Simple Summary
Autonomic ocular control is mediated by sympathetic, parasympathetic, and primary trigeminal afferent nerve fibers. Intrinsic choroidal neurons (ICN) contribute to this complex neuronal network. Vasoactive intestinal peptide (VIP), a major transmitter of ICN, mediates choroidal vasodilation and, thus, potentially choroidal thickness and intraocular pressure (IOP). Therefore, it was the aim of the present study to investigate the choroidal VIP level (VIPchor) in the presence of an increased atmospheric pressure in a chicken model. Chicken choroidal whole mounts were exposed to ambient pressure (n = 20) and 40 mmHg (n = 20) in a PC-controlled, open chamber system for 24 and 72 h, respectively. VIPchor was significantly increased after exposure to 40 mmHg compared to exposure to ambient pressure. This increase of the VIPchor level, representing the intracellular choroidal VIP level, might argue for a retention of VIP within the neurons, consequently decreasing vasodilatation and choroid thickness.
Abstract
Purpose: Autonomic control is important in maintaining ocular integrity. As recent data suggested that intrinsic choroidal neurons (ICN), an intrinsic choroidal autonomic control, may regulate choroidal thickening via release of the vasodilative vasoactive intestinal peptide (VIP), it was the aim of the study to investigate the level of choroidal VIP (VIPchor) in the presence of an increased atmospheric pressure in a chicken model. Methods: Chicken choroidal whole mounts were exposed to ambient pressure (n = 20) and 40 mm Hg (n = 20) in a PC-controlled, open chamber system for 24 and 72 h, respectively. The VIP concentration was analyzed by ELISA, and the total protein concentration was measured by the BCA assay. Statistical analysis was done using an unpaired two-tailed t-test. Results: The pressurization systems enabled choroidal whole mount pressurization (40 mm Hg) with humidifying, pressure, temperature, and gas exchange. Overall, the VIPchor level concentration was significantly increased at 40 mmHg compared to the ambient pressure (30.09 ± 7.18 pg vs. 20.69 ± 3.24 pg; p < 0.0001). Subgroup analysis yielded a significantly increased VIPchor level at 40 mmHg compared to the ambient pressure after 24 h (28.42 ± 6.03 pg vs. 20.76 ± 4.06 pg; p = 0.005) and 72 h (31.77 ± 7.82 pg vs. 20.61 ± 2.12 pg; p = 0.002), respectively. The VIPchor elevation at 40 mm Hg ranged between 1.37- (24 h) and 1.54-fold (72 h) compared to the ambient pressure. No difference was observed between the VIPchor level at 24 h and 72 h (p > 0.05). Conclusions: The increase of the total choroidal VIP level, representing the intracellular VIP content, in the presence of an increased ambient pressure argues for a retention of VIP within the neurons, decreasing both vasodilatation and, consequently, choroid thickness. This finding might be a passive or even active function of ICN in the regulation of choroidal thickness, ocular integrity and IOP.
1. Introduction
Autonomic control of the eye is mediated by a complex neuronal meshwork of sympathetic [1] and parasympathetic fibers [2,3,4] and primary afferent nerve fibers of the trigeminal nerve [5]. This neuronal network regulates accommodation, pupil size and ocular blood flow [6,7,8,9]. Furthermore, intraocular pressure (IOP) and aqueous humor (AH) production are controlled by fine autonomic innervation [9,10]. Intrinsic choroidal neurons (ICN) are assumed to be a fourth important autonomic component of ocular neuronal regulation [11].
ICN were described in 1859 for the first time [12]. These neurons were localized within the choroid, mainly co-expressing the transmitters, vasoactive intestinal polypeptide (VIP) and nitric oxide (NO) [13,14,15]. A topographic analysis yielded a temporal and suprachoroidal accumulation [5,13,16,17,18]. ICN are assumed to contribute to the complex neuronal autonomic network within the choroid analog to the myenteric plexus (Auerbach Plexus) [19]. Interestingly, only humans and species with high visual acuity (i.e., birds) show large amounts of ICN [20,21]. As classical animal models (e.g., rat and mouse) lack or even show low numbers of ICN [14,22,23], the avian model seems to be predisposed toward analysis of ICN [5,21,24]. The function of ICN has been elusive until now. Close neuronal contacts of ICN were observed for blood vessels, non-vascular smooth muscle cells (NVSMC) and choroidal melanocytes [20,25,26,27]. It is supposed that ICN are involved in ‘choroidal accommodation’ and the regulation of choroidal blood flow [28,29,30]. Considering this topographic aspect and their content of transmitters, ICN might regulate vessel diameters (vasodilation) and the consecutive choroidal thickness. Recent data suggest that the regulation of choroidal thickness might attribute to IOP control [16]. A circadian analysis of VIP concentration yielded an increased level in the evening compared to the morning [16]. This VIP release over the night is parallel with the circadian changes of the choroid (i.e., increased during the night) [31] and antithetical changes of IOP (i.e., lower at night) [32]. It can be assumed that this nocturnal VIP release contributes to the regulation of ocular integrity by choroidal thickening as compensation of a lower IOP. Circadian changes of IOP were shown in several studies throughout different species, including humans [33,34,35], birds [32], smaller mammals [36] and rodents [37].
IOP regulation is complex and still under investigation. The level of IOP is the steady state of the production in the nonpigmented ciliary body and outflow of the aqueous humor (AH, trabecular meshwork and suprachoroidal) [38]. As (I) circadian changes of the IOP, choroidal thickness and level of VIP were known, and (II) a link between the level of IOP and VIP was supposed, it was the aim of the present study to investigate the level of choroidal VIP (VIPchor) dependent on different levels of ambient pressure in an in vitro avian model.
