiswanger, K.; Marazita, M.L. Intraobserver Error Associated with Measurements of the Hand. Am. J. Phys. Anthropol. 2005, 17, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Mays, S.; Zakrzewski, S.; Field, S. The Relationship between Dental Wear and Age at Death in British Archaeological Human skeletal Remains: A Re-evaluation of the ‘Brothwell chart’. J. Archaeol. Sci. Rep. 2022, 46, 103707. [Google Scholar] [CrossRef]
- Scott, E.C. Dental Wear Scoring Technique. Am. J. Phys. Anthropol. 1979, 51, 213–218. [Google Scholar] [CrossRef]
- Littleton, J.; Scott, R.; McFarlane, G.; Walshe, K. Hunter-gatherer Variability: Dental Wear in South Australia. Am. J. Phys. Anthropol. 2013, 152, 273–286. [Google Scholar] [CrossRef]
- Flensborg, G. Health and Disease of Hunter-gatherer Groups from the Eastern Pampa–Patagonia Transition (Argentina) during the Late Holocene. Anthropol. Sci. 2016, 124, 29–44. [Google Scholar] [CrossRef]
- Tomczyk, J.; Zalewska, M. Mechanical and Chemical Dental Wear in Historical Population from the Syrian lower Euphrates Valley. Arch. Oral Biol. 2016, 62, 49–57. [Google Scholar] [CrossRef]
- Michael, D.E.; Iliadis, E.; Manolis, S.K. Using Dental and Activity Indicators in Order to Explore Possible Sex Differences in an Adult Rural Medieval Population from Thebes (Greece). Anthropol. Rev. 2017, 80, 427–447. [Google Scholar] [CrossRef]
- Crittenden, A.N.; Sorrentino, J.; Moonie, S.A.; Peterson, M.; Mabulla, A.; Ungar, P.S. Oral Health in Transition: The Hadza Foragers of Tanzania. PLoS ONE 2017, 12, e0172197. [Google Scholar] [CrossRef]
- Michael, D.E.; Eliopoulos, C.; Manolis, S.K. Exploring Sex Differences in Diets and Activity Patterns Through Dental and Skeletal Studies in Populations from Ancient Corinth, Greece. Homo 2017, 68, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Hillson, S. Dental Anthropology; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Martin, R.; Saller, K. Lehrbuch der Antropologie; Gustav Fischer Verlag: Stuttgart, Germany, 1957. [Google Scholar]
- Bräuer, G. Osteometrie. In Anthropologie, 4th ed.; Knussmann, R., Ed.; G. Fischer: Stuttgart, Germany, 1988; pp. 160–232. [Google Scholar]
- Cunningham, D.J. The Evolution of the Eyebrow Region of the Forehead, with Special Reference to the Excessive Supraorbital Development in the Neanderthal Race. Earth Environ. Sci. Trans. R. Soc. Edinb. 1908, 46, 283–311. [Google Scholar] [CrossRef]
- Piontek, J. Biologia Populacji Pradziejowych. Zarys Metodyczny; Wydawnictwo Naukowe, Uniwersytet im. Adama Mickiewicza w Poznaniu: Poznań, Poland, 1996; ISBN 83-232-0979-0. [Google Scholar]
