Simple Summary
Diseases that are characterized by inflammation such as sepsis, fatty liver or severe COVID-19 are more critical in the elderly population. Endotoxin tolerance is a tolerance towards pathogen components like lipopolysaccharides from bacterial cell walls. Tolerance is developed by the host to avoid strong inflammatory responses when repeatedly being in contact with these endotoxins. We hypothesized that aging is linked to a loss of this tolerance and to elevated fat content in the liver, potentially contributing to an increased risk of inflammatory diseases in the elderly. In this study, mice underwent a treatment protocol that allowed us to study the markers of tolerance in the blood and in the tissues. Based on the markers found in the blood serum and the lung tissue, the old mice showed a clear potential for endotoxin tolerance. They also showed a different fat composition in their liver compared to the young mice. Thus, we conclude that endotoxin tolerance is not necessarily affected by advanced age, but that changes in metabolic tissue homeostasis may lead to an altered immune response in old individuals. An understanding of how the immune response changes with advanced age and changed fat metabolism is important in order to understand age-related inflammatory diseases, which will help in the development of therapies for these diseases.
Abstract
(1) Background: Aging is linked to an altered immune response and metabolism. Inflammatory conditions, such as sepsis, COVID-19, and steatohepatitis are more prevalent in the elderly and steatosis is linked both to severe COVID-19 and sepsis. We hypothesized that aging is linked to a loss of endotoxin tolerance, which normally protects the host from excessive inflammation, and that this is accompanied by elevated levels of hepatic lipids. (2) Methods: An in vivo lipopolysaccharide (LPS) tolerance model in young and old mice was used and the cytokine serum levels were measured by ELISA. Cytokine and toll-like receptor gene expression was determined by qPCR in the lungs and the liver; hepatic fatty acid composition was assessed by GC–MS. (3) Results: The old mice showed a distinct potential for endotoxin tolerance as suggested by the serum cytokine levels and gene expression in the lung tissue. Endotoxin tolerance was less pronounced in the livers of the aged mice. However, the fatty acid composition strongly differed in the liver tissues of the young and old mice with a distinct change in the ratio of C18 to C16 fatty acids. (4) Conclusions: Endotoxin tolerance is maintained in advanced age, but changes in the metabolic tissue homeostasis may lead to an altered immune response in old individuals.
1. Introduction
The SARS-CoV-2 pandemic has substantially raised awareness that the immune response changes with age: severe COVID-19 was initially linked to advanced age and hyperinflammation, which is strongly determined by the myeloid cells [1,2,3]. Sepsis, pneumonia, cardiovascular disease, diabetes mellitus, and Alzheimer’s disease also have an inflammatory basis and are more readily and severely found in the elderly [4,5,6]. With increasing age, subcutaneous fat depots decrease, and more fat is stored in the visceral depots, e.g., in the liver tissue, leading to inflammatory diseases such as non-alcoholic steatohepatitis (NASH) [4,7]. Non-alcoholic fatty liver disease (NAFLD) has been found to be associated with sepsis mortality and changes in fatty acid metabolism [8]. Furthermore, in addition to old age, NAFLD—comprising both hepatic steatosis and NASH—as well as impaired metabolic health have been associated with severe or fatal COVID-19 cases independent of the metabolic syndrome [9,10,11,12]. Both conditions, hepatic steatosis and NASH, are associated with an increased gut permeability, endotoxemia, and inflammation [13,14] and NASH is more commonly found in old patients [15].
Endotoxin tolerance represents a mechanism to avoid an exuberant innate immune response by an impaired pro-inflammatory response of myeloid cells after an initial exposure to the endotoxin lipopolysaccharide (LPS), leading to reduced levels of tumor necrosis factor (TNF), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in the cells, serum, and, in accordance, also in the mRNA levels of Tnf and Il1b in the liver and lung tissue [16,17,18,19,20,21]. Endotoxin tolerance is a hallmark of the late phase of sepsis, where the macrophages and other immune cells experience a shift from a pro-inflammatory phenotype towards an anti-inflammatory phenotype. Thus, cells such as macrophages secrete less TNF and IL-1β, but more IL-10 [21,22,23]. Macrophages from aged individuals, however, show a pro-inflammatory phenotype, leading to an increased inflammatory response to an LPS stimulus [24]. One of these pro-inflammatory cytokines is IL-6 that is upregulated in serum with increasing age [25]. Additional to its role during inflammatory responses, IL-6 has also been identified as a key player in regulating lipid metabolism by stimulating lipolysis and fat oxidation in humans [26].
Considering that senior citizens are one of the most vulnerable groups in this SARS-CoV-2 pandemic as well as in other acute inflammatory diseases such as sepsis, lung and cardiovascular diseases, communicable diseases, and NASH [5,15,27], we hypothesized that old individuals have lost the potential to develop endotoxin tolerance leading to excessive inflammation and cytokine storms. We also hypothesized that this changed immune response is accompanied by an increased level of hepatic fatty acids (FA), due to a strong link between liver inflammation and hepatic fatty acid content [28].
To test the aforementioned hypotheses, we used an in vivo LPS tolerance model in young and old mice and assessed the cytokine levels in serum and the cytokine mRNA expression in the lungs and the liver as organs strongly affected by systemic inflammation.
