Circle(s) of Life: The Circadian Clock from Birth to Death
Abstract
Simple Summary
Abstract
1. Introduction
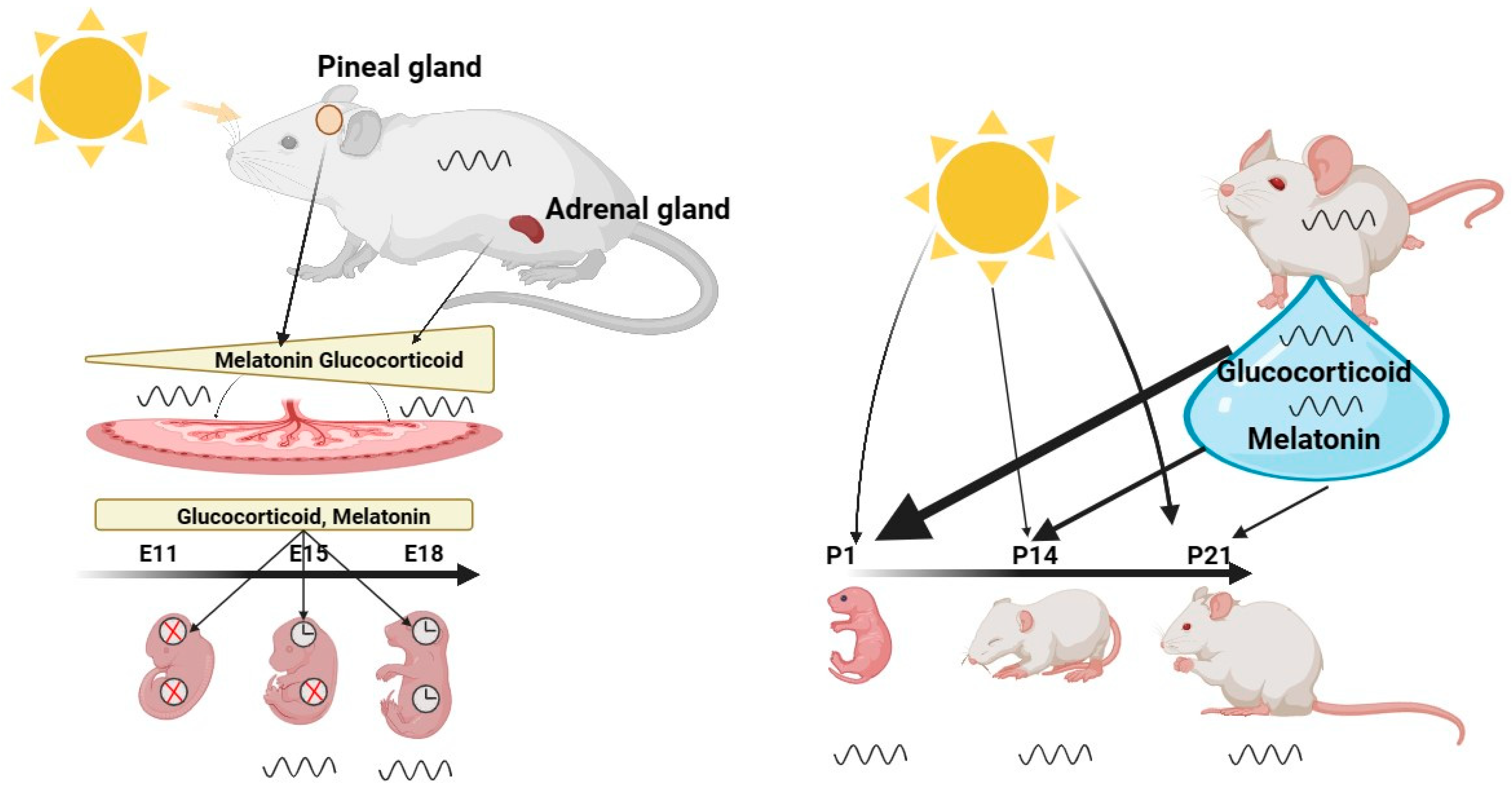
2. Prenatal and Infancy
3. Childhood and Adolescence
4. Menopause

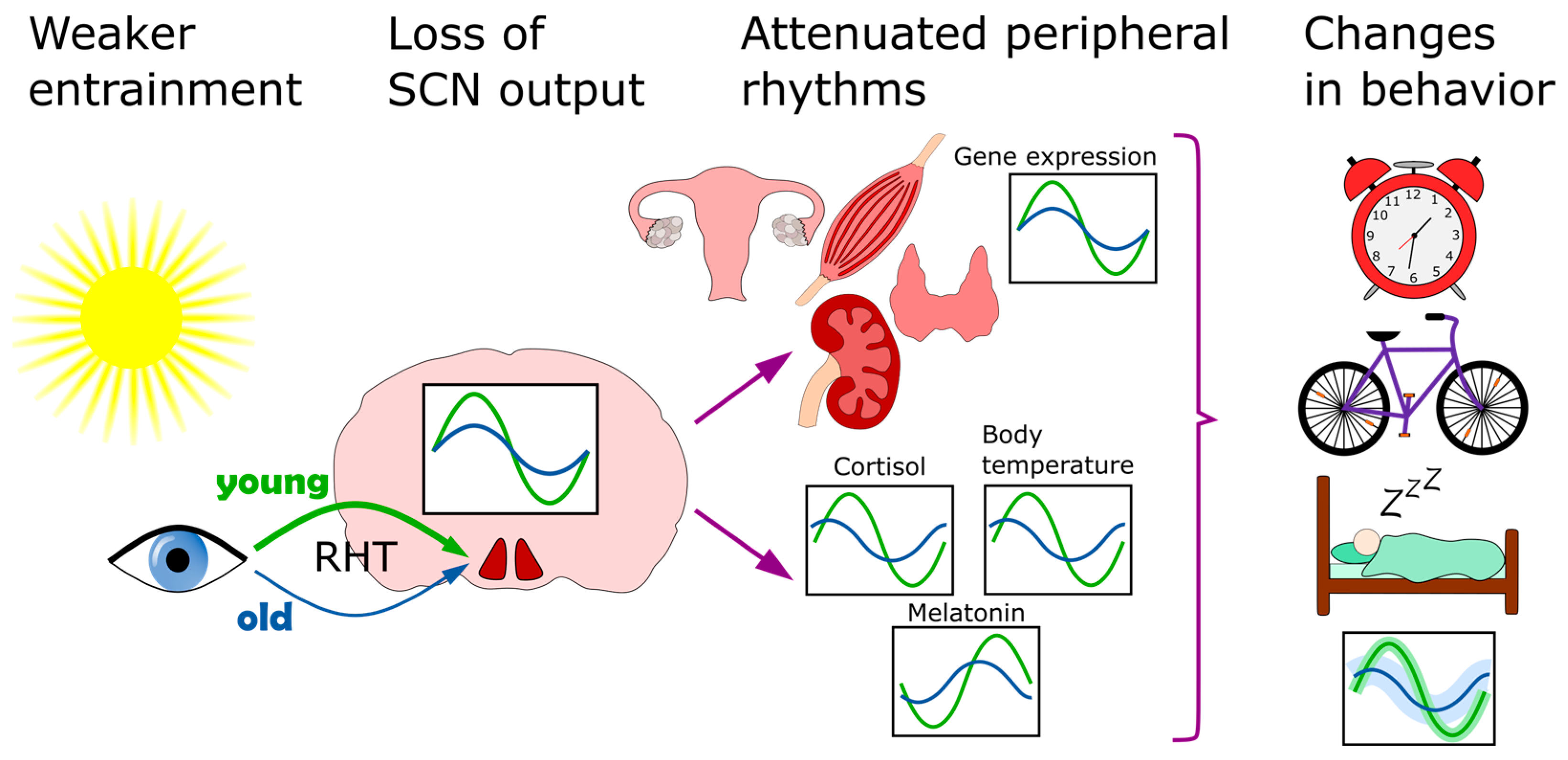
5. Old Age
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cox, K.H.; Takahashi, J.S. Circadian Clock Genes and the Transcriptional Architecture of the Clock Mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Okamoto-Uchida, Y.; Izawa, J.; Nishimura, A.; Hattori, A.; Suzuki, N.; Hirayama, J. Post-Translational Modifications Are Required for Circadian Clock Regulation in Vertebrates. Curr. Genom. 2019, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Finger, A.-M.; Kramer, A. Peripheral Clocks Tick Independently of Their Master. Genes Dev. 2021, 35, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.; Foster, R.G.; Jagannath, A. Photic Entrainment of the Circadian System. Int. J. Mol. Sci. 2022, 23, 729. [Google Scholar] [CrossRef] [PubMed]
- von Gall, C. The Effects of Light and the Circadian System on Rhythmic Brain Function. Int. J. Mol. Sci. 2022, 23, 2778. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Entrainment of the Mouse Circadian Clock: Effects of Stress, Exercise, and Nutrition. Free Radic. Biol. Med. 2018, 119, 129–138. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian Rhythms and Exercise-Re-Setting the Clock in Metabolic Disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Zordan, M.A.; Rosato, E.; Piccin, A.; Foster, R. Photic Entrainment of the Circadian Clock: From Drosophila to Mammals. Semin. Cell Dev. Biol. 2001, 12, 317–328. [Google Scholar] [CrossRef]
- Albrecht, U. The Circadian Clock, Metabolism and Obesity. Obes. Rev. 2017, 18 (Suppl. 1), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of Circadian Rhythms in Health and Disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; Lam, M.T.Y.; Panda, S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer 2019, 5, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Schwartz, W.J. Maternal Coordination of the Fetal Biological Clock in Utero. Science 1983, 220, 969–971. [Google Scholar] [CrossRef]
- Ono, M.; Ando, H.; Daikoku, T.; Fujiwara, T.; Mieda, M.; Mizumoto, Y.; Iizuka, T.; Kagami, K.; Hosono, T.; Nomura, S.; et al. The Circadian Clock, Nutritional Signals and Reproduction: A Close Relationship. Int. J. Mol. Sci. 2023, 24, 1545. [Google Scholar] [CrossRef]
- Kovanen, L.; Saarikoski, S.T.; Aromaa, A.; Lönnqvist, J.; Partonen, T. ARNTL (BMAL1) and NPAS2 Gene Variants Contribute to Fertility and Seasonality. PLoS ONE 2010, 5, e10007. [Google Scholar] [CrossRef]
- Jud, C.; Albrecht, U. Circadian Rhythms in Murine Pups Develop in Absence of a Functional Maternal Circadian Clock. J. Biol. Rhythm. 2006, 21, 149–154. [Google Scholar] [CrossRef]
- Astiz, M.; Oster, H. Feto-Maternal Crosstalk in the Development of the Circadian Clock System. Front. Neurosci. 2021, 14, 631687. [Google Scholar] [CrossRef]
- Bates, K.; Herzog, E.D. Maternal-Fetal Circadian Communication During Pregnancy. Front. Endocrinol. 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Han, L.W.; Gao, C.; Mao, Q. An Update on Expression and Function of P-Gp/Abcb1 and Bcrp/Abcg2 in the Placenta and Fetus. Expert Opin. Drug Metab. Toxicol. 2018, 14, 817–829. [Google Scholar] [CrossRef]
- Staud, F.; Karahoda, R. Trophoblast: The Central Unit of Fetal Growth, Protection and Programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of Serum Melatonin Level and Its Relationship to Feto-Placental Unit during Pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Naitoh, N.; Watanabe, Y.; Matsumura, K.; Murai, I.; Kobayashi, K.; Imai-Matsumura, K.; Ohtuka, H.; Takagi, K.; Miyake, Y.; Satoh, K.; et al. Alteration by Maternal Pinealectomy of Fetal and Neonatal Melatonin and Dopamine D1 Receptor Binding in the Suprachiasmatic Nuclei. Biochem. Biophys. Res. Commun. 1998, 253, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Antle, M.C.; LeSauter, J.; Silver, R. Neurogenesis and Ontogeny of Specific Cell Phenotypes within the Hamster Suprachiasmatic Nucleus. Dev. Brain Res. 2005, 157, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.E.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.M. Role of Melatonin in Embryo Fetal Development. J. Med. Life 2014, 7, 488–492. [Google Scholar] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and Stable Circadian Rhythms Optimize Maternal, Placental and Fetal Physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef]
- Lee, C.K.; Moon, D.H.; Shin, C.S.; Kim, H.; Yoon, Y.D.; Kang, H.S.; Lee, B.J.; Kang, S.G. Circadian Expression of Mel1a and PL-II Genes in Placenta: Effects of Melatonin on the PL-II Gene Expression in the Rat Placenta. Mol. Cell. Endocrinol. 2003, 200, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Schwartz, W.J. Maternal Suprachiasmatic Nuclei Are Necessary for Maternal Coordination of the Developing Circadian System. J. Neurosci. 1986, 6, 2724–2729. [Google Scholar] [CrossRef]
- Grosse, J.; Davis, F.C. Transient Entrainment of a Circadian Pacemaker during Development by Dopaminergic Activation in Syrian Hamsters. Brain Res. Bull. 1999, 48, 185–194. [Google Scholar] [CrossRef]
- Viswanathan, N.; Weaver, D.R.; Reppert, S.M.; Davis, F.C. Entrainment of the Fetal Hamster Circadian Pacemaker by Prenatal Injections of the Dopamine Agonist SKF 38393. J. Neurosci. 1994, 14, 5393–5398. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Ballard, P.L. Glucocorticoid Regulation of Lung Maturation. Mead Johns. Symp. Perinat. Dev. Med. 1987, 30, 22–27. [Google Scholar]
- Majumdar, A.P.; Nielsen, H. Influence of Glucocorticoids on Prenatal Development of the Gut and Pancreas in Rats. Scand. J. Gastroenterol. 1985, 20, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Venihaki, M.; Carrigan, A.; Dikkes, P.; Majzoub, J.A. Circadian Rise in Maternal Glucocorticoid Prevents Pulmonary Dysplasia in Fetal Mice with Adrenal Insufficiency. Proc. Natl. Acad. Sci. USA 2000, 97, 7336–7341. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Suthers, K.; Ferrucci, L.; Simonsick, E.M.; Ble, A.; Schrager, M.; Bandinelli, S.; Lauretani, F.; Giannelli, S.V.; Penninx, B.W. Hypercortisolemic Depression Is Associated with the Metabolic Syndrome in Late-Life. Psychoneuroendocrinology 2007, 32, 151–159. [Google Scholar] [CrossRef]
