Epigenetic Regulation of miR-25 and Lnc107153 on Expression of Seasonal Estrus Key Gene CHGA in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Expression Assay
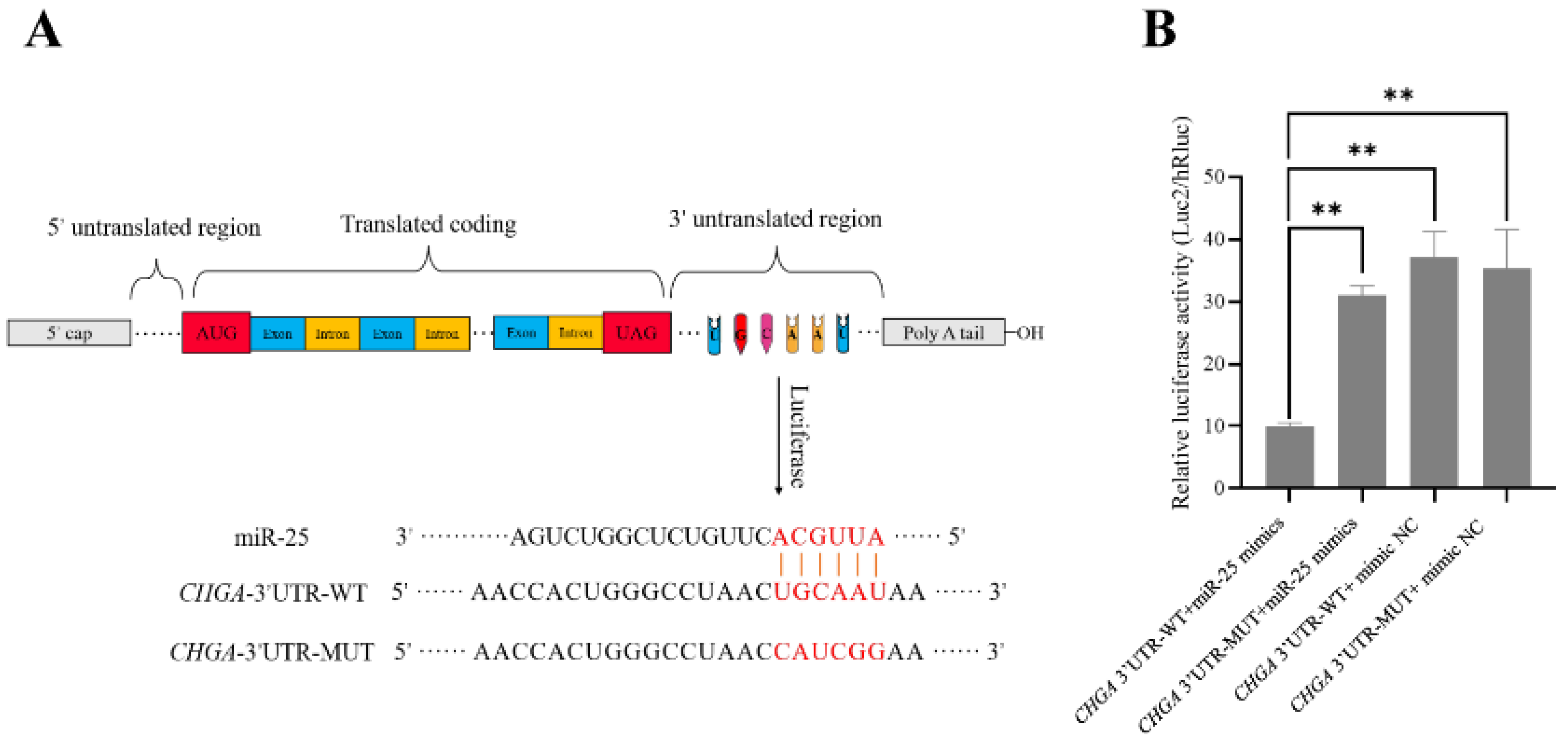
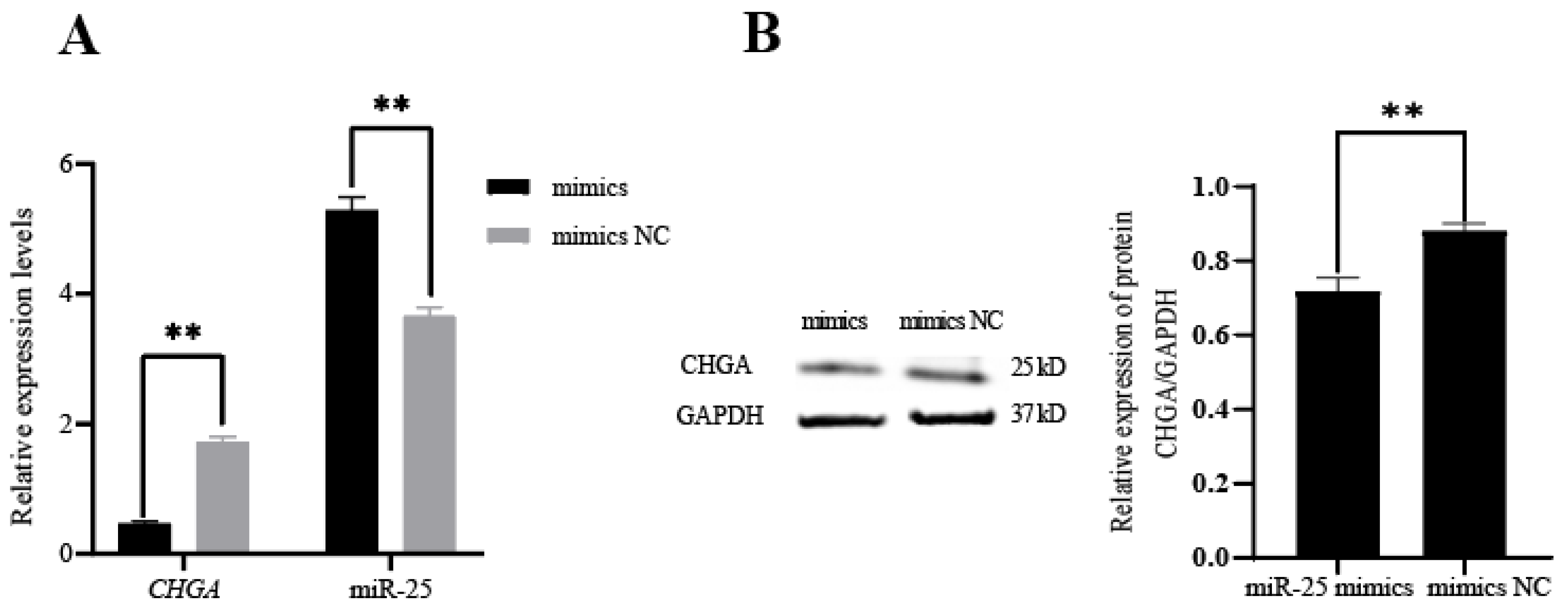
2.2. Identification of the Targeting Relationship between miR-25 and CHGA
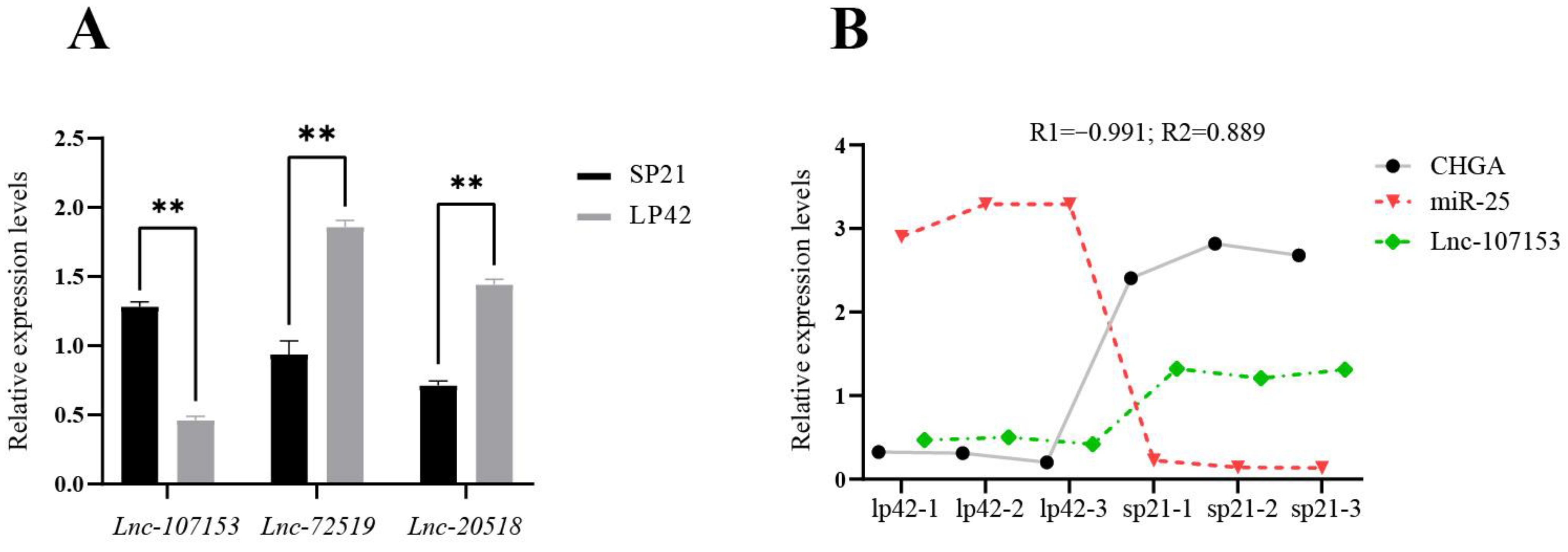
2.3. Screening and Identification of LncRNA Targeted by miR-25
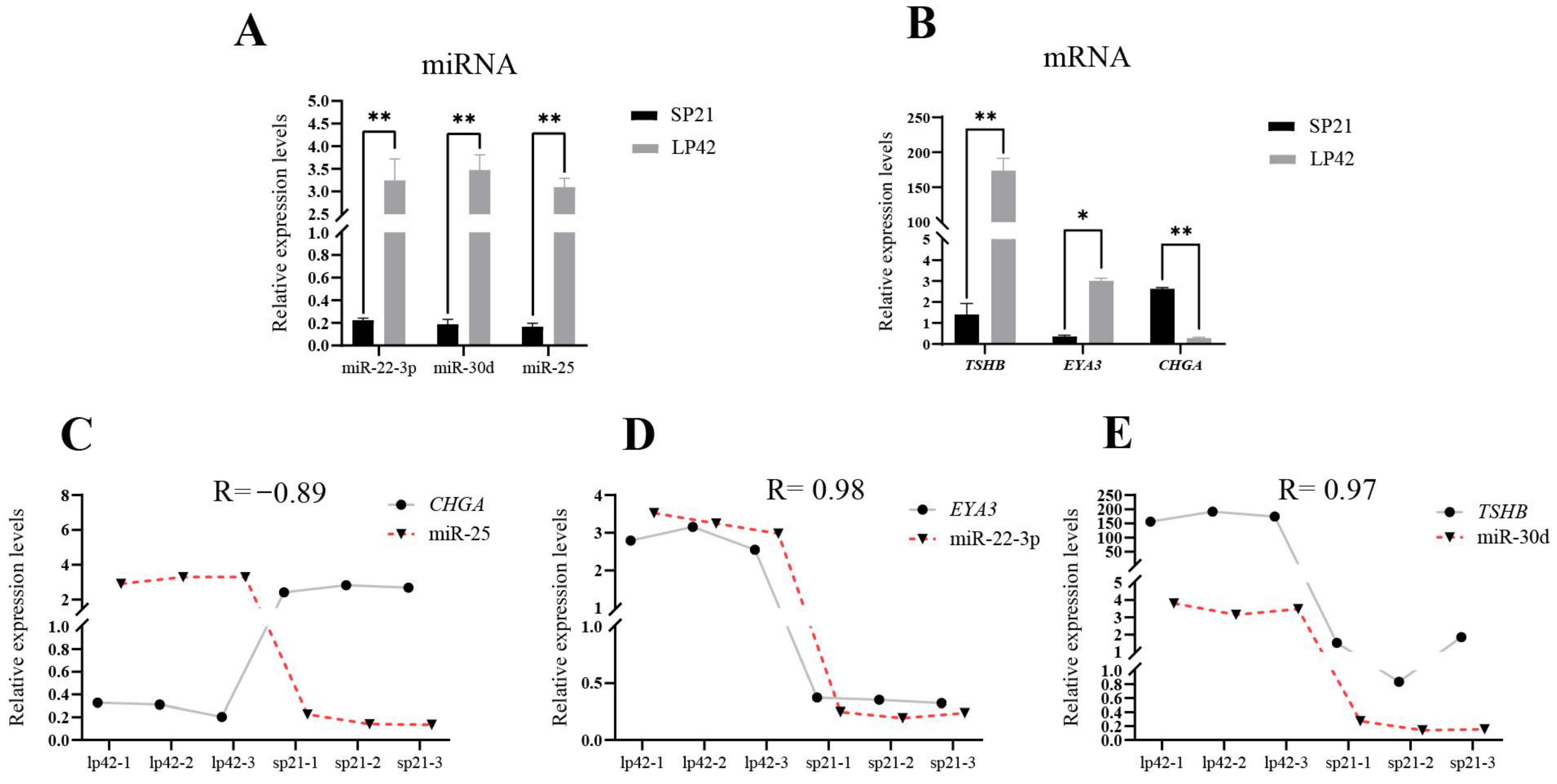
3. Results
3.1. Prediction of the Targeting Relationship between the Key Genes and miRNA in Sheep PT
3.2. Identification of the Targeting Relationship between miR-25 and CHGA
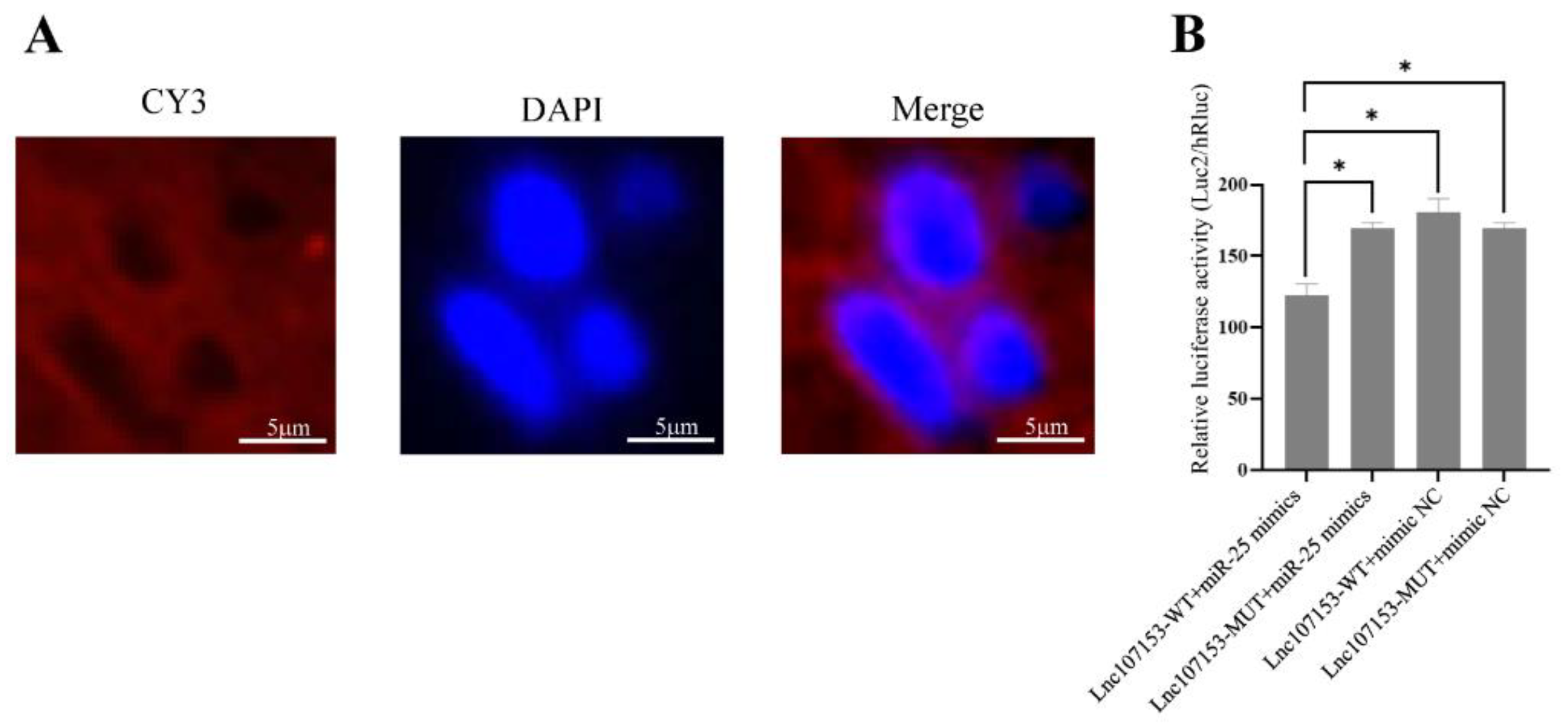
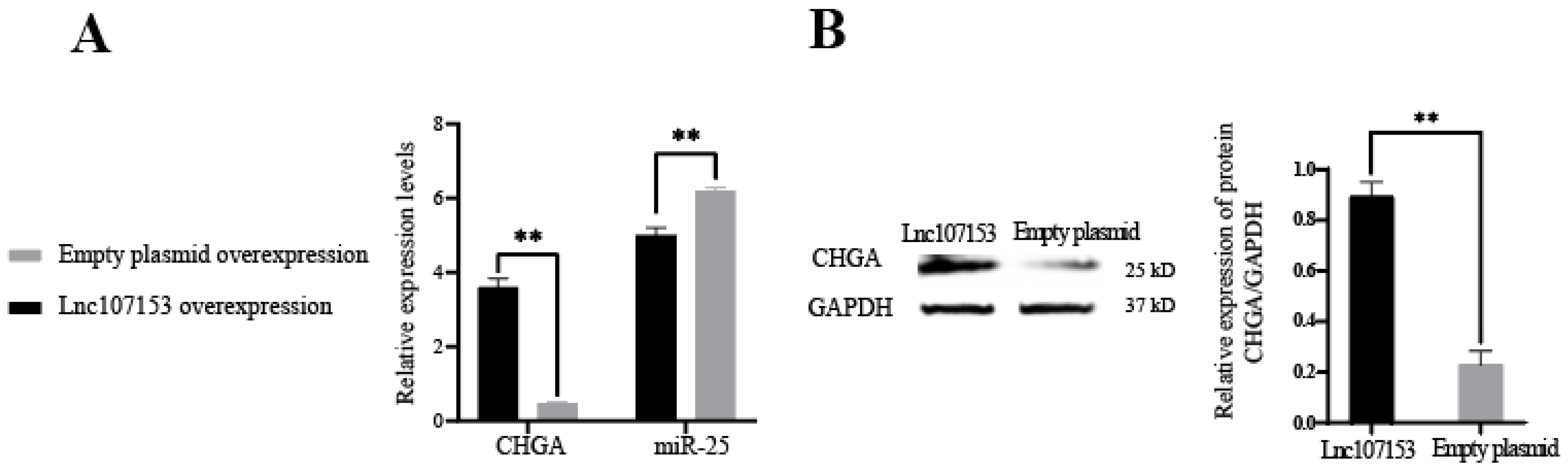
3.3. Screening and Identification of LncRNA Targeted by miR-25
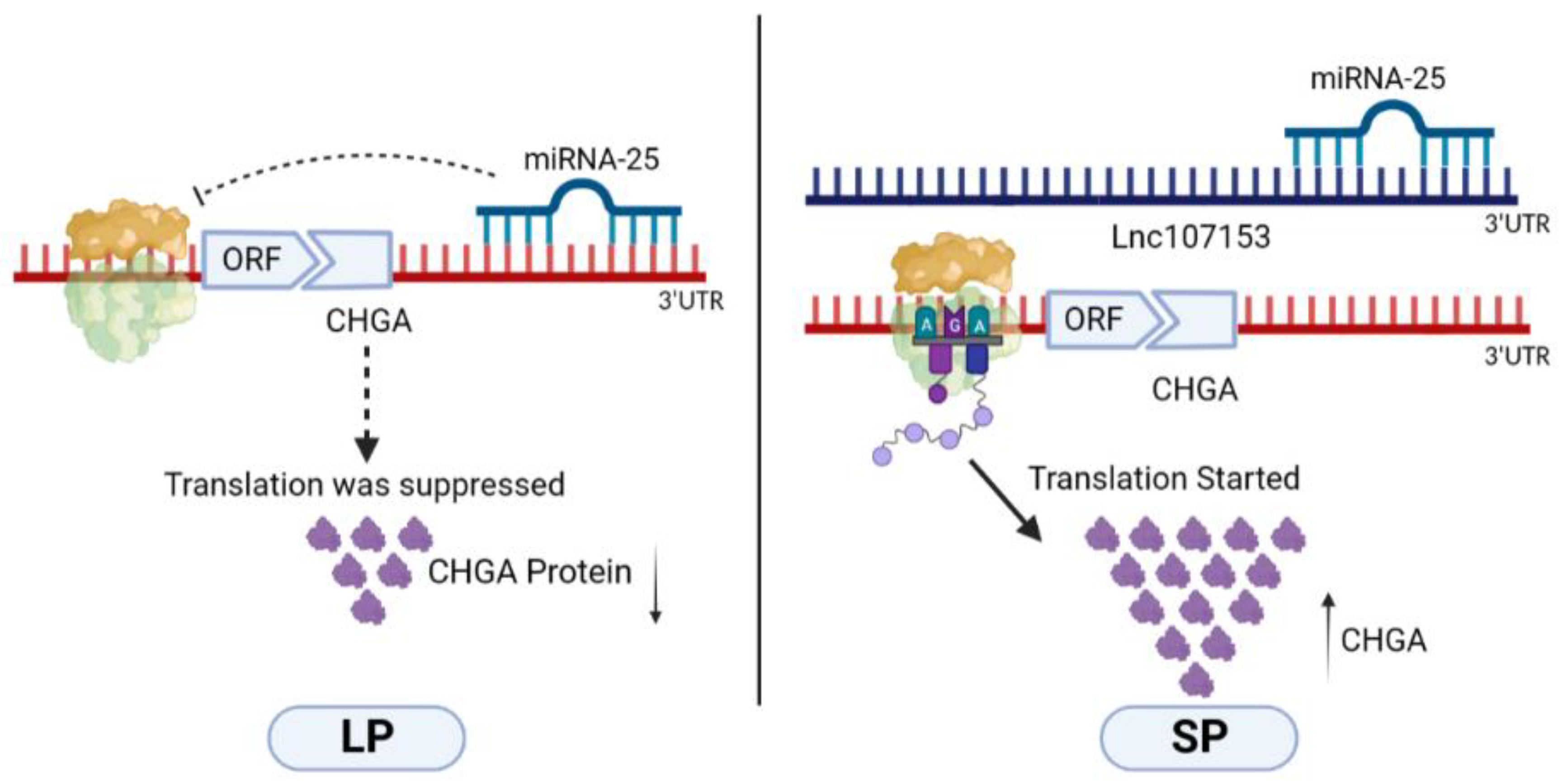
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebling, F.J.; Foster, D.L. Photoperiod requirements for puberty differ from those for the onset of the adult breeding season in female sheep. J. Reprod. Fertil. 1988, 84, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Casellas, J.; Id-Lahoucine, S.; Martin de Hijas-Villalba, M. Analysis of lambing distribution in the Ripollesa sheep breed. II. Environmental and genetic sources of variation. Animal 2019, 13, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Notter, D.R. Genetic improvement of reproductive efficiency of sheep and goats. Anim. Reprod. Sci. 2012, 130, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H. Melatonin-dependent timing of seasonal reproduction by the pars tuberalis: Pivotal roles for long daylengths and thyroid hormones. J. Neuroendocrinol. 2012, 24, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Wyse, C.A.; Birnie, M.J.; Dupre, S.M.; Loudon, A.S.; Lincoln, G.A.; Hazlerigg, D.G. A molecular switch for photoperiod responsiveness in mammals. Curr. Biol. 2010, 20, 2193–2198. [Google Scholar] [CrossRef]
