Simple Summary
We found higher levels of six biomarkers significantly involved in cardiovascular pathology, i.e., irisin, periostin, osteoglycin, interleukin 18, high mobility group box 1 and proprotein convertase subtilisin/kexin type 9 in the serum at the protein level, and in the tissue at both the protein and mRNA levels of patients with AS (N = 60). Higher levels of all factors were found in DAS patients’ serum than in normal C (Ν = 22). All biomarkers were seen in the aortic valve cusps with DAS, but no trace of PCR mRNA was found in the five transplantation valves.
Abstract
Background and Aim. Degenerative Aortic Stenosis (DAS) is a common disease that causes substantial morbidity and mortality worldwide, especially in the older population. Our aim was to further investigate novel serum and tissue biomarkers to elucidate biological processes involved in this entity. Material and Methods. We evaluated the expression of six biomarkers significantly involved in cardiovascular pathology, i.e., irisin, periostin, osteoglycin, interleukin 18, high mobility group box 1 and proprotein convertase subtilisin/kexin type 9 in the serum at the protein level, and in the tissue at both the protein and mRNA levels of patients with AS (N = 60). Five normal valves obtained after transplantation from hearts of patients with idiopathic dilated cardiomyopathy were also studied. Serum measurements were also performed in 22 individuals without valvular disease who served as controls (C). Results. Higher levels of all factors were found in DAS patients’ serum than in normal C. IHC and PCR mRNA tissue analysis showed the presence of all biomarkers in the aortic valve cusps with DAS, but no trace of PCR mRNA was found in the five transplantation valves. Moreover, periostin serum levels correlated significantly with IHC and mRNA tissue levels in AS patients. Conclusion. We showed that six widely prevalent biomarkers affecting the atherosclerotic process were also involved in DAS, suggesting a strong osteogenic and pro-inflammatory profile, indicating that aortic valve calcification is a multifactorial biological process.
1. Introduction
The interest in degenerative aortic stenosis (DAS) remains unabated, since it is an increasingly frequent cause of cardiac morbidity and mortality [1,2]. In a U.S. population study, its prevalence was 2.8% for peoples aged > 75 years [3]. It is correlated to atherosclerotic vascular disease, with many factors being prevalent in both conditions [1,2]. However, Ortlepp et al. [4] point out, that the predictive power of risk factors is not equal for the two entities. It is currently being accepted that DAS is not a passive process, but involves many mechanisms, such as lipoprotein profile derangement, oxidation, inflammation and valvular cell apoptosis [5,6,7]. All these are compounded by hemodynamic factors, since the initial valve sclerosis causes flow turbulence and nonlinear flow promoting further progress of the sclerosis/calcification process [1,6].
Livia Passos et al. [8] argue that cardiovascular calcification is an inflammatory disease, through crosstalk between innate and adaptive immune cell components.
A great number of biomarkers has been studied in DAS.
The purpose of our study is to identify additional serum and tissue biomarkers involved in patients with DAS, and also to investigate the correlation between their tissue and serum levels. Since 2015 we have been investigating tissue and serum biomarkers in this entity. We have shown that patients with DAS express more pro-inflammatory, calcification, fibrosis, proliferation and apoptosis biomarkers. We have also shown that Toll-like receptors and interleukin-37 are differentially expressed in aortic compared to mitral valves, indicating a higher pro-calcific and pro-inflammatory process in the aortic valve [7], in addition to the hemodynamic factors and the turbulent aortic flow.
Trying to obtain further insight in the calcification process we measured six biomarkers in the tissue of stenotic aortic valves excised at surgery for aortic valve replacement and compared them to normal aortic valves obtained at cardiac transplantation.
These six biomarkers were also measured in the serum of patients and controls without any cardiovascular disease; the reasons for their inclusion are explained below.
1.1. Irisin Levels
The myokine irisin, which is cleaved from the plasma membrane FNDC5, is more highly expressed in cardiac muscle than in skeletal [9]. It is also highly elevated in patients with heart failure and preserved ejection fraction (EF) more than in those with reduced EF; in the former, it correlated with total antioxidant capacity [10]. Irisin is increased in acute heart failure and is an independent factor for 1 year all-cause mortality [11]. However, it is decreased in myocardial infarction, both clinical and experimental [12,13]. It is correlated negatively to coronary artery disease severity [14]. Patients in the higher SYNTAX score levels were older and had lower irisin levels than younger ones. However, it has also been reported to be cardioprotective [15].
Irisin has been found to promote osteoblast proliferation and differentiation via the MAP kinase pathways by Qiao et al. [16] who postulated its possible use in osteopenia. It increases sclerostin expression in osteocytes to induce bone resorption. It mediates this effect via αV integrin receptors [17]. In our previous study we have found sclerostin to be increased in DAS [5]. Irisin administration has been proposed for treatment of osteoporosis by Colaioanni et al. [18].
1.2. Periostin
Hakuno et al. [19] found PN to be increased in degenerative or rheumatically affected heart valves. The same authors also found that in wild type mice a high fat diet markedly increased its expression in both AV and MV together with the fibrotic markers MMP2 and MMP13. It is associated with myocardial fibrosis in human heart failure [20]. It was increased together with MMP2 activity. It is increased in hypertrophic mice hearts together with interstitial fibrosis [21]. It decreases together with a reduction in myocardial fibrosis in hearts unloaded both clinically (LVAD) and experimentally in mice (aortic arch de-banding) [22]. It is also a potential marker biomarker for coronary artery disease with acute heart failure [23]. Furthermore patients with CAD had higher levels than controls at similar ages (around 63 y).