2. Materials and Methods
2.1. Tissue
Forty eyes of 20 chickens (Gallus domesticus, type Ross, aged 6–8 weeks) were enucleated after slaughtering, which was performed for commercial purposes at a local farm.
All actions concerning the animals were performed according to the European and national legislation for animal welfare [39,40]. The animals were slaughtered between 8 a.m. and 10 a.m. to avoid circadian fluctuations in the VIP concentration within the obtained tissues. Choroidal whole mounts were prepared in DMEM/Ham’s F12 medium.
2.2. Preparation of Choroidal Whole Mounts
Choroidal whole mounts were prepared as described earlier [16]. Briefly, the eyeballs were opened along the ora serrata, and the retinal pigment epithelium, retina and vitreous body were removed by blunt tweezers. If any retinal pigment epithelium remained, this tissue was removed carefully by cotton swabs. Choroidal whole mounts were prepared after separation of the choroids from the sclera and pecten oculi using tweezers and scissors to cut the connecting structures. The tissues were rinsed in phosphate-buffered saline (PBS; PAN Biotech, Aiden Bach, Germany). All choroids were transferred to a 6-well plate containing 8 mL of culture medium (DMEM/HAM’s F12 with 8 g/L glucose and 1× N2 supplement; Thermo Fisher Scientific, Schwerte, Germany) and incubated at 37 °C.
2.3. Pressurization of Choroidal Whole Mounts
The experiments were done subdividing the choroidal whole mounts into two groups: (1) exposed to ambient pressure (n = 20, pressure group) and (2) exposed to a pressure of twice the upper range of a regulated human IOP (i.e., 40 mm Hg, n = 20, control). Considering a time-dependent, yet not a circadian, effect, experiments with choroidal whole mounts were performed for 24 h (n = 10) and 72 h (n = 10), respectively. Keeping a constant temperature of 37 °C, the experiments were performed within an incubator. After 24 h, the culture medium was replaced. Considering inter-individual differences in tissue compositions, the choroidal whole mounts of the left eye were assigned to the pressure group and that of the right eye to the control group.
2.4. Enzyme-Linked Immunosorbent Assay
After the incubation, the choroidal whole mounts were homogenized by the Precellys 24 homogenizer and lysing kit (Bertin; Frankfurt, Germany) in PBS with 3 cycles of 2 × 30 s at 5000 rpm. The choroidal homogenates were centrifuged at 14,000 rpm (21,913× g) for 10 min (4 °C), and the supernatant was frozen immediately at −80 °C. VIP concentrations in the supernatant were analyzed using an enzyme-linked immunosorbent assay, namely the Chicken VIP Peptide ELISA Kit (Chicken VIP Peptides ELISA Kit, WUHAN EIAAB SCIENCE CO., LTD, Wuhan, China) according to the manufacturer’s protocol. The total protein concentration was measured by the Micro BCA Protein Assay (Thermo Fisher Scientific, Schwerte, Germany). The VIP and total protein levels were measured using the MULTISCAN SPECTRUM and Skanlt software V 2.2 (both by Thermo Fisher Scientific, Schwerte, Germany).
2.5. Statistical Analysis
Normal Gaussian distribution was tested using the Kolmogorov–Smirnov test. Group comparisons were performed using an unpaired two-tailed t-test. A p-value of <0.05 was considered statistically significant. Choroidal VIP concentrations were presented as the mean ± standard deviation. Statistical power analysis was performed to determine the sample size needed for significant data and yielded a minimum of 6 specimen (alpha: 0.05, power: 90%).
3. Results
3.1. Pressurization System
The construction of the pressurization system was done within the scope of contract work at the Department of Process Machinery and Plant Engineering Friedrich-Alexander-University-Erlangen-Nürnberg (FAU). The pressurization system consists of four parts (Figure 1 and Figure 2e). The chamber itself houses four 6-well-plates; a gas container (comprising a gas mixture of 74% nitrogen, 21% oxygen and 5% carbon dioxide); the control unit that regulates the flow of gas and a computer. The specially designed software operates the system and documents the pressure and the temperature every minute.
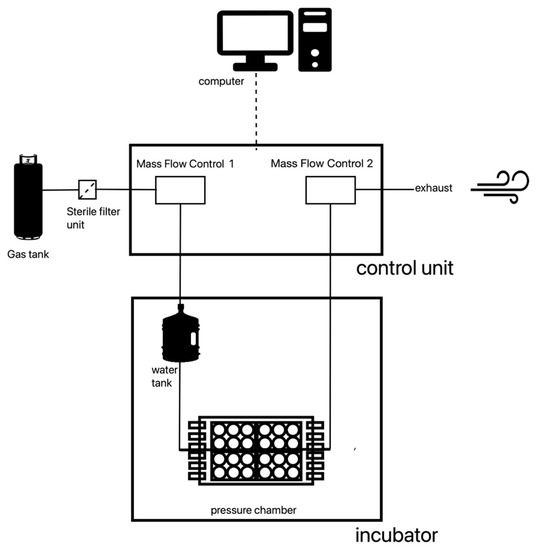
Figure 1.
Pressurization system—schematic illustration.

Figure 2.