- Dupre, S.M.; Miedzinska, K.; Duval, C.V.; Yu, L.; Goodman, R.L.; Lincoln, G.A.; Davis, J.R.; McNeilly, A.S.; Burt, D.D.; Loudon, A.S. Identification of Eya3 and TAC1 as long-day signals in the sheep pituitary. Curr. Biol. 2010, 20, 829–835. [Google Scholar] [CrossRef]
- Hanon, E.A.; Lincoln, G.A.; Fustin, J.M.; Dardente, H.; Masson-Pevet, M.; Morgan, P.J.; Hazlerigg, D.G. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 2008, 18, 1147–1152. [Google Scholar] [CrossRef]
- Lomet, D.; Cognie, J.; Chesneau, D.; Dubois, E.; Hazlerigg, D.; Dardente, H. The impact of thyroid hormone in seasonal breeding has a restricted transcriptional signature. Cell Mol. Life Sci. 2018, 75, 905–919. [Google Scholar] [CrossRef]
- Wood, S.H.; Christian, H.C.; Miedzinska, K.; Saer, B.R.; Johnson, M.; Paton, B.; Yu, L.; McNeilly, J.; Davis, J.R.; McNeilly, A.S.; et al. Binary Switching of Calendar Cells in the Pituitary Defines the Phase of the Circannual Cycle in Mammals. Curr. Biol. 2015, 25, 2651–2662. [Google Scholar] [CrossRef]
- Borah, B.K.; Renthlei, Z.; Tripathi, A.; Trivedi, A.K. Molecular and epigenetic regulation of seasonal reproduction in Terai tree frog (Polypedates teraiensis). Photochem. Photobiol. Sci. 2022, 21, 1067–1076. [Google Scholar] [CrossRef]
