Larval Dispersal Modeling Reveals Low Connectivity among National Marine Protected Areas in the Yellow and East China Seas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
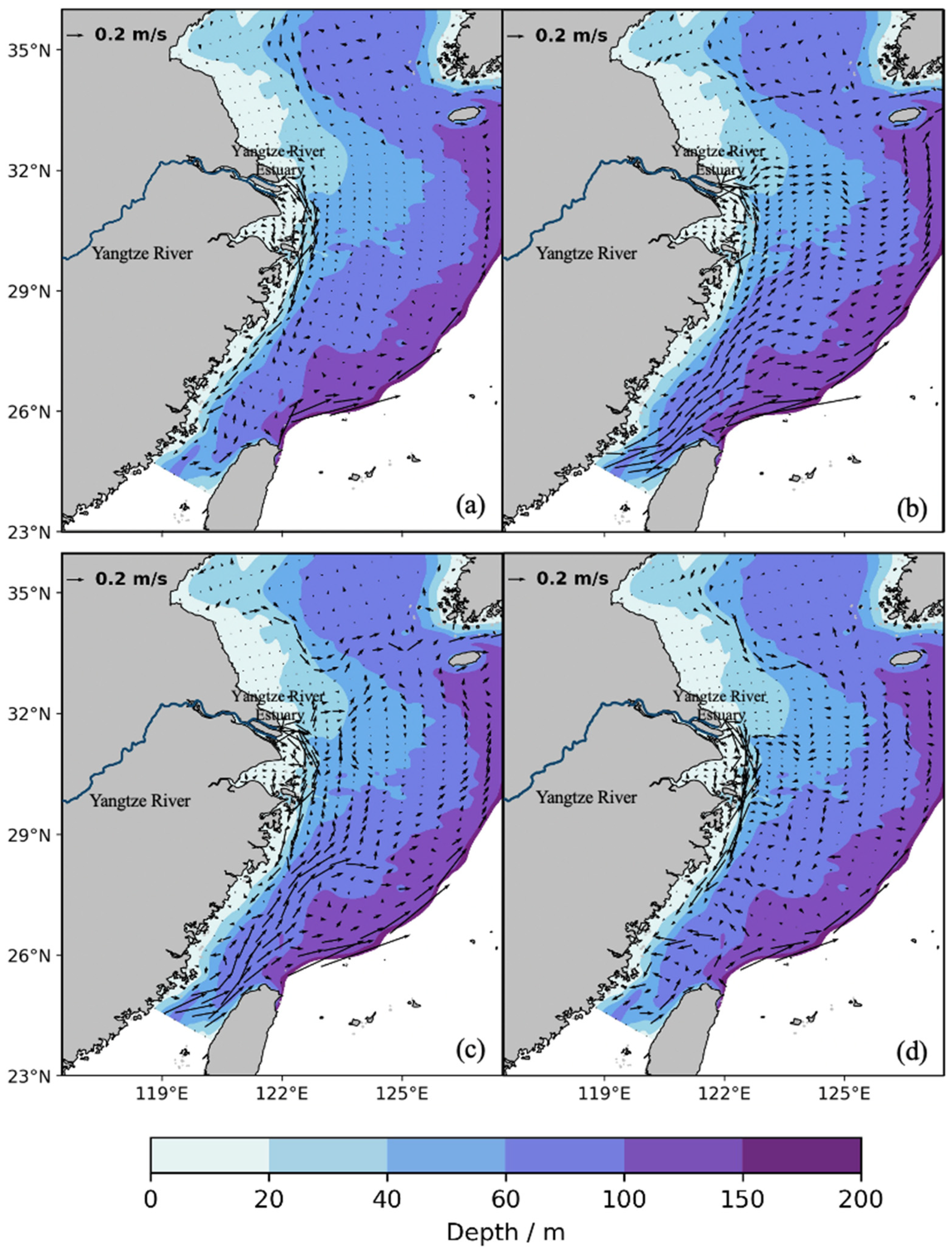
2.2. Ocean Model Configuration
2.3. Lagrangian Particle Dispersion Model
3. Results
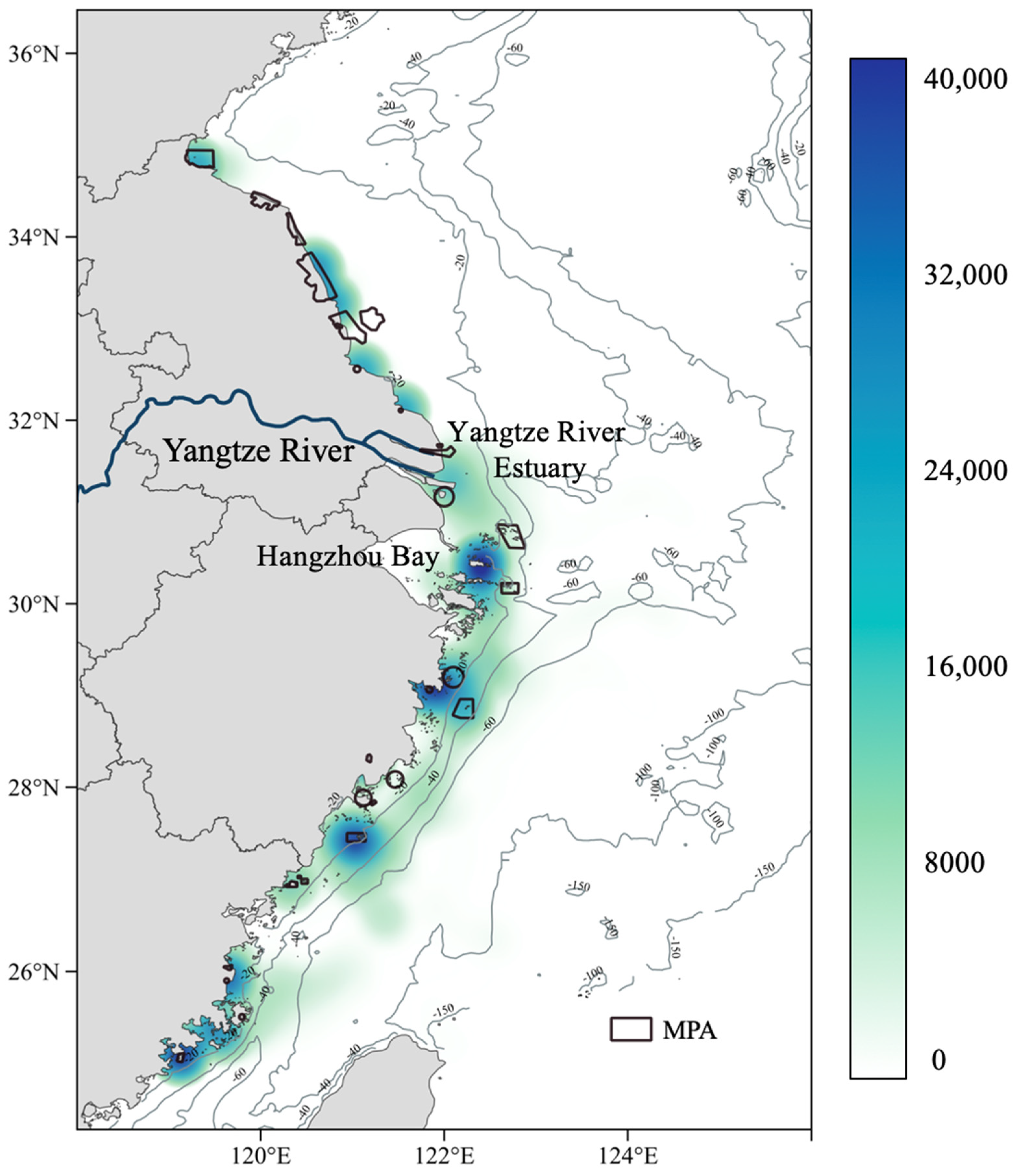
3.1. Larval Dispersal
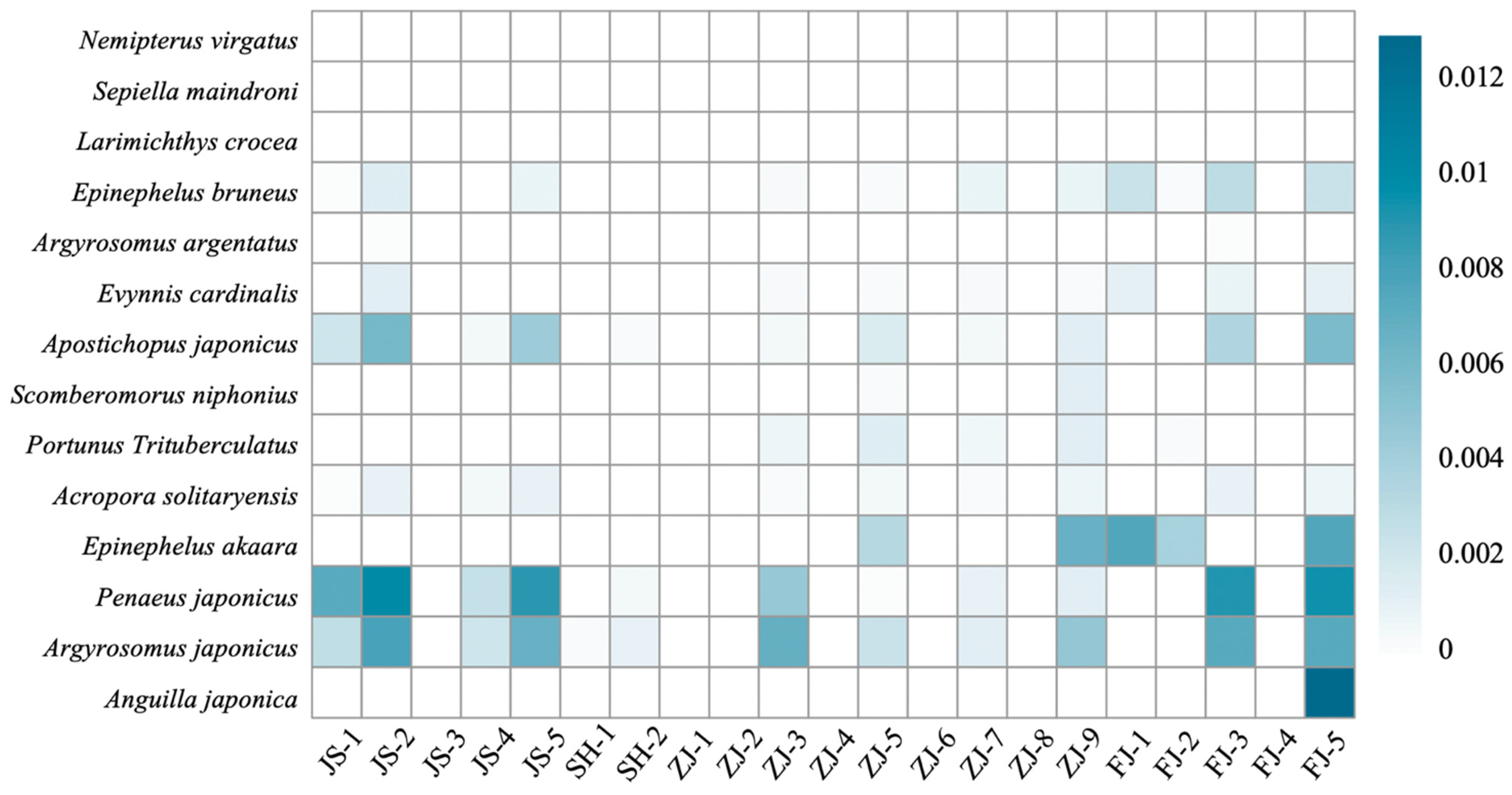
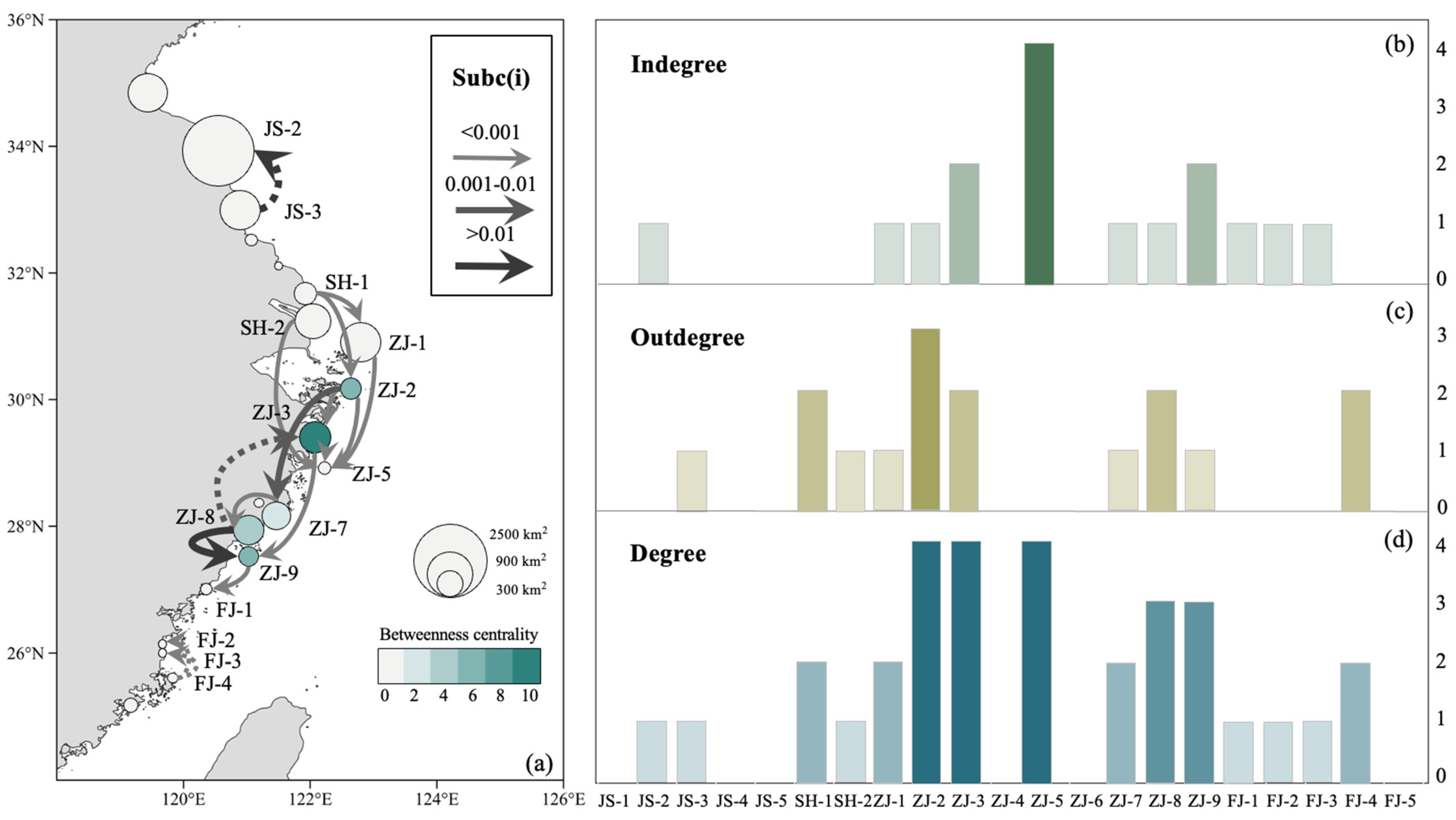
3.2. MPA Ecological Connectivity Analysis
4. Discussion
4.1. Model Strengths and Limitations
4.2. Ecological Connectivity between the MPAs
4.3. Future Planning of the MPA Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, T.; Baird, A.; Bellwood, D.; Card, M.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, C.; Kleypas, J.; Lough, J. Climate change, human impact, and the resilience of coral reefs. Aust. Inst. Mar. Sci. 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, X. Evaluation of the Management Effectiveness of Marine Protected Areas: A Brief Review of Recent Research Progress. J. Oceanol. Limnol. 2019, 26, 286–294. [Google Scholar]
- Woodley, S.; Bertzky, B.; Crawhall, N.; Dudley, N.; Sandwith, T. Meeting Aichi Target 11: What does success look like for Protected Area systems? Parks 2012, 18, 23–36. [Google Scholar]
- Harrison, H.B.; Williamson, D.H.; Evans, R.D.; Almany, G.R.; Thorrold, S.R.; Russ, G.R.; Feldheim, K.A.; van Herwerden, L.; Planes, S.; Srinivasan, M.; et al. Larval Export from Marine Reserves and the Recruitment Benefit for Fish and Fisheries. Curr. Biol. 2012, 22, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Olds, A.; Connolly, R.; Pitt, K.; Maxwell, P. Habitat connectivity improves reserve performance. Conserv. Lett. 2012, 5, 56–63. [Google Scholar] [CrossRef]
- Kalinina, M.U.N. Body Releases Draft Plan to Put Biodiversity on Path to Recovery by 2050. Available online: https://www.pewtrusts.org/en/research-and-analysis/articles/2021/07/12/un-body-releases-draft-plan-to-put-biodiversity-on-path-to-recovery-by-2050 (accessed on 18 June 2022).
- Assis, J.; Fragkopoulou, E.; Serrão, E.A.; Horta e Costa, B.; Gandra, M.; Abecasis, D. Weak biodiversity connectivity in the European network of no-take marine protected areas. Sci. Total Environ. 2021, 773, 145664. [Google Scholar] [CrossRef]
- Andrello, M.; Mouillot, D.; Beuvier, J.; Albouy, C.; Thuiller, W.; Manel, S. Low Connectivity between Mediterranean Marine Protected Areas: A Biophysical Modeling Approach for the Dusky Grouper Epinephelus marginatus. PLoS ONE 2013, 8, e68564. [Google Scholar] [CrossRef]
- Cowen, R.; Lwiza, K.; Sponaugle, S.; Olson, D. Connectivity of Marine Populations: Open or Closed? Science 2000, 287, 857–859. [Google Scholar] [CrossRef]
