Tau; One Protein, So Many Diseases
Abstract
Simple Summary
Abstract
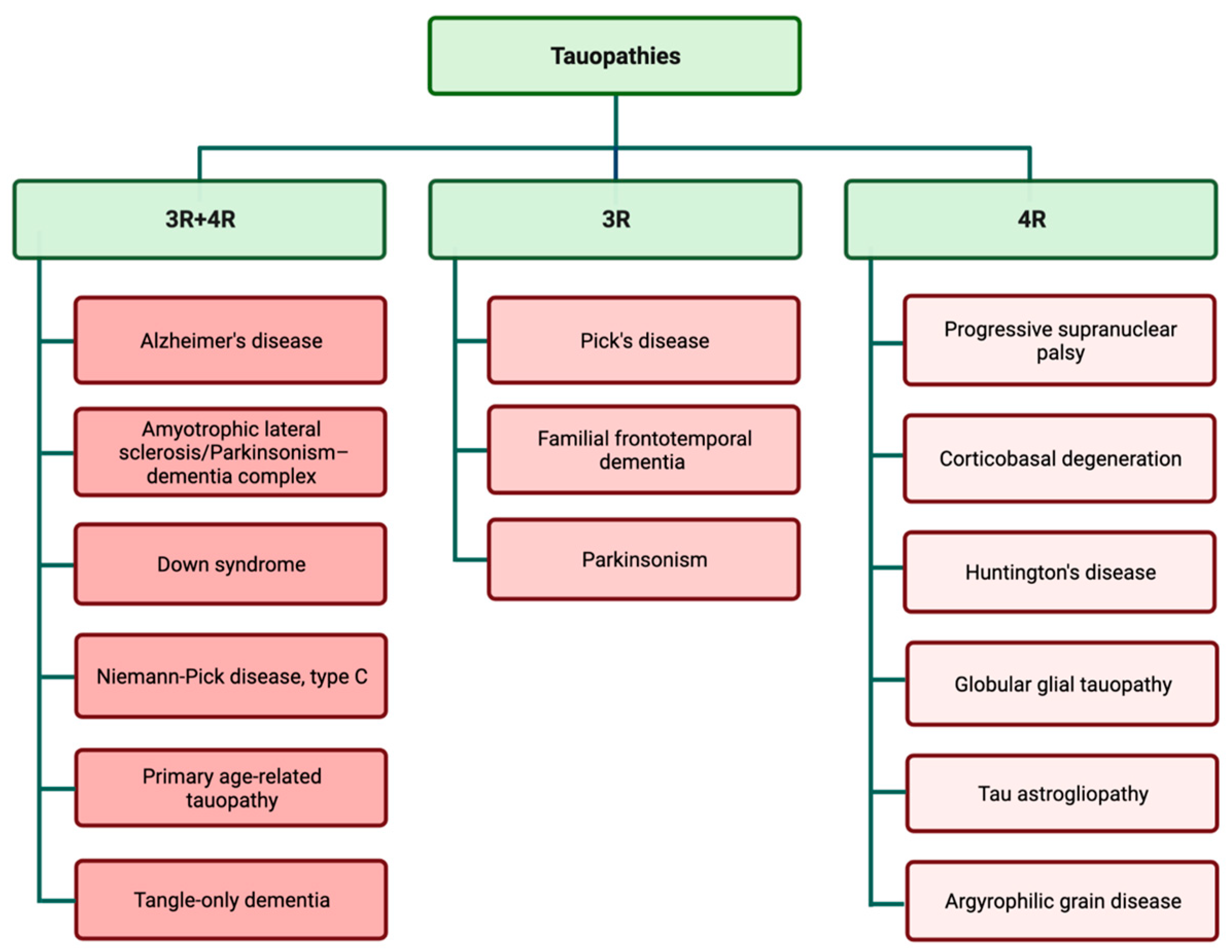
1. Tauopathies: Types and Importance
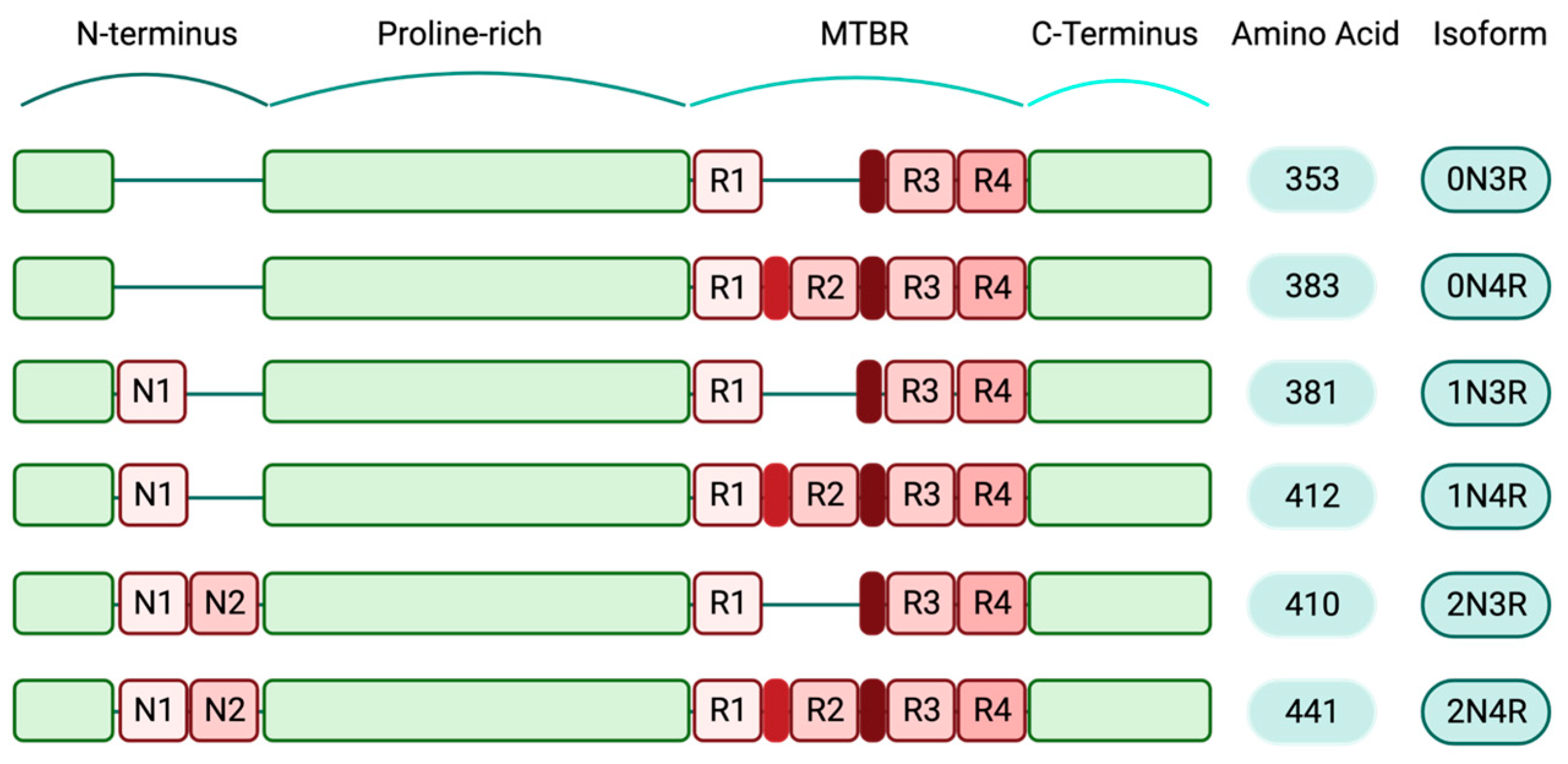
2. Tau
3. Physiologic Tau
4. Pathologic Tau
5. One Protein and Various Conformers
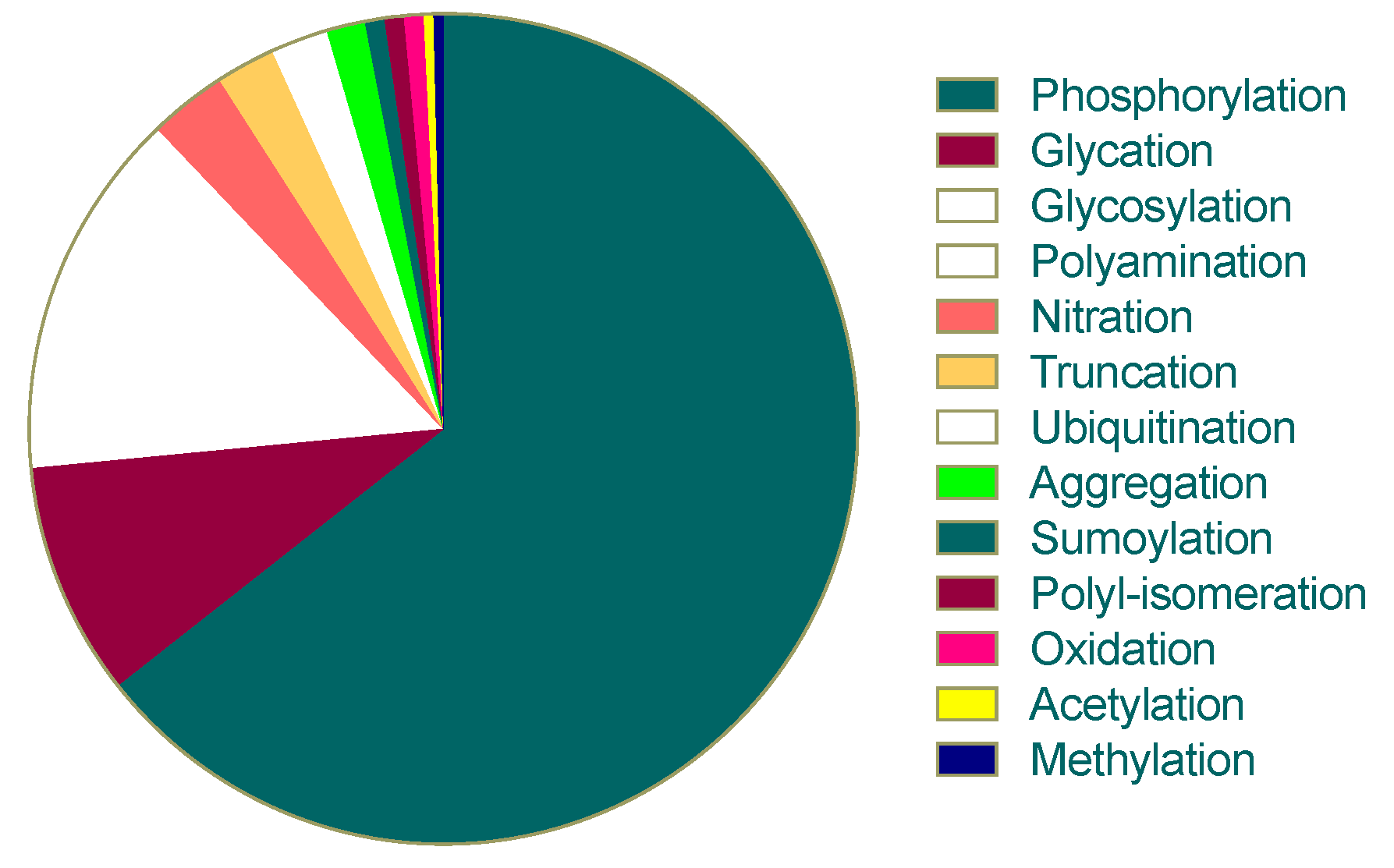
6. Abnormal Post-Translational Modifications of Tau
7. Inducers of Pathologic Tau, Upstream Mechanisms
8. Pathologic Tau and Cell Toxicity, Downstream Events of Tau Dysfunction
9. Diagnosis and Therapeutic Strategies for Tauopathies
10. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sexton, C.; Snyder, H.; Beher, D.; Boxer, A.L.; Brannelly, P.; Brion, J.P.; Buée, L.; Cacace, A.M.; Chételat, G.; Citron, M.; et al. Current Directions in Tau Research: Highlights from Tau 2020. Alzheimer’s Dement. 2021, 18, 988–1007. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Ruiz, A.; Sánchez-Juan, P. Genetic Architecture of Primary Tauopathies. Neuroscience, 2022; in press. [Google Scholar] [CrossRef]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Dirk Keene, C.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.P.; Stewart, W.; et al. The First NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dementia. Available online: https://www.who.int/health-topics/dementia#tab=tab_2 (accessed on 27 August 2022).
