Simple Summary
The problem of antimicrobial resistance represents a real danger to mankind. There is a crucial necessity to search for new antibiotics. The current advances in sequencing techniques and bioinformatics represent a critical tool for finding out the hidden biosynthetic capabilities within bacterial genomes and finding out novel active secondary metabolites. In this work, we managed to recover the Streptomyces vinaceusdrappus strain AC-40, a rhizobacterium with potent and broad-spectrum antimicrobial activity. We made whole genome sequencing and genome mining for the genes and gene clusters related to beneficial traits such as the production of secondary metabolites and plant growth promotion.
Abstract
Streptomyces are factories of antimicrobial secondary metabolites. We isolated a Streptomyces species associated with the Pelargonium graveolens rhizosphere. Its total metabolic extract exhibited potent antibacterial and antifungal properties against all the tested pathogenic microbes. Whole genome sequencing and genome analyses were performed to take a look at its main characteristics and to reconstruct the metabolic pathways that can be associated with biotechnologically useful traits. AntiSMASH was used to identify the secondary metabolite gene clusters. In addition, we searched for known genes associated with plant growth-promoting characteristics. Finally, a comparative and pan-genome analysis with three closely related genomes was conducted. It was identified as Streptomyces vinaceusdrappus strain AC-40. Genome mining indicated the presence of several secondary metabolite gene clusters. Some of them are identical or homologs to gene clusters of known metabolites with antimicrobial, antioxidant, and other bioactivities. It also showed the presence of several genes related to plant growth promotion traits. The comparative genome analysis indicated that at least five of these gene clusters are highly conserved through rochei group genomes. The genotypic and phenotypic characteristics of S. vinaceusdrappus strain AC-40 indicate that it is a promising source of beneficial secondary metabolites with pharmaceutical and biotechnological applications.
1. Introduction
The overuse and misuse of the currently available antibiotics have led to a fast increase in the resistance to these antibiotics with a heavy impact on human public health and national economies. As the problem of antimicrobial resistance becomes more serious, finding novel antibiotics has become more urgent than it has ever been [1,2].
Actinobacteria are reactors for production of the active secondary metabolites [3]. Several natural products have been derived from actinomycetes [3,4]. Over the last few years, actinomycetes took place in the fields of biotechnology, pharmacy, and agriculture [5].
Streptomyces represents the biggest genus in the phylum of Actinobacteria. They are high GC content, Gram-positive, and spore-forming bacteria. This genus is highly represented in the environment. From different soil and marine ecosystems [6,7] to extreme environments such as deserts and volcanic environments, different species of Streptomyces have been found [8,9]. With over 600 known species, the Streptomyces is a genus greatly known for the production of active compounds. Several bioactive molecules have been previously isolated from Streptomyces species. Antibacterial, antifungal, antitumor, anti-inflammatory, and immunosuppressive are all reported activities of secondary metabolites from the Streptomyces species [10]. Genome mining indicated that the genome of a single Streptomyces species can harbor from 25 up to 70 biosynthetic gene clusters (BGCs). Most of these BGCs are silent under standard laboratory conditions [5].
Streptomyces produces a chemically diverse collection of secondary metabolites with antimicrobial activity. Polyketides, non-ribosomal peptides, siderophores, terpenes, alkaloids, lanthipeptides, and others are among the most common classes of Streptomyces antimicrobial secondary metabolites [11]. The first antimicrobial compounds to be isolated from Streptomyces were actinomycin and streptothricin in the first half of the 20th century. Currently, Streptomyces species are a source of several known antibiotics, such as streptomycin, tetracycline chloramphenicol, clindamycin, lincomycin, erythromycin, kanamycin, and many others [12]. From 2015, more than 70 Streptomyces species with over 170 novel bioactive metabolites were isolated from terrestrial ecosystems. Many of these compounds had antimicrobial activity even against multidrug-resistant pathogens such as krisynomycin (B and C), picolinamycin, nybomycin D, puromycin B–E, ulleungmycin (A and B), streptoone A, and others [5].
Streptomyces is the most bacterial genus to be isolated as plant-associated actinobacteria [13]. It was reported to play an important role in the biological control of plant pathogens by producing antimicrobial molecules that fight against plant-infectious microbes. Some of their metabolites act as active plant growth promoters [14]. This makes the plant-associated Streptomyces a very promising source to search for novel antimicrobial metabolites. Some Streptomyces have also the capacity to produce enzymes with extracellular cell wall-degrading activity such as chitinases and β-1,3-glucanase that increases the plant’s resistance to diseases [15].
In this work, we aimed to investigate the genomic characteristics and the biosynthetic gene clusters of the biologically active Streptomyces vinaceusdrappus strain AC-40 that was isolated from the rhizosphere of the Pelargonium graveolens plant.
2. Materials and Methods
2.1. Isolation and Purification of the Strain AC-40
The strain AC-40 was isolated from the rhizosphere of three-month-old Pelargonium graveolens. A total of 5 g of Pelargonium graveolens root sample was placed in falcon tubes with 45 mL of 0.85% NaCl and vigorously mixed using a vortex for 1 min. Tenfold serial dilutions of the microbial suspensions were plated onto starch casein agar medium (SCA) supplemented with nystatin (100 µg/mL) to prevent fungal growth. The plates were incubated at 30 °C for 7 days. Typical actinomycetes colonies were picked and purified by streaking onto the SCA medium for 7 more days until full growth was observed. The pure colonies were preserved in Luria–Bertani (LB) broth medium supplemented with 20% glycerol at −20 °C for future use.