1.3. Osteoglycin
The same pattern for PN was found for OGN, which is implicated in matrix homeostasis. It modulates fibrosis and inflammation. Deckx et al. [24] found that its levels were higher in patients with AS with less severe myocardial fibrosis, in whom it was negatively correlated with collagen content in the myocardium, but they did not measure it in the valves. Van Aelst et al. [25] found that it prevents cardiac dilation and dysfunction after myocardial infarction through infarct collagen strengthening. Zuo et al. [26] have found that osteoglycin attenuates cardiac fibrosis; it could be an antifibrotic, but is also pro-calcific by suppressing myofibroblast proliferation. Circulating osteoglycin and NGAL/MMP9 complex concentrations predict 1 y MACE after coronary arteriography [27]. It was statistically slightly higher in CAD patients aged 70 vs. 65 y [28] Tanaka et al. have stated that it is a humoral bone anabolic factor [28]
1.4. Interleukin 18
IL-18 is a dominant pro-inflammatory cytokine. In the heart, it is produced by infiltrating neutrophils, resident macrophages, endothelial cells, smooth muscle cells and cardiomyocytes [29]. In the non-rheumatic aortic valve, increased tissue levels have been found as compared to controls [30], and are correlated to advanced clinical severity. It promotes myofibroblast activation of porcine valvular interstitial cells [31]. Interestingly, the administration of increased doses of IL-18 upregulated the expression of osteopontin [32] which we have found to be pro-osteogenic [5]. It is prospectively and independently associated with CVD risk [33] in patients of similar ages (52.5 y).
1.5. HMGB1
The high mobility group box 1 (HMGB1) which is also a pro-inflammatory factor has been implicated in the pathogenesis of DAS. It has been found to be increased in the calcific regions; the same was found in regard to TLR by Shen et al. [34], who postulate that TLR4 may function as an essential mediator of HMGB1-induced calcification and in the activation of p38 and NFkB. Wang et al. [35] found that HMGB1 protein and TLR4 are upregulated in vitro by HMGB1 in aortic valvular interstitial cells. We have described in detail the role of TLRs in aortic and mitral valve stenosis [7]. Its increase in the serum is related to severity of coronary artery stenosis [36].
1.6. PCSK9
Recent experimental studies have demonstrated that PCSK9 might directly promote inflammation, apoptotic cell death and endothelial dysfunction in the atherosclerotic process and plaque formation [37] and is associated with the severity of CAD [38] in hypercholesterolemia, cardiovascular inflammation and diabetes [39,40,41].
Wang et al. [42] and Poggio et al. [43] found lower calcium content in aortic valves of PCSK9−/− mice than wild type animals. The former were resistant to production of aortic calcification with two pro-calcification diets (β-glycero–phosphate and ascorbic acid). The same authors found that human calcified aortic valves expressed higher PCSK9 than non-calcified ones. Interestingly, Salaun et al. [44] found that elevated plasma levels of PCSK9 were a risk factor for hemodynamic deterioration of surgically implanted bioprosthetic aortic valves. The same held true for LP-PLA2 and HoMA. PCSK9, apart from its action on LDL receptors, can promote apoptotic cell death of neurons [45].
2. Material and Methods
2.1. Ethics Statement
This study was approved by the Ethics Committees of the Onassis Cardiac Surgery Center (OCSC) and conformed to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients.
2.2. Study Population
The present, prospective, open-label study, extends the results of our previously published studies [5,7]. The age of the 60 DAS patients was 66.1 ± 12.5 years, 50% were women. Echocardiography was obtained in all patients. Aortic valve area was 0.9 to 0.5 cm [2]. Patients were taking antihypertensive (75%) and antilipidemic (80%) drugs during the last two years. However, none had significant coronary artery disease necessitating concomitant aortocoronary bypass surgery. Patients with rheumatic cardiac disease, bicuspid aortic valve or connective tissue disorders were excluded. The excised valve cusps were harvested during surgery. Blood was collected from AS patients before valve surgery and from twenty-two (22) healthy subjects without any chronic cardiovascular or metabolic disease, and not receiving any long-term medication, who served as healthy control group (C), for comparison of serum biomarkers; mean age was 34.4 ± 7.5 years, 50% were women. None were steadily employing radioactive drugs.
We also obtained 5 aortic valves from patients undergoing cardiac transplantation, 3 men and 2 women (min age 48.4), because of terminal heart failure due to idiopathic dilated cardiomyopathy, who did not show any sclerotic or calcific aortic valve changes.
2.3. Blood Analysis
Blood samples were collected by venipuncture after subjects were fasted overnight. Serum was collected and stored at −80 °C, analysis was performed in duplicate; dilution was assessed as per protocol. We used commercially available enzyme immunoassay kits for irisin (FNDC5) (EK-067-29, Phoenix Pharmaceuticals, California, CA, USA); periostin (PN) (EHPOSTN, Thermoscientific, Germany); osteoglycin (OGN) (CSB EL016314HU, Cusabio, Houston, TX, USA); IL-18 (DY318-05, R&D, Minneapolis, MN, USA); high-mobility group box 1, HMGB1 (LS-F11641, Lifespan Ltd., Seattle, DC, USA); PCSK9 (DPC900, R&D, Minneapolis, MN, USA) and quantified each protein according to the protocol of the manufacturer with an ELISA reader system (Spectramax 190; Molecular Devices, Sunnyvale, Calif, CA, USA).
2.4. Valve Cusp Immunohistochemistry and Quantitative Morphometrical Analysis
Aortic valve cusps were excised and one part of each valve tissue was placed in a container for immunohistochemistry (IHC) analysis at the pathology department of the OCSC and the Biomedical Research Foundation of Academy of Athens according to our previous protocol. The protocol of IHC has been described and validated in our lab [46]; FNDC-5 (PA5-62368, 5 μg/mL, Invitrogen, CA, USA); PN (PA5-82458, 5 μg/mL, Invitrogen, CA, USA); OGN (PA5-48255, 5 μg/mL, Invitrogen, CA, USA); IL-18 (PA5-79479, Invitrogen, CA, USA) HMGB1 (PA5-80691, 5 μg/mL, Invitrogen, CA, USA); PCSK9 (PA5-79789, 5 μg/mL, Invitrogen, CA, USA) were used for IHC. IHC was performed according to the manufacturer’s protocol by using the development kit (Zytochem Plus; Zytomed system, Germany). Appropriate isotype negative controls were performed at the same concentrations as the primary antibodies. Microscopic investigation of the IHC sections was performed with stereology upright Leica DMRA2 camera, and were analyzed by stereo-investigator 10 program (version 10.1, MBF Bioscience, Microbrightfield. Inc., Willinston, VT, USA) in order to quantify the extent of the tissue covered by each antibody.