- He, X.; Liu, Q.; Li, X.; Guo, X.; Wang, X.; Hu, W.; Di, R.; Chu, M. Molecular cloning and epigenetic change detection of Kiss1 during seasonal reproduction in Chinese indigenous sheep. Reprod. Fertil. Dev. 2018, 30, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.J. Circannual and circadian rhythms of hypothalamic DNA methyltransferase and histone deacetylase expression in male Siberian hamsters (Phodopus sungorus). Gen. Comp. Endocrinol. 2017, 243, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.J.; Prendergast, B.J. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc. Natl. Acad. Sci. USA 2013, 110, 16651–16656. [Google Scholar] [CrossRef]
- Xia, Q.; Chu, M.; He, X.; Liu, Q.; Zhang, X.; Zhang, J.; Guo, X.; Di, R. Identification of Photoperiod-Induced LncRNAs and mRNAs in Pituitary Pars Tuberalis of Sheep. Front. Vet. Sci. 2021, 8, 644474. [Google Scholar] [CrossRef]
- Lindner, M.; Laine, V.N.; Verhagen, I.; Viitaniemi, H.M.; Visser, M.E.; van Oers, K.; Husby, A. Rapid changes in DNA methylation associated with the initiation of reproduction in a small songbird. Mol. Ecol. 2021, 30, 3645–3659. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Wei, S.; Bradford, K.J. Delay of germination1 (DOG1) regulates both seed dormancy and flowering time through microRNA pathways. Proc. Natl. Acad. Sci. USA 2016, 113, E2199–E2206. [Google Scholar] [CrossRef]
- Longpre, K.M.; Kinstlinger, N.S.; Mead, E.A.; Wang, Y.; Thekkumthala, A.P.; Carreno, K.A.; Hot, A.; Keefer, J.M.; Tully, L.; Katz, L.S.; et al. Seasonal variation of urinary microRNA expression in male goats (Capra hircus) as assessed by next generation sequencing. Gen. Comp. Endocrinol. 2014, 199, 1–15. [Google Scholar] [CrossRef]
- Yang, H.; Fu, L.; Luo, Q.; Li, L.; Zheng, F.; Liu, X.; Zhao, Z.; Wang, Z.; Xu, H. Comparative analysis and identification of differentially expressed micrornas in the hypothalamus of Kazakh sheep exposed to different photoperiod conditions. Biochemistry 2021, 86, 1315–1325. [Google Scholar] [CrossRef]