- Watson, J.; Hays, C.; Raimondi, P.; Mitarai, S.; Dong, C.; McWilliams, J.; Blanchette, C.; Caselle, J.; Siegel, D. Currents connecting communities: Nearshore community similarity and ocean circulation. Ecology 2011, 92, 1193–1200. [Google Scholar] [CrossRef]
- Ponchon, A.; Aulert, C.; Le Guillou, G.; Gallien, F.; Peron, C.; Gremillet, D. Spatial overlaps of foraging and resting areas of black-legged kittiwakes breeding in the English Channel with existing marine protected areas. Mar. Biol. 2017, 164, 119. [Google Scholar] [CrossRef]
- Thorrold, S.R.; Jones, G.P.; Hellberg, M.E.; Burton, R.S.; Swearer, S.E.; Neigel, J.E.; Morgan, S.G.; Warner, R.R. Quantifying larval retention and connectivity in marine populations with artificial and natural markers. Bull. Mar. Sci. 2002, 70, 291–308. [Google Scholar]
- Gillanders, B.M. Using elemental chemistry of fish otoliths to determine connectivity between estuarine and coastal habitats. Estuar. Coast. Shelf Sci. 2005, 64, 47–57. [Google Scholar] [CrossRef]
- Campana, S.E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef]
- Standish, J.D.; Sheehy, M.; Warner, R.R. Use of otolith natal elemental signatures as natural tags to evaluate connectivity among open-coast fish populations. Mar. Ecol. Prog. Ser. 2008, 356, 259–268. [Google Scholar] [CrossRef]
- Li, Y.; Han, Z.; Song, N.; Gao, T.-X. New evidence to genetic analysis of small yellow croaker (Larimichthys polyactis) with continuous distribution in China. Biochem. Syst. Ecol. 2013, 50, 331–338. [Google Scholar] [CrossRef]
- Planes, S.; Jones, G.P.; Thorrold, S.R. Larval dispersal connects fish populations in a network of marine protected areas. Proc. Natl. Acad. Sci. USA 2009, 106, 5693–5697. [Google Scholar] [CrossRef]
- Andrello, M.; Mouillot, D.; Somot, S.; Thuiller, W.; Manel, S. Additive effects of climate change on connectivity between marine protected areas and larval supply to fished areas. Divers. Distrib. 2015, 21, 139–150. [Google Scholar] [CrossRef]
- Xing, Q.; Yu, H.; Yu, H.; Sun, P.; Liu, Y.; Ye, Z.; Li, J.; Tian, Y. A comprehensive model-based index for identification of larval retention areas: A case study for Japanese anchovy Engraulis japonicus in the Yellow Sea. Ecol. Indic. 2020, 116, 106479. [Google Scholar] [CrossRef]
- Roberts, K.; Cook, C.; Beher, J.; Treml, E. Assessing the current state of ecological connectivity in a large marine protected area system. Conserv. Biol. 2020, 35, 699–710. [Google Scholar] [CrossRef]