- Sardi, M.L.; Ramírez Rozzi, F.; Pucciarelli, H.M. The Neolithic Transition in Europe and North Africa. The Functional Craneology Contribution. Anthropol. Anz. 2004, 62, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 1 March 2022).
- Alvesalo, L. Human Sex Chromosomes in Oral and Craniofacial Growth. Arch. Oral Biol. 2009, 54, S18–S24. [Google Scholar] [CrossRef]
- Garvin, H.M.; Ruff, C.B. Sexual Dimorphism in Skeletal Browridge and Chin Morphologies Determined Using a New Quantitative Method. Am. J. Phys. Anthropol. 2012, 147, 661–670. [Google Scholar] [CrossRef]
- Perlaza, N.A. Sex Determination from the Frontal Bone: A Geometric Morphometric Study. J. Forensic Sci. 2014, 59, 1330–1332. [Google Scholar] [CrossRef]
- Rosas, A.; Bastir, M. Thin-plate Spline Analysis of Allometry and Sexual Dimorphism in the Human Craniofacial Complex. Am. J. Phys. Anthropol. 2002, 117, 236–245. [Google Scholar] [CrossRef]
- Schlager, S.; Rüdell, A. Sexual Dimorphism and Population Affinity in the Human Zygomatic Structure—Comparing Surface to Outline Data. Anat. Rec. 2017, 300, 226–237. [Google Scholar] [CrossRef]
- Bulygina, E.; Mitteroecker, P.; Aiello, L. Ontogeny of Facial Dimorphism and Patterns of Individual Development within One Human Population. Am. J. Phys. Anthropol. 2006, 131, 432–443. [Google Scholar] [CrossRef]
- Freidline, S.E.; Gunz, P.; Hublin, J.-J. Ontogenetic and Static Allometry in the Human Face: Contrasting Khoisan and Inuit. Am. J. Phys. Anthropol. 2015, 158, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, L.T. Growth Patterns in the Modern Human Skeleton. Am. J. Phys. Anthropol. 1998, 105, 57–72. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 40th ed.; Churchill Livingstone Elsevier: London, UK, 2008. [Google Scholar]
- Toro-Ibacachea, V.; Muñozd, V.Z.; O’Higgins, P. The Relationship between Skull Morphology, Masticatory Muscle Force and Cranial Skeletal Deformation during Biting. Ann. Anat. 2016, 203, 59–68. [Google Scholar] [CrossRef]
- Athreya, S. The Frontal Bone in the Genus Homo: A Survey of Functional and Phylogenetic Sources of Variation. J. Anthropol. Sci. 2012, 90, 59–80. [Google Scholar] [CrossRef]
- Haavikko, K. The Formation and the Alveolar and Clinical Eruption of the Permanent Teeth. Proc. Finn. Dent. Soc. 1970, 66, 101–170. [Google Scholar]
- Schour, I.; Massler, M. Studies in Tooth Development: The Growth Pattern of Human Teeth. J. Am. Dent. Assoc. 1940, 27, 1918–1931. [Google Scholar] [CrossRef]
- Lovejoy, C.O. Dental Wear in the Libben Population: Its Functional Pattern and Role in the Determination of Adult Skeletal Age at Death. Am. J. Phys. Anthropol. 1985, 68, 47–56. [Google Scholar] [CrossRef]
- Dreier, F.G. Age at Death Estimates for the Prehistoric Arikara Using Molar Attrition Rates: A New Quantification Method. Int. J. Osteoarchaeol. 1994, 4, 137–148. [Google Scholar] [CrossRef]
