Stem Anatomy Confirms Tingia unita Is a Progymnosperm
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
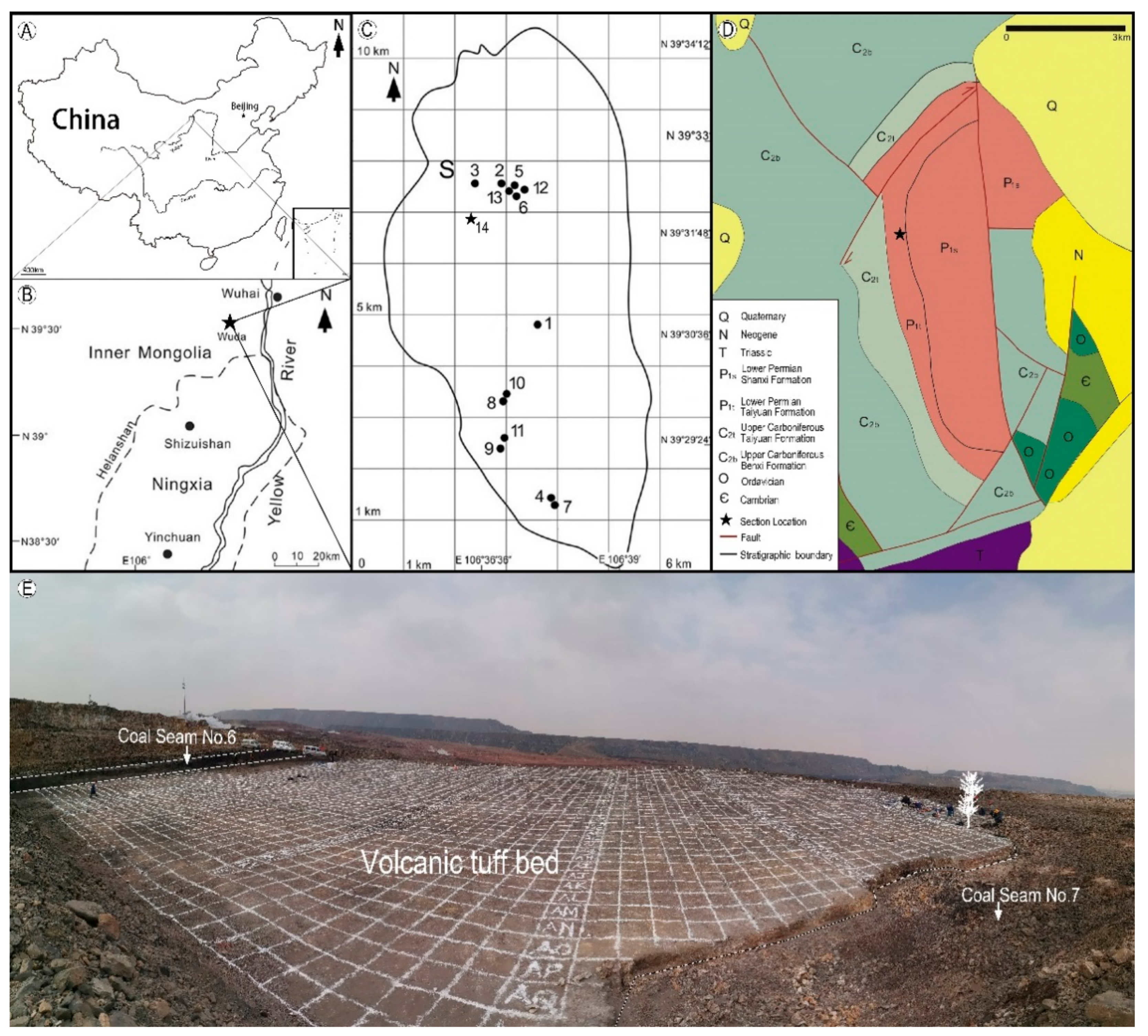
3. Results
3.1. Systematics
3.2. Description
3.2.1. The Compound Leaf and Strobilus
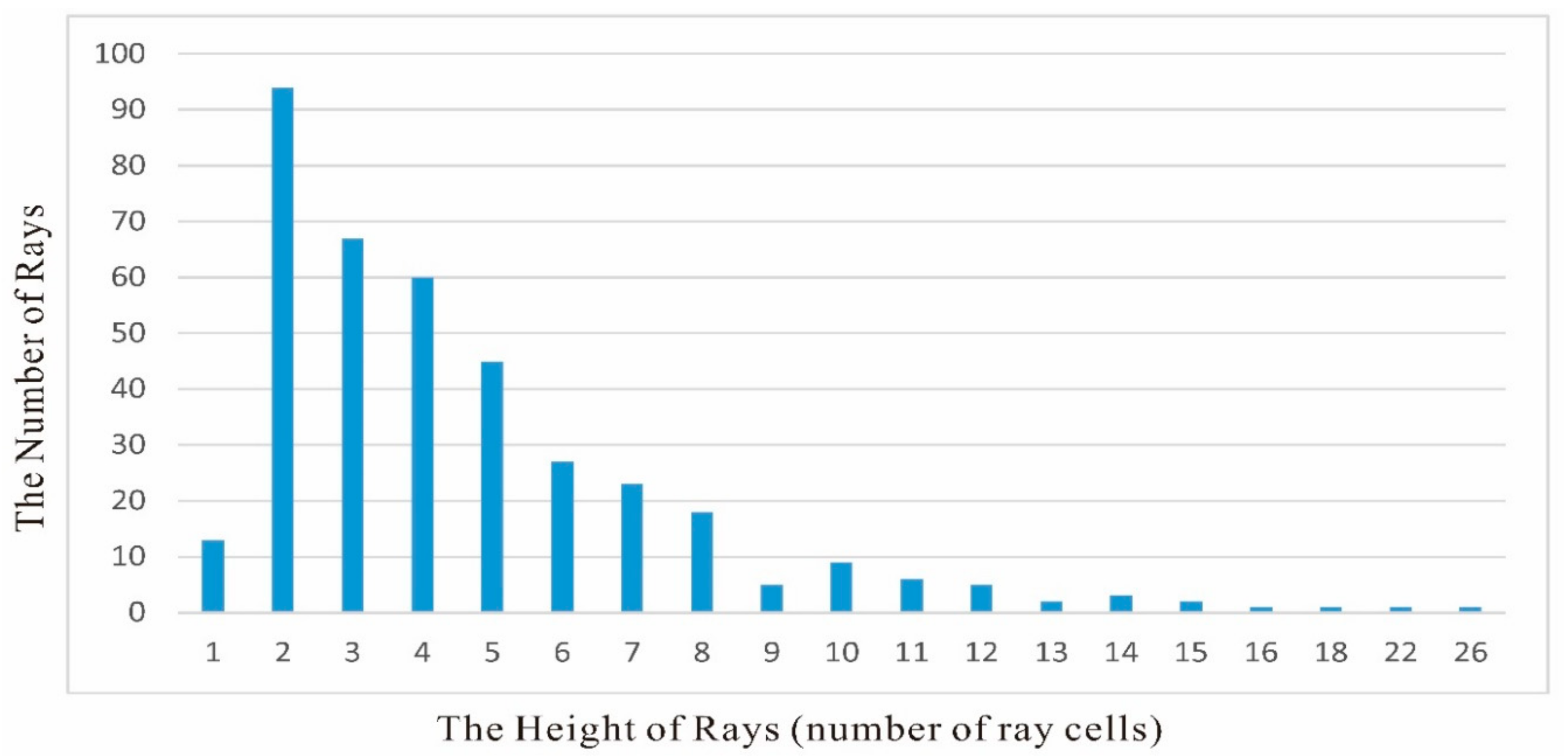
3.2.2. Stem Anatomy
Pith
Primary Xylem
Secondary Xylem
Cortex
Leaf Trace
4. Comparison and Discussion
4.1. Comparison with Tingia unita and the Relationship among These Stems
4.2. Comparison with Species from the Same Collection Area
4.3. Comparison and Discussion with Other Species
4.4. Comparison and Discussion with Other Progymnosperms
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Li, N.; Yang, X.J.; Wang, Y.D.; Fu, X.P.; Li, Y.; Zheng, S.L. Early Triassic Scalaroxylon in Nei Mongol and its evolutionary siginificance. Acta Palaeontol. Sin. 2006, 45, 332–344. [Google Scholar]
- Yang, X.J.; Wang, Y.D.; Zhang, W. Occurrences of Early Cretaceous fossil woods in China: Implications for paleoclimates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 385, 213–220. [Google Scholar] [CrossRef]
- Gnaedinger, S.C.; Zavattieri, A.M. Coniferous woods from the Upper Triassic of southwestern Gondwana, Tronquimalal Group, Neuquén Basin, Mendoza Province, Argentina. J. Paleontol. 2020, 94, 387–416. [Google Scholar] [CrossRef]
- Wan, M.L.; Wang, J.; Shi, T.M.; Wang, K.Y.; Tang, P.; Wang, J. Megaporoxylon sinensis sp. nov., a new coniferous trunk from the Upper Triassic of northern Bogda Mountains, northwestern China. Rev. Palaeobot. Palynol. 2021, 295, 104536. [Google Scholar] [CrossRef]
- Sternberg, K. Versuch Einer Geologisch-Botanischen Darstellung der Flora der Vorwelt I (2); Fleischer, Friedrich: Leipzig, Germany, 1821; p. 33. [Google Scholar]
- Bek, J.; Wang, J. A comparative study on in situ spores of some Paleozoic noeggerathialeans and their implcations for dispersed spore assemblages. Rev. Palaeobot. Palynol. 2021, 294, 104379. [Google Scholar] [CrossRef]
- Wang, J.; Hilton, J.; Pfefferkorn, H.W.; Wang, S.J.; Zhang, Y.; Bek, J.; Pšenička, J.; Seyfullah, L.J.; Dilcher, D. Ancient noeggerathialean reveals the seed plant sister group diversified alongside the primary seed plant radiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2013442118. [Google Scholar] [CrossRef]
- Šimůnek, Z.; Bek, J. Noeggerathiaceae from the Carboniferous basins of the Bohemian Massif. Rev. Palaeobot. Palynol. 2003, 125, 249–284. [Google Scholar] [CrossRef]
