C-Type Natriuretic Peptide Acts as a Microorganism-Activated Regulator of the Skin Commensals Staphylococcus epidermidis and Cutibacterium acnes in Dual-Species Biofilms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultivation
2.2. Natriuretic Peptide
2.3. Mono-Species Biofilm Growth on Polytetrafluoroethylene Cubes
2.4. Mono-Species and Dual-Species Biofilm Growth on Glass Microfiber Filters
2.5. Study of Planktonic Cultures and Biofilm Growth Kinetics
2.6. Confocal Laser Scanning Microscopy of FISH-Stained Biofilms
2.7. Study of Differential Gene Expression
2.8. Read Preprocessing and Quality Control
2.9. Quantitative PCR
2.10. In Silico Protein Sequence Analysis
2.11. Microbiological Statistics
3. Results
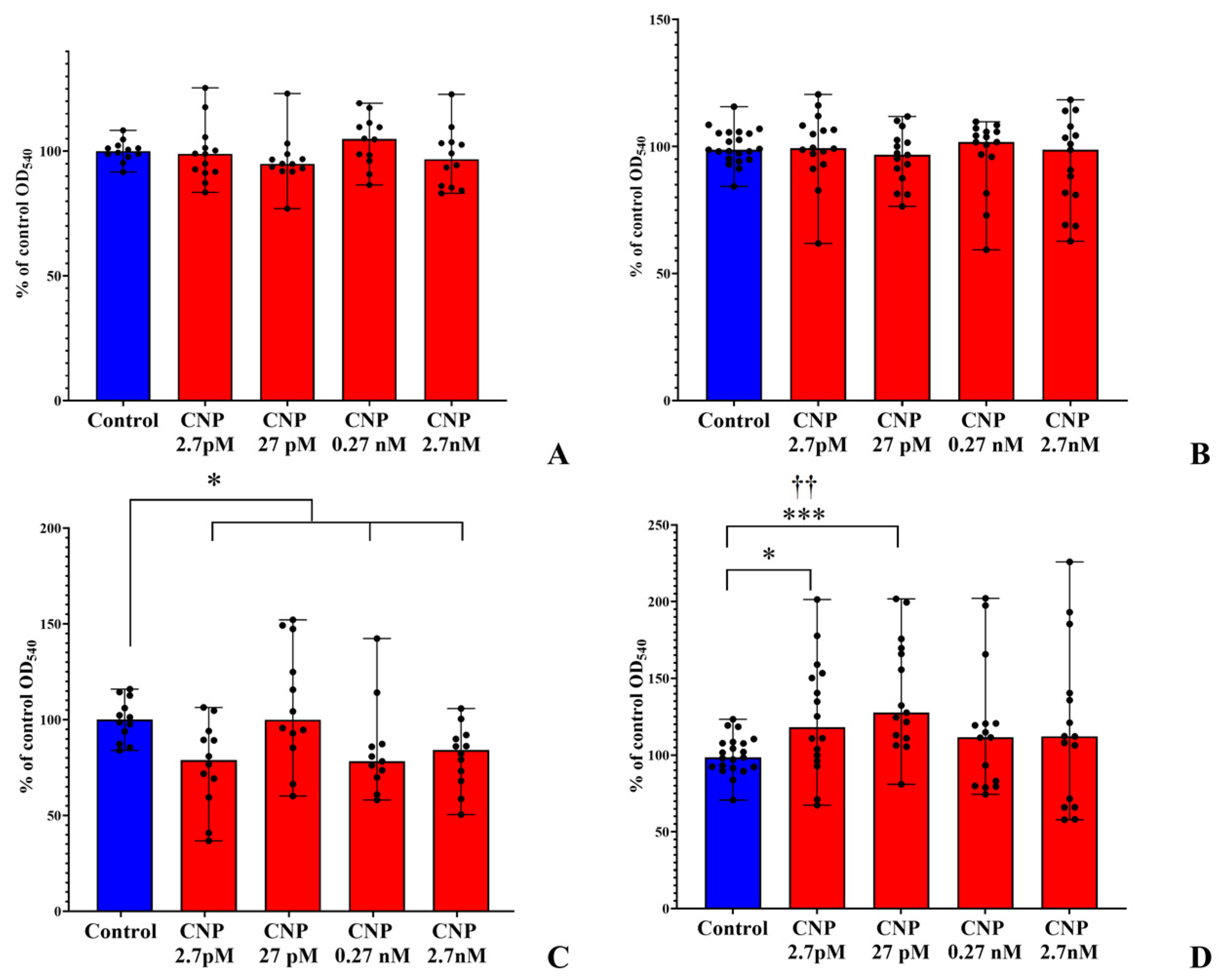
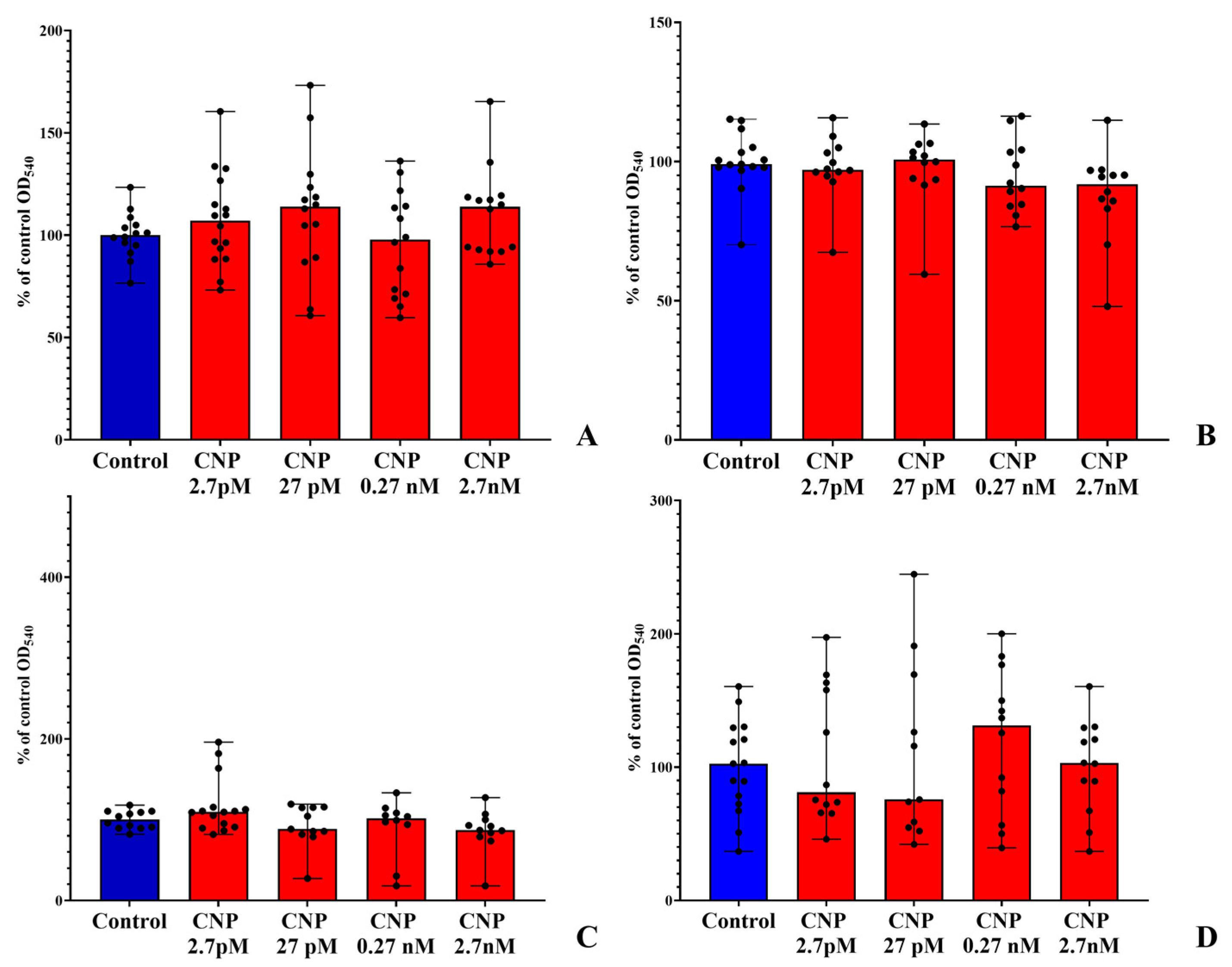
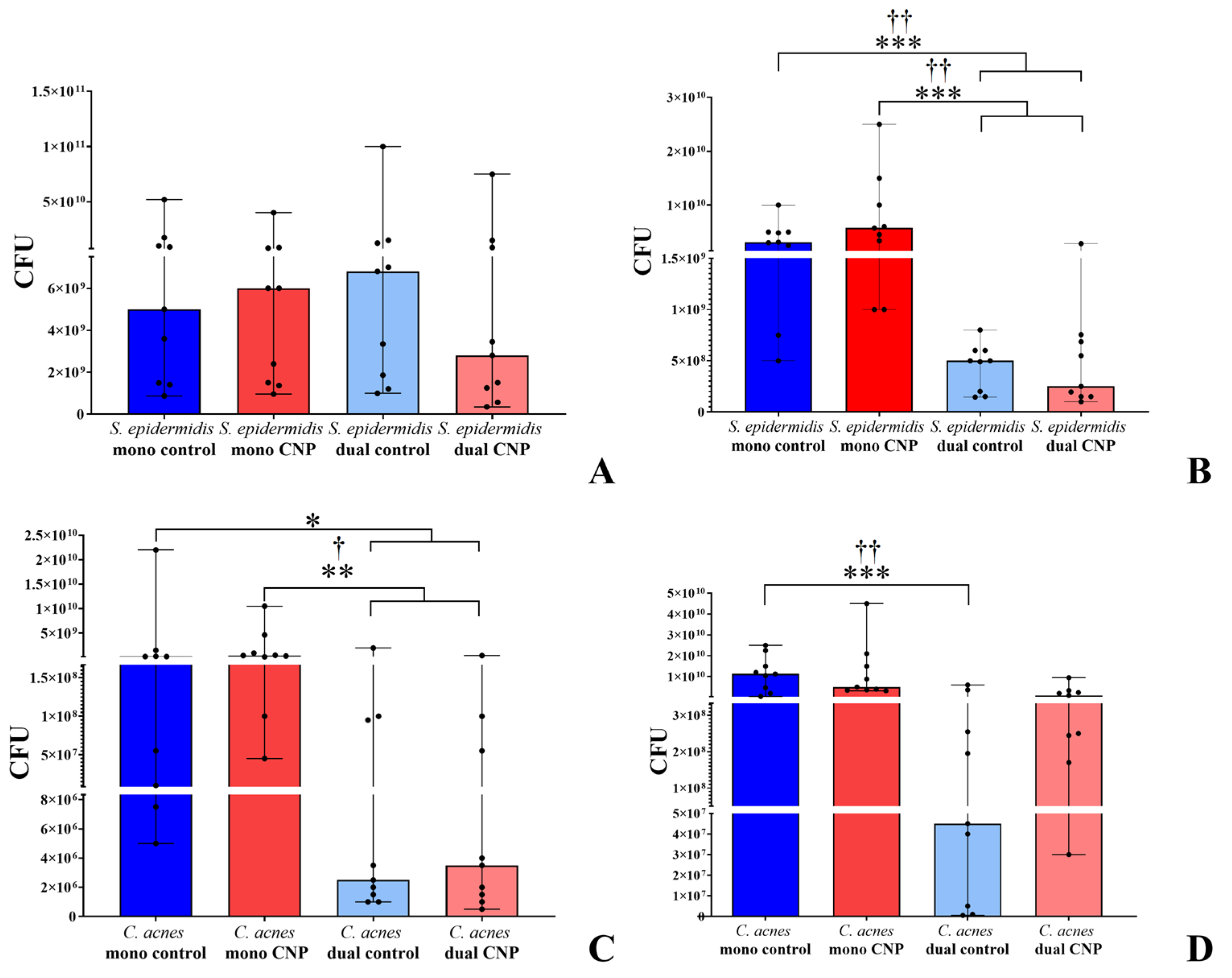
3.1. Biofilm Growth on PTFE Cubes
3.2. Biofilms on Glass Microfiber Filters
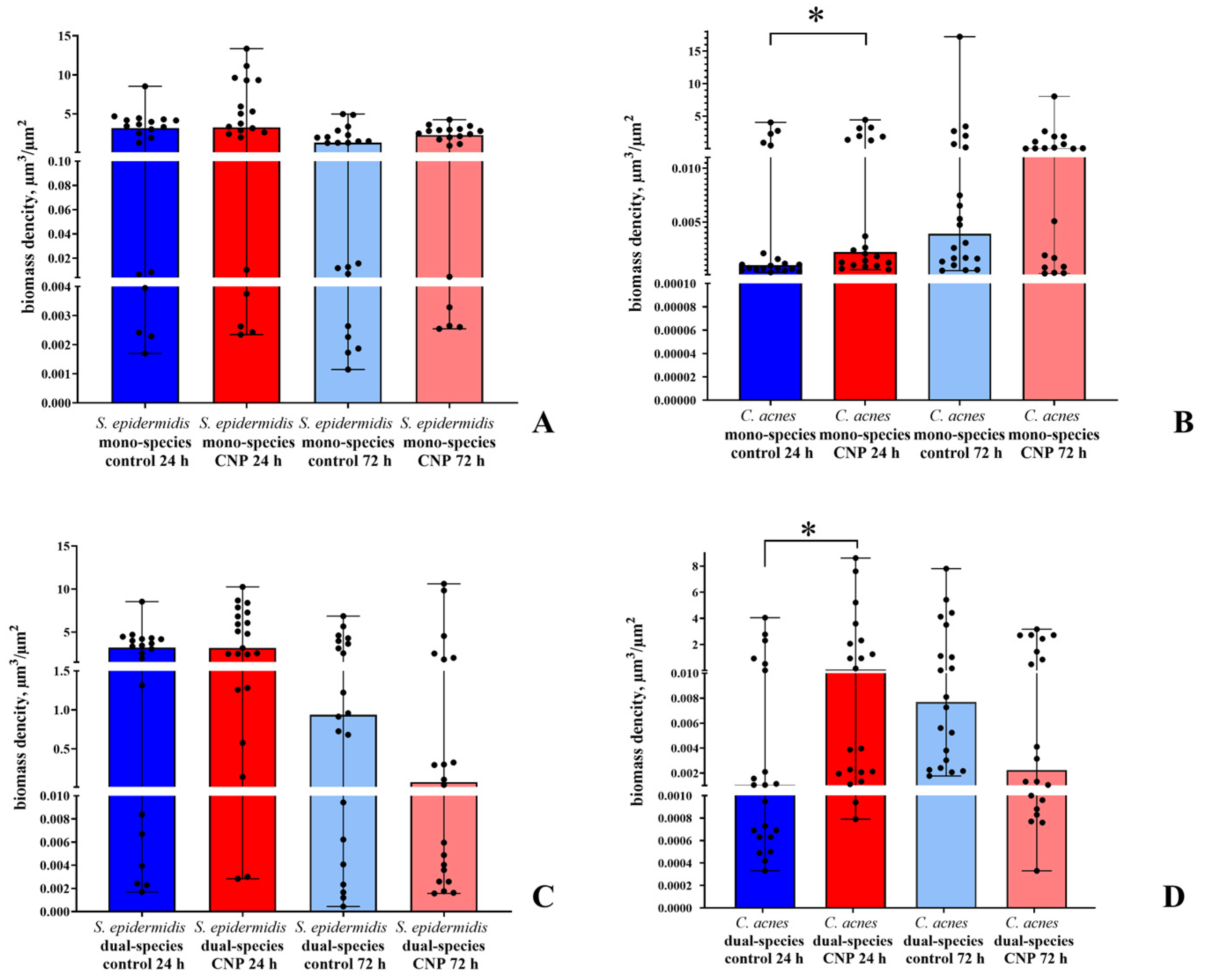
3.3. Confocal Microscopy of Biofilms
3.4. Study of Growth Kinetics
3.5. Differential Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.M. Non-surface attached bacterial aggregates: A ubiquitous third lifestyle. Front. Microbiol. 2020, 11, 557035. [Google Scholar] [CrossRef]
- Singh, A.; Amod, A.; Pandey, P.; Bose, P.; Pingali, M.S.; Shivalkar, S.; Varadwaj, P.K.; Sahoo, A.K.; Samanta, S.K. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 2022, 17, 022003. [Google Scholar] [CrossRef]
- Hansa, R.K.; Khan, M.; Frangie, M.; Gilmore, D.; Shelton, R.; Savenka, A.; Basnakian, A.; Shuttleworth, S.; Smeltzer, M.; Alam, M. 4-4-(Anilinomethyl)-3-[4-(trifluoromethyl) phenyl]-1H-pyrazol-1-ylbenzoic acid derivatives as potent anti-gram-positive bacterial agents. Eur. J. Med. Chem. 2021, 219, 113402. [Google Scholar] [CrossRef]
