Tackling Inequalities in Oral Health: Bone Augmentation in Dental Surgery through the 3D Printing of Poly(ε-caprolactone) Combined with 20% Tricalcium Phosphate
Abstract
Simple Summary
Abstract
1. Introduction
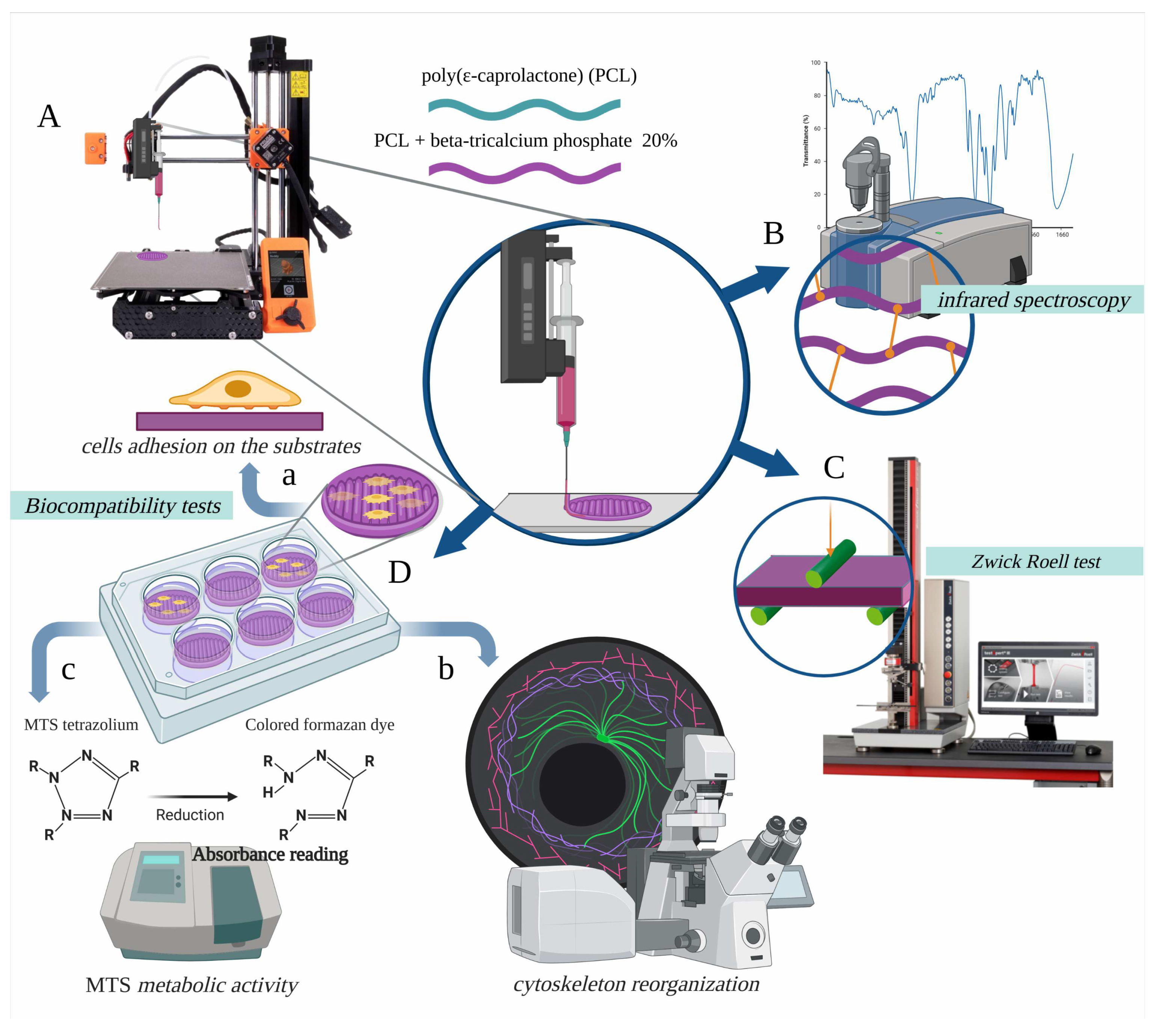
2. Materials and Methods
2.1. Sample Materials, Sample Geometries, and FDM Process
2.2. Sterilisation of the Samples
2.3. Sample Analysis by Infrared Spectroscopy
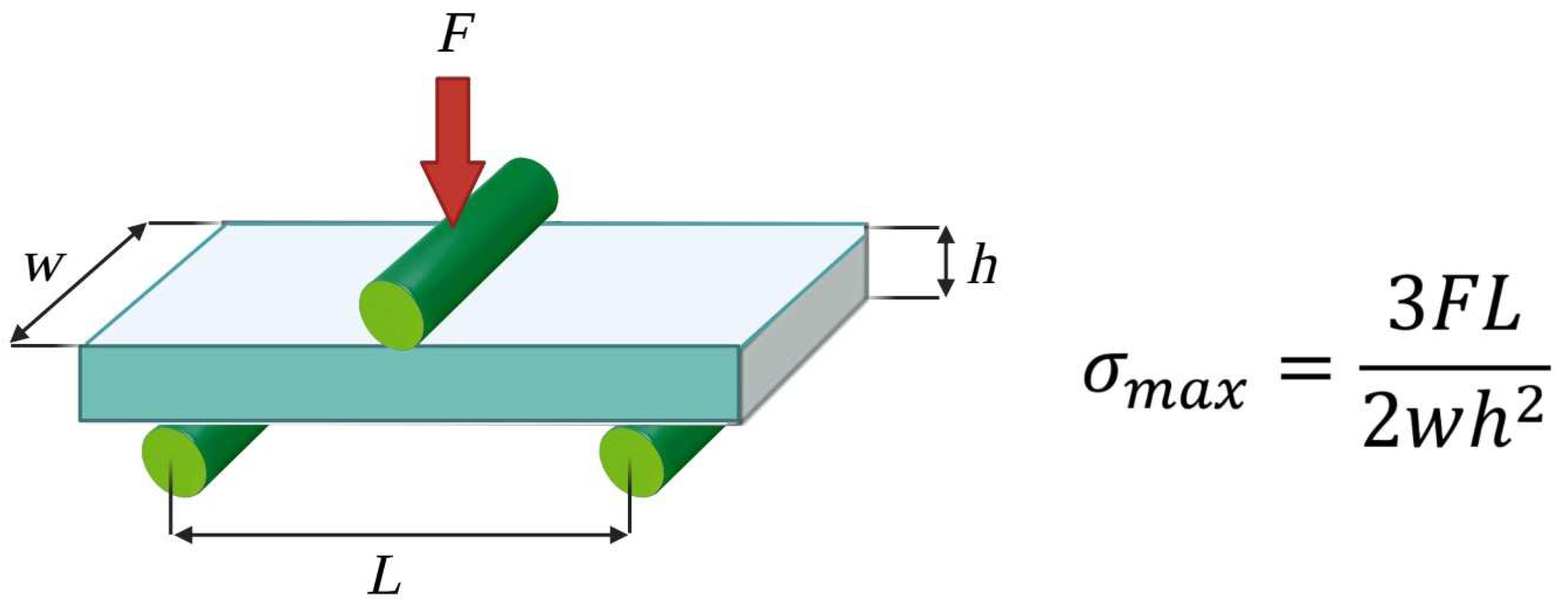
2.4. Static Mechanical Tests
2.5. Biocompatibility Tests: Preosteoblast Cell Line
2.6. Biocompatibility Tests: Cell Adhesion on the Substrate
2.7. Biocompatibility Tests: F-Actin Labelling and FilaQuant Software
2.8. Biocompatibility Tests: Evaluation of the Metabolic Activity of Viable Cells on Substrates (MTS Assay)
2.9. Statistical Analysis
3. Results
3.1. Weight Evaluation
3.2. Infrared Spectroscopy
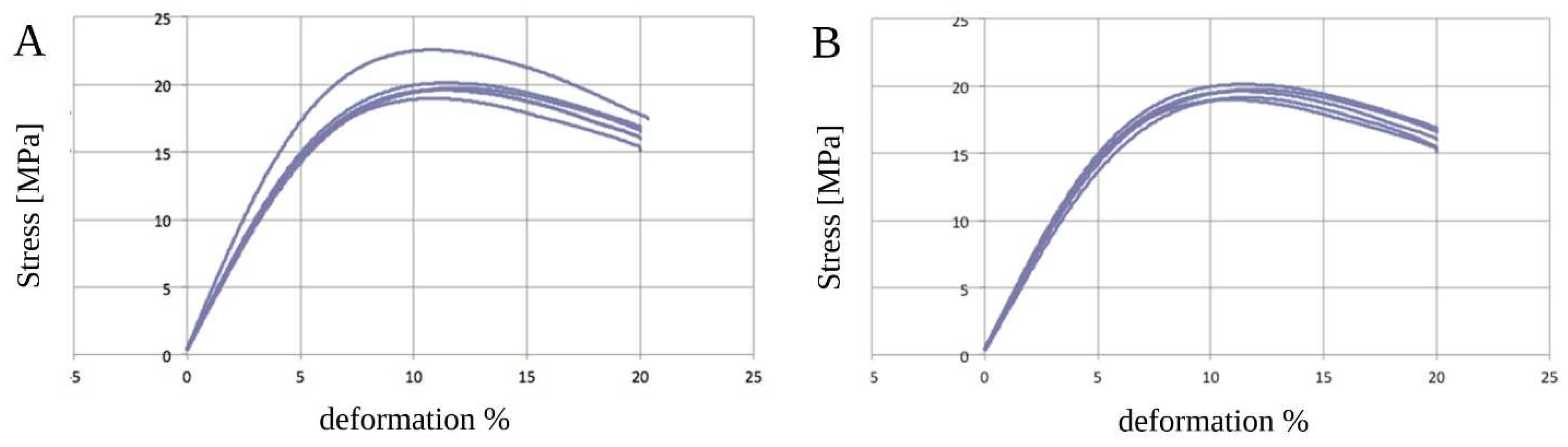
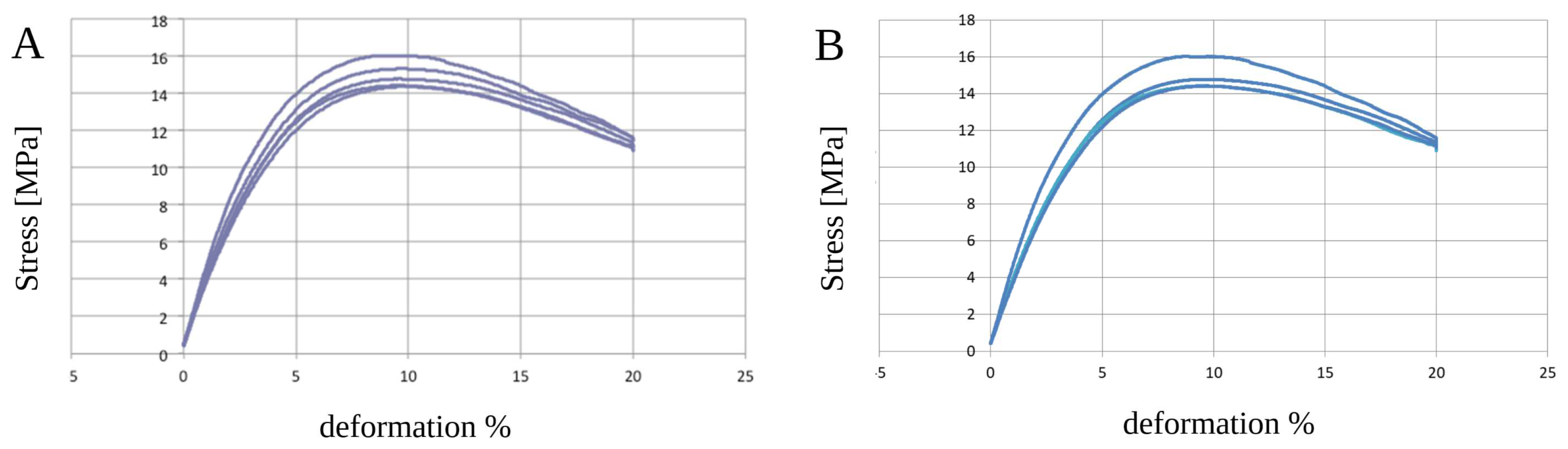
3.3. Static Mechanical Tests—Three-Point Bending Zwick Roell Test
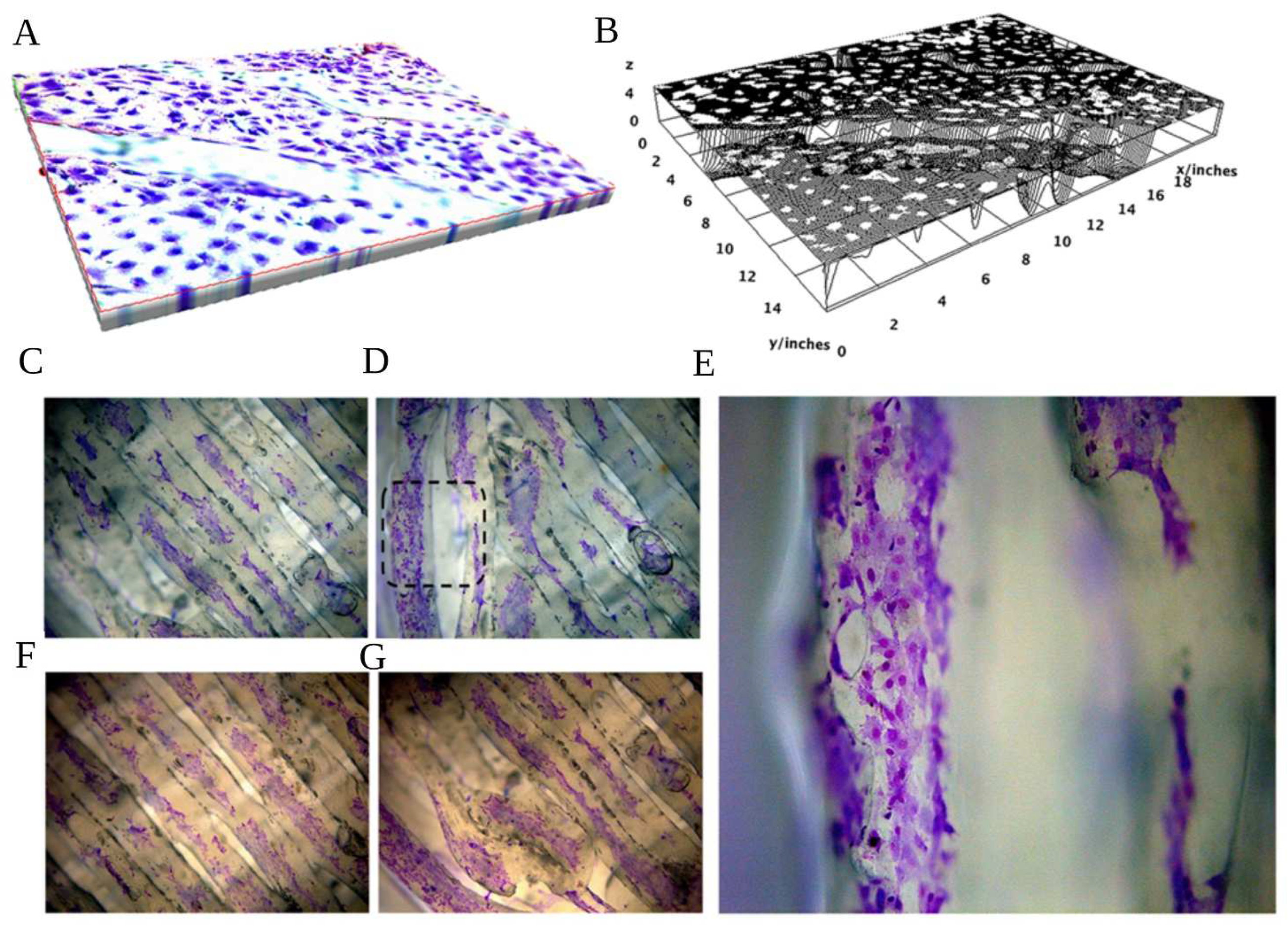
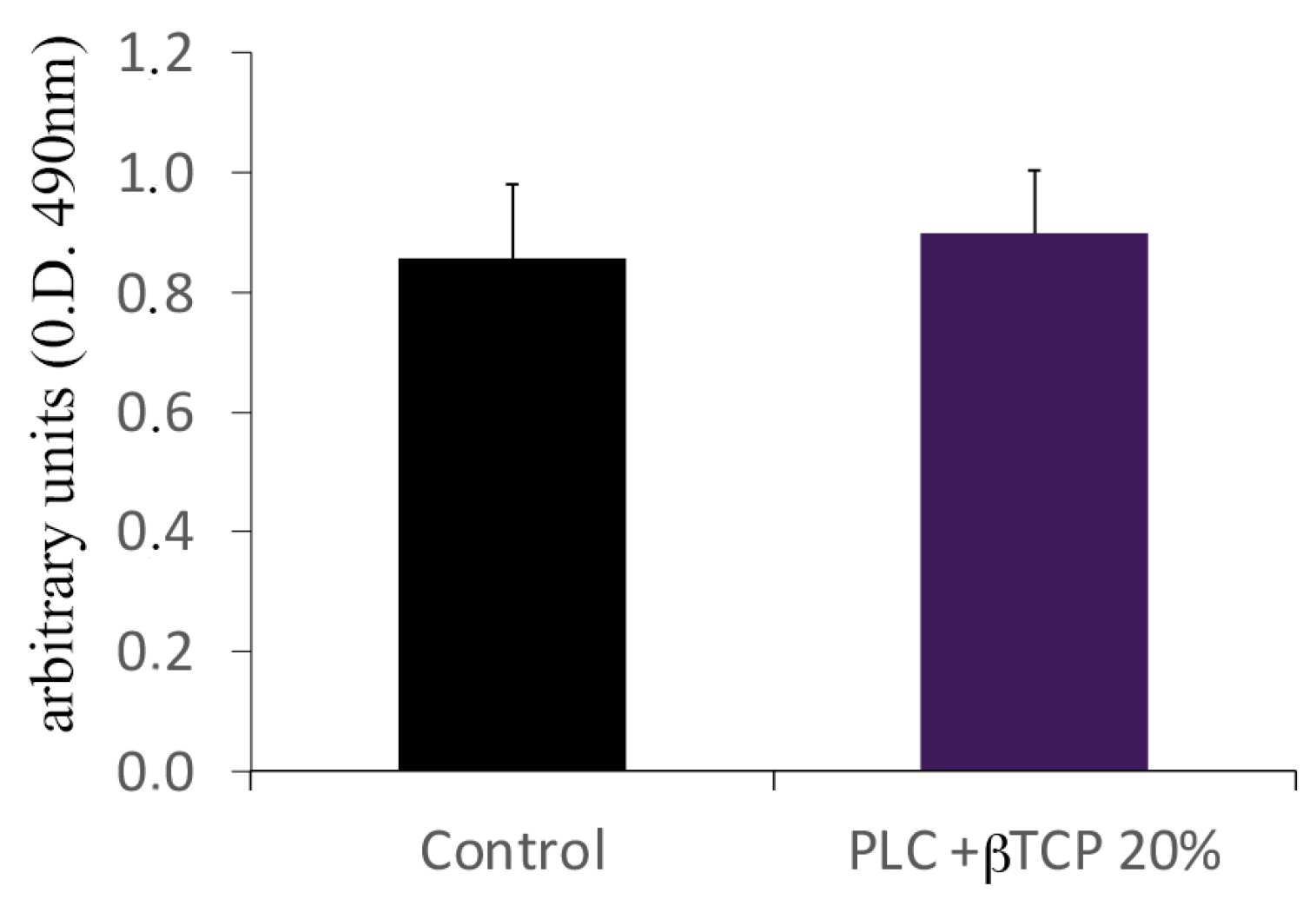
3.4. Biocompatibility Test
3.5. Actin Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holt, R.; Roberts, G.; Scully, C. ABC of Oral Health. Oral Health and Disease. BMJ 2000, 320, 1652–1655. [Google Scholar] [CrossRef]
- Paganini-Hill, A.; White, S.C.; Atchison, K.A. Dental Health Behaviors, Dentition, and Mortality in the Elderly: The Leisure World Cohort Study. J. Aging Res. 2011, 2011, 156061. [Google Scholar] [CrossRef]
