Figure 1.
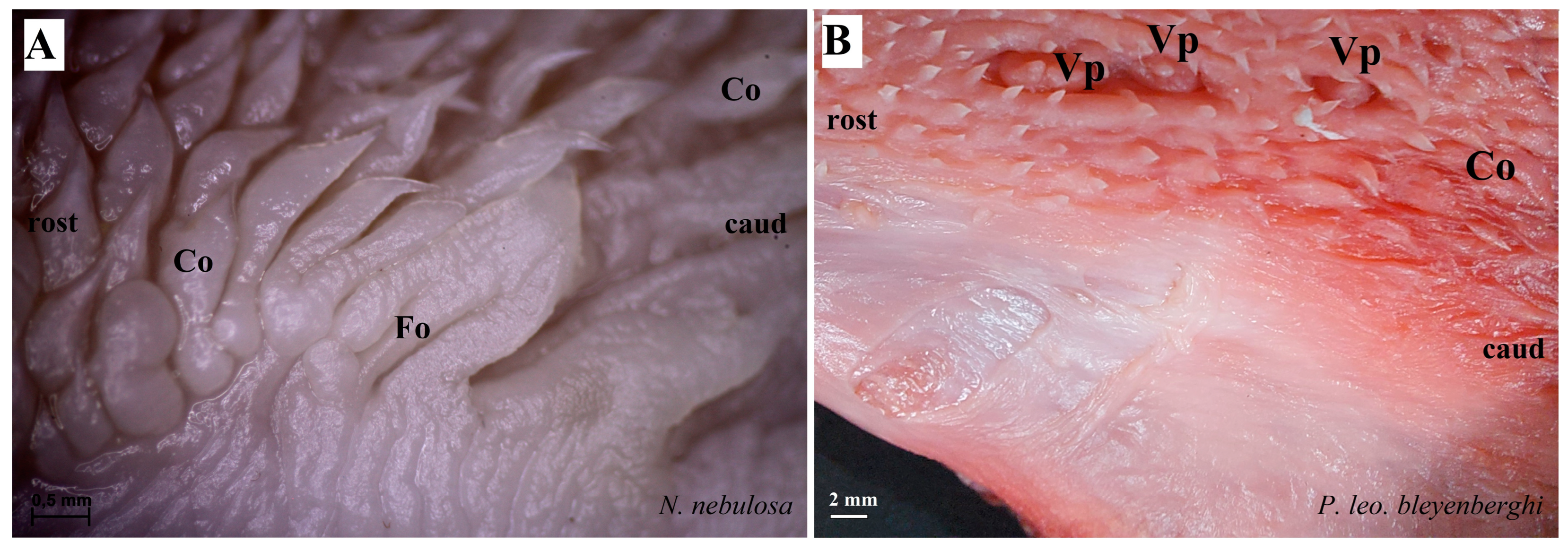
Macroscopic view of the dorsal surface of the tongue of N. nebulosa. Division of the tongue into apex (a), body (b) and root (c). See the areas (rectangles) for the collection of samples for histological and histochemical analyses. Abbreviations: Co—conical papillae; FiI, FiII, FiIII, FiIV and FiV—types of filiform papillae; Fu—fungiform papillae; Fo—foliate papillae.
Figure 1.
Macroscopic view of the dorsal surface of the tongue of N. nebulosa. Division of the tongue into apex (a), body (b) and root (c). See the areas (rectangles) for the collection of samples for histological and histochemical analyses. Abbreviations: Co—conical papillae; FiI, FiII, FiIII, FiIV and FiV—types of filiform papillae; Fu—fungiform papillae; Fo—foliate papillae.
Figure 2.
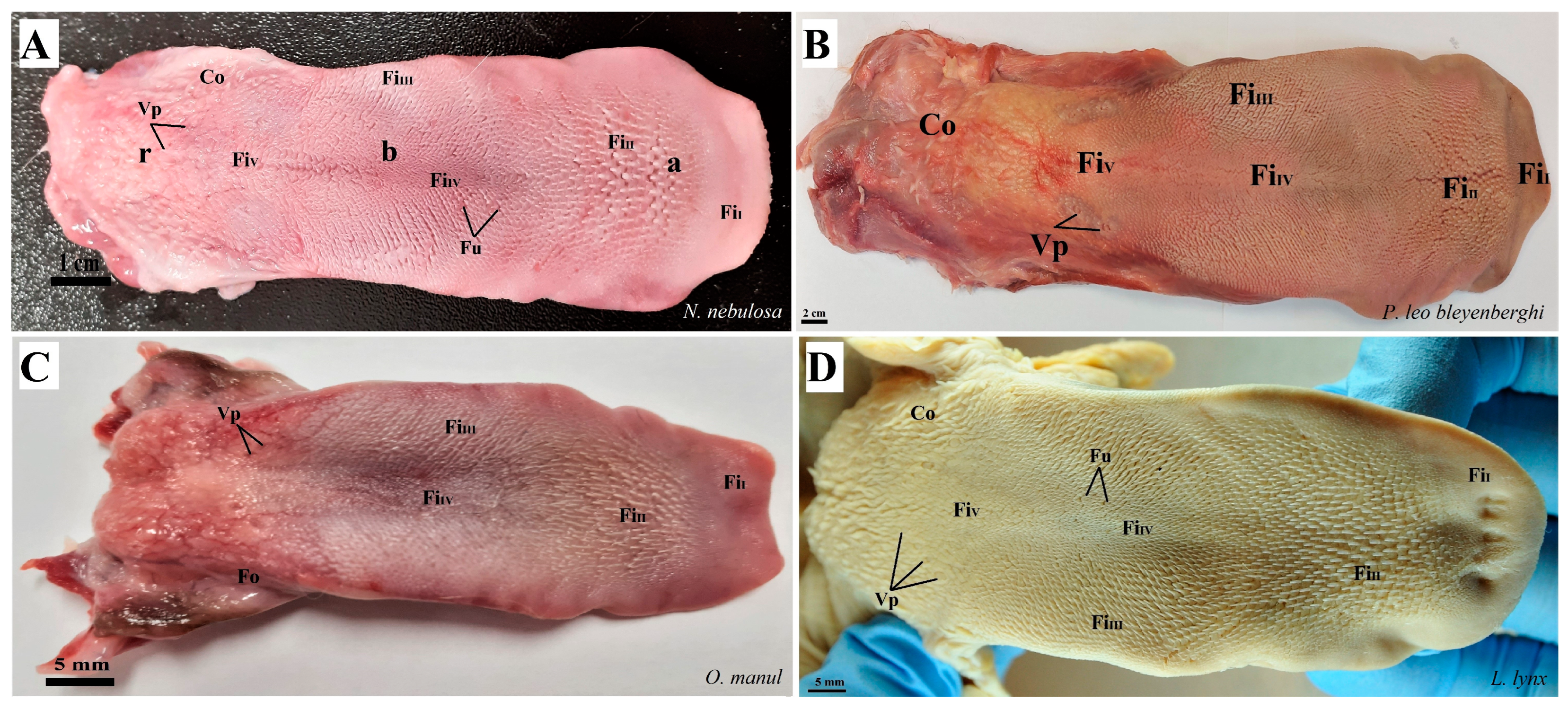
Photograph of the dorsal surface of the tongue of four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). Bar = 2 cm (B); bar = 1 cm (A); bar = 5 mm (C,D). Abbreviations: a—apex of the tongue, b—body of the tongue, FiI—filiform papillae subtype I (with caudal orientation of these papillae), FiII—filiform papillae subtype II (with caudal orientation of these papillae), FiIII—filiform papillae subtype III (with medio-caudal orientation of these papillae), FiIV—filiform papillae subtype IV (with caudal orientation of these papillae), FiV—filiform papillae subtype V (with caudal orientation of these papillae), Fu—fungiform papilla, r—root of the tongue, Co—small conical papilla, Vp—vallate papilla.
Figure 2.
Photograph of the dorsal surface of the tongue of four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). Bar = 2 cm (B); bar = 1 cm (A); bar = 5 mm (C,D). Abbreviations: a—apex of the tongue, b—body of the tongue, FiI—filiform papillae subtype I (with caudal orientation of these papillae), FiII—filiform papillae subtype II (with caudal orientation of these papillae), FiIII—filiform papillae subtype III (with medio-caudal orientation of these papillae), FiIV—filiform papillae subtype IV (with caudal orientation of these papillae), FiV—filiform papillae subtype V (with caudal orientation of these papillae), Fu—fungiform papilla, r—root of the tongue, Co—small conical papilla, Vp—vallate papilla.
Figure 3.