- Miles, A.E.W. The Miles Method of Assessing Age from Tooth Wear Revisited. J. Archaeol. Sci. 2001, 28, 973–982. [Google Scholar] [CrossRef]
- Molnar, S. Human Tooth Wear, Tooth Function, and Cultural Variability. Am. J. Phys. Anthropol. 1971, 34, 175–190. [Google Scholar] [CrossRef]
- Smith, B.H. Patterns of Molar Wear in Hunter-gatherers and Agriculturists. Am. J. Phys. Anthropol. 1984, 63, 39–56. [Google Scholar] [CrossRef]
- Barbour, M.E.; Rees, G.D. The Role of Erosion, Abrasion and Attrition in Tooth Wear. J. Clin. Dent. 2006, 17, 88–93. [Google Scholar] [PubMed]
- Lucas, P.W.; van Casteren, A.; Al-Fadhalah, K.; Almusallam, A.S.; Henry, A.G.; Michael, S.; Watzke, J.; Reed, D.A.; Diekwisch, T.G.H.; Strait, D.S.; et al. The Role of Dust, Grit and Phytoliths in Tooth Wear. Ann. Zool. Fennici. 2014, 51, 143–152. [Google Scholar] [CrossRef]
- Deter, C.A. Correlation between Dental Occlusal Wear and Approximal Facet Length. Int. J. Osteoarchaeol. 2012, 22, 708–717. [Google Scholar] [CrossRef]
- Smith, B.H. Development and Evolution of the Helicoidal Plane of Dental Occlusion. Am. J. Phys. Anthropol. 1986, 69, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.C.; Brown, T. Development of the Helicoidal Plane. Hum. Evol. 1986, 5, 385–398. [Google Scholar] [CrossRef]
- Kaifu, Y. Changes in the Pattern of Tooth Wear from Prehistoric to Recent Periods in Japan. Am. J. Phys Anthropol. 1999, 109, 485–499. [Google Scholar] [CrossRef]
- Senator, M.; Kwiatkowska, B.; Gronkiewicz, S. Height of Skull Base as an Indicator of Living Conditions in Historical Native Populations from Europe, Australia and Africa. Homo 2009, 60, 535–549. [Google Scholar] [CrossRef]
- Turner, C. Dental anthropological indications of agriculture among the Jomon people of Central Japan. Am. J. Phys. Anthropol. 1979, 51, 619–636. [Google Scholar] [CrossRef]
- Lukacs, J. Dental Paleopathology: Methods for Reconstructing Dietary Patterns. In Reconstruction of Life from the Skeleton; Kennedy, K., Iscan, M.Y., Eds.; Liss: New York, NY, USA, 1989; pp. 261–286. [Google Scholar]
- Molnar, P. Dental Wear and Oral Pathology: Possible Evidence and Consequences of Habitual Use of Teeth in a Swedish Neolithic Sample. Am. J. Phys. Anthropol. 2008, 136, 423–431. [Google Scholar] [CrossRef]
- Clement, A.; Hillson, S.; de la Torre, I.; Townsend, G. Tooth Use in Aboriginal Australia. Archaeol. Int. 2007, 11, 37–40. [Google Scholar] [CrossRef]