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of U.S. Edentulism Prevalence Following 5 Decades of Decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef]
- Shah, N.; Sundaram, K.R. Impact of Socio-Demographic Variables, Oral Hygiene Practices, Oral Habits and Diet on Dental Caries Experience of Indian Elderly: A Community-Based Study. Gerodontology 2004, 21, 43–50. [Google Scholar] [CrossRef]
- Qin, X.; Zi, H.; Zeng, X. Changes in the Global Burden of Untreated Dental Caries from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study. Heliyon 2022, 8, e10714. [Google Scholar] [CrossRef]
- Elani, H.W.; Harper, S.; Allison, P.J.; Bedos, C.; Kaufman, J.S. Socio-Economic Inequalities and Oral Health in Canada and the United States. J. Dent. Res. 2012, 91, 865–870. [Google Scholar] [CrossRef]
- Tchicaya, A.; Lorentz, N. Socioeconomic Inequalities in the Non-Use of Dental Care in Europe. Int. J. Equity Health 2014, 13, 7. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- De Angelis, N.; Benedicenti, S.; Zekiy, A.; Amaroli, A. Current Trends in Bone Augmentation Techniques and Dental Implantology: An Editorial Overview. J. Clin. Med. 2022, 11, 4348. [Google Scholar] [CrossRef]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-Term Effectiveness of Maxillary Sinus Floor Augmentation: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2019, 46 (Suppl. 2), 307–318. [Google Scholar] [CrossRef]