Pressurization system. (a) Pressurization chamber with lid; clasps closed. (b) Pressurization chamber without lid; clasps open. (c) Pressurization chamber control unit. (d) Pressurization chamber control unit; German translations: Druck = pressure, Durchfluss = flow. (e) Pressurization chamber inside of an incubator.
The gas is released from its container through a two-step pressure reducer. Before entering the chamber, the gas passes through a sterile filter, the first MFC vent (mass flow control) and a water tank. Then, the humidified and warmed gas enters the chamber. Immediately after the chamber, the gas passes a second MFC vent. The outflow of the second MFC vent is controlled by the computer. Through this outflow control, the pressure of 40 mm Hg is maintained consistently over an extended period. To ensure that the fluctuations in pressure are at a bare minimum, a computer program, which supervised the experiment, recorded the pressure in the system every minute. If a deviation of 1 mm Hg over a period longer than one minute was recorded, this section of the experiment was repeated. The pressure chamber is placed in an incubator to maintain a constant temperature of 37 °C.
3.2. VIPchor Concentration
The total VIPchor concentration was significantly increased at 40 mm Hg compared to the ambient pressure (p < 0.0001; Table 1). We observed the overall VIPchor to be increased 1.45-fold at 40 mmHg compared to the ambient pressure (Table 1).

Table 1.
VIPchor concentration after incubation at the ambient pressure (control) and 40 mm Hg for 24 h and 72 h: mean VIPchor level (pg/ug total protein) and standard deviation; n—sample size; Chicken VIP Peptides ELISA Kit, WUHAN EIAAB SCIENCE CO., LTD, Wuhan, China, was used.
The subgroup analysis yielded a significantly increased mean VIPchor concentration after 24 h at 40 mmHg (28.42 ± 6.03 pg VIP/µg total protein) compared to the ambient pressure (20.76 ± 4.06 pg VIP/µg total protein, p < 0.005). In addition, the mean VIPchor concentration was significantly increased after 72 h of pressure incubation at 40 mmHg (31.77 ± 7.82 pg VIP/µg total protein) compared to the ambient pressure (20.61 ± 2.12 pg VIP/ug total protein; p = 0.002). The VIP increase was 1.37-fold (after 24 h) and 1.54-fold (after 72 h) at 40 mm Hg compared to the ambient pressure (Table 1). The VIPchor concentration (72 h) was not significantly different compared to the VIPchor level (24 h) in the presence of 40 mm Hg (p > 0.05).
4. Discussion
Intraocular pressure is known to fluctuate over a 24-h period [32,34,41]. Although fluctuations of a few mm Hg are considered normal [32], the maintenance of a constant IOP is paramount to sustain ocular homeostasis, function and efficiency [38,42]. These circadian fluctuations of IOP have been described in humans [33,34] and throughout different animals [36,37]. In young chickens, IOP tends to decrease during the night and reach its peak during the day [32,43]. Experimental studies showed that choroidal thickness [31,44] and the release of VIP by ICN [16] follow the circadian rhythm in a chicken model.
Considering VIP as a potent vasodilator, consecutively increasing the choroidal blood flow [45,46,47], the aim of this study was to investigate the level of choroidal VIP in the context of different ambient pressures in an in vitro chicken model. Exposing choroidal whole mounts to a pressure of 40 mm Hg, we observed a significantly increased overall mean VIPchor concentration compared to the ambient pressure (1.37-fold). This increase of the mean VIPchor concentration lasted over the whole experimental observation period of 72 h compared to the ambient pressure (1.54-fold).
A multitude of laboratory devices have been developed for the treatment of cells and different human and animal tissues with increased ambient pressure in order to investigate their response to hypertensive conditions [48]. Commercially available systems providing pressure, temperature and gas exchange control are often not in the budget for small laboratories. A simple and inexpensive way to exert pressure stress is a pressurized chamber designed as a closed system [49]. However, closed systems do not provide gas exchange, leading to hypoxic conditions and pH shifts in culture mediums [50]. The design of our self-constructed pressurization system provides pressure control and a constant temperature, humidification, and gas exchange. Therefore, this device may be an affordable alternative to high-priced commercial systems.
Chicken models have already been used in a variety of different ophthalmological research topics, such as corneas disease, retinopathies, ophthalmological tumors, or glaucoma [51,52]. Especially, light-induced glaucoma (LIG) has been studied in chicken models [53,54,55,56]. Of interest, the use of avian models in the research of ICN was first established by Bergua et al. [5] by introducing the duck Carina moschata. This experimental study provided the first evidence of similar numbers of ICN in avian choroids compared to humans.
Subsequent studies yielded similar amounts of ICN in different avian models, yet not in rats, mice, or rabbits [5,11,14,20]. Several homologies between human and chicken ICN predisposed chicken as the animal model in the research of ICN: the basic anatomic characteristics (i.e., shape and choroidal localization); neuronal synaptic interactions (sympathetic, parasympathetic, trigeminal neurons and inter-neuronal ICN interactions); major transmitters (VIP and NO) [13,14] and targets (blood vessels and NVSMC) [11,20,24,25,57,58].
First described by Müller in 1959 [12], ICN contribute to the complex autonomic ocular control system. Parasympathetic input is mediated by the ciliary [2,5] and the pterygopalatine ganglia (PPG) [3,58], while the superior ciliary ganglion (SCG) contributes to sympathetic choroidal innervation [1,4]. In addition, the primary afferent nerve fibers of the trigeminal ganglion provide neuronal input to this autonomic regulation [5]. Similar to other organs (e.g., the gut) [59], the choroid seems to have its own intrinsic neurons [16,30,60], yet, their specific function is still elusive. As their major neurotransmitters (VIP and NO) are known to be vasoactive agents [15,45,46], a regulation of the choroidal blood flow [20] and, thus, choroidal thickness [32,61] and, potentially, the IOP can be assumed [6,16,20,47].