- Elbasuney, S.; Yehia, M.; Ismael, S.; Al-Hazmi, N.E.; El-Sayyad, G.S.; Tantawy, H. Potential impact of reduced graphene oxide incorporated metal oxide nanocomposites as antimicrobial, and antibiofilm agents against pathogenic microbes: Bacterial protein leakage reaction mechanism. J. Clust. Sci. 2022, 1–18. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dey, A.; Sarkar, T.; Ray, R.R.; Rebezov, M.; Shariati, M.A.; Thiruvengadam, M.; Simal-Gandara, J. Immobilized enzymes as potent antibiofilm agent. Biotechnol. Prog. 2022, 38, e3281. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014, 5, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Racine, P.J.; Janvier, X.; Clabaut, M.; Catovic, C.; Souak, D.; Boukerb, A.M.; Groboillot, A.; Konto-Ghiorghi, Y.; Duclairoir-Poc, C.; Lesouhaitier, O.; et al. Dialog between skin and its microbiota: Emergence of “Cutaneous Bacterial Endocrinology”. Exp. Dermatol. 2020, 29, 790–800. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Ziganshin, R.H.; Zdorovenko, E.L.; Klimko, A.I.; Ianutsevich, E.A.; Danilova, O.A.; Tereshina, V.M.; Gorbachevskii, M.V.; Ovcharova, M.A.; Nevolina, E.D.; et al. Epinephrine extensively changes the biofilm matrix composition in Micrococcus luteus C01 isolated from human skin. Front. Microbiol. 2022, 13, 1003942. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.; Clamens, T.; Tahrioui, A.; Desriac, F.; Rodrigues, S.; Rosay, T.; Harmer, N.; Diaz, S.; Barreau, M.; Racine, P.-J.; et al. Pseudomonas aeruginosa biofilm dispersion by the human atrial natriuretic peptide. Adv. Sci. 2022, 9, 2103262. [Google Scholar] [CrossRef]
- Reading, N.C.; Rasko, D.A.; Torres, A.G.; Sperandio, V. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. PNAS 2006, 106, 5889–5894. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sperandio, V. Indolesignaling at the host-microbiota-pathogeninterface. MBio 2019, 10, e01031-19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Russell, R.M.; Pifer, R.; Menezes-Garcia, Z.; Cuesta, S.; Narayanan, S.; MacMillan, J.B.; Sperandio, V. The serotonin neurotransmitter modulates virulence of enteric pathogens. Cell Host Microbe 2020, 28, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Clabaut, M.; Suet, A.; Racine, P.-J.; Tahrioui, A.; Verdon, J.; Barreau, M.; Maillot, O.; Le Tirant, A.; Karsybayeva, M.; Kremser, C.; et al. Effect of 17β-estradiol on a human vaginal Lactobacillus crispatus strain. Sci. Rep. 2021, 11, 7133. [Google Scholar] [CrossRef] [PubMed]