- Bucchi, C.; del Fabbro, M.; Arias, A.; Fuentes, R.; Mendes, J.M.; Ordonneau, M.; Orti, V.; Manzanares-Céspedes, M.C. Multicenter Study of Patients’ Preferences and Concerns Regarding the Origin of Bone Grafts Utilized in Dentistry. Patient Prefer. Adherence 2019, 13, 179–185. [Google Scholar] [CrossRef]
- Jodati, H.; Yılmaz, B.; Evis, Z. A Review of Bioceramic Porous Scaffolds for Hard Tissue Applications: Effects of Structural Features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone Substitutes: An Update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of Bone Graft and Bone Substitutes with an Emphasis on Fracture Surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Sanz-Martín, I.; Ortiz-Vigón, A.; Molina, A.; Sanz, M. Complications in Bone-Grafting Procedures: Classification and Management. Periodontology 2000 2022, 88, 86–102. [Google Scholar] [CrossRef]
- Hardwick, R.; Hayes, B.K.; Flynn, C. Devices for Dentoalveolar Regeneration: An Up-To-Date Literature Review. J. Periodontol. 1995, 66, 495–505. [Google Scholar] [CrossRef]
- Kadkhodazadeh, M.; Amid, R.; Moscowchi, A. Management of Extensive Peri-Implant Defects with Titanium Meshes. Oral. Maxillofac. Surg. 2021, 25, 561–568. [Google Scholar] [CrossRef]
- Rasia-dal Polo, M.; Poli, P.-P.; Rancitelli, D.; Beretta, M.; Maiorana, C. Alveolar Ridge Reconstruction with Titanium Meshes: A Systematic Review of the Literature. Med. Oral. Patol. Oral Cir. Bucal 2014, 19, e639–e646. [Google Scholar] [CrossRef]