- Sjölin, K.; Kultima, K.; Larsson, A.; Freyhult, E.; Zjukovskaja, C.; Alkass, K.; Burman, J. Distribution of Five Clinically Important Neuroglial Proteins in the Human Brain. Mol. Brain 2022, 15, 52. [Google Scholar] [CrossRef]
- Blennow, K.; Wallin, A.; Agren, H.; Spenger, C.; Siegfried, J.; Vanmechelen, E. Tau Protein in Cerebrospinal Fluid: A Biochemical Marker for Axonal Degeneration in Alzheimer Disease? Mol. Chem. Neuropathol. 1995, 26, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and Tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.L.; Harris, P.; Kosik, K.S.; Kurnit, D.M.; Donlon, T.A. Identification of CDNA Clones for the Human Microtubule-Associated Protein Tau and Chromosomal Localization of the Genes for Tau and Microtubule-Associated Protein 2. Brain Res. 1986, 387, 271–280. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.; Jakes, R.; Rutherford, D. Multiple Isoforms of Human Microtubule-Associated Protein Tau: Sequences and Localization in Neurofibrillary Tangles of Alzheimer’s Disease. Neuron 1989, 3, 519–526. [Google Scholar] [CrossRef]
- Hefti, M.M.; Farrell, K.; Kim, S.H.; Bowles, K.R.; Fowkes, M.E.; Raj, T.; Crary, J.F. High-Resolution Temporal and Regional Mapping of MAPT Expression and Splicing in Human Brain Development. PLoS ONE 2018, 13, e0195771. [Google Scholar] [CrossRef]
- Hanger, D.; Anderton, B.; Medicine, W.N.-T. Tau Phosphorylation: The Therapeutic Challenge for Neurodegenerative Disease. Trends Mol. Biol. 2009, 15, 112–119. [Google Scholar] [CrossRef]
- Hirokawa, N.; Takemura, R. Molecular Motors and Mechanisms of Directional Transport in Neurons. Nat. Rev. Neurosci. 2005, 6, 201–214. [Google Scholar] [CrossRef]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.; Lee, G. The Balance Between τ Protein’s Microtubule Growth and Nucleation Activities: Implications for the Formation of Axonal Microtubules. J. Neurochem. 1993, 61, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Drubin, D.; Kirschner, M.W. Tau Protein Function in Living Cells. J. Cell Biol. 1986, 103, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Run, X.; Liang, Z.; Li, Y.; Liu, F.; Liu, Y.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.X. Developmental Regulation of Tau Phosphorylation, Tau Kinases, and Tau Phosphatases. J. Neurochem. 2009, 108, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Moraga, D.M.; Nuñez, P.; Garrido, J.; Maccioni, R.B. A τ Fragment Containing a Repetitive Sequence Induces Bundling of Actin Filaments. J. Neurochem. 1993, 61, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Elie, A.; Prezel, E.; Guérin, C.; Denarier, E.; Ramirez-Rios, S.; Serre, L.; Andrieux, A.; Fourest-Lieuvin, A.; Blanchoin, L.; Arnal, I. Tau Co-Organizes Dynamic Microtubule and Actin Networks. Sci. Rep. 2015, 5, 9967. [Google Scholar] [CrossRef] [PubMed]
- Correas, I.; Padilla, R.; Journal, J.A.-B. The Tubulin-Binding Sequence of Brain Microtubule-Associated Proteins, Tau and MAP-2, Is Also Involved in Actin Binding. Biochem. J