VIP is of special interest, as it was observed in the PPG and the ICN, providing a special form of intrinsic and extrinsic regulation [28]. VIP, first described in 1970 [62], was characterized in the porcine intestine as a 28 amino acid residue polypeptide with systemic vasodilation properties. VIP is attributed to the PACAP (pituitary adenylate cyclase-activating peptide) family [63], with its gene completely analyzed in 1985 by Tsukada [64]. Showing strong structural similarities to glucagon and secretin, VIP is derived from a precursor protein, namely the Pre-Pro VIP [65]. The Pre-Pro VIP gene is found on chromosome 6 in humans (6q25.2), has a length of 9 kb and is divided by six introns [66]. These introns code for the known cleavage products within the Pre-Pro-VIP, such as the 5’untranslated region and PHM (exon 4). The VIP itself is encoded on exon 5. Due to the presence of an intron within the 3’untranslated region, it is also assumed that the introns of the gene divide the precursor peptide into functional zones [64,67]. PHM is considered to be the human counterpart to PHI-27 [62]. PHI-27, being expressed in the porcine intestines [68], acts as an inhibitor of the binding of VIP to its receptors [69]. VIP can activate three G-protein-coupled receptors (GPCR; VPAC1, VPAC2 and PAC1). VPAC1 and VPAC2 induce the cAMP-PKA pathway, whereas PAC1 mediates their functional activity via PKA and PLC [67,70].
Overall, VIP is known to be a potent neurotransmitter further involved in the regulation of circadian rhythms [71], hormones [72,73], the intestines [74,75] and cancer [76,77,78]. As these GPCRs were observed in different immune cells (e.g., macrophages, CD4+ T cells and CD8+ T cells lymphocytes) [79,80,81], an involvement in the immune response can be assumed.
Focusing on the function of VIP within the choroid, smooth muscle relaxation and vasodilation are characteristics predisposing a regulatory function within the choroidal blood flow [13,14,15]. VIP-positive neurons were observed to form a perivascular plexus around arteries in ducks [5]. The presence of a single base membrane and a full glia coating provide morphological findings for an additional mechanosensory function [16,25]. Furthermore, ICN were observed in regions underlying retinal areas with high visual acuity (subfoveal) [14,21]. It is assumed that ICN contribute to choroidal accommodation by their quick intrinsic plexus with a dual function via a passive (mechanosensory) and an active (vasodilative) component [20,82,83]. Regulatory interactions between nerve cells and the vasculature are also known in the central nervous system, as microglia were shown to regulate the cerebral blood flow (CBF) through PANX1-P2Y12 coupling and contribute to the “vascular reactivity” of the CBF [84].
Considering these different aspects and a correlation between the blood flow and IOP [6], we assume there is a link between the VIP, choroidal vasodilation, blood flow and IOP. The choroid is a dense vascular network responsible for about 85% of the ocular vascular supply [85,86].
Clinical studies offer that choroidal thickening might compensate (passive) or even influence (active) IOP [86,87,88,89,90]; thus, a link between IOP (i.e., ambient pressure in in vitro experiments) and choroidal thickness (i.e., choroidal VIP as correlated for vasodilative function) can be assumed. Patients with an increased IOP, namely ocular hypertension, showed a choroidal thinning [91,92]. Decreasing the IOP by a surgical method (trabeculectomy) resulted in an increase of subfoveal and peripapillary choroidal thickness in patients with increased IOP and neurodegeneration of the optic nerve head (glaucoma) [90,93,94,95,96]. Interestingly, subfoveal choroidal thickness was observed to decrease significantly in the presence of an acute IOP increase (time range 2 h) in healthy humans, probably mediated by a fast molecular mechanism [88].
5. Limitations of the Study
The present study is not without limitations. The present experimental set-up was performed as an in vitro experiment in a chicken model. Thus, we used atmospheric pressure (as correlated for IOP) and an animal model for the analysis of VIP in ICN research. This set-up does not claim to represent the complex dynamic physiological conditions (e.g., blood flow and hormones) within an in vivo chicken or even human eye. Further studies are necessary to investigate the clinical implications of VIP in regulating IOP and choroidal thickness.
6. Conclusions
In the present study, we observed an increase in the total choroidal VIP level, representing the intracellular VIP content, in the presence of an increased ambient pressure. Thus, we assume that VIP is retained within the neurons, decreasing both vasodilation and, consequently, the choroid thickness. This finding might be a passive or even active function of ICN in the regulation of choroidal thickness, ocular integrity and IOP.