- De Angelis, N.; De Lorenzi, M.; Benedicenti, S. Surgical Combined Approach for Alveolar Ridge Augmentation with Titanium Mesh and RhPDGF-BB: A 3-Year Clinical Case Series. Int. J. Periodontics Restor. Dent. 2015, 35, 231–237. [Google Scholar] [CrossRef]
- Jacobsen, H.C.; Wahnschaff, F.; Trenkle, T.; Sieg, P.; Hakim, S.G. Oral Rehabilitation with Dental Implants and Quality of Life Following Mandibular Reconstruction with Free Fibular Flap. Clin. Oral Investig. 2016, 20, 187–192. [Google Scholar] [CrossRef]
- Xiao, Y. Bone Tissue Engineering for Dentistry and Orthopaedics. Biomed. Res. Int. 2014, 2014, 241067. [Google Scholar] [CrossRef]
- Offner, D.; de Grado, G.F.; Meisels, I.; Pijnenburg, L.; Fioretti, F.; Benkirane-Jessel, N.; Musset, A.-M. Bone Grafts, Bone Substitutes and Regenerative Medicine Acceptance for the Management of Bone Defects among French Population: Issues about Ethics, Religion or Fear? Cell. Med. 2019, 11, 215517901985766. [Google Scholar] [CrossRef]
- Hartmann, A.; Seiler, M. Minimizing Risk of Customized Titanium Mesh Exposures—A Retrospective Analysis. BMC Oral Health 2020, 20, 36. [Google Scholar] [CrossRef]
- De Angelis, N.; Felice, P.; Pellegrino, G.; Camurati, A.; Gambino, P.; Angelis, N. De Guided Bone Regeneration with and without a Bone Substitute at Single Post-Extractive Implants: 1-Year Post-Loading Results from a Pragmatic Multicentre Randomised Controlled Trial. Eur. J. Oral Implantol. 2011, 4, 313–325. [Google Scholar] [PubMed]
