Current and Potential Future Global Distribution of the Raisin Moth Cadra figulilella (Lepidoptera: Pyralidae) under Two Different Climate Change Scenarios
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. Distribution Data
2.1.2. Climate Data
2.2. Bioclimatic Modeling
2.2.1. CLIMEX Model
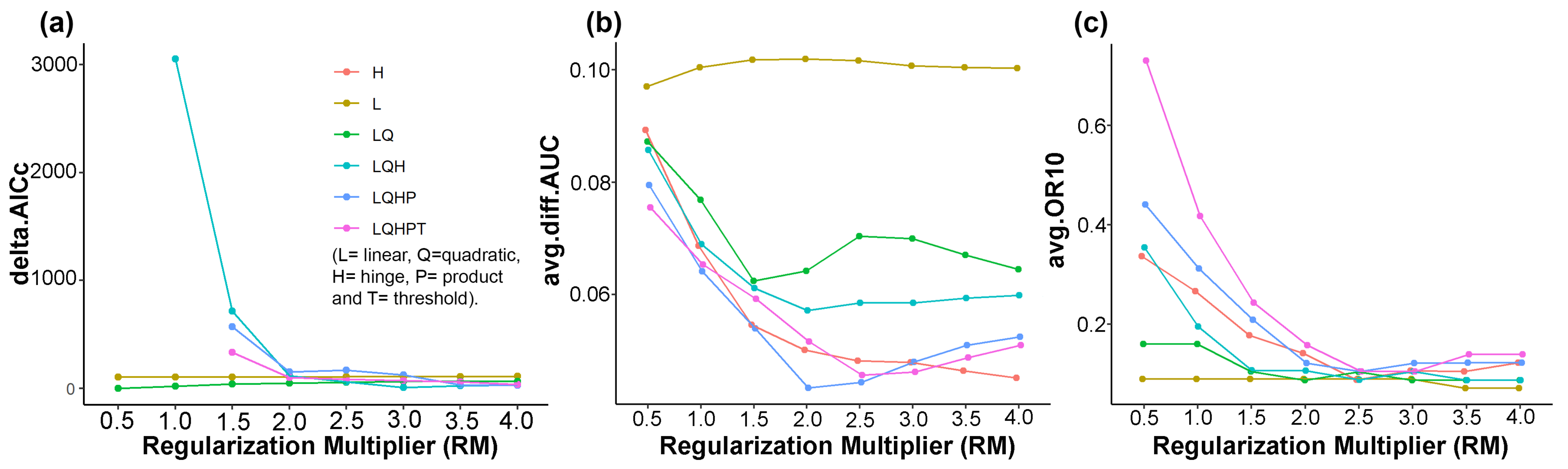
2.2.2. MaxEnt Model
3. Results
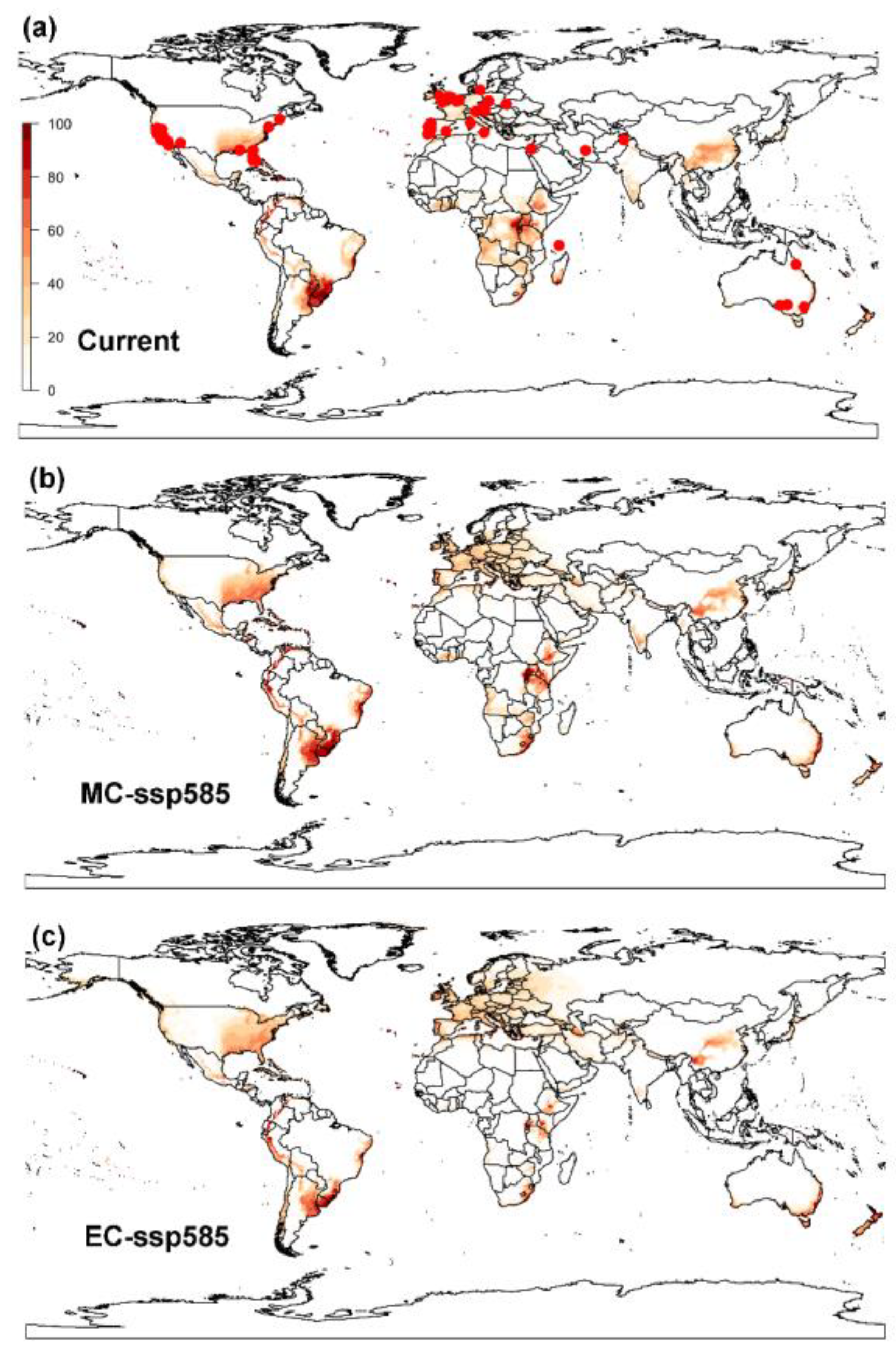
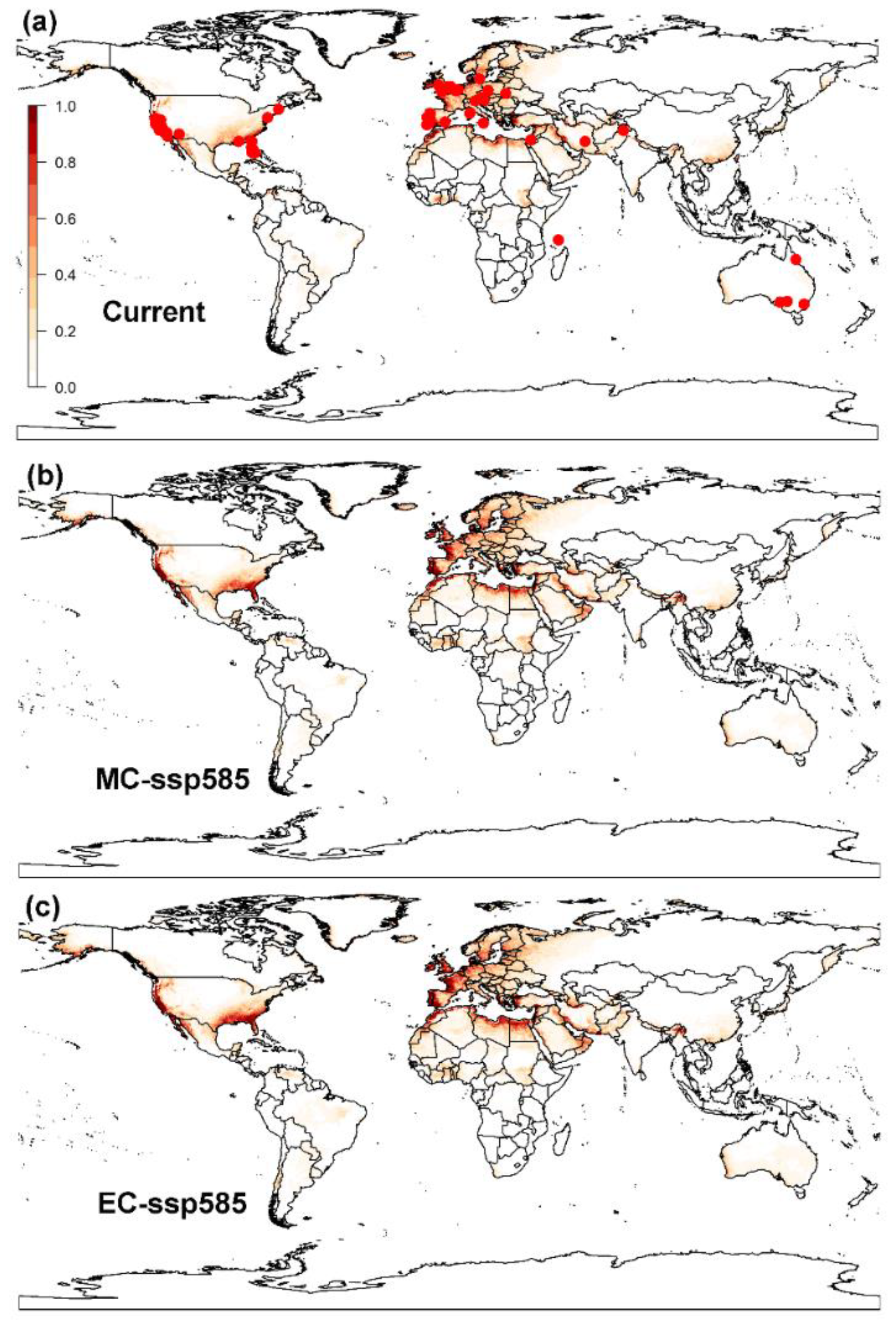
3.1. Projected Potential Distribution of the Raisin Moth under Current Climate Conditions
3.1.1. CLIMEX Model
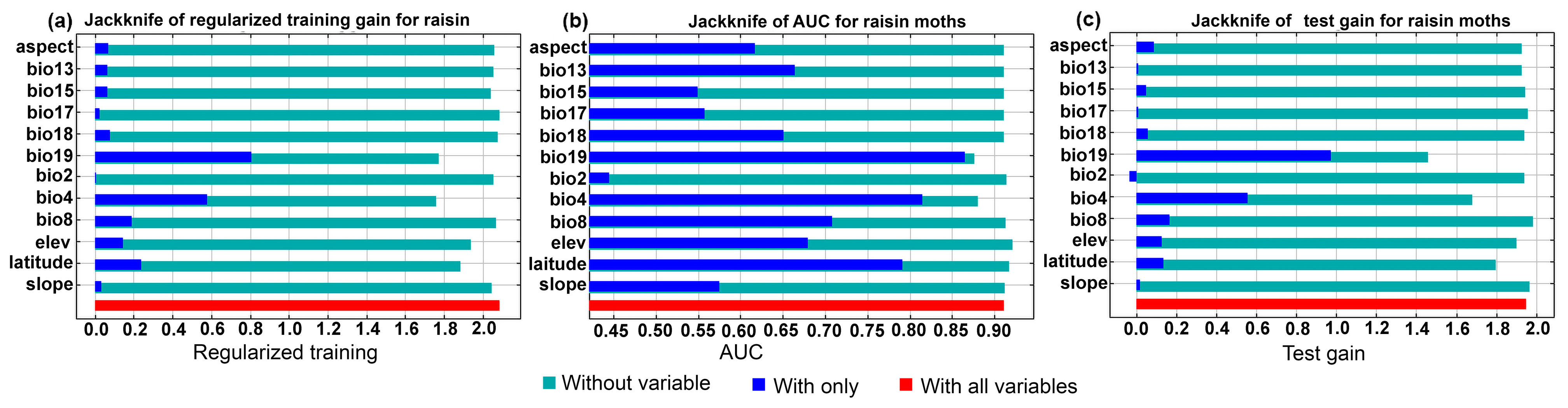
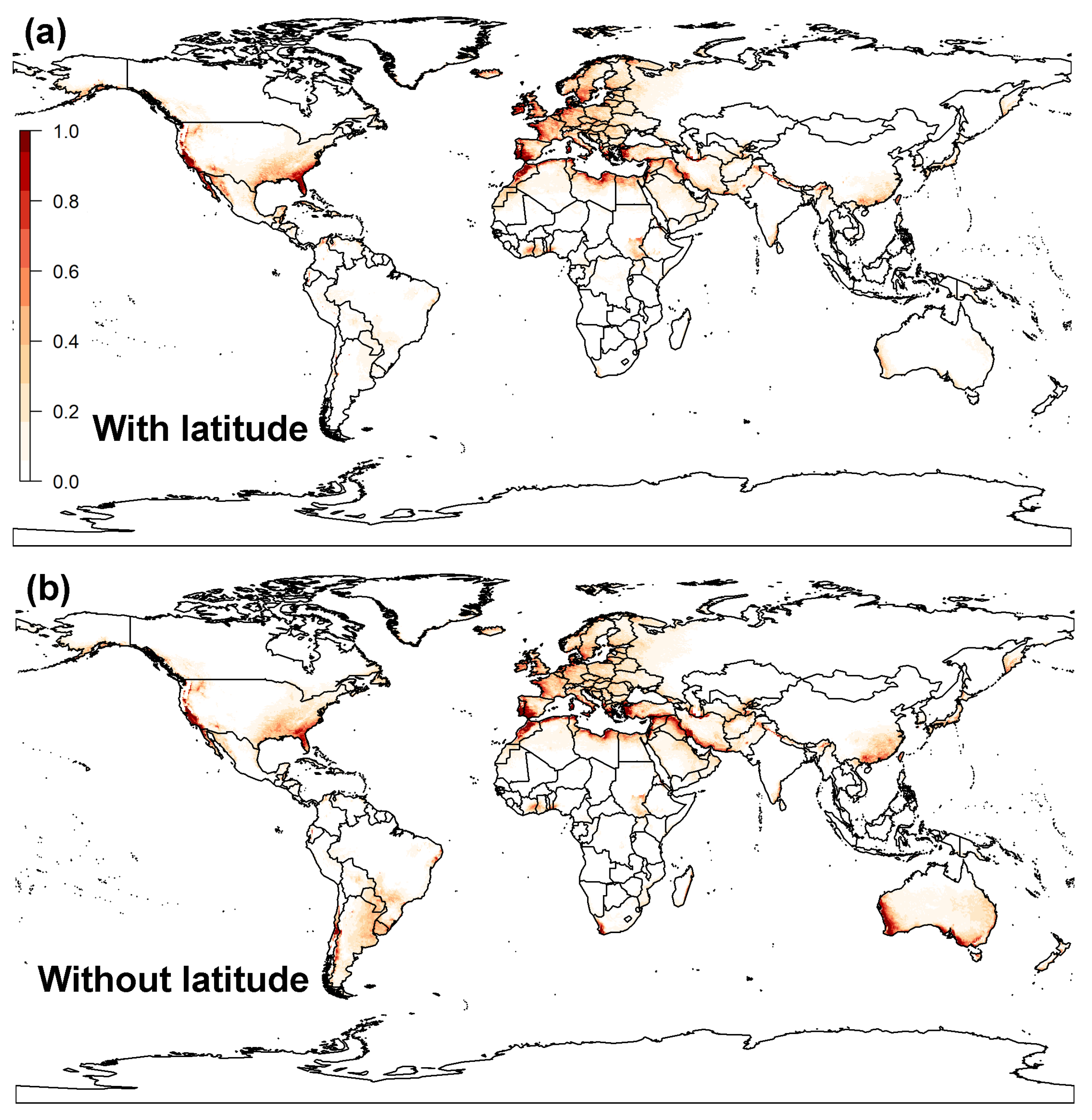
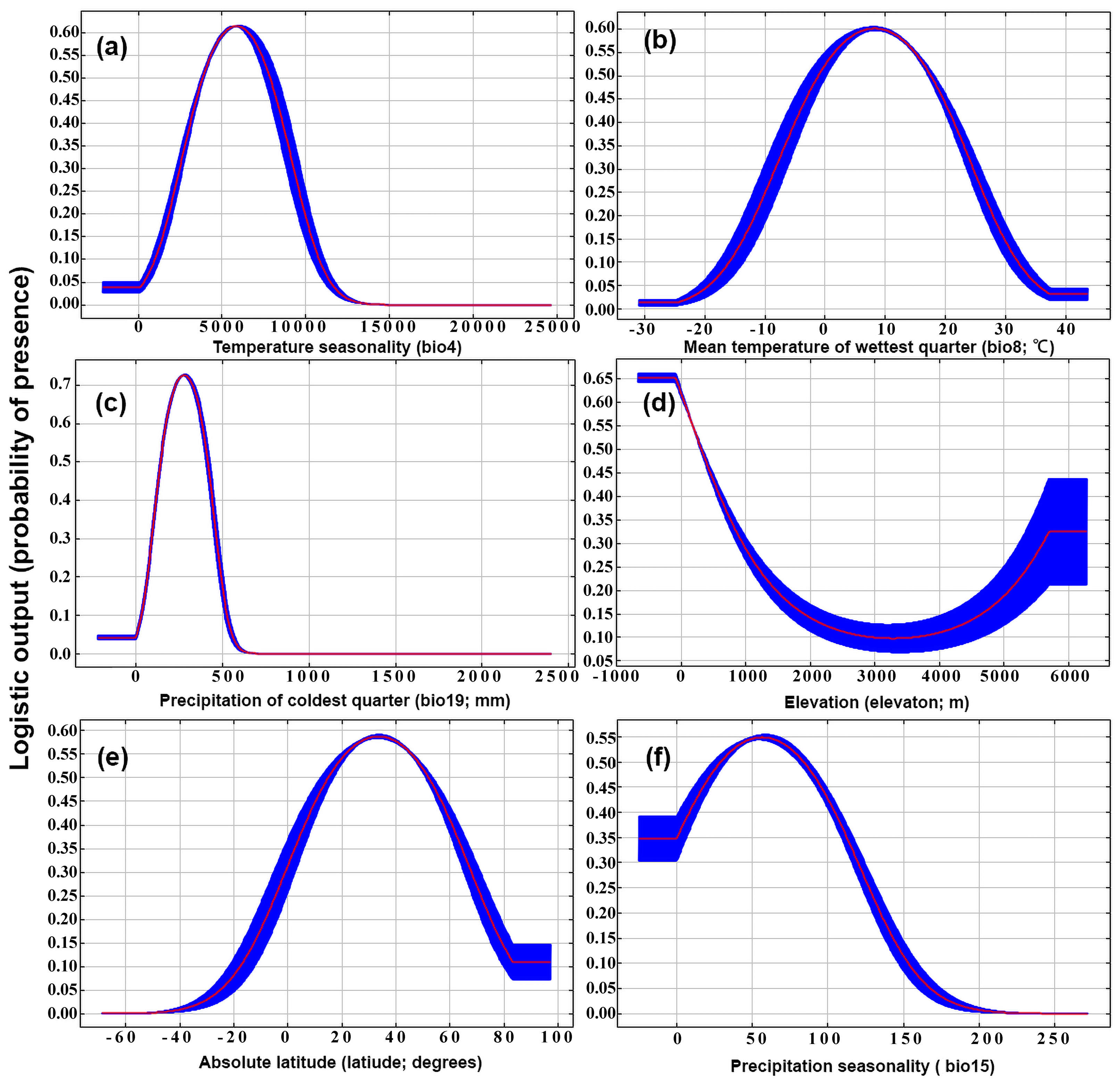
3.1.2. MaxEnt Model
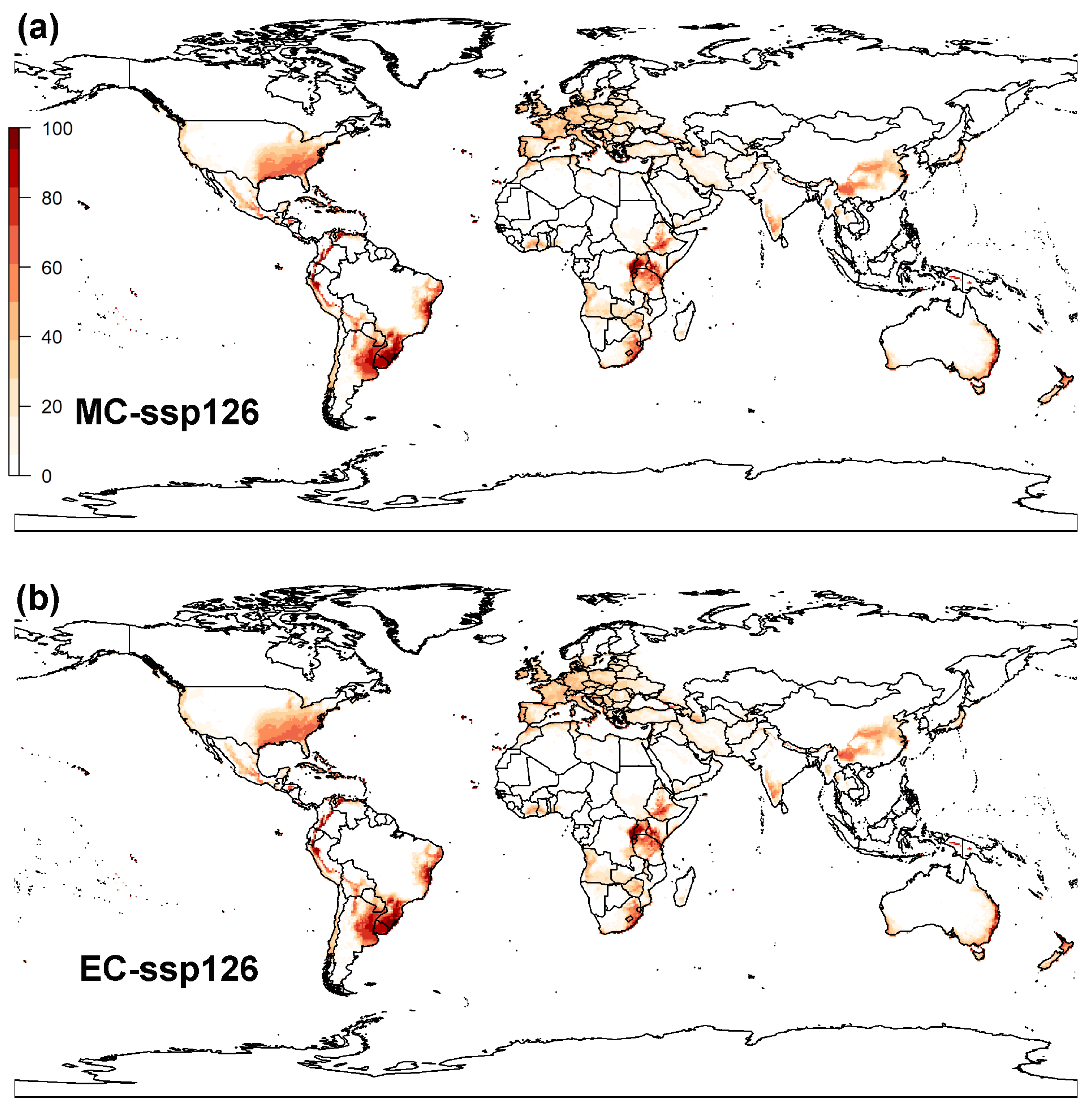
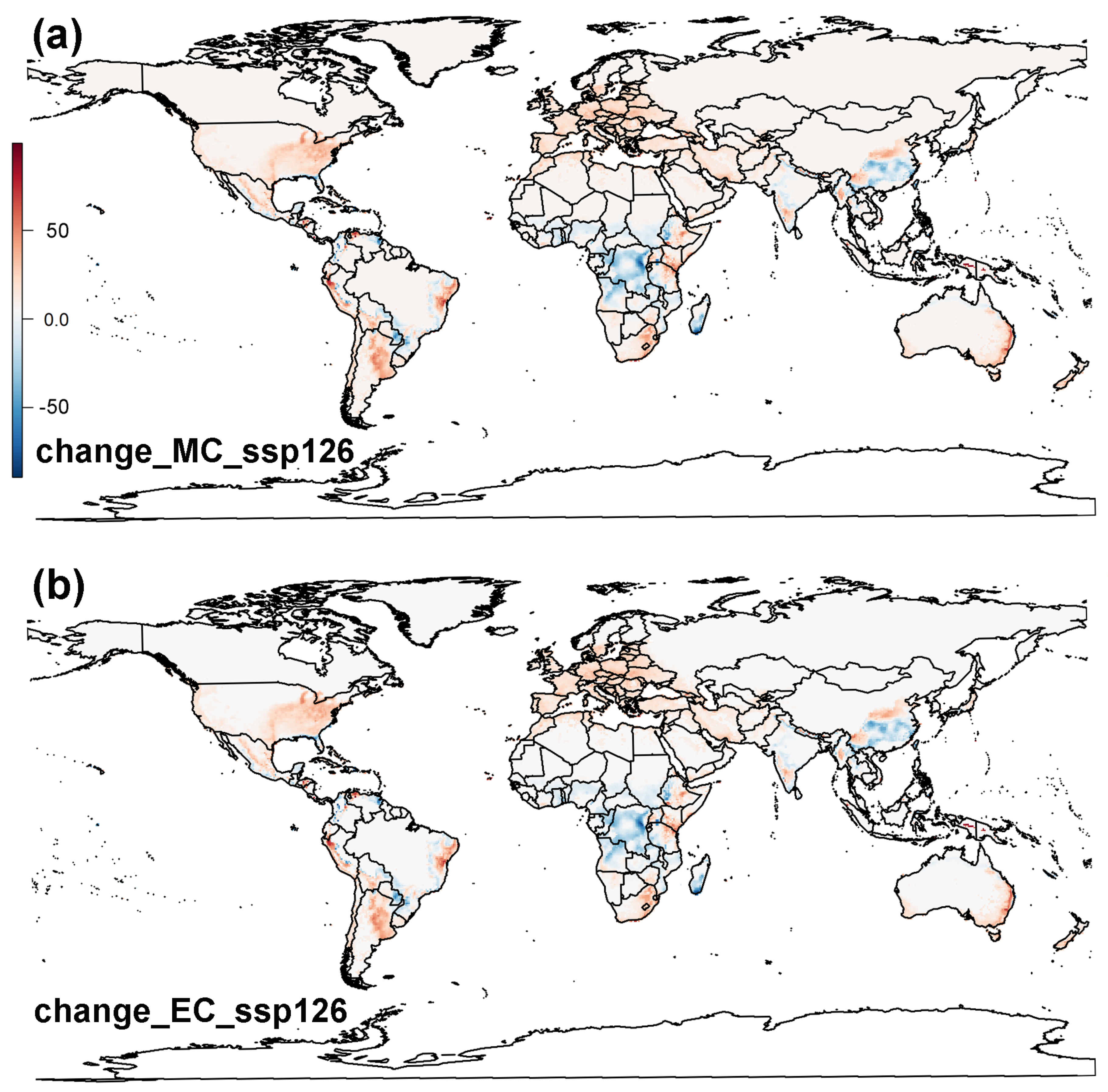
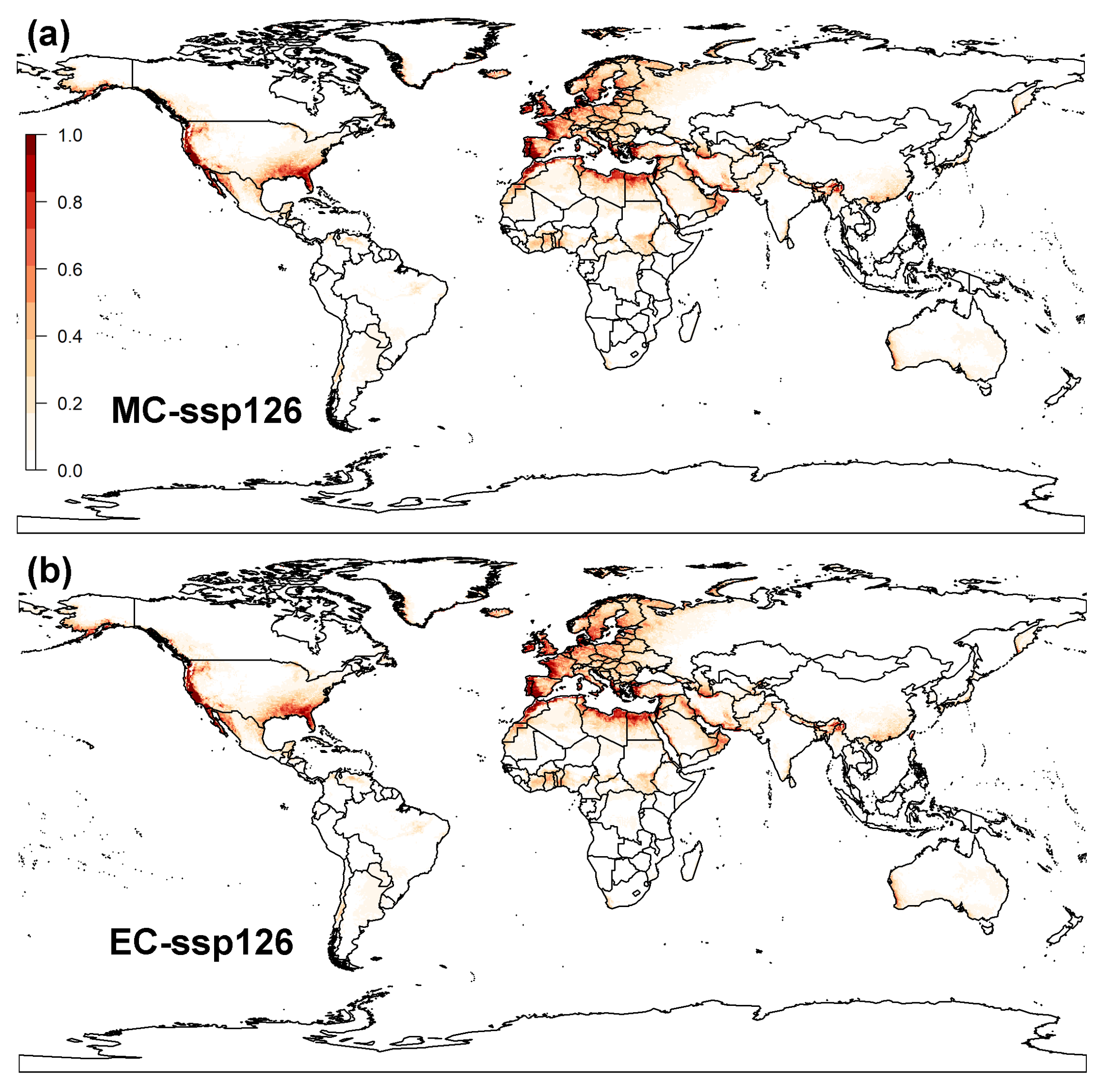
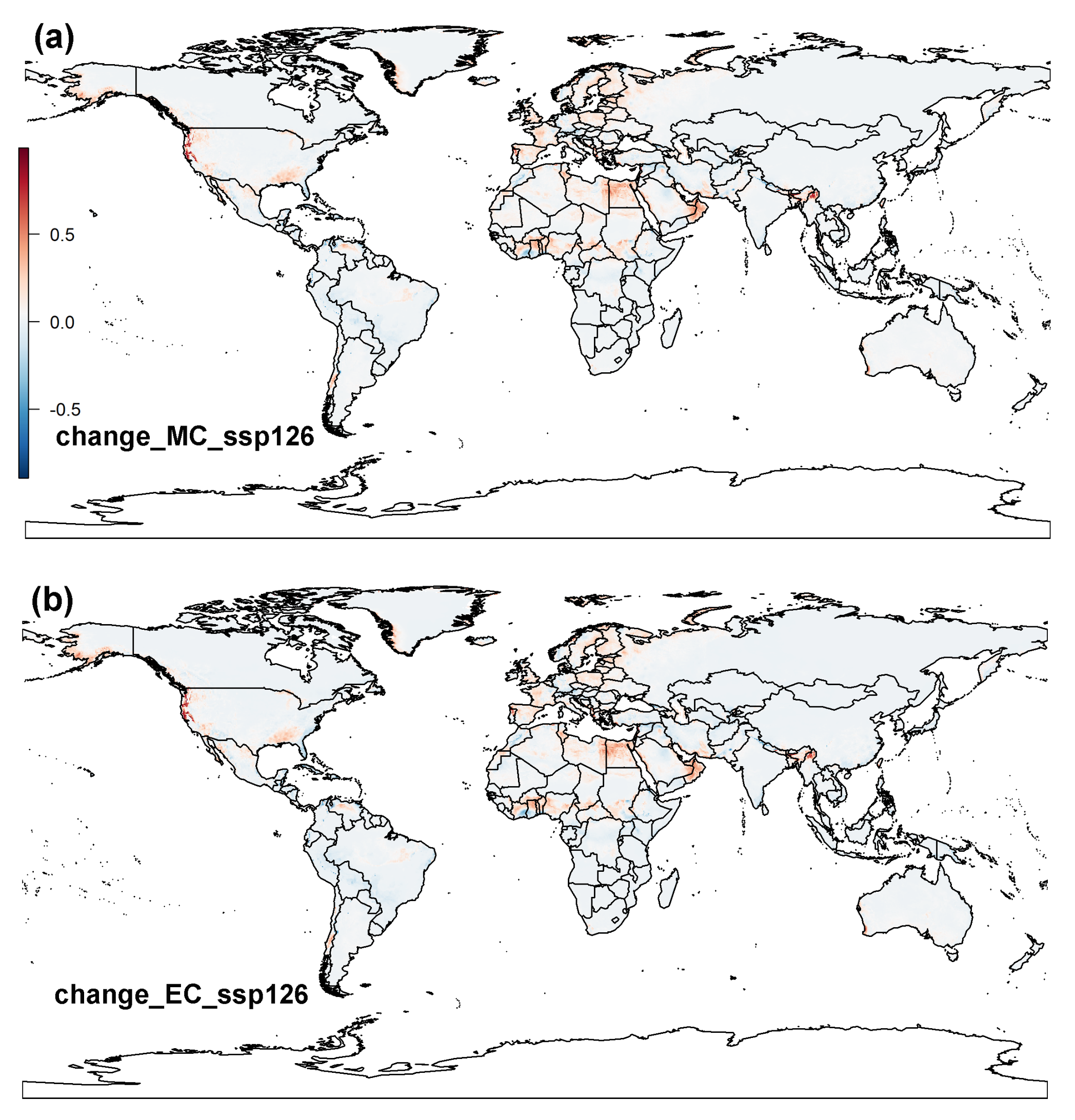
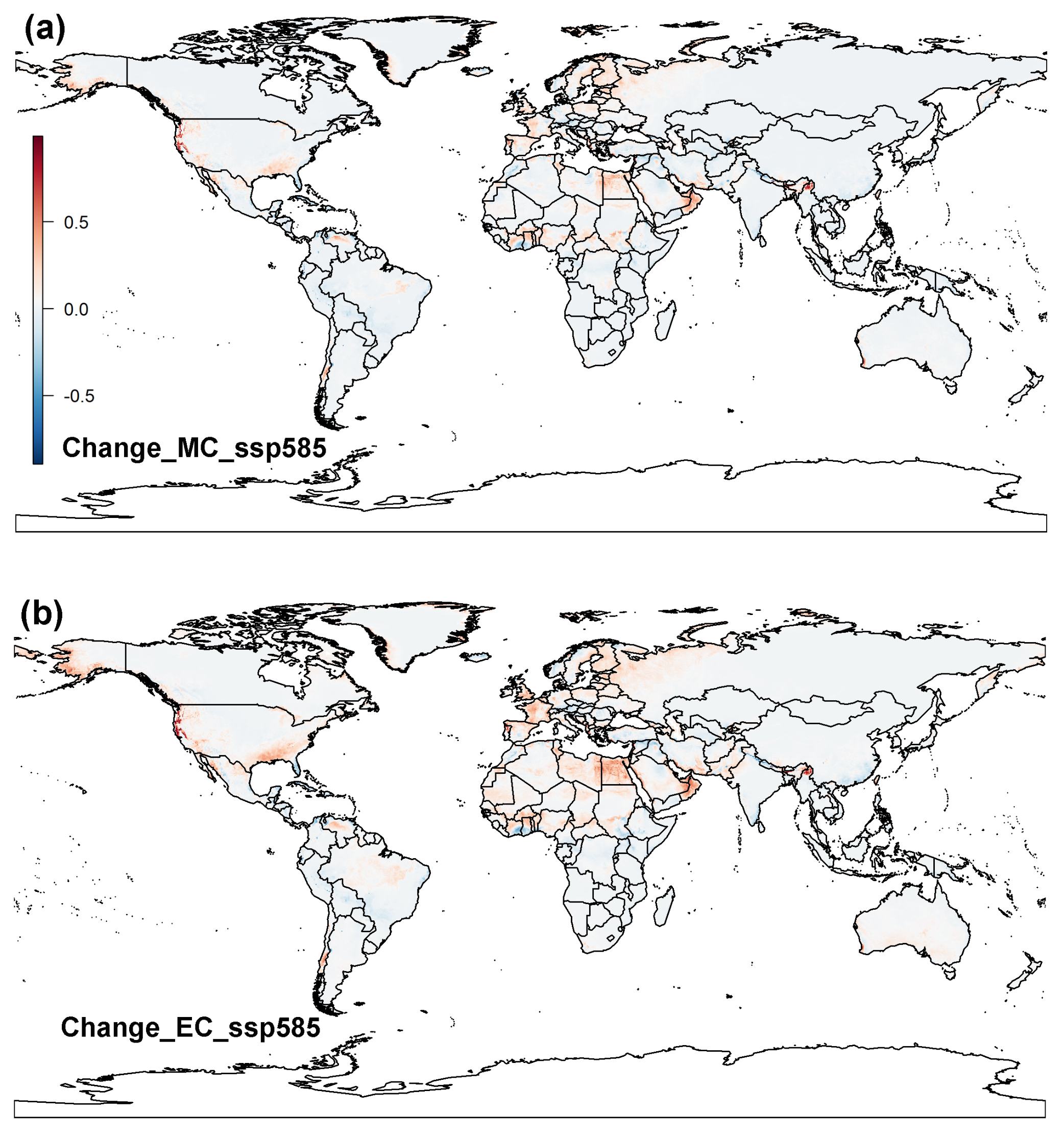
3.2. Projected Potential Distribution of Raisin Moth under Climate Change Scenarios
3.2.1. CLIMEX Model
3.2.2. MaxEnt Model
3.3. Global Distribution Prediction and the Dynamics Shift under Two SDMs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDMs | Species distribution models |
| CRU | Climate Research Unit |
| CMIP | Coupled Model Intercomparison Project |
| GCMs | Global Climate Models |
| SSP | Shared Socioeconomic Pathways |
| ELEV | Elevation |
| ASPE | Aspect |
| SLOP | Slope |
| EI | Ecoclimatic Index |
| RM | Regularization Multiplier |
| FC | Feature Combination |
| L | Linear |
| Q | Quadratic |
| H | Hinge |
| P | Product |
| T | Threshold |
| AICc | Akaike information criterion correction |
| AUC | Area Under the receiver operating characteristic Curve |
| ROC | Receiver Operating Characteristic |
| OR | Omission Rate |
| MC | Mid-Century |
| EC | End of this Century |
Appendix A
| No. | Model | Institute/Country | Reference |
|---|---|---|---|
| 1 | ACCESS-ESM1-5 | CSIRO-ARCCSS/Australia | [56] |
| 2 | BCC-CSM2-MR | BCC/China | [57] |
| 3 | CanESM5 | BCC/CCCma/Canada | [58] |
| 4 | CanESM5-CanOE | BCC/CCCma/Canada | [58] |
| 5 | CMCC-ESM2 | CMCC/Italy | [59] |
| 6 | CNRM-CM6-1 | CNRM-CERFACS/France | [60] |
| 7 | CNRM-CM6-1-HR | CNRM-CERFACS/France | [60] |
| 8 | CNRM-ESM2-1 | CNRM-CERFACS/France | [61] |
| 9 | EC-Earth3-Veg | EC-Earth-Consortium/Sweden | [62] |
| 10 | EC-Earth3-Veg-LR | EC-Earth-Consortium/Sweden | [62] |
| 11 | FIO-ESM-2-0 | FIO-QLNM/China | [63] |
| 12 | GISS-E2-1-G | NASA-GISS/USA | [64] |
| 13 | GISS-E2-1-H | NASA-GISS/USA | [64] |
| 14 | HadGEM3-GC31-LL | MOHC/UK | [65] |
| 15 | INM-CM4-8 | INM/Russia | [66] |
| 16 | INM-CM5-0 | INM/Russia | [67] |
| 17 | IPSL-CM6A-LR | IPSL/France | [68] |
| 18 | MIROC-ES2L | MIROC/Japan | [69] |
| 19 | MIROC6 | MIROC/Japan | [70] |
| 20 | MPI-ESM1-2-HR | MPIM/Germany | [71] |
| 21 | MPI-ESM1-2-LR | MPIM/Germany | [72] |