2. Materials and Methods
2.1. In Vivo LPS Tolerance Model
Mice were held in IVC cages under controlled conditions of temperature, humidity, 12/12 h light/dark cycle and with food and water ad libitum. Young (10–12 weeks) and old (82–102 weeks) wild-type mice (carrying a LysM-Cre but no flox, B6.129P2-Lyz2tm1(cre)Ifo/J, The Jackson Laboratory) (males and females) were injected (i.p.) either with NaCl (0.9%, control) or a low dose of LPS (0.5 mg/kg body weight) on three consecutive days, followed by a high dose of LPS (5 mg/kg body weight) or NaCl on the fourth day according to their treatment group (Figure 1A). All injections were done at the same time between 8 and 9 a.m. A dose of 5 mg/kg LPS is commonly used to induce a robust but non-lethal inflammation in C57BL/6 mice [29,30]. The repeated exposure to a low dose of LPS, as seen in the tolerant group (tol), induces tolerance (Figure 1B) and is commonly used in both murine [31,32,33] and human studies [19,20,34,35,36,37,38]. Injections were made with ultrapure LPS from Salmonella minnesota R595 (# tlrl-smlps, Invivogen, Toulouse, France).
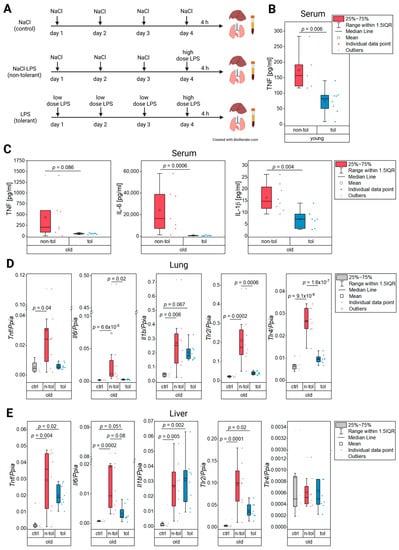
Figure 1.
In vivo LPS tolerance model (A), protein levels of inflammatory cytokines in serum (B,C), and relative gene expression in lung and liver tissue (D,E). (A) Mice were injected (i.p.) with NaCl (0.9%), a low dose of LPS (0.5 mg/kg body weight), or a high dose of LPS (5 mg/kg body weight) on four consecutive days. Liver, lung, and serum were harvested 4 h after the final injection. (B) Protein levels of TNF were determined by ELISA in young non-tolerant and tolerant mice. (C) Protein levels of TNF, IL-6, and IL-1β were determined by ELISA in old non-tolerant and tolerant mice. (D) Relative expression of inflammatory markers Tnf, Il6, and Il1b as well as toll-like receptors in lung and (E) liver tissue.
The livers, lungs, and serum were harvested 4 h after the final injection. The livers and lungs were immediately placed in liquid nitrogen after being harvested and stored at −80 °C until further use. Serum was also stored at −80 °C until further use. For reasons of feasibility, injections were carried out in 2 separate cohorts. This resulted in sample sizes between 7 and 8 animals (Supplementary Table S1). To ensure large enough sample sizes with mice of a certain age at a specific time point, both males and females were used (Supplementary Table S1). The local animal welfare committee (application no. 06-2016) approved all experiments.
2.2. Quantitative RT-PCR
RNA was isolated from small pieces of frozen liver and lung tissue with the QIAzol method (#79306, QIAGEN, Germantown, MD, USA). Preceding the precipitation of lung RNA, a final concentration of 2.5 M of ammonium acetate (#09691-250ML, Sigma, Taufkirchen, Germany) was added for a better yield. Any residual genomic DNA was removed by a DNase digestion with the DNA free kit (#AM 1906, Ambion, Waltham, MA, USA). An amount of 1 µg of the obtained RNA was reverse transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (#4368813, Applied Biosystems, Waltham, MA, USA) and the RNaseOUT Recombinant Ribonuclease Inhibitor (#10777019, Invitrogen, Waltham, MA, USA). qPCR measurements were carried out on CFX96 touch (BioRad, Feldkirchen, Germany) devices using HOT FIREPol EvaGreen qPCR Supermix (#08-25-00020, Solis Biodyne, Tartu, Estonia). To generate a standard curve, a plasmid dilution series of known concentrations containing the respective PCR-product was run alongside the samples. Tlr4 constituted the only exception and was quantified using the delta Ct method. The quantities of all markers measured in the samples were normalized against Ppia. The gene Ppia was chosen among 4 other commonly used housekeeping genes on grounds of its stability throughout all age and treatment groups using the software tool geNorm [24,39]. Primer sequences are listed in Supplementary Table S2.
2.3. ELISA
Protein concentrations of TNF, IL-1β, and IL-6 in serum were determined by LEGEND MAX™ Mouse TNF-α ELISA Kit (#430907, Biolegend, San Diego, CA, USA), IL-1 beta Mouse ELISA Kit (#BMS6002, Invitrogen, Toulouse, France), and Interleukin-6 (mouse) ELISA Kit (#583371, Cayman Chemicals, Ann Arbor, MI, USA), respectively. Kits were used according to the manufacturers’ instructions with adjustments in serum dilution. Absorbance measurements were carried out in a GloMax Discover Microplate reader (Promega, Walldorf, Germany). For IL-6, 4 of the 7 samples in the old non-tolerant treatment group were higher than the highest concentration of the standard curve (1500 pg/mL) despite being diluted by a factor of 10.