- Tumedei, M.; Mourão, C.F.; D’Agostino, S.; Dolci, M.; Di Cosola, M.; Piattelli, A.; Lucchese, A. Histological and Histomorphometric Effectiveness of the Barrier Membranes for Jawbone Regeneration: An Overview of More Than 30 Years’ Experience of Research Results of the Italian Implant Retrieval Center (1988–2020). Appl. Sci. 2021, 11, 2438. [Google Scholar] [CrossRef]
- Corsalini, M.; D’Agostino, S.; Favia, G.; Dolci, M.; Tempesta, A.; Di Venere, D.; Limongelli, L.; Capodiferro, S. A Minimally Invasive Technique for Short Spiral Implant Insertion with Contextual Crestal Sinus Lifting in the Atrophic Maxilla: A Preliminary Report. Healthcare 2020, 24, 11. [Google Scholar] [CrossRef]
- De Santis, D.; Umberto, L.; Dario, D.; Paolo, F.; Zarantonello, M.; Alberti, C.; Verlato, G.; Gelpi, F. Custom Bone Regeneration (CBR): An Alternative Method of Bone Augmentation-A Case Series Study. J. Clin. Med. 2022, 11, 4739. [Google Scholar] [CrossRef]
- De Angelis, N.; Solimei, L.; Pasquale, C.; Alvito, L.; Lagazzo, A.; Barberis, F. Microscopical Analysis of Explanted Titanium Alloy Customised Meshes for Bone Augmentation: A Case Series Study. Discov. Mater. 2022, 2, 9. [Google Scholar] [CrossRef]
- De Angelis, N.; Solimei, L.; Pasquale, C.; Alvito, L.; Lagazzo, A.; Barberis, F. Applied Sciences Mechanical Properties and Corrosion Resistance of TiAl6V4 Alloy Produced with SLM Technique and Used for Customized Mesh in Bone Augmentations. Appl. Sci. 2021, 11, 5622. [Google Scholar] [CrossRef]