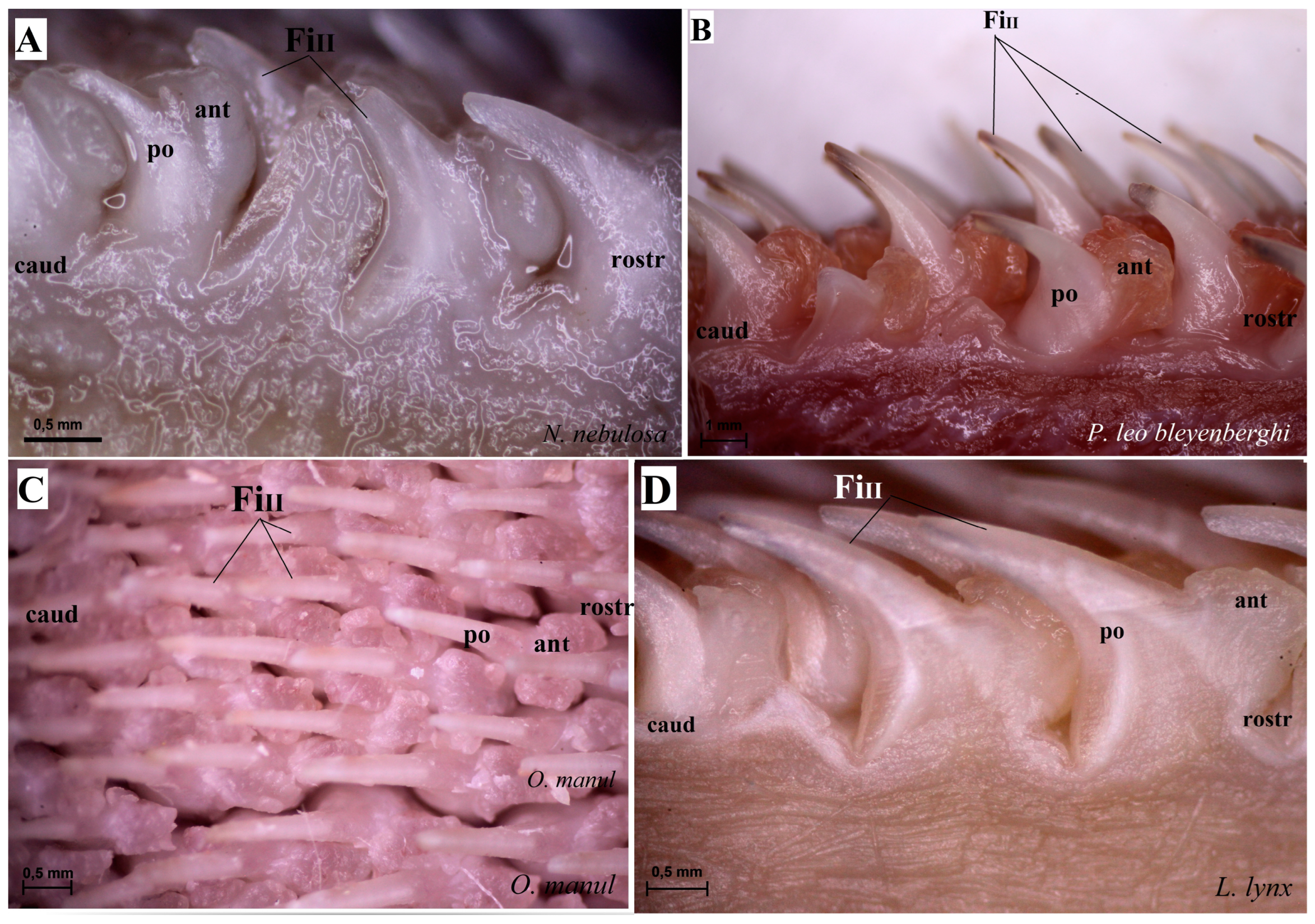
Stereoscopic view of the mechanical lingual papillae from dorsal surface of the rostral part of the tongue of four species of wild Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). Bar = 0.5 mm (A,C,D); bar = 1 mm (B). Abbreviations: ant—anterior part of the mechanical lingual papillae (prominent stratum granulosum), caud—caudal orientation of the mechanical lingual papillae, FiII—filiform papillae subtype II, po—posterior part of mechanical lingual papillae (prominent stratum corneum), rostr—rostral orientation of the mechanical lingual papillae.
Figure 3.
Stereoscopic view of the mechanical lingual papillae from dorsal surface of the rostral part of the tongue of four species of wild Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). Bar = 0.5 mm (A,C,D); bar = 1 mm (B). Abbreviations: ant—anterior part of the mechanical lingual papillae (prominent stratum granulosum), caud—caudal orientation of the mechanical lingual papillae, FiII—filiform papillae subtype II, po—posterior part of mechanical lingual papillae (prominent stratum corneum), rostr—rostral orientation of the mechanical lingual papillae.
Figure 4.
Stereoscopic view of the dorsal surface (A,C,E–G) and the ventral surface (B,D) of the lingual apex of N. nebulosa (A,E), P. leo bleyenberghi (B,F), O. manul (C) and L. lynx (D,G). Bar = 2 mm (A,D); bar = 1 cm (B); bar = 5 mm (C); bar = 2 mm (A,D). Abbreviations: a—apex of the tongue, bv—blood vessel visible on the ventral surface of the tongue, lma—lateral margin of the lingual apex (purple arrow), vs.—ventral surface of the tongue.
Figure 4.
Stereoscopic view of the dorsal surface (A,C,E–G) and the ventral surface (B,D) of the lingual apex of N. nebulosa (A,E), P. leo bleyenberghi (B,F), O. manul (C) and L. lynx (D,G). Bar = 2 mm (A,D); bar = 1 cm (B); bar = 5 mm (C); bar = 2 mm (A,D). Abbreviations: a—apex of the tongue, bv—blood vessel visible on the ventral surface of the tongue, lma—lateral margin of the lingual apex (purple arrow), vs.—ventral surface of the tongue.
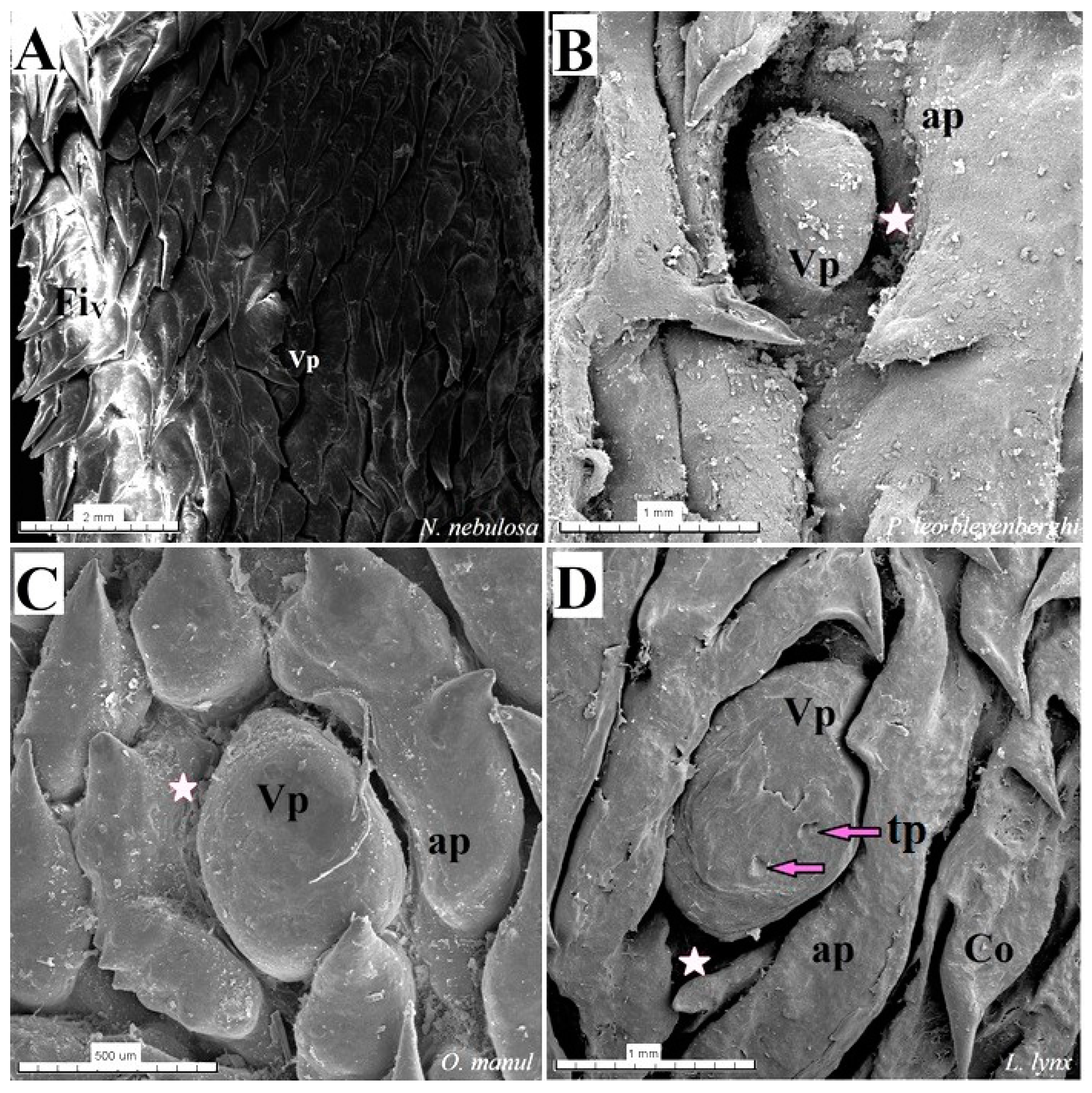
Figure 5.