- Clabaut, M.; Boukerb, A.M.; Ben Mlouka, A.; Suet, A.; Tahrioui, A.; Verdon, J.; Barreau, M.; Maillot, O.; Le Tirant, A.; Karsybayeva, M.; et al. Variability of the response of human vaginal Lactobacillus crispatus to 17β-estradiol. Sci. Rep. 2021, 11, 11533. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.A. Effect of β-estradiol on mono-and mixed-species biofilms of human commensal bacteria Lactobacillus paracasei AK508 and Micrococcus luteus C01 on different model surfaces. Coatings 2022, 12, 436. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Lesouhaitier, O.; Netrusov, A.I.; Plakunov, V.K.; Feuilloley, M.G.J. Regulation of formation of monospecies and binary biofilms by human skin microbiota components, Staphylococcus epidermidis and Staphylococcus aureus, by human natriuretic peptides. Microbiology 2018, 87, 597–609. [Google Scholar] [CrossRef]
- Ovcharova, M.A.; Geraskina, O.; Danilova, N.; Botchkova, E.; Martyanov, S.; Feofanov, A.; Plakunov, V.; Gannesen, A. Atrial natriuretic peptide affects skin commensal Staphylococcus epidermidis and Cutibacterium acnes dual-species biofilms. Microorganisms 2021, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Diuvenji, E.V.; Nevolina, E.D.; Mart’Yanov, S.V.; Zhurina, M.A.; Kalmantaeva, O.V.; Makarova, M.A.; Botchkova, E.A.; Firstova, V.V.; Plakunov, V.K.; Gannesen, A.V. Binary biofilms of Staphylococcus aureus 209P and Kytococcus schroeteri H01: Dualistic role of kytococci and cell adhesion alterations in the presence of the A-type natriuretic peptide. Microbiology 2022, 91, 563–576. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 191, 341–366. [Google Scholar]
- Veron, W.; Lesouhaitier, O.; Pennanec, X.; Rehel, K.; Leroux, P.; Orange, N.; Feuilloley, M.G.J. Natriuretic peptides affect Pseudomonas aeruginosa and specifically modify lipopolysaccharide biosynthesis. FEBS J. 2007, 274, 5852–5864. [Google Scholar] [CrossRef] [PubMed]