2.5. RNA Isolation and qRT PCR Analysis
Total RNA was extracted using Trizol reagent (Sigma, Saint Louis, MO, USA) according to the manufacturer’s instructions [46]. The RNA quality was assessed with agarose gel electrophoresis and quantitated spectrophotometrically. cDNA was synthesized by RT (MMLV, reverse transcriptase; Sigma), and real-time quantitative polymerase chain reaction was performed by using SYBR Green (Invitrogen, Life Technologies, New York, NY, USA). The primers synthesized by Origine (Herford, Germany) were used as documented in Table 1. The thermal cycling protocol was performed according to our lab protocol [5,7].

Table 1.
Primers sequences. Abbreviations: Irisin (FNDC5); periostin (PN); osteoglycin (OGN); interleukin 18 (IL-18); high-mobility group box 1 (HMGB1); proprotein convertase subtilisin kexin 9 (PCSK9).
We also measured the 6 biomarkers already discussed in the 5 aortic valves explanted from patients who had undergone cardiac transplantation by RNA isolation and qRT analysis.
All had idiopathic dilated cardiomyopathy without coronary artery disease at arteriography: 2 were women aged 28 and 56 years, and were 3 men aged 48, 54 and 58 years.
2.6. Statistical Analysis
Shapiro–Wilks test for normality showed that none of the variables had normal distribution. Thus, Univariate and Multivariate analysis, one-way ANOVA or t-test were inappropriate tests for the analysis. We performed non-parametric tests instead. The Mann–Whitney test for evaluating the patients versus control serum biomarkers showed significant differences between all serum markers. All correlations were performed with non-parametric Spearman’s rho. Alpha was set at 0.05. Statistics were performed with SPSS28.
3. Results
3.1. Serum Findings
(a) The non-parametric Mann–Whitney test showed significant differences in all serum biomarkers between patients and the control group. (Figure 1, Table 2).
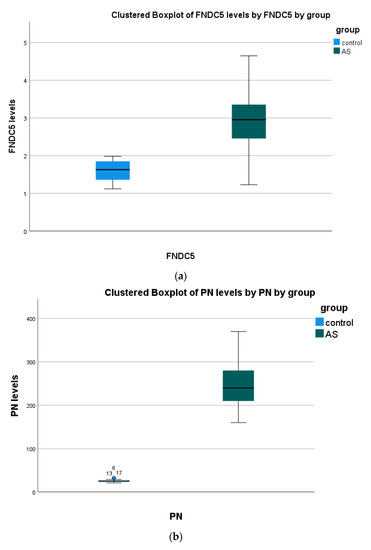
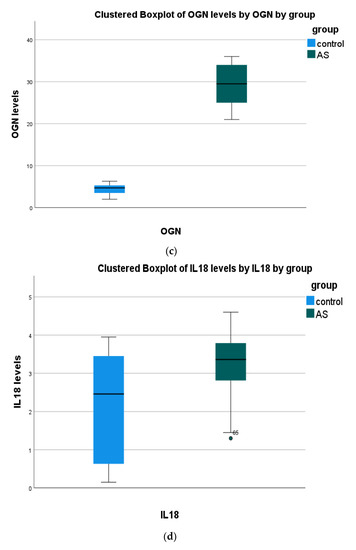
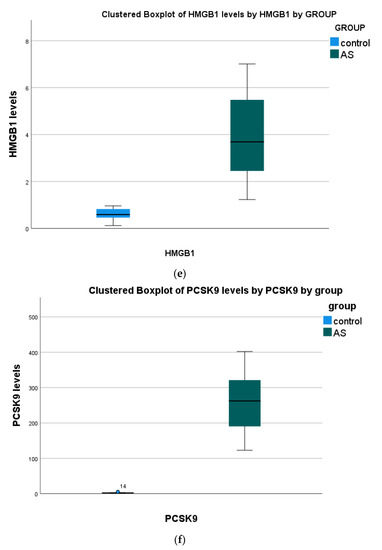
Figure 1.
Serum biomarkers’ concentration in all study groups. Data presented as boxplots: Blue color, Control; Green color, (AS) aortic stenosis patients. Abbreviations: (a) irisin (FNDC5) (b) periostin (PN); (c) osteoglycin (OGN); (d) interleukin 18 (IL-18); (e) high-mobility group box 1 (HMGB1); (f) proprotein convertase subtilisin kexin 9 (PCSK9).

Table 2.
Statistical analysis of serum biomarkers in all study groups. Mann–Whitney test for all markers measured in the serum of both groups (control and AS); alpha was set at 0.05 C and AS. Abbreviations: C (control); AS (aortic stenosis patients); Irisin (FNDC5); periostin (PN); osteoglycin (OGN); interleukin 18 (IL-18); high-mobility group box 1 (HMGB1); proprotein convertase subtilisin kexin 9 (PCSK9).
(b) Positive correlations were found in HMGB1 with PCSK9 and PN vs. PSCK9. Negative correlations were observed in OGN with PN and IL-18.
Any other correlation was found insignificant (p > 0.05).
(c) Positive correlations were found in HMGB1 with PCSK9 and PN with PSCK9. Negative correlations were observed in OGN with PN and IL-18.
(d) Any other correlation was found insignificant (p > 0.05).
3.2. Tissue vs. Serum
(a) Of all biomarkers found significantly correlated only PN tissue levels correlate with the same marker’s levels detected in serum.
(b) For all other tissue markers described in Table 3, the correlations were found in non-identical markers: (positive) tissue HMGB1 with serum OGN, tissue PCSK9 with serum OGN, (negative) tissue IL-18 with serum PCSK9 and OGN, tissue HMGB1 with serum IL-18, tissue PCSK9 with serum HMGB1 and IL-18. (Table 3)

Table 3.
