Effect of Rice Straw and Stubble Burning on Soil Physicochemical Properties and Bacterial Communities in Central Thailand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Management Practices and Fire Measurements
2.3. Sample Collection and Analysis
2.4. DNA Extraction, Bacterial 16s Amplification, and Sequencing
2.5. Bacterial Taxonomic and Functional Identification
2.6. Statistical Analysis
3. Results
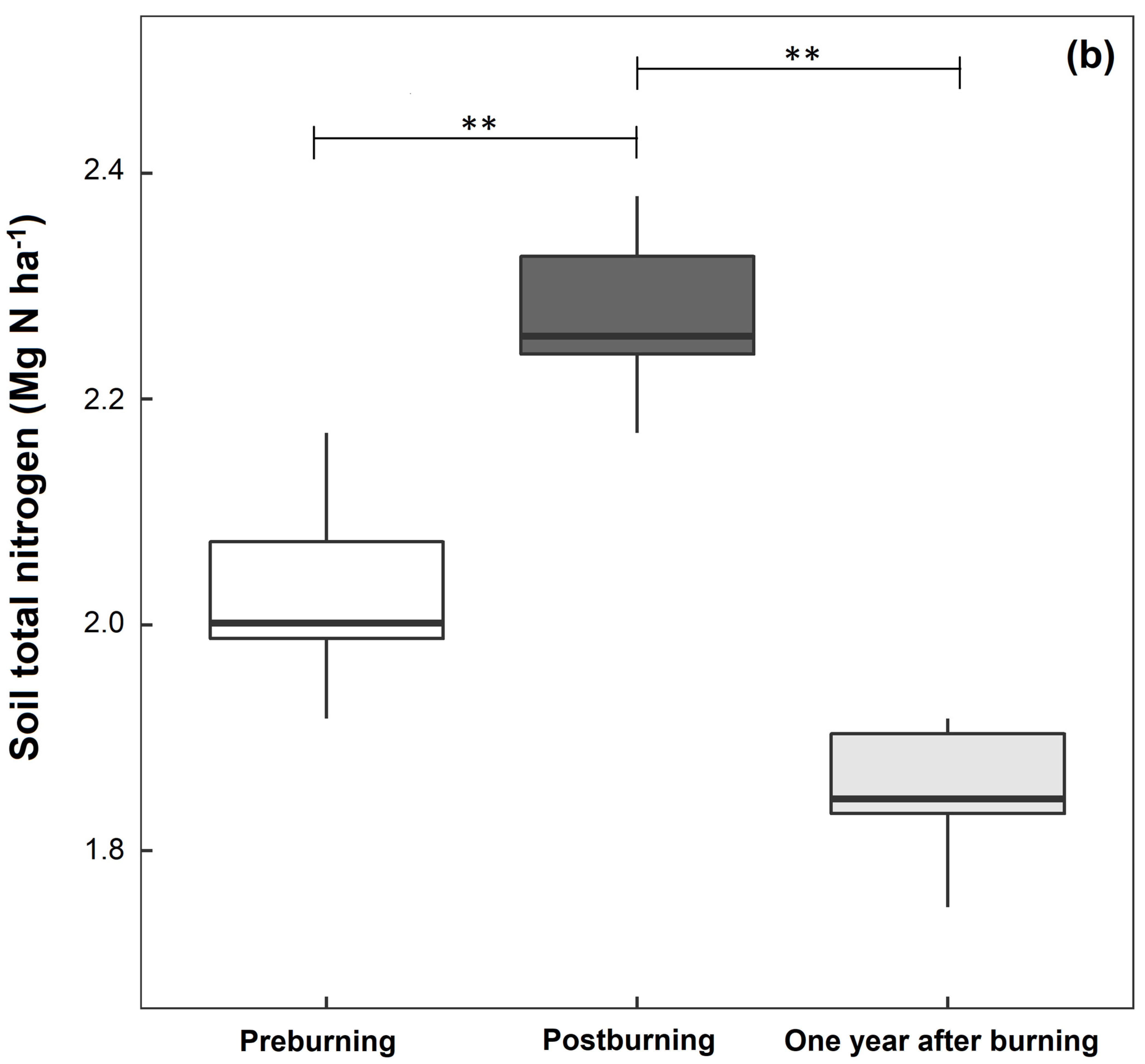
3.1. Soil Physical and Chemical Properties
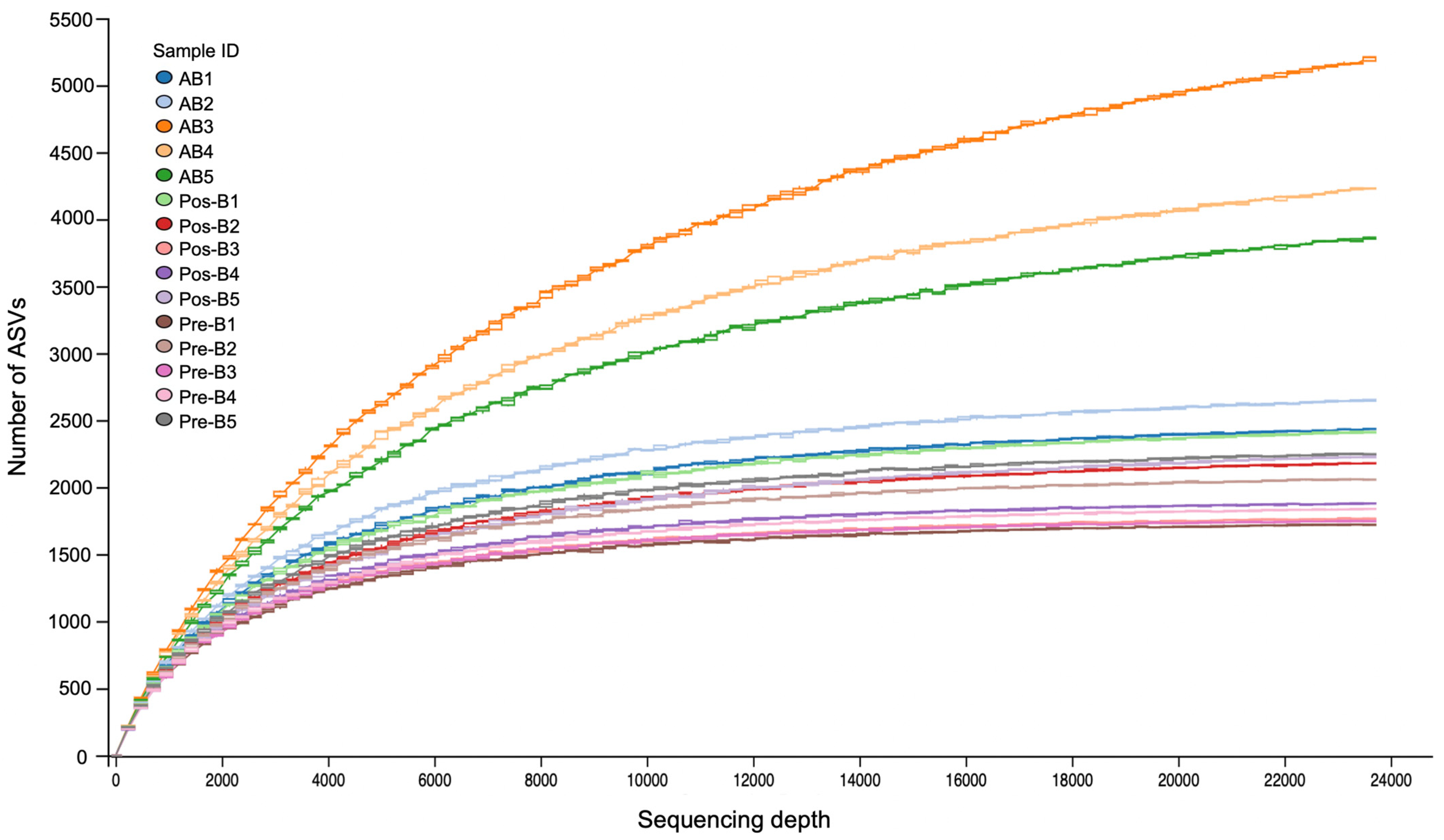
3.2. Overview of the Sequencing Analysis