2.4. Lipid Measurements
The frozen liver samples were lyophilized for 24 h with an ALPHA 2–4 LD plus lyophilizer (Christ, Osterode am Harz, Germany). The dried samples were pulverized using a ball mill (MM 400, Retsch, Germany) for 30 s at 20 Hz. An amount of 30 µL of methyl-nonadecanoat (Roth, Karlsruhe, Germany, stock solution 0.15 mg/mL in chloroform) was added to the homogenized liver samples as an internal standard and then resuspended in 600 μL methanol:chloroform:water solution (1:3:2). The mixture was shaken and incubated three times for 10 min each on ice. The mixture was then centrifuged for 11,000× g for 5 min at 4 °C. After phase separation, 20 μL of the lower organic phase was mixed with 60 µL TMSH (Merck, Darmstadt, Germany). All samples were analyzed by GC-TOF-MS (7890B/7200, Agilent, Santa Clara, CA, USA). One ml of the derivatized samples was injected at 250 °C in splitless mode with a helium gas flow of 1 mL/min. Chromatography was performed with a Zebron Capillary GC-Column (ZB-Semi Volatiles, length 30 m, diameter 0.25 mm, film thickness 0.25 μm, Phenomenex, Aschaffenburg, Germany). The temperature gradient was set to 60 °C for 1 min followed by a linear ramp of 10 °C/min to 320 °C and holding this temperature for 3 min. Throughout the run, the transfer line, source, and the quadrupole were set to 290 °C, 230 °C, and 150 °C, respectively. The ionization was performed with electron impact (EI) ionization and recorded in scan mode with 6.5 spectra/s.
The raw data were processed by MassHunter Qualitative Analysis software (Agilent, B.07.00) and MassHunter Quantitative Analysis software for QTOF (Agilent, B.08.00). Standard compounds were obtained from Roth (Karlsruhe, Germany) or Sigma (Taufkirchen, Germany) and used for identification and confirmation of the chromatographic peaks. Peak areas were normalized with the internal standard and dry weight of the respective compound.
2.5. Statistical Analysis
The statistical analysis was carried out with the software R 4.1.2 (http://www.R-project.org/, packages: ‘car’, ’FSA’, ‘tidyverse’, ‘writexl’, accessed on 2 January 2022). Data were tested for normal distribution with the Shapiro–Wilk test and for homogeneity of variance with the Fligner–Killeen test. Based on the outcome of these tests, the quantities of the ELISA results of non-tolerant and tolerant mice were either analyzed by a Student’s t-test, Welch’s t-test or Mann–Whitney U test. To compare expressed mRNA levels of Tnf, Il6, Il1b, Tlr2, and Tlr4 between treatments in lung and liver tissue of old mice, either a Kruskal–Wallis followed by a post hoc test after Dunn [40] with a p-value adjustment after Holm [41] or a one-way analysis of variance (ANOVA) followed by a Tukey HSD post hoc test was carried out.
A two-factorial ANOVA (factor one: age, factor two: LPS pre-treatment, factor three: interaction of age and LPS pre-treatment) was used to analyze the normalized fatty acid data as well as the normalized qPCR data from genes involved in the fatty acid metabolism. It was also applied to the normalized qPCR data in lung and liver tissue of young and old mice of cytokines and toll-like receptors. Only the groups of non-tolerant and tolerant mice were included in the model. Control groups were only depicted to visualize the baseline levels. Where the two-factorial ANOVA revealed significant differences, either a one-way ANOVA with Tukey HSD post hoc or Kruskal–Wallis test with Dunn test was used to compare the levels of the various measured parameters between the groups including controls. The choice of tests was based on the fulfillment of the respective test assumptions. The significance threshold for all tests was set to p ≤ 0.05. Graphs were generated with OriginPro 2022b 9.9.5.167 (Academic). Individual parameters were analyzed for sex specific differences, but none were found compared to combining both sexes in the data sets.
3. Results
3.1. Serum
The TNF protein levels in blood serum from the young mice treated according to Figure 1A were significantly reduced in the tolerant compared to the non-tolerant mice (Figure 1B) indicating an LPS tolerance as was expected through the pre-treatment with LPS [19,20,36]. In the old mice, the protein blood levels of the inflammatory cytokines IL-6 and IL-1β were reduced in the mice of the tolerant treatment group. Despite the mean and median TNF levels of the tolerant group being lower than those of the non-tolerant group, a Welch’s t-test did not generate a significant difference between the treatment groups. The combined cytokine results, however, show endotoxin tolerance in the old mice (Figure 1C).
3.2. Lung
The cytokine levels from the serum suggested endotoxin tolerance in the old mice. Similar findings were made with Tnf, where the expression in the tolerant mice was just as low as it was in the control group while the non-tolerant mice had a significantly increased expression (Figure 1D). The same expression pattern was observable for Il6 where statistical significance supported the expression decrease in the tolerant mice compared to the non-tolerant ones (Figure 1D). Il1b expression, on the other hand, was not altered by tolerance in the old mice as its expression was almost as high as in the mice of the non-tolerant group (Figure 1D). The pathogen recognition receptors Tlr2 and Tlr4 were both significantly upregulated in the non-tolerant old mice, yet showed a significant decrease in the group that received a low LPS dose pre-treatment before the final high dose compared to the animals that only received the high dose (Figure 1A,D). This significant decrease of the pathogen recognition receptors’ expression in the group of tolerant old mice again indicated that this group had a clearly suppressed immune response to a sublethal dose of LPS.
Gene expression data for the young and old mice is depicted in Supplementary Figure S1.
3.3. Liver
In the liver tissue, the evidence for tolerance in the old mice was not as pronounced as it was in the lung tissue as Il1b was not expressed in lower levels in the tolerant mice than in the non-tolerant ones (Figure 1E). Even though Tnf and Il6 appeared to be decreased in the tolerant group, it was not supported by statistical evidence (Figure 1E). A similar pattern was observed for Tlr2, while Tlr4 did not have any expression differences between the treatments (Figure 1E). The gene expression data for the young and old mice is depicted in Supplementary Figure S1.