Author Contributions
Conceptualization, B.H., E.P. and M.Z.; methodology, B.H., E.P. and M.Z.; software, M.Z.; validation, B.H.; formal analysis, M.Z. and E.P.; investigation, E.P.; resources, S.K. and U.S.-S.; data curation, M.Z. and E.P.; writing—original draft preparation, E.P.; writing—review and editing, B.H., E.P., M.Z., U.S.-S. and S.K.; visualization, E.P. and M.Z.; supervision, B.H. and A.B. and project administration, B.H. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Ethical approval was not needed, as the animal organs were purchased from a local poultry farm that breeds and slaughters chickens for commercial purposes.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Institute of Anatomy at the Friedrich-Alexander-University-Erlangen-Nürnberg (FAU) and, namely, its former chairwoman Stefanie Kuerten, as well as laboratory assistants Anita Hecht und Hedwig Simowsky, for their close collaboration in the preparational part of the study. To the Department of Ophthalmology, we give additional thanks to Edith Monczak for administrative support and the entire lab team for being there for inquiries. The present work was performed in fulfillment of the requirements for obtaining a Dr. med. degree for Evgeny Privalov. The Department of Ophthalmology is part of the Universität of Erlangen-Nürnberg, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Germany.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guglielmone, R.; Cantino, D. Autonomic innervation of the ocular choroid membrane in the chicken: A fluorescence-histochemical and electron-microscopic study. Cell Tissue Res. 1982, 222, 417–431. [Google Scholar] [CrossRef]
- Bill, A. Effects of some neuropeptides on the uvea. Exp. Eye Res. 1991, 53, 3–11. [Google Scholar] [CrossRef]
- Cuthbertson, S.; Jackson, B.; Toledo, C.; Fitzgerald, M.E.; Shih, Y.F.; Zagvazdin, Y.; Reiner, A. Innervation of orbital and choroidal blood vessels by the pterygopalatine ganglion in pigeons. J. Comp. Neurol. 1997, 386, 422–442. [Google Scholar] [CrossRef]
- Kirby, M.L.; Diab, I.M.; Mattio, T.G. Development of adrenergic innervation of the iris and fluorescent ganglion cells in the choroid of the chick eye. Anat. Rec. 1978, 191, 311–319. [Google Scholar] [CrossRef]
- Bergua, A.; Mayer, B.; Neuhuber, W.L. Nitrergic and VIPergic neurons in the choroid and ciliary ganglion of the duck Anis carina. Anat. Embryol. 1996, 193, 239–248. [Google Scholar] [CrossRef]
- Bill, A.; Sperber, G.O. Control of retinal and choroidal blood flow. Eye 1990, 4 Pt 2, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Troger, J.; Kieselbach, G.; Teuchner, B.; Kralinger, M.; Nguyen, Q.A.; Haas, G.; Yayan, J.; Göttinger, W.; Schmid, E. Peptidergic nerves in the eye, their source and potential pathophysiological relevance. Brain Res. Rev. 2007, 53, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Ten Tusscher, M.P.; Beckers, H.J.; Vrensen, G.F.; Klooster, J. Peripheral neural circuits regulating IOP? A review of its anatomical backbone. Doc. Ophthalmol. 1994, 87, 291–313. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic control of the eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef]
- Lapalus, P.; Elena, P.P. Neurotransmitters and intraocular pressure. Fundam. Clin. Pharmacol. 1988, 2, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Stübinger, K.; Brehmer, A.; Neuhuber, W.L.; Reitsamer, H.; Nickla, D.; Schrödl, F. Intrinsic choroidal neurons in the chicken eye: Chemical coding and synaptic input. Histochem. Cell. Biol. 2010, 134, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Müller, H. Ueber glatte Muskeln und Nervengeflechte der Choroidea im menschlichen Auge. Verh. Phys.-Med. Ges. Würzburg 1859, 10, 179–192. [Google Scholar]
- Miller, A.S.; Coster, D.J.; Costa, M.; Furness, J.B. Vasoactive intestinal polypeptide immunoreactive nerve fibres in the human eye. Aust. J. Ophthalmol. 1983, 11, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Flügel, C.; Tamm, E.R.; Mayer, B.; Lütjen-Drecoll, E. Species differences in choroidal vasodilative innervation: Evidence for specific intrinsic nitrergic and VIP-positive neurons in the human eye. Investig. Ophthalmol. Vis. Sci. 1994, 35, 592–599. [Google Scholar]
- Bergua, A.; Junemann, A.; Naumann, G.O. NADPH-D reactive choroid ganglion cells in the human. Klin. Monbl. Augenheilkd. 1993, 203, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Jessberger, C.; Hermann, F.; Zenkel, M.; Kaser-Eichberger, A.; Bergua, A.; Junemann, A.G.; Schrodl, F.; Neuhuber, W. VIP changes during daytime in chicken intrinsic choroidal neurons. Exp. Eye Res. 2018, 170, 8–12. [Google Scholar] [CrossRef]
- Schrödl, F.; Brehmer, A.; Neuhuber, W.L. Intrinsic choroidal neurons in the duck eye express galanin. J. Comp. Neurol. 2000, 425, 24–33. [Google Scholar] [CrossRef]
- Triviño, A.; De Hoz, R.; Salazar, J.J.; Ramírez, A.I.; Rojas, B.; Ramírez, J.M. Distribution and organization of the nerve fiber and ganglion cells of the human choroid. Anat. Embryol. 2002, 205, 417–430. [Google Scholar] [CrossRef]
