Promoter Cis-Element Analyses Reveal the Function of αVPE in Drought Stress Response of Arabidopsis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Drought Treatment
2.3. Promoter Sequence Analysis
2.4. Co-Expression Network Modeling
2.5. Expression Analysis of AtVPEs upon Drought Treatment
2.6. Plant Water Status
2.7. Biochemical Assays
2.8. Statistical Analysis
3. Results
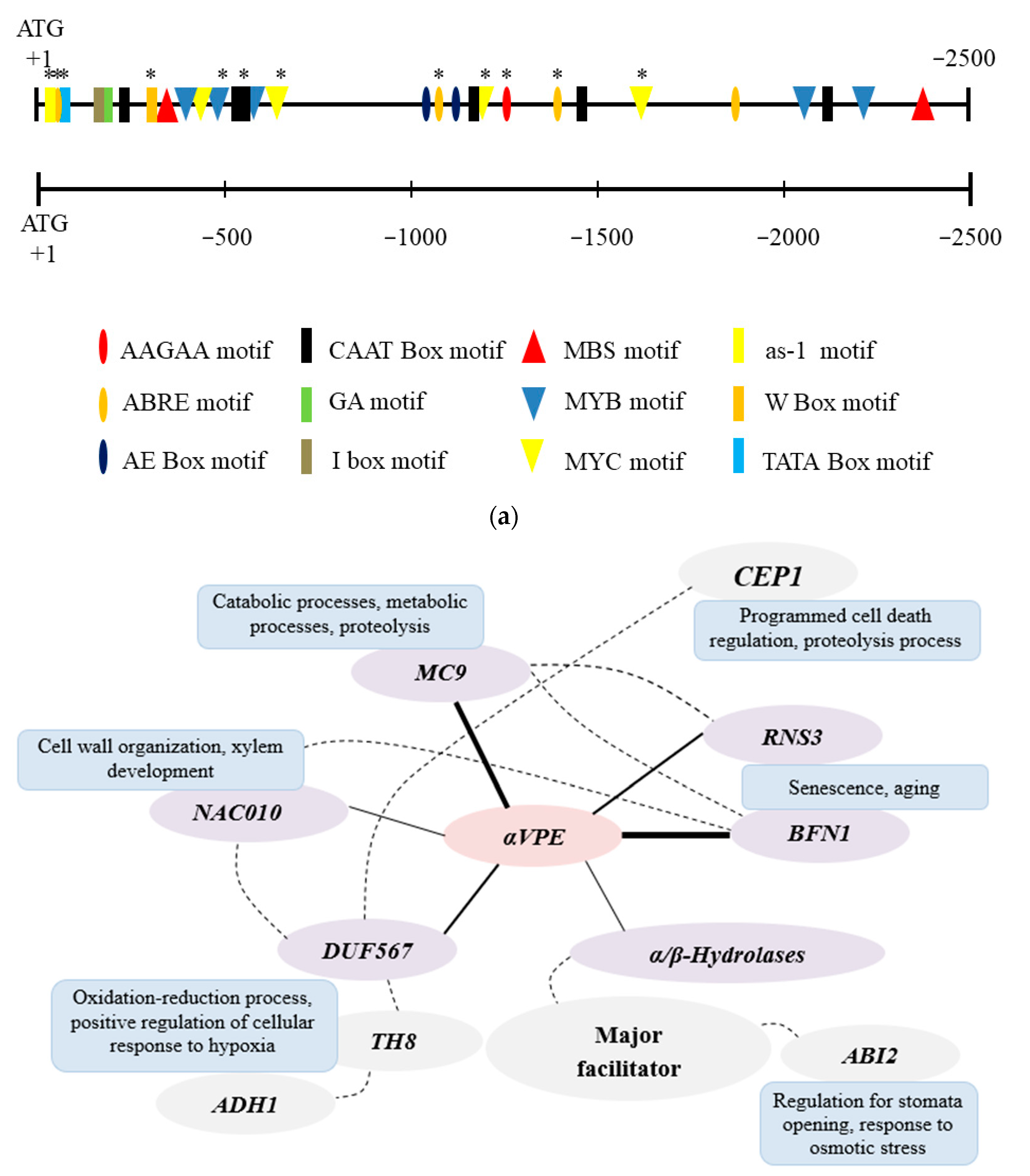
3.1. Analyses of Cis-Elements in the Promoter and Co-Expression Network of αVPE
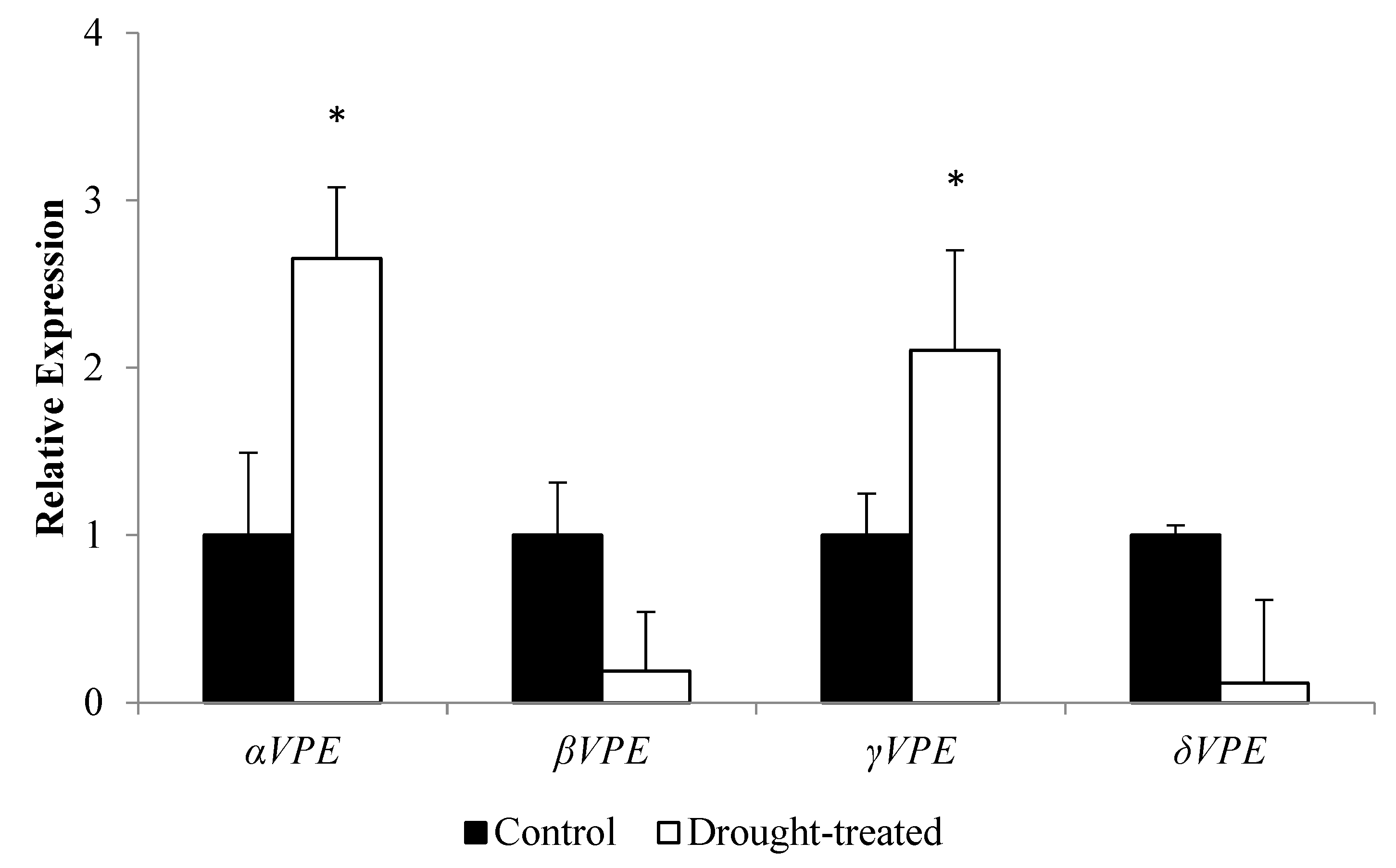
3.2. Expression Profile of the AtVPE Gene Family towards Drought Stress
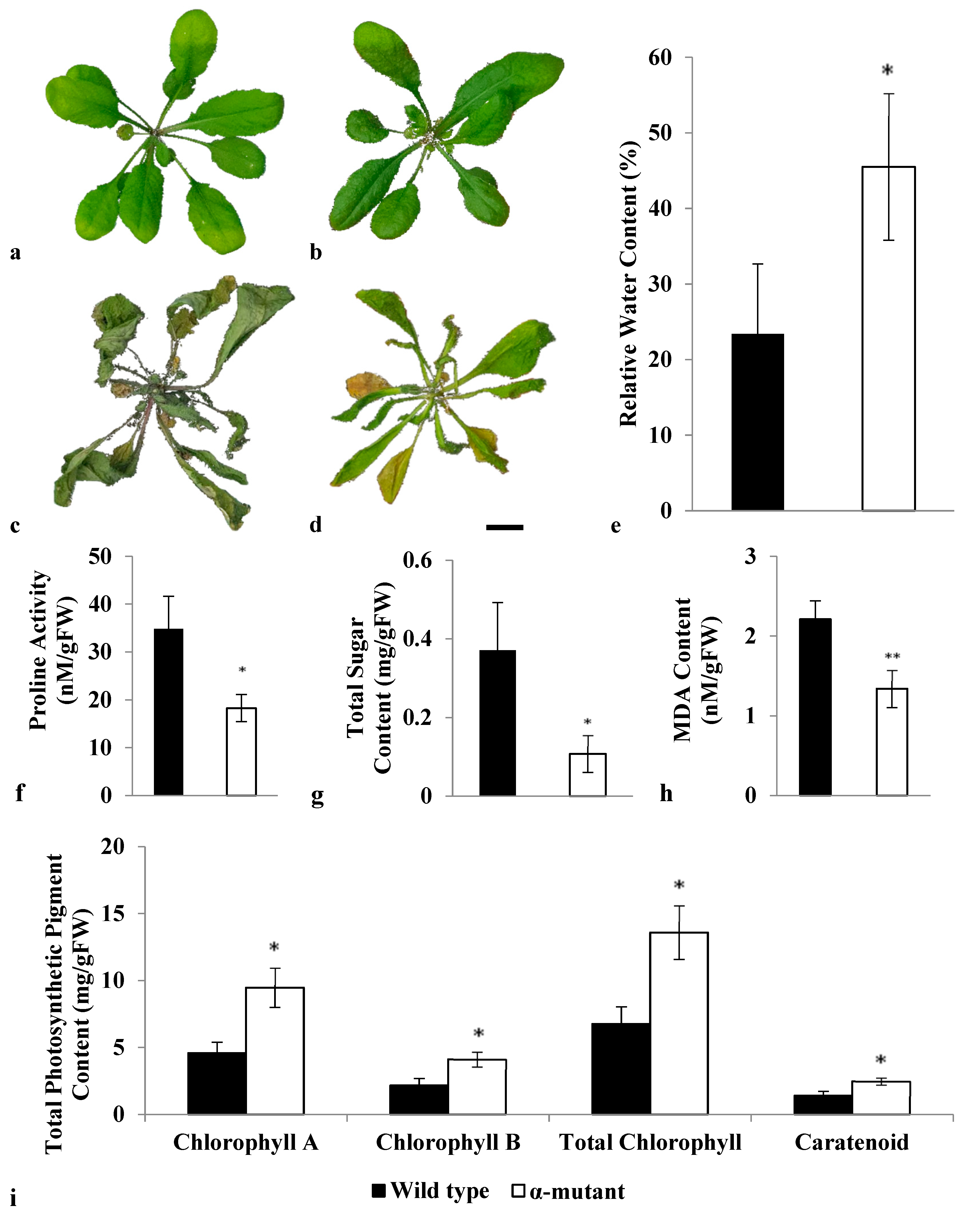
3.3. Morphological and Physiological Responses of Both Wild Type and Alpha Mutant towards Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eun, C.H.; Kim, S.U.; Kim, I.J. Regulatory cis-elements on citrus peel-specific expressed gene, CuCRTISO-like, promoter respond to hormones and abiotic stresses in transgenic Arabidopsis. Plant Biotechnol. Rep. 2017, 11, 63–69. [Google Scholar] [CrossRef]
- Li, R.; Zhu, F.; Duan, D. Function analysis and stress-mediated cis-element identification in the promoter region of VqMYB15. Plant Signal. Behav. 2020, 15, 1773664. [Google Scholar] [CrossRef] [PubMed]
- Sazegari, S.; Niazi, A.; Ahmadi, F.S. A study on the regulatory network with promoter analysis for Arabidopsis DREB-genes. Bioinformation 2015, 11, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rani, V. Computational methods to dissect cis-regulatory transcriptional networks. J. Biosci. 2007, 32, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, F.; Jia, D.; Li, J.; Zhang, Y.; Jia, C.; Wang, D.; Pan, H. Cloning and functional analysis of the promoter of a stress-inducible gene (ZmRXO1) in maize. Plant Mol. Biol. Rep. 2015, 33, 200–208. [Google Scholar] [CrossRef]