- Fraser, R.; Ingram, M.C.; Anderson, N.H.; Morrison, C.; Davies, E.; Connell, J.M.C. Cortisol Effects on Body Mass, Blood Pressure, and Cholesterol in the General Population. Hypertension 1999, 33, 1364–1368. [Google Scholar] [CrossRef]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder—Translating Findings From Humans to Animal Models and Back. Front. Psychiatry 2020, 10, 974. [Google Scholar] [CrossRef]
- Khan, Q.U.; Zaffar, S.; Rehan, A.M.; Rashid, R.R.; Ashraf, H.; Hafeez, F. Relationship of Major Depression with Body Mass Index and Salivary Cortisol. Cureus 2023, 12, e6577. [Google Scholar] [CrossRef] [PubMed]
- Wharfe, M.D.; Mark, P.J.; Wyrwoll, C.S.; Smith, J.T.; Yap, C.; Clarke, M.W.; Waddell, B.J. Pregnancy-Induced Adaptations of the Central Circadian Clock and Maternal Glucocorticoids. J. Endocrinol. 2016, 228, 135–147. [Google Scholar] [CrossRef]
- Seckl, J.R. Glucocorticoids, Feto-Placental 11 Beta-Hydroxysteroid Dehydrogenase Type 2, and the Early Life Origins of Adult Disease. Steroids 1997, 62, 89–94. [Google Scholar] [CrossRef]
- Nyirenda, M.J.; Lindsay, R.S.; Kenyon, C.J.; Burchell, A.; Seckl, J.R. Glucocorticoid Exposure in Late Gestation Permanently Programs Rat Hepatic Phosphoenolpyruvate Carboxykinase and Glucocorticoid Receptor Expression and Causes Glucose Intolerance in Adult Offspring. J. Clin. Investig. 1998, 101, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.J.; Waddell, B.J. Dual Function of 11beta-Hydroxysteroid Dehydrogenase in Placenta: Modulating Placental Glucocorticoid Passage and Local Steroid Action. Biol. Reprod. 1999, 60, 234–240. [Google Scholar] [CrossRef]
- Gatford, K.L.; Simmons, R.A.; De Blasio, M.J.; Robinson, J.S.; Owens, J.A. Review: Placental Programming of Postnatal Diabetes and Impaired Insulin Action after IUGR. Placenta 2010, 31, S60–S65. [Google Scholar] [CrossRef]
- Gatford, K.L.; Mohammad, S.N.B.; Harland, M.L.; De Blasio, M.J.; Fowden, A.L.; Robinson, J.S.; Owens, J.A. Impaired β-Cell Function and Inadequate Compensatory Increases in β-Cell Mass after Intrauterine Growth Restriction in Sheep. Endocrinology 2008, 149, 5118–5127. [Google Scholar] [CrossRef]
- Zimmermann, C.A.; Arloth, J.; Santarelli, S.; Löschner, A.; Weber, P.; Schmidt, M.V.; Spengler, D.; Binder, E.B. Stress Dynamically Regulates Co-Expression Networks of Glucocorticoid Receptor-Dependent MDD and SCZ Risk Genes. Transl. Psychiatry 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Cintra, A.; Solfrini, V.; Bunnemann, B.; Okret, S.; Bortolotti, F.; Gustafsson, J.A.; Fuxe, K. Prenatal Development of Glucocorticoid Receptor Gene Expression and Immunoreactivity in the Rat Brain and Pituitary Gland: A Combined in Situ Hybridization and Immunocytochemical Analysis. Neuroendocrinology 1993, 57, 1133–1147. [Google Scholar] [CrossRef]
- Matthews, S.G. Antenatal Glucocorticoids and Programming of the Developing CNS. Pediatr. Res. 2000, 47, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Pesonen, A.-K.; O’Reilly, J.R.; Tuovinen, S.; Lahti, M.; Kajantie, E.; Villa, P.M.; Laivuori, H.; Hämäläinen, E.; Seckl, J.R.; et al. Maternal Depressive Symptoms throughout Pregnancy Are Associated with Increased Placental Glucocorticoid Sensitivity. Psychol. Med. 2015, 45, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and Fetal Programming Part 1: Outcomes. Nat. Rev. Endocrinol. 2014, 10, 391–402. [Google Scholar] [CrossRef]
- Moisiadis, V.G.; Matthews, S.G. Glucocorticoids and Fetal Programming Part 2: Mechanisms. Nat. Rev. Endocrinol. 2014, 10, 403–411. [Google Scholar] [CrossRef]
- Astiz, M.; Heyde, I.; Fortmann, M.I.; Bossung, V.; Roll, C.; Stein, A.; Grüttner, B.; Göpel, W.; Härtel, C.; Obleser, J.; et al. The Circadian Phase of Antenatal Glucocorticoid Treatment Affects the Risk of Behavioral Disorders. Nat. Commun. 2020, 11, 3593. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Chavan, R.; Fonseca Costa, S.S.; Brenna, A.; Ripperger, J.A.; Albrecht, U. REV-ERBα Influences the Stability and Nuclear Localization of the Glucocorticoid Receptor. J. Cell Sci. 2016, 129, 4143–4154. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.; Van Eekelen, J.A.M.; Levine, S.; De Kloet, E.R. Ontogeny of the Type 2 Glucocorticoid Receptor in Discrete Rat Brain Regions: An Immunocytochemical Study. Dev. Brain Res. 1988, 42, 119–127. [Google Scholar] [CrossRef]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Kellendonk, C.; Reichardt, H.M.; Schütz, G.; Schibler, U. Resetting of Circadian Time in Peripheral Tissues by Glucocorticoid Signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef] [PubMed]
- Čečmanová, V.; Houdek, P.; Šuchmanová, K.; Sládek, M.; Sumová, A. Development and Entrainment of the Fetal Clock in the Suprachiasmatic Nuclei: The Role of Glucocorticoids. J. Biol. Rhythm. 2019, 34, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Houdek, P.; Sumová, A. In Vivo Initiation of Clock Gene Expression Rhythmicity in Fetal Rat Suprachiasmatic Nuclei. PLoS ONE 2014, 9, e107360. [Google Scholar] [CrossRef]
- Ashwood, P.J.; Crowther, C.A.; Willson, K.J.; Haslam, R.R.; Kennaway, D.J.; Hiller, J.E.; Robinson, J.S. Neonatal Adrenal Function after Repeat Dose Prenatal Corticosteroids: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2006, 194, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Erni, K. Prenatal Stress: Long-Term Effects of Psychobiological Reactivity on Acute Psychosocial Stress and Anxiety in Ten-Year-Old-Children; Cuvillier Verlag: Goettingen, Germany, 2012; ISBN 978-3-7369-4022-2. [Google Scholar]
- Alexander, N.; Rosenlöcher, F.; Stalder, T.; Linke, J.; Distler, W.; Morgner, J.; Kirschbaum, C. Impact of Antenatal Synthetic Glucocorticoid Exposure on Endocrine Stress Reactivity in Term-Born Children. J. Clin. Endocrinol. Metab. 2012, 97, 3538–3544. [Google Scholar] [CrossRef] [PubMed]
- Engeland, W.C.; Arnhold, M.M. Neural Circuitry in the Regulation of Adrenal Corticosterone Rhythmicity. Endocrine 2005, 28, 325–332. [Google Scholar] [CrossRef]
- Lemos, D.R.; Downs, J.L.; Urbanski, H.F. Twenty-Four-Hour Rhythmic Gene Expression in the Rhesus Macaque Adrenal Gland. Mol. Endocrinol. 2006, 20, 1164–1176. [Google Scholar] [CrossRef]