- Kolmer, W.; Lauber, H. Die Aderhaut (Chorioidea). In Haut Und Sinnesorgane: Auge; Kolmer, W., Lauber, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1936; pp. 91–134. [Google Scholar] [CrossRef]
- Schroedl, F. Neuropeptides in the Eye; Research Signpost: Thiruvananthapuram, India, 2009. [Google Scholar]
- Schroedl, F.; Egle De Stefano, M.; Reese, S.; Brehmer, A.; Neuhuber, W.L. Comparative anatomy of nitrergic intrinsic choroidal neurons (ICN) in various avian species. Exp. Eye Res. 2004, 78, 187–196. [Google Scholar] [CrossRef]
- Beatie, J.C.; Stilwell, D.L., Jr. Innervation of the eye. Anat. Rec. 1961, 141, 45–61. [Google Scholar] [CrossRef]
- Flügel-Koch, C.; Kaufman, P.; Lütjen-Drecoll, E. Association of a choroidal ganglion cell plexus with the fovea centralis. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4268–4272. [Google Scholar]
- Schrödl, F.; Schweigert, M.; Brehmer, A.; Neuhuber, W.L. Intrinsic Neurons in the Duck Choroid are Contacted by CGRP-Immunoreactive Nerve Fibres: Evidence for a Local Pre-central Reflex Arc in the Eye. Exp. Eye Res. 2001, 72, 137–146. [Google Scholar] [CrossRef]
- May, C.A.; Neuhuber, W.; Lütjen-Drecoll, E. Immunohistochemical classification and functional morphology of human choroidal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 361–367. [Google Scholar] [CrossRef]
- Matsusaka, T. Cytoarchitecture of choroidal melanocytes. Exp. Eye Res. 1982, 35, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.A. Vasoactive intestinal polypeptide and the ocular innervation. Investig. Ophthalmol. Vis. Sci. 1986, 27, 951–957. [Google Scholar]
- Lütjen-Drecoll, E. Choroidal innervation in primate eyes. Exp. Eye Res. 2006, 82, 357–361. [Google Scholar] [CrossRef]
- Flügel-Koch, C.; May, C.A.; Lütjen-Drecoll, E. Presence of a contractile cell network in the human choroid. Ophthalmologica 1996, 210, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wildsoet, C.; Wallman, J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp. Eye Res. 1998, 66, 163–181. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wildsoet, C.; Wallman, J. A The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Exp. Eye Res. 1998, 66, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Sit, A.J.; Weinreb, R.N. Variation of 24-hour intraocular pressure in healthy individuals: Right eye versus left eye. Ophthalmology 2005, 112, 1670–1675. [Google Scholar] [CrossRef]
- Henkind, P.; Leitman, M.; Weitzman, E. The diurnal curve in man: New observations. Investig. Ophthalmol. 1973, 12, 705–707. [Google Scholar]
- Frampton, P.; Da Rin, D.; Brown, B. Diurnal variation of intraocular pressure and the overriding effects of sleep. Am. J. Optom. Physiol. Opt. 1987, 64, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.G.; Johnson, E.C.; Morrison, J.C. Circadian rhythm of intraocular pressure in the rat. Curr. Eye Res. 1996, 15, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Del Sole, M.J.; Sande, P.H.; Bernades, J.M.; Aba, M.A.; Rosenstein, R.E. Circadian rhythm of intraocular pressure in cats. Vet. Ophthalmol. 2007, 10, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B. Inraocular Pressure Variations; A Risk Factor for Glaucoma. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2017; Volume 105. [Google Scholar]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; Miranda Chueca, M.; et al. Slaughter of animals: Poultry. Efsa J. 2019, 17, e05849. [Google Scholar] [CrossRef]
- Federal Ministry of Food, Agriculture and Consumer Protection. Animal Welfare Act (Germany). 1972. Available online: https://www.animallaw.info/statute/germany-cruelty-german-animal-welfare-act (accessed on 20 March 2022).
- Terauchi, R.; Ogawa, S.; Noro, T.; Ito, K.; Kato, T.; Tatemichi, M.; Nakano, T. Seasonal Fluctuation in intraocular pressure and retinal nerve fiber layer thinning in primary open-angle glaucoma. Ophthalmol. Glaucoma 2021, 4, 373–381. [Google Scholar] [CrossRef]
- Acott, T.S.; Kelley, M.J.; Keller, K.E.; Vranka, J.A.; Abu-Hassan, D.W.; Li, X.; Aga, M.; Bradley, J.M. Intraocular pressure homeostasis: Maintaining balance in a high-pressure environment. J. Ocul. Pharmacol. Ther. 2014, 30, 94–101. [Google Scholar] [CrossRef]
- Wahl, C.; Li, T.; Howland, H.C. Intraocular pressure fluctuations of growing chick eyes are suppressed in constant light conditions. Exp. Eye Res. 2016, 148, 52–54. [Google Scholar] [CrossRef]
- Brown, J.S.; Flitcroft, D.I.; Ying, G.S.; Francis, E.L.; Schmid, G.F.; Quinn, G.E.; Stone, R.A. In vivo human choroidal thickness measurements: Evidence for diurnal fluctuations. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5–12. [Google Scholar] [CrossRef]
- Henning, R.J.; Sawmiller, D.R. Vasoactive intestinal peptide: Cardiovascular effects. Cardiovasc. Res. 2001, 49, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef]