2.2. Solvent Extraction of the Strain AC-40 Bioactive Metabolites
Submerged fermentation with starch casein broth (SCB) medium was used to produce metabolites of the strain AC-40. A single colony of the isolated strain was inoculated in 250 mL of sterilized SCB medium and kept on a rotary shaker at 150 rpm, and 30 °C for 7 days. After fermentation, the supernatant was centrifuged at 2325× g (4000 rpm) for 15 min to remove the cell mass, and the crude cell-free supernatant was filter-sterilized using 0.22 µm filters. An equal volume of ethyl acetate was used to extract the cell-free supernatant. The top organic layer was collected in a beaker and evaporated at 30 °C using a rotor evaporator.
2.3. Antimicrobial Activity Screening of the Strain AC-40 Bioactive Metabolites
The antimicrobial activity of the cell-free supernatant of the broth culture and the ethyl acetate extract of the strain AC-40 was assessed against Staphylococcus aureus ATCC 6538, MRSA, Bacillus cereus ATCC 33018, Pseudomonas aeruginosa ATCC 27853, Escherichia coli (O157:H7), and Candida albicans ATCC 60193. Antimicrobial activity screening was analyzed using the agar well diffusion method on Mueller–Hinton agar (MHA) medium as follows: Bacterial and fungal pathogens (100 µL) were inoculated over the surface of MHA plates, 9 mm wells were prepared using a sterilized cork borer. The residual material resulting after the drying of the ethyl acetate extract of AC-40 was dissolved in 10% dimethyl sulfoxide (DMSO) with a concentration of 10 mg/mL then 50 µL was used in each well. An amount of 10% DMSO was used as a negative control, and gentamycin of a concentration of 10 μg/mL was used as a positive control for both Gram-positive and Gram-negative bacterial pathogens. Itraconazole of a concentration of 10 μg/mL was used as a positive control for fungal microbial strains. All assays were performed in triplicates.
2.4. Genomic DNA Extraction, and Sequencing of the Strain AC-40
A previously incubated SCB culture of the strain AC-40 at 30 °C for 7 days was used for genomic DNA extraction. A cell pellet of 1–5 × 106 cells was prepared by centrifugation for 10 min at 5000× g (7500 rpm). The genomic DNA was extracted using the QIAamp® DNA Minikit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. The purity of the extracted DNA was determined using NanoDrop 1000 Spectrophotometer V3.8 (Thermo Fisher Scientific Inc., Waltham, MA, USA). A260/A280 ratio of ~1.8 indicated a pure DNA extraction. The genome was sequenced using the Illumina MiSeq platform at the Genomics unit (57357, Egypt), following the standard Illumina protocols. The preparation of the library was carried out utilizing the Nextera XT DNA Library preparation kit (Illumina, San Diego, CA, USA).
2.5. Genome Assembly, Scaffolding, and Annotation of the Strain AC-40
The paired-ended reads with an average length of 277 bases were filtered using Trimmomatic, version 0.38.1 [16], with an adaptor cutting option, a sliding window of 4 bases, and a minimum quality of 20. The reads were assembled using the Unicycler version 0.4.8 assembler [17] on Pathosystems Resource Integration Center (PATRIC) server (www.patricbrc.org (accessed on 1 July 2022)), currently (https://www.bv-brc.org (accessed on 1 July 2022)). A minimum contig size of 1000 bp was used. For the reference-guided genome scaffolding of the draft genome, the similar genome finder (a part of PATRIC services) was used to compute genome distance estimation using Mash/MinHash algorithm [18] with all public genomes on the PATRIC platform. The most similar complete genome was used as a reference for the reference-guided genome scaffolding carried out using RagTag version 2.1.0 [19]. The genome was annotated using rapid annotations using subsystems technology (RAST) [20] and the rapid prokaryotic genome annotation (Prokka) [21].
2.6. Strain AC-40 Typing and Phylogeny
For typing the strain AC-40, the Type Strain Genome Server (TYGS) was used [22]. The phylogenetic tree was constructed using a concatenated sequence of the 16S rRNA gene sequence (~1500 pb), gyrB (~2000 pb), and rpoB (~1700 pb) sequences of the strain AC-40 and its most closely related type strains were aligned using MUSCLE [23]. The maximum likelihood phylogenetic tree was constructed using the Molecular Evolutionary Genetics Analysis software (MegaX) [24]. The Kimura 2-parameter model [25] and 1000-bootstrap test were used. The average nucleotide identity (ANI) of the genomes was calculated using the JSpecies Web Service [26]. In silico DNA–DNA hybridization (isDDH) of genomes was compared using the Genome-to-Genome Distance Calculator (GGDC) [27].
2.7. General Genome Feature and Pathways of the Strain AC-40
The annotated proteins from the genome of the strain AC-40 were analyzed on a subsystem level using the comprehensive genome analysis pipeline (a part of PATRIC serves). The GhostKOALA was used for KO annotation and reconstruction of the KEGG pathways [28].