The Spearman’s rho coefficients, p-value and 95% Confidence Intervals of every significant correlation between and within the tissues under investigation.
(c) The insignificant correlations were not included in Table 3.
3.3. Immunohistochemistry Biomarkers in Aortic Valve Cusps
Immunohistochemistry staining was performed (Figure 2). Biomarker tissue presence was confirmed in all AVC samples.
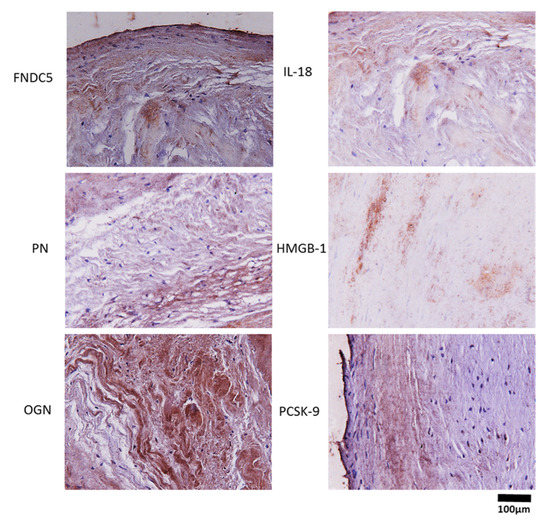
Figure 2.
Immunohistochemistry staining in the AS group. Representative photos of tissue biomarkers from aortic valve cusps. Nuclei stained with celestine blue are shown in blue, expression of biomarkers shown in brown. 40× magnification. Abbreviations: irisin (FNDC5); periostin (PN); osteoglycin (OGN); interleukin 18 (IL-18); high-mobility group box 1 (HMGB1); proprotein convertase subtilisin kexin 9 (PCSK9).
3.4. mRNA Expression of Inflammation and Calcification Biomarkers in AS Patients
Tissue mRNA levels of all biomarkers was present in AVC (Figure 3).
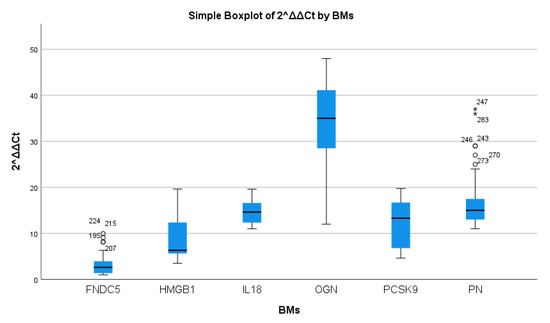
Figure 3.
mRNA expression of tissue biomarker concentrations in AS patients. Data presented as boxplots; Abbreviations: irisin (FNDC5); periostin (PN); osteoglycin (OGN); interleukin 18 (IL-18); high-mobility group box 1 (HMGB1); proprotein convertase subtilisin kexin 9 (PCSK9). Non-normality confirmed by outliers imposed non-parametric evaluation opted herein.
The highest expression was found for osteoglycin, while FNDC5 was only mildly elevated
None of the valves from patients with cardiac transplantation had any expression of mRNA of the above biomarkers.
The five valves from the cardiac transplantation hearts showed no expression at all.
3.5. Tissue Biomarkers Correlations
We found significant correlations between most tissue biomarkers.
4. Discussion
In this study we continue the investigation of the calcification process through the novel body of biomarkers examined in DAS [5,7]. We investigated six factors, both in serum and valve tissue, of which some have been very scantily studied. All of them have also been involved in CAD. Thus, we did not consider it practical to add a comparison Group with CAD only and without DAS.
We obtained valves from five patients undergoing cardiac transplantation, which did not show any expression of RNA of these biomarkers. With regard to the serum biomarkers, it must be realized that the control individuals were younger. However, this difference reflects a real-life situation, that individuals develop DAS later in life. It must be realized that DAS and CAD co-exist in around 50% of patients, as presented by Ortlep et al. [4]. However, none of our patients needed any concomitant surgery for CAD, thus excluding significant disease.
Our study may offer another biomarker, periostin, which may in the future provide prognostic information. Up to now, NT-proBNP [47] and BNP [48] have provided data with regard to intervention time because they reflect myocardial stress which would prompt information. A valve-specific marker such as periostin may provide means of following the course of sclerosis to stress.
Again, we must stress that we did not perform a population study so as to assess the importance of these biomarkers for predicting the presence of DAS.
Νew technologies to measure early calcification and inflammation are available. Dweck et al. have provided data from position emission tomography in vivo [49] in patents with DAS.
It must be noted that we did not find any microscopic evidence of changes in the normal aortic valves obtained at cardiac transplantation, nor by mRNA PCR, which is more reliable than immunohistochemistry [50].
Our findings confirm the association of PCSK9 with valve tissue calcification, and supports the postulations that PCSK9 inhibitors or drugs preventing its production by the liver could be a consideration for prevention of the course towards AS once aortic valve sclerosis has been diagnosed. In fact, in the FOURIER study, PCSK9 inhibition was associated with a lower hazard of new or worsening stenosis AS [51]. This is especially relevant since initial trials with statins have not been successful in preventing AS [52,53]. While there was some promise in a rosuvastatin trial with echo measurements [54], this was not validated in the ASTRONOMER TRIAL [55]. A reason might be that statins do lower cholesterol but increase PCSK9 levels [56]. Thus, well-controlled therapeutic trials with PCSK9 inhibitors are warranted.
In the coronary arteries statins actually promote calcification, while decreasing atherosclerotic plaque burden and fibrosis [57]. This action may explain their lack of influence on the progression of aortic calcification. We found that the PCSK9 receptor is strongly expressed in the aortic valve both by IHC and mRNA analysis and increased in the serum as compared to controls. This supports the population study of Perrot et al. [58] who found that AS was less frequent in carriers of the PCSK9 R461 loss-of-function variants. They also found increased expression of PCSK9 in calcified human valves.
The high interrelation between PCSK9 and aortic valve calcification has already been stressed [59]. Additionally, this family of drugs antagonizes apoptosis; [45,56] endothelial apoptosis [60] is found in endothelial cells in DAS.