- Rouf, S.; Malik, A.; Singh, N.; Raina, A.; Naveed, N.; Siddiqui, M.I.H.; Haq, M.I.U. Additive Manufacturing Technologies: Industrial and Medical Applications. Sustain. Oper. Comput. 2022, 3, 258–274. [Google Scholar] [CrossRef]
- Bardot, M.; Schulz, M.D. Biodegradable Poly(Lactic Acid) Nanocomposites for Fused Deposition Modeling 3D Printing. Nanomaterials 2020, 10, 2567. [Google Scholar] [CrossRef]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D Printing of Hydroxyapatite Polymer-Based Composites for Bone Tissue Engineering. J. Polym. Eng. 2017, 37, 741–746. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Biodegradable Polymers for Biomedical Additive Manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (∊-Caprolactone) Fiber: An Overview. J. Eng. Fiber Fabr. 2014, 9, 155892501400900. [Google Scholar] [CrossRef]
- Åkerlund, E.; Diez-Escudero, A.; Grzeszczak, A.; Persson, C. The Effect of PCL Addition on 3D-Printable PLA/HA Composite Filaments for the Treatment of Bone Defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef]
- ISO 14937:2009; Sterilization of Health Care Products—General Requirements for Characterization of a Sterilizing Agent and the Development, Validation and Routine Control of a Sterilization Process for Medical Devices. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/44954.html (accessed on 11 January 2023).