- Rosay, T.; Bazire, A.; Diaz, S.; Clamens, T.; Blier, A.-S.; Mijouin, L.; Hoffmann, B.; Sergent, J.-A.; Bouffartigues, E.; Boireau, W.; et al. Pseudomonas aeruginosa expresses a functional human natriuretic peptide receptor ortholog: Involvement in biofilm formation. MBio 2015, 6, e01033-15. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Boutcher, S.H.; Maw, G.J.; Taylor, N.A. Forehead skin temperature and thermal sensation during exercise in cool and thermoneutral environments. ASEM 1996, 66, 1058–1062. [Google Scholar]
- Lange-Asschenfeldt, B.; Marenbach, D.; Lang, C.; Patzelt, A.; Ulrich, M.; Maltusch, A.; Terhorst, D.; Stockfleth, E.; Sterry, W.; Lademann, J. Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol. Physiol. 2011, 24, 305–311. [Google Scholar] [CrossRef]
- Ikeda, K.; Ikeda, T.; Onizuka, T.; Terashi, H.; Fukuda, T. C-type natriuretic peptide concentrations in the plasma and cerebrospinal fluid of patients with subarachnoid hemorrhage. Crit Care 2000, 5, 37. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Schelkunov, M.I.; Geras’ kina, O.V.; Makarova, N.E.; Sukhacheva, M.V.; Danilova, N.D.; Ovcharova, M.A.; Mart, S.V.; Pankratov, T.A.; Muzychenko, D.S.; et al. Epinephrine affects gene expression levels and has a complex effect on biofilm formation in Micrococcus luteus strain C01 isolated from human skin. Biofilm 2021, 3, 100058. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B: Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ommen, P.; Zobek, N.; Meyer, R.L. Quantification of biofilm biomass by staining: Non-toxic safranin can replace the popular crystal violet. J. Microbiol. Methods 2017, 141, 87–89. [Google Scholar] [CrossRef]
- Kragh, K.N.; Alhede, M.; Kvich, L.; Bjarnsholt, T. Into the well—A close look at the complex structures of a microtiter biofilm and the crystal violet assay. Biofilm 2019, 1, 100006. [Google Scholar] [CrossRef]