- Oster, H. The Genetic Basis of Circadian Behavior. Genes Brain Behav. 2006, 5 (Suppl. 2), 73–79. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Mendez, N.; Abarzua-Catalan, L.; Vilches, N.; Valenzuela, G.J.; Seron-Ferre, M. A Circadian Clock Entrained by Melatonin Is Ticking in the Rat Fetal Adrenal. Endocrinology 2011, 152, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Seron-Ferre, M.; Valenzuela, G.J.; Torres-Farfan, C. Circadian Clocks during Embryonic and Fetal Development. Birth Defects Res. C Embryo Today 2007, 81, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, C.M.; Menaker, M. Effect of Transplanting Suprachiasmatic Nuclei from Donors of Different Ages into Completely SCN Lesioned Hamsters. J. Neural Transplant. Plast. 1993, 4, 257–265. [Google Scholar] [CrossRef]
- Silver, R.; Lehman, M.N.; Gibson, M.; Gladstone, W.R.; Bittman, E.L. Dispersed Cell Suspensions of Fetal SCN Restore Circadian Rhythmicity in SCN-Lesioned Adult Hamsters. Brain Res. 1990, 525, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Wreschnig, D.; Dolatshad, H.; Davis, F.C. Embryonic Development of Circadian Oscillations in the Mouse Hypothalamus. J. Biol. Rhythm. 2014, 29, 299–310. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.-K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE Real-Time Reporting of Circadian Dynamics Reveals Persistent Circadian Oscillations in Mouse Peripheral Tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, D.; Achten, C.; Dallmann, F.; Oster, H. Embryonic Development and Maternal Regulation of Murine Circadian Clock Function. Chronobiol. Int. 2015, 32, 416–427. [Google Scholar] [CrossRef]
- Kennaway, D.J. The Role of Circadian Rhythmicity in Reproduction. Hum. Reprod. Update 2005, 11, 91–101. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, G.T.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.; Takao, M.; de Wit, J.; Verkerk, A.; Eker, A.P.; van Leenen, D.; et al. Mammalian Cry1 and Cry2 Are Essential for Maintenance of Circadian Rhythms. Nature 1999, 398, 627–630. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Voultsios, A.; Varcoe, T.J.; Moyer, R.W. Melatonin and Activity Rhythm Responses to Light Pulses in Mice with the Clock Mutation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1231–R1240. [Google Scholar] [CrossRef]
- Cowden, K.D.; Simon, M.C. The BHLH/PAS Factor MOP3 Does Not Participate in Hypoxia Responses. Biochem. Biophys. Res. Commun. 2002, 290, 1228–1236. [Google Scholar] [CrossRef]
- Roh, J.H.; Huang, Y.; Bero, A.W.; Kasten, T.; Stewart, F.R.; Bateman, R.J.; Holtzman, D.M. Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of β-Amyloid in Mice with Alzheimer’s Disease Pathology. Sci. Transl. Med. 2012, 4, 150ra122. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Campbell, S.S.; Zone, S.E.; Cooper, F.; DeSano, A.; Murphy, P.J.; Jones, B.; Czajkowski, L.; Ptček, L.J. Familial Advanced Sleep-Phase Syndrome: A Short-Period Circadian Rhythm Variant in Humans. Nat. Med. 1999, 5, 1062–1065. [Google Scholar] [CrossRef]
- Swaab, D.F.; Hofman, M.A.; Honnebier, M.B. Development of Vasopressin Neurons in the Human Suprachiasmatic Nucleus in Relation to Birth. Brain Res. Dev. Brain Res. 1990, 52, 289–293. [Google Scholar] [CrossRef]
- Perez-Catalan, N.A.; Doe, C.Q.; Ackerman, S.D. The Role of Astrocyte-Mediated Plasticity in Neural Circuit Development and Function. Neural Dev. 2021, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Greiner, P.; Houdek, P.; Sládek, M.; Sumová, A. Early Rhythmicity in the Fetal Suprachiasmatic Nuclei in Response to Maternal Signals Detected by Omics Approach. PLoS Biol. 2022, 20, e3001637. [Google Scholar] [CrossRef] [PubMed]
- Sládek, M.; Jindráková, Z.; Bendová, Z.; Sumová, A. Postnatal Ontogenesis of the Circadian Clock within the Rat Liver. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1224–R1229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Davis, F.C. Developmental Expression of Clock Genes in the Syrian Hamster. Dev. Brain Res. 2005, 158, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Agathagelidis, M.; Lee, C.; Korf, H.-W.; von Gall, C. Differential Maturation of Circadian Rhythms in Clock Gene Proteins in the Suprachiasmatic Nucleus and the Pars Tuberalis during Mouse Ontogeny. Eur. J. Neurosci. 2009, 29, 477–489. [Google Scholar] [CrossRef]
- Shimomura, H.; Moriya, T.; Sudo, M.; Wakamatsu, H.; Akiyama, M.; Miyake, Y.; Shibata, S. Differential Daily Expression of Per1 and Per2 MRNA in the Suprachiasmatic Nucleus of Fetal and Early Postnatal Mice. Eur. J. Neurosci. 2001, 13, 687–693. [Google Scholar] [CrossRef]
- Ohta, H.; Xu, S.; Moriya, T.; Iigo, M.; Watanabe, T.; Nakahata, N.; Chisaka, H.; Hanita, T.; Matsuda, T.; Ohura, T.; et al. Maternal Feeding Controls Fetal Biological Clock. PLoS ONE 2008, 3, e2601. [Google Scholar] [CrossRef]
- McNeill, D.S.; Sheely, C.J.; Ecker, J.L.; Badea, T.C.; Morhardt, D.; Guido, W.; Hattar, S. Development of Melanopsin-Based Irradiance Detecting Circuitry. Neural Dev. 2011, 6, 8. [Google Scholar] [CrossRef]
- Tarttelin, E.E.; Bellingham, J.; Hankins, M.W.; Foster, R.G.; Lucas, R.J. Neuropsin (Opn5): A Novel Opsin Identified in Mammalian Neural Tissue. FEBS Lett. 2003, 554, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Wu, V.; Donovan, M.; Majumdar, S.; Rentería, R.C.; Porco, T.; Van Gelder, R.N.; Copenhagen, D.R. Melanopsin-Dependent Light Avoidance in Neonatal Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17374–17378. [Google Scholar] [CrossRef] [PubMed]
- Kandel, G.L.; Bedell, H.; Walker, R.; Wolf, B.M. Negative Phototaxis in Pigmented, Albinotic and RCS Rat Pups Measured with a New Technique. Vision Sci. 1987, 1, 357–366. [Google Scholar]
- Crawford, M.L.; Marc, R.E. Light Transmission of Cat and Monkey Eyelids. Vision Res. 1976, 16, 323–324. [Google Scholar] [CrossRef]
- Crozier, W.J.; Pincus, G. Photic Stimulation of Young Rats. J. Gen. Psychol. 1937, 17, 105–111. [Google Scholar] [CrossRef]
- Polese, D.; Riccio, M.L.; Fagioli, M.; Mazzetta, A.; Fagioli, F.; Parisi, P.; Fagioli, M. The Newborn’s Reaction to Light as the Determinant of the Brain’s Activation at Human Birth. Front. Integr. Neurosci. 2022, 16, 933426. [Google Scholar] [CrossRef]
- Glotzbach: Development of the Human Retinohypothalamic Tract-Google Scholar. Available online: https://scholar.google.com/scholar_lookup?journal=Soc+Neurosci&title=Development+of+the+human+retinohypothalamic+tract&author=SF+Glotzbach&author=P+Sollars&author=M+Pagano&volume=18&publication_year=1992&pages=857& (accessed on 19 January 2023).