- He, X.Y.; Di, R.; Guo, X.F.; Cao, X.H.; Zhou, M.; Li, X.Y.; Xia, Q.; Wang, X.Y.; Zhang, J.L.; Zhang, X.S.; et al. Transcriptomic Changes of Photoperiodic Response in the Hypothalamus Were Identified in Ovariectomized and Estradiol-Treated Sheep. Front. Mol. Biosci. 2022, 9, 848144. [Google Scholar] [CrossRef]
- He, X.Y.; Tao, L.; Zhong, Y.J.; Di, R.; Xia, Q.; Wang, X.Y.; Guo, X.F.; Gan, S.Q.; Zhang, X.S.; Zhang, J.L.; et al. Photoperiod induced the pituitary differential regulation of lncRNAs and mRNAs related to reproduction in sheep. PeerJ 2021, 9, e10953. [Google Scholar] [CrossRef]
- Karsch, F.J.; Bittman, E.L.; Foster, D.L.; Goodman, R.L.; Legan, S.J.; Robinson, J.E. Neuroendocrine basis of seasonal reproduction. Recent Prog. Horm. Res. 1984, 40, 185–232. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Clay, C.M.; Caraty, A.; Clarke, I.J. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 2007, 148, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.R.; Davalos, A.; Kiltschewskij, D.; Crespo, M.C.; Cairns, M.; Andres-Leon, E.; Soler-Rivas, C. RNA-Seq, Bioinformatic Identification of Potential MicroRNA-like Small RNAs in the Edible Mushroom Agaricus bisporus and Experimental Approach for Their Validation. Int. J. Mol. Sci. 2022, 23, 4923. [Google Scholar] [CrossRef]
- de Reviers, M.M.; Tillet, Y.; Pelletier, J. Melatonin binding sites in the brain of sheep exposed to light or pinealectomized. Neurosci. Lett. 1991, 121, 17–20. [Google Scholar] [CrossRef]