2.8. Screening the Genome of the Strain AC-40 for Genes Related to Beneficial Traits
AntiSMASH bacterial version 6.0 [29] was used to scan the genome of S. vinaceusdrappus strain AC-40 for the presence of biosynthetic gene clusters (BGCs) responsible for the biosynthetic process of bioactive secondary metabolites. Furthermore, genes successfully annotated by Prokka were manually screened for gene products with plant growth-promoting (PGP) traits, such as genes incorporated in nitrogen fixation, phosphate solubilization, iron sequestration, and phytohormones production. We also searched for gene products that serve as biocatalysts, such as proteases, lipases, chitinases, and catalase production [30].
2.9. Pan-Genome and Comparative Genome Analysis
To identify the number of core genes, accessory genes, and the gene presence–absence distribution between the genome of the strain AC-40 and other genomes of the same phylogenetic group, a pan-genome analysis was conducted using Roary pan-genome pipeline [31].
The proteome comparison tool on PATRIC was used to compare the percentage identity between the protein sequences from other genomes referenced to those of the AC-40. The orthovenn2 web server was used to visualize and compare the orthologous protein clusters across these genomes [32].
The different BGCs detected with AntiSMASH in the genome of AC-40 were compared to the other genomes in the same phylogenetic group to investigate their conservation through the group.
3. Results
3.1. General Features and Antimicrobial Activity of the Strain AC-40
The culture of the strain AC-40 on SCA, after 7 days, appeared as raised, rough, buff colonies. The culture had the distinctive earthy smell of geosmin-producing actinomycetes (Figure 1a). The catalase activity of the culture was positive. The cell-free supernatant and the ethyl acetate extract of strain AC-40 showed broad-spectrum antimicrobial activity against all indicator strains (Figure 1b). Both the cell-free liquid culture and total metabolic extract showed inhibitory effects significantly equivalent and sometimes higher than the positive control. This makes the AC-40 a potential source of antibacterial and antifungal compounds.
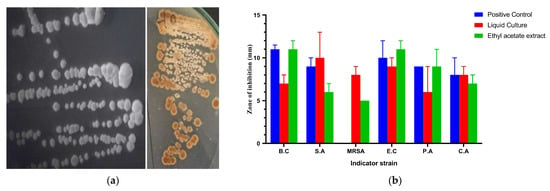
Figure 1.
(a) The colony morphology of the strain AC-40 on starch casein agar; (b) zone of inhibition in (mm) produced by the cell-free supernatant and ethyl acetate extract of the strain AC-40 against B.C (Bacillus cereus), S.A (Staphylococcus aureus), MRSA (methicillin-resistant Staphylococcus aureus), E.C (Escherichia coli), P.A (Pseudomonas aeruginosa), and CA (Candida albicans). An amount of 10% DMSO was used as a negative control, and gentamycin (10 μg/mL) was used as a positive control.
3.2. AC-40 Genome Assembly, Scaffolding, and Annotation
After the genomic DNA extraction, sequencing, and the de novo assembly of the genome of the strain AC-40, a total of 1048 contigs covering 7,493,232 bp were obtained. The average GC% was 72.41. Using the similar genome finder tool, the genome of Streptomyces sp. Osf17 (7,967,258 bp) was found to be the closest genome to the genome of strain AC-40 with a distance of 0.00944662. The genome of Streptomyces sp. Osf17 (GCA_019029505.1) is a two contigs genome (7,883,883 bp and 83,375 bp) sequenced with the PacBio Sequel technology. By using the genome of Streptomyces sp. Osf17 as a reference for the reference-guided scaffolding, one scaffold representing the bacterial chromosome was obtained. Genome annotation was conducted using two different platforms. The number of annotated CDSs was higher in the case of the RAST platform than it was in Prokka. A total of 7241 CDS (5059 functional), 35 transfer RNA (tRNA) genes, and 3 ribosomal RNA (rRNA) genes were annotated with RAST. A total of 6983 CDS (3303 functional), 82 tRNA genes, and 3 rRNA genes were annotated with Prokka. The genome map is illustrated in Figure 2.
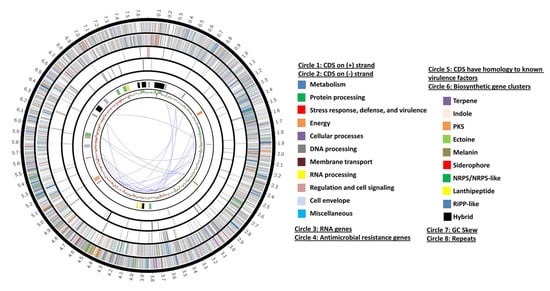
Figure 2.
A circos diagram represents the genome map of the strain AC-40’s bacterial chromosome indicating the RAST, AntiSMASH annotations, GC Skew, and the repeated regions.
3.3. AC-40 Typing and Phylogeny
The TYGS indicated a close relationship between the genome of AC-40 and Streptomyces species. The top hit was Streptomyces vinaceusdrappus (Streptomyces rochei group).
The phylogenetic tree based on the 16S rRNA, gyrB, and rpoB gene partial sequences located the AC-40 within the S. rochei group (Figure 3). The ANI, DDH, and ∆GC% values comparisons between other genomes and the genome of AC-40 were calculated to reinforce the strain typing. We identified the isolate AC-40 as S. vinaceusdrappus strain AC-40.