- Chander, N.G.; Jayaraman, V.; Sriram, V. Comparison of ISO and ASTM Standards in Determining the flexural Strength of Denture Base Resin. Eur. Oral. Res. 2019, 53, 137. [Google Scholar] [CrossRef]
- Dlgs 46/97. Available online: https://www.parlamento.it/parlam/leggi/deleghe/97046dl.htm (accessed on 11 January 2023).
- EUR-Lex—31993L0042—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=CELEX%3A31993L0042 (accessed on 11 January 2023).
- Chlebus, E.; Kuźnicka, B.; Kurzynowski, T.; Dybała, B. Microstructure and Mechanical Behaviour of Ti-6Al-7Nb Alloy Produced by Selective Laser Melting. Mater. Charact. 2011, 62, 488–495. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Nano-Hydroxyapatite Composite Biomaterials for Bone Tissue Engineering—A Review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef]
- Dellinger, J.G.; Eurell, J.A.C.; Jamison, R.D. Bone Response to 3D Periodic Hydroxyapatite Scaffolds with and without Tailored Microporosity to Deliver Bone Morphogenetic Protein 2. J. Biomed. Mater. Res. A 2006, 76A, 366–376. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Agas, D.; Hanna, R.; Benedicenti, S.; de Angelis, N.; Sabbieti, M.G.; Amaroli, A. Photobiomodulation by Near-Infrared 980-Nm Wavelengths Regulates Pre-Osteoblast Proliferation and Viability through the PI3K/Akt/Bcl-2 Pathway. Int. J. Mol. Sci. 2021, 22, 7586. [Google Scholar] [CrossRef]
- Kharitonova, M.A.; Vasiliev, J.M. Length Control Is Determined by the Pattern of Cytoskeleton. J. Cell. Sci. 2004, 117, 1955–1960. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Singh, G.K.; Daus, G.P.; Allender, M.; Ramey, C.T.; Martin, E.K., Jr.; Perry, C.; De Los Reyes, A.; Vedamuthu, I.P. Social Determinants of Health in the United States: Addressing Major Health Inequality Trends for the Nation, 1935–2016. Int. J. MCH AIDS 2017, 6, 139–164. [Google Scholar] [CrossRef]
- Hughes, R.C. Egalitarian Provision of Necessary Medical Treatment. J. Ethics 2020, 24, 55–78. [Google Scholar] [CrossRef]
- Tipnis, N.P.; Burgess, D.J. Sterilization of Implantable Polymer-Based Medical Devices: A Review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef]
- Kahnert, A.; Seiler, P.; Stein, M.; Aze, B.; McDonnell, G.; Kaufmann, S.H.E. Decontamination with Vaporized Hydrogen Peroxide Is Effective against Mycobacterium Tuberculosis. Lett. Appl. Microbiol. 2005, 40, 448–452. [Google Scholar] [CrossRef]
- Holy, C.E.; Cheng, C.; Davies, J.E.; Shoichet, M.S. Optimizing the Sterilization of PLGA Scaffolds for Use in Tissue Engineering. Biomaterials 2000, 22, 25–31. [Google Scholar] [CrossRef]
- Gülden, M.; Jess, A.; Kammann, J.; Maser, E.; Seibert, H. Cytotoxic Potency of H2O2 in Cell Cultures: Impact of Cell Concentration and Exposure Time. Free. Radic. Biol. Med. 2010, 49, 1298–1305. [Google Scholar] [CrossRef]
- Wiseman, J.; Rawther, T.; Langbart, M.; Kernohan, M.; Ngo, Q. Sterilization of Bedside 3D-Printed Devices for Use in the Operating Room. Ann. 3D Print. Med. 2022, 5, 100045. [Google Scholar] [CrossRef]