| 22 | MRI-ESM2-0 | MRI/Japan | [73] |
| 23 | UKESM1-0-LL | MOHC/UK | [74] |
| Environmental Variables | Abbreviation | Unites | Range |
|---|---|---|---|
| Annual Mean Temperature | bio1 | Degrees Celsius | −26.9–31.4 |
| Mean Diurnal Range (Mean of monthly (max temp-min temp)) | bio2 | Degrees Celsius | 0.9–21.1 |
| Isothermality (BIO2/BIO7) (×100) | bio3 | / | 8–95 |
| Temperature Seasonality (standard deviation ×100) | bio4 | / | 72–22,673 |
| Max Temperature of Warmest Month | bio5 | Degrees Celsius | −5.9–48.9 |
| Min Temperature of Coldest Month | bio6 | Degrees Celsius | −54.7–25.8 |
| Temperature Annual Range | bio7 | Degrees Celsius | 5.3–72.5 |
| Mean Temperature of Wettest Quarter | bio8 | Degrees Celsius | −25.1–37.5 |
| Mean Temperature of Driest Quarter | bio9 | Degrees Celsius | −45.0–36.4 |
| Mean Temperature of Warmest Quarter | bio10 | Degrees Celsius | −9.7–38.0 |
| Mean Temperature of Coldest Quarter | bio11 | Degrees Celsius | −48.8–28.9 |
| Annual Precipitation | bio12 | Millimeters | 0–9916 |
| Precipitation of Wettest Month | bio13 | Millimeters | 0–2088 |
| Precipitation of Driest Month | bio14 | Millimeters | 0–652 |
| Precipitation Seasonality (Coefficient of Variation) | bio15 | / | 0–261 |
| Precipitation of Wettest Quarter | bio16 | Millimeters | 0–5043 |
| Precipitation of Driest Quarter | bio17 | Millimeters | 0–2159 |
| Precipitation of Warmest Quarter | bio18 | Millimeters | 0–4001 |
| Precipitation of Coldest Quarter | bio19 | Millimeters | 0–3985 |
| Elevation | ELEV | Meters a.s.l. | −352–6251 |
| Aspect | ASPECT | Degrees | 0–360 |
| Slope | SLOP | rad | 0–7.893677 |
| Latitude | Lat | / | −59.42–83.58 |


References
- Blumberg, D. Date palm arthropod pests and their management in Israel. Phytoparasitica 2008, 36, 411–448. [Google Scholar] [CrossRef]
- Kellen, W.R.; Hoffmann, D.F. Occurrence of two baculoviruses in Cadra Figulilella (Lepidoptera: Pyralidae). J. Invertebr. Pathol. 1984, 43, 439–440. [Google Scholar] [CrossRef]
- Cox, P.D. The influence of temperature and humidity on the life-cycles of Ephestia Figulilella Gregson Ephestia Calidella (Guenée) (Lepidoptera: Phycitidae). J. Stored Prod. Res. 1974, 10, 43–55. [Google Scholar] [CrossRef]
- Assari, M.J.; Khajepour, S. Population fluctuation of Ephestia Figulilella Kerman Prov. Iran. Arch. Phytopathol. Plant Prot. 2013, 46, 862–867. [Google Scholar] [CrossRef]
- Burks, C.S.; Johnson, J.A. Biology, behavior, and ecology of stored fruit and nut insects. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 26–32. [Google Scholar]
- Perring, T.M.; El-Shafie, H.A.F.; Wakil, W. Carob Moth, Lesser Date Moth, and Raisin Moth. In Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges; Springer: Berlin/Heidelberg, Germany, 2015; pp. 109–167. [Google Scholar] [CrossRef]