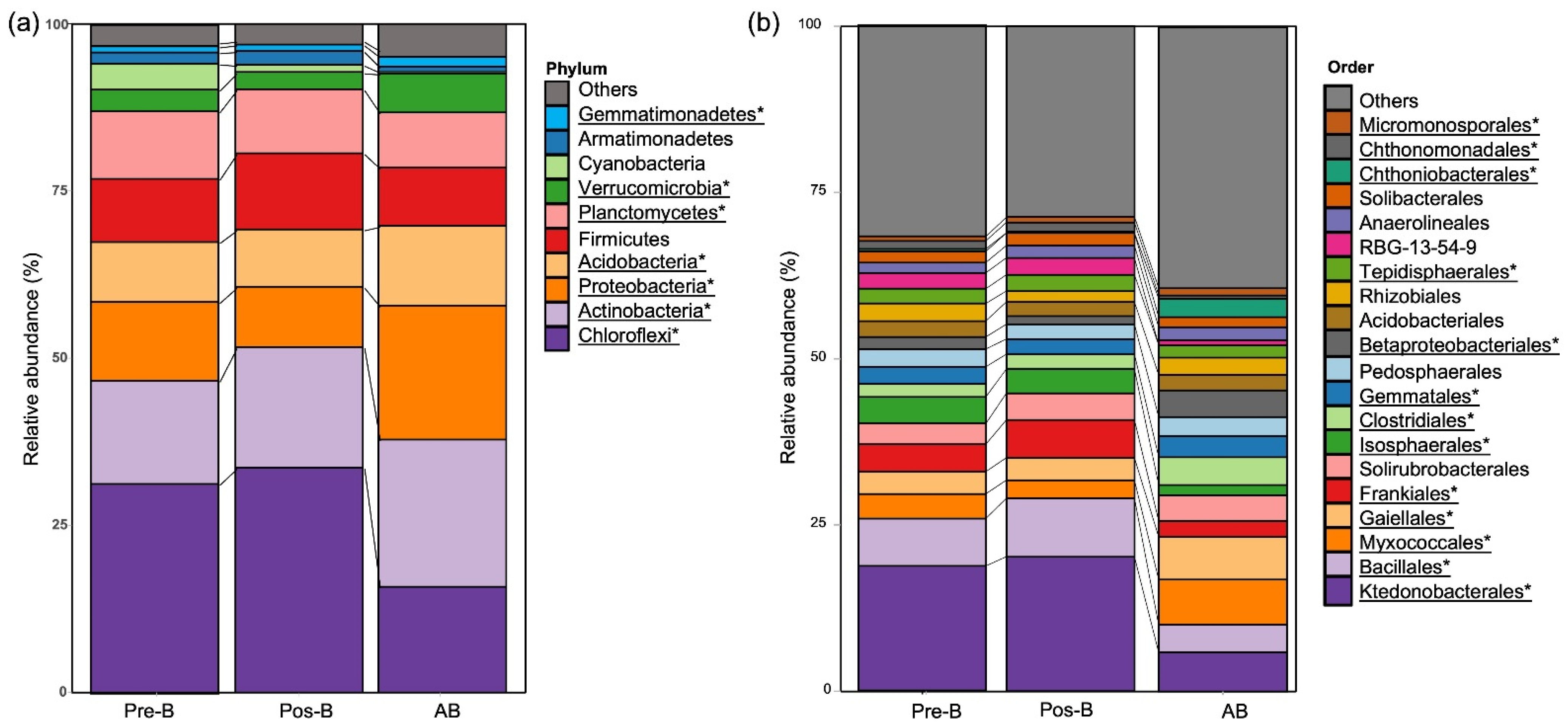
3.3. Taxonomic Distribution
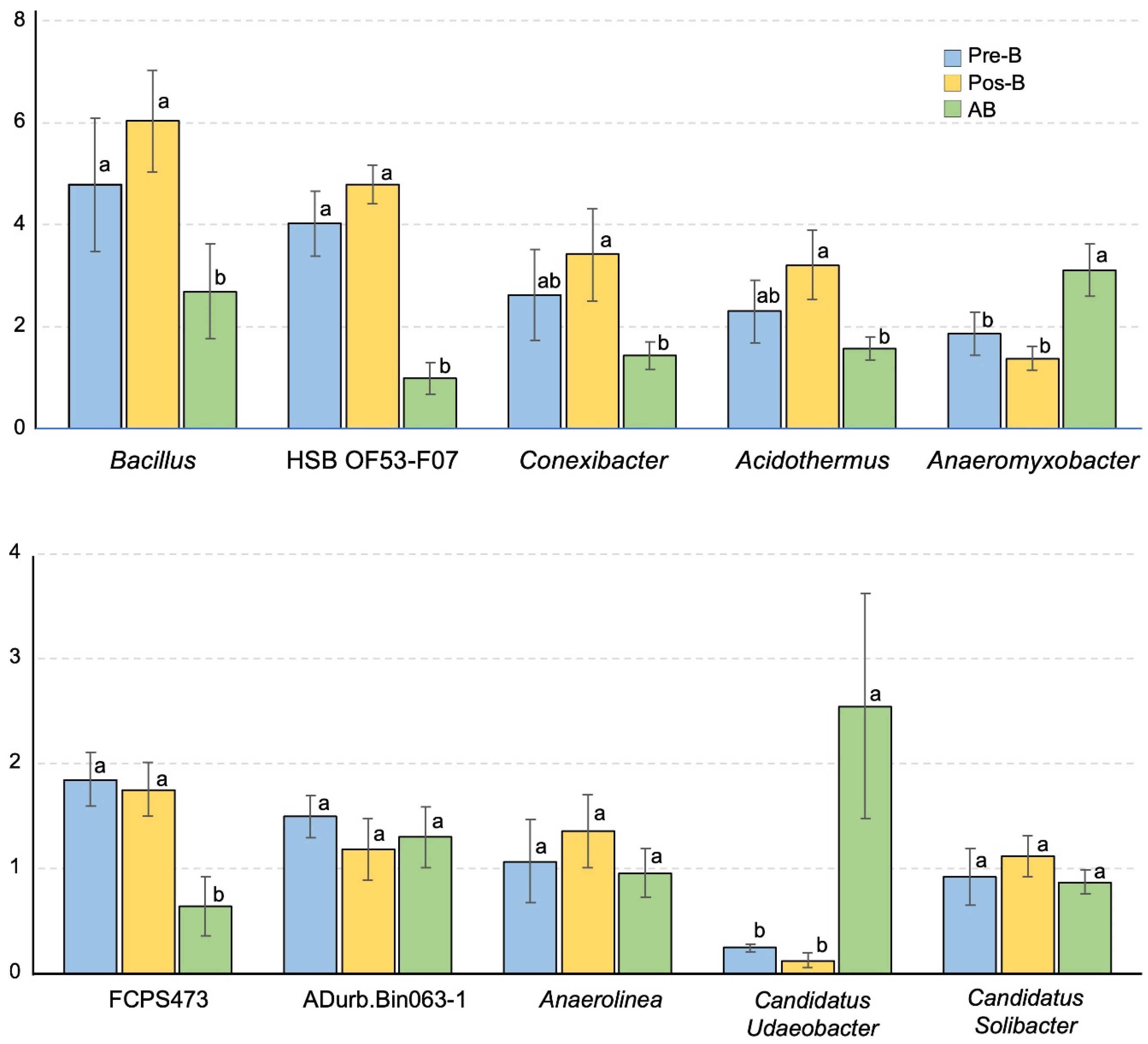
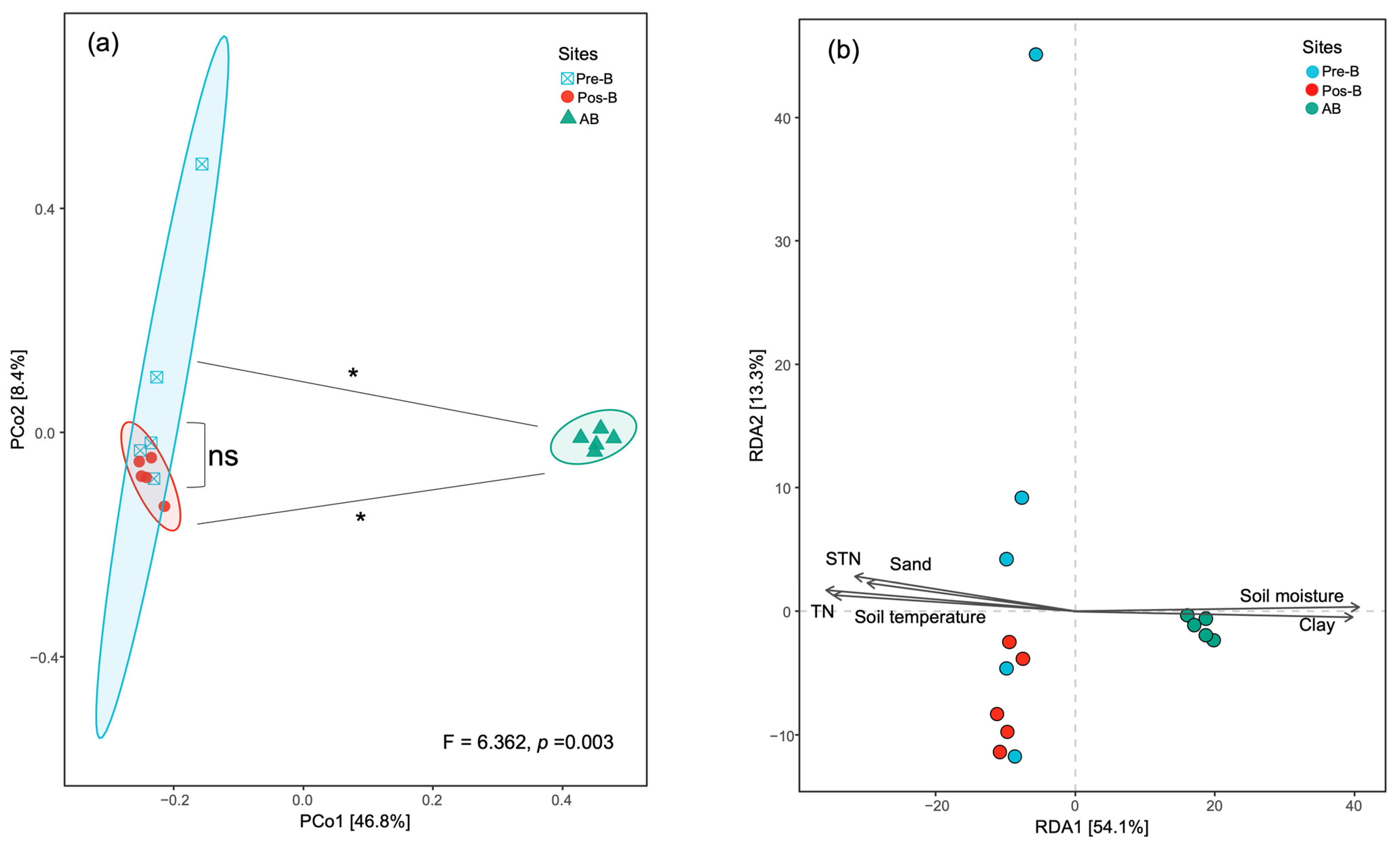
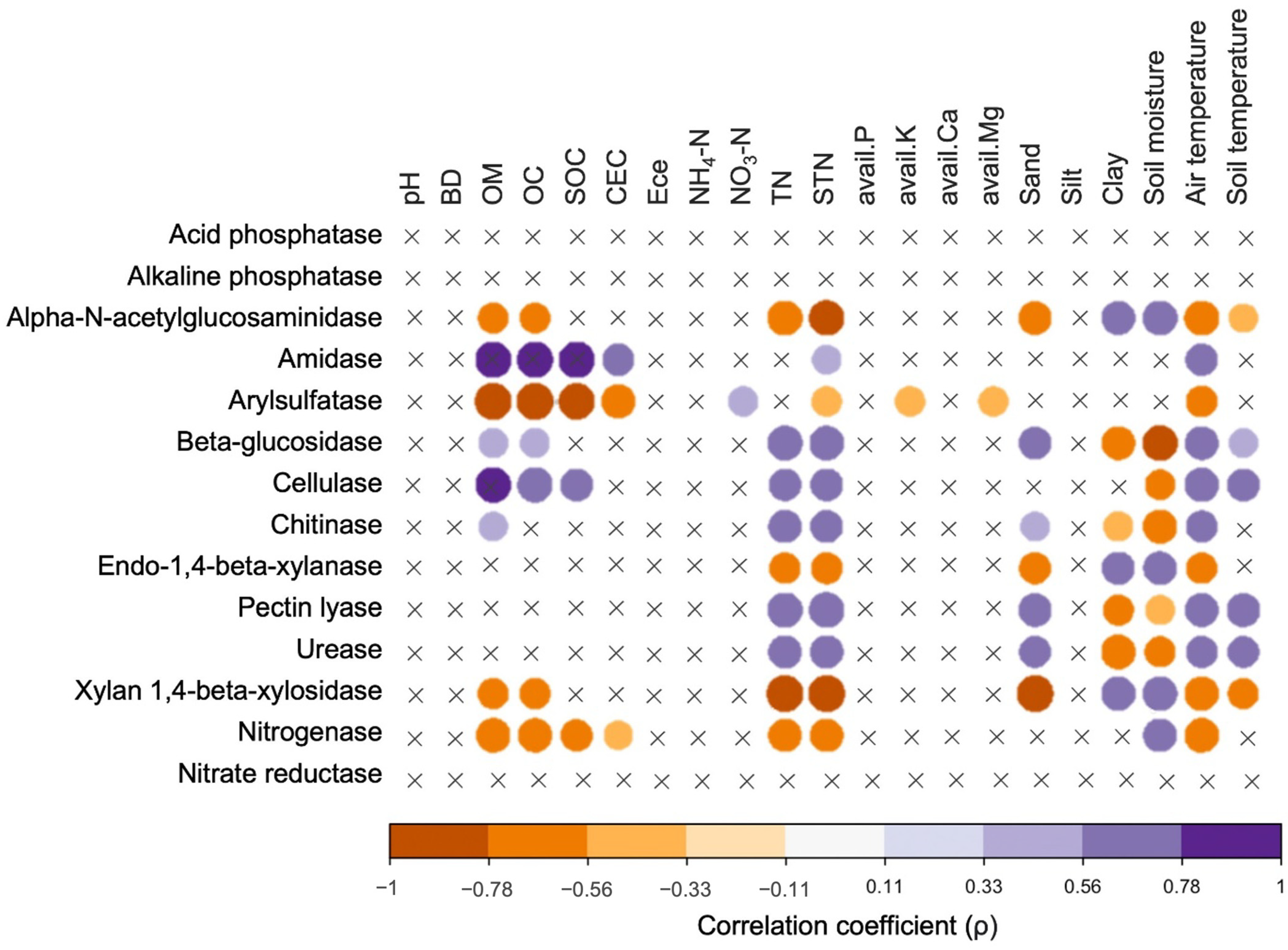
3.4. Bacterial Diversity, Community Compositions, and Correlations to Soil Properties
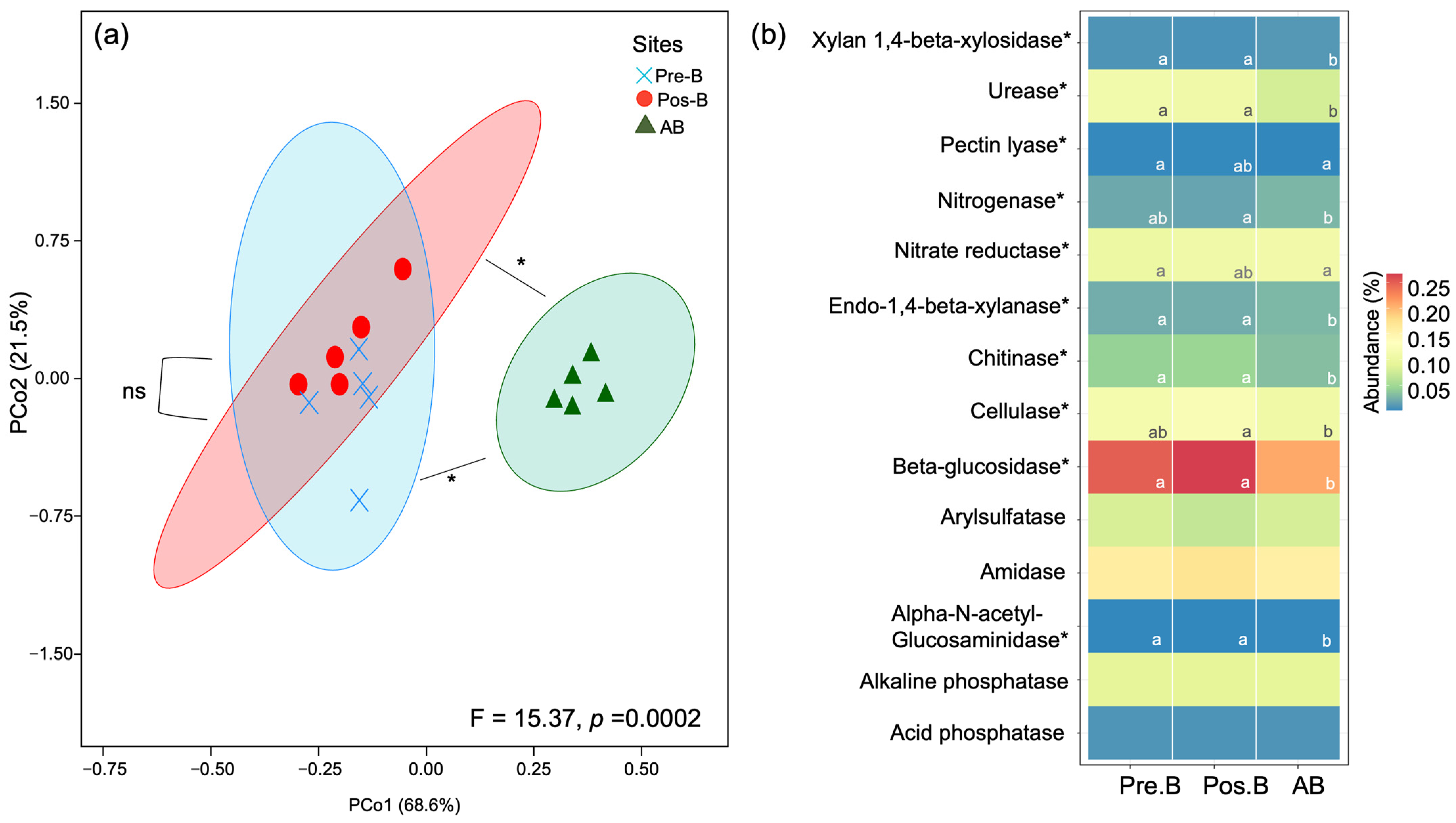
3.5. Predictive Functions
4. Discussion
4.1. Effects of Burning Rice Straw and Stubble on Soil Physicochemical Properties
4.2. Soil Bacterial Community Composition and Diversity Responses Immediately after Burning
4.3. Changes in Soil Physicochemical Properties and Soil Bacterial Community Composition and Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tipayarom, D.; Oanh, N.K. Effects from open rice straw burning emission on air quality in the Bangkok metropolitan region. Sci. Asia 2007, 33, 339–345. [Google Scholar] [CrossRef]
- Oanh, N.T.K.; Ly, B.T.; Tipayarom, D.; Manandhar, B.R.; Prapat, P.; Simpson, C.D.; Liu, L.J.S. Characterization of particulate matter emission from open burning of rice straw. Atmos. Environ. 2011, 45, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Gadde, B.; Menke, C.; Wassmann, R. Rice straw as a renewable energy source in India, Thailand, and the Philippines: Overall potential and limitations for energy contribution and greenhouse gas mitigation. Biomass Bioenergy 2009, 33, 1532–1546. [Google Scholar] [CrossRef]
- Office of Agricultural Economics (OAE). Agricultural Statistics of Thailand 2021. Centre for Agricultural Information, Office of Agricultural Economics (in Thai), 2021. Available online: https://www.oae.go.th/assets/portals/1/files/jounal/2565/yearbook2564.pdf (accessed on 21 August 2022).