Since an altered hepatic fatty acid (FA) content typically reflects inflammatory processes in the liver [28,42], FA profiles were determined in both the young and old animals. Despite the FA content in the liver tissue not showing statistical differences between the non-tolerant and tolerant mice, according to a one-factorial ANOVA, there was an observable change between the livers of the young and old mice (Figure 2). The total amount of FAs was significantly affected by the LPS treatment, as determined by the two-factorial ANOVA, with the tolerant animals having lower levels than the non-tolerant ones as depicted in Figure 2A (Supplementary Table S3). The total FA content also appeared to be elevated in the aged mice, albeit not significantly. There were distinct changes in the FA composition between the age groups and treatment groups (Figure 2B,C,E–I, Supplementary Table S3): in the young individuals C18 FAs made up a higher percentage of the total FAs, while C16 FAs dominated in the old mice (Figure 2B,C). Accordingly, one of the most abundant C18 FAs—stearic acid (C18:0)—was decreased percentage wise in the old mice, while the two most abundant C16 FAs—palmitic acid (C16:0) and palmitoleic acid (C16:1)—were increased (Figure 2F–H). Contradictorily, oleic acid (C18:1) increased in the old animals while linoleic acid (C18:2) was not affected by age (Figure 2D,E, Supplementary Table S3). Because both the FAs, however, contribute relatively little to the total percentage of the C18 FAs compared to stearic acid, it is still significantly affected by old age leading to lower levels (Figure 2B).
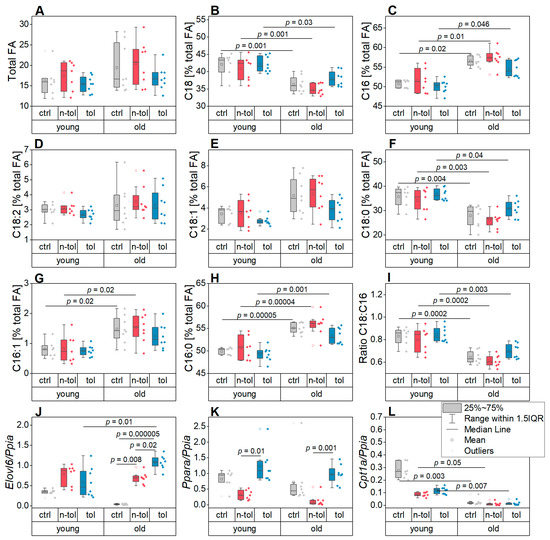
Figure 2.
Fatty acids (FAs) and relative gene expression of relevant markers in liver tissue. Data are shown for young and old mice as well as control (ctrl), non-tolerant (n-tol), and tolerant (tol) mice in each age group. (A) Total amount of FAs. (B,C) Sum of all (B) C18 and (C) C16 FAs shown as percentage of the total amount of FAs, respectively. (D–H) The C18 and C16 FAs with the highest relative concentrations per mg dry tissue weight shown as percentage of total FA amount: (D) linoleic acid (C18:2), (E) oleic acid (C18:1), (F) stearic acid (C18:0), (G) palmitoleic acid (C16:1), and (H) palmitic acid (C16:0). (I) Ratio of C18/C16 FAs. (J–L) Relative expression of genes involved in lipid synthesis and lysis: (J) Elovl6, (K) Ppara, (L) and Cpt1a.
The C18:C16 fatty acid ratio—frequently found to be altered in inflammatory events in the liver—was clearly higher in the young mice than the old ones, suggesting an altered fatty acid elongation activity (Figure 2J, Supplementary Table S3) [28,43,44]. This was supported by the expression of the lipogenic elongase Elovl6 in the liver that was significantly affected by both age and the interaction of age and treatment (Figure 2J, Supplementary Table S3).
FA abundance is determined by both lipogenesis and lipolysis. We therefore assessed the gene expression of Ppara and Cpt1a, both of which are involved in FA catabolism (Figure 2K,L). Ppara had distinctly higher expression levels in the tolerant mice compared to the non-tolerant mice and was affected by age according to the two-factorial ANOVA (Supplementary Table S3). Cpt1a was also differentially expressed in the young mice compared to the old ones (Supplementary Table S3), the latter showing a higher expression (Figure 2L). The expression patterns of these FA catabolism markers suggest that they contributed to the reduced total amount of FAs in the younger age group as well as in the tolerant mice.
4. Discussion
Advanced age is often accompanied by impaired physical and mental health as well as an increased mortality from communicable infectious diseases [45,46]. A key player causing age-associated degenerative diseases and increased severity is inflammation, both acute and chronically low-grade [45,46,47]. In fact, severe COVID-19 was initially linked to advanced age and hyperinflammation, which is strongly determined by myeloid cells [1,2,3]. The hypothesis of an overexuberant immune response to SARS-CoV-2 as a main cause of death, however, has been challenged by studies determining immunosuppression as a critical factor in severe COVID-19 cases [48,49]. Moreover, endotoxin tolerance hallmarks are induced by SARS-CoV-2 proteins and are found in COVID-19 patients [48,49,50]. Along with the SARS-CoV-2 pandemic came a stream of research identifying the virus and its effects. Specifically, factors and causes justifying the variety in severity started drawing attention. Advanced age, obesity, type 2 diabetes, smoking, hepatic steatosis, steatohepatitis, and cardiovascular disease are all independently considered risk factors for a severe outcome of COVID-19. These pathologic conditions are often associated with an increased circulation of bacterial products such as LPS [9,10,50]. The increased circulation of endotoxins can lead to an endotoxin tolerance causing an impaired immune response and thus ineffective clearing of pathogens [50]. Endotoxemia and inflammation due to an increased gut permeability are also symptoms of hepatic steatosis and NASH [13,14]. Since these diseases are characterized by an increased level of lipids in the liver—NASH is more commonly found in old patients [15] and a strong link between liver inflammation and hepatic fatty acid content has been shown [28,42]—we analyzed inflammatory markers on a protein and mRNA level as well as the hepatic fatty acid (FA) composition.