- Hatsugai, N.; Yamada, K.; Goto-Yamada, S.; Hara-Nishimura, I. Vacuolar processing enzyme in plant programmed cell death. Front. Plant Sci. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.C.; Kisku, A.V.; Sarin, N.B. Understanding the gamma-vacuolar processing enzyme gene regulation by promoter-GUS fusion approach. Plant Arch. 2018, 18, 679–689. [Google Scholar]
- Kuroyanagi, M.; Yamada, K.; Hatsugai, N.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J. Biol. Chem. 2005, 280, 32914–32920. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yamada, K.; Hiraiwa, N.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J. 1999, 19, 43–53. [Google Scholar] [CrossRef]
- Alpuerto, J.B.; Mukherjee, A.; Kitazumi, A.; Alyokhin, A.; De Koeyer, D.; de los Reyes, B.G. Impaired expressions of the beta and delta isoforms of vacuolar processing enzymes compromise the basal defenses of Arabidopsis thaliana against the phloem-feeding insect Myzus persicae. Acta Physiol. Plant. 2017, 39, 233. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaga, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, T.; Okamura, Y.; Ito, S.; Tadaka, S.; Aoki, Y.; Shirota, M.; Kinoshita, K. ATTED-II in 2014: Evaluation of gene coexpression in agriculturally important plants. Plant Cell Physiol. 2014, 55, e6. [Google Scholar] [CrossRef] [PubMed]
- Wan-Abdullah, W.M.A.N.; Saidi, N.B.; Yusof, M.T.; Wee, C.Y.; Loh, H.S.; Ong-Abdullah, J.; Lai, K.S. Vacuolar Processing Enzymes Modulating Susceptibility Response to Fusarium oxysporum f. sp. cubense Tropical Race 4 Infections in Banana. Front. Plant Sci. 2022, 12, 769855. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.S.; Yusoff, K.; Maziah, M. Extracellular matrix as the early structural marker for Centella asiatica embryogenic tissues. Biol. Plant. 2011, 55, 549–553. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- GarcÍa-Mata, C.; Lamattina, L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 2001, 126, 1196–1204. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, C.; Xie, J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit. Postharvest Biol. Technol. 2011, 62, 115–120. [Google Scholar] [CrossRef]