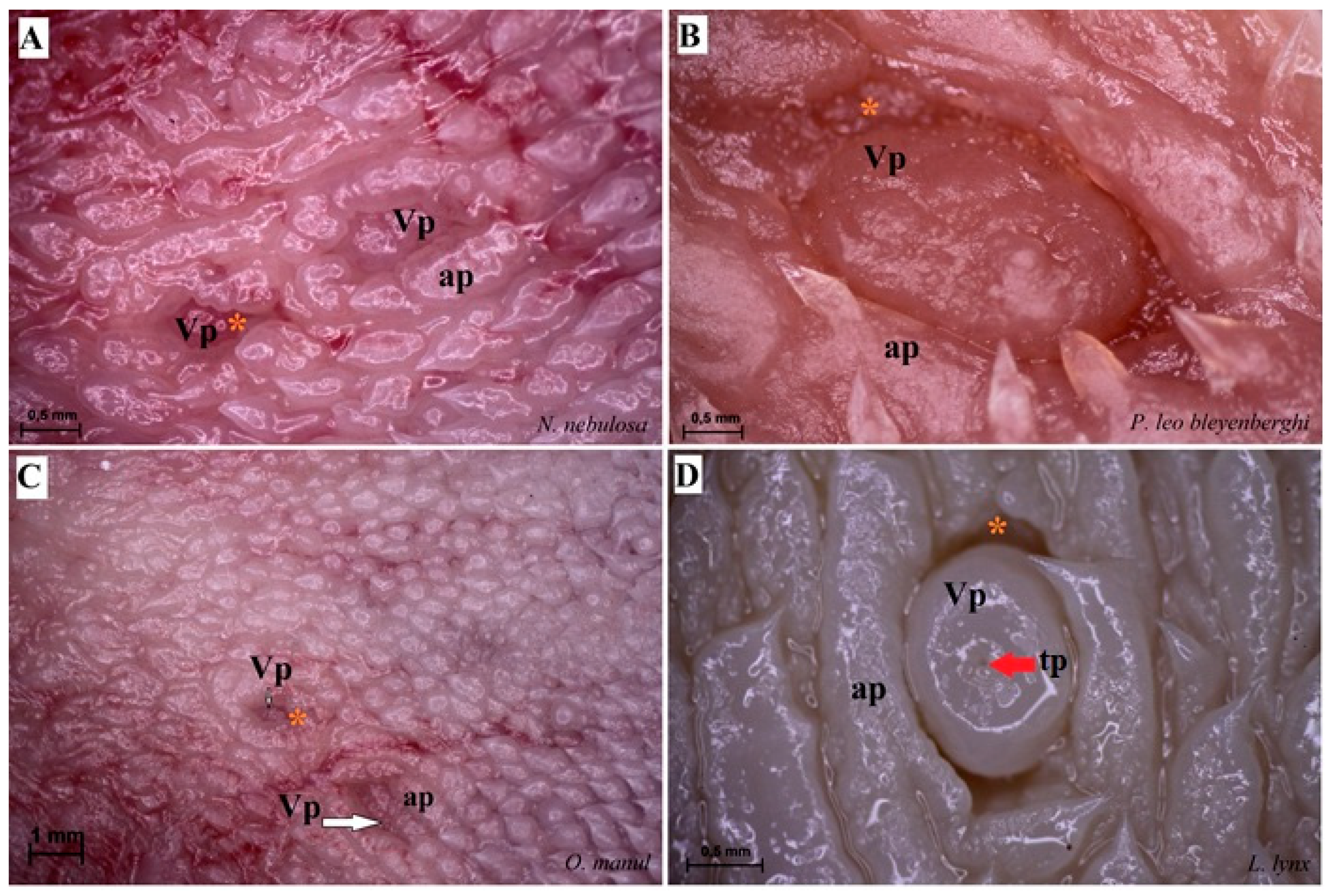
Stereoscopic view of the vallate papillae of the tongue of the four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). (A) Two vallate papillae encircled by papillary groove (asterisk). (B) Magnification of the elongate vallate papilla with irregular dorsal surface. (C) Two vallate papillae among the numerous filiform papillae. (D) Magnification of the round shape vallate papilla with well-visible taste pore (red arrow) of the taste bud on the dorsal surface of the vallum of the papilla. Bar = 1 mm (C); bar = 0.5 mm (A,B,D). Abbreviations: ap—annular pad with irregular surface, tp—taste pore of the taste bud (red arrow), Vp—vallate papilla (white arrow), * (orange asterisk)—groove of the vallate papilla.
Figure 5.
Stereoscopic view of the vallate papillae of the tongue of the four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). (A) Two vallate papillae encircled by papillary groove (asterisk). (B) Magnification of the elongate vallate papilla with irregular dorsal surface. (C) Two vallate papillae among the numerous filiform papillae. (D) Magnification of the round shape vallate papilla with well-visible taste pore (red arrow) of the taste bud on the dorsal surface of the vallum of the papilla. Bar = 1 mm (C); bar = 0.5 mm (A,B,D). Abbreviations: ap—annular pad with irregular surface, tp—taste pore of the taste bud (red arrow), Vp—vallate papilla (white arrow), * (orange asterisk)—groove of the vallate papilla.
Figure 6.
Stereoscopic view of the lateral left (A,B,D) and right (C) lingual surface between the body and root of the tongue in four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). (A) Several smooth folds separated by parallel grooves within the foliate papillae area. (B) Lack of the foliate papillae. (C) Smooth folds separated by five grooves, which form the foliate papillae. (D) Lack of the foliate papillae. Bar = 2 mm (B,D); bar = 0.5 mm (A,C). Abbreviations: caud—caudal orientation of the mechanical lingual papillae, Co—conical papilla, Fo—foliate papilla, rostr—rostral orientation of the mechanical lingual papilla, Vp—vallate papilla.
Figure 6.
Stereoscopic view of the lateral left (A,B,D) and right (C) lingual surface between the body and root of the tongue in four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). (A) Several smooth folds separated by parallel grooves within the foliate papillae area. (B) Lack of the foliate papillae. (C) Smooth folds separated by five grooves, which form the foliate papillae. (D) Lack of the foliate papillae. Bar = 2 mm (B,D); bar = 0.5 mm (A,C). Abbreviations: caud—caudal orientation of the mechanical lingual papillae, Co—conical papilla, Fo—foliate papilla, rostr—rostral orientation of the mechanical lingual papilla, Vp—vallate papilla.
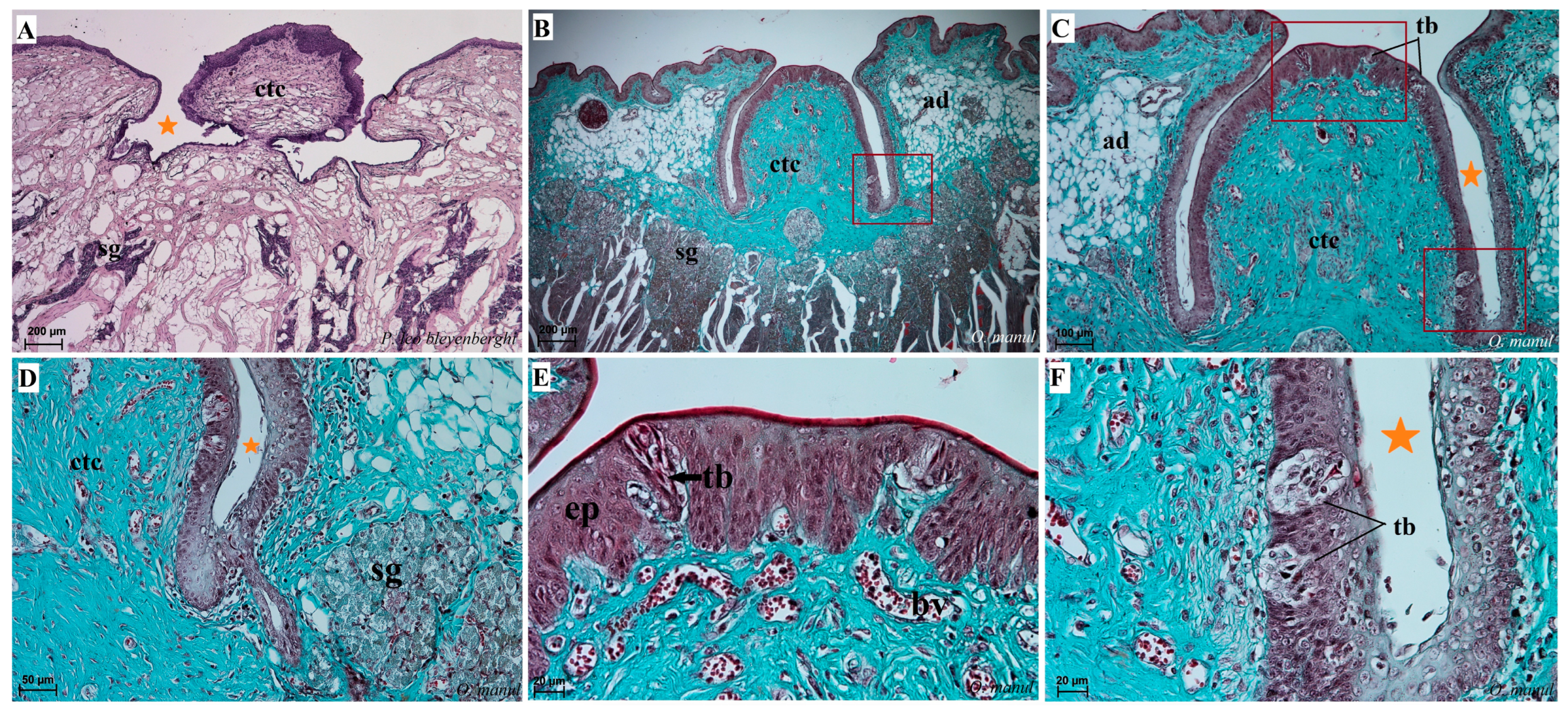
Figure 7.
Macroscopic view (A–C,E) and histological examination (D,F–H) of the lyssa of O. manul (A,B,D,G), P. leo bleyenberghi (C,E,F) and L. lynx (H). (A,C) Ventral smooth surface of the tongue with lyssa (orange arrow). (B) Magnification (black rectangle) of the ventral surface of the tongue with well-defined lyssa. (D) Transverse section of the lyssa. (E) Well-defined lyssa, which is elongated in shape. (F) Transverse section of the lyssa; Masson–Goldner trichrome staining. (G) Transverse section of the lyssa; Azan trichrome staining. (H) Transverse section of lyssa; H&E staining. Bar = 5 mm (A,C); bar = 2 mm (B,E); bar = 100 µm (D,F,G), bar = 200 µm (H). Abbreviations: ad—adipocytes of the lyssa, c—capsule of the lyssa, caud—caudal orientation, ct—connective tissue, m—muscle fibers, dost—dorsal orientation, rostr—rostral orientation, vent—ventral surface of the tongue.
Figure 7.
Macroscopic view (A–C,E) and histological examination (D,F–H) of the lyssa of O. manul (A,B,D,G), P. leo bleyenberghi (C,E,F) and L. lynx (H). (A,C) Ventral smooth surface of the tongue with lyssa (orange arrow). (B) Magnification (black rectangle) of the ventral surface of the tongue with well-defined lyssa. (D) Transverse section of the lyssa. (E) Well-defined lyssa, which is elongated in shape. (F) Transverse section of the lyssa; Masson–Goldner trichrome staining. (G) Transverse section of the lyssa; Azan trichrome staining. (H) Transverse section of lyssa; H&E staining. Bar = 5 mm (A,C); bar = 2 mm (B,E); bar = 100 µm (D,F,G), bar = 200 µm (H). Abbreviations: ad—adipocytes of the lyssa, c—capsule of the lyssa, caud—caudal orientation, ct—connective tissue, m—muscle fibers, dost—dorsal orientation, rostr—rostral orientation, vent—ventral surface of the tongue.