- Maguire, B.A.; Wild, D.G. The roles of proteins L28 and L33 in the assembly and function of Escherichia coli ribosomes in vivo. Mol. Microbiol. 1997, 23, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Graziano, V.; Capel, M.S. A role for proteins S3 and S14 in the 30 S ribosomal subunit. J. Biol. Chem. 1986, 261, 15049–15052. [Google Scholar] [CrossRef]
- Fan, S.H.; Ebner, P.; Reichert, S.; Hertlein, T.; Zabel, S.; Lankapalli, A.K.; Nieselt, K.; Ohlsen, K.; Götz, F. MpsAB is important for Staphylococcus aureus virulence and growth at atmospheric CO2 levels. Nat. Commun. 2019, 10, 3627. [Google Scholar] [CrossRef]
- Short, B.; Delaney, C.; McKloud, E.; Brown, J.L.; Kean, R.; Litherland, G.J.; Williams, C.; Martin, S.L.; MacKay, W.G.; Ramage, G. Investigating the transcriptome of Candida albicans in a dual-species Staphylococcus aureus biofilm model. Front. Cell. Infect. Microbiol. 2021, 11, 791523. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, L.; Deng, Y.; Zhang, H.; Xia, M.; Wei, X.; Yang, Y.; Hu, T. RNase III coding genes modulate the cross-kingdom biofilm of Streptococcus mutans and Candida albicans. Front. Microbiol. 2022, 13, 957879. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Li, X.; Liu, Q.; Lu, J.; Wang, T.; Zhang, Q. Antifungal and antibiofilm efficacy of paeonol treatment against biofilms comprising Candida albicans and/or Cryptococcus neoformans. Front. Cell Infect. Microbiol. 2022, 12, 884793. [Google Scholar] [CrossRef]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida albicans mycofilms support Staphylococcus aureus colonization and enhances miconazole resistance in dual-species interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, L.E. Dentures are a reservoir for respiratory pathogens. J. Prosthodont. 2016, 25, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, N.K.; Sundaram, V.K.; Danthi, P.S.; Barakat, R.; Solomon, S.; Mondal, M.; Carre, I.; El Jalkh, T.; Padilla-Ferrer, A.; Grenier, J.; et al. RNA-Seq is not required to determine stable reference genes for qPCR normalization. PLoS Comput. Biol. 2022, 18, e1009868. [Google Scholar] [CrossRef] [PubMed]