- Lee, M.R.; Kim, C.S.; Park, T.; Choi, Y.S.; Lee, K.H. Optimization of the ninhydrin reaction and development of a multiwell plate-based high-throughput proline detection assay. Anal. Biochem. 2018, 556, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods Enzymol; Douce, R., Packer, L., Eds.; Academic Press Inc.: New York, NY, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Leonard, J.M.; Monteros, A.; Liu, P.P.; Nonogaki, H. A novel endo-β-mannanase gene in tomato LeMAN5 is associated with anther and pollen development. Plant Physiol. 2004, 134, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, H.K.; Dong, Q.L.; Zhang, Y.Y.; Wang, Y.M.; Li, H.Y.; Xing, G.J.; Li, Q.Y.; Dong, Y.S. Genome-wide analysis and expression profiling under heat and drought treatments of HSP70 gene family in soybean (Glycine max L.). Front. Plant Sci. 2015, 6, 773. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Sharma, D.; Kaul, S.; Dhar, M.K. Identification and in silico characterization of cis-acting elements of genes involved in carotenoid biosynthesis in tomato. 3 Biotech 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, Q.; Bai, R.; Li, R.; Chen, L.; Xu, Y.; Zhang, M.; Duan, D. The effect of transcription factor MYB14 on defense mechanisms in Vitis quinquangularis-Pingyi. Int. J. Mol. Sci. 2020, 21, 706. [Google Scholar] [CrossRef] [PubMed]
- Bhati, J.; Chaduvula, K.P.; Rai, A.; Gaikwad, K.; Soma Marla, S.; Kumar, S. In-Silico Prediction and Functional Analysis of Salt Stress Responsive Genes in Rice (Oryza sativa). J. Rice Res. 2016, 4, 164. [Google Scholar] [CrossRef]