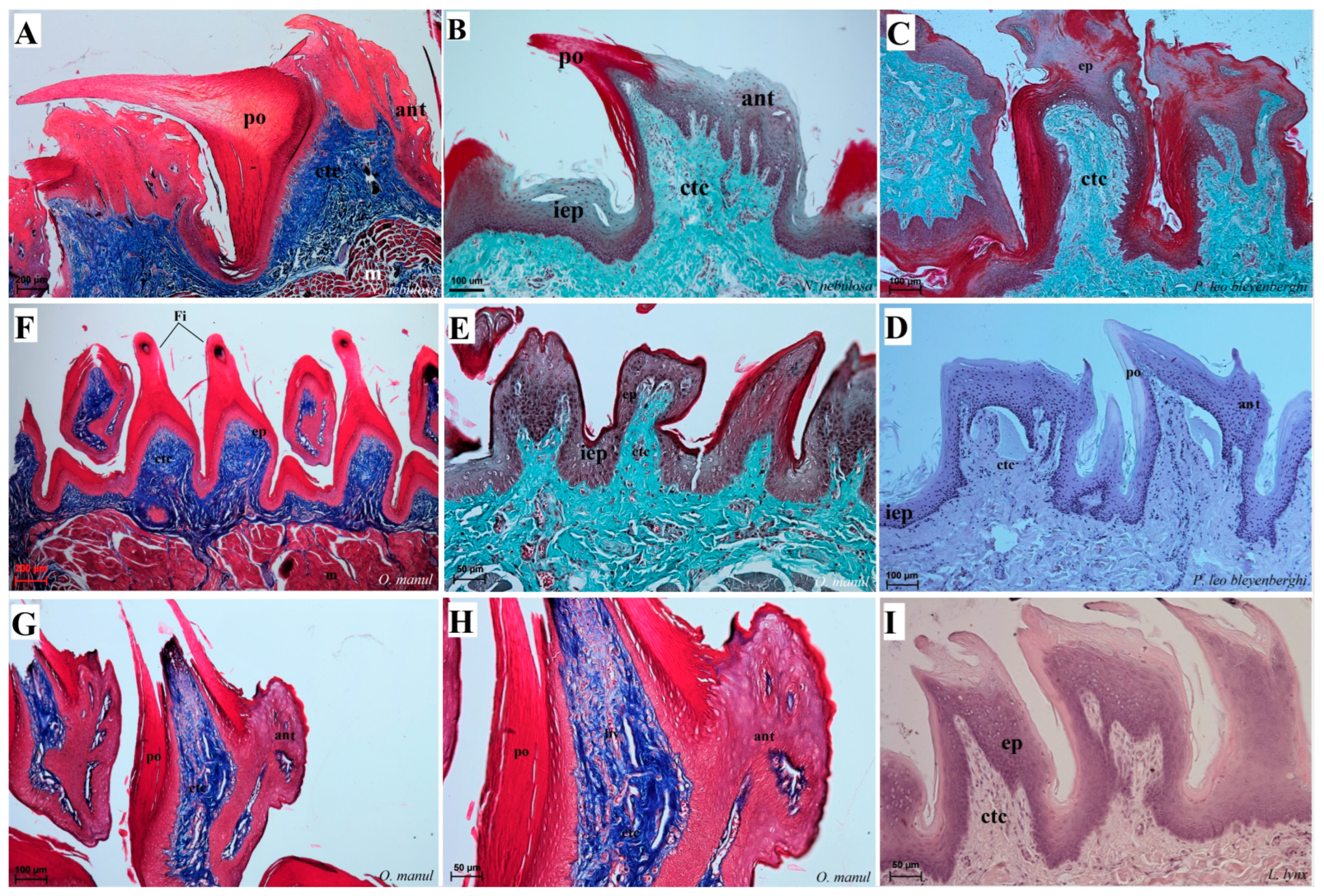
Figure 8.
Histological structure of the filiform papillae of the tongue of the four undomesticated species of Felidae: N. nebulosa (A,B), P. leo bleyenberghi (C,D), O. manul (E–H) and L. lynx (I). (A) Longitudinal section of the filiform papillae with well-defined stratum granulosum and stratum corneum of the papillary stratified squamous epithelium. (B) Longitudinal section of the filiform papillae with interpapillary epithelium. (C,F) Rostral view of the filiform papillae. (D,E–I) Longitudinal section of the filiform papillae with the enlarged stratum granulosum. Azan trichrome staining (A,F,G,H); Masson–Goldner trichrome staining (B,C,E); H&E staining (D,I). Bar = 200 µm (A,F); bar = 100 µm (B,C,D,G); bar = 50 µm (E,H,I). Abbreviations: ant—anterior part of the filiform papillae (prominent stratum granulosum), ctc—connective tissue core, ep—epithelium, Fi—filiform papilla, iep—interpapillary epithelium, m—muscle fibers, po—posterior part of filiform papillae (prominent stratum corneum).
Figure 8.
Histological structure of the filiform papillae of the tongue of the four undomesticated species of Felidae: N. nebulosa (A,B), P. leo bleyenberghi (C,D), O. manul (E–H) and L. lynx (I). (A) Longitudinal section of the filiform papillae with well-defined stratum granulosum and stratum corneum of the papillary stratified squamous epithelium. (B) Longitudinal section of the filiform papillae with interpapillary epithelium. (C,F) Rostral view of the filiform papillae. (D,E–I) Longitudinal section of the filiform papillae with the enlarged stratum granulosum. Azan trichrome staining (A,F,G,H); Masson–Goldner trichrome staining (B,C,E); H&E staining (D,I). Bar = 200 µm (A,F); bar = 100 µm (B,C,D,G); bar = 50 µm (E,H,I). Abbreviations: ant—anterior part of the filiform papillae (prominent stratum granulosum), ctc—connective tissue core, ep—epithelium, Fi—filiform papilla, iep—interpapillary epithelium, m—muscle fibers, po—posterior part of filiform papillae (prominent stratum corneum).
![Biology 12 00516 g008 Biology 12 00516 g008]()
Figure 9.
Histological picture of the fungiform papillae of the tongue of undomesticated species of Felidae: N. nebulosa (A,B) and O. manul (C,D). (A) Longitudinal section of the fungiform papilla with several taste buds within the epithelium. (B) Magnification of the fungiform papillae with two elongated-shaped taste buds (green arrow). Thin keratinized layer is visible (white asterisk). (C) Longitudinal section of dome-shaped fungiform papilla with numerous blood vessels within the connective tissue core of this papilla. Single taste bud present within the epithelium as well as a thin keratinized layer (white asterisk). (D) More elongated fungiform papillae with two taste buds. Azan trichrome staining (A,B,D); Masson–Goldner trichrome (C). Bar = 100 µm (A); bar = 50 µm (D); bar = 20 µm (B,C). Abbreviations: bv—blood vessels, ctc—connective tissue core, ep—epithelium, tb—taste bud, tp—taste pore.
Figure 9.
Histological picture of the fungiform papillae of the tongue of undomesticated species of Felidae: N. nebulosa (A,B) and O. manul (C,D). (A) Longitudinal section of the fungiform papilla with several taste buds within the epithelium. (B) Magnification of the fungiform papillae with two elongated-shaped taste buds (green arrow). Thin keratinized layer is visible (white asterisk). (C) Longitudinal section of dome-shaped fungiform papilla with numerous blood vessels within the connective tissue core of this papilla. Single taste bud present within the epithelium as well as a thin keratinized layer (white asterisk). (D) More elongated fungiform papillae with two taste buds. Azan trichrome staining (A,B,D); Masson–Goldner trichrome (C). Bar = 100 µm (A); bar = 50 µm (D); bar = 20 µm (B,C). Abbreviations: bv—blood vessels, ctc—connective tissue core, ep—epithelium, tb—taste bud, tp—taste pore.
Figure 10.
Histological pictures of the vallate papillae of the tongue of P. leo bleyenberghi (A) and O. manul (B–F). (A–C) Longitudinal section of the vallate papilla with papillary groove (orange asterisk). (D,F) Magnification of the lateral epithelium of the papillae with several taste buds. (E) Magnification of the dorsal epithelium of the vallate papilla. H&E staining (A); Masson–Goldner trichrome staining (B–F). Bar = 200 µm (A,B); bar = 100 µm (C); bar = 50 µm (D); bar = 20 µm (E,F). Abbreviations: ad—adipocytes, bv—blood vessels, ctc—connective tissue core, ep—epithelium, m—muscle fibers, sg—serous glands, tb—taste bud.
Figure 10.
Histological pictures of the vallate papillae of the tongue of P. leo bleyenberghi (A) and O. manul (B–F). (A–C) Longitudinal section of the vallate papilla with papillary groove (orange asterisk). (D,F) Magnification of the lateral epithelium of the papillae with several taste buds. (E) Magnification of the dorsal epithelium of the vallate papilla. H&E staining (A); Masson–Goldner trichrome staining (B–F). Bar = 200 µm (A,B); bar = 100 µm (C); bar = 50 µm (D); bar = 20 µm (E,F). Abbreviations: ad—adipocytes, bv—blood vessels, ctc—connective tissue core, ep—epithelium, m—muscle fibers, sg—serous glands, tb—taste bud.
Figure 11.
Histological pictures of the foliate papillae area of the tongue of N. nebulosa (A) and O. manul (B–D). (A) Lack of taste buds within the lateral side of the tongue within the epithelium of the foliate papillae. (B,C) Foliate papillae with several well-visible elongated taste buds beneath the papillae serous lingual glands are present. Deep sulcus of the foliate papilla (orange asterisk). (D) Magnification of the foliate papilla fold with several taste buds (orange arrow). Azan trichrome staining (A,B); Masson–Goldner trichrome staining (C,D). Bar = 100 µm (A–C); bar = 50 µm (D). Abbreviations: ad—adipocytes, bv—blood vessels, ctc—connective tissue core, ep—epithelium, m—muscle fibers, sg—serous glands, tb—taste bud.
Figure 11.
Histological pictures of the foliate papillae area of the tongue of N. nebulosa (A) and O. manul (B–D). (A) Lack of taste buds within the lateral side of the tongue within the epithelium of the foliate papillae. (B,C) Foliate papillae with several well-visible elongated taste buds beneath the papillae serous lingual glands are present. Deep sulcus of the foliate papilla (orange asterisk). (D) Magnification of the foliate papilla fold with several taste buds (orange arrow). Azan trichrome staining (A,B); Masson–Goldner trichrome staining (C,D). Bar = 100 µm (A–C); bar = 50 µm (D). Abbreviations: ad—adipocytes, bv—blood vessels, ctc—connective tissue core, ep—epithelium, m—muscle fibers, sg—serous glands, tb—taste bud.