- Arunrat, N.; Pumijumnong, N. Practices for reducing greenhouse gas emissions from rice production in Northeast Thailand. Agriculture 2017, 7, 4. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Hatano, R. Impact of burning on soil organic carbon of maize-upland rice system in Mae Chaem Basin of Northern Thailand. Geoderma 2021, 392, 115002. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Hatano, R. Effects of fire on soil organic carbon, soil total nitrogen, and soil properties under rotational shifting cultivation in northern Thailand. J. Environ. Manag. 2022, 302, 113978. [Google Scholar] [CrossRef]
- Dooley, S.R.; Treseder, K.K. The effect of fire on microbial biomass: A meta-analysis of field studies. Biogeochemistry 2012, 109, 49–61. [Google Scholar] [CrossRef]
- Mickovski, M. Effect of burned straw on the microflora of the soil. Annu. Fac. Agric. Univ. Skopje 1967, 20, 55–68. [Google Scholar]
- Biederbeck, V.O.; Campbell, C.A.; Bowren, K.E.; Schnitzer, M.; McIver, R.N. Effect of Burning Cereal Straw on Soil Properties and Grain Yields in Saskatchewan. Soil Sci. Soc. Am. J. 1980, 44, 103–111. [Google Scholar] [CrossRef]
- Kumar, A.; Kushwaha, K.K.; Singh, S.; Shivay, Y.S.; Meena, M.C.; Nain, L. Effect of paddy straw burning on soil microbial dynamics in sandy loam soil of Indo-Gangetic plains. Environ. Technol. Innov. 2019, 16, 100469. [Google Scholar] [CrossRef]
- Luo, X.S.; Han, S.; Lai, S.S.; Huang, Q.Y.; Chen, W.L. Long-term straw returning affects Nitrospira-like nitrite oxidizing bacterial community in a rapeseed-rice rotation soil. J. Basic Microbiol. 2017, 57, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Zhang, H.; Shen, Y.; Li, G.; Wang, H.; Cheng, W. Mitigation of heavy metal accumulation in rice grain with silicon in animal manure fertilized field. Environ. Eng. Manag. J. 2016, 15, 2223–2229. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Guo, L.J.; Zheng, S.X.; Cao, C.G.; Li, C.F. Tillage practices and straw-returning methods affect topsoil bacterial community and organic C under a rice-wheat cropping system in central China. Sci. Rep. 2016, 6, 33155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, T.; Xun, W.; Huang, X.; Huang, Q.; Ran, W.; Shen, B.; Zhang, R.; Shen, Q. Influence of straw incorporation with and without straw decomposer on soil bacterial community structure and function in a rice-wheat cropping system. Appl. Microbiol. Biotechnol. 2017, 101, 4761–4773. [Google Scholar] [CrossRef] [PubMed]
- National Soil Survey Center. Soil Survey Center. Soil Survey Laboratory Methods Manual. In Soil Survey Investigations Report No. 42; Version 3.0; Natural Conservation Service: Washington, DC, USA, 1996. [Google Scholar]
- United States Department of Agriculture (USDA). Diagnosis and Improvement of Saline and Alkali Soils, Agriculture. Handbook No. 60; U.S. Salinity Laboratory; Government Printing Office: Washington, DC, USA, 1954.