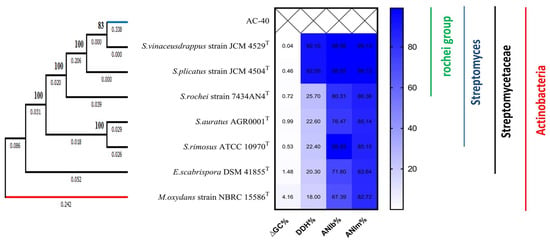
Figure 3.
A 1000 bootstrap, un-rooted, maximum likelihood phylogenetic tree based on the 16S rRNA, gyrB, and rpoB gene partial sequences between Streptomyces vinaceusdrappus strain AC-40 and the closest related Streptomyces type strains; S. vinaceusdrappus strain JCM 4529, S. plicatus strain JCM 4504, S. rochei strain 7434AN4, S. auratus AGR0001, S. rimosus ATCC 10970, and Embleya scabrispora DSM 41855. Microbacterium oxydans strain NBRC 15586 was used to root the tree. The branch lengths are displayed under the branch. The bootstrap values are displayed beside the nodes.
3.4. Streptomyces vinaceusdrappus Strain AC-40 Genome Features and Pathways Reconstruction
A subsystem is a group of proteins that work together to carry out a certain biological function or structural complex. PATRIC comprehensive genome analysis service provides a subsystem analysis of prokaryotic genomes. The subsystem analysis of S. vinaceusdrappus strain AC-40 showed that from the 7241 annotated CDS only 2023 CDS were assigned as “in a subsystem” (Figure 4a). A total of 2530 protein products distributed over 11 functional categories were annotated on KEGG using the GhostKOALA tool (Figure 4b). The 2530 annotated gene products are involved in 327 pathways over 59 complete KEGG modules, including carbohydrate metabolism (13 modules), carbon fixation (2 modules), nitrogen metabolism (1 module), sulfur metabolism (1 module), ATP synthesis (5 modules), lipid metabolism (3 modules), nucleotide metabolism (9 modules), amino acid metabolism (13 modules), cofactors and vitamins biosynthesis (10 modules), terpenoids and polyketides (2 modules). Among the metabolic modules that were found complete within the genome of the S. vinaceusdrappus strain AC-40 were the dissimilatory nitrate reduction to ammonia, the assimilatory sulfate reduction, and crassulacean acid metabolism.
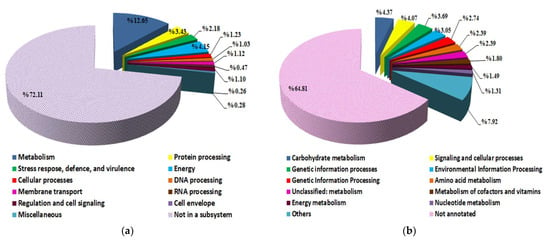
Figure 4.
(a) a subsystem analysis by PATRIC platform indicates the number of genes incorporated in specific biological process within the genome of S. vinaceusdrappus strain AC-40; (b) a functional categorization of KEGG annotations of the genome of S. vinaceusdrappus strain AC-40 using the GhostKOALA tool.
3.5. Streptomyces vinaceusdrappus Strain AC-40 Secondary Metabolites-Related Gene Clusters
A total of 27 biosynthetic gene clusters (BGCs) were detected within the genome of S. vinaceusdrappus strain AC-40 using AntiSMASH bacterial version 6.0 (Table 1).

Table 1.
The biosynthetic gene clusters (BGCs) detected with AntiSMASH within the genome of Streptomyces vinaceusdrappus strain AC-40.
Some of the BGCs were highly related to known metabolites with antibacterial and antifungal properties such as candicidin, albaflavenone, and streptothricin (Figure 5). Other clusters related to known active metabolites such as the carotene derivative isorenieratene, ectoine, hopene, coelichelin, coelibactin, desferrioxamine, SapB, and geosmin were detected. Some of the clusters showed small or no relevance to known metabolites. The type of these clusters included terpene, T2PKS, NRPS, RiPP-like, lanthipeptides, siderophores, or hybrid gene clusters.
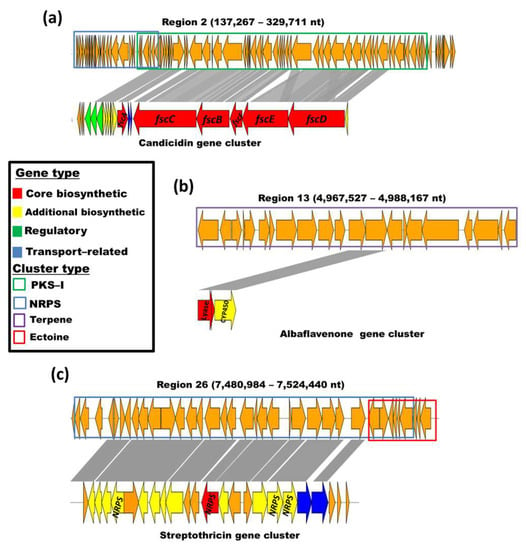
Figure 5.
Similarity to known antimicrobial metabolites BGCs in three of the regions detected with AntiSMASH in the genome of Streptomyces vinaceusdrappus strain AC-40: (a) candicidin; (b) albaflavenone; (c) streptothricin. The characteristics of the clusters and gene types are indicated in the box.