- Wang, J.; Pfefferkorn, H.W.; Bek, J. Paratingia wudensis sp. nov., a whole noeggerathialean plant preserved in an earliest Permian air fall tuff in Inner Mongolia, China. Am. J. Bot. 2009, 96, 1676–1689. [Google Scholar] [CrossRef]
- Wang, J. Tingia unita sp. nov. (Noeggerathiales) with strobilus from the Lower Permian of Wuda, Inner Mongolia, China. Chin. Sci. Bull. 2006, 51, 2624–2633. [Google Scholar] [CrossRef]
- Wang, J.; Wan, S.; Kerp, H.; Bek, J.; Wang, S.J. A whole noeggerathialean plant Tingia unita Wang from the earliest Permian peat-forming flora, Wuda Coalfield, Inner Mongolia. Rev. Palaeobot. Palynol. 2021, 294, 104204. [Google Scholar] [CrossRef]
- Wang, J.; Pfefferkorn, H.W.; Zhang, Y.; Feng, Z. Permian vegetational Pompeii from Inner Mongolia and its implications for landscape paleoecology and paleobiogeography of Cathaysia. Proc. Natl. Acad. Sci. USA 2012, 109, 4927–4932. [Google Scholar] [CrossRef]
- Wang, J.; Pfefferkorn, H.W.; Feng, Z. Noeggerathiales as coal-forming plants in Cathaysia: Conclusions from an Early Permian vegetational Pompeii in Inner Mongolia. Chin. Sci. Bull. 2014, 59, 2785–2792. [Google Scholar] [CrossRef]
- Wang, J.; Pfefferkorn, H.W.; Opluštil, S.; Kerp, H. Permian “vegetational Pompeii”: A peat-forming in situ preserved forest from the Wuda Coalfield, Inner Mongolia, China–Introduction to a volume of detailed studies. Rev. Palaeobot. Palynol. 2021, 294, 104502. [Google Scholar] [CrossRef]
- He, X.Z.; Zhou, W.M.; Li, D.D.; Wang, S.J.; Hilton, J.; Wang, J. A 298-million-year-old gleicheniaceous fern from China. Rev. Palaeobot. Palynol. 2021, 294, 104355. [Google Scholar] [CrossRef]
- Schmitz, M.D.; Pfefferkorn, H.W.; Shen, S.Z.; Wang, J. A volcanic tuff near the Carboniferous–Permian boundary, Taiyuan Formation, North China: Radioisotopic dating and global correlation. Rev. Palaeobot. Palynol. 2021, 294, 104244. [Google Scholar] [CrossRef]
- Hass, H.; Rowe, N.P. Thin sections and wafering. In Fossil Plants and Spores: Modern Techniques; Jones, T.P., Rowe, N.P., Eds.; The Geological Society of London: London, UK, 1990; pp. 76–81. [Google Scholar]
- Galtier, J. Morphology and Phylogenetic Relationships of Early Pteridosperms. In Origin and Evolution of Gymnosperms; Beck, C.B., Ed.; Columbia University Press: New York, NY, USA, 1988; pp. 135–176. [Google Scholar]
- Galtier, J. On the Earliest Arborescent Gymnosperms. Cour. Forsch. Inst. Senckenberg 1992, 147, 119–125. [Google Scholar]