- Hardin, A.M.; Knigge, R.P.; Oh, H.S.; Valiathan, M.; Duren, D.L.; McNulty, K.P.; Middleton, K.M.; Sherwood, R.J. Estimating Craniofacial Growth Cessation: Comparison of Asymptote—And Rate-based Methods. Cleft Palate Craniofac. J. 2022, 59, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Krumholt, L.; Roed-Petersen, B.; Pindborg, J.J. Eruption Times of the Permanent Teeth in 622 Ugandan Children. Arch. Oral. Biol. 1971, 16, 1281–1288. [Google Scholar] [CrossRef]
- Diamanti, J.; Townsend, G.C. New Standards for Permanent Tooth Emergence in Australian Children. Aust. Dent. J. 2003, 48, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.J. Form and Patterning of Anterior Tooth Wear among Aboriginal Human Groups. Am. J. Phys. Anthropol. 1981, 54, 555–564. [Google Scholar] [CrossRef]
- Katz, D.C.; Grote, M.N.; Weaver, T.D. Changes in Human Skull Morphology across the Agricultural Transition are Consistent with Softer Diets in Preindustrial Farming Groups. Proc. Natl. Acad. Sci. USA 2017, 114, 9050–9055. [Google Scholar] [CrossRef]
- Gonzalez, P.N.; Perez, S.I.; Bernal, V. Ontogeny of Robusticity of Craniofacial Traits in Modern Humans: A Study of South American Populations. Am. J. Phys. Anthropol. 2010, 42, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Viðarsdóttir, U.S.; O’Higgins, P.; Stringer, C. A Geometric Morphometric Study of Regional Differences in the Ontogeny of the Modern Human Facial Skeleton. J. Anat. 2002, 201, 211–229. [Google Scholar] [CrossRef]
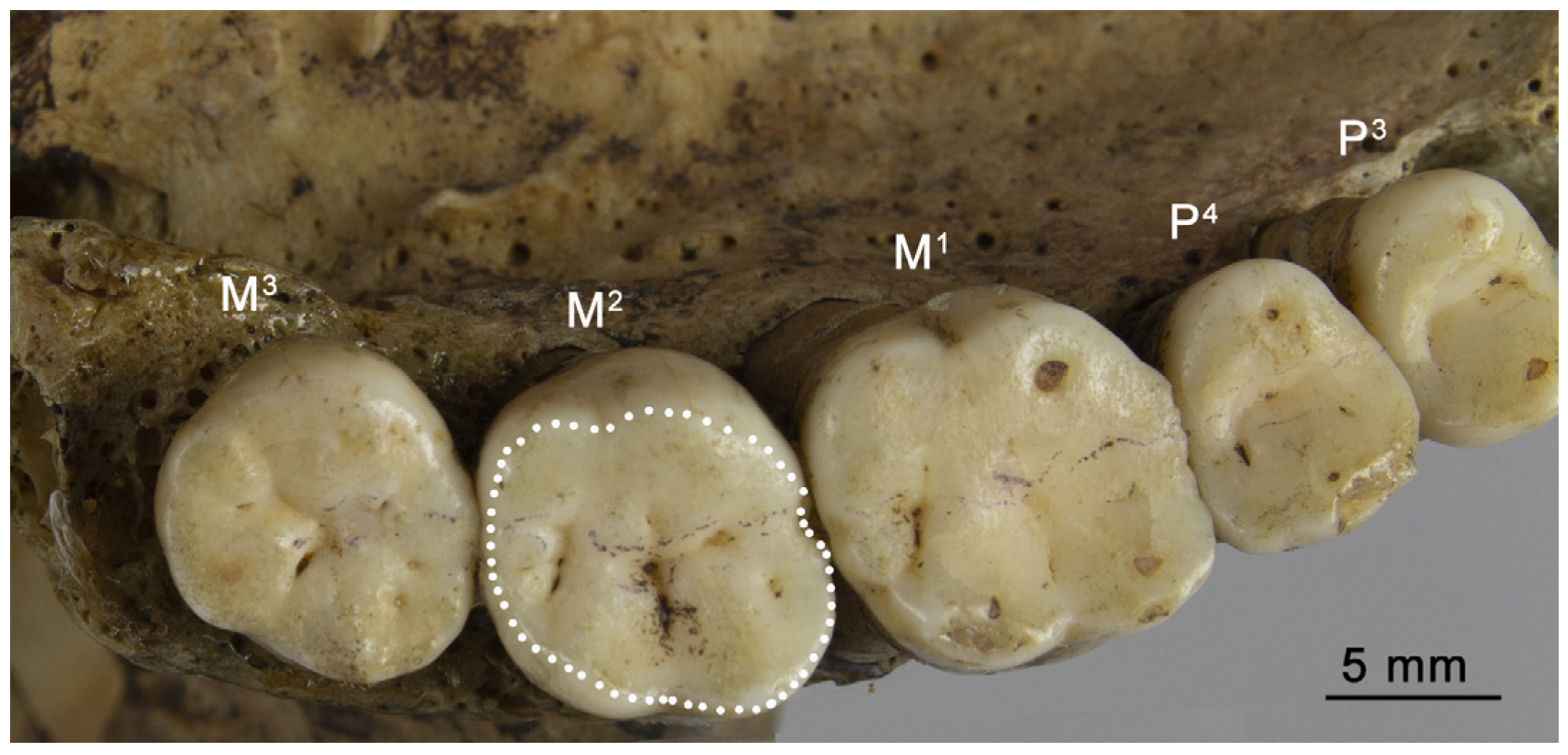
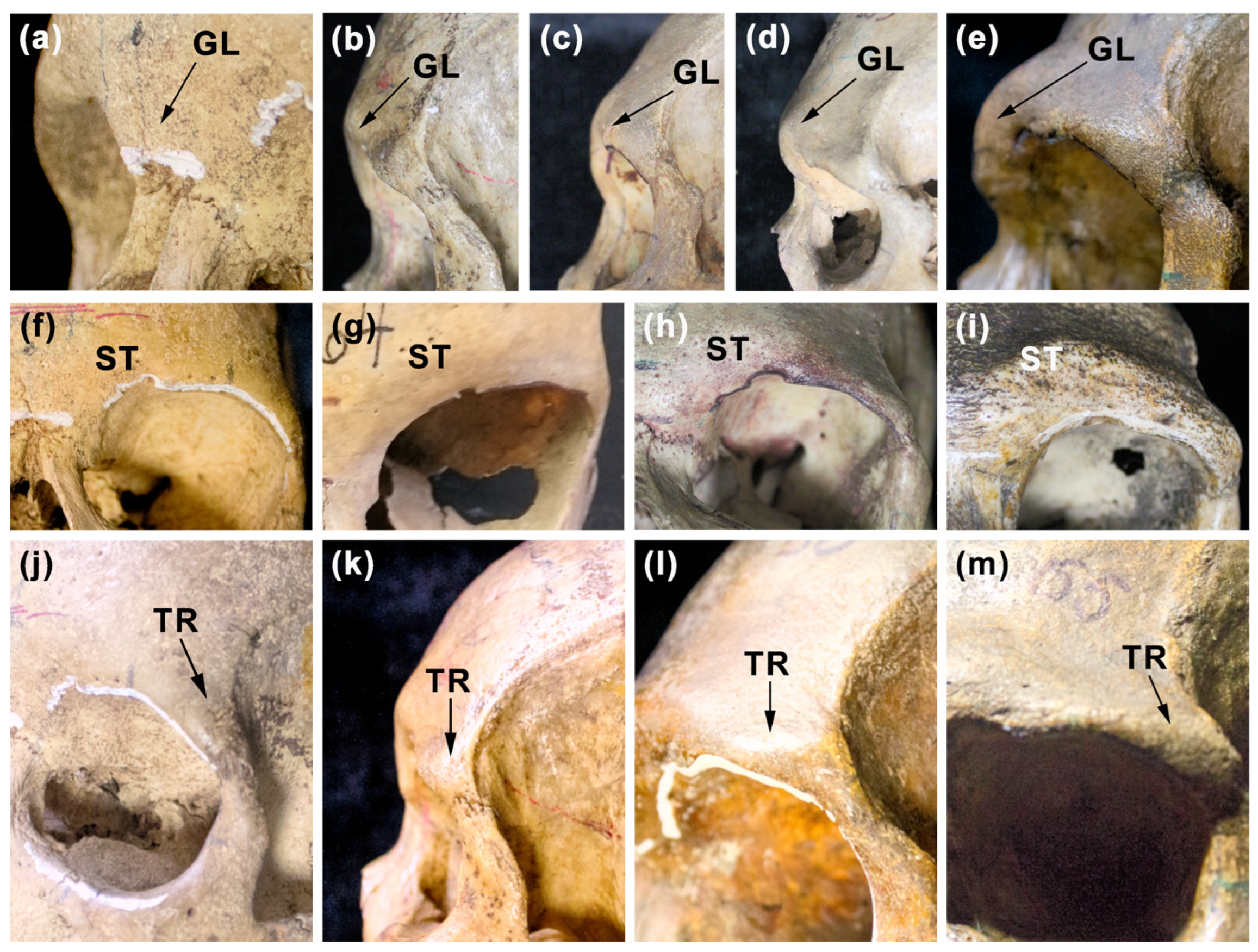
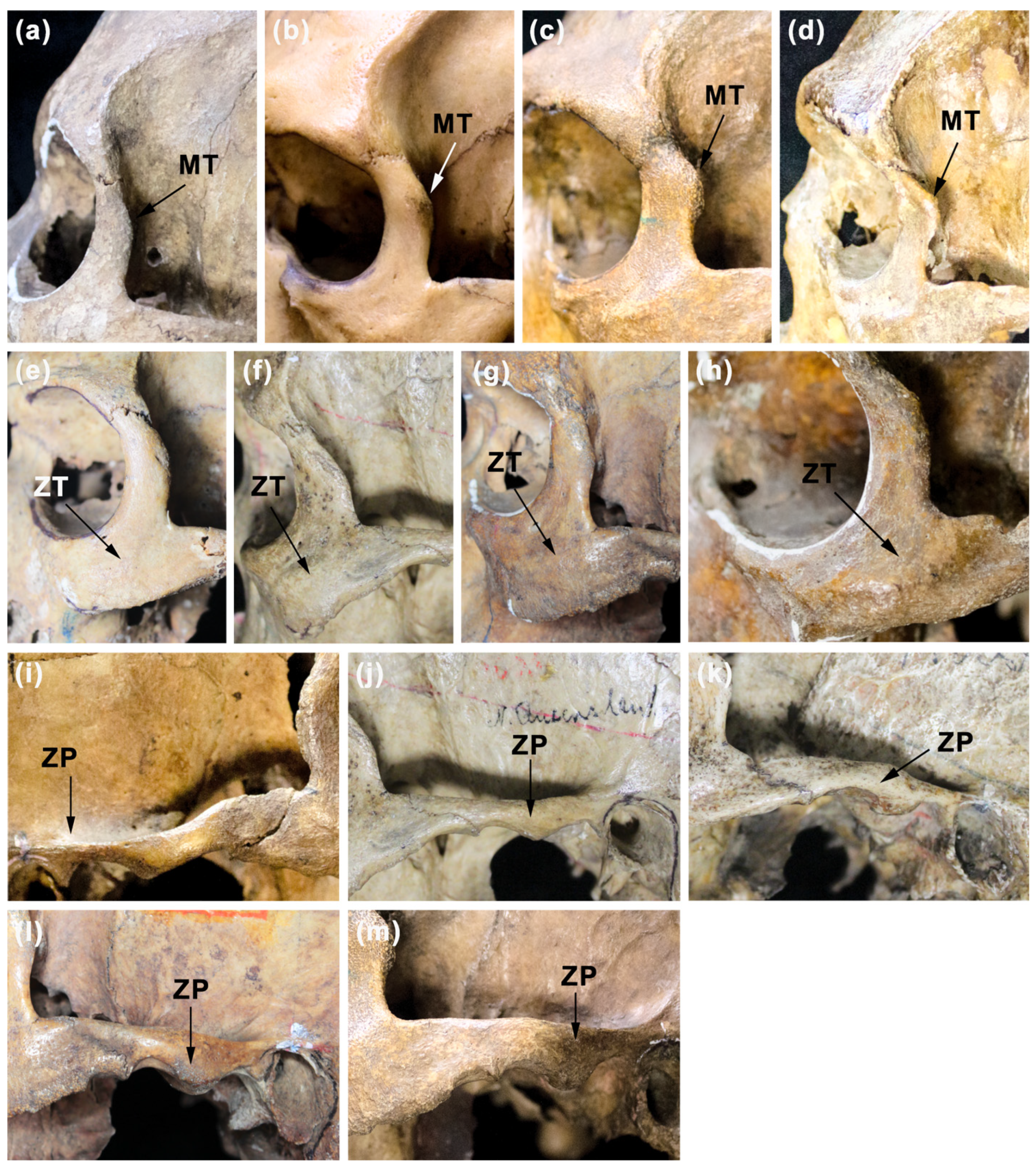
| Sample | M1 | M2 |
|---|---|---|
| All Females | 21 | 26 |
| Africans | 14 | 18 |
| Only M1 or M2 present | 1 | 5 |
| M1 and M2 * | 13 | 13 |
| Australians | 7 | 8 |
| Only M1 or M2 present | 1 | 2 |
| M1 and M2 * | 6 | 6 |
| All Males | 48 | 50 |
| Africans | 31 | 31 |
| Only M1 or M2 present | 2 | 2 |
| M1 and M2 * | 29 | 29 |
| Australians | 17 | 19 |
| Only M1 or M2 present | 1 | 3 |
| M1 and M2 * | 16 | 16 |
| Trait | Definition and Meaning |
| SFS—measure of the size of the facial skeleton (mm) | Geometric mean of the following measurements (see Figure S1): nasion hormion length, nasal height (nasion—nasospinale chord, M 55), orbital height (M 52), outer biorbital width (frontomalare temporale—frontomalare temporale chord, M 43) and bimaxillary width (zygomaxillare—zygomaxillare chord, M 46). M—the measurements were taken according to Martin and Saller’s [69] definitions (see also [53,70]) except the nasion-hormion chord (the hormion point was placed at the crossing at a right angle to the sagittal midline with line runs through the point marked as the most posterior part of the vomer). The higher value of SFS means the greater size of the facial skeleton. |