- Donohoe, H.C.; Simmons, P.; Barnes, D.F.; Kaloostian, G.H.; Heinrich, C. Biology of the Raisin Moth; Technical Report 9781119130536; United States Department of Agriculture: Washington, DC, USA, 1949; Volume 994.
- Velcheva, N.; Atanassov, A. Records of Cadra Figulilella (Gregson 1871) (Pyralidae, Lepidoptera) Contrib. Its Parasit. Assam. Bulgaria. Bulg. J. Agric. Sci. 2015, 21, 1254–1256. [Google Scholar]
- Carpenter, J.B.; McMillen, J.M.; Wengert, E.M.; Elmer, H.S. Pests and Diseases of the Date Palm (No. 526–528); US Department of Agriculture, Science and Education Administration: Washington, DC, USA, 1978.
- Oiv, F. FAO-OIV Focus Table and Dried Grapes; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; Volume I7042, pp. 1–64. [Google Scholar]
- Zhang, W.; Bai, M.; Lu, X.; Guo, Q.; Leng, J.; Luo, C.; Luo, R. Grape varieties and key cultivation techniques suitable for cultivation in thermal area. Bot. Res. 2022, 11, 659–668. [Google Scholar] [CrossRef]
- Ma, G.; Ma, C.S. Potential distribution of invasive crop pests under climate change: Incorporating mitigation responses of insects into prediction models. Curr. Opin. Insect. Sci. 2022, 49, 15–21. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Kriticos, D.; Maywald, G.F.; Yonow, T.; Zurcher, E.J.; Herrmann, N.I.; Sutherst, R.W. CLIMEX Version 4: Exploring the Effects of Climate on Plants, Animals and Diseases; CSIRO: Canberra, Australia, 2015. [Google Scholar]
- Venette, R.C.; Kriticos, D.J.; Magarey, R.D.; Koch, F.H.; Baker, R.H.; Worner, S.P.; Raboteaux, N.N.; McKenney, D.W.; Dobesberger, E.J.; Yemshanov, D.; et al. Pest risk maps for invasive alien species: A roadmap for improvement. BioScience 2010, 60, 349–362. [Google Scholar] [CrossRef]
- Tonnang, H.E.; Hervé, B.D.; Biber-Freudenberger, L.; Salifu, D.; Subramanian, S.; Ngowi, V.B.; Guimapi, R.Y.; Anani, B.; Kakmeni, F.M.; Affognon, H.; et al. Advances in crop insect modelling methods—Towards a whole system approach. Ecol. Model. 2017, 354, 88–103. [Google Scholar] [CrossRef]
- Maino, J.L.; Kong, J.D.; Hoffmann, A.A.; Barton, M.G.; Kearney, M.R. Mechanistic models for predicting insect responses to climate change. Curr. Opin. Insect. Sci. 2016, 17, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Neven, L.G.; Zhu, H.; Zhang, R. Assessing the global risk of establishment of Cydia Pomonella (Lepidoptera: Tortricidae) Using CLIMEX MaxEnt Niche Model. J. Econ. Entomol. 2015, 108, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, D.E.; Lee, H.; Jung, S.; Lee, W.H. Ensemble evaluation of the potential risk areas of yellow-legged hornet distribution. Environ. Monit. Assess. 2021, 193, 601. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; Rwomushana, I.; Chipabika, G.; Day, R. Comparing, evaluating and combining statistical species distribution models and CLIMEX to forecast the distributions of emerging crop pests. Pest Manag. Sci. 2022, 78, 671–683. [Google Scholar] [CrossRef]