PCSK9 inhibitors alleviate oxidation and inflammation [61].
Another candidate drug family would be SGLT2 inhibitors. Interestingly, they have been found to attenuate the secretion of IL1β and IL-18 [59] by repressing the HMGB1-TLR4 receptor axis [62]. They also antagonize many inflammatory interleukins [63,64,65]. They are also considered powerful antioxidants [66]. Possibly, if these families of drugs for PCSK9 and SGLT2 are given together they could exert a synergistic effect.
Another practical aspect of our finding is if serum levels could predict or follow the course of aortic valve sclerosis towards actual stenosis, by a set of easily measured biomarkers. This held true for periostin in our patients.
We tried to address the mechanisms of DAS. The fact that none of these biomarkers are found in the tissue of normal aortic valves in control patients undergoing cardiac transplantation of a similar age to those with AVR suggests that this is not a problem regulated only by age. As it regards serum levels, all our control patients had much lower levels than those with DAS, which is not surprising. Furthermore, biomarkers in DAS are legion and concern all aspects of inflammation, oxidative stress, pro-calcification effects and lipid metabolism.
It should also be stressed that they all have been associated with CAD as well, since approximately half of the patients with DAS have CVD as well. As already stated, coronary artery disease is strongly associated with DAS in many studies considering the age of our patients; its prevalence could be estimated between 41% and 51% [4,67].
Indeed, non-obstructive aortic valve calcification has become a window into significant coronary artery disease [68].
However, since as already stated, many biomarkers are being studied, we did not address any other correlations in the tissue which would create confusion, since it would be difficult to find practical explanations and promote far-fetched postulation.
We believe that our findings add impetus to the efforts towards preventing the progression of aortic sclerosis to frank DAS by drugs affecting causative factors, such as hyperlipidemia, inflammation, oxidation and endothelial apoptosis. This is a realistic goal in parallel to CAD, where, although interventional and surgical therapies have attained excellent results, efforts at prevention continue unabated.
5. Study Limitations
The lower serum levels of most markers in our controls may be because they are of younger age and there is an absence of comorbidities. However, we do not consider that we have a new diagnostic and pragmatic biomarker, but we have investigated the pathobiology of this entity.
6. Conclusions
Patients with stenotic aortic valves express higher pro-inflammatory, calcification, fibrosis, proliferation and apoptosis-expressing markers in their serum than normal controls. They are all strongly expressed in calcified, but not normal, valves. We found that PN concentration is an important finding that can lead us to a consideration of the prognostic role of serum biomarkers in the course of this pathological process. Our findings point toward a higher pro-calcification and pro-inflammatory profile in DAS patients. We believe that our findings provide interesting data for the diagnosis and prevention of aortic sclerosis, and possibly treatment of mild AS.
Author Contributions
Data curation, and management activities to annotate (produce metadata), scrub and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later re-use: A.K., S.G., I.L. and C.K.; Formal analysis and application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data: A.K. and S.G.; Funding acquisition, acquisition of the financial support for the project leading to this publication: D.V.C.; Investigation, conducting the research and investigation process, specifically performing the experiments, or data/evidence collection: A.K., L.K., L.T. and D.V.C.; Methodology, development or design of methodology, creation of models: A.K. and D.V.C.; Project administration, management and coordination responsibility for the research activity planning and execution: A.K., L.T. and D.V.C.; Resources, provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools: A.K., S.G., I.L. and L.T.; Software, programming, software development, designing computer programs, implementation of the computer code and supporting algorithms, testing of existing code components: A.K., S.G., I.L. and C.K.; Supervision, oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team: A.K. and D.V.C.; Validation, verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs: A.K., S.G., I.L., C.K., L.K. and D.V.C.; Visualization, preparation, creation and/or presentation of the published work, specifically visualization/data presentation: A.K. and D.V.C.; Writing—original draft, preparation, creation and/or presentation of the published work, specifically writing the initial draft (including substantive translation): A.K., S.G., I.L., C.K. and D.V.C.; Writing—review and editing, preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision—including pre- or post-publication stages: A.K. and D.V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific additional grant from funding agencies in the public, commercial or not-for-profit sectors.
Institutional Review Board Statement
This was approved by the University of Athens on 12 February 2014, Protocol number 2257.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data is not available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
AS, aortic stenosis; AVC, aortic valve cusp; CAD, coronary artery disease; CVD, cardiovascular disease; FNDC5, irisin; PN, periostin; OGN, osteoglycin; IL-18, interleukin 18; HMGB-1, high mobility group box 1; IHC, immunochemistry; PCSK9, proprotein convertase subtilisin/kexin type 9; qRT-PCR, quantitative reverse transcription polymerase chain reaction.