- Galtier, J.; Meyer-Berthaud, B. The diversification of early arborescent seed ferns. J. Torrey Bot. Soc. 2006, 133, 7–19. [Google Scholar] [CrossRef]
- Taylor, T.N.; Taylor, E.L.; Krings, M. Paleobotany, the Biology and Evolution of Fossil Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 1–1230. [Google Scholar]
- Wang, D.M.; Liu, L. A new Late Devonian genus with seed plant affinities. BMC Evol. Biol. 2015, 15, 28. [Google Scholar] [CrossRef]
- Boyce, C.K.; DiMichele, W.A. Arborescent lycopsid productivity and lifespan: Constraining the possibilities. Rev. Palaeobot. Palynol. 2016, 227, 97–110. [Google Scholar] [CrossRef]
- D’Antonio, M.P.; Boyce, C.K.; Zhou, W.M.; Pfefferkorn, H.W.; Wang, J. Primary tissues dominated ground-level trunk diameter in Sigillaria: Evidence from the Wuda Tuff, Inner Mongolia. J. Geol. Soc. 2022, 179, jgs2021-021. [Google Scholar] [CrossRef]
- Wang, S.J.; Wang, J.; Liu, L.; Hilton, J. Stem diversity of the marattialean tree fern family Psaroniaceae from the earliest Permian Wuda Tuff Flora. Rev. Palaeobot. Palynol. 2021, 294, 104378. [Google Scholar] [CrossRef]
- Zhou, W.M.; Wan, S.; Wan, M.L.; Hilton, J.; Pšenička, J.; Wang, J. Yangopteris ascendens (Halle) gen. et comb. nov., a climbing alethopterid pteridosperm from the Asselian (earliest Permian) Wuda Tuff Flora. Rev. Palaeobot. Palynol. 2021, 294, 104282. [Google Scholar] [CrossRef]
- Galtier, J.; Schneider, J.L.; Grauvogel-Stamm, L. Arborescent gymnosperms and occurrence of Protopitys from the Lower Carboniferous of the Vosges, France. Rev. Palaeobot. Palynol. 1998, 99, 203–215. [Google Scholar] [CrossRef]
- Scheckler, S.E.; Galtier, J. Tyloses and ecophysiology of the early Carboniferous progymnosperm tree Protopitys buchiana. Ann. Bot. 2003, 91, 739–747. [Google Scholar] [CrossRef]
- Decombeix, A.L.; Meyer-Berthaud, B.; Galtier, J.; Talent, J.A.; Mawson, R. Arborescent lignophytes in the Tournaisian vegetation of Queensland (Australia): Palaeoecological and palaeogeographical significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 301, 39–55. [Google Scholar] [CrossRef]
- Decombeix, A.L.; Galtie, J.; Prestianni, C. The Early Carboniferous progymnosperm Protopitys: New data on vegetative and fertile structures, and on its geographic and stratigraphic distribution. Hist. Biol. 2014, 27, 345–354. [Google Scholar] [CrossRef]