| R-RFS—index of the relative robusticity of the facial skeleton (1/mm) | The sum of the grades of massiveness of all examined regions of the facial skeleton standardized to the size of the facial skeleton divided by the number of these regions, R-RFS = (GL/SFS + ST/SFS + TR/SFS + MT/SFS + ZT/SFS + ZP/SFS)/6. The higher the value of R-RFS, the greater the relative general massiveness of the facial skeleton. |
| Facial region | Definition and the scale of its expression |
| GL—glabella | The area at the glabella located above the nasal bones between the left and right arcus superciliaris (see, e.g., [71]). Scale according to Buikstra and Ubelaker [52] includes five grades from the weakest (1) to the strongest (5)—see Figure 2. |
| ST—superciliary ridge | The supraorbital region placed laterally from the glabella (see, e.g., [40]). Scale according to Nowaczewska et al. ([38], p. 112) includes four grades from the weakest (1) to the strongest (4)—see Figure 2. |
| TR—trigone | The most lateral portion of the supraorbital region (see, e.g., [71]). Scale according to Lahr [3] with modification concerning the exclusion of the assessment of the malar portion located nearest to the trigone includes four grades from the weakest (1) to the strongest (4)—see Figure 2. |
| MT—malar tubercle | The region of the posterior margin of the frontal process of the zygomatic (malar) bone, where the presence of spina of this process can be observed (see, e.g., [72]). Scale according to Piontek ([72], p. 83) with modification by adding the new grade between the original second and third grade includes four grades from the weakest (1) to the strongest (4)—see Figure 3. |
| ZT—zygomaxillary tuberosity | The region of the body of the zygomatic bone between its orbital and inferior margin where tuberosity or surface elevation can be observed. Scale according to Lahr ([3], p. 346–347) includes four grades from the weakest (1) to the strongest (4)—see Figure 3. |
| ZP—zygomatic process of temporal bone | The process of the temporal bone connecting this bone with the zygomatic bone (see, e.g., [53]). Scale according to Ferembach et al. [54] includes five grades from the weakest (1) to the strongest (5)—see Figure 3. |
| Relative robusticity of the examined facial regions (1/mm) | The massiveness of the examined facial region (GL, ST, TR, MT, ZT, ZP) in relation to the size of the facial skeleton (SFS): GL/SFS, ST/SFS, TR/SFS, MT/SFS, ZT/SFS, ZP/SFS. The higher value = the greater relative robusticity of the examined region of the facial skeleton. |
| Trait/Sample (Number of Specimens) | Minimum Value | Maximum Value | Mean | Standard Deviation |
|---|---|---|---|---|
| TOCA/SFS (mm) | ||||
| M1 Females (21) | 1.32 | 1.96 | 1.58 | 0.16 |
| M1 Males (48) | 1.20 | 1.99 | 1.56 | 0.17 |