References
- Otto, C.M.; Prendergast, B. Aortic-Valve Stenosis—From Patients at Risk to Severe Valve Obstruction. N. Engl. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Benamer, H.; Auffret, V.; Cayla, G.; Chevalier, B.; Commeau, P.; Dupouy, P.; Eltchaninoff, H.; Gilard, M.; Guerin, P.; Lung, B.; et al. Position papier français (GACI) pour l’implantation de valve aortique percutanée (TAVI). Arch. Mal. Coeur Vaiss. Prat. 2018, 2018, 32–40. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Ortlepp, J.R.; Schmitz, F.; Bozoglu, T.; Hanrath, P.; Hoffmann, R. Cardiovascular risk factors in patients with aortic stenosis predict prevalence of coronary artery disease but not of aortic stenosis: An angiographic pair matched case-control study. Heart 2003, 89, 1019–1022. [Google Scholar] [CrossRef]
- Kapelouzou, A.; Tsourelis, L.; Kaklamanis, L.; Degiannis, D.; Kogerakis, N.; Cokkinos, D.V. Serum and tissue biomarkers in aortic stenosis. Glob. Cardiol. Sci. Pract. 2015, 2015, 49. [Google Scholar] [CrossRef]
- Helske, S.; Kupari, M.; Lindstedt, K.A.; Kovanen, P.T. Aortic valve stenosis: An active atheroinflammatory process. Curr. Opin. Infect. Dis. 2007, 18, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Kapelouzou, A.; Kontogiannis, C.; Tsilimigras, D.I.; Georgiopoulos, G.; Kaklamanis, L.; Tsourelis, L.; Cokkinos, D.V. Differential expression patterns of Toll Like Receptors and Interleukin-37 between calcific aortic and mitral valve cusps in humans. Cytokine 2019, 116, 150–160. [Google Scholar] [CrossRef]
- Passos, L.S.; Lupieri, A.; Becker-Greene, D.; Aikawa, E. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis 2020, 306, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, I.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef]
- Silvestrini, A.; Bruno, C.; Vergani, E.; Venuti, A.; Favuzzi, A.M.R.; Guidi, F.; Nicolotti, N.; Meucci, E.; Mordente, A.; Mancini, A. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: A pilot study. PLoS ONE 2019, 14, e0210320. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Gao, R.; Bei, Y.; Li, J.; Zhang, H.; Zhou, Y.; Yao, W.; Xu, D.; Zhou, F.; Jin, M.; et al. Serum Irisin Predicts Mortality Risk in Acute Heart Failure Patients. Cell. Physiol. Biochem. 2017, 42, 615–622. [Google Scholar] [CrossRef]
- Emanuele, E.; Minoretti, P.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Garatachea, N.; Lucia, A. Serum Irisin Levels, Precocious Myocardial Infarction, and Healthy Exceptional Longevity. Am. J. Med. 2014, 127, 888–890. [Google Scholar] [CrossRef]
- Kuloglu, T.; Aydin, S.; Eren, M.N.; Yilmaz, M.; Sahin, I.; Kalayci, M.; Sarman, E.; Kaya, N.; Yilmaz, O.F.; Turk, A.; et al. Irisin: A potentially candidate marker for myocardial infarction. Peptides 2014, 55, 85–91. [Google Scholar] [CrossRef]
- Efe, T.H.; Açar, B.; Ertem, A.G.; Yayla, K.G.; Algül, E.; Yayla, K.G.; Ünal, S.; Bilgin, M.; Çimen, T.; Kirbaş, Ö.; et al. Serum Irisin Level Can Predict the Severity of Coronary Artery Disease in Patients with Stable Angina. Korean Circ. J. 2017, 47, 44–49. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, H.; Zhang, S.; Du, J.; Zhuang, S.; Zhao, T.C. Irisin Ameliorates Hypoxia/Reoxygenation-Induced Injury through Modulation of Histone Deacetylase 4. PLoS ONE 2016, 11, e0166182. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.J.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via αV Integrin receptors. Cell 2018, 175, 1756–1768. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. N. Y. Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, D.; Kimura, N.; Yoshioka, M.; Mukai, M.; Kimura, T.; Okada, Y.; Yozu, R.; Shukunami, C.; Hiraki, Y.; Kudo, A.; et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2010, 120, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, H.; Xia, W.; Chen, X.; Zhu, S.; Zhang, S.; Shao, Y.; Ma, W.; Yang, D.; Zhang, J. Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. J. Cardiol. 2014, 63, 373–378. [Google Scholar] [CrossRef]
- Oka, T.; Xu, J.; Kaiser, R.A.; Melendez, J.; Hambleton, M.; Sargent, M.A.; Lorts, A.; Brunskill, E.W.; Dorn, G.W.; Conway, S.J.; et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ. Res. 2007, 101, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Stansfield, W.E.; Andersen, N.M.; Tang, R.-H.; Selzman, C.H. Periostin Is a Novel Factor in Cardiac Remodeling After Experimental and Clinical Unloading of the Failing Heart. Ann. Thorac. Surg. 2009, 88, 1916–1921. [Google Scholar] [CrossRef]
- Qiu, X.; Ma, F.; Zhang, H. Circulating Levels of IL-13, TGF-β1, and Periostin as Potential Biomarker for Coronary Artery Disease with Acute Heart Failure. Evid. Based Complement. Altern. Med. 2021, 2021, 1690421. [Google Scholar] [CrossRef] [PubMed]
- Deckx, S.; Heggermont, W.; Carai, P.; Rienks, M.; Dresselaers, T.; Himmelreich, U.; van Leeuwen, R.; Lommen, W.; van der Velden, J.; Gonzalez, A.; et al. Osteoglycin prevents the development of age-related diastolic dysfunction during pressure overload by reducing cardiac fibrosis and inflammation. Matrix Biol. 2018, 66, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.N.; Voss, S.; Carai, P.; Van Leeuwen, R.; Vanhoutte, D.; Sanders-van Wijk, S.; Eurlings, L.; Swinnen, M.; Verheyen, F.K.; Verbeken, E.; et al. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ. Res. 2015, 116, 425–436. [Google Scholar] [CrossRef]