- Meijer, J.H.; Schwartz, W.J. In Search of the Pathways for Light-Induced Pacemaker Resetting in the Suprachiasmatic Nucleus. J. Biol. Rhythm. 2003, 18, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.D.; Wright, K.P.; Spencer, R.L.; Vetter, C.; Hicks, L.M.; Jenni, O.G.; LeBourgeois, M.K. Development of the Circadian System in Early Life: Maternal and Environmental Factors. J. Physiol. Anthropol. 2022, 41, 22. [Google Scholar] [CrossRef]
- Pundir, S.; Mitchell, C.J.; Thorstensen, E.B.; Wall, C.R.; Perrella, S.L.; Geddes, D.T.; Cameron-Smith, D. Impact of Preterm Birth on Glucocorticoid Variability in Human Milk. J. Hum. Lact. 2018, 34, 130–136. [Google Scholar] [CrossRef]
- Sánchez, C.L.; Cubero, J.; Sánchez, J.; Franco, L.; Rodríguez, A.B.; Rivero, M.; Barriga, C. Evolution of the Circadian Profile of Human Milk Amino Acids during Breastfeeding. J. Appl. Biomed. 2013, 11, 59–70. [Google Scholar] [CrossRef]
- França, E.L.; Nicomedes, T.d.R.; Calderon, I.d.P.; França, A.C.H. Time-Dependent Alterations of Soluble and Cellular Components in Human Milk. Biol. Rhythm. Res. 2010, 41, 333–347. [Google Scholar] [CrossRef]
- Illnerová, H.; Buresová, M.; Presl, J. Melatonin Rhythm in Human Milk. J. Clin. Endocrinol. Metab. 1993, 77, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.M.; Kakulas, F.; Hepworth, A.R.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. The Effects of Leptin on Breastfeeding Behaviour. Int. J. Environ. Res. Public Health 2015, 12, 12340–12355. [Google Scholar] [CrossRef]
- Cubero, J.; Narciso, D.; Terrón, P.; Rial, R.; Esteban, S.; Rivero, M.; Parvez, H.; Rodríguez, A.B.; Barriga, C. Chrononutrition Applied to Formula Milks to Consolidate Infants’ Sleep/Wake Cycle. Neuro Endocrinol. Lett. 2007, 28, 360–366. [Google Scholar] [PubMed]
- Cubero, J.; Valero, V.; Sánchez, J.; Rivero, M.; Parvez, H.; Rodríguez, A.B.; Barriga, C. The Circadian Rhythm of Tryptophan in Breast Milk Affects the Rhythms of 6-Sulfatoxymelatonin and Sleep in Newborn. Neuro Endocrinol. Lett. 2005, 26, 657–661. [Google Scholar]
- Reppert, S.M.; Klein, D.C. Transport of Maternal[3H]Melatonin to Suckling Rats and the Fate of [3H]Melatonin in the Neonatal Rat. Endocrinology 1978, 102, 582–588. [Google Scholar] [CrossRef]
- Pundir, S.; Wall, C.R.; Mitchell, C.J.; Thorstensen, E.B.; Lai, C.T.; Geddes, D.T.; Cameron-Smith, D. Variation of Human Milk Glucocorticoids over 24 Hour Period. J. Mammary Gland. Biol. Neoplasia 2017, 22, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.; Nolvi, S.; Härkönen, J.; Aatsinki, A.-K.; Karlsson, L.; Karlsson, H.; Uusitupa, H.-M. Associations between Maternal Socioeconomic, Psychosocial and Seasonal Factors, Infant Characteristics and Human Milk Cortisol Concentrations. Am. J. Hum. Biol. 2021, 33, e23561. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Slupsky, C.M.; Aatsinki, A.-K.; Sinkkonen, J.; Karlsson, L.; Linderborg, K.M.; Yang, B.; Karlsson, H.; Kailanto, H.-M. Human Milk Metabolome Is Associated with Symptoms of Maternal Psychological Distress and Milk Cortisol. Food Chem. 2021, 356, 129628. [Google Scholar] [CrossRef]
- Angelucci, L.; Patacchioli, F.R.; Chierichetti, C.; Laureti, S. Perinatal Mother-Offspring Pituitary-Adrenal Interrelationship in Rats: Corticosterone in Milk May Affect Adult Life. Endocrinol. Exp. 1983, 17, 191–205. [Google Scholar] [PubMed]
- Hirotsu, C.; Tufik, S.; Andersen, M.L. Interactions between Sleep, Stress, and Metabolism: From Physiological to Pathological Conditions. Sleep Sci. 2015, 8, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Patterson, Z.; Abizaid, A. Stress Induced Obesity: Lessons from Rodent Models of Stress. Front. Neurosci. 2013, 7, 130. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- de Weerth, C.; Zijl, R.H.; Buitelaar, J.K. Development of Cortisol Circadian Rhythm in Infancy. Early Hum. Dev. 2003, 73, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kiess, W.; Meidert, A.; Dressendörfer, R.A.; Schriever, K.; Kessler, U.; König, A.; Schwarz, H.P.; Strasburger, C.J. Salivary Cortisol Levels throughout Childhood and Adolescence: Relation with Age, Pubertal Stage, and Weight. Pediatr. Res. 1995, 37, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Holbrook, J.; Le, T.B.; Chung, A.; Davis, E.P.; Glynn, L.M. Cortisol in Human Milk Predicts Child BMI. Obesity 2016, 24, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Nolvi, S.; Uusitupa, H.-M.; Bridgett, D.J.; Pesonen, H.; Aatsinki, A.-K.; Kataja, E.-L.; Korja, R.; Karlsson, H.; Karlsson, L. Human Milk Cortisol Concentration Predicts Experimentally Induced Infant Fear Reactivity: Moderation by Infant Sex. Dev. Sci. 2018, 21, e12625. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.L.; Chun, L.E.; Hartsock, M.J.; Woodruff, E.R. Glucocorticoid Hormones Are Both a Major Circadian Signal and Major Stress Signal: How This Shared Signal Contributes to a Dynamic Relationship between the Circadian and Stress Systems. Front. Neuroendocrinol. 2018, 49, 52–71. [Google Scholar] [CrossRef]
- Kim, J.J.; Yoon, K.S. Stress: Metaplastic Effects in the Hippocampus. Trends Neurosci. 1998, 21, 505–509. [Google Scholar] [CrossRef]
- Brandner, S.; Schroeter, S.; Çalışkan, G.; Salar, S.; Kobow, K.; Coras, R.; Blümcke, I.; Hamer, H.; Schwarz, M.; Buchfelder, M.; et al. Glucocorticoid Modulation of Synaptic Plasticity in the Human Temporal Cortex of Epilepsy Patients: Does Chronic Stress Contribute to Memory Impairment? Epilepsia 2022, 63, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Smits, M.; Spence, W.; Srinivasan, V.; Cardinali, D.P.; Lowe, A.D.; Kayumov, L. Dim Light Melatonin Onset (DLMO): A Tool for the Analysis of Circadian Phase in Human Sleep and Chronobiological Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1–11. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the Human Circadian Clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Simpkin, C.T.; Jenni, O.G.; Carskadon, M.A.; Wright, K.P.; Akacem, L.D.; Garlo, K.G.; LeBourgeois, M.K. Chronotype Is Associated with the Timing of the Circadian Clock and Sleep in Toddlers. J. Sleep Res. 2014, 23, 397–405. [Google Scholar] [CrossRef]
- LeBourgeois, M.K.; Carskadon, M.A.; Akacem, L.D.; Simpkin, C.T.; Wright, K.P.; Achermann, P.; Jenni, O.G. Circadian Phase and Its Relationship to Nighttime Sleep in Toddlers. J. Biol. Rhythm. 2013, 28, 322–331. [Google Scholar] [CrossRef]
- Lassonde, J.M.; Rusterholz, T.; Kurth, S.; Schumacher, A.M.; Achermann, P.; LeBourgeois, M.K. Sleep Physiology in Toddlers: Effects of Missing a Nap on Subsequent Night Sleep. Neurobiol. Sleep Circadian Rhythm. 2016, 1, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Akacem, L.D.; Simpkin, C.T.; Carskadon, M.A.; Wright, K.P.; Jenni, O.G.; Achermann, P.; LeBourgeois, M.K. The Timing of the Circadian Clock and Sleep Differ between Napping and Non-Napping Toddlers. PLoS ONE 2015, 10, e0125181. [Google Scholar] [CrossRef]