- Thomas, G.W. Soil pH and Soil Acidity. Method of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Inc.: Madison, WI, USA; ASA Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Bray, R.A.; Kurtz, L.T. Determination of total organic and available form of phosphorus in soil. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 Years of Serving the Community with Ribosomal RNA Gene Reference Databases and Tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Role of Enzymes in Maintaining Soil Health. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–42. [Google Scholar]
- Wickham, H. ggplot2; Springer: New York, NY, USA, 2009. [Google Scholar]
- Ribeiro Filho, A.A.; Adams, C.; Manfrendini, S.; Aguilar, R.; Neves, W. Dynamics of soil chemical properties in shifting cultivation systems in the tropics: A Meta analysis. Soil Use Manag. 2015, 31, 474–482. [Google Scholar] [CrossRef]
- Granged, A.J.P.; Zavala, L.M.; Jordan, A.; Moreno, G.B. Post- fire evolution of soil properties and vegetation cover in a Mediterranean heathland after experimental burning: A 3-year study. Geoderma 2011, 164, 85–94. [Google Scholar] [CrossRef]
- Raison, R.J. Modification of the soil environment by vegetation fires, with particular reference to nitrogen transformations: A review. Plant Soil. 1979, 51, 73–108. [Google Scholar] [CrossRef]
- Zavala, L.; De Celis, R.; Jordán, A. How wildfires affect soil properties. Brief Rev. Cuad. Investig. Geográf. 2014, 40, 311–332. [Google Scholar]
- Nigussie, A.; Kissi, E. Impact of biomass burning on physicochemical properties of Nitisol in the southwestern Ethiopia. Asian J. Agric. Res. 2011, 5, 223–233. [Google Scholar] [CrossRef]
- Gangwar, K.S.; Singh, K.K.; Sharma, S.K.; Tomar, O.K. Alternative tillage and crop residue management in wheat after rice in sandy loam soils of Indo-Gangetic plains. Soil Tillage Res. 2006, 88, 242–252. [Google Scholar] [CrossRef]
- Pellegrini, A.F.; Ahlström, A.; Hobbie, S.E.; Reich, P.B.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nature 2018, 553, 194. [Google Scholar] [CrossRef] [PubMed]
- Parro, K.; Köster, K.; Jõgiste, K.; Seglinš, K.; Sims, A.; Stanturf, J.A.; Metslaid, M. Impact of post-fire management on soil respiration, carbon and nitrogen content in a managed hemiboreal forest. J. Environ. Manag. 2019, 233, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Knicker, H.; Müller, P.; Hilscher, A. How useful is chemical oxidation with dichromate for the determination of “Black Carbon” in fire affected soils? Geoderma 2007, 142, 178–196. [Google Scholar] [CrossRef]
- Riggan, P.J.; Lockwood, R.N.; Jacks, P.M.; Colver, C.G.; Weirich, F.; DeBano, L.F.; Brass, J.A. Effects of Fire Severity on Nitrate Mobilization in Watersheds Subject to Chronic Atmospheric Deposition. Environ. Sci. Technol. 1994, 28, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Hui, D.; Luo, Y. Fire effects on nitrogen pools and dynamics in terrestrial ecosystems: A meta-analysis. Ecological 2001, 11, 711–730. [Google Scholar] [CrossRef]
- Neary, D.G.; Ryan, K.C.; DeBano, L.F. Wildland Fire in Ecosystems: Effects of Fire on Soils and Water; General Technical Report RMRS-GTR-42; United States Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2005; Volume 4, pp. 1–262.
- Delač, D.; Pereira, P.; Bogunović, I.; Kisić, I. Short-Term Effects of Pile Burn on N Dynamic and N Loss in Mediterranean Croatia. Agronomy 2020, 10, 1340. [Google Scholar] [CrossRef]
- Caon, L.; Vallejo, V.R.; Coen, R.J.; Geissen, V. Effects of wildfire on soil nutrients in Mediterranean ecosystems. Earth Sci. Rev. 2014, 139, 47–58. [Google Scholar] [CrossRef]
- Covington, W.W.; DeBano, L.F.; Huntsberger, T.G. Soil nitrogen changes associated with slash pile burning in pinyon-juniper woodlands. For. Sci. 1991, 37, 347–355. [Google Scholar]
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (red pine) wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Oguntunde, P.G.; Abiodun, B.J.; Ajayi, A.E.; van de Giesen, N. Effects of charcoal production on soil physical properties in Ghana. J. Plant Nutr. Soil Sci. 2008, 171, 591–596. [Google Scholar] [CrossRef]
- Ayodele, A.; Oguntunde, P.; Joseph, A.; De Souza Dias Junios, M. Numerical analysis of the impact of charcoal production on soil hydrological behavior, runoff response and erosion susceptibility. Rev. Bras. Cienc. Solo 2009, 33, 137–145. [Google Scholar] [CrossRef]
- Giovannini, G.; Lucchesi, S.; Giachetti, M. Effect of heating on some physical and chemical parameters related to soil aggregation and erodibility. Soil Sci. 1988, 146, 255–261. [Google Scholar] [CrossRef]
- Fonseca, F.; de Figueiredo, T.; Nogueira, C.; Queirós, A. Effect of prescribed fire on soil properties and soil erosion in a Mediterranean mountain area. Geoderma 2017, 307, 172–180. [Google Scholar] [CrossRef]
- Neill, C.; Patterson, W.A.; Crary, D.W. Responses of soil carbon, nitrogen and cations to the frequency and seasonality of prescribed burning in a Cape Cod oak-pine forest. For. Ecol. Manag. 2007, 250, 234–243. [Google Scholar] [CrossRef]
- Stock, W.D.; Lewis, O.A.M. Soil Nitrogen and the Role of Fire as a Mineralizing Agent in a South African Coastal Fynbos Ecosystem. J. Ecol. 1986, 74, 317–328. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C. Plant ash and heat intensity eff ects on chemical and physical properties of two contrasting soils. Arid Land Res. Manag. 2003, 17, 23–41. [Google Scholar] [CrossRef]
- Johnson, B.G.; Johnson, D.W.; Chambers, J.C.; Blank, R.R. Fire effects on the mobilization and uptake of nitrogen by cheatgrass (Bromus tectorum L.). Plant Soil 2011, 341, 437–445. [Google Scholar] [CrossRef]
- Castelli, L.; Lazzari, M. Impact of fire on soil nutrients in central semiarid Argentina. Arid Land Res. Manag. 2002, 16, 349–364. [Google Scholar] [CrossRef]