| M2 Females (26) | 1.09 | 1.76 | 1.41 | 0.19 |
| M2 Males (50) | 1.01 | 1.80 | 1.43 | 0.19 |
| TOCA (mm2) | ||||
| M1 Females (21) | 80.84 | 115.36 | 96.73 | 9.12 |
| M1 Males (48) | 75.22 | 133.51 | 100.98 | 12.42 |
| M2 Females (26) | 66.37 | 107.79 | 87.08 | 11.56 |
| M2 Males (50) | 63.70 | 121.33 | 92.99 | 13.67 |
| SFS (mm) | ||||
| M1 Females (21) | 57.29 | 65.92 | 61.18 | 2.35 |
| M1 Males (48) | 58.15 | 68.90 | 64.74 | 2.59 |
| M2 Females (26) | 58.35 | 65.92 | 61.76 | 2.29 |
| M2 Males (50) | 58.15 | 68.90 | 64.76 | 2.52 |
| Samples (N) | GL (1–5) % | ST (1–4) % | TR (1–4) % | MT (1–4) % | ZT (1–4) % | ZP (1–5) % |
|---|---|---|---|---|---|---|
| M1 Females (21) | 1—76.19 | 1—71.43 | 1—85.71 | 1—52.38 | 1—90.48 | 1—38.10 |
| 2—14.29 | 2—19.05 | 2—14.29 | 2—42.86 | 2—9.52 | 2—42.86 | |
| 3—9.52 | 3—4.76 | 3—0.00 | 3—4.76 | 3—0.00 | 3—19.05 | |
| 4—0.00 | 4—4.76 | 4—0.00 | 4—0.00 | 4—0.00 | 4—0.00 | |
| 5—0.00 | 5—0.00 | |||||
| M1 Males (48) | 1—37.50 | 1—25.00 | 1—50.00 | 1—12.50 | 1—66.67 | 1—2.08 |
| 2—29.17 | 2—33.33 | 2—27.08 | 2—50.00 | 2—14.58 | 2—20.83 | |
| 3—18.75 | 3—18.75 | 3—16.67 | 3—27.08 | 3—16.67 | 3—43.75 | |
| 4—6.25 | 4—22.92 | 4—6.25 | 4—10.42 | 4—2.08 | 4—25.00 | |
| 5—8.33 | 5—8.33 | |||||
| M2 Females (26) | 1—80.77 | 1—65.38 | 1—73.08 | 1—53.85 | 1—80.77 | 1—26.92 |
| 2—7.69 | 2—23.08 | 2—26.92 | 2—38.46 | 2—19.23 | 2—50.00 | |
| 3—11.54 | 3—7.69 | 3—0.00 | 3—3.85 | 3—0.00 | 3—23.08 | |
| 4—0.00 | 4—3.85 | 4—0.00 | 4—3.85 | 4—0.00 | 4—0.00 | |
| 5—0.00 | 5—0.00 | |||||
| M2 Males (50) | 1—32.00 | 1—20.00 | 1—44.00 | 1—10.00 | 1—62.00 | 1—2.00 |
| 2—28.00 | 2—34.00 | 2—26.00 | 2—50.00 | 2—14.00 | 2—18.00 | |
| 3—20.00 | 3—20.00 | 3—20.00 | 3—28.00 | 3—22.00 | 3—44.00 | |
| 4—10.00 | 4—26.00 | 4—10.00 | 4—12.00 | 4—2.00 | 4—28.00 | |
| 5—10.00 | 5—8.00 |
| Sample (N)/Traits | Correlation Coefficient | p-Value | Sample (N)/Traits | Correlation Coefficient | p-Value |
|---|---|---|---|---|---|
| M1 Females (21) | M1 Males (48) | ||||
| R-RFS | r = 0.28 | 0.218 | R-RFS | rs = 0.26 | 0.076 |
| GL/SFS | rs = 0.58 | 0.006 * | GL/SFS | rs = 0.17 | 0.243 |
| ST/SFS | rs = 0.39 | 0.080 | ST/SFS | rs = 0.22 | 0.128 |
| TR/SFS | rs = 0.35 | 0.115 | TR/SFS | rs = 0.30 | 0.035 * |
| MT/SFS | rs = 0.24 | 0.286 | MT/SFS | rs = −0.06 | 0.697 |
| ZT/SFS | rs = 0.29 | 0.199 | ZT/SFS | rs = 0.11 | 0.454 |
| ZP/SFS | rs = −0.02 | 0.924 | ZP/SFS | r = 0.18 | 0.211 |
| M2 Females (26) | M2 Males (50) | ||||
| R-RFS | r = 0.33 | 0.103 | R-RFS | rs = 0.33 | 0.021 * |
| GL/SFS | rs = 0.35 | 0.082 | GL/SFS | rs = 0.22 | 0.131 |
| ST/SFS | rs = 0.14 | 0.346 | ST/SFS | rs = 0.30 | 0.037 * |
| TR/SFS | rs = 0.13 | 0.530 | TR/SFS | rs = 0.35 | 0.013 * |