- Giannoumis, M.; Mok, E.; Borkhoff, C.M.; Birken, C.S.; Maguire, J.; Parkin, P.C.; Li, P.; Constantin, E. Association of Accelerometry-Derived Social Jetlag and Sleep with Temperament in Children Less than 6 Years of Age. J. Clin. Sleep Med. 2022, 18, 1993–1999. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social Jetlag: Misalignment of Biological and Social Time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Caliandro, R.; Streng, A.A.; van Kerkhof, L.W.M.; van der Horst, G.T.J.; Chaves, I. Social Jetlag and Related Risks for Human Health: A Timely Review. Nutrients 2021, 13, 4543. [Google Scholar] [CrossRef] [PubMed]
- Hartstein, L.E.; Behn, C.D.; Akacem, L.D.; Stack, N.; Wright, K.P.; LeBourgeois, M.K. High Sensitivity of Melatonin Suppression Response to Evening Light in Preschool-Aged Children. J. Pineal. Res. 2022, 72, e12780. [Google Scholar] [CrossRef]
- Eto, T.; Kitamura, S.; Nishimura, K.; Takeoka, K.; Nishimura, Y.; Lee, S.-I.; Ohashi, M.; Shikano, A.; Noi, S.; Higuchi, S. Circadian Phase Advances in Children during Camping Life According to the Natural Light-Dark Cycle. J. Physiol. Anthropol. 2022, 41, 42. [Google Scholar] [CrossRef]
- Falbe, J.; Davison, K.K.; Franckle, R.L.; Ganter, C.; Gortmaker, S.L.; Smith, L.; Land, T.; Taveras, E.M. Sleep Duration, Restfulness, and Screens in the Sleep Environment. Pediatrics 2015, 135, e367–e375. [Google Scholar] [CrossRef] [PubMed]
- Hagenauer, M.H.; Perryman, J.I.; Lee, T.M.; Carskadon, M.A. Adolescent Changes in the Homeostatic and Circadian Regulation of Sleep. Dev. Neurosci. 2009, 31, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Micic, G.; Lovato, N.; Gradisar, M.; Ferguson, S.A.; Burgess, H.J.; Lack, L.C. The Etiology of Delayed Sleep Phase Disorder. Sleep Med. Rev. 2016, 27, 29–38. [Google Scholar] [CrossRef]
- Schröder, C.M. Désordre circadien du sommeil de l’adolescent: Rôle du multimédia. Bull. De L’académie Natl. De Médecine 2015, 199, 1099–1113. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Vieira, C.; Acebo, C. Association between Puberty and Delayed Phase Preference. Sleep 1993, 16, 258–262. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A Marker for the End of Adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Acebo, C.; Richardson, G.S.; Tate, B.A.; Seifer, R. An Approach to Studying Circadian Rhythms of Adolescent Humans. J. Biol. Rhythm. 1997, 12, 278–289. [Google Scholar] [CrossRef]
- Mong, J.A.; Cusmano, D.M. Sex Differences in Sleep: Impact of Biological Sex and Sex Steroids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150110. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Silver, R. Minireview: The Neuroendocrinology of the Suprachiasmatic Nucleus as a Conductor of Body Time in Mammals. Endocrinology 2007, 148, 5640–5647. [Google Scholar] [CrossRef]
- Baker, F.C.; Driver, H.S. Circadian Rhythms, Sleep, and the Menstrual Cycle. Sleep Med. 2007, 8, 613–622. [Google Scholar] [CrossRef]
- Hagenauer, M.H.; Lee, T.M. The Neuroendocrine Control of the Circadian System: Adolescent Chronotype. Front. Neuroendocrinol. 2012, 33, 211–229. [Google Scholar] [CrossRef]
- Jenni, O.G.; Achermann, P.; Carskadon, M.A. Homeostatic Sleep Regulation in Adolescents. Sleep 2005, 28, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, E.J.; Joyce, D.S.; Rissman, A.J.; Burgess, H.J.; Colwell, C.S.; Lack, L.C.; Gradisar, M. Electric Lighting, Adolescent Sleep and Circadian Outcomes, and Recommendations for Improving Light Health. Sleep Med. Rev. 2022, 64, 101667. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening Exposure to a Light-Emitting Diodes (LED)-Backlit Computer Screen Affects Circadian Physiology and Cognitive Performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.C.; Dolinoy, D.; Peterson, K.E.; O’Brien, L.M.; Chervin, R.D.; Cantoral, A.; Tellez-Rojo, M.M.; Solano-Gonzalez, M.; Goodrich, J. Adolescent Sleep Timing and Dietary Patterns in Relation to DNA Methylation of Core Circadian Genes: A Pilot Study of Mexican Youth. Epigenetics 2021, 16, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Weinert, D.; Eimert, H.; Erkert, H.G.; Schneyer, U. Resynchronization of the Circadian Corticosterone Rhythm after a Light/Dark Shift in Juvenile and Adult Mice. Chronobiol. Int. 1994, 11, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Gau, S.-F.; Soong, W.-T. The Transition of Sleep-Wake Patterns in Early Adolescence. Sleep 2003, 26, 449–454. [Google Scholar] [CrossRef]
- Carrell, S.E.; Maghakian, T.; West, J.E. A’s from Zzzz’s? The Causal Effect of School Start Time on the Academic Achievement of Adolescents. Am. Econ. J. Econ. Policy 2011, 3, 62–81. [Google Scholar] [CrossRef]
- Kirby, M.; Maggi, S.; D’Angiulli, A. School Start Times and the Sleep–Wake Cycle of Adolescents: A Review and Critical Evaluation of Available Evidence. Educ. Res. 2011, 40, 56–61. [Google Scholar] [CrossRef]
- Kelley, P.; Lockley, S.W.; Kelley, J.; Evans, M.D.R. Is 8:30 a.m. Still Too Early to Start School? A 10:00 a.m. School Start Time Improves Health and Performance of Students Aged 13–16. Front. Hum. Neurosci. 2017, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- van der Lely, S.; Frey, S.; Garbazza, C.; Wirz-Justice, A.; Jenni, O.G.; Steiner, R.; Wolf, S.; Cajochen, C.; Bromundt, V.; Schmidt, C. Blue Blocker Glasses as a Countermeasure for Alerting Effects of Evening Light-Emitting Diode Screen Exposure in Male Teenagers. J. Adolesc. Health 2015, 56, 113–119. [Google Scholar] [CrossRef]
- Ellis, S.; Franks, D.W.; Nattrass, S.; Cant, M.A.; Bradley, D.L.; Giles, D.; Balcomb, K.C.; Croft, D.P. Postreproductive Lifespans Are Rare in Mammals. Ecol. Evol. 2018, 8, 2482–2494. [Google Scholar] [CrossRef]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The Role of Androgens and Estrogens on Healthy Aging and Longevity. J. Gerontol. Ser. A 2012, 67, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Yopo Díaz, M. The Biological Clock: Age, Risk, and the Biopolitics of Reproductive Time. Sex Roles 2021, 84, 765–778. [Google Scholar] [CrossRef]
- Wagner, M.; Huinink, J.; Liefbroer, A.C. Running out of Time? Understanding the Consequences of the Biological Clock for the Dynamics of Fertility Intentions and Union Formation. Demogr. Res. 2019, 40, 1–26. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Barad, D.H. The Role of Androgens in Follicle Maturation and Ovulation Induction: Friend or Foe of Infertility Treatment? Reprod. Biol. Endocrinol. 2011, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Kuo, T.-H.; Chen, C.-C.; Jian, C.-Y.; Chen, C.-W.; Wang, K.-L.; Kuo, Y.-C.; Shen, H.-Y.; Hsia, S.-M.; Wang, P.S.; et al. Downregulation of Testosterone Production through Luteinizing Hormone Receptor Regulation in Male Rats Exposed to 17α-Ethynylestradiol. Sci. Rep. 2020, 10, 1576. [Google Scholar] [CrossRef]
- Lispi, M.; Drakopoulos, P.; Spaggiari, G.; Caprio, F.; Colacurci, N.; Simoni, M.; Santi, D. Testosterone Serum Levels Are Related to Sperm DNA Fragmentation Index Reduction after FSH Administration in Males with Idiopathic Infertility. Biomedicines 2022, 10, 2599. [Google Scholar] [CrossRef] [PubMed]
- Schill, W.B. Fertility and Sexual Life of Men after Their Forties and in Older Age. Asian J. Androl. 2001, 3, 1–7. [Google Scholar] [PubMed]
- Fahrenkrug, J.; Georg, B.; Hannibal, J.; Hindersson, P.; Gräs, S. Diurnal Rhythmicity of the Clock Genes Per1 and Per2 in the Rat Ovary. Endocrinology 2006, 147, 3769–3776. [Google Scholar] [CrossRef] [PubMed]