3.6. Streptomyces vinaceusdrappus Strain AC-40 Genes Related to Plant Growth Promotion Traits
The functional annotation by Prokka revealed the presence of several genes within the genome of S. vinaceusdrappus strain AC-40 whose annotated products can have PGP characteristics (Table 2). Those genes are related to processes that play a helpful role in plant nutrition, such as nitrogen assimilation, phosphate solubilization, and iron sequestration via siderophores. Chitin is the major component of the cell wall of several phytopathogens, especially fungi [33]. A set of genes responsible for the production of chitinases and other lytic enzymes such as proteases and lipases were detected within the genome of the S. vinaceusdrappus strain AC-40. That suggests a strong antifungal activity of this strain. The production of catalases and peroxidases by rhizobacteria is a very helpful mechanism in plant oxidative stress tolerance [33]. A set of genes responsible for the peroxidase/catalase activity were also detected within the genome of the AC-40.

Table 2.
Some genes related to the plant growth promotion and their representation within Streptomyces vinaceusdrappus strain AC-40 according to Prokka annotations.
3.7. Pan-Genome and Comparative Genomics
The pan-genome analysis of the S. vinaceusdrappus strain AC-40 and other related three members of S. rochei group genomes (S. plicatus strain JCM 4504 (GCA_014650135.1), S. vinaceusdrappus strain JCM 4529 (ASM1465021v1), and S. rochei strain 7434AN4 (GCA_008064995.1) showed the presence of 246 core genes shared within the four genomes and 15638 accessory genes distributed within them. Most of the core genes are incorporated in protein processing, cell defense, and stress response (Figure 6a). The KEGG mapper reconstruction of the core indicated that these genes are incorporated in 90 different pathways but there was only one complete central carbohydrate metabolism module representing the phosphoribosyl-diphosphate (PRPP) biosynthesis in prokaryotes. The gene presence–absence matrix showed a unique print of the AC-40 genome which was more related to genomes of S. plicatus strain JCM 4504 and S. vinaceusdrappus strain JCM 4529 than the genome of S. rochei strain 7434AN4 (Figure 6b).
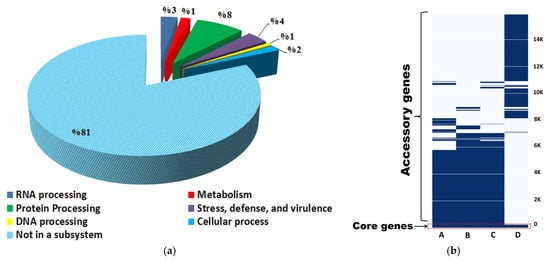
Figure 6.
(a) Subsystem analysis of the rochei group’s core genome indicates the number of genes incorporated in specific biological process; (b) gene presence–absence matrix in the four genomes A (S. vinaceusdrappus strain AC-40), B (S. vinaceusdrappus strain JCM 4529), C (S. plicatus strain JCM 4504), and D (S. rochei strain 7434AN4).
The proteome comparison tool on PATRIC showed that most of the proteins in S. vinaceusdrappus strain JCM 4529, and S. plicatus strain JCM 4504 had similarity >90% to those of S. vinaceusdrappus strain AC-40. However, S. rochei strain 7434AN4 showed similarities of 80% or lower on the protein level (Figure 7a). The orthologous cluster protein analysis showed that 4058 clusters are shared between all four genomes. Only seven protein clusters (26 protein sequences) were unique to the genome of AC-40 and not annotated on Swiss-Prot (Figure 7b).
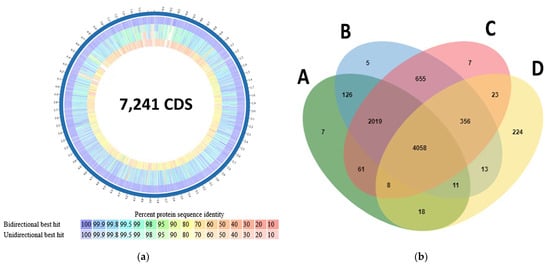
Figure 7.
(a) Proteome similarity analysis carried out by the proteome comparison tool on PATRIC between—as ordered from the outside to the inside—S. vinaceusdrappus strain AC-40 as a reference, S. vinaceusdrappus strain JCM 4529, S. plicatus strain JCM 4504, and S. rochei strain 7434AN4; (b) A Venn diagram indicated the shared orthologous protein clusters among A (S. vinaceusdrappus strain AC-40), B (S. vinaceusdrappus strain JCM 4529), C (S. plicatus strain JCM 4504), and D (S. rochei strain 7434AN4).
Comparing the biosynthetic genes clusters between the four genomes, most of the clusters were found to be conserved among S. vinaceusdrappus strain AC-40, S. vinaceusdrappus strain JCM 4529, and S. plicatus strain JCM 4504 with few differences. Region 11, a Lanthipeptide class-V BGC, was found within the genome of the S. vinaceusdrappus strain AC-40. This cluster showed no relevance to any known clusters. Instead, within the genomes of S. vinaceusdrappus strains JCM4529 and S. plicatus strain JCM4504, there was a hybrid gene cluster of the type PKS-like/furan/lanthipeptide class-v. The BGC in region 26 within the genome of the S. vinaceusdrappus strain AC-40 was not detected within the genome of S. plicatus strain JCM4504. This gene cluster was highly similar to that of the known antimicrobial metabolite streptothricin. Region 27, representing the hybrid PKS-II/butyrolactone cluster within the genome of S. vinaceusdrappus strain AC-40, was not found within the genomes of S. vinaceusdrappus strains JCM4529 and S. plicatus strain JCM4504. However, the sequence of the PKS-II part of the cluster was highly similar to a single PKS-II gene cluster found within the genomes of S. vinaceusdrappus strains JCM4529 and S. plicatus strain JCM4504.