- Suma, P.; La Pergola, A.; Bella, S.; Russo, A. Olfactometer responses of a wild strain of the parasitic wasp Venturia Canescens (Hymenoptera, Ichneumonidae) Obtained Its Nat. Host Cadra Figulilella (Lepidoptera, Pyralidae) Odours Three Stored Food Prod. Infested Pyralid Pests. J. Stored Prod. Res. 2014, 59, 55–60. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- University of East Anglia Climatic Research Unit; Harris, I.C.; Jones, P.D.; Osborn, T. CRU TS4.05: Climatic Research Unit (CRU) Time-Series (TS) Version 4.05 of High-Resolution Gridded Data of Month-by-Month Variation in Climate (January 1901–December 2020); NERC EDS Centre for Environmental Data Analysis: Chilton, UK, 2021. [Google Scholar]
- Sockman, K.W.; Hurlbert, A.H. How the effects of latitude on daylight availability may have influenced the evolution of migration and photoperiodism. Funct. Ecol. 2020, 34, 1752–1766. [Google Scholar] [CrossRef]
- Cox, P.D. The influence of photoperiod on the life-cycles of Ephestia Calidella (Guenée) Ephestia Figulilella Gregson (Lepidoptera: Phycitidae). J. Stored Prod. Res. 1975, 11, 75–85. [Google Scholar] [CrossRef]
- Sutherst, R.W. Prediction of species geographical ranges. J. Biogeogr. 2003, 30, 805–816. [Google Scholar] [CrossRef]
- Avila, G.A.; Davidson, M.; Van Helden, M.; Fagan, L. The potential distribution of the Russian wheat aphid (Diuraphis Noxia): Updat. Distrib. Model Incl. Irrig. Improv. Model Fit Predict. Potential Spread. Bull. Entomol. Res. 2018, 109, 90–101. [Google Scholar] [CrossRef]
- Siebert, S.; Henrich, V.; Frenken, K.; Burke, J. Global Map of Irrigation Areas Version 5; Rheinische Friedrich-Wilhelms: Bonn, Germany; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 2, pp. 1299–1327. [Google Scholar]
- Cox, P.D.; Crawford, L.A.; Gjestrud, G.; Bell, C.H.; Bowley, C.R. The influence of temperature and humidity on the life-cycle of Corcyra Cephalonica (Stainton) (Lepidoptera: Pyralidae). Bull. Entomol. Res. 1981, 71, 171–181. [Google Scholar] [CrossRef]
- Wiik, L.; Ewaldz, T. Impact of temperature and precipitation on yield and plant diseases of winter wheat in Southern Sweden 1983–2007. Crop Prot. 2009, 28, 952–962. [Google Scholar] [CrossRef]
- Ahmad, T.R.; Ali, M.A. Forecasting emergence and flight of some Ephestia Spp. (Lep., Pyralidae) Based Pheromone Trapp. Degree-day Accumulations. J. Appl. Entomol. 1995, 119, 611–614. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, R. Multimodel Inference: Understanding AIC and BIC in model selection. Sociol. Methods Res 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological niches and geographic distributions (MPB-49). In Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic predictors for supporting ecological applications in the conterminous United States. U. S. Geol. Surv. Data Ser. 2012, 691, 4–9. [Google Scholar]