- Arefin, S.; Bhuiyan, M.F.H.; Akther, J.; Prodhan, S.H.; Hoque, H. The dynamics of cis-regulatory elements in promoter regions of tomato sucrose transporter genes. Int. J. Veg. Sci. 2021, 27, 167–186. [Google Scholar] [CrossRef]
- Basyuni, M.; Wati, R.; Sulistiyono, N.; Oku, H.; Baba, S.; Sagami, H. Isolation and analysis of a multifunctional triterpene synthase KcMS promoter region from mangrove plant Kandelia candel. IOP Conf. Ser. Earth Environ. Sci. 2018, 130, 012013. [Google Scholar] [CrossRef]
- Yamada, K.; Basak, A.K.; Goto-Yamada, S.; Tarnawska-Glatt, K.; Hara-Nishimura, I. Vacuolar processing enzymes in the plant life cycle. New Phytol. 2020, 226, 21–31. [Google Scholar] [CrossRef]
- Ye, H.; Ren, F.; Guo, H.; Guo, L.; Bai, J.; Wang, Y. Identification of key genes and transcription factors in ageing Arabidopsis papilla cells by transcriptome analysis. Plant Physiol. Biochem. 2020, 147, 1–9. [Google Scholar] [CrossRef]
- Rojas, B.E.; Hartman, M.D.; Figueroa, C.M.; Iglesias, A.A. Proteolytic cleavage of Arabidopsis thaliana phosphoenol pyruvate carboxykinase-1 modifies its allosteric regulation. J. Exp. Bot. 2021, 72, 2514–2524. [Google Scholar] [CrossRef]
- Marzec-Schmidt, K.; Ludwików, A.; Wojciechowska, N.; Kasprowicz-Maluśki, A.; Mucha, J.; Bagniewska-Zadworna, A. Xylem cell wall formation in pioneer roots and stems of Populus trichocarpa (Torr. & Gray). Front. Plant Sci. 2019, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- Baig, A. Role of Arabidopsis LOR1 (LURP-one related one) in basal defense against Hyaloperonospora arabidopsidis. Physiol. Mol. Plant Pathol. 2018, 103, 71–77. [Google Scholar] [CrossRef]
- Ding, X.; Chen, L.; Guo, J.; Gai, J.; Yang, S. A small RNA of miR2119b from soybean CMS line acts as a negative regulator of male fertility in transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 167, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Calderón, A.; Sevilla, F.; Jiménez, A. Redox Protein Thioredoxins: Function Under Salinity, Drought and Extreme Temperature Conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D., Palma, J., Corpas, F., Eds.; Springer: Cham, Switzerland, 2018; pp. 123–162. [Google Scholar] [CrossRef]