- Duguy, B.; Rovira, P.; Vallejo, R. Land-use history and fire effects on soil fertility in eastern Spain. Eur. J. Soil Sci. 2007, 58, 83–91. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Dowd, S.; Sun, Y.; Allen, V. Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol. Biochem. 2008, 40, 2762–2770. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.J.; Su, J.Q.; Sun, G.X.; Wu, J.X.; Wei, W.X. Increased microbial functional diversity under long-term organic and integrated fertilization in a paddy soil. Appl. Microbiol. Biotechnol. 2018, 102, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.D.; Shestak, C.J.; Hubbert, K.R.; Knapp, E.E. Soil physical properties regulate lethal heating during burning of woody residues. Soil Sci. Soc. Am. J. 2010, 74, 947–955. [Google Scholar] [CrossRef]
- Whelan, R.J.; Landedyk, W.; Pashby, A.S. The effects of Wildfire on arthropod Populations in Jerrah Banksia Woodland. West. Aust. Nat. 1980, 14, 214–220. [Google Scholar]
- Badía, D.; Martí, C.; Aguirre, A.J.; Aznar, J.M.; González-Pérez, J.A.; De la Rosa, J.M.; León, J.; Ibarra, P.; Teresa Echeverría, T. Wildfire effects on nutrients and organic carbon of a Rendzic Phaeozem in NE Spain: Changes at cm-scale topsoil. CATENA 2014, 113, 267–275. [Google Scholar] [CrossRef]
- Armas-Herrera, C.M.; Martí, C.; Badía, D.; Ortiz-Perpiñá, O.; Girona-García, A.; Mora, J.L. Short-term and midterm evolution of topsoil organic matter and biological properties after prescribed burning for pasture recovery (Tella, Central Pyrenees, Spain). Land Degrad. Dev. 2018, 29, 1545–1554. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Niu, S. Differential responses of the acidobacterial community in the topsoil and subsoil to fire disturbance in Pinus tabulaeformis stands. PeerJ 2019, 7, e8047. [Google Scholar] [CrossRef]
- Li, W.T.; Chen, X.F.; Liu, M.; Kuzyakov, Y.; Jiang, C.Y.; Wu, M.; Li, Z.P. Shifts in microbial communities with increasing soil fertility across a chronosequence of paddy cultivation in subtropical China. Appl. Soil Ecol. 2017, 120, 153–159. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Q.; Li, Z.; Cheng, W.; Sun, J.; Guo, Z.; Li, Y.; Zhou, J.; Meng, D.; Li, H.; et al. Environmental factors shaping the diversity of bacterial communities that promote rice production. BMC Microbiol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Herlambang, A.; Murwantoko, M.; Istiqomah, I. Dynamic change in bacterial communities in the integrated rice– fish farming system in Sleman, Yogyakarta, Indonesia. Aquac. Res. 2021, 52, 5566–5578. [Google Scholar] [CrossRef]
- Chen, L.; Xu, J.; Wan, W.; Xu, Z.; Hu, R.; Zhang, Y.; Zheng, J.; Gu, Z. The Microbiome Structure of a Rice-Cray fish Integrated Breeding Model and Its Association with Cray fish Growth and Water Quality. Microbiol. Spectr. 2022, 10, e0220421. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Song, J.; Kim, B.Y.; Kim, M.S.; Joa, J.H.; Weon, H.Y. Characterization of the bacterial and archaeal communities in rice field soils subjected to long-term fertilization practices. J. Microbiol. 2012, 50, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Wu, M.; Li, Z.; Lin, X.; Feng, Y. Important ecophysiological roles of non-dominant Actinobacteria in plant residue decomposition, especially in less fertile soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Bukar, M.; Sodipo, O.; Dawkins, K.; Ramirez, R.; Kaldapa, J.T.; Tarfa, M.; Esiobu, N. Microbiomes of Top and Sub-Layers of Semi-Arid Soils in North-Eastern Nigeria Are Rich in Firmicutes and Proteobacteria with Surprisingly High Diversity of Rare Species. Adv. Microbiol. 2019, 9, 102–118. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Shridhar, B.S. Review: Nitrogen fixing microorganisms. Int. J. Microbiol. Res. 2012, 3, 46–52. [Google Scholar]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Aislabie, J.; Deslippe, J.; Dymond, J. Soil microbes and their contribution to soil services. In Ecosystem Services in New Zealand: Conditions and Trends; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. [Google Scholar]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Stinca, A.; Ravo, M.; Marzaioli, R.; Marchese, G.; Cordella, A.; Rutigliano, F.A.; Esposito, A. Changes in Multi-Level Biodiversity and Soil Features in a Burned Beech Forest in the Southern Italian Coastal Mountain. Forests 2020, 11, 983. [Google Scholar] [CrossRef]
- Mohagheghi, A.; Grohmann, K.; Himmel, M.; Leighton, L.; Updegraff, D.M. Isolation and characterization of Acidothermus cellulolyticus gen. nov., sp. nov., a new genus of thermophilic, acidophilic, cellulolytic bacteria. Int. J. Syst. Evol. Microbiol. 1986, 36, 435–443. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; Gomez-Brandon, M.; Rousk, J.; Bååth, E. Adaptation of soil microbial communities to temperature: Comparison of fungi and bacteria in a laboratory experiment. Glob. Chang. Biol. 2009, 15, 2950–2957. [Google Scholar] [CrossRef]
- Stott, D.E.; Andrews, S.S.; Liebig, M.A.; Wienhold, B.J.; Karlen, D.L. Evaluation of β-Glucosidase Activity as a Soil Quality Indicator for the Soil Management Assessment Framework. Soil Sci. Am. J. 2010, 74, 107–119. [Google Scholar]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J.; Bååth, E. Soil microbial recolonisation after a fire in a Mediterranean forest. Biol. Fertil. Soils 2011, 47, 261–272. [Google Scholar] [CrossRef]
- Thirukkumaran, C.M.; Parkinson, D. Microbial respiration, biomass, metabolic quotient and litter decomposition in a lodgepole pine forest floor amended with nitrogen and phosphorous fertilizers. Soil Biol. Biochem. 2000, 32, 59–66. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, O.; Steinberger, Y. Effects of forest wildfire on soil microbial community activity and chemical components on a Temporal-seasonal scale. Plant Soil. 2012, 360, 243–257. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Monciardini, P.; Cavaletti, L.; Schumann, P.; Rohde, M.; Donadio, S. Conexibacter woesei gen. nov., sp. nov., a novel representative of a deep evolutionary line of descent within the class Actinobacteria. Int. J. Syst. Evol. Microbiol. 2003, 53, 569–576. [Google Scholar] [CrossRef]
- Seki, T.; Matsumoto, A.; Shimada, R.; Inahashi, Y.; Omura, S.; Takahashi, Y. Conexibacter arvalis sp. nov., isolated from a cultivated field soil sample. Int. J. Syst. Evol. Microbiol. 2012, 62, 2400–2404. [Google Scholar] [CrossRef]