- Nilsson, S.F.; Bill, A. Vasoactive intestinal polypeptide (VIP): Effects in the eye and on regional blood flows. Acta Physiol. Scand. 1984, 121, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.A.; Zambrano, S.; Anumolu, P.; Allen, A.C.; Sonoqui, L.; Moreno, M.R. Device-based in vitro techniques for mechanical stimulation of vascular cells: A review. J. Biomech. Eng. 2015, 137, 040801. [Google Scholar] [CrossRef]
- Brown, T.D. Techniques for mechanical stimulation of cells in vitro: A review. J. Biomech. 2000, 33, 3–14. [Google Scholar] [CrossRef]
- Hasel, C.; Dürr, S.; Brüderlein, S.; Melzner, I.; Möller, P. A cell-culture system for long-term maintenance of elevated hydrostatic pressure with the option of additional tension. J. Biomech. 2002, 35, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Wisely, C.E.; Sayed, J.A.; Tamez, H.; Zelinka, C.; Abdel-Rahman, M.H.; Fischer, A.J.; Cebulla, C.M. The chick eye in vision research: An excellent model for the study of ocular disease. Prog. Retin. Eye Res. 2017, 61, 72–97. [Google Scholar] [CrossRef]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef]
- Jensen, L.S.; Matson, W.E. Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science 1957, 125, 741. [Google Scholar] [CrossRef]
- Lauber, J.K.; Shutze Jv Mcginnis, J. Effects of exposure to continuous light on the eye of the growing chick. Proc. Soc. Exp. Biol. Med. 1961, 106, 871–872. [Google Scholar] [CrossRef]
- Smith, M.E.; Becker, B.; Podos, S. Light-induced angle-closure glaucoma in the domestic fowl. Investig. Ophthalmol. 1969, 8, 213–221. [Google Scholar]
- Kinnear, A.; Lauber, J.K.; Boyd, T.A. Genesis of light-induced avian glaucoma. Investig. Ophthalmol. 1974, 13, 872–875. [Google Scholar]
- Schrödl, F.; Tines, R.; Brehmer, A.; Neuhuber, W.L. Intrinsic choroidal neurons in the duck eye receive sympathetic input: Anatomical evidence for adrenergic modulation of nitrergic functions in the choroid. Cell Tissue Res. 2001, 304, 175–184. [Google Scholar] [CrossRef]
- Neuhuber, W.; Schrödl, F. Autonomic control of the eye and the iris. Auton. Neurosci. 2011, 165, 67–79. [Google Scholar] [CrossRef]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Schrödl, F.; De Laet, A.; Tassignon, M.J.; Van Bogaert, P.P.; Brehmer, A.; Neuhuber, W.L.; Timmermans, J.P. Intrinsic choroidal neurons in the human eye: Projections, targets, and basic electrophysiological data. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3705–3712. [Google Scholar] [CrossRef]
- Papastergiou, G.I.; Schmid, G.F.; Riva, C.E.; Mendel, M.J.; Stone, R.A.; Laties, A.M. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp. Eye Res. 1998, 66, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Said, S.I.; Mutt, V. Polypeptide with broad biological activity: Isolation from small intestine. Science 1970, 169, 1217–1218. [Google Scholar] [CrossRef]
- Takei, Y. Chapter 18—Secretin (Pituitary Adenylate Cyclase-Activating Polypeptide) Family. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, Chile, 2016; pp. 140–141, e18-1–e18-2. [Google Scholar] [CrossRef]
- Tsukada, T.; Horovitch, S.J.; Montminy, M.R.; Mandel, G.; Goodman, R.H. Structure of the human vasoactive intestinal polypeptide gene. DNA 1985, 4, 293–300. [Google Scholar] [CrossRef]
- Itoh, N.; Obata, K.; Yanaihara, N.; Okamoto, H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature 1983, 304, 547–549. [Google Scholar] [CrossRef]
- Fahrenkrug, J. VIP and PACAP. Results Probl. Cell. Differ. 2010, 50, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Fujimori, N.; Ito, T.; Nakamura, T.; Oono, T.; Nakamura, K.; Suzuki, K.; Jensen, R.; Takayanagi, R. Vasoactive Intestinal Peptide (VIP) and VIP Receptors-Elucidation of Structure and Function for Therapeutic Applications. Int. J. Clin. Med. 2011, 2, 500–508. [Google Scholar] [CrossRef]
- Tatemoto, K.; Mutt, V. Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon--secretin family. Proc. Natl. Acad. Sci. USA 1981, 78, 6603–6607. [Google Scholar] [CrossRef] [PubMed]
- Bataille, D.; Gespach, C.; Laburthe, M.; Amiranoff, B.; Tatemoto, K.; Vauclin, N.; Mutt, V.; Rosselin, G. Porcine peptide having N-terminal histidine and C-terminal isoleucine amide (PHI). FEBS Lett. 1980, 114, 240–242. [Google Scholar] [CrossRef]
- Langer, I.; Jeandriens, J.; Couvineau, A.; Sanmukh, S.; Latek, D. Signal Transduction by VIP and PACAP Receptors. Biomedicines 2022, 10, 406. [Google Scholar] [CrossRef]
- Fahrenkrug, J. Transmitter role of vasoactive intestinal peptide. Pharm. Toxicol. 1993, 72, 354–363. [Google Scholar] [CrossRef]
- Gozes, I.; Brenneman, D.E. VIP: Molecular biology and neurobiological function. Mol. Neurobiol. 1989, 3, 201–236. [Google Scholar] [CrossRef]
- Weick, R.F.; Stobie, K.M. Role of VIP in the regulation of LH secretion in the female rat. Neurosci. Biobehav. Rev. 1995, 19, 251–259. [Google Scholar] [CrossRef]