- Liu, G.; Bracco, A.; Quattrini, A.; Herrera, S. Kilometer-Scale Larval Dispersal Processes Predict Metapopulation Connectivity Pathways for Paramuricea biscaya in the Northern Gulf of Mexico. Front. Mar. Sci. 2021, 8, 790927. [Google Scholar] [CrossRef]
- Ospina-Alvarez, A.; Weidberg, N.; Aiken, C.M.; Navarrete, S.A. Larval transport in the upwelling ecosystem of central Chile: The effects of vertical migration, developmental time and coastal topography on recruitment. Prog. Oceanogr. 2018, 168, 82–99. [Google Scholar] [CrossRef]
- Lopera, L.; Cardona, Y.; Zapata-Ramírez, P.A. Circulation in the Seaflower Reserve and Its Potential Impact on Biological Connectivity. Front. Mar. Sci. 2020, 7, 385. [Google Scholar] [CrossRef]
- Buonaccorsi, V.P.; Kimbrell, C.A.; Lynn, E.A.; Vetter, R.D. Limited realized dispersal and introgressive hybridization influence genetic structure and conservation strategies for brown rockfish, Sebastes auriculatus. Conserv. Genet. 2005, 6, 697–713. [Google Scholar] [CrossRef]
- Crochelet, E.; Roberts, J.; Lagabrielle, E.; Obura, D.; Petit, M.; Chabanet, P. A model-based assessment of reef larvae dispersal in the Western Indian Ocean reveals regional connectivity patterns—Potential implications for conservation policies. Reg. Stud. Mar. Sci. 2016, 7, 159–167. [Google Scholar] [CrossRef]
- Di Franco, A.; Gillanders, B.; Benedetto, G.; Pennetta, A.; De Leo, G.; Guidetti, P. Dispersal Patterns of Coastal Fish: Implications for Designing Networks of Marine Protected Areas. PLoS ONE 2012, 7, e31681. [Google Scholar] [CrossRef] [PubMed]
- Bohorquez, J.J.; Xue, G.; Frankstone, T.; Grima, M.M.; Kleinhaus, K.; Zhao, Y.; Pikitch, E.K. China’s little-known efforts to protect its marine ecosystems safeguard some habitats but omit others. Sci. Adv. 2021, 7, eabj1569. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, M.; Zeng, C.; Cheng, S.; Wang, Z.; Liu, S.; Zou, C.; Ye, S.; Zhu, Z.; Cao, L. Assessing the management effectiveness of China’s marine protected areas: Challenges and recommendations. Ocean Coast. Manag. 2022, 224, 106172. [Google Scholar] [CrossRef]
- Deng, B.; Ji, H.; He, Y.; Ji, X. The Development of the National Marine Reserve Area in East China Sea District. Ocean Dev. Manag. 2017, 34, 64–67. [Google Scholar]
- Chen, J.; Ma, J.; Xu, K.; Liu, Y.; Cao, W.; Wei, T.; Zhao, B.; Chen, Z. Provenance discrimination of the clay sediment in the western Taiwan Strait and its implication for coastal current variability during the late-Holocene. Holocene 2016, 27, 110–121. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, P. Numerical modelling study of tidal energy and dissipation in the East China Seas. J. Xiamen Univ. 2020, 59, 61–70+151–152. [Google Scholar]