S. rochei strain 7434AN4 had the lowest relation to the BGCs in the S. vinaceusdrappus strain AC-40 (Figure 8).
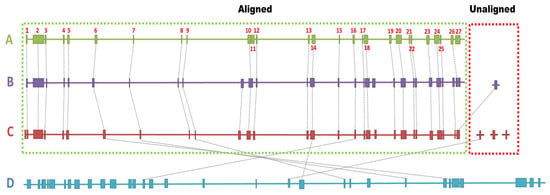
Figure 8.
A relation between 27 biosynthetic gene clusters among A (S. vinaceusdrappus strain AC-40) as a reference, B (S. vinaceusdrappus strain JCM 4529), C (S. plicatus strain JCM 4504), and D (S. rochei strain 7434AN4). The labels aligned and unaligned indicate the contigs from genomes B and C that were successfully and unsuccessfully aligned on the genome A as reference for reference-guided scaffolding.
4. Discussion
Rhizobacteria play an important role in plant growth under both normal and challenging environmental circumstances [30]. Those bacteria also play an important role in the biocontrol process against plant pathogens by producing antimicrobial metabolites [34]. The interaction between the plant and its associated bacteria can also take a deeper connection where some endophytic bacteria can synthesize one or more of their host’s metabolites [35].
AC-40 was isolated from the rhizosphere of Pelargonium graveolens, a medicinal, aromatic plant belonging to the family Geraniaceae. It is known for its antibacterial, antifungal, and antioxidant activities [36].
The closely related genome of Streptomyces sp. Osf17 which showed the lowest genomic distance between all publicly available genomes on PATRIC has been isolated from the Algerian desert and was reported for its potent antimicrobial activity [9]. This may suggest the ability of AC-40 to survive in extreme conditions. The genome of AC-40 showed the presence of several genes responsible for stress response, including those of the sigmaB stress response regulator.
Phylogenetic analysis showed that the isolate is closely grouped with S. vinaceusdrappus (S. rochei group). Most of the members of the rochei group were reported as antibiotic-producing Streptomyces [37,38]. The genomes of this group of Streptomyces are understudied. The genome list on NCBI of the members from this group is quite short. The S. vinaceusdrappus and S. plicatus genome list contain only one genome for each.
The reconstruction of the KEGG pathways indicated the presence of some interesting, complete modules. The dissimilatory nitrate reduction to ammonia is a beneficial ability for the microorganism and plant. It converts the nitrate and nitrite compounds into the less mobile ammonia compound. It serves for longer availability of nitrogen for both the microorganism and plant [39]. The hydrogen sulfide produced from the act as a signaling molecule that can promote plant growth and germination [40]. Crassulacean acid metabolism (CAM) is believed to be another mode of photosynthesis in some plants that have been found long ago as an evolutionary response to challenging climate conditions [41]. Half of this mode takes place in the dark and the other half during the day. Interestingly, we have found a complete set of genes code for the CAM pathway’s enzymes in the dark and a nearly complete set of its enzymes during the day in our Streptomyces sp. AC-40. We have also found this to be common in several public genomes belonging to different species from the Streptomyces genus. Liu et al. (2016) have described the same notice in the sponge-active symbiotic filamentous bacteria of the genus “Entotheonella” (a phylum of Tectomicrobia) [42].
The AntiSMASH results indicated the presence of several BGCs. The largest number of those BGCs was of the type of NRPS and terpenes. PKS-I and PKS-II are significantly represented in the terrestrial and aquatic environments and strongly related to the biosynthesis of antimicrobial metabolites in actinobacteria, especially the Streptomyces species [43]. Six of the PKS and PKS-like gene clusters were found within the genome of AC-40.
At least three of the detected gene clusters are related to known potent antimicrobials. Streptothricin is an N-glycoside antibiotic that has been found in different Streptomyces species with broad-spectrum activity against both Gram-positive and Gram-negative bacteria [44]. Candicidin is a polyene antifungal originally derived from Streptomyces griseus and clinically approved against Candida albicans vaginal infections [45]. Albaflavenone is a sesquiterpene antibiotic originally derived from Streptomyces coelicolor A3(2) [46]. These results can explain the potent and broad antimicrobial activity of S. vinaceusdrappus strain AC-40. The same results were reported from the closely related Streptomyces sp. Osf17 [9].
Other BGCs are related to other active metabolites, including isorenieratene, which was reported for its strong antioxidant and protection against photooxidation ultraviolet wave damage [47]. Ectoine is a bacterial natural osmolyte produced by many extremophiles. It plays an important role in bacterial survival in high osmotic environments. Ectoine was reported for its ability to protect the biological membrane (e.g., skin) from extreme conditions such as dryness, heat, UV, and surfactants [48,49]. With three siderophore BGCs, the siderophore peptide “coelichelin” and the zincophore “coelibactin”, which are detected in our strain and other related strains, are thought to be highly adapted to limited-nutrient conditions [50,51].