- Albertini, A.; Simeoni, F.; Galbiati, M.; Bauer, H.; Tonelli, C.; Cominelli, E. Involvement of the vacuolar processing enzyme γVPE in response of Arabidopsis thaliana to water stress. Biol. Plant. 2014, 58, 531–538. [Google Scholar] [CrossRef]
- Cilliers, M.; van Wyk, S.G.; van Heerden, P.D.R.; Kunert, K.J.; Vorster, B.J. Identification and changes of the drought-induced cysteine protease transcriptome in soybean (Glycine max) root nodules. Environ. Exp. Bot. 2018, 148, 59–69. [Google Scholar] [CrossRef]
- Chowdhury, J.; Karim, M.; Khaliq, Q.; Ahmed, A.; Mondol, A.M. Effect of drought stress on water relation traits of four soybean genotypes. SAARC J. Agric. 2018, 15, 163–175. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of drought on soluble sugars and free proline content in selected Arabidopsis mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.U.; Hussain, A.; Lee, I.J.; Yun, B.W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Li, Y.; Li, X.; Zhang, J. Proline metabolism-related gene expression in four potato genotypes in response to drought stress. Biol. Plant. 2019, 63, 757–764. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis SMALL AUXIN UP RNA32 Protein Regulates ABA-Mediated Responses to Drought Stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Lou, D.; Wang, H.; Liang, G.; Yu, D. OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front. Plant Sci. 2017, 8, 993. [Google Scholar] [CrossRef]