- Narouei-Khandan, H.A.; Worner, S.P.; Viljanen, S.L.; van Bruggen, A.H.; Jones, E.E. Projecting the suitability of global and local habitats for myrtle rust (Austropuccinia Psidii) Using Model Consensus. Plant Pathol. 2020, 69, 17–27. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef]
- Nawrot, J. Effect of temperature and relative humidity on population parameters for almond moth (Cadra Cautella Wlk.)(Lepid. Phycitidae). Pr. Nauk. Inst. Ochr. Roślin 1979, 21, 41–52. [Google Scholar]
- Mohandass, S.; Arthur, F.H.; Zhu, K.Y.; Throne, J.E. Biology and management of Plodia Interpunctella (Lepidoptera: Pyralidae) Stored Prod. J. Stored Prod. Res. 2007, 43, 302–311. [Google Scholar] [CrossRef]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Wang, K.; Sun, J.; Cheng, G.; Jiang, H. Effect of altitude and latitude on surface air temperature across the Qinghai-Tibet Plateau. J. Mt. Sci. 2011, 8, 808–816. [Google Scholar] [CrossRef]
- Jones, V.P.; Hilton, R.; Brunner, J.F.; Bentley, W.J.; Alston, D.G.; Barrett, B.; Van Steenwyk, R.A.; Hull, L.A.; Walgenbach, J.F.; Coates, W.W.; et al. Predicting the emergence of the codling moth, Cydia Pomonella(Lepidoptera: Tortricidae), A Degree-Day Scale North America. Pest Manag. Sci. 2013, 69, 1393–1398. [Google Scholar] [CrossRef]
- Zou, Y.; Ge, X.; Guo, S.; Zhou, Y.; Wang, T.; Zong, S. Impacts of climate change and host plant availability on the global distribution of Brontispa Longissima (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2019, 76, 244–256. [Google Scholar] [CrossRef]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Dillon, G.R.; De Ruffray, P.; Vidal, C.; Pierrat, J.C.; Gégout, J.C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.; Xiang, W.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Zhu, L.; Hoffmann, A.A.; Li, S.; Ma, C. Extreme climate shifts pest dominance hierarchy through thermal evolution and transgenerational plasticity. Funct. Ecol. 2021, 35, 1524–1537. [Google Scholar] [CrossRef]
- Ma, G.; Bai, C.M.; Rudolf, V.H.W.; Ma, C.S. Night warming alters mean warming effects on predator–prey interactions by modifying predator demographics and interaction strengths. Funct. Ecol. 2021, 35, 2094–2107. [Google Scholar] [CrossRef]
- Wang, B.X.; Hof, A.R.; Ma, C.S. Impacts of climate change on crop production, pests and pathogens of wheat and rice. Front. Agric. Sci. Eng. 2022, 9, 4–18. [Google Scholar] [CrossRef]
- Soderstrom, E.L.; Lovitt, A.E. Interspecific competition of almond moth, Indian meal moth, and raisin moth in Malathion-treated and untreated almonds. J. Econ. Entomol. 1973, 66, 742–745. [Google Scholar] [CrossRef]