Lanthipeptides are RiPPs that produce a wide range of bioactivities [52]. Class I and II lanthipeptides were usually reported for their broad antimicrobial activity through binding to lipid II, inhibiting cell wall synthesis [53]. There was a complete lanthipeptide class I lanthipeptide gene cluster detected within our isolate AC-40 genome with no relevance to any known lanthipeptide, suggesting its novelty. Unlike classes I and II lanthipeptides, classes III and V usually play biological functions [53]. S. vinaceusdrappus strain AC-40 showed the presence of two lanthipeptides class III gene clusters. One of them belongs to the known lanthipeptide SapB. It plays an important morphogenetic function in the formation of aerial mycelium [53,54].
5. Conclusions
Rhizoactinobacteria are good producers of antimicrobial secondary metabolites for self-defense and competing with other microbes for space and nutrients, including the host-pathogenic ones. This represents a bright side for the microorganism–host interaction.
The rhizobacterium S. vinaceusdrappus strain AC-40 is an active producer of antimicrobial secondary metabolites. Its genome is quite interesting for carrying several secondary metabolite-related gene clusters which have little or no relation to known ones, which make it a potential producer for novel antimicrobial metabolites. Some of those gene clusters were found to be conserved or semi-conserved in the S. rochei group, which make this group interesting for deeper investigation.
Author Contributions
Conceptualization, A.M.S. and T.R.E.; data curation, A.M.S., I.S. and H.L.K.; formal analysis, A.M.S. and T.R.E.; investigation, A.M.S., E.N. and T.R.E.; methodology, A.M.S. and T.R.E.; project administration, T.R.E.; resources, A.M.S., I.S., H.L.K., M.A.S., A.A., M.S.R.R. and T.R.E.; software, A.M.S.; supervision, T.R.E.; validation, A.M.S. and T.R.E.; visualization, A.M.S.; writing—original draft, A.M.S.; writing—review and editing, A.M.S., I.S., H.L.K., M.A.S. and T.R.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data were uploaded to the NCBI GenBank under the accession numbers; BioProject (PRJNA879080), BioSample (SAMN30790593), SRA (SRR21521741), and Genome (CP104697).
Acknowledgments
Authors are thankful to the Researchers Supporting Project number (RSP2023R491), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Hoffman, P.S. Antibacterial discovery: 21st century challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef]
- Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Editorial: Actinobacteria: Prolific Producers of Bioactive Metabolites. Front. Microbiol. 2020, 11, 1612. [Google Scholar] [CrossRef]
- Bundale, S.; Singh, J.; Begde, D.; Nashikkar, N.; Upadhyay, A. Rare actinobacteria: A potential source of bioactive polyketides and peptides. World J. Microbiol. Biotechnol. 2019, 35, 92. [Google Scholar] [CrossRef]
- Donald, L.; Pipite, A.; Subramani, R.; Owen, J.; Keyzers, R.A.; Taufa, T. Streptomyces: Still the Biggest Producer of New Natural Secondary Metabolites, a Current Perspective. Microbiol. Res. 2022, 13, 418–465. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Sedeek, A.M.; Ismail, M.M.; Elsayed, T.R.; Ramadan, M.A. Recent methods for discovering novel bioactive metabolites, specifically antimicrobial agents, from marine-associated micro-organisms. Lett. Appl. Microbiol. 2022, 75, 511–525. [Google Scholar] [CrossRef]
- Jia, F.; Liu, C.; Zhao, J.; Zhang, Y.; Li, L.; Zhou, S.; Shen, Y.; Wang, X.; Xiang, W. Streptomyces vulcanius sp. nov., a novel actinomycete isolated from volcanic sediment. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 15–21. [Google Scholar] [CrossRef]
- Zerouki, C.; Bensalah, F.; Kuittinen, S.; Pappinen, A.; Turunen, O. Whole-genome sequencing of two Streptomyces strains isolated from the sand dunes of Sahara. BMC Genom. 2021, 22, 578. [Google Scholar] [CrossRef]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Lee, N.; Hwang, S.; Kim, J.; Cho, S.; Palsson, B.; Cho, B.K. Mini review: Genome mining approaches for the identification of secondary metabolite biosynthetic gene clusters in Streptomyces. Comput. Struct. Biotechnol. J. 2020, 18, 1548–1556. [Google Scholar] [CrossRef]
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040–1048. [Google Scholar] [CrossRef]