- Stidd, B.M.; Phillips, T.L. Johnhallia Lacunosa gen. et sp. n.: A New Pteridosperm from the Middle Pennsylvanian of Indiana. J. Paleontol. 1982, 56, 1093–1102. [Google Scholar]
- He, J.; Wang, S.J.; Hilton, J.; Shao, L.Y. Xuanweioxylon scalariforme gen. et sp. nov.: Novel Permian coniferophyte stems with scalariform bordered pitting on secondary xylem tracheids. Rev. Palaeobot. Palynol. 2013, 197, 152–165. [Google Scholar] [CrossRef]
- Yang, Y.; He, X.Y.; Hilton, J.; Zhao, F.G.; Chen, X.S.; Wang, S.J. Xuanweioxylon damogouense sp. nov., a gymnosperm stem from the Lopingian (late Permian) of southwestern China and its systematic and paleoecological implications. Rev. Palaeobot. Palynol. 2019, 269, 94–103. [Google Scholar] [CrossRef]
- Cribbs, J.E. Cordaites missouriense from the Lower Carboniferous of Missouri. Am. J. Bot. 1935, 22, 427–438. [Google Scholar] [CrossRef]
- Wan, M.L.; Yang, W.; He, X.Z.; Zhou, W.M.; Liu, L.J.; Wang, J. Yangquanoxylon miscellum gen. nov. et sp. nov., a gymnospermous wood from the Upper Pennsylvanian–lower Permian Taiyuan Formation of Yangquan City, Shanxi Province, with reference to the palaeoclimate in North China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 479, 115–125. [Google Scholar] [CrossRef]
- Walton, J. XV.—On Protopitys (Göppert): With a Description of a Fertile Specimen Protopitys scotica sp. nov. From the Calciferous Sandstone Series of Dunbartonshire. Trans. R. Soc. Edinb. 1957, 63, 333–340. [Google Scholar] [CrossRef]
- Galtier, J.; Scott, A. On Eristophyton and other gymnosperms from the Lower Carboniferous of Castelton Bay, East Lothian, Scotland. Geobios 1990, 23, 5–19. [Google Scholar] [CrossRef]
- Tian, B.L.; Li, H.Q. A new special petrified stem, Guizhouoxylon dahebianense gen. et sp. nov., from Upper Permian in Shuicheng District, Guizhou, China. Acta Palaeontol. Sin. 1992, 31, 336–345. [Google Scholar]
- Kurzawe, F.; Iannuzzi, R.; Merlotti, S. On the Permian permineralized woods of the “fossil flora of the coal measures of Brazil” (D. White, 1908): Taxonomic re-evaluation. Palaeobotanist 2012, 61, 57–65. [Google Scholar] [CrossRef]
- Artabe, A.E.; Zamuner, A.B.; Stevenson, D.W. A new genus of Late Cretaceous cycad stem from Argentina, with reappraisal of known forms. Alcheringa 2005, 29, 87–100. [Google Scholar] [CrossRef]
- Ryberg, P.E.; Taylor, E.L.; Taylor, T.N. Secondary Phloem Anatomy of Cycadeoidea (Bennettitales). Am. J. Bot. 2007, 94, 791–798. [Google Scholar] [CrossRef]