- Wang, R.; Gao, M.; Ji, S.; Wang, S.; Meng, Y.; Zhou, Z. Carbon allocation, osmotic adjustment, antioxidant capacity and growth in cotton under long-term soil drought during flowering and boll-forming period. Plant Physiol. Biochem. 2016, 107, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, H.; Wang, H.; Zhang, P.; Shi, C. A novel sucrose transporter gene IbSUT4 involves in plant growth and response to abiotic stress through the ABF-dependent ABA signaling pathway in Sweetpotato. BMC Plant Biol. 2020, 20, 157. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Adhikari, A.; Lee, I.J.; Loake, G.J.; Yun, B.W. A Novel DUF569 Gene is a positive regulator of the drought stress response in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 5316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.-N.; Wan Abdullah, W.M.A.N.; Wee, C.-Y.; Balia Yusof, Z.N.; Yap, W.-S.; Cheng, W.-H.; Baharum, N.A.; Ong-Abdullah, J.; Loh, J.-Y.; Lai, K.-S. Promoter Cis-Element Analyses Reveal the Function of αVPE in Drought Stress Response of Arabidopsis. Biology 2023, 12, 430. https://doi.org/10.3390/biology12030430
Tang C-N, Wan Abdullah WMAN, Wee C-Y, Balia Yusof ZN, Yap W-S, Cheng W-H, Baharum NA, Ong-Abdullah J, Loh J-Y, Lai K-S. Promoter Cis-Element Analyses Reveal the Function of αVPE in Drought Stress Response of Arabidopsis. Biology. 2023; 12(3):430. https://doi.org/10.3390/biology12030430
Chicago/Turabian StyleTang, Chu-Nie, Wan Muhamad Asrul Nizam Wan Abdullah, Chien-Yeong Wee, Zetty Norhana Balia Yusof, Wai-Sum Yap, Wan-Hee Cheng, Nadiya Akmal Baharum, Janna Ong-Abdullah, Jiun-Yan Loh, and Kok-Song Lai. 2023. "Promoter Cis-Element Analyses Reveal the Function of αVPE in Drought Stress Response of Arabidopsis" Biology 12, no. 3: 430. https://doi.org/10.3390/biology12030430
APA StyleTang, C.-N., Wan Abdullah, W. M. A. N., Wee, C.-Y., Balia Yusof, Z. N., Yap, W.-S., Cheng, W.-H., Baharum, N. A., Ong-Abdullah, J., Loh, J.-Y., & Lai, K.-S. (2023). Promoter Cis-Element Analyses Reveal the Function of αVPE in Drought Stress Response of Arabidopsis. Biology, 12(3), 430. https://doi.org/10.3390/biology12030430