In this study the potential of the old mice to develop an endotoxin tolerance was investigated as well as the fatty acid composition of livers from the young and old mice. In accordance with previous findings [20,36], an LPS pre-exposure decreased the serum levels of the inflammatory cytokine TNF in the young mice demonstrating an acquired endotoxin tolerance. The old mice also showed this endotoxin tolerance by a suppressed secretion of the pro-inflammatory proteins IL-6, and IL-1β in the tolerant mice with a similar tendency for TNF. This observation is in accordance with Kohman et al. [51], who found protein levels of TNF, IL-6, and IL-1β to be increased in the spleen of young (4 months) and middle-aged (12 months) male BALB/c mice after an acute LPS injection, while the levels were reduced after a repeated LPS administration. An expected higher secretion of serum TNF in the non-tolerant old mice compared to the young ones after receiving their single high LPS dose [24] was met for most data points (young: 174.1 ± 30.2 (sem), old: 434.2 ± 183.8 (sem)).
The acquired endotoxin tolerance in the old mice was not only supported by the serum protein levels of pro-inflammatory markers, but also in the expression of these markers in the lung and liver tissue as previously reported for young mice [19,20,36], albeit the effect was not as pronounced in the liver as it was in the lung tissue. While the endotoxin tolerance was observable in the Tnf and Il6 expression in the lung of the old individuals, it was not statistically supported in the liver. Despite the pro-inflammatory Il1b not supporting an endotoxin tolerance, the expression of Tlr2 in both tissue types and Tlr4 in the lung tissue strongly did. Similar to Sun et al. (2012), who reported that the ability of LPS to induce TLR2 and TLR4 was reduced in peritoneal macrophages after LPS restimulation, we also observed the repeated LPS treatment affecting Tlr2 and Tlr4 expression in the lung tissue [52].
The results failing to support an endotoxin tolerance for each inflammatory marker in each tissue at the exact time point the samples were taken was to be expected, as each parameter has a different kinetic and metabolism. Thus, as the lung and the liver tissue metabolize substances very differently and are, therefore, differently affected by them, it was not surprising that we saw discrepancies in the gene expressions between these tissue types.
Overall, the protein and mRNA levels indicated the general potential for endotoxin tolerance in the old mice, yet not to the same degree in each marker and tissue.
A change between the age groups in the overall accumulation of FAs in the liver tissue as well as in the ratio of C18 to C16 FAs was observed. This not only indicated a fundamental change in the fatty acid composition of the liver, but by extension also a changed fluidity of the cell membranes and energy metabolism [53,54]. The increased level of palmitic acid in old mice may be a key parameter causing inflammation in aged individuals, since palmitic acid has been shown to polarize monocytes to the inflammatory M1 macrophage phenotype in healthy midlife human males [55]. The two-way ANOVA in our study not only identified age, but also treatment, to affect the total FA level. Compared to non-tolerant mice, tolerant individuals showed a reduced FA level. This may be explained by the declining IL-6 levels in endotoxin tolerance, since IL-6 is involved in regulating lipid metabolism by stimulating lipolysis and fat oxidation in humans [26]. Higher levels of IL-6, as observed in aged humans [25], may also contribute to the altered FA profile in the livers of aged mice [56].
In accordance with previous studies, old mice showed a relative increase in oleic acid resulting in a lower ratio of the unsaturated stearic acid to the monosaturated oleic acid, as observed in patients with hepatocellular cancer or NASH [28,57,58].
Altered C18:C16 ratios and the expression of the fatty acid elongase ELOVL6 have been reported to contribute to NASH [28,43,44]. Depending on the mouse model, either elevated [28] or decreased [59] ratios have been observed, while human data indicate that reduced C18:C16 are found in steatohepatitis [60]. Interestingly, it has been shown that an age-associated upregulation of circulating C16:0 can promote pro-inflammatory monocyte polarization. However, this potential link between hepatic FA metabolism and age-dependent macrophage polarization requires further investigation [55].
Overall, we found that old mice showed a clear potential for endotoxin tolerance in terms of systemic cytokine levels as well as cytokine expression in the lung and the liver. The higher total content of hepatic fatty acids and the changed ratio of C18 to C16, observed in aged mice, may modulate immune responses in this tissue.
5. Conclusions
We conclude that the acquisition of endotoxin tolerance is not decreased in old age and thus an altered immune response is not necessarily linked to an altered cytokine expression, but to a modified metabolism including a changed hepatic fatty acid composition. Further studies are needed to reveal the underlying mechanisms in endotoxin tolerance in old mice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12040530/s1, Table S1: Sample sizes of sample types, age groups, and LPS treatments; Table S2: Real-Time PCR primers and conditions; Table S3: Analyses of hepatic fatty acid abundance and gene expression with a two-factorial ANOVA. Figure S1: Relative gene expression in lung (A–E) and liver (F–J) tissue. Data are shown for young and old mice as well as control (ctrl), non-tolerant (n-tol), and tolerant (tol) mice in each age group. Note: Results of two-factorial ANOVA in Supplementary Table S3.