- Everaert, C.; Luypaert, M.; Maag, J.L.; Cheng, Q.X.; Dinger, M.E.; Hellemans, J.; Mestdagh, P. Benchmarking of RNA-sequencing analysis workflows using whole-transcriptome RT-qPCR expression data. Sci. Rep. 2017, 7, 1559. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T. Do results obtained with RNA-sequencing require independent verification? Biofilm 2021, 3, 100043. [Google Scholar] [CrossRef]



| The Program | Version | Parameters Applied in the Study 1 |
|---|---|---|
| Trimmomatic | 0.39 | ILLUMINACLIP: [adapters file path]:2:30:10:1: TRUE TRAILING:3 SLIDINGWINDOW:4:15 AVGQUAL:20 MINLEN:30 |
| BWA | 0.7.17 | |
| Salmon | 1.3.0 | --libType A --gcBias --numGibbsSamples 20 |
| sva | 3.38 | |
| DESeq2 | 1.22.2 |
| № | Reagent | V, µL |
|---|---|---|
| 1 | dNTP, 2.5 мM | 2.5 |
| 2 | 10× PCR buffer B | 2.5 |
| 3 | MgCl2, 25 мM | 2.5 |
| 4 | Primer mix, 10 pM/µL | 2 |
| 5 | SynTaq DNA polymerase, 5 U/µL | 0.5 |
| 6 | dd H2O | 15 |
| 7 | DNA matrix | 0.2 |
| Without Pre-Adhesion | With Pre-Adhesion | ||||||
|---|---|---|---|---|---|---|---|
| Growth Rate, h−1 | Generation Time, h | Maximal OD540 | Growth Rate, h−1 | Generation Time, h | Maximal OD540 | ||
| S. epidermidis mono-species | Control | 0.25 | 2.8 | 0.86 | 0.17 | 3.94 | 0.8 |
| CNP | 0.22 | 3.19 | 0.84 | 0.2 | 3.51 | 0.84 | |
| C. acnes mono-species | Control | 0.11 | 6.58 | 1.12 | 0.1 | 7.44 | 0.9 |
| CNP | 0.13 | 5.42 | 1.14 | 0.09 | 7.74 | 0.82 | |
| Dual-species | Control | 0.18 | 3.85 | 0.97 | 0.18 | 3.75 | 0.82 |
| CNP | 0.16 | 4.23 | 0.93 | 0.21 | 3.32 | 0.84 | |
| Locus Tag | Protein Description | Log2 (Expression Level Ratio 1) | Standard Error of log2 (Expression Ratio) | p-Value | q-Value |
|---|---|---|---|---|---|
| B6C95_03235 | Hypothetical protein | −4.02808 | 0.600497 | 4.59 × 10−7 | 0.000553 |
| B6C95_04760 | Hypothetical protein | −7.89188 | 1.204172 | 1.04 × 10−8 | 2.52 × 105 |
| Locus Tag | Protein Description | Log2 (Expression Level Ratio) | Standard Error of log2 (Expression LEVEL Ratio) | p-Value | q-Value |
|---|---|---|---|---|---|
| C. acnes | |||||