- Zuo, C.; Li, X.; Huang, J.; Chen, D.; Ji, K.; Yang, Y.; Xu, T.; Zhu, D.; Yan, C.; Gao, P. Osteoglycin attenuates cardiac fibrosis by suppressing cardiac myofibroblast proliferation and migration through antagonizing lysophosphatidic acid 3/matrix metalloproteinase 2/epidermal growth factor receptor signalling. Cardiovasc. Res. 2018, 114, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.M.; Akkerhuis, K.M.; Meilhac, O.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; van Geuns, R.-J.; Piquer, D.; Merle, D.; du Paty, E.; Galéa, P.; et al. Circulating Osteoglycin and NGAL/MMP9 Complex Concentrations Predict 1-Year Major Adverse Cardiovascular Events after Coronary Angiography. Arter. Thromb. Vasc. Biol. 2014, 34, 1078–1084. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Matsumoto, E.; Higashimaki, Y.; Katagiri, T.; Sugimoto, T.; Seino, S.; Kaji, H. Role of Osteoglycin in the Linkage between Muscle and Bone. J. Biol. Chem. 2012, 287, 11616–11628. [Google Scholar] [CrossRef]
- Wang, M.; Markel, T.A.; Meldrum, D.R. Interleukin 18 in the heart. Shock 2008, 30, 3–10. [Google Scholar] [CrossRef]
- Naito, Y.; Tsujino, T.; Wakabayashi, K.; Matsumoto, M.; Ohyanagi, M.; Mitsuno, M.; Miyamoto, Y.; Hao, H.; Hirota, S.; Okamura, H.; et al. Increased interleukin-18 expression in nonrheumatic aortic valve stenosis. Int. J. Cardiol. 2010, 144, 260–263. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, J.; Jiang, L.; Zhang, B.; Zhu, D.; Wu, Y. Interleukin 18 promotes myofibroblast activation of valvular interstitial cells. Int. J. Cardiol. 2016, 221, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Vazquez, R.; Khojeini, E.V.; Patel, C.; Venkataramani, R.; Larson, D.F. IL-18 induction of osteopontin mediates cardiac fibrosis and diastolic dysfunction in mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H76–H85. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, B.J.; Papacosta, O.; Owen, C.G.; Wannamethee, S.G.; Humphries, S.E.; Woodward, M.; Lennon, L.T.; Thomson, A.; Welsh, P.; Rumley, A.; et al. Interleukin 18 and coronary heart disease: Prospective study and systematic review. Atherosclerosis 2011, 217, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhou, J.; Wang, C.; Xu, G.; Wu, Y.; Hu, Z. High mobility group box 1 induces calcification of aortic valve interstitial cells via toll-like receptor 4. Mol. Med. Rep. 2017, 15, 2530–2536. [Google Scholar] [CrossRef]
- Wang, B.; Li, F.; Zhang, C.; Wei, G.; Liao, P.; Dong, N. High-mobility group box-1 protein induces osteogenic phenotype changes in aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2016, 151, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, H.; Bai, Q.; Zhou, X.; Xu, C.; Lu, Z.; Cui, B.; Wen, H. Increased serum HMGB1 is related to the severity of coronary artery stenosis. Clin. Chim. Acta 2009, 406, 139–142. [Google Scholar] [CrossRef]
- Cheng, J.M.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; Boersma, E.; van Geuns, R.-J.; Serruys, P.W.; Kardys, I.; Akkerhuis, K.M. PCSK9 in relation to coronary plaque inflammation: Results of the ATHEROREMO-IVUS study. Atherosclerosis 2016, 248, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xing, C.Y.; Zhao, K.; Deng, J.; Olmedo, D.A.; Ma, Z.; Zhang, M.; Wang, Y. Associations of pro-protein convertase subtilisin-like kexin type 9, soluble low-density lipoprotein receptor and coronary artery disease: A case-control study. Int. J. Cardiol. 2022, 350, 9–15. [Google Scholar] [CrossRef]
- Donato, L.J.; Saenger, A.K.; Train, L.J.; Kotzer, K.E.; Lagerstedt, S.A.; Hornseth, J.M.; Basu, A.; Winters, J.L.; Baudhuin, L.M. Genetic and biochemical analyses in dyslipidemic patients undergoing LDL apheresis. J. Clin. Apher. 2014, 29, 256–265. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Camici, G.G.; Dallegri, F.; Vecchie, A.; Carbone, F.; Bonaventura, A. Treatment with Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors to Reduce Cardiovascular Inflammation and Outcomes. Curr. Med. Chem. 2017, 24, 1403–1416. [Google Scholar] [CrossRef]
- Vavlukis, M.; Kedev, S. Effects of High Intensity Statin Therapy in the Treatment of Diabetic Dyslipidemia in Patients with Coronary Artery Disease. Curr. Pharm. Des. 2018, 24, 427–441. [Google Scholar] [CrossRef]
- Wang, W.-G.; He, Y.-F.; Chen, Y.-L.; Zhao, F.-M.; Song, Y.-Q.; Zhang, H.; Ma, Y.-H.; Guan, X.; Zhang, W.-Y.; Chen, X.-L.; et al. Proprotein convertase subtilisin/kexin type 9 levels and aortic valve calcification: A prospective, cross sectional study. J. Int. Med. Res. 2016, 44, 865–874. [Google Scholar] [CrossRef]
- Poggio, P.; Songia, P.; Cavallotti, L.; Barbieri, S.S.; Zanotti, I.; Arsenault, B.J.; Valerio, V.; Ferri, N.; Capoulade, R.; Camera, M. PCSK9 Involvement in Aortic Valve Calcification. J. Am. Coll. Cardiol. 2018, 72, 3225–3227. [Google Scholar] [CrossRef] [PubMed]
- Salaun, E.; Mahjoub, H.; Dahou, A.; Mathieu, P.; Larose, É.; Després, J.-P.; Rodés-Cabau, J.; Arsenault, B.J.; Puri, R.; Clavel, M.-A.; et al. Hemodynamic Deterioration of Surgically Implanted Bioprosthetic Aortic Valves. J. Am. Coll. Cardiol. 2018, 72, 241–251. [Google Scholar] [CrossRef]