Author Contributions
Conceptualization, A.K.K. and J.H.; methodology, A.A.W., A.K.K. and J.H.; validation, A.A.W., A.K.K., J.H., S.M.K. and A.H.; formal analysis, A.A.W.; investigation, A.A.W., T.M.L., C.R. and B.H.J.; resources, A.K.K., B.H.J. and S.M.K.; data curation, A.A.W., C.R., B.H.J. and A.H.; writing—original draft preparation, A.A.W.; writing—review and editing, A.A.W., A.K.K. and J.H.; visualization, A.A.W.; supervision, A.K.K.; project administration, A.A.W., A.K.K. and J.H.; funding acquisition, A.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), grant number KI702. We acknowledge support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and Saarland University within the ‘Open Access Publication Funding’ program.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Landesamt für Verbraucherschutz Saarland (application no. 06-2016 and date of 24 March 2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Acknowledgments
The authors thank Eva Dilly for her support in animal maintenance and Lisa Flöck for the qPCR measurements of Cpt1a, Elovl6, and Ppara.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Szabo, P.A.; Dogra, P.; Gray, J.I.; Wells, S.B.; Connors, T.J.; Weisberg, S.P.; Krupska, I.; Matsumoto, R.; Poon, M.M.L.; Idzikowski, E.; et al. Longitudinal Profiling of Respiratory and Systemic Immune Responses Reveals Myeloid Cell-Driven Lung Inflammation in Severe COVID-19. Immunity 2021, 54, 797–814.e6. [Google Scholar] [CrossRef]
- Kiemer, A.K. Blockade of an Innate Immune Amplifier to Fight Immune Hyperactivation in COVID-19? EBioMedicine 2020, 61, 103086–103087. [Google Scholar] [CrossRef]
- Martin, T.R.; Wurfel, M.M.; Zanoni, I.; Ulevitch, R. Targeting Innate Immunity by Blocking CD14: Novel Approach to Control Inflammation and Organ Dysfunction in COVID-19 Illness. EBioMedicine 2020, 57, 102836–102837. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in Age-Related Chronic Inflammatory Diseases. NPJ Aging Mech. Dis. 2016, 2, 16018–16026. [Google Scholar] [CrossRef]
- Janssens, J.-P.; Williams, L. Pneumonia in the Elderly (Geriatric) Population. Curr. Opin. Pulm. Med. 2005, 11, 226–230. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Tardif, J.C.; Libby, P.; Camici, G.G. Inflamm-Ageing: The Role of Inflammation in Age-Dependent Cardiovascular Disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Pirtskhalava, T.; Han, J.; Karagiannides, I. Adipogenesis and Aging: Does Aging Make Fat Go MAD? Exp. Gerontol. 2002, 37, 757–767. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Cui, P.; Zhou, Y.; Liu, C.; Wu, X.; Ji, Y.; Wang, S.; Cheng, B.; Ye, H.; et al. TREM2 Sustains Macrophage-Hepatocyte Metabolic Coordination in Nonalcoholic Fatty Liver Disease and Sepsis. J. Clin. Investig. 2021, 131, e135197. [Google Scholar] [CrossRef]
- Medeiros, A.K.; Barbisan, C.C.; Cruz, I.R.; de Araújo, E.M.; Libânio, B.B.; Albuquerque, K.S.; Torres, U.S. Higher Frequency of Hepatic Steatosis at CT among COVID-19-Positive Patients. Abdom. Radiol. 2020, 45, 2748–2754. [Google Scholar] [CrossRef]
- Ghoneim, S.; Butt, M.U.; Hamid, O.; Shah, A.; Asaad, I. The Incidence of COVID-19 in Patients with Metabolic Syndrome and Non-Alcoholic Steatohepatitis: A Population-Based Study. Metabol. Open 2020, 8, 100057. [Google Scholar] [CrossRef]
- Mahamid, M.; Nseir, W.; Khoury, T.; Mahamid, B.; Nubania, A.; Sub-Laban, K.; Schifter, J.; Mari, A.; Sbeit, W.; Goldin, E. Nonalcoholic Fatty Liver Disease Is Associated with COVID-19 Severity Independently of Metabolic Syndrome: A Retrospective Case-Control Study. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Roca-Fernández, A.; Dennis, A.; Nicholls, R.; McGonigle, J.; Kelly, M.; Banerjee, R.; Banerjee, A.; Sanyal, A.J. Hepatic Steatosis, Rather Than Underlying Obesity, Increases the Risk of Infection and Hospitalization for COVID-19. Front. Med. 2021, 8, 636637. [Google Scholar] [CrossRef] [PubMed]
- Kessoku, T.; Kobayashi, T.; Imajo, K.; Tanaka, K.; Yamamoto, A.; Takahashi, K.; Kasai, Y.; Ozaki, A.; Iwaki, M.; Nogami, A.; et al. Endotoxins and Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2021, 12, 770986. [Google Scholar] [CrossRef] [PubMed]
- Nier, A.; Huber, Y.; Labenz, C.; Michel, M.; Bergheim, I.; Schattenberg, J.M. Adipokines and Endotoxemia Correlate with Hepatic Steatosis in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 699. [Google Scholar] [CrossRef]
- Frith, J.; Day, C.P.; Henderson, E.; Burt, A.D.; Newton, J.L. Non-Alcoholic Fatty Liver Disease in Older People. Gerontology 2009, 55, 607–613. [Google Scholar] [CrossRef]
- Venet, F.; Demaret, J.; Gossez, M.; Monneret, G. Myeloid Cells in Sepsis-Acquired Immunodeficiency. Ann. N. Y. Acad. Sci. 2021, 1499, 3–17. [Google Scholar] [CrossRef]