- Sellix, M.T. Circadian Clock Function in the Mammalian Ovary. J. Biol. Rhythm. 2015, 30, 7–19. [Google Scholar] [CrossRef]
- Kriegsfeld, L.J.; Silver, R. The Regulation of Neuroendocrine Function: Timing Is Everything. Horm. Behav. 2006, 49, 557–574. [Google Scholar] [CrossRef]
- Stigliani, S.; Massarotti, C.; De Leo, C.; Maccarini, E.; Sozzi, F.; Cagnacci, A.; Anserini, P.; Scaruffi, P. Fifteen Year Regional Center Experience in Sperm Banking for Cancer Patients: Use and Reproductive Outcomes in Survivors. Cancers 2021, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.D.; Hansen, A.; Ord, T.; Bebas, P.; Chappell, P.E.; Giebultowicz, J.M.; Williams, C.; Moss, S.; Sehgal, A. The Circadian Clock Protein BMAL1 Is Necessary for Fertility and Proper Testosterone Production in Mice. J. Biol. Rhythm. 2008, 23, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Boden, M.J.; Varcoe, T.J.; Voultsios, A.; Kennaway, D.J. Reproductive Biology of Female Bmal1 Null Mice. Reproduction 2010, 139, 1077–1090. [Google Scholar] [CrossRef]
- Chappell, P.E.; White, R.S.; Mellon, P.L. Circadian Gene Expression Regulates Pulsatile Gonadotropin-Releasing Hormone (GnRH) Secretory Patterns in the Hypothalamic GnRH-Secreting GT1-7 Cell Line. J. Neurosci. 2003, 23, 11202–11213. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Boden, M.J.; Voultsios, A. Reproductive Performance in Female Clock Delta19 Mutant Mice. Reprod. Fertil. Dev. 2004, 16, 801–810. [Google Scholar] [CrossRef]
- Miller, B.H.; Olson, S.L.; Turek, F.W.; Levine, J.E.; Horton, T.H.; Takahashi, J.S. Circadian Clock Mutation Disrupts Estrous Cyclicity and Maintenance of Pregnancy. Curr. Biol. 2004, 14, 1367–1373. [Google Scholar] [CrossRef]
- Dolatshad, H.; Campbell, E.A.; O’Hara, L.; Maywood, E.S.; Hastings, M.H.; Johnson, M.H. Developmental and Reproductive Performance in Circadian Mutant Mice. Hum. Reprod. 2006, 21, 68–79. [Google Scholar] [CrossRef]
- Ratajczak, C.K.; Boehle, K.L.; Muglia, L.J. Impaired Steroidogenesis and Implantation Failure in Bmal1−/− Mice. Endocrinology 2009, 150, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Boden, M.J.; Varcoe, T.J.; Kennaway, D.J. Circadian Regulation of Reproduction: From Gamete to Offspring. Prog. Biophys. Mol. Biol. 2013, 113, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Steinlechner, S. Low Reproductive Success in Per1 and Per2 Mutant Mouse Females Due to Accelerated Ageing? Reproduction 2008, 135, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, C.; Li, Y.; Jiang, H.; Yang, P.; Tang, J.; Xu, Y.; Wang, H.; He, Y. Loss-of-Function Mutations with Circadian Rhythm Regulator Per1/Per2 Lead to Premature Ovarian Insufficiency. Biol. Reprod. 2019, 100, 1066–1072. [Google Scholar] [CrossRef]
- Chu, A.; Zhu, L.; Blum, I.D.; Mai, O.; Leliavski, A.; Fahrenkrug, J.; Oster, H.; Boehm, U.; Storch, K.-F. Global but Not Gonadotrope-Specific Disruption of Bmal1 Abolishes the Luteinizing Hormone Surge without Affecting Ovulation. Endocrinology 2013, 154, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M.; Weaver, D.R. Differential Functions of MPer1, MPer2, and MPer3 in the SCN Circadian Clock. Neuron 2001, 30, 525–536. [Google Scholar] [CrossRef]
- Sellix, M.T.; Yoshikawa, T.; Menaker, M. A Circadian Egg Timer Gates Ovulation. Curr. Biol. 2010, 20, R266–R267. [Google Scholar] [CrossRef]
- de la Iglesia, H.O.; Schwartz, W.J. Minireview: Timely Ovulation: Circadian Regulation of the Female Hypothalamo-Pituitary-Gonadal Axis. Endocrinology 2006, 147, 1148–1153. [Google Scholar] [CrossRef]
- Barbieri, R.L. The Endocrinology of the Menstrual Cycle. Methods Mol. Biol. 2014, 1154, 145–169. [Google Scholar] [CrossRef]
- Ohara, T.; Nakamura, T.; Nakamura, W.; Tokuda, I. Modeling Circadian Regulation of Ovulation Timing: Age-Related Disruption of Estrous Cyclicity. Sci. Rep. 2020, 10, 16767. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Hoffmann, H.M. Role of Core Circadian Clock Genes in Hormone Release and Target Tissue Sensitivity in the Reproductive Axis. Mol. Cell. Endocrinol. 2020, 501, 110655. [Google Scholar] [CrossRef] [PubMed]
- Lejri, I.; Grimm, A.; Eckert, A. Mitochondria, Estrogen and Female Brain Aging. Front. Aging Neurosci. 2018, 10, 124. [Google Scholar] [CrossRef]
- Mobbs, C.V.; Flurkey, K.; Gee, D.M.; Yamamoto, K.; Sinha, Y.N.; Finch, C.E. Estradiol-Induced Adult Anovulatory Syndrome in Female C57BL/6J Mice: Age-Like Neuroendocrine, but Not Ovarian, Impairments1. Biol. Reprod. 1984, 30, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Finch, C.E. The Menopause and Aging, a Comparative Perspective. J. Steroid Biochem. Mol. Biol. 2014, 142, 132–141. [Google Scholar] [CrossRef]
- Gill, S.; Sharpless, J.L.; Rado, K.; Hall, J.E. Evidence That GnRH Decreases with Gonadal Steroid Feedback but Increases with Age in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2002, 87, 2290–2296. [Google Scholar] [CrossRef]
- Ottowitz, W.E.; Dougherty, D.D.; Fischman, A.J.; Hall, J.E. [18F]2-Fluoro-2-Deoxy-d-Glucose Positron Emission Tomography Demonstration of Estrogen Negative and Positive Feedback on Luteinizing Hormone Secretion in Women. J. Clin. Endocrinol. Metab. 2008, 93, 3208–3214. [Google Scholar] [CrossRef]
- Wise, P.M. Alterations in Proestrous LH, FSH, and Prolactin Surges in Middle-Aged Rats. Proc. Soc. Exp. Biol. Med. 1982, 169, 348–354. [Google Scholar] [CrossRef]
- Cruz, G.; Fernandois, D.; Paredes, A.H. Ovarian Function and Reproductive Senescence in the Rat: Role of Ovarian Sympathetic Innervation. Reproduction 2017, 153, R59–R68. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M. Estrogens And Age-Related Memory Decline in Rodents: What Have We Learned and Where do We Go From Here? Horm. Behav. 2009, 55, 2–23. [Google Scholar] [CrossRef]
- Lloyd, J.M.; Hoffman, G.E.; Wise, P.M. Decline in Immediate Early Gene Expression in Gonadotropin-Releasing Hormone Neurons during Proestrus in Regularly Cycling, Middle-Aged Rats. Endocrinology 1994, 134, 1800–1805. [Google Scholar] [CrossRef]
- Krajnak, K.; Rosewell, K.L.; Wise, P.M. Fos-Induction in Gonadotropin-Releasing Hormone Neurons Receiving Vasoactive Intestinal Polypeptide Innervation Is Reduced in Middle-Aged Female Rats. Biol. Reprod. 2001, 64, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Gerhold, L.M.; Rosewell, K.L.; Wise, P.M. Suppression of Vasoactive Intestinal Polypeptide in the Suprachiasmatic Nucleus Leads to Aging-Like Alterations in CAMP Rhythms and Activation of Gonadotropin-Releasing Hormone Neurons. J. Neurosci. 2005, 25, 62–67. [Google Scholar] [CrossRef]
- Anzalone, C.R.; Hong, L.-S.; Lu, J.K.H.; LaPolt, P.S. Influences of Age and Ovarian Follicular Reserve on Estrous Cycle Patterns, Ovulation, and Hormone Secretion in the Long-Evans Rat1. Biol. Reprod. 2001, 64, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B.; Cargill, S.L.; Anderson, G.B.; Carey, J.R. Transplantation of Young Ovaries to Old Mice Increased Life Span in Transplant Recipients. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1207–1211. [Google Scholar] [CrossRef]
- Klein, N.A.; Illingworth, P.J.; Groome, N.P.; McNeilly, A.S.; Battaglia, D.E.; Soules, M.R. Decreased Inhibin B Secretion Is Associated with the Monotropic FSH Rise in Older, Ovulatory Women: A Study of Serum and Follicular Fluid Levels of Dimeric Inhibin A and B in Spontaneous Menstrual Cycles. J. Clin. Endocrinol. Metab. 1996, 81, 2742–2745. [Google Scholar] [CrossRef]