- Jiang, X. The Numerical Simulation of Circulations, Mesoscale Eddies and Submesoscale Processes in the East China Sea. Ph.D. Thesis, Nanjing University of Information Science & Technology, Nanjing, China, 2022. [Google Scholar]
- Zhang, K.; Li, A.; Zhang, J.; Lu, J.; Wang, H. Seasonal variations in the surficial sediment grain size in the East China Sea continental shelf and their implications for terrigenous sediment transport. J. Oceanogr. 2020, 76, 1–14. [Google Scholar] [CrossRef]
- Rassweiler, A.; Ojea, E.; Costello, C. Strategically designed marine reserve networks are robust to climate change driven shifts in population connectivity. Environ. Res. Lett. 2020, 15, 034030. [Google Scholar] [CrossRef]
- Shchepetkin, A.F.; McWilliams, J.C. The regional oceanic modeling system (ROMS): A split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Model. 2005, 9, 347–404. [Google Scholar] [CrossRef]
- Chapman, D.C. Numerical Treatment of Cross-Shelf Open Boundaries in a Barotropic Coastal Ocean Model. J. Phys. Oceanogr. 1985, 15, 1060–1075. [Google Scholar] [CrossRef]
- Carter, G.S.; Merrifield, M.A. Open boundary conditions for regional tidal simulations. Ocean Model. 2007, 18, 194–209. [Google Scholar] [CrossRef]
- Treml, E.A.; Roberts, J.J.; Chao, Y.; Halpin, P.N.; Possingham, H.P.; Riginos, C. Reproductive Output and Duration of the Pelagic Larval Stage Determine Seascape-Wide Connectivity of Marine Populations. Integr. Comp. Biol. 2012, 52, 525–537. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, C.; Jiang, Z.; Cao, L. Conservation gap analysis of threatened fish in the East China Sea and adjacent sea areas. J. Trop. Oceanogr. 2023, 46, 66–82. [Google Scholar]
- Schill, S.; Raber, G.; Roberts, J.; Treml, E.; Brenner, J.; Halpin, P. No Reef Is an Island: Integrating Coral Reef Connectivity Data into the Design of Regional-Scale Marine Protected Area Networks. PLoS ONE 2015, 10, e0144199. [Google Scholar] [CrossRef]
- Becker, B.J.; Levin, L.A.; Fodrie, F.J.; McMillan, P.A. Complex larval connectivity patterns among marine invertebrate populations. Proc. Natl. Acad. Sci. USA 2007, 104, 3267–3272. [Google Scholar] [CrossRef]
- Kininmonth, S.J.; De’ath, G.; Possingham, H.P. Graph theoretic topology of the Great but small Barrier Reef world. Theor. Ecol. 2010, 3, 75–88. [Google Scholar] [CrossRef]
- Treml, E.; Halpin, P.; Urban, D.; Pratson, L. Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landsc. Ecol. 2008, 23, 19–36. [Google Scholar] [CrossRef]