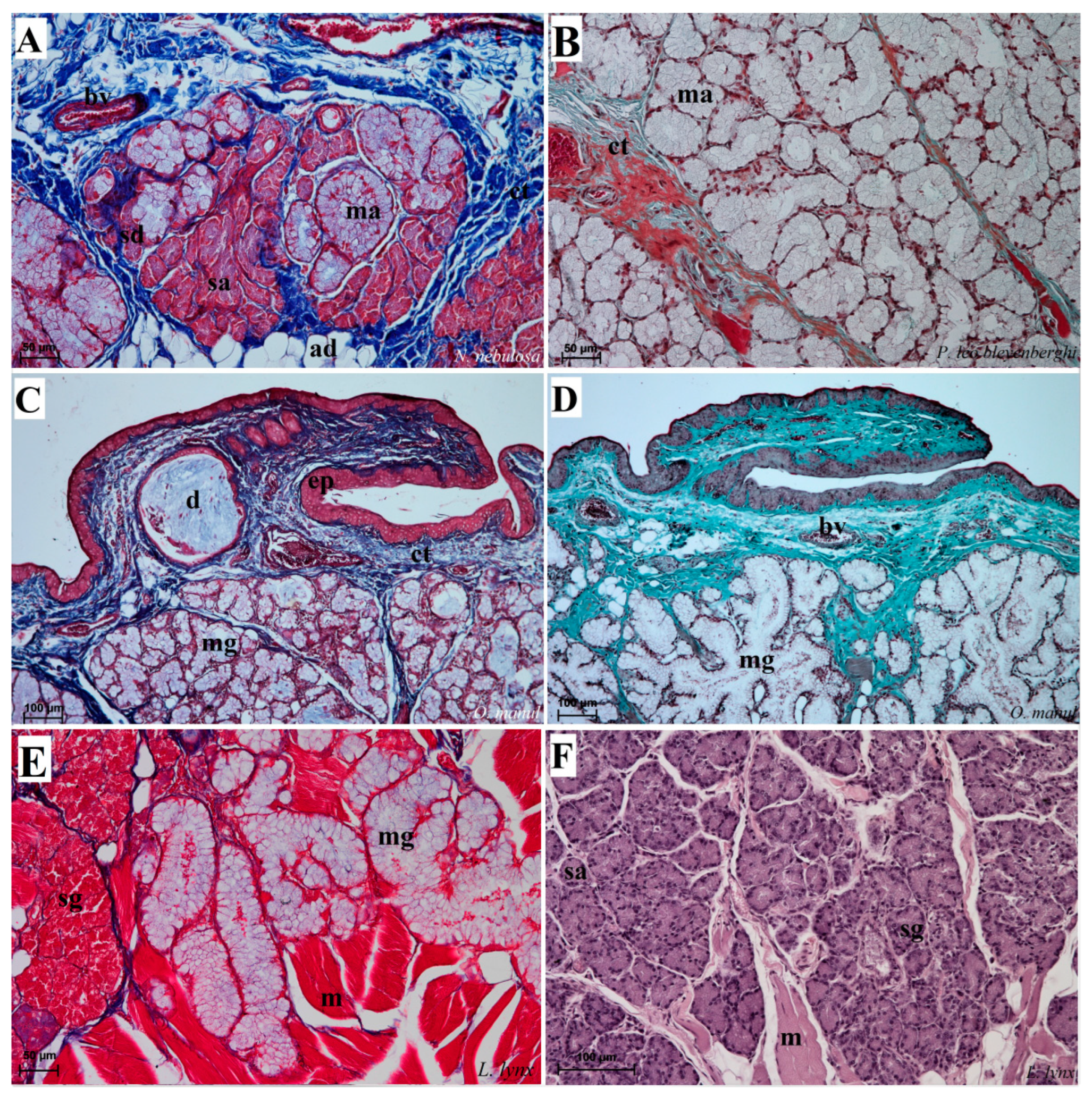
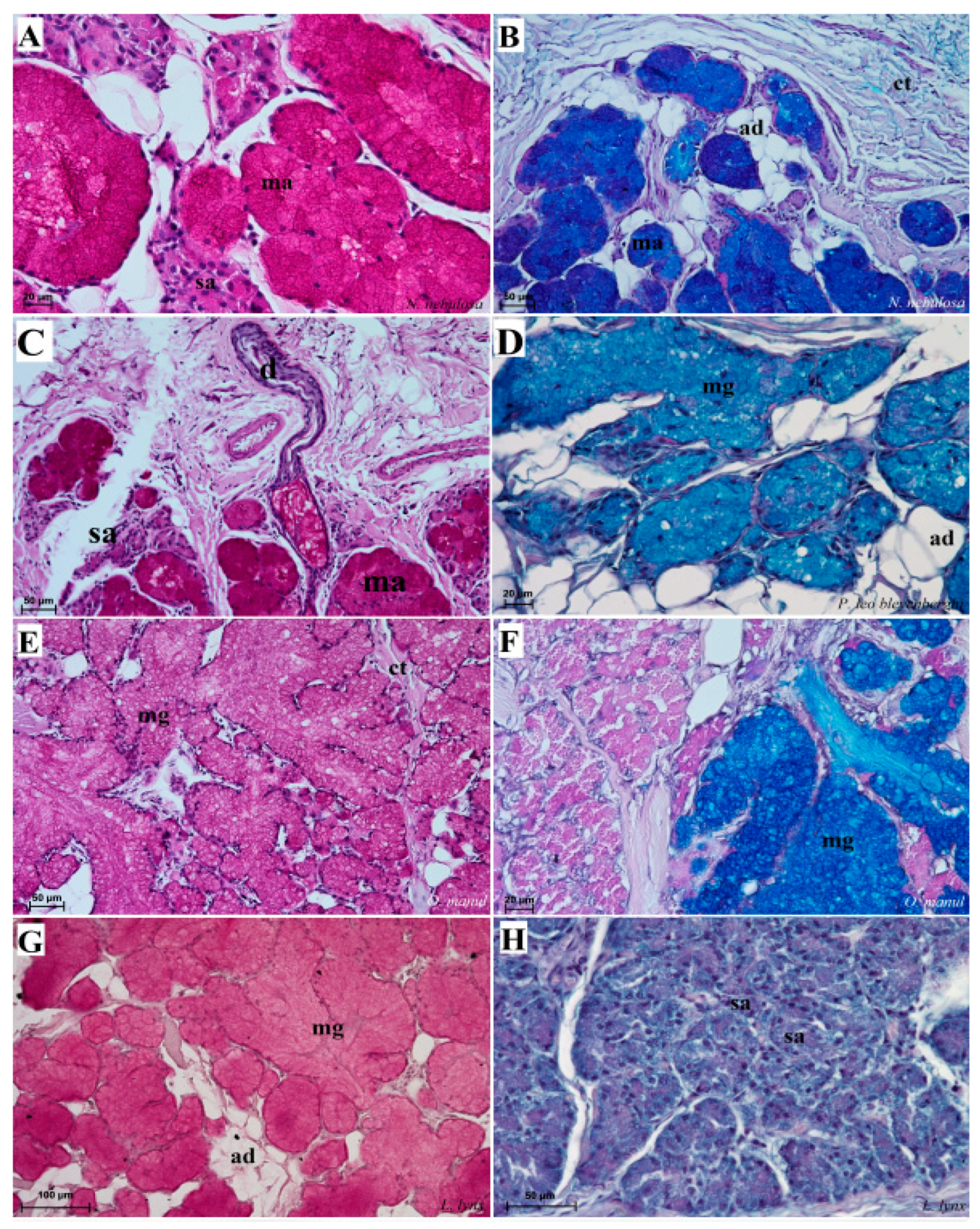
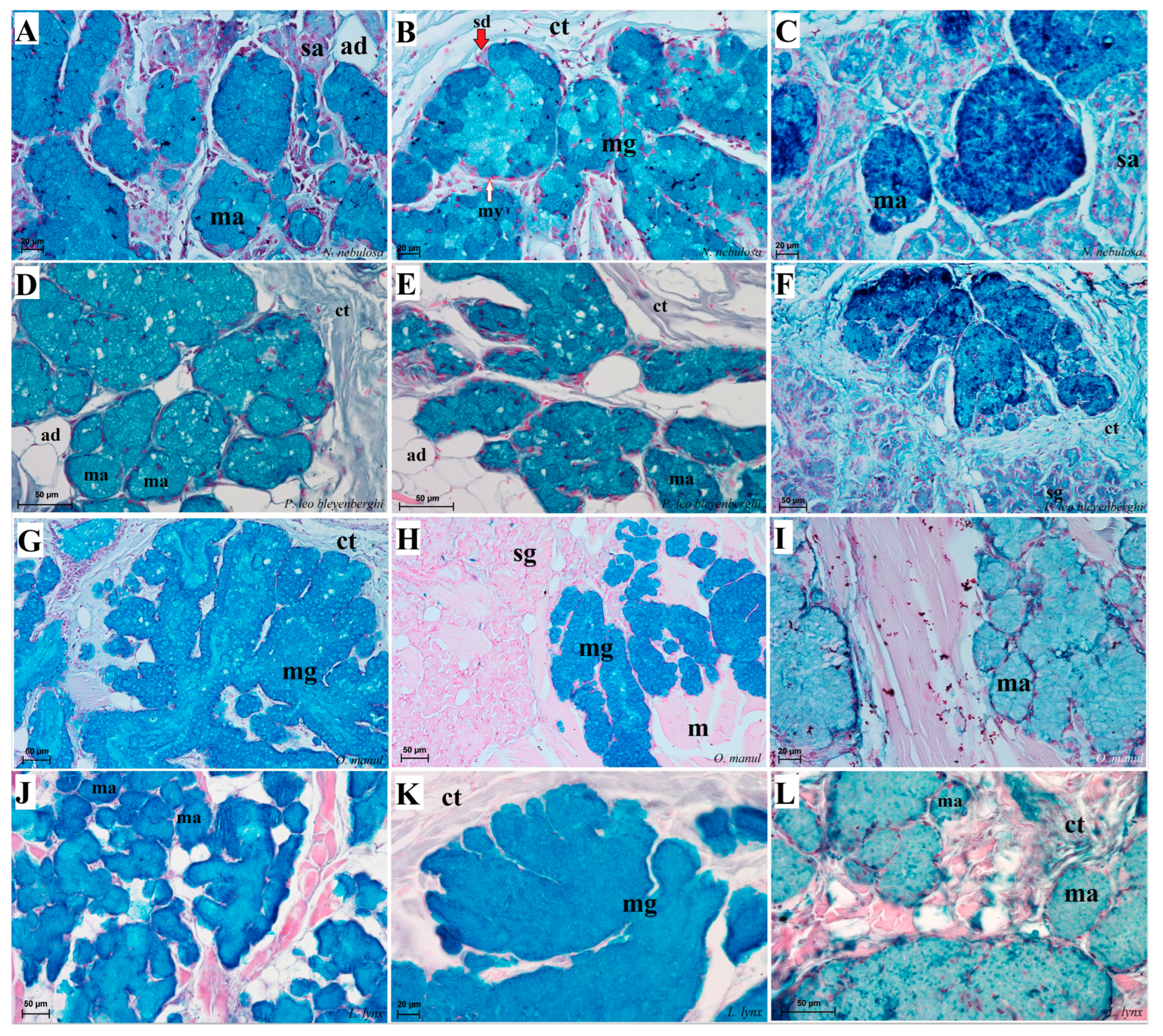
Figure 12.