- Johnson, J.A.; Wofford, P.L.; Whitehand, L.C. Effect of diet and temperature on development rates, survival, and reproduction of the Indian meal moth (Lepidoptera: Pyralidae). J. Econ. Entomol. 1992, 85, 561–566. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Lissovsky, A.A.; Dudov, S. Species-Distribution Modeling: Advantages and limitations of Its application. 2. MaxEnt. Biol. Bull. Rev. 2021, 11, 265–275. [Google Scholar] [CrossRef]
- Ziehn, T.; Chamberlain, M.A.; Law, R.M.; Lenton, A.; Bodman, R.W.; Dix, M.; Stevens, L.; Wang, Y.P.; Srbinovsky, J. The Australian earth system model: ACCESS-ESM1. 5. J. South. Hemisph. Earth Syst. Sci. 2020, 70, 193–214. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L. The Beijing Climate Center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Swart, N.C.; Cole, J.N.S.; Kharin, V.V.; Lazare, M.; Scinocca, J.F.; Gillett, N.P.; Anstey, J.; Arora, V.; Christian, J.R.; Hanna, S. The Canadian earth system model version 5 (CanESM5. 0.3). Geosci. Model Dev. 2019, 12, 4823–4873. [Google Scholar] [CrossRef]
- Lovato, T.; Peano, D.; Butenschön, M.; Materia, S.; Iovino, D.; Scoccimarro, E.; Fogli, P.G.; Cherchi, A.; Bellucci, A.; Gualdi, S. CMIP6 Simulations With the CMCC Earth System Model (CMCC-ESM2). J. Adv. Model. Earth Syst. 2022, 14, e2021MS002814. [Google Scholar] [CrossRef]
- Voldoire, A.; Saint-Martin, D.; Sénési, S.; Decharme, B.; Alias, A.; Chevallier, M.; Colin, J.; Guérémy, J.; Michou, M.; Moine, M. Evaluation of CMIP6 deck experiments with CNRM-CM6-1. J. Adv. Model. Earth Syst. 2019, 11, 2177–2213. [Google Scholar] [CrossRef]
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M. Evaluation of CNRM earth system model, CNRM-ESM2-1: Role of earth system processes in present-day and future climate. J. Adv. Model. Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef]
- Wyser, K.; Kjellström, E.; Koenigk, T.; Martins, H.; Döscher, R. Warmer climate projections in EC-Earth3-Veg: The role of changes in the greenhouse gas concentrations from CMIP5 to CMIP6. Environ. Res. Lett. 2020, 15, 54020. [Google Scholar] [CrossRef]
- Bracegirdle, T.J.; Holmes, C.R.; Hosking, J.S.; Marshall, G.J.; Osman, M.; Patterson, M.; Rackow, T. Improvements in circumpolar Southern Hemisphere extratropical atmospheric circulation in CMIP6 compared to CMIP5. Earth Space Sci. 2020, 7, e2019EA001065. [Google Scholar] [CrossRef]
- Kelley, M.; Schmidt, G.A.; Nazarenko, L.S.; Bauer, S.E.; Ruedy, R.; Russell, G.L.; Ackerman, A.S.; Aleinov, I.; Bauer, M.; Bleck, R. GISS-E2. 1: Configurations and climatology. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002025. [Google Scholar] [CrossRef]
- Roberts, M.J.; Baker, A.; Blockley, E.W.; Calvert, D.; Coward, A.; Hewitt, H.T.; Jackson, L.C.; Kuhlbrodt, T.; Mathiot, P.; Roberts, C.D. Description of the resolution hierarchy of the global coupled HadGEM3-GC3. 1 model as used in CMIP6 HighResMIP experiments. Geosci. Model Dev. 2019, 12, 4999–5028. [Google Scholar] [CrossRef]