- DePaolo, L.V. Age-Associated Increases in Serum Follicle-Stimulating Hormone Levels on Estrus Are Accompanied by a Reduction in the Ovarian Secretion of Inhibin. Exp. Aging Res. 1987, 13, 3–7. [Google Scholar] [CrossRef]
- Scarbrough, K.; Wise, P.M. Age-Related Changes in Pulsatile Luteinizing Hormone Release Precede the Transition to Estrous Acyclicity and Depend upon Estrous Cycle History. Endocrinology 1990, 126, 884–890. [Google Scholar] [CrossRef]
- Matt, D.W.; Gilson, M.P.; Sales, T.E.; Krieg, R.J.; Kerbeshian, M.C.; Veldhuis, J.D.; Evans, W.S. Characterization of Attenuated Proestrous Luteinizing Hormone Surges in Middle-Aged Rats by Deconvolution Analysis. Biol. Reprod. 1998, 59, 1477–1482. [Google Scholar] [CrossRef]
- Rahman, S.A.; Grant, L.K.; Gooley, J.J.; Rajaratnam, S.M.W.; Czeisler, C.A.; Lockley, S.W. Endogenous Circadian Regulation of Female Reproductive Hormones. J. Clin. Endocrinol. Metab. 2019, 104, 6049–6059. [Google Scholar] [CrossRef]
- Bremner, W.J.; Vitiello, M.V.; Prinz, P.N. Loss of Circadian Rhythmicity in Blood Testosterone Levels with Aging in Normal Men*. J. Clin. Endocrinol. Metab. 1983, 56, 1278–1281. [Google Scholar] [CrossRef]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; Beersma, D.G.; van den Hoofdakker, R.H. All Night Spectral Analysis of EEG Sleep in Young Adult and Middle-Aged Male Subjects. Neurobiol. Aging 1989, 10, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Zitting, K.-M.; Chinoy, E.D. Aging and Circadian Rhythms. Sleep Med. Clin. 2015, 10, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.A.; Roozendaal, B.; Swaab, D.F.; Goudsmit, E.; Mirmiran, M. Vasoactive Intestinal Polypeptide Neuron Changes in the Senile Rat Suprachiasmatic Nucleus. Neurobiol. Aging 1988, 9, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Effects of Aging on Central and Peripheral Mammalian Clocks. Proc. Natl. Acad. Sci. USA 2002, 99, 10801–10806. [Google Scholar] [CrossRef] [PubMed]
- Buijink, M.R.; Michel, S. A Multi-Level Assessment of the Bidirectional Relationship between Aging and the Circadian Clock. J. Neurochem. 2021, 157, 73–94. [Google Scholar] [CrossRef]
- Simonneaux, V.; Piet, R. Neuroendocrine Pathways Driving Daily Rhythms in the Hypothalamic Pituitary Gonadal Axis of Female Rodents. Curr. Opin. Physiol. 2018, 5, 99–108. [Google Scholar] [CrossRef]
- Buijs, R.M.; Soto-Tinoco, E.; Kalsbeek, A. Circadian Control of Neuroendocrine Systems. In Neuroanatomy of Neuroendocrine Systems; Grinevich, V., Dobolyi, Á., Eds.; Masterclass in Neuroendocrinology; Springer International Publishing: Cham, Switzerland, 2021; pp. 297–315. ISBN 978-3-030-86630-3. [Google Scholar]
- Williams III, W.P.; Kriegsfeld, L.J. Circadian Control of Neuroendocrine Circuits Regulating Female Reproductive Function. Front. Endocrin. 2012, 3, 60. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.P.; Jarjisian, S.G.; Mikkelsen, J.D.; Kriegsfeld, L.J. Circadian Control of Kisspeptin and a Gated GnRH Response Mediate the Preovulatory Luteinizing Hormone Surge. Endocrinology 2011, 152, 595–606. [Google Scholar] [CrossRef]
- Karatsoreos, I.N. Circadian Regulation of Brain and Behavior: A Neuroendocrine Perspective. Curr. Top. Behav. Neurosci. 2019, 43, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Yamazaki, S.; Arble, D.M.; Menaker, M.; Block, G.D. Resetting of Central and Peripheral Circadian Oscillators in Aged Rats. Neurobiol. Aging 2008, 29, 471–477. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Wright, K.M.; Rand, K.A.; Kermany, A.; Noto, K.; Curtis, D.; Varner, N.; Garrigan, D.; Slinkov, D.; Dorfman, I.; et al. Estimates of the Heritability of Human Longevity Are Substantially Inflated Due to Assortative Mating. Genetics 2018, 210, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- de Groot, L.C.P.M.G.; Verheijden, M.W.; de Henauw, S.; Schroll, M.; van Staveren, W.A. SENECA Investigators Lifestyle, Nutritional Status, Health, and Mortality in Elderly People across Europe: A Review of the Longitudinal Results of the SENECA Study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Simon, M.; Zhao, Y.; Ablaeva, J.; Corson, N.; Choi, Y.; Yamada, K.Y.H.; Schork, N.J.; Hood, W.R.; Hill, G.E.; et al. Comparative Transcriptomics Reveals Circadian and Pluripotency Networks as Two Pillars of Longevity Regulation. Cell Metab. 2022, 34, 836–856.e5. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Mechanisms Linking Circadian Clocks, Sleep, and Neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Kettner, N.M. The Circadian Clock in Cancer Development and Therapy. Prog. Mol. Biol. Transl. Sci. 2013, 119, 221–282. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian Rhythms and the Molecular Clock in Cardiovascular Biology and Disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Costello, H.M.; Gumz, M.L. Circadian Rhythm, Clock Genes, and Hypertension: Recent Advances in Hypertension. Hypertension 2021, 78, 1185–1196. [Google Scholar] [CrossRef]
- Panagiotou, M.; Michel, S.; Meijer, J.H.; Deboer, T. The Aging Brain: Sleep, the Circadian Clock and Exercise. Biochem. Pharmacol. 2021, 191, 114563. [Google Scholar] [CrossRef]
- Maiese, K. Cognitive Impairment and Dementia: Gaining Insight through Circadian Clock Gene Pathways. Biomolecules 2021, 11, 1002. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Dumont, M.; Duffy, J.F.; Steinberg, J.D.; Richardson, G.S.; Brown, E.N.; Sánchez, R.; Ríos, C.D.; Ronda, J.M. Association of Sleep-Wake Habits in Older People with Changes in Output of Circadian Pacemaker. Lancet 1992, 340, 933–936. [Google Scholar] [CrossRef]
- Touitou, Y.; FèVre, M.; Lagoguey, M.; Carayon, A.; Bogdan, A.; Reinberg, A.; Beck, H.; Cesselin, F.; Touitou, C. Age-and Mental Health-Related Circadian Rhythms of Plasma Levels of Melatonin, Prolactin, Luteinizing Hormone and Follicle-Stimulating Hormone in Man. J. Endocrinol. 1981, 91, 467–475. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Lushington, K.; Dawson, D.; Lack, L.; van den Heuvel, C.; Rogers, N. Urinary 6-Sulfatoxymelatonin Excretion and Aging: New Results and a Critical Review of the Literature. J. Pineal. Res. 1999, 27, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Leproult, R.; Kupfer, D.J. Effects of Gender and Age on the Levels and Circadian Rhythmicity of Plasma Cortisol. J. Clin. Endocrinol. Metab. 1996, 81, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Hurd, M.W.; Ralph, M.R. The Significance of Circadian Organization for Longevity in the Golden Hamster. J. Biol. Rhythm. 1998, 13, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Scarbrough, K.; Hinkle, D.A.; Wise, P.M. Fetal Grafts Containing Suprachiasmatic Nuclei Restore the Diurnal Rhythm of CRH and POMC MRNA in Aging Rats. Am. J. Physiol. 1997, 273, R1764–R1770. [Google Scholar] [CrossRef]
- Li, H.; Satinoff, E. Fetal Tissue Containing the Suprachiasmatic Nucleus Restores Multiple Circadian Rhythms in Old Rats. Am. J. Physiol. 1998, 275, R1735–R1744. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Zhou, J.-N.; Van Heerikhuize, J.; Jockers, R.; Swaab, D.F. Decreased MT1 Melatonin Receptor Expression in the Suprachiasmatic Nucleus in Aging and Alzheimer’s Disease. Neurobiol. Aging 2007, 28, 1239–1247. [Google Scholar] [CrossRef]