| HMPREF9571_ RS01535 | TIGR03773 family transporter- associated surface protein | −4.54224 | 0.606570082 | 5.22661 × 10−9 | 1.3051 × 10−5 |
| HMPREF9571_ RS02495 | Metal ABC transporter permease | −3.45326 | 0.607693267 | 5.41364 × 10−5 | 0.02703573 |
| HMPREF9571_ RS06980 | Choice-of-anchor M domain- containing protein | −3.83189 | 0.543910001 | 1.92397 × 10−7 | 0.00018143 |
| HMPREF9571_ RS11165 | 50S ribosomal protein L28 | −10.5324 | 1.839060929 | 2.17973 × 10−7 | 0.00018143 |
| HMPREF9571_ RS11175 | 30S ribosomal protein S14 | −4.65725 | 0.720441212 | 3.84643 × 10−7 | 0.00024011 |
| S. epidermidis | |||||
| B6C95_06200 | Hypothetical protein (Na-translocating system protein MpsB) | −5.14976 | 0.986638 | 2.6 × 10−5 | 0.031301 |
| B6C95_06205 | NADH-quinone oxidoreductase subunit L | −4.89638 | 0.922791 | 2.42 × 10−5 | 0.031301 |
| RNA-Seq | qPCR | ||||||
|---|---|---|---|---|---|---|---|
| Locus Tag | Protein Description | Log2 | |||||
| B6C95_03235 | Hypothetical protein | 4.02808 | no reaction | 12.1 | 1.8 | −1.3 | 1.3 |
| B6C95_04760 | Hypothetical protein | 7.89188 | −1.4 | 5 | 1.2 | −3.3 | −1.7 |
| RNA-Seq | qPCR | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus Tag | Protein Description | Log2 (Expression Level Ratio) | May 2022 | September 2022 | ||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 5 | 6 | |||
| C. acnes | ||||||||||||
| HMPREF9571_ RS01535 | TIGR03773 family transporter-associated surface protein | −4.54224 | 3 | 2.3 | −1.5 | 1.6 | 2.9 | 3.3 | −2.9 | 1.6 | −8.3 | 2.3 |
| HMPREF9571_ RS02495 | Metal ABC transporter permease | −3.45326 | 5.4 | 1.8 | −1.3 | 1.7 | 26.4 | 4.2 | −3 | 1.5 | −33 | 1.9 |
| HMPREF9571_ RS06980 | Choice-of-anchor M domain-containing protein | −3.83189 | 4.9 | 1.4 | −1.5 | 2 | 16.7 | 3.8 | −1.8 | 2 | −20 | 3.5 |
| HMPREF9571_ RS11165 | 50S ribosomal protein L28 | −10.5324 | 2.7 | 3.3 | −2.3 | 1.9 | −12.5 | 1.6 | ||||
| HMPREF9571_ RS11175 | 30S ribosomal protein S14 | −4.65725 | 3.9 | 1.6 | −1.5 | 1.3 | 7.8 | 2.9 | −2.5 | no reaction | −8.3 | 1.9 |
| S. epidermidis | ||||||||||||