Histological pictures of the lingual glands of the root of the tongue in four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C,D) and L. lynx (E,F). (A) Mucoserous glands with presence of mucous and serous acini. Some mucous acini with serous demilunes are visible. (B) Serous glands beneath vallate papilla. (C) Longitudinal section of the mechanical lingual papilla and cross-section of the duct of the lingual glands as well as mucoserous glands with the dominance of mucous acini. (D) Mucoserous glands with dominance of mucous acini. (E) Mucoserous glands of the root of the tongue. (F) Serous glands in the tongue root. Azan trichrome staining (A,C,E); Masson–Goldner trichrome (B,D); H&E staining (E). Bar = 100 µm (C,D,F); bar = 50 µm (A,B,E). Abbreviations: ad—adipocytes, bv—blood vessel, ep—epithelium, ct—connective tissue, m—muscle fibers, ma—mucous acini, mg—mucoserous glands with dominant mucous acini (ma), sa—serous acini, sg—serous glands, sd—serous demilunes.
Figure 12.
Histological pictures of the lingual glands of the root of the tongue in four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C,D) and L. lynx (E,F). (A) Mucoserous glands with presence of mucous and serous acini. Some mucous acini with serous demilunes are visible. (B) Serous glands beneath vallate papilla. (C) Longitudinal section of the mechanical lingual papilla and cross-section of the duct of the lingual glands as well as mucoserous glands with the dominance of mucous acini. (D) Mucoserous glands with dominance of mucous acini. (E) Mucoserous glands of the root of the tongue. (F) Serous glands in the tongue root. Azan trichrome staining (A,C,E); Masson–Goldner trichrome (B,D); H&E staining (E). Bar = 100 µm (C,D,F); bar = 50 µm (A,B,E). Abbreviations: ad—adipocytes, bv—blood vessel, ep—epithelium, ct—connective tissue, m—muscle fibers, ma—mucous acini, mg—mucoserous glands with dominant mucous acini (ma), sa—serous acini, sg—serous glands, sd—serous demilunes.
![Biology 12 00516 g012 Biology 12 00516 g012]()
Figure 13.
Histochemical analysis (PAS staining, PAS-AB pH2.5 staining) of the mucous synthesis in the posterior lingual glands of the four species of undomesticated Felidae: N. nebulosa (A,B), P. leo bleyenberghi (C,D), O. manul (E,F) and L. lynx (G,H). (A) Strong positive reaction (+++) in mucous acini (magenta) and weakly positive reaction (+) in serous acini. (B) Strong positive reaction (+++) in mucous acini (dark blue). (C) Positive reaction (++) in mucous acini (magenta). (D) Positive reaction (++) (blue) in mucous acini. (E) Positive reaction (++) in mucous acini (light magenta). (F) Strong positive reaction (+++) (dark blue) in mucous acini and negative reaction (−) in serous acini. (G) Weakly positive reaction (+) (light magenta) in mucous acini. (H) Negative reaction (−) in serous acini. PAS staining (A,C,E,G); PAS-AB pH2.5 staining (B,D,F,H). Bar = 100 µm (G); bar = 50 µm (B–E,H); bar = 20 µm (A,F). Abbreviations: ct—connective tissue, m—muscle fibers, ma—mucous acini, mg—mucoserous glands with the dominance of mucous acini, sa—serous acini, sg—serous glands.
Figure 13.
Histochemical analysis (PAS staining, PAS-AB pH2.5 staining) of the mucous synthesis in the posterior lingual glands of the four species of undomesticated Felidae: N. nebulosa (A,B), P. leo bleyenberghi (C,D), O. manul (E,F) and L. lynx (G,H). (A) Strong positive reaction (+++) in mucous acini (magenta) and weakly positive reaction (+) in serous acini. (B) Strong positive reaction (+++) in mucous acini (dark blue). (C) Positive reaction (++) in mucous acini (magenta). (D) Positive reaction (++) (blue) in mucous acini. (E) Positive reaction (++) in mucous acini (light magenta). (F) Strong positive reaction (+++) (dark blue) in mucous acini and negative reaction (−) in serous acini. (G) Weakly positive reaction (+) (light magenta) in mucous acini. (H) Negative reaction (−) in serous acini. PAS staining (A,C,E,G); PAS-AB pH2.5 staining (B,D,F,H). Bar = 100 µm (G); bar = 50 µm (B–E,H); bar = 20 µm (A,F). Abbreviations: ct—connective tissue, m—muscle fibers, ma—mucous acini, mg—mucoserous glands with the dominance of mucous acini, sa—serous acini, sg—serous glands.
![Biology 12 00516 g013 Biology 12 00516 g013]()
Figure 14.
Histochemical analysis (AB pH 1.0, AB, pH 2.5 and HDI staining) of the mucous synthesis in the posterior lingual glands of the four species of undomesticated Felidae: N. nebulosa (A–C), P. leo bleyenberghi (D–F), O. manul (G–I) and L. lynx (J–L). (A,D,G,J) Mucoserous glands (with dominance of mucous acini). Strong positive reaction (+++) in mucous acini. (B,E,H,K) Strong positive reaction (+++)—blue color in mucous acini, while negative reaction in serous cells of serous demilunes (B). (C,F,I,L) Strong positive reaction (+++) in mucous acini (C,F), positive reaction (+) in mucous acini (I,L) and negative reaction (−) in serous acini (C,F). AB pH2.5 staining (A,D,G,J); AB pH1.0 staining (B,E,H,K); HDI staining (C,F,I,L). Bar = 50 µm (D–H,J,L); bar = 20 µm (A–C,I,K). Abbreviations: ct—connective tissue, m—muscle fibers, ma—mucous acini (round secretory units), mg—mucoserous glands with the dominance of mucous acini, my—myoepithelial cell (white arrow), sa—serous acini, sd—serous demilunes (red arrow), sg—serous glands.
Figure 14.
Histochemical analysis (AB pH 1.0, AB, pH 2.5 and HDI staining) of the mucous synthesis in the posterior lingual glands of the four species of undomesticated Felidae: N. nebulosa (A–C), P. leo bleyenberghi (D–F), O. manul (G–I) and L. lynx (J–L). (A,D,G,J) Mucoserous glands (with dominance of mucous acini). Strong positive reaction (+++) in mucous acini. (B,E,H,K) Strong positive reaction (+++)—blue color in mucous acini, while negative reaction in serous cells of serous demilunes (B). (C,F,I,L) Strong positive reaction (+++) in mucous acini (C,F), positive reaction (+) in mucous acini (I,L) and negative reaction (−) in serous acini (C,F). AB pH2.5 staining (A,D,G,J); AB pH1.0 staining (B,E,H,K); HDI staining (C,F,I,L). Bar = 50 µm (D–H,J,L); bar = 20 µm (A–C,I,K). Abbreviations: ct—connective tissue, m—muscle fibers, ma—mucous acini (round secretory units), mg—mucoserous glands with the dominance of mucous acini, my—myoepithelial cell (white arrow), sa—serous acini, sd—serous demilunes (red arrow), sg—serous glands.
![Biology 12 00516 g014 Biology 12 00516 g014]()
Figure 15.
Histological analysis of the dorsal surface of root of the tongue with the aggregation of the lymphocytes and macrophages of N. nebulosa (A,B) and L. lynx (C,D). (A–C) Aggregation of lymphocytes within the root of the tongue. (D) Magnification of the venule containing numerous erythrocytes surrounded by aggregates of the leukocytes (mainly macrophages and lymphocytes). Azan trichrome staining (A); Masson–Goldner trichrome staining (B); H&E staining (C,D). Bar = 100 µm (A–C); bar = 50 µm (D). Abbreviations: bv—blood vessel, ct—connective tissue, ep—epithelium, la—aggregation of the lymphoid tissue, m—muscle fibers.
Figure 15.
Histological analysis of the dorsal surface of root of the tongue with the aggregation of the lymphocytes and macrophages of N. nebulosa (A,B) and L. lynx (C,D). (A–C) Aggregation of lymphocytes within the root of the tongue. (D) Magnification of the venule containing numerous erythrocytes surrounded by aggregates of the leukocytes (mainly macrophages and lymphocytes). Azan trichrome staining (A); Masson–Goldner trichrome staining (B); H&E staining (C,D). Bar = 100 µm (A–C); bar = 50 µm (D). Abbreviations: bv—blood vessel, ct—connective tissue, ep—epithelium, la—aggregation of the lymphoid tissue, m—muscle fibers.
Figure 16.