- Fan, H.; Cook, J.A. Molecular Mechanism of Endotoxin Tolerance. J. Endotoxin Res. 2004, 10, 71–84. [Google Scholar] [CrossRef]
- Liu, D.; Cao, S.; Zhou, Y.; Xiong, Y. Recent Advances in Endotoxin Tolerance. J. Cell. Biochem. 2019, 120, 56–70. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Kiemer, A.K. Glucocorticoid-Induced Leucine Zipper (GILZ) in Immuno Suppression: Master Regulator or Bystander? Oncotarget 2015, 6, 38446–38457. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Kessler, S.M.; Bruscoli, S.; Huwer, H.; Riccardi, C.; Kiemer, A.K. Glucocorticoid-Induced Leucine Zipper: A Critical Factor in Macrophage Endotoxin Tolerance. J. Immunol. 2015, 194, 6057–6067. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Vaporidi, K.; Tsatsanis, C. Regulation of Endotoxin Tolerance and Compensatory Anti-Inflammatory Response Syndrome by Non-Coding RNAs. Front. Immunol. 2018, 9, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Pena, O.M.; Hancock, D.G.; Lyle, N.H.; Linder, A.; Russell, J.A.; Xia, J.; Fjell, C.D.; Boyd, J.H.; Hancock, R.E.W. An Endotoxin Tolerance Signature Predicts Sepsis and Organ Dysfunction at Initial Clinical Presentation. EBioMedicine 2014, 1, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Valbuena Perez, J.V.; Linnenberger, R.; Dembek, A.; Bruscoli, S.; Riccardi, C.; Schulz, M.H.; Meyer, M.R.; Kiemer, A.K.; Hoppstädter, J. Altered Glucocorticoid Metabolism Represents a Feature of Macroph-Aging. Aging Cell 2020, 19, e13156. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef]
- van Hall, G.; Steensberg, A.; Sacchetti, M.; Fischer, C.; Keller, C.; Schjerling, P.; Hiscock, N.; Møller, K.; Saltin, B.; Febbraio, M.A.; et al. Interleukin-6 Stimulates Lipolysis and Fat Oxidation in Humans. J. Clin. Endocrinol. Metab. 2003, 88, 3005–3010. [Google Scholar] [CrossRef]
- Glynn, J.R.; Moss, P.A.H. Systematic Analysis of Infectious Disease Outcomes by Age Shows Lowest Severity in School-Age Children. Sci. Data 2020, 7, 329–342. [Google Scholar] [CrossRef]
- Kessler, S.M.; Simon, Y.; Gemperlein, K.; Gianmoena, K.; Cadenas, C.; Zimmer, V.; Pokorny, J.; Barghash, A.; Helms, V.; van Rooijen, N.; et al. Fatty Acid Elongation in Non-Alcoholic Steatohepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2014, 15, 5762–5773. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Huh, Y.; Keep, R.F.; Smith, D.E. Impact of Lipopolysaccharide-Induced Inflammation on the Disposition of the Aminocephalosporin Cefadroxil. Antimicrob. Agents Chemother. 2013, 57, 6171–6178. [Google Scholar] [CrossRef]
- Kinoshita, M.; Miyazaki, H.; Nakashima, H.; Nakashima, M.; Nishikawa, M.; Ishikiriyama, T.; Kato, S.; Iwaya, K.; Hiroi, S.; Shinomiya, N.; et al. In Vivo Lipopolysaccharide Tolerance Recruits CD11b+ Macrophages to the Liver with Enhanced Bactericidal Activity and Low Tumor Necrosis Factor-Releasing Capability, Resulting in Drastic Resistance to Lethal Septicemia. J. Innate Immun. 2017, 9, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, M.; Liao, Y.; Yang, J.; Gao, J. Endotoxin Tolerance Induced by Lipopolysaccharide Preconditioning Protects against Surgery-Induced Cognitive Impairment in Aging Mice. Mol. Med. Rep. 2018, 17, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Kim, J.; Remick, D.G. Chronic Pulmonary LPS Tolerance Induces Selective Immunosuppression While Maintaining the Neutrophilic Response. Shock 2010, 33, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Draisma, A.; Pickkers, P.; Bouw, M.; van der Hoeven, J. Development of Endotoxin Tolerance in Humans in Vivo. Crit. Care Med. 2009, 37, 1261–1267. [Google Scholar] [CrossRef]
- Draisma, A.; de Goeij, M.; Wouters, C.W.; Riksen, N.P.; Oyen, W.J.G.; Rongen, G.A.; Boerman, O.C.; van Deuren, M.; van der Hoeven, J.G.; Pickkers, P. Endotoxin Tolerance Does Not Limit Mild Ischemia-Reperfusion Injury in Humans In Vivo. Innate Immun. 2009, 15, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Dembek, A.; Laggai, S.; Kessler, S.M.; Czepukojc, B.; Simon, Y.; Kiemer, A.K.; Hoppstädter, J. Hepatic Interleukin-6 Production Is Maintained during Endotoxin Tolerance and Facilitates Lipid Accumulation. Immunobiology 2017, 222, 786–796. [Google Scholar] [CrossRef]
- Rosenzweig, H.L.; Lessov, N.S.; Henshall, D.C.; Minami, M.; Simon, R.P.; Stenzel-Poore, M.P. Endotoxin Preconditioning Prevents Cellular Inflammatory Response during Ischemic Neuroprotection in Mice. Stroke 2004, 35, 2576–2581. [Google Scholar] [CrossRef]
- Li, L.; Ng, D.S.W.; Mah, W.C.; Almeida, F.F.; Rahmat, S.A.; Rao, V.K.; Leow, S.C.; Laudisi, F.; Peh, M.T.; Goh, A.M.; et al. A Unique Role for P53 in the Regulation of M2 Macrophage Polarization. Cell Death Differ. 2015, 22, 1081–1093. [Google Scholar] [CrossRef]