- Guidetti, P.; Milazzo, M.; Bussotti, S.; Molinari, A.; Murenu, M.; Pais, A.; Spanò, N.; Balzano, R.; Agardy, T.; Boero, F.; et al. Italian marine reserve effectiveness: Does enforcement matter? Biol. Conserv. 2008, 141, 699–709. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E.; Dendrinos, P.; Di Franco, A.; Ferretti, F.; Foley, D.; Fraschetti, S.; Friedlander, A.; Garrabou, J.; Güçlüsoy, H.; et al. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 2012, 7, e32742. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, A.F.; Arnaud-Haond, S.; Hernández-García, E.; Eguíluz, V.M.; Serrão, E.A.; Duarte, C.M. Network analysis identifies weak and strong links in a metapopulation system. Proc. Natl. Acad. Sci. USA 2008, 105, 18824–18829. [Google Scholar] [CrossRef]
- Balbar, A.C.; Metaxas, A. The current application of ecological connectivity in the design of marine protected areas. Glob. Ecol. Conserv. 2019, 17, e00569. [Google Scholar] [CrossRef]
- Bode, M.; Bode, L.; Armsworth, P. Larval dispersal reveals regional sources and sinks in the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2006, 308, 17–25. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The Igraph Software Package for Complex Network Research. InterJ. Complex Syst. 2005, 1695, 1–9. [Google Scholar]
- Werner, F.; Cowen, R. Coupled Biological and Physical Models: Present Capabilities and Necessary Developments for Future Studies of Population Connectivity. Oceanography 2007, 20, 54–69. [Google Scholar] [CrossRef]
- Pairaud, I.; Gatti, J.; Bensoussan, N.; Verney, R.; Garreau, P. Hydrology and circulation in a coastal area off Marseille: Validation of a nested 3D model with observations. J. Mar. Syst. 2011, 88, 20–33. [Google Scholar] [CrossRef]
- Cowen, R.; Srinivasan, A. Scaling of Connectivity in Marine Populations. Science 2006, 311, 522–527. [Google Scholar] [CrossRef]
- Treml, E.; Halpin, P. Marine population connectivity identifies ecological neighbors for conservation planning in the Coral Triangle. Conserv. Lett. 2012, 5, 441–449. [Google Scholar] [CrossRef]
- Allendorf, F.; Luikart, G. Conservation and Genetics of Populations; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mumby, P.; Edwards, I.; Eakin, C.M.; Skirving, W.; Edwards, H.; Enríquez, S.; Iglesias-Prieto, R.; Cherubin, L.; Stevens, J. Reserve design for uncertain responses of coral reefs to climate change. Ecol. Lett. 2011, 14, 132–140. [Google Scholar] [CrossRef]
- Zeng, X.; Liang, J.; Zeng, J. Evaluating the effectiveness of three national marine protected areas (MPAs) in the Yangtze River delta. Front. Mar. Sci. 2022, 9, 1393. [Google Scholar] [CrossRef]
- López-Sanz, A.; Stelzenmüller, V.; Maynou, F.; Sabatés, A. The influence of environmental characteristics on fish larvae spatial patterns related to a marine protected area: The Medes islands (NW Mediterranean). Estuar. Coast. Shelf Sci. 2011, 92, 521–533. [Google Scholar] [CrossRef]