Scanning electron microscopic (SEM) pictures of the filiform papillae of the tongue of the four undomesticated species of Felidae: N. nebulosa (A–C), P. leo bleyenberghi (D–F), O. manul (G–I) and L. lynx (J–L). (A,B) Filiform papillae subtype I and II between the apex and body of the tongue. (C) Filiform papillae subtype III from lateral part of the body of the tongue. (D) Largest filiform papillae subtype II with the best-developed anterior part of each papilla. (E,F,I,K) Filiform papillae subtype III without additional projections. (G) Small filiform papillae subtype I with main base of papillae and several additional projections on the anterior part of main papilla. (H) High filiform papillae subtype II between the apex and body of the tongue. (J) Largest filiform papillae subtype II. (L) Short filiform papillae subtype V between body and root of the tongue. Bar = 2 mm (A,B,D,E,H); bar = 1 mm (C,F,J,L); bar = 500 µm (I,K); bar = 200 µm (G). Abbreviations: ant—anterior part of the mechanical lingual papillae, ap—additional projection (purple arrows), caud—caudal orientation of the papillae, ec—exfoliated cell (orange arrow), FiI—filiform papillae subtype I, FiII —filiform papillae subtype II, FiIII—filiform papillae subtype III, FiIV—filiform papillae subtype V, FiV —filiform papillae subtype V, mp—main part of the lingual mechanical papilla subtype FiI, rostr—rostral orientation of the papillae.
Figure 16.
Scanning electron microscopic (SEM) pictures of the filiform papillae of the tongue of the four undomesticated species of Felidae: N. nebulosa (A–C), P. leo bleyenberghi (D–F), O. manul (G–I) and L. lynx (J–L). (A,B) Filiform papillae subtype I and II between the apex and body of the tongue. (C) Filiform papillae subtype III from lateral part of the body of the tongue. (D) Largest filiform papillae subtype II with the best-developed anterior part of each papilla. (E,F,I,K) Filiform papillae subtype III without additional projections. (G) Small filiform papillae subtype I with main base of papillae and several additional projections on the anterior part of main papilla. (H) High filiform papillae subtype II between the apex and body of the tongue. (J) Largest filiform papillae subtype II. (L) Short filiform papillae subtype V between body and root of the tongue. Bar = 2 mm (A,B,D,E,H); bar = 1 mm (C,F,J,L); bar = 500 µm (I,K); bar = 200 µm (G). Abbreviations: ant—anterior part of the mechanical lingual papillae, ap—additional projection (purple arrows), caud—caudal orientation of the papillae, ec—exfoliated cell (orange arrow), FiI—filiform papillae subtype I, FiII —filiform papillae subtype II, FiIII—filiform papillae subtype III, FiIV—filiform papillae subtype V, FiV —filiform papillae subtype V, mp—main part of the lingual mechanical papilla subtype FiI, rostr—rostral orientation of the papillae.
![Biology 12 00516 g016a Biology 12 00516 g016a]()
![Biology 12 00516 g016b Biology 12 00516 g016b]()
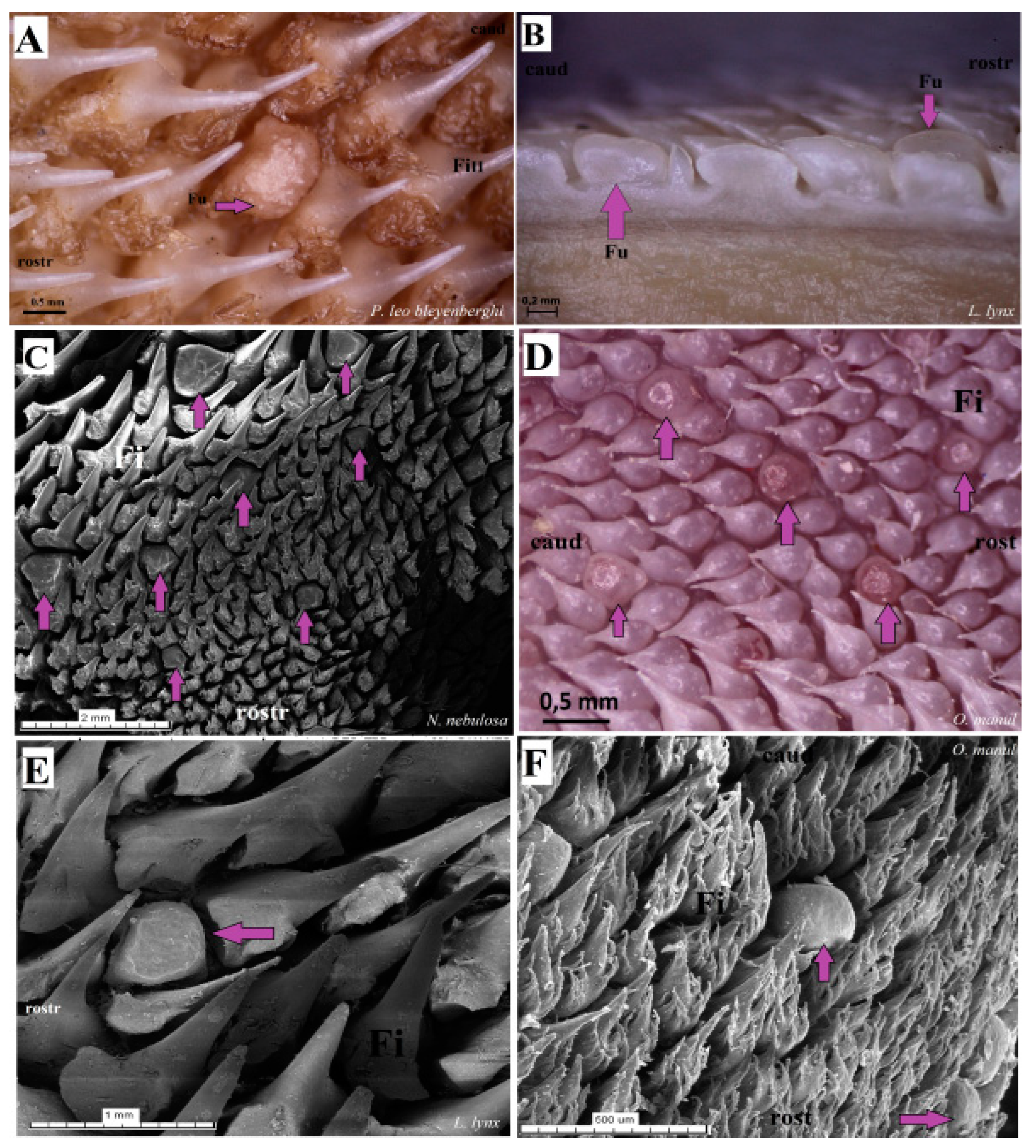
Figure 17.
Macroscopic view (A,B,D) and scanning electron microscopy (SEM) (C,E,F) pictures of the fungiform papillae of the undomesticated species of Felidae. (A) Dorsal view of the one dome-shaped fungiform papilla (purple arrow) of the P. leo bleyenberghi with several filiform papillae. (B) Longitudinal section of the body of the tongue of L. lynx with well-visible sunken-shaped fungiform papillae between filiform papillae. (C) Eight sunken-shaped fungiform papillae within the filiform papillae on the dorsal surface of the body of tongue of N. nebulosa. (D) Five round-shaped fungiform papillae of the tongue of O. manul. (E) SEM picture of the one sunken-shaped fungiform papillae of the tongue of L. lynx. (F) Structure of the two smooth dome-shaped fungiform papillae of O. manul SEM. Bar = 2 mm (C); bar = 1 mm (E); bar = 0.5 mm (A,D,F); bar = 0.2 mm (B). Abbreviations: caud—caudal orientation of the filiform papillae, Fi—filiform papilla, Fu—fungiform papilla (purple arrow), rostr—rostral orientation of the filiform papillae.
Figure 17.
Macroscopic view (A,B,D) and scanning electron microscopy (SEM) (C,E,F) pictures of the fungiform papillae of the undomesticated species of Felidae. (A) Dorsal view of the one dome-shaped fungiform papilla (purple arrow) of the P. leo bleyenberghi with several filiform papillae. (B) Longitudinal section of the body of the tongue of L. lynx with well-visible sunken-shaped fungiform papillae between filiform papillae. (C) Eight sunken-shaped fungiform papillae within the filiform papillae on the dorsal surface of the body of tongue of N. nebulosa. (D) Five round-shaped fungiform papillae of the tongue of O. manul. (E) SEM picture of the one sunken-shaped fungiform papillae of the tongue of L. lynx. (F) Structure of the two smooth dome-shaped fungiform papillae of O. manul SEM. Bar = 2 mm (C); bar = 1 mm (E); bar = 0.5 mm (A,D,F); bar = 0.2 mm (B). Abbreviations: caud—caudal orientation of the filiform papillae, Fi—filiform papilla, Fu—fungiform papilla (purple arrow), rostr—rostral orientation of the filiform papillae.
![Biology 12 00516 g017 Biology 12 00516 g017]()
Figure 18.
Scanning electron microscopic (SEM) pictures of the vallate papillae of the tongue of the four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). (A) An elongated-shaped vallate papilla. (B) Well-visible deep papillary groove and oval- shaped vallate papilla. (C) Vallate papillae encircled by an irregular annular pad. (D) Two taste pores on the dorsal surface of the vallate papilla. Bar = 2 mm (A); bar = 1 mm (B,D); bar = 500 (C). Abbreviations: ap—annular pad with irregular surface with exfoliated cells, Co—conical papilla, tp—taste pore of the taste bud (purple arrow), Vp—vallate papilla, * (asterisk)—groove of the vallate papilla.
Figure 18.
Scanning electron microscopic (SEM) pictures of the vallate papillae of the tongue of the four species of undomesticated Felidae: N. nebulosa (A), P. leo bleyenberghi (B), O. manul (C) and L. lynx (D). (A) An elongated-shaped vallate papilla. (B) Well-visible deep papillary groove and oval- shaped vallate papilla. (C) Vallate papillae encircled by an irregular annular pad. (D) Two taste pores on the dorsal surface of the vallate papilla. Bar = 2 mm (A); bar = 1 mm (B,D); bar = 500 (C). Abbreviations: ap—annular pad with irregular surface with exfoliated cells, Co—conical papilla, tp—taste pore of the taste bud (purple arrow), Vp—vallate papilla, * (asterisk)—groove of the vallate papilla.