- Hoppstädter, J.; Valbuena Perez, J.V.; Linnenberger, R.; Dahlem, C.; Legroux, T.M.; Hecksteden, A.; Tse, W.K.F.; Flamini, S.; Andreas, A.; Herrmann, J.; et al. The Glucocorticoid-Induced Leucine Zipper Mediates Statin-Induced Muscle Damage. FASEB J. 2020, 34, 4684–4701. [Google Scholar] [CrossRef]
- Jean Dunn, O. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Kröhler, T.; Kessler, S.M.; Hosseini, K.; List, M.; Barghash, A.; Patial, S.; Laggai, S.; Gemperlein, K.; Haybaeck, J.; Müller, R.; et al. The MRNA-Binding Protein TTP/ZFP36 in Hepatocarcinogenesis and Hepatocellular Carcinoma. Cancers 2019, 11, 1754. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, T.; Atsumi, A.; Matsumori, R.; Nie, T.; Shinozaki, H.; Suzuki-Kemuriyama, N.; Kuba, M.; Nakagawa, Y.; Ishii, K.; Shimada, M.; et al. Elovl6 Promotes Nonalcoholic Steatohepatitis. Hepatology 2012, 56, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Laggai, S.; Kessler, S.M.; Boettcher, S.; Lebrun, V.; Gemperlein, K.; Lederer, E.; Leclercq, I.A.; Mueller, R.; Hartmann, R.W.; Haybaeck, J.; et al. The IGF2 MRNA Binding Protein P62/IGF2BP2-2 Induces Fatty Acid Elongation as a Critical Feature of Steatosis. J. Lipid Res. 2014, 55, 1087–1097. [Google Scholar] [CrossRef]
- Neves, J.; Sousa-Victor, P. Regulation of Inflammation as an Anti-Aging Intervention. FEBS J. 2020, 287, 43–52. [Google Scholar] [CrossRef]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-Associated Chronic Diseases Require Age-Old Medicine: Role of Chronic Inflammation. Prev. Med. 2012, 54, 29–37. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef]
- Avendaño-Ortiz, J.; Lozano-Rodríguez, R.; Martín-Quirós, A.; Maroun-Eid, C.; Terrón-Arcos, V.; Montalbán-Hernández, K.; Valentín, J.; Muñoz del Val, E.; García-Garrido, M.A.; del Balzo-Castillo, Á.; et al. SARS-CoV-2 Proteins Induce Endotoxin Tolerance Hallmarks: A Demonstration in Patients with COVID-19. J. Immunol. 2021, 207, 162–174. [Google Scholar] [CrossRef]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe Immunosuppression and Not a Cytokine Storm Characterizes COVID-19 Infections. JCI Insight 2020, 5, e140329. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Preexisting and Inducible Endotoxemia as Crucial Contributors to the Severity of COVID-19 Outcomes. PLoS Pathog. 2021, 17, e1009306. [Google Scholar] [CrossRef]
- Kohman, R.A.; Crowell, B.; Kusnecov, A.W. Differential Sensitivity to Endotoxin Exposure in Young and Middle-Age Mice. Brain Behav. Immun. 2010, 24, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.; Yang, M.F.; Shu, W.; Sun, M.J.; Xu, Y. Effects of Aging on Endotoxin Tolerance Induced by Lipopolysaccharides Derived from Porphyromonas Gingivalis and Escherichia Coli. PLoS ONE 2012, 7, e39224. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Siegel, D.; Aaronson, L.R. Effects of Temperature Acclimation on Neurospora Phospholipids. Fatty Acid Desaturation Appears to Be a Key Element in Modifying Phospholipid Fluid Properties. Biochim. Biophys. Acta 1981, 665, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, T. Role of Fatty Acid Elongase Elovl6 in the Regulation of Energy Metabolism and Pathophysiological Significance in Diabetes. Diabetol. Int. 2021, 12, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Pararasa, C.; Ikwuobe, J.; Shigdar, S.; Boukouvalas, A.; Nabney, I.T.; Brown, J.E.; Devitt, A.; Bailey, C.J.; Bennett, S.J.; Griffiths, H.R. Age-Associated Changes in Long-Chain Fatty Acid Profile during Healthy Aging Promote pro-Inflammatory Monocyte Polarization via PPARγ. Aging Cell 2016, 15, 128–139. [Google Scholar] [CrossRef]
- Lehrskov, L.L.; Christensen, R.H. The Role of Interleukin-6 in Glucose Homeostasis and Lipid Metabolism. Semin. Immunopathol. 2019, 41, 491–499. [Google Scholar] [CrossRef]
- Wood, C.B.; Habib, N.A.; Apostolov, K.; Thompson, A.; Barker, W.; Hershman, M.; Blumgart, L.H. Reduction in the Stearic to Oleic Acid Ratio in Human Malignant Liver Neoplasms. Eur. J. Surg. Oncol. 1985, 11, 347–348. [Google Scholar]
- Puri, P.; Wiest, M.M.; Cheung, O.; Mirshahi, F.; Sargeant, C.; Min, H.K.; Contos, M.J.; Sterling, R.K.; Fuchs, M.; Zhou, H.; et al. The Plasma Lipidomic Signature of Nonalcoholic Steatohepatitis. Hepatology 2009, 50, 1827–1838. [Google Scholar] [CrossRef]
- Wang, L.; Xu, F.; Song, Z.; Han, D.; Zhang, J.; Chen, L.; Na, L. A High Fat Diet with a High C18:0/C16:0 Ratio Induced Worse Metabolic and Transcriptomic Profiles in C57BL/6 Mice. Lipids Health Dis. 2020, 19, 172. [Google Scholar] [CrossRef]
- Yamada, K.; Mizukoshi, E.; Sunagozaka, H.; Arai, K.; Yamashita, T.; Takeshita, Y.; Misu, H.; Takamura, T.; Kitamura, S.; Zen, Y.; et al. Characteristics of Hepatic Fatty Acid Compositions in Patients with Nonalcoholic Steatohepatitis. Liver Int. 2015, 35, 582–590. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).