- Talia, P.; Sede, S.M.; Campos, E.; Rorig, M.; Principi, D.; Tosto, D.; Hopp, H.E.; Grasso, D.; Cataldi, A. Biodiversity characterization of cellulolytic bacteria present on native Chaco soil by comparison of ribosomal RNA genes. Res. Microbiol. 2012, 163, 221–232. [Google Scholar] [CrossRef]
- Sanford, R.A.; Wagner, D.D.; Wu, Q.; Chee-Sanford, J.C.; Thomas, S.H.; Cruz-García, C.; Rodríguez, G.; Massol-Deyá, A.; Krishnani, K.K.; Ritalahti, K.M.; et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl. Acad. Sci. USA 2012, 109, 19709–19714. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Itoh, H.; Shiratori, Y.; Isobe, K.; Otsuka, S.; Senoo, K. Predominant but previously-overlooked prokaryotic drivers of reductive nitrogen transformation in paddy soils, revealed by metatranscriptomics. Microbes Environ. 2017, 32, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liao, Y.; Xu, Y.; Dang, Z.; Zhu, X.; Ji, G. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: A review. Bioresour. Technol. 2020, 314, 123759. [Google Scholar] [CrossRef] [PubMed]
- Onley, J.R.; Ahsan, S.; Sanford, R.A.; Löffler, F.E. Denitrification by Anaeromyxobacter dehalogenans, a common soil bacterium lacking the nitrite reductase genes nirS and nirK. Appl. Environ. Microbiol. 2017, 84, e01985-17. [Google Scholar] [CrossRef] [PubMed]
- Willms, I.M.; Rudolph, A.Y.; Göschel, I.; Bolz, S.H.; Schneider, D.; Penone, C.; Poehlein, A.; Schöning, I.; Nacke, H. Globally abundant ‘Candidatus Udaeobacter’ benefits from release of antibiotics in soil and potentially performs trace gas scavenging. Msphere 2020, 5, e00186-20. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.E.; Handley, K.M.; Carini, P.; Gilbert, J.A.; Fierer, N. Genome reduction in an abundant and ubiquitous soil bacterium ‘Candidatus Udaeobacter copiosus’. Nat. Microbiol. 2016, 2, 16198. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Bibi, A. Fungal cellulase; production and applications: Minireview. Int. J. Health Life-Sci. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Masepohl, B.; Forchhammer, K. Chapter 9—Regulatory cascades to express nitrogenases. In Biology of the Nitrogen Cycle; Elsevier: Amsterdam, The Netherlands, 2007; pp. 131–145. [Google Scholar]


| Variables | Preburning | Postburning | One Year after Burning |
|---|---|---|---|
| Soil moisture (%) | 45.1–48.4 a | 44.5–46.0 a | 49.0–55.3 a |
| Soil temperature (°C) | 25.7–26.5 a | 25.9–26.8 a | 25.2–26.1 a |
| Fire temperature in the litter layer (°C) | 415.5–469.5 | ||
| Soil Properties | Preburning | Postburning | One Year after Burning |
|---|---|---|---|
| pH (1:1) | 5.08 ± 0.03 a | 6.89 ± 0.16 b | 5.15 ± 0.01 a |
| Bulk density (g cm−1) | 1.42 ± 0.01 a | 1.40 ± 0.03 a | 1.41 ± 0.01 a |
| Organic matter (%) | 4.15 ± 0.05 a | 4.22 ± 0.10 a | 4.13 ± 0.03 a |
| Organic carbon (%) | 2.39 ± 0.03 a | 2.44 ± 0.07 a | 2.38 ± 0.04 a |
| CEC (meq 100 g−1) | 24.10 ± 0.68 a | 27.16 ± 0.96 a | 25.01 ± 0.51 a |
| ECe (dS m−1) | 2.45 ± 0.05 a | 5.24 ± 0.64 b | 3.11 ± 0.04 a |
| NH4N (mg kg−1) | 48.48 ± 1.59 a | 58.06 ± 2.69 b | 51.34 ± 1.88 a |
| NO3N (mg kg−1) | 55.22 ± 2.69 a | 19.90 ± 2.84 b | 57.59 ± 3.66 a |
| Total nitrogen (%) | 0.29 ± 0.01 a | 0.32 ± 0.03 b | 0.26 ± 0.03 a |
| Available P (mg kg−1) | 50.52 ± 1.24 a | 56.73 ± 3.28 b | 53.36 ± 2.11 a |
| Available K (mg kg−1) | 297.37 ± 19.35 a | 505.91 ± 13.10 b | 319.37 ± 21.33 a |
| Available Ca (mg kg−1) | 1766.81 ± 96.58 a | 2191.13 ± 65.86 b | 1898.81± 77.11 a |
| Available Mg (mg kg−1) | 288.67 ± 23.16 a | 365.73 ± 21.06 b | 312.67± 16.66 a |
| Sand (%) | 36.20 ± 2.01 a | 37.21 ± 1.98 a | 35.68 ± 2.33 a |
| Silt (%) | 36.19 ± 2.54 a | 35.29 ± 2.66 a | 34.91 ± 2.21 a |
| Clay (%) | 27.61 ± 1.88 a | 27.50 ± 2.69 a | 29.41 ± 1.73 a |
| Texture | Clay Loam | Clay Loam | Clay Loam |
| Site | Observed Richness | Chao-1 | Shannon | Simpson |
|---|---|---|---|---|
| Preburning | 1936.80 ± 203.24 b | 1954.39 ± 243.83 b | 7.03 ± 0.10 b | 0.9984 ± 0.0001 b |
| Postburning | 2114.60 ± 273.81 b | 2169.11 ± 315.25 b | 7.09 ± 0.12 b | 0.9984 ± 0.0002 b |
| One year after burning | 3761.40 ± 1203.85 a | 4176.56 ± 1547.81 a | 7.66 ± 0.30 a | 0.9991 ± 0.0002 a |
| Soil Properties | Correlation Coefficient | p-Value |
|---|---|---|
| pH | 0.0274 | 0.259 |
| Bulk density | −0.1338 | 0.975 |
| Organic matter | 0.2153 | 0.056 |
| Organic carbon | 0.2357 | 0.052 |
| Soil organic carbon | 0.1802 | 0.071 |
| CEC | −0.0739 | 0.712 |
| Ece | −0.0194 | 0.452 |
| NH4N | −0.1115 | 0.813 |
| NO3N | 0.0291 | 0.283 |
| Total nitrogen | 0.3780 | 0.010 * |
| Soil total nitrogen | 0.4268 | 0.006 * |
| Available P | −0.1008 | 0.812 |
| Available K | 0.0292 | 0.260 |
| Available Ca | −0.0704 | 0.758 |
| Available Mg | −0.0252 | 0.510 |
| Sand | 0.3377 | 0.011 * |
| Silt | 0.1602 | 0.094 |
| Clay | 0.7666 | 0.001 * |
| Soil moisture | 0.7223 | 0.001 * |
| Air temperature | 0.1176 | 0.142 |
| Soil temperature | 0.2259 | 0.028 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunrat, N.; Sereenonchai, S.; Sansupa, C.; Kongsurakan, P.; Hatano, R. Effect of Rice Straw and Stubble Burning on Soil Physicochemical Properties and Bacterial Communities in Central Thailand. Biology 2023, 12, 501. https://doi.org/10.3390/biology12040501
Arunrat N, Sereenonchai S, Sansupa C, Kongsurakan P, Hatano R. Effect of Rice Straw and Stubble Burning on Soil Physicochemical Properties and Bacterial Communities in Central Thailand. Biology. 2023; 12(4):501. https://doi.org/10.3390/biology12040501
Chicago/Turabian StyleArunrat, Noppol, Sukanya Sereenonchai, Chakriya Sansupa, Praeploy Kongsurakan, and Ryusuke Hatano. 2023. "Effect of Rice Straw and Stubble Burning on Soil Physicochemical Properties and Bacterial Communities in Central Thailand" Biology 12, no. 4: 501. https://doi.org/10.3390/biology12040501
APA StyleArunrat, N., Sereenonchai, S., Sansupa, C., Kongsurakan, P., & Hatano, R. (2023). Effect of Rice Straw and Stubble Burning on Soil Physicochemical Properties and Bacterial Communities in Central Thailand. Biology, 12(4), 501. https://doi.org/10.3390/biology12040501