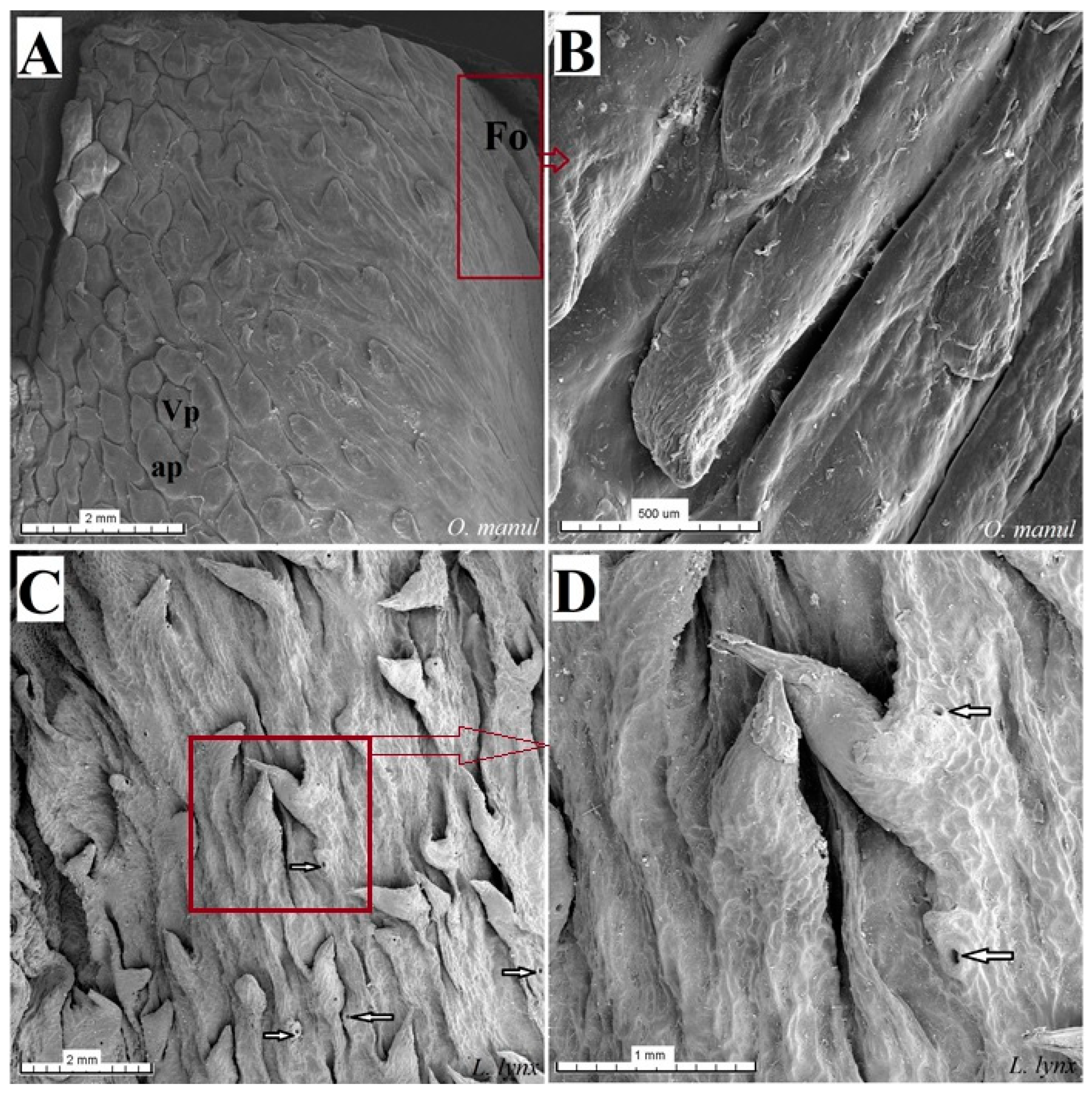
Figure 19.
Scanning electron microscopy (SEM) pictures of the foliate papillae area of the tongue of O. manul (A,B) and lateral area of the tongue between the body and root of the L. lynx (C,D). (A) Foliate papillae area on the right part of the sample (maroon rectangle) and vallate papillae surrounded by the annular pad. (B) Magnification of the folds and grooves which form the foliate papilla. (C) Several round openings (white arrows) of the glandular ducts of posterior lingual glands on the lateral posterior surface of the tongue and several well-visible conical papillae. (D) Magnification of the opening of the lingual gland ducts and two conical lingual papillae. Bar = 2 mm (A,C); bar = 1 mm (D); bar = 500 µm (B). Abbreviations: ap—annular pad, Fo—foliate papilla, Vp—vallate papilla.
Figure 19.
Scanning electron microscopy (SEM) pictures of the foliate papillae area of the tongue of O. manul (A,B) and lateral area of the tongue between the body and root of the L. lynx (C,D). (A) Foliate papillae area on the right part of the sample (maroon rectangle) and vallate papillae surrounded by the annular pad. (B) Magnification of the folds and grooves which form the foliate papilla. (C) Several round openings (white arrows) of the glandular ducts of posterior lingual glands on the lateral posterior surface of the tongue and several well-visible conical papillae. (D) Magnification of the opening of the lingual gland ducts and two conical lingual papillae. Bar = 2 mm (A,C); bar = 1 mm (D); bar = 500 µm (B). Abbreviations: ap—annular pad, Fo—foliate papilla, Vp—vallate papilla.
Table 1.
Felids described in current study.
Table 1.
Felids described in current study.
| Species | Prey
[8] | Habitat
[8] | Collection
of Research Material | Age/Sex
of Animal | The IUCN Red List of Threatened
Species/CITES Status |
|---|
| Pantherinae |
Clouded leopard
(Neofelis nebulosa
Griffith, 1821) | birds, monkeys, pigs, cattle, young buffalo, goats, deer and porcupines | both arboreal and terrestrial; various kinds of forests | Zoo Wrocław
(Poland) | 5 years, 6 months, 24 days/female | Vulnerable (VU)
CITES II |
Katanga lion
(Panthera leo bleyenberghi
Lönnberg, 1914) | wildebeests, impalas, other antelopes, giraffes, buffalo, wild hogs, zebras and carrions | terrestrial; grassy plains, savannahs, woodlands and scrub country | Zoo Wrocław
(Poland) | 13 years, 3 months, 14 days/male
14 years, 9 months,
28 days/female | Vulnerable (VU)
CITES II |
| Felinae |
Eurasian lynx
(Lynx lynx
Linnaeus, 1758) | hares, small ungulates, rodents, pikas and birds | terrestrial; mostly forests | ZOO Ljubljana
(Slovenia) | 7 years/female | Least concern (LC)
CITES II |
Pallas’s cat
(Otocolobus manul
Pallas, 1776) | pikas, small rodents and ground-dwelling birds | terrestrial; steppes, deserts and rocky country | Zoo Wrocław
(Poland) | 4 years, 6 months, 8 days/male | Least concern (LC) |
Table 2.
Macroscopic measurements of the tongue, the number of vallate papillae and the length of lyssa in the four felids described in this study.
Table 2.
Macroscopic measurements of the tongue, the number of vallate papillae and the length of lyssa in the four felids described in this study.
| Species | Length
(cm) | Width (cm) | Thickness (cm) | Number of Vallate Papillae | Lyssa
Length (cm) |
|---|
| Apex | Body | Root | Apex | Body | Root |
|---|
| N. nebulosa | 12 | 1.8 | 2.5 | 3 | 0.3 | 0.7 | 1.2 | 4 (2 on the right and 2 on the left) | 1.7 |
| P. leo bleyenberghi | 44 ♂ | 6 | 7.1 | 9 | 1.3 | 2.5 | 3.6 | 7 (3 on the right and 4 on the left) | 2.9 |
| 33 ♀ | 5 | 5.9 | 6 | 0.9 | 3.0 | 4.1 | 5 (3 on the right and 2 on the left) | 1.9 |
| L. lynx | 10 | 2.6 | 3.8 | 4 | 0.3 | 0.6 | 2 | 9 (5 on the right and 4 on the left) | 1.6 |
| O. manul | 7 | 1.2 | 2.2 | 2 | 0.2 | 0.4 | 0.7 | 6 (2 on the right and 4 on the left) | 1.4 |
Table 3.
Measurement (height and width (µm)) of the mechanical filiform papillae and gustatory papillae in the four felid species described in this study.
Table 3.
Measurement (height and width (µm)) of the mechanical filiform papillae and gustatory papillae in the four felid species described in this study.
| Species | Mechanical Filiform Papillae | Gustatory Papillae |
|---|
| FiI | FiII | FiIII | FiIV | FiV | Fu | Vp | Fo |
|---|
| N. nebulosa | height | 138.75 ± 15.73 | 1248.16 ± 77.09 | 541.91 ± 55.08 | 371.83 ± 157.86 | 413.75 ± 139.11 | 647.92 ± 184.55 | 513.66 ± 51.02 | +/− |
| width | 121.75 ± 20.43 | 182.91 ± 39.47 | 254.33 ± 27.64 | 200.5 ± 41.70 | 261.5 ± 103.06 | 407.91 ± 157.26 | 546.08 ± 93.50 | +/− |
| P. leo bleyenberghi (♀ and ♂) | height | 181.41 ± 47.25 | 3650.41 ± 687.53 | 1567.66 ± 257.97 | 1041.41 ± 172.64 | 270.33 ± 48.33 | 605.91 ± 113.8 | 2428.58 ± 579.50 | − |
| width | 180.08 ± 25.07 | 1352.08 ± 90.22 | 729.33 ± 72.39 | 278.25 ± 64.68 | 331.25 ± 70.49 | 579.08 ± 63.19 | 2040.25 ± 545.78 | − |
| L. lynx | height | 88.41 ± 32.57 | 1106.08 ± 144.06 | 530.75 ± 160.28 | 219.16 ± 86.82 | 367.66 ± 180.89 | 370.5 ± 79.32 | 428.28 ± 40.80 | − |
| width | 75.91 ± 22.02 | 238.25 ± 50.03 | 232.16 ± 47.12 | 211.58 ± 61.74 | 321.91 ± 135.16 | 464.75 ± 120.30 | 672.66 ± 121.81 | − |
| O. manul | height | 124.91 ± 51.25 | 1651.08 ± 397.87 | 512.16 ± 74.31 | 216.75 ± 49.57 | 257.41 ± 99.56 | 246.08 ± 73.51 | 509.5 ± 52.55 | 457.25 ± 31.13 |
| width | 105.75 ± 18.84 | 290.91 ± 58.51 | 196.41 ± 50.67 | 232.51 ± 43.01 | 273.08 ± 82.99 | 284.5 ± 42.12 | 526.33 ± 227.90 | 574.91 ± 48.75 |