- Kysenius, K.; Muggalla, P.; Mätlik, K.; Arumäe, U.; Huttunen, H.J. PCSK9 regulates neuronal apoptosis by adjusting ApoER2 levels and signaling. Cell. Mol. Life Sci. 2012, 69, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Weber, M.; Arnold, R.; Rau, M.; Brandt, R.; Berkovitsch, A.; Mitrovic, V.; Hamm, C. Relation of N-Terminal Pro–B-Type Natriuretic Peptide to Severity of Valvular Aortic Stenosis. Am. J. Cardiol. 2004, 94, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.J.; Clavel, M.-A.; Pibarot, P.; Dweck, M.R. Timing of intervention in aortic stenosis: A review of current and future strategies. Heart 2018, 104, 2067–2076. [Google Scholar] [CrossRef]
- Dweck, M.R.; Jones, C.; Joshi, N.V.; Fletcher, A.M.; Richardson, H.; White, A.; Marsden, M.; Pessotto, R.; Clark, J.C.; Wallace, W.A.; et al. Assessment of Valvular Calcification and Inflammation by Positron Emission Tomography in Patients with Aortic Stenosis. Circulation 2012, 125, 76–86. [Google Scholar] [CrossRef]
- Awan, M.S.; Irfan, B.; Zahid, I.; Mirza, Y.; Ali, S.A. Comparison of Polymerase Chain Reaction and Immunohistochemistry Assays for Analysing Human Papillomavirus Infection in Oral Squamous Cell Carcinoma. J. Clin. Diagn. Res. 2017, 11, XC10–XC13. [Google Scholar] [CrossRef]
- Bergmark, B.A.; O’Donoghue, M.L.; Murphy, S.A.; Kuder, J.F.; Ezhov, M.V.; Ceška, R.; Gouni-Berthold, I.; Jensen, H.K.; Tokgozoglu, S.L.; Mach, F.; et al. An Exploratory Analysis of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibition and Aortic Stenosis in the FOURIER Trial. JAMA Cardiol. 2020, 5, 709–713. [Google Scholar] [CrossRef]
- Cowell, S.J.; Newby, D.E.; Prescott, R.J.; Bloomfield, P.; Reid, J.; Northridge, D.B.; Boon, N.A. A Randomized Trial of Intensive Lipid-Lowering Therapy in Calcific Aortic Stenosis. N. Engl. J. Med. 2005, 352, 2389–2397. [Google Scholar] [CrossRef]
- Rossebø, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Bärwolf, C.; Holme, I.; Kesäniemi, Y.A.; et al. Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef]
- Moura, L.M.; Ramos, S.F.; Zamorano, J.L.; Barros, I.M.; Azevedo, L.F.; Rocha-Gonçalves, F.; Rajamannan, N.M. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J. Am. Coll. Cardiol. 2007, 49, 554–561. [Google Scholar] [CrossRef]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J.; ASTRONOMER Investigators. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef]
- Cokkinos, D.V.; Cokkinos, P.; Kolovou, G. Proprotein convertase subtilisin/kexin type 9 inhibitors: New insights into cardiovascular atherosclerotic pathophysiology with therapeutic implications. Arch. Cardiovasc. Dis. 2019, 112, 455–458. [Google Scholar] [CrossRef]
- Ferencik, M.; Chatzizisis, Y.S. Statins and the coronary plaque calcium “paradox”: Insights from non-invasive and invasive imaging. Atherosclerosis 2015, 241, 783–785. [Google Scholar] [CrossRef]
- Perrot, N.; Valerio, V.; Moschetta, D.; Boekholdt, S.M.; Dina, C.; Chen, H.Y.; Abner, E.; Martinsson, A.; Manikpurage, H.D.; Rigade, S.; et al. Genetic and In Vitro Inhibition of PCSK9 and Calcific Aortic Valve Stenosis. JACC Basic Transl. Sci. 2020, 5, 649–661. [Google Scholar] [CrossRef]
- Sundararaman, S.S.; Döring, Y.; van Der Vorst, E.P. PCSK9: A multi-faceted protein that is involved in cardiovascular biology. Biomedicines 2021, 9, 793. [Google Scholar] [CrossRef]
- Lee, Y.S.; Chou, Y.Y. Pathogenetic mechanism of senile calcific aortic stenosis: The role of apoptosis. Chin. Med. J. 1998, 111, 934–939. [Google Scholar]
- Punch, E.; Klein, J.; Diaba-Nuhoho, P.; Morawietz, H.; Garelnabi, M. Effects of PCSK9 Targeting: Alleviating Oxidation, Inflammation, and Atherosclerosis. J. Am. Heart Assoc. 2022, 11, e023328. [Google Scholar] [CrossRef] [PubMed]
- Jigheh, Z.A.; Haghjo, A.G.; Argani, H.; Roshangar, L.; Rashtchizadeh, N.; Sanajou, D.; Ahmad, S.N.S.; Rashedi, J.; Dastmalchi, S.; Abbasi, M.M. Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran. J. Basic Med. Sci. 2019, 22, 384–390. [Google Scholar] [CrossRef]
- Sukhanov, S.; Higashi, Y.; Yoshida, T.; Mummidi, S.; Aroor, A.R.; Russell, J.J.; Bender, S.B.; DeMarco, V.G.; Chandrasekar, B. The SGLT2 inhibitor Empagliflozin attenuates interleukin-17A-induced human aortic smooth muscle cell proliferation and migration by targeting TRAF3IP2/ROS/NLRP3/Caspase-1-dependent IL-1β and IL-18 secretion. Cell. Signal. 2021, 77, 109825. [Google Scholar] [CrossRef]
- Uthman, L.; Homayr, A.; Hollmann, M.W.; Zuurbier, C.J.; Weber, N.C. Administration of SGLT2 inhibitor empagliflozin against TNF-α induced endothelial dysfunction in human venous and arterial endothelial cells. FASEB J. 2018, 32, 569.4. [Google Scholar] [CrossRef]
- Pirklbauer, M.; Sallaberger, S.; Staudinger, P.; Corazza, U.; Leierer, J.; Mayer, G.; Schramek, H. Empagliflozin Inhibits IL-1β-Mediated Inflammatory Response in Human Proximal Tubular Cells. Int. J. Mol. Sci. 2021, 22, 5089. [Google Scholar] [CrossRef]
- Tsai, K.-F.; Chen, Y.-L.; Chiou, T.; Chu, T.-H.; Li, L.-C.; Ng, H.-Y.; Lee, W.-C.; Lee, C.-T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef]
- Kvidal, P.; Bergström, P.R.; Hörte, L.G.; Ståhle, E. Observed and relative survival after aortic valve replacement. J. Am. Coll. Cardiol. 2000, 35, 747–756. [Google Scholar] [CrossRef]
- Adler, Y.; Vaturi, M.; Herz, I.; Iakobishvili, Z.; Toaf, J.; Fink, N.; Battler, A.; Sagie, A. Nonobstructive aortic valve calcification: A window to significant coronary artery disease. Atherosclerosis 2002, 161, 193–197. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).