- Philippe, M.; Cuny, G.; Bashforth, A. Ecpagloxylon mathiesenii gen. nov. et sp. nov., a Jurassic wood from Greenland with several primitive angiosperm features. Plant Syst. Evol. 2010, 287, 153–165. [Google Scholar] [CrossRef]
- Sze, H.C. On the structure and relationship of Phoroxylon scalariforme Sze. Acta Palaeontol. Sin. 1954, 4, 527–539. [Google Scholar]
- Philippe, M.; Torres, T.; Zhang, W.; Zheng, S.L. Sahnioxylon, a Mesozoic wood with a loose distribution: China, India and wester Antarctica. Bull. De La Soc. Geol. De Fr. 1999, 170, 513–519. [Google Scholar]
- Zheng, S.L.; Li, Y.; Zhang, W.; Wang, Y.D.; Yang, X.J.; Li, N.; Fu, X.P. Jurassic fossil wood of Sahnioxylon from western Liaoning, China and special references to its systematic affinity. Glob. Geol. 2005, 24, 209–218, (In Chinese with English abstract). [Google Scholar]
- Vozenin-Serra, C.; Pons, D. Intérêts phylogénétique et paléoécologique des structures ligneuses homoxylées découvertes dans le Crétacé inférieur du Tibet méridional. Palaeontogr. Abt. B Paläophytologie 1990, 216, 107–127. [Google Scholar]
- Beck, C.B. Tetraxylopteris schmidtii gen. et sp. nov., a probable pteridosperm precursor from the Devonian of New York. Am. J. Bot. 1957, 44, 350–367. [Google Scholar] [CrossRef]
- Matten, L.C.; Banks, H.P. Triloboxylon ashlandicum gen. and sp. n. from the Upper Devonian of New York. Am. J. Bot. 1966, 53, 1020–1028. [Google Scholar] [CrossRef]
- Scheckler, S.E. A fertile axis of Triloboxylon ashlandicum, a progymnosperm from the Upper Devonian of New York. Am. J. Bot. 1975, 62, 923–934. [Google Scholar] [CrossRef]
- Dannenhoffer, J.M.; Stein, W.; Bonamo, P.M. The primary body of Rellimia thomsonii: Integrated perspective based on organically connected specimens. Int. J. Plant Sci. 2007, 168, 491–506. [Google Scholar] [CrossRef]
- Hirmer, M. Noeggerathiineae. Die Karbon-Flora des Saargebietes, Abt. 3. Filicales und Verwandte I. Palaeontographica, 1940, Suppl.-Bd. IX; Hirmer, M., Gothan, P., Eds.; Schweizerbart Science: Stuttgart, Germany, 1940; pp. 1–44. [Google Scholar]
- Leary, R.L.; Pfefferkorn, H.W. An Early Pennsylvanian flora with Megalopteris and Noeggerathiales from west-central Illinois. Ill. State Geol. Surv. Circ. 1977, 500, 1–77. [Google Scholar]
- Beck, C.B. Archaeopteris and Its Role in Vascular Plant Evolution. In Paleobotany, Paleobotany and Evolution (v. 1); Niklas, K.J., Ed.; Praeger Press: New York, NY, USA, 1981; pp. 193–230. [Google Scholar]