| MT/SFS | rs = 0.04 | 0.841 | MT/SFS | rs = −0.20 | 0.173 |
| ZT/SFS | rs = 0.23 | 0.257 | ZT/SFS | rs = 0.10 | 0.509 |
| ZP/SFS | rs = 0.19 | 0.346 | ZP/SFS | rs = 0.12 | 0.395 |
| GL | TOCA M1 | SFS | |
| GL | - | 0.43 (0.0503) | 0.14 (0.547) |
| TOCA M1 | 0.44 (0.051) | - | −0.04 (0.858) |
| SFS | 0.17 (0.461) | −0.11 (0.632) | - |
| ST | TOCA M1 | SFS | |
| ST | - | 0.17 (0.450) | 0.22 (0.343) |
| TOCA M1 | 0.18 (0.427) | - | −0.04 (0.858) |
| SFS | 0.23 (0.332) | −0.08 (0.729) | - |
| TR | TOCA M1 | SFS | |
| TR | - | 0.07 (0.772) | 0.07 (0.772) |
| TOCA M1 | 0.07 (0.768) | - | −0.04 (0.858) |
| SFS | 0.07 (0.768) | −0.05 (0.846) | - |
| MT | TOCA M1 | SFS | |
| MT | - | 0.16 (0.483) | 0.16 (0.483) |
| TOCA M1 | 0.17 (0.471) | - | −0.04 (0.858) |
| SFS | 0.17 (0.471) | −0.07 (0.771) | - |
| ZT | TOCA M1 | SFS | |
| ZT | - | −0.08 (0.729) | −0.13 (0.563) |
| TOCA M1 | −0.09 (0.729) | - | −0.04 (0.858) |
| SFS | −0.14 (0.563) | −0.05 (0.858) | - |
| ZP | TOCA M1 | SFS | |
| ZP | - | −0.09 (0.713) | 0.04 (0.871) |
| TOCA M1 | −0.09 (0.725) | - | −0.04 (0.858) |
| SFS | 0.03 (0.886) | −0.04 (0.872) | - |
| GL | TOCA M2 | SFS | |
| GL | - | 0.38 (0.053) | 0.21 (0.304) |
| TOCA M2 | 0.37 (0.071) | - | 0.13 (0.526) |
| SFS | 0.17 (0.405) | 0.06 (0.793) | - |
| ST | TOCA M2 | SFS | |
| ST | - | 0.13 (0.536) | 0.15 (0.469) |
| TOCA M2 | 0.11 (0.601) | - | 0.13 (0.526) |
| SFS | 0.13 (0.522) | 0.11 (0.589) | - |
| TR | TOCA M2 | SFS | |
| TR | - | 0.11 (0.593) | 0.03 (0.889) |
| TOCA M2 | 0.11 (0.611) | - | 0.13 (0.526) |
| SFS | 0.02 (0.944) | 0.13 (0.542) | - |
| MT | TOCA M2 | SFS | |
| MT | - | 0.04 (0.847) | 0.11 (0.582) |
| TOCA M2 | 0.03 (0.905) | - | 0.13 (0.526) |
| SFS | 0.11 (0.604) | 0.13 (0.546) | - |
| ZT | TOCA M2 | SFS | |
| ZT | - | 0.18 (0.391) | 0.03 (0.875) |
| TOCA M2 | 0.17 (0.408) | - | 0.13 (0.526) |
| SFS | 0.01 (0.963) | 0.13 (0.547) | - |
| ZP | TOCA M2 | SFS | |
| ZP | - | 0.16 (0.434) | 0.01 (0.980) |
| TOCA M2 | 0.16 (0.442) | - | 0.13 (0.526) |
| SFS | −0.02 (0.939) | 0.13 (0.532) | - |
| GL | TOCA M1 | SFS | |
| GL | - | 0.23 (0.114) | 0.21 (0.159) |
| TOCA M1 | 0.14 (0.335) | - | 0.55 (0.000) * |
| SFS | 0.10 (0.513) | 0.53 (0.000) * | - |
| ST | TOCA M1 | SFS | |
| ST | - | 0.31 (0.032) | 0.38 (0.007) * |
| TOCA M1 | 0.13 (0.391) | - | 0.55 (0.000) * |
| SFS | 0.27 (0.067) | 0.49 (0.000) * | - |
| TR | TOCA M1 | SFS | |
| TR | - | 0.41 (0.004) * | 0.31 (0.034) * |
| TOCA M1 | 0.30 (0.038) * | - | 0.55 (0.000) * |
| SFS | 0.11 (0.480) | 0.49 (0.001) * | - |
| MT | TOCA M1 | SFS | |
| MT | - | 0.05 (0.744) | 0.26 (0.071) |
| TOCA M1 | −0.12 (0.422) | - | 0.55 (0.000) * |
| SFS | 0.28 (0.053) | 0.56 (0.000) * | - |
| ZT | TOCA M1 | SFS | |