- Hofman, M.A.; Swaab, D.F. Alterations in Circadian Rhythmicity of the Vasopressin-Producing Neurons of the Human Suprachiasmatic Nucleus (SCN) with Aging. Brain Res. 1994, 651, 134–142. [Google Scholar] [CrossRef]
- Zhou, J.N.; Hofman, M.A.; Swaab, D.F. VIP Neurons in the Human SCN in Relation to Sex, Age, and Alzheimer’s Disease. Neurobiol. Aging 1995, 16, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B.; van Gool, W.A.; Swaab, D.F.; Hoogendijk, J.E.; Mirmiran, M. Changes in Vasopressin Cells of the Rat Suprachiasmatic Nucleus with Aging. Brain Res. 1987, 409, 259–264. [Google Scholar] [CrossRef]
- Palomba, M.; Nygård, M.; Florenzano, F.; Bertini, G.; Kristensson, K.; Bentivoglio, M. Decline of the Presynaptic Network, Including GABAergic Terminals, in the Aging Suprachiasmatic Nucleus of the Mouse. J. Biol. Rhythm. 2008, 23, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Satinoff, E.; Li, H.; Tcheng, T.K.; Liu, C.; McArthur, A.J.; Medanic, M.; Gillette, M.U. Do the Suprachiasmatic Nuclei Oscillate in Old Rats as They Do in Young Ones? Am. J. Physiol. 1993, 265, R1216–R1222. [Google Scholar] [CrossRef]
- Watanabe, A.; Shibata, S.; Watanabe, S. Circadian Rhythm of Spontaneous Neuronal Activity in the Suprachiasmatic Nucleus of Old Hamster in Vitro. Brain Res. 1995, 695, 237–239. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Nakamura, W.; Yamazaki, S.; Kudo, T.; Cutler, T.; Colwell, C.S.; Block, G.D. Age-Related Decline in Circadian Output. J. Neurosci. 2011, 31, 10201–10205. [Google Scholar] [CrossRef]
- Farajnia, S.; Michel, S.; Deboer, T.; vanderLeest, H.T.; Houben, T.; Rohling, J.H.T.; Ramkisoensing, A.; Yasenkov, R.; Meijer, J.H. Evidence for Neuronal Desynchrony in the Aged Suprachiasmatic Nucleus Clock. J. Neurosci. 2012, 32, 5891–5899. [Google Scholar] [CrossRef]
- Nakamura, T.J.; Takasu, N.N.; Nakamura, W. The Suprachiasmatic Nucleus: Age-Related Decline in Biological Rhythms. J. Physiol. Sci. 2016, 66, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Scheuermaier, K.; Laffan, A.M.; Duffy, J.F. Light Exposure Patterns in Healthy Older and Young Adults. J. Biol. Rhythm. 2010, 25, 113–122. [Google Scholar] [CrossRef]
- Kessel, L.; Lundeman, J.H.; Herbst, K.; Andersen, T.V.; Larsen, M. Age-Related Changes in the Transmission Properties of the Human Lens and Their Relevance to Circadian Entrainment. J. Cataract Refract. Surg. 2010, 36, 308–312. [Google Scholar] [CrossRef]
- Biello, S.M. Circadian Clock Resetting in the Mouse Changes with Age. Age 2009, 31, 293–303. [Google Scholar] [CrossRef]
- Jiang, Z.; Zou, K.; Liu, X.; Gu, H.; Meng, Y.; Lin, J.; Shi, W.; Yu, C.; Jin, L.; Wang, L.; et al. Aging Attenuates the Ovarian Circadian Rhythm. J. Assist. Reprod. Genet. 2021, 38, 33–40. [Google Scholar] [CrossRef]
- Lee, J.; Sul, H.J.; Choi, H.; Oh, D.H.; Shong, M. Loss of Thyroid Gland Circadian PER2 Rhythmicity in Aged Mice and Its Potential Association with Thyroid Cancer Development. Cell Death Dis. 2022, 13, 898. [Google Scholar] [CrossRef]
- Silva, B.S.d.A.; Uzeloto, J.S.; Lira, F.S.; Pereira, T.; Coelho-E-Silva, M.J.; Caseiro, A. Exercise as a Peripheral Circadian Clock Resynchronizer in Vascular and Skeletal Muscle Aging. Int. J. Environ. Res. Public Health 2021, 18, 12949. [Google Scholar] [CrossRef]
- Schmitt, E.E.; Johnson, E.C.; Yusifova, M.; Bruns, D.R. The Renal Molecular Clock: Broken by Aging and Restored by Exercise. Am. J. Physiol. Renal Physiol. 2019, 317, F1087–F1093. [Google Scholar] [CrossRef]
- Bruns, D.R.; Yusifova, M.; Marcello, N.A.; Green, C.J.; Walker, W.J.; Schmitt, E.E. The Peripheral Circadian Clock and Exercise: Lessons from Young and Old Mice. J. Circadian Rhythm. 2020, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.G.; Jung, H.J.; Kim, S.; Arulkumar, R.; Kim, D.H.; Park, D.; Chung, H.Y. Regulation of Circadian Genes Nr1d1 and Nr1d2 in Sex-Different Manners during Liver Aging. Int. J. Mol. Sci. 2022, 23, 10032. [Google Scholar] [CrossRef] [PubMed]
- Valentinuzzi, V.S.; Scarbrough, K.; Takahashi, J.S.; Turek, F.W. Effects of Aging on the Circadian Rhythm of Wheel-Running Activity in C57BL/6 Mice. Am. J. Physiol. 1997, 273, R1957–R1964. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.A.; Powell, A.; Allen, G.C.; Earnest, D.J. Development of an Age-Dependent Cognitive Index: Relationship between Impaired Learning and Disturbances in Circadian Timekeeping. Front. Aging Neurosci. 2022, 14, 991833. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X.; Belsky, D.W.; McCall, W.V.; Liu, Y.; Su, S. Blunted Rest-Activity Circadian Rhythm Is Associated with Increased Rate of Biological Aging: An Analysis of NHANES 2011-2014. J. Gerontol. A Biol. Sci. Med. Sci. 2022, glac199. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Ayalon, L. Diagnosis and Treatment of Sleep Disorders in Older Adults. Am. J. Geriatr. Psychiatry 2009, 7, 98–105. [Google Scholar] [CrossRef]
- Dijk, D.J.; Duffy, J.F.; Czeisler, C.A. Contribution of Circadian Physiology and Sleep Homeostasis to Age-Related Changes in Human Sleep. Chronobiol. Int. 2000, 17, 285–311. [Google Scholar] [CrossRef]
- Hayashi, Y.; Endo, S. All-Night Sleep Polygraphic Recordings of Healthy Aged Persons: REM and Slow-Wave Sleep. Sleep 1982, 5, 277–283. [Google Scholar] [CrossRef]
- Lo, J.C.; Groeger, J.A.; Cheng, G.H.; Dijk, D.-J.; Chee, M.W.L. Self-Reported Sleep Duration and Cognitive Performance in Older Adults: A Systematic Review and Meta-Analysis. Sleep Med. 2016, 17, 87–98. [Google Scholar] [CrossRef]
- Sloane, P.D.; Williams, C.S.; Mitchell, C.M.; Preisser, J.S.; Wood, W.; Barrick, A.L.; Hickman, S.E.; Gill, K.S.; Connell, B.R.; Edinger, J.; et al. High-Intensity Environmental Light in Dementia: Effect on Sleep and Activity. J. Am. Geriatr. Soc. 2007, 55, 1524–1533. [Google Scholar] [CrossRef]
- Liu, C.-R.; Liou, Y.M.; Jou, J.-H. Ambient Bright Lighting in the Morning Improves Sleep Disturbances of Older Adults with Dementia. Sleep Med. 2022, 89, 1–9. [Google Scholar] [CrossRef]
- Hjetland, G.J.; Kolberg, E.; Pallesen, S.; Thun, E.; Nordhus, I.H.; Bjorvatn, B.; Flo-Groeneboom, E. Ambient Bright Light Treatment Improved Proxy-Rated Sleep but Not Sleep Measured by Actigraphy in Nursing Home Patients with Dementia: A Placebo-Controlled Randomised Trial. BMC Geriatr. 2021, 21, 312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejniczak, I.; Pilorz, V.; Oster, H. Circle(s) of Life: The Circadian Clock from Birth to Death. Biology 2023, 12, 383. https://doi.org/10.3390/biology12030383
Olejniczak I, Pilorz V, Oster H. Circle(s) of Life: The Circadian Clock from Birth to Death. Biology. 2023; 12(3):383. https://doi.org/10.3390/biology12030383
Chicago/Turabian StyleOlejniczak, Iwona, Violetta Pilorz, and Henrik Oster. 2023. "Circle(s) of Life: The Circadian Clock from Birth to Death" Biology 12, no. 3: 383. https://doi.org/10.3390/biology12030383
APA StyleOlejniczak, I., Pilorz, V., & Oster, H. (2023). Circle(s) of Life: The Circadian Clock from Birth to Death. Biology, 12(3), 383. https://doi.org/10.3390/biology12030383