- Zeng, C.; Rowden, A.; Clark, M.; Gardner, J. Population genetic structure and connectivity of deep-sea stony corals (O. scleractinia) in the New Zealand region: Implications for the conservation and management of Vulnerable Marine Ecosystem. Evol. Appl. 2017, 10, 1040–1054. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Chen, C.; Ge, J.; Ji, R.; Tian, R.; Xue, P.; Xu, L. Dispersal and survival of chub mackerel (Scomber japonicus) larvae in the East China Sea. Ecol. Model. 2014, 283, 70–84. [Google Scholar] [CrossRef]
- Tang, Y.; Huangfu, J.; Huang, R.; Chen, W. Surface warming reacceleration in offshore China and its interdecadal effects on the East Asia–Pacific climate. Sci. Rep. 2020, 10, 14811. [Google Scholar] [CrossRef]
- Lacroix, G.; Barbut, L.; Volckaert, F. Complex effect of projected sea temperature and wind change on flatfish dispersal. Glob. Chang. Biol. 2017, 24, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Legrand, T.; Di Franco, A.; Ser-Giacomi, E.; Caló, A.; Rossi, V. A multidisciplinary analytical framework to delineate spawning areas and quantify larval dispersal in coastal fish. Mar. Environ. Res. 2019, 151, 104761. [Google Scholar] [CrossRef]
- Gaines, S.; White, C.; Carr, M.; Palumbi, S. Designing Marine Reserve Networks for Both Conservation and Fisheries Management. Proc. Natl. Acad. Sci. USA 2010, 107, 18286–18293. [Google Scholar] [CrossRef]
- Bode, M.; Leis, J.; Mason, L.; Williamson, D.; Harrison, H.; Choukroun, S.; Jones, G. Successful validation of a larval dispersal model using genetic parentage data. PLoS Biol. 2019, 17, e3000380. [Google Scholar] [CrossRef]
- Wu, C.; Dong, Z.; Chi, C.; Ding, F. Reproductive and spawning habits of Sepiella maindroni off Zhejiang, China. Oceanol. Limnol. Sin. 2010, 41, 39–46. [Google Scholar]
- Wang, B.; Sun, P.; Zhang, Z.; Ma, J. Biological characteristics of Epinephelus brunneus and preliminary experiment of indoor culture. Fish. Mod. 2006, 1, 28–29. [Google Scholar]
- Zhuang, P. Yangtze Estuary Fishes; Shanghai Science and Technology Press: Shanghai, China, 2006; Volume 10, pp. 338–340. [Google Scholar]
- Wang, B.; Zhang, X.; Qu, X.; Ruan, S.; Qu, Y. Biological characteristics and technologies of seed production of Japanese croaker (N. japanica). Prog. Fish. Sci. 2002, 4, 13–19. [Google Scholar]
- Cai, Y.; Chen, Z.; Xu, S.; Zhang, K. Tempo-spatial distribution of Evynnis cardinalis in Beibu Gulf. South China Fish. Sci. 2017, 13, 1–10. [Google Scholar]
- Tang, Q. Regional Oceanography of China Seas-Fisheries Oceanography; China Ocean Press: Beijing, China, 2012; Volume 6, pp. 166–169. [Google Scholar]