- Supong, K.; Thawai, C.; Choowong, W.; Kittiwongwattana, C.; Thanaboripat, D.; Laosinwattana, C.; Koohakan, P.; Parinthawong, N.; Pittayakhajonwut, P. Antimicrobial compounds from endophytic Streptomyces sp. BCC72023 isolated from rice (Oryza sativa L.). Res. Microbiol. 2016, 167, 290–298. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Ruangwong, O.U.; Kunasakdakul, K.; Daengsuwan, W.; Wonglom, P.; Pitija, K.; Sunpapao, A. A Streptomyces rhizobacterium with antifungal properties against spadix rot in flamingo flowers. Physiol. Mol. Plant Pathol. 2022, 117, 101784. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Alonge, M.; Soyk, S.; Ramakrishnan, S.; Wang, X.; Goodwin, S.; Sedlazeck, F.J.; Lippman, Z.B.; Schatz, M.C. RaGOO: Fast and accurate reference-guided scaffolding of draft genomes. Genome Biol. 2019, 20, 224. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1473. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wen, W.; Qin, M.; He, Y.; Xu, D.; Li, L. Biosynthetic Mechanisms of Secondary Metabolites Promoted by the Interaction Between Endophytes and Plant Hosts. Front. Microbiol. 2022, 13, 2584. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Marshall, V.; Hamidpour, R. Pelargonium graveolens (Rose Geranium)-A Novel Therapeutic Agent for Antibacterial, Antioxidant, Antifungal and Diabetics. Arch. Cancer Res. 2017, 5, 134. [Google Scholar] [CrossRef]
- Arakawa, K.; Kodama, K.; Tatsuno, S.; Ide, S.; Kinashi, H. Analysis of the loading and hydroxylation steps in lankamycin biosynthesis in Streptomyces rochei. Antimicrob. Agents Chemother. 2006, 50, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, H.; Li, S.; Xiao, J.; Zhang, G.; Zhu, Y.; Niu, S.; Ju, J.; Zhang, C. Characterization of the amicetin biosynthesis gene cluster from Streptomyces vinaceusdrappus NRRL 2363 implicates two alternative strategies for amide bond formation. Appl. Environ. Microbiol. 2012, 78, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, U.; Augustin, J.; Spröer, C.; Gelbrecht, J.; Schumann, P.; Ulrich, A. Taxonomic characterisation of Proteus terrae sp. nov., a N2O-producing, nitrate-ammonifying soil bacterium. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 108, 1457–1468. [Google Scholar] [CrossRef]
- Dooley, F.D.; Nair, S.P.; Ward, P.D. Increased Growth and Germination Success in Plants following Hydrogen Sulfide Administration. PLoS ONE 2013, 8, e62048. [Google Scholar] [CrossRef]
- Heyduk, K. Evolution of Crassulacean acid metabolism in response to the environment: Past, present, and future. Plant Physiol. 2022, 190, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.; Feng, G.; Li, Z. New Genomic Insights into “Entotheonella” Symbionts in Theonella swinhoei: Mixotrophy, Anaerobic Adaptation, Resilience, and Interaction. Front. Microbiol. 2016, 7, 1333. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, A.M.; Ismail, M.M.; Elsayed, T.R.; Ramadan, M.A. Evaluation of the Marine Bacterial Population in the Great Bitter Lake, Egypt, as a Source of Antimicrobial Secondary Metabolites. Fermentation 2022, 8, 309. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, M.; Zhang, J.; Wei, S.; Wu, W. Two new members of streptothricin class antibiotics from Streptomyces qinlingensis sp. nov. J. Antibiot. 2007, 60, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.A.; Campelo-Diez, A.B. Candicidin biosynthesis in Streptomyces griseus. Appl. Microbiol. Biotechnol. 2003, 60, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lin, X.; Lei, L.; Lamb, D.C.; Kelly, S.L.; Waterman, M.R.; Cane, D.E. Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor A3(2). J. Biol. Chem. 2008, 283, 8183–8189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, M.; Yang, J.; Chen, J.; Xie, B.; Sun, Z. Potential TSPO Ligand and Photooxidation Quencher Isorenieratene from Arctic Ocean Rhodococcus sp. B7740. Mar. Drugs 2019, 17, 316. [Google Scholar] [CrossRef]
- Bilstein, A.; Heinrich, A.; Rybachuk, A.; Mösges, R. Ectoine in the Treatment of Irritations and Inflammations of the Eye Surface. Biomed Res. Int. 2021, 2021, 8885032. [Google Scholar] [CrossRef]
- Graf, R.; Anzali, S.; Buenger, J.; Pfluecker, F.; Driller, H. The multifunctional role of ectoine as a natural cell protectant. Clin. Dermatol. 2008, 26, 326–333. [Google Scholar] [CrossRef]
- Challis, G.L.; Ravel, J. Coelichelin, a new peptide siderophore encoded by the Streptomyces coelicolor genome: Structure prediction from the sequence of its non-ribosomal peptide synthetase. FEMS Microbiol. Lett. 2000, 187, 111–114. [Google Scholar] [CrossRef]
- Zhao, B.; Moody, S.C.; Hider, R.C.; Lei, L.; Kelly, S.L.; Waterman, M.R.; Lamb, D.C. Structural Analysis of Cytochrome P450 105N1 Involved in the Biosynthesis of the Zincophore, Coelibactin. Int. J. Mol. Sci. 2012, 13, 8500–8513. [Google Scholar] [CrossRef] [PubMed]
- Lagedroste, M.; Reiners, J.; Knospe, C.V.; Smits, S.H.J.; Schmitt, L. A Structural View on the Maturation of Lanthipeptides. Front. Microbiol. 2020, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, J.D.; Süssmuth, R.D. Matters of class: Coming of age of class III and IV lanthipeptides. RSC Chem. Biol. 2020, 1, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Kodani, S.; Hudson, M.E.; Durrant, M.C.; Buttner, M.J.; Nodwell, J.R.; Willey, J.M. The SapB morphogen is a lantibiotic-like peptide derived from the product of the developmental gene ramS in Streptomyces coelicolor. Proc. Natl. Acad. Sci. USA 2004, 101, 11448–11453. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).