- Lyubarova, A.; Snigirevsky, S.M. New investigations of Upper Devonian wood from the north of the European part of Russia. Acta Palaeobot. 2020, 60, 143–155. [Google Scholar] [CrossRef]
- Decombeix, A.L.; MeyerLBerthaud, B.A. Callixylon (Archaeopteridales, Progymnospermopsida) trunk with preserved secondary phloem from the Late Devonian of Morocco. Am. J. Bot. 2013, 100, 2219–2230. [Google Scholar] [CrossRef]
- Tanrattana, M.; Meyer-Berthaud, B.; Decombeix, A.L. Callixylon wendtii sp. nov., a new species of archaeopteridalean progymnosperm from the Late Devonian of Anti-Atlas, Morocco. Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 108, 373–385. [Google Scholar]
- Matten, L.C. Actinoxylon banksii gen. et sp. nov.: A progymnosperm from the Middle Devonian of New York. Am. J. Bot. 1968, 55, 773–782. [Google Scholar] [CrossRef]
- Høeg, O.A. The Downtonian and Devonian flora of Spitsbergen. In Norges Svalbard-Og Ishavs-Undersøkelser: Skrifter; I Kommission Hos Jacob Dybwad: Oslo, Norway, 1942; Volume 83, pp. 1–229. [Google Scholar]
- Read, C.B. A Devonian flora from Kentucky. J. Paleontol. 1936, 10, 213–227. [Google Scholar]
- Namboodiri, K.K.; Beck, C.B. A comparative study of the primary vascular system of conifers. III. Stelar evolution in gymnosperms. Am. J. Bot. 1968, 55, 464–472. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, X.M. Wood Anatomy and Ultrastructure of Gymnospermous Woods in China; China Forestry Publishing House Press: Beijing, China, 1994; p. 154. (In Chinese) [Google Scholar]
- Stewart, W.N.; Rothwell, G.W. Paleobotany and the Evolution of Plants, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Beck, C.B. An Introduction to Plant Structure and Development: Plant Anatomy for the Twenty-First Century, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, S.-J.; Wang, J. Stem Anatomy Confirms Tingia unita Is a Progymnosperm. Biology 2023, 12, 494. https://doi.org/10.3390/biology12040494
Yang Y, Wang S-J, Wang J. Stem Anatomy Confirms Tingia unita Is a Progymnosperm. Biology. 2023; 12(4):494. https://doi.org/10.3390/biology12040494
Chicago/Turabian StyleYang, Yang, Shi-Jun Wang, and Jun Wang. 2023. "Stem Anatomy Confirms Tingia unita Is a Progymnosperm" Biology 12, no. 4: 494. https://doi.org/10.3390/biology12040494
APA StyleYang, Y., Wang, S.-J., & Wang, J. (2023). Stem Anatomy Confirms Tingia unita Is a Progymnosperm. Biology, 12(4), 494. https://doi.org/10.3390/biology12040494