- Volodin, E. The mechanisms of cloudiness evolution responsible for equilibrium climate sensitivity in climate model INM-CM4-8. Geophys. Res. Lett. 2021, 48, e2021GL096204. [Google Scholar] [CrossRef]
- Volodin, E. The mechanism of 60-year and 15-year Arctic climate oscillations in climate model INM-CM5-0. In EGU General Assembly Conference Abstracts; Institute of Numerical Mathematics, Russian Academy of Science: Moscow, Russia, 2020; p. 7265. [Google Scholar]
- Bonnet, R.; Boucher, O.; Deshayes, J.; Gastineau, G.; Hourdin, F.; Mignot, J.; Servonnat, J.; Swingedouw, D. Presentation and Evaluation of the IPSL-CM6A-LR Ensemble of Extended Historical Simulations. J. Adv. Model. Earth Syst. 2021, 13, e2021MS002565. [Google Scholar] [CrossRef]
- Hajima, T.; Watanabe, M.; Yamamoto, A.; Tatebe, H.; Noguchi, M.A.; Abe, M.; Ohgaito, R.; Ito, A.; Yamazaki, D.; Okajima, H. Development of the MIROC-ES2L earth system model and the evaluation of biogeochemical processes and feedbacks. Geosci. Model Dev. 2020, 13, 2197–2244. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Müller, W.A.; Jungclaus, J.H.; Mauritsen, T.; Baehr, J.; Bittner, M.; Budich, R.; Bunzel, F.; Esch, M.; Ghosh, R.; Haak, H.; et al. A higher-resolution version of the Max Planck Institute Earth System Model (MPI-ESM1.2-HR). J. Adv. Model. Earth Syst. 2018, 10, 1383–1413. [Google Scholar] [CrossRef]
- Mauritsen, T.; Bader, J.; Becker, T.; Behrens, J.; Bittner, M.; Brokopf, R.; Brovkin, V.; Claussen, M.; Crueger, T.; Esch, M. Developments in the MPI-M Earth System Model version 1.2 (MPI-ESM1.2) and its response to increasing CO2. J. Adv. Model. Earth Syst. 2019, 11, 998–1038. [Google Scholar] [CrossRef] [PubMed]
- Yukimoto, S.; Kawai, H.; Koshiro, T.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M. The Meteorological Research Institute Earth System Model version 2.0, MRI-ESM2.0: Description and basic evaluation of the physical component. J. Meteorol. Soc. Jpn. 2019, 97, 931–965. [Google Scholar] [CrossRef]
- Sellar, A.A.; Jones, C.G.; Mulcahy, J.P.; Tang, Y.; Yool, A.; Wiltshire, A.; O’connor, F.M.; Stringer, M.; Hill, R.; Palmieri, J. UKESM1: Description and evaluation of the UK Earth System Model. J. Adv. Model. Earth Syst. 2019, 11, 4513–4558. [Google Scholar] [CrossRef]
| Parameters | Descriptions | C. figulilella |
|---|---|---|
| Temperature | ||
| DV0 | Lower temperature threshold (°C) | 13 |
| DV1 | Lower optimum temperature (°C) | 15 |
| DV2 | Upper optimum temperature (°C) | 30 |
| DV3 | Upper temperature threshold (°C) | 36 |
| Moisture | ||
| SM0 | Lower soil moisture threshold (°C) | 0.25 |
| SM1 | Lower optimal soil moisture (°C) | 0.8 |
| SM2 | Upper optimal soil moisture (°C) | 1.5 |
| SM3 | Upper soil moisture threshold (°C) | 2.5 |