- Gozes, I.; Shani, Y.; Rostène, W.H. Developmental expression of the VIP-gene in brain and intestine. Brain Res. 1987, 388, 137–148. [Google Scholar] [CrossRef]
- Talbot, J.; Hahn, P.; Kroehling, L.; Nguyen, H.; Li, D.; Littman, D.R. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 2020, 579, 575–580. [Google Scholar] [CrossRef]
- Asano, S.; Yamasaka, M.; Ozasa, K.; Sakamoto, K.; Hayata-Takano, A.; Nakazawa, T.; Hashimoto, H.; Waschek, J.A.; Ago, Y. Vasoactive intestinal peptide-VIPR2 signaling regulates tumor cell migration. Front. Oncol. 2022, 12, 852358. [Google Scholar] [CrossRef]
- Siddappa, P.K.; Vege, S.S. Vasoactive Intestinal Peptide-Secreting Tumors: A Review. Pancreas 2019, 48, 1119–1125. [Google Scholar] [CrossRef]
- Ravindranathan, S.; Passang, T.; Li, J.M.; Wang, S.; Dhamsania, R.; Ware, M.B.; Zaidi, M.Y.; Zhu, J.; Cardenas, M.; Liu, Y.; et al. Targeting vasoactive intestinal peptide-mediated signaling enhances response to immune checkpoint therapy in pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 6418. [Google Scholar] [CrossRef]
- Gurusamy, M.; Tischner, D.; Shao, J.; Klatt, S.; Zukunft, S.; Bonnavion, R.; Günther, S.; Siebenbrodt, K.; Kestner, R.I.; Kuhlmann, T.; et al. G-protein-coupled receptor P2Y10 facilitates chemokine-induced CD4 T cell migration through autocrine/paracrine mediators. Nat. Commun. 2021, 12, 6798. [Google Scholar] [CrossRef]
- Sun, C.; Wang, B.; Hao, S. Adenosine-A2A Receptor Pathway in Cancer Immunotherapy. Front. Immunol. 2022, 13, 837230. [Google Scholar] [CrossRef]
- Boularan, C.; Kehrl, J.H. Implications of non-canonical G-protein signaling for the immune system. Cell Signal. 2014, 26, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Woodman, E.C.; Read, S.A.; Collins, M.J. Axial length and choroidal thickness changes accompanying prolonged accommodation in myopes and emmetropes. Vis. Res. 2012, 72, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Woodman-Pieterse, E.C.; Read, S.A.; Collins, M.J.; Alonso-Caneiro, D. Regional Changes in Choroidal Thickness Associated With Accommodation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Okojie, K.A.; Sharma, K.; Lentferink, D.H.; Sun, Y.Y.; Chen, H.R.; Uweru, J.O.; Amancherla, S.; Calcuttawala, Z.; Campos-Salazar, A.B.; et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 2021, 12, 5289. [Google Scholar] [CrossRef]
- Reiner, A.; Fitzgerald, M.E.C.; Del Mar, N.; Li, C. Neural control of choroidal blood flow. Prog. Retin. Eye Res. 2018, 64, 96–130. [Google Scholar] [CrossRef] [PubMed]
- Goharian, I.; Sehi, M. Is There Any Role for the Choroid in Glaucoma? J. Glaucoma 2016, 25, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Friedman, D.S.; Congdon, N.G. Possible mechanisms of primary angle-closure and malignant glaucoma. J. Glaucoma 2003, 12, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Jiang, R.; Ren, X.L.; Chen, J.D.; Shi, H.L.; Xu, L.; Wei, W.B.; Jonas, J.B. Intraocular pressure elevation and choroidal thinning. Br. J. Ophthalmol. 2016, 100, 1676–1681. [Google Scholar] [CrossRef]
- Zhang, X.; Cole, E.; Pillar, A.; Lane, M.; Waheed, N.; Adhi, M.; Magder, L.; Quigley, H.; Saeedi, O. The Effect of Change in Intraocular Pressure on Choroidal Structure in Glaucomatous Eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3278–3285. [Google Scholar] [CrossRef]
- Usui, S.; Ikuno, Y.; Uematsu, S.; Morimoto, Y.; Yasuno, Y.; Otori, Y. Changes in axial length and choroidal thickness after intraocular pressure reduction resulting from trabeculectomy. Clin. Ophthalmol. 2013, 7, 1155–1161. [Google Scholar] [CrossRef]
- Bayraktar, S.; İpek, A.; Takmaz, T.; Yildiz Tasci, Y.; Gezer, M.C. Ocular blood flow and choroidal thickness in ocular hypertension. Int. Ophthalmol. 2022, 42, 1357–1368. [Google Scholar] [CrossRef]
- Hata, M.; Hirose, F.; Oishi, A.; Hirami, Y.; Kurimoto, Y. Changes in choroidal thickness and optical axial length accompanying intraocular pressure increase. Jpn. J. Ophthalmol. 2012, 56, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, W.; Gao, X.; Li, Z.; Huang, W.; Li, X.; Zhou, M.; Zhang, X. Changes in Choroidal Thickness After Trabeculectomy in Primary Angle Closure Glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2608–2613. [Google Scholar] [CrossRef]
- Saeedi, O.; Pillar, A.; Jefferys, J.; Arora, K.; Friedman, D.; Quigley, H. Change in choroidal thickness and axial length with change in intraocular pressure after trabeculectomy. Br. J. Ophthalmol. 2014, 98, 976–979. [Google Scholar] [CrossRef]
- Kadziauskiene, A.; Kuoliene, K.; Asoklis, R.; Lesinskas, E.; Schmetterer, L. Changes in choroidal thickness after intraocular pressure reduction following trabeculectomy. Acta Ophthalmol. 2016, 94, 586–591. [Google Scholar] [CrossRef]
- Kara, N.; Baz, O.; Altan, C.; Satana, B.; Kurt, T.; Demirok, A. Changes in choroidal thickness, axial length, and ocular perfusion pressure accompanying successful glaucoma filtration surgery. Eye 2013, 27, 940–945. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).