- Anil, L.; KL, V. Three Dimensional Printed Scaffolds and Biomaterials for Periodontal Regeneration-an Insight. Int. J. Mol. Biol. Open. Access 2020, 5, 73–77. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhao, J.; Hussain, M.; Wang, M. Additive Manufacturing in Orthopedics: A Review. ACS Biomater. Sci. Eng. 2022, 8, 1367–1380. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Amaroli, A.; De Angelis, N.; Benedicenti, S. Effects of Photobiomodulation on Bone Defects Grafted with Bone Substitutes: A Systematic Review of in Vivo Animal Studies. J. Biophotonics 2021, 14, e202000267. [Google Scholar] [CrossRef]
- Ambriz, X.; de Lanerolle, P.; Ambrosio, J.R. The Mechanobiology of the Actin Cytoskeleton in Stem Cells during Differentiation and Interaction with Biomaterials. Stem Cells Int. 2018, 2018, 2891957. [Google Scholar] [CrossRef]
- Marchetti, L.; Sabbieti, M.G.; Agas, D.; Menghi, M.; Materazzi, G.; Menghi, G.; Hurley, M.M. PGF2α Increases FGF-2 and FGFR2 Trafficking in Py1a Rat Osteoblasts via Clathrin Independent and Importin β Dependent Pathway. J. Cell. Biochem. 2006, 97, 1379–1392. [Google Scholar] [CrossRef]


| Polymer | Printer | Nozzle Temperature | Print Bed | Printing Speed |
|---|---|---|---|---|
| PCL | Prusa Mini | 110 °C | 30 °C | 60 mm/s |
| PCL+β-TCP 20% | Prusa Mini | 110 °C | 30 °C | 60 mm/s |
| Material | Weight Before (g) | Weight After (g) |
|---|---|---|
| PCL | 2.11 ± 0.01 | 2.12 ± 0.01 |
| PCL+ β-TCP 20% | 2.26 ± 0.01 | 2.27 ± 0.01 |
| PCL before Sterilization | ||||
| Ef | sfM | efM | ||
| MPa | MPa | % | ||
| PCL (mean ± standard deviation) | 338.5 ± 30.0 | 20.6 ± 1.0 | 11.4 ± 0.3 | |
| PCL after sterilization | ||||
| Ef | sfM | efM | ||
| MPa | MPa | % | ||
| PCL (mean ± standard deviation) | 301.2 ± 19.6 | 19.4 ± 0.4 | 11.3 ± 0.2 | |
| PCL+β-TCP 20% before sterilization | ||||
| Ef | sfM | efM | ||
| Mpa | Mpa | % | ||
| PCL+β-TCP 20% (mean ± standard deviation) | 382.1 ± 21.0 | 15.3 ± 0.5 | 9.5 ± 0.4 | |
| PCL+β-TCP 20% after sterilization | ||||
| Ef | sfM | efM | ||
| Mpa | Mpa | % | ||
| PCL+β-TCP 20% (mean ± standard deviation) | 335.5 ± 6.9 | 14.5 ± 0.1 | 9.6 ± 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Angelis, N.; Amaroli, A.; Sabbieti, M.G.; Cappelli, A.; Lagazzo, A.; Pasquale, C.; Barberis, F.; Agas, D. Tackling Inequalities in Oral Health: Bone Augmentation in Dental Surgery through the 3D Printing of Poly(ε-caprolactone) Combined with 20% Tricalcium Phosphate. Biology 2023, 12, 536. https://doi.org/10.3390/biology12040536
De Angelis N, Amaroli A, Sabbieti MG, Cappelli A, Lagazzo A, Pasquale C, Barberis F, Agas D. Tackling Inequalities in Oral Health: Bone Augmentation in Dental Surgery through the 3D Printing of Poly(ε-caprolactone) Combined with 20% Tricalcium Phosphate. Biology. 2023; 12(4):536. https://doi.org/10.3390/biology12040536
Chicago/Turabian StyleDe Angelis, Nicola, Andrea Amaroli, Maria Giovanna Sabbieti, Alessia Cappelli, Alberto Lagazzo, Claudio Pasquale, Fabrizio Barberis, and Dimitrios Agas. 2023. "Tackling Inequalities in Oral Health: Bone Augmentation in Dental Surgery through the 3D Printing of Poly(ε-caprolactone) Combined with 20% Tricalcium Phosphate" Biology 12, no. 4: 536. https://doi.org/10.3390/biology12040536
APA StyleDe Angelis, N., Amaroli, A., Sabbieti, M. G., Cappelli, A., Lagazzo, A., Pasquale, C., Barberis, F., & Agas, D. (2023). Tackling Inequalities in Oral Health: Bone Augmentation in Dental Surgery through the 3D Printing of Poly(ε-caprolactone) Combined with 20% Tricalcium Phosphate. Biology, 12(4), 536. https://doi.org/10.3390/biology12040536