- Misztal, T.; Romanowicz, K.; Barcikowski, B. Melatonin—A modulator of the GnRH/LH axis in sheep. Reprod. Biol. 2002, 2, 267–275. [Google Scholar]
- Ortavant, R.; Bocquier, F.; Pelletier, J.; Ravault, J.P.; Thimonier, J.; Volland-Nail, P. Seasonality of reproduction in sheep and its control by photoperiod. Aust. J. Biol. Sci. 1988, 41, 69–85. [Google Scholar] [CrossRef]
- Woodfill, C.J.; Wayne, N.L.; Moenter, S.M.; Karsch, F.J. Photoperiodic synchronization of a circannual reproductive rhythm in sheep: Identification of season-specific time cues. Biol. Reprod. 1994, 50, 965–976. [Google Scholar] [CrossRef]
- Saenz de Miera, C.; Monecke, S.; Bartzen-Sprauer, J.; Laran-Chich, M.P.; Pevet, P.; Hazlerigg, D.G.; Simonneaux, V. A circannual clock drives expression of genes central for seasonal reproduction. Curr. Biol. 2014, 24, 1500–1506. [Google Scholar] [CrossRef]
- Wood, S.H.; Hindle, M.M.; Mizoro, Y.; Cheng, Y.; Saer, B.R.C.; Miedzinska, K.; Christian, H.C.; Begley, N.; McNeilly, J.; McNeilly, A.S.; et al. Circadian clock mechanism driving mammalian photoperiodism. Nat. Commun. 2020, 11, 4291. [Google Scholar] [CrossRef]
- Saenz de Miera, C.; Hanon, E.A.; Dardente, H.; Birnie, M.; Simonneaux, V.; Lincoln, G.A.; Hazlerigg, D.G. Circannual variation in thyroid hormone deiodinases in a short-day breeder. J. Neuroendocrinol. 2013, 25, 412–421. [Google Scholar] [CrossRef]
- Nakao, N.; Ono, H.; Yamamura, T.; Anraku, T.; Takagi, T.; Higashi, K.; Yasuo, S.; Katou, Y.; Kageyama, S.; Uno, Y.; et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 2008, 452, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Haugg, E.; Borner, J.; Diedrich, V.; Herwig, A. Comparative transcriptomics of the Djungarian hamster hypothalamus during short photoperiod acclimation and spontaneous torpor. FEBS Open Bio. 2022, 12, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Hazlerigg, D.G.; Ebling, F.J. Thyroid hormone and seasonal rhythmicity. Front. Endocrinol. 2014, 5, 19. [Google Scholar] [CrossRef]