| ZT | - | 0.28 (0.056) | 0.33 (0.020) * |
| TOCA M1 | 0.12 (0.424) | - | 0.55 (0.000) * |
| SFS | 0.23 (0.127) | 0.51 (0.000) * | - |
| ZP | TOCA M1 | SFS | |
| ZP | - | 0.19 (0.187) | 0.29 (0.043) * |
| TOCA M1 | 0.04 (0.790) | - | 0.55 (0.000) * |
| SFS | 0.23 (0.123) | 0.53 0.000) * | - |
| GL | TOCA M2 | SFS | |
| GL | - | 0.31 (0.030) * | 0.15 (0.307) |
| TOCA M2 | 0.27 (0.057) | - | 0.55 (0.000) * |
| SFS | −0.03 (0.855) | 0.54 (0.000) * | - |
| ST | TOCA M2 | SFS | |
| ST | - | 0.38 (0.010) * | 0.35 (0.013) * |
| TOCA M2 | 0.24 (0.095) | - | 0.55 (0.000) * |
| SFS | 0.18 (0.210) | 0.48 (0.001) * | - |
| TR | TOCA M2 | SFS | |
| TR | - | 0.49 (0.000) * | 0.24 (0.094) |
| TOCA M2 | 0.44 (0.002) * | - | 0.55 (0.000) * |
| SFS | −0.04 (0.804) | 0.51 (0.000) * | - |
| MT | TOCA M2 | SFS | |
| MT | - | −0.03 (0.825) | 0.21 (0.149) |
| TOCA M2 | −0.18 (0.220) | - | 0.55 (0.000) * |
| SFS | 0.27 (0.062) | 0.57 (0.000) * | - |
| ZT | TOCA M2 | SFS | |
| ZT | - | 0.30 (0.036) * | 0.24 (0.094) |
| TOCA M2 | 0.21 (0.158) | - | 0.55 (0.000) * |
| SFS | 0.10 (0.516) | 0.52 (0.000) * | - |
| ZP | TOCA M2 | SFS | |
| ZP | - | 0.26 (0.070) | 0.24 (0.100) |
| TOCA M2 | 0.16 (0.275) | - | 0.55 (0.000) * |
| SFS | 0.12 (0.429) | 0.52 (0.000) * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowaczewska, W.; Górka, K.; Cieślik, A. Assessment of the Relationship between the Total Occlusal Area of the Human Permanent Upper First and Second Molars and the Robusticity of the Facial Skeleton in Sex-Different Cranial Samples of Homo Sapiens: A Preliminary Study. Biology 2023, 12, 566. https://doi.org/10.3390/biology12040566
Nowaczewska W, Górka K, Cieślik A. Assessment of the Relationship between the Total Occlusal Area of the Human Permanent Upper First and Second Molars and the Robusticity of the Facial Skeleton in Sex-Different Cranial Samples of Homo Sapiens: A Preliminary Study. Biology. 2023; 12(4):566. https://doi.org/10.3390/biology12040566
Chicago/Turabian StyleNowaczewska, Wioletta, Katarzyna Górka, and Agata Cieślik. 2023. "Assessment of the Relationship between the Total Occlusal Area of the Human Permanent Upper First and Second Molars and the Robusticity of the Facial Skeleton in Sex-Different Cranial Samples of Homo Sapiens: A Preliminary Study" Biology 12, no. 4: 566. https://doi.org/10.3390/biology12040566
APA StyleNowaczewska, W., Górka, K., & Cieślik, A. (2023). Assessment of the Relationship between the Total Occlusal Area of the Human Permanent Upper First and Second Molars and the Robusticity of the Facial Skeleton in Sex-Different Cranial Samples of Homo Sapiens: A Preliminary Study. Biology, 12(4), 566. https://doi.org/10.3390/biology12040566