| Cold stress | ||
| TTCS | Cold stress temperature threshold (°C) | 0 |
| THCS | Cold stress temperature rate (week−1) | −0.001 |
| Heat stress | ||
| TTHS | Heat stress temperature threshold (°C) | 36 |
| THHS | Heat stress temperature rate (week−1) | 0.0001 |
| Dry stress | ||
| SMDS | Dry stress threshold | 0.02 |
| HDS | Dry stress rate (week−1) | −0.05 |
| Wet stress | ||
| SMWS | Wet stress threshold | 2.5 |
| HWS | Wet stress rate (week−1) | 0.0015 |
| Hot-Wet stress | ||
| TTHW | Hot-wet maximum temperature threshold (°C) | 23 |
| MTHW | Hot-wet moisture threshold | 1.35 |
| PHW | Hot-wet stress accumulation rate (week−1) | 0.075 |
| PDD | Effective accumulated temperature (degree-days) | 292 |
| Variable | Descriptions | Percent Contribution | Permutation Importance |
|---|---|---|---|
| bio4 | Temperature Seasonality (standard deviation ×100) | 23.3 | 37.1 |
| bio8 | Mean Temperature of Wettest Quarter | 18.3 | 1.6 |
| bio19 | Precipitation of Coldest Quarter | 17.7 | 32.8 |
| elev | Elevation | 14 | 5 |
| latitude | Latitude | 12 | 9.6 |
| bio15 | Precipitation Seasonality (Coefficient of Variation) | 3 | 2.5 |
| bio17 | Precipitation of Driest Quarter | 2.8 | 0.5 |
| bio2 | Mean Diurnal Range (Mean of monthly (max temp–min temp)) | 2.6 | 0.7 |
| aspect | Aspect | 2.2 | 0.4 |
| bio13 | Precipitation of Wettest Month | 2.2 | 4.7 |
| slope | Slope | 1 | 2 |
| bio18 | Precipitation of Warmest Quarter | 0.9 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-X.; Zhu, L.; Ma, G.; Najar-Rodriguez, A.; Zhang, J.-P.; Zhang, F.; Avila, G.A.; Ma, C.-S. Current and Potential Future Global Distribution of the Raisin Moth Cadra figulilella (Lepidoptera: Pyralidae) under Two Different Climate Change Scenarios. Biology 2023, 12, 435. https://doi.org/10.3390/biology12030435
Wang B-X, Zhu L, Ma G, Najar-Rodriguez A, Zhang J-P, Zhang F, Avila GA, Ma C-S. Current and Potential Future Global Distribution of the Raisin Moth Cadra figulilella (Lepidoptera: Pyralidae) under Two Different Climate Change Scenarios. Biology. 2023; 12(3):435. https://doi.org/10.3390/biology12030435
Chicago/Turabian StyleWang, Bing-Xin, Liang Zhu, Gang Ma, Adriana Najar-Rodriguez, Jin-Ping Zhang, Feng Zhang, Gonzalo A. Avila, and Chun-Sen Ma. 2023. "Current and Potential Future Global Distribution of the Raisin Moth Cadra figulilella (Lepidoptera: Pyralidae) under Two Different Climate Change Scenarios" Biology 12, no. 3: 435. https://doi.org/10.3390/biology12030435
APA StyleWang, B.-X., Zhu, L., Ma, G., Najar-Rodriguez, A., Zhang, J.-P., Zhang, F., Avila, G. A., & Ma, C.-S. (2023). Current and Potential Future Global Distribution of the Raisin Moth Cadra figulilella (Lepidoptera: Pyralidae) under Two Different Climate Change Scenarios. Biology, 12(3), 435. https://doi.org/10.3390/biology12030435