- Li, R.; Zhao, J.; Huang, L.; Yi, Y.; Li, A.; Li, D.; Tao, M.; Liu, Y. Antimicrobial peptide CGA-N12 decreases the Candida tropicalis mitochondrial membrane potential via mitochondrial permeability transition pore. Biosci. Rep. 2020, 40, BSR20201007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Mir, S.A.; Hightower, C.M.; Miramontes-Gonzalez, J.P.; Maihofer, A.X.; Chen, Y.; Mahata, S.K.; Nievergelt, C.M.; Schork, N.J.; Freedman, B.I.; et al. Molecular Mechanism for Hypertensive Renal Disease: Differential Regulation of Chromogranin A Expression at 3′-Untranslated Region Polymorphism C+87T by MicroRNA-107. J. Am. Soc. Nephrol. 2015, 26, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xu, Y.; Chen, Y.; Lv, H.; Chen, G.; Chen, Y. Construction of miRNA-mRNA-TF Regulatory Network for Diagnosis of Gastric Cancer. Biomed. Res. Int. 2021, 2021, 9121478. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zheng, X.; Zhang, L.; Ai, X.; Venkataramani, V.; Kilic, E.; Hermann, D.M.; Majid, A.; Bahr, M.; Doeppner, T.R. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J. Extracell. Vesicles. 2020, 10, e12024. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, R.; Li, M.; Ye, H.; Wu, C.; Wang, C.; Li, S.; Tan, L.; Mai, D.; Li, G.; et al. Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat. Commun. 2019, 10, 1858. [Google Scholar] [CrossRef]