- Oh, C.-W. Population Biology of the Swimming Crab Portunus Trituberculatus (Miers, 1876) (Decapoda, Brachyura) on the Western Coast of Korea, Yellow Sea. Crustaceana 2011, 84, 1251–1267. [Google Scholar]
- Ukawa, M.; Higuchi, M.; Mito, S. Spawning habits and early life history of a serranid fish, Epinephelus akaara (TEMMINCK et SCHLEGEL). Jpn. J. Ichthyol. 1966, 13, 156–161. [Google Scholar]
- Zou, Y. Special Aquaculture; China Agriculture Press: Beijing, China, 2002; Volume 7, pp. 30–31. [Google Scholar]
- Cai, X.; Lin, Q.; Wan, W. On the Development of the Prawn Penaeus penicillatus in Comparison with Both P. japonicus and P. orientalis. J. Xiamen Univ. (Nat. Sci.) 1981, 2, 243–252. [Google Scholar]
- Zhang, Y.; Huang, H.; Huang, J.; Yuan, T. Experimental cultivation of coral larvae in Xisha Islands. Ocean. Dev. Manag. 2013, 30, 78–82. [Google Scholar]

| Scientific Name | Classification | Category | PLD (Days) | Depth (m) | Spawning Window |
|---|---|---|---|---|---|
| Nemipterus virgatus | Actinopterygii | Threatened | 75 | 25 | April–May |
| Sepiella maindroni | Cephalopoda | Economic | 45 | 10 | April–May |
| Epinephelus bruneus | Actinopterygii | Threatened | 45 | 20 | May–June |
| Larimichthys crocea | Actinopterygii | Threatened | 33 | 15 | April–May |
| Argyrosomus argentatus | Actinopterygii | Economic | 33 | 50 | June–August |
| Argyrosomus japonicus | Actinopterygii | Threatened | 33 | 100 | January–March |
| Evynnis cardinalis | Actinopterygii | Threatened | 30 | 45 | November–January |
| Apostichopus japonicus | Holothuroidea | Threatened | 20 | 10 | May–June |
| Scomberomorus niphonius | Actinopterygii | Economic | 19 | 20 | May–June |
| Portunus Trituberculatus | Malacostraca | Economic | 17 | 20 | April–June |
| Epinephelus akaara | Actinopterygii | Threatened | 15 | 25 | April–June |
| Anguilla japonica | Actinopterygii | Threatened | 10 | 3 | November–December |
| Penaeus japonicus | Crustacea | Economic | 10 | 20 | December–March |
| Acropora solitaryensis | Anthozoa | Threatened | 7 | 5 | April–May |
| MPA | Area (km2) | Acropora solitaryensis | Apostichopus japonicus | Epinephelus akaara | Epinephelus bruneus | Nemipterus virgatus | Scomberomorus niphonius | Argyrosomus japonicus | Larimichthys crocea | Evynnis cardinalis | Portunus Trituberculatus | Anguilla japonica | Argyrosomus argentatu | Sepiella maindroni | Penaeus japonicus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JS-1 | 514.55 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| JS-2 | 2472.6 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||
| JS-3 | 780 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| JS-4 | 47.1 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| JS-5 | 15.46 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| SH-1 | 241.55 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| SH-2 | 423.2 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||||||
| ZJ-1 | 549 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| ZJ-2 | 218.4 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| ZJ-3 | 484.78 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| ZJ-4 | 44.19 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| ZJ-5 | 57 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| ZJ-6 | 30.8 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| ZJ-7 | 306.69 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| ZJ-8 | 311.04 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| ZJ-9 | 201.06 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| FJ-1 | 67.83 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| FJ-2 | 22.6 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| FJ-3 | 24.44 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| FJ-4 | 34.9 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| FJ-5 | 69.11 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Distribution points | 2 | 5 | 11 | 11 | 14 | 14 | 15 | 19 | 21 | 21 | 21 | 21 | 21 | 21 | |
| Number of Released particles | 2000 | 5000 | 11,000 | 11,000 | 14,000 | 14,000 | 15,000 | 19,000 | 21,000 | 21,000 | 21,000 | 21,000 | 21,000 | 21,000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Chen, Y.; Wang, Z.; Zhao, F.; Zhong, Y.; Zeng, C.; Cao, L. Larval Dispersal Modeling Reveals Low Connectivity among National Marine Protected Areas in the Yellow and East China Seas. Biology 2023, 12, 396. https://doi.org/10.3390/biology12030396
Lu J, Chen Y, Wang Z, Zhao F, Zhong Y, Zeng C, Cao L. Larval Dispersal Modeling Reveals Low Connectivity among National Marine Protected Areas in the Yellow and East China Seas. Biology. 2023; 12(3):396. https://doi.org/10.3390/biology12030396
Chicago/Turabian StyleLu, Jiaying, Yuanjie Chen, Zihan Wang, Feng Zhao, Yisen Zhong, Cong Zeng, and Ling Cao. 2023. "Larval Dispersal Modeling Reveals Low Connectivity among National Marine Protected Areas in the Yellow and East China Seas" Biology 12, no. 3: 396. https://doi.org/10.3390/biology12030396
APA StyleLu, J., Chen, Y., Wang, Z., Zhao, F., Zhong, Y., Zeng, C., & Cao, L. (2023). Larval Dispersal Modeling Reveals Low Connectivity among National Marine Protected Areas in the Yellow and East China Seas. Biology, 12(3), 396. https://doi.org/10.3390/biology12030396