| B6C95_06200 | Hypothetical protein (Na-translocating system protein MpsB) | −5.14976 | 12.1 | 2.6 | −25 | −1.6 | −100 | |||||
| B6C95_06205 | NADH-quinone oxidoreductase subunit L | −4.89638 | −5.5 | −5.5 | 2.9 | 1.5 | 2.4 | no reaction | −1.8 | 1.9 | −3.6 | −25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovcharova, M.A.; Schelkunov, M.I.; Geras’kina, O.V.; Makarova, N.E.; Sukhacheva, M.V.; Martyanov, S.V.; Nevolina, E.D.; Zhurina, M.V.; Feofanov, A.V.; Botchkova, E.A.; et al. C-Type Natriuretic Peptide Acts as a Microorganism-Activated Regulator of the Skin Commensals Staphylococcus epidermidis and Cutibacterium acnes in Dual-Species Biofilms. Biology 2023, 12, 436. https://doi.org/10.3390/biology12030436
Ovcharova MA, Schelkunov MI, Geras’kina OV, Makarova NE, Sukhacheva MV, Martyanov SV, Nevolina ED, Zhurina MV, Feofanov AV, Botchkova EA, et al. C-Type Natriuretic Peptide Acts as a Microorganism-Activated Regulator of the Skin Commensals Staphylococcus epidermidis and Cutibacterium acnes in Dual-Species Biofilms. Biology. 2023; 12(3):436. https://doi.org/10.3390/biology12030436
Chicago/Turabian StyleOvcharova, Maria A., Mikhail I. Schelkunov, Olga V. Geras’kina, Nadezhda E. Makarova, Marina V. Sukhacheva, Sergey V. Martyanov, Ekaterina D. Nevolina, Marina V. Zhurina, Alexey V. Feofanov, Ekaterina A. Botchkova, and et al. 2023. "C-Type Natriuretic Peptide Acts as a Microorganism-Activated Regulator of the Skin Commensals Staphylococcus epidermidis and Cutibacterium acnes in Dual-Species Biofilms" Biology 12, no. 3: 436. https://doi.org/10.3390/biology12030436
APA StyleOvcharova, M. A., Schelkunov, M. I., Geras’kina, O. V., Makarova, N. E., Sukhacheva, M. V., Martyanov, S. V., Nevolina, E. D., Zhurina, M. V., Feofanov, A. V., Botchkova, E. A., Plakunov, V. K., & Gannesen, A. V. (2023). C-Type Natriuretic Peptide Acts as a Microorganism-Activated Regulator of the Skin Commensals Staphylococcus epidermidis and Cutibacterium acnes in Dual-Species Biofilms. Biology, 12(3), 436. https://doi.org/10.3390/biology12030436