- Chang, Z.; Huang, R.; Fu, W.; Li, J.; Ji, G.; Huang, J.; Shi, W.; Yin, H.; Wang, W.; Meng, T.; et al. The Construction and Analysis of ceRNA Network and Patterns of Immune Infiltration in Colon Adenocarcinoma Metastasis. Front. Cell Dev. Biol. 2020, 8, 688. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Bahrami, A.; Hasankhani, A.; Kioumarsi, H.; Nouralizadeh, R.; Abdulkareem, S.A.; Ghafouri, F.; Barkema, H.W. lncRNA-miRNA-mRNA ceRNA Network Involved in Sheep Prolificacy: An Integrated Approach. Genes 2022, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, F.; Han, G. LncRNA PSMB8-AS1 acts as ceRNA of miR-22-3p to regulate DDIT4 expression in glioblastoma. Neurosci. Lett. 2020, 728, 134896. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ye, Z.; Zhao, X.; Ji, Z. SP1-induced up-regulation of lncRNA SNHG14 as a ceRNA promotes migration and invasion of clear cell renal cell carcinoma by regulating N-WASP. Am. J. Cancer Res. 2017, 7, 2515–2525. [Google Scholar]
- Yao, X.; Gao, X.; Bao, Y.; El-Samahy, M.A.; Yang, J.; Wang, Z.; Li, X.; Zhang, G.; Zhang, Y.; Liu, W.; et al. lncRNA FDNCR promotes apoptosis of granulosa cells by targeting the miR-543-3p/DCN/TGF-beta signaling pathway in Hu sheep. Mol. Ther. Nucleic. Acids. 2021, 24, 223–240. [Google Scholar] [CrossRef]
| Genes/LncRNAs | Primer Sequences (5′-3′) | Length (bp) | Amplification Efficiency |
|---|---|---|---|
| EYA3 | F: ACCCTTCTCCAAGCCCATC | 232 | 97% |
| R: ACTGTTGGGTCCTTTCCATACTT | |||
| TSHβ | F: CCTAACCATCAACACCACCAT | 211 | 98% |
| R: ATTACACTTGCCACACTTACAGC | |||
| CHGA | F: GAATAAAGGGGACACTGAGGTGAT | 112 | 96% |
| R: TCCTCGGAGCGTCTCAAAAC | |||
| RPL19 | F: TCTAAGAAGATTGACCGCCACAT | 240 | 99% |
| R: CTCCTTGGACAGAGTCTTGATGATT | |||
| Lnc107153 | F: AATATGGGTCTGATGGTGGTAGC | 187 | 98% |
| R: TGCCTTTGCCATATAACGTAGC | |||
| Lnc72519 | F: ATGAAGTGATGGGACCAGATG | 149 | 97% |
| R: GATGAAGCACAAGCTGGAATC | |||
| Lnc20518 | F: CCACAAGGGAAGCCCATAGT | 244 | 97% |
| R: AGGTGACTTCCAAATCTTGGCT |
| miRNAs | Mature Sequences (5′-3′) | Forward Primer Sequences (5′-3″) |
|---|---|---|
| miR-25 | AUUGCACUUGUCUCGGUCUGA | F: GATTGCACTTGTCTCGGT |
| miR-30d | UGUAAACAUCCCCGACUGG | F: GCAGTGTAAACATCCCCGA |
| miR-22-3p | AAGCUGCCAGUUGAAGAACUG | F: GAAGCTGCCAGTTGAAGA |
| u6 | - | F: CTCGCTTCGGCAGCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, R.; Fan, Y.; He, X.; Liu, Q.; Wang, X.; Gong, Y.; Mwacharo, J.M.; Wei, C.; Liu, Y.; Chu, M. Epigenetic Regulation of miR-25 and Lnc107153 on Expression of Seasonal Estrus Key Gene CHGA in Sheep. Biology 2023, 12, 250. https://doi.org/10.3390/biology12020250
Di R, Fan Y, He X, Liu Q, Wang X, Gong Y, Mwacharo JM, Wei C, Liu Y, Chu M. Epigenetic Regulation of miR-25 and Lnc107153 on Expression of Seasonal Estrus Key Gene CHGA in Sheep. Biology. 2023; 12(2):250. https://doi.org/10.3390/biology12020250
Chicago/Turabian StyleDi, Ran, Yekai Fan, Xiaoyun He, Qiuyue Liu, Xiangyu Wang, Yiming Gong, Joram Mwashigadi Mwacharo, Caihong Wei, Yufang Liu, and Mingxing Chu. 2023. "Epigenetic Regulation of miR-25 and Lnc107153 on Expression of Seasonal Estrus Key Gene CHGA in Sheep" Biology 12, no. 2: 250. https://doi.org/10.3390/biology12020250
APA StyleDi, R., Fan, Y., He, X., Liu, Q., Wang, X., Gong, Y., Mwacharo, J. M., Wei, C., Liu, Y., & Chu, M. (2023). Epigenetic Regulation of miR-25 and Lnc107153 on Expression of Seasonal Estrus Key Gene CHGA in Sheep. Biology, 12(2), 250. https://doi.org/10.3390/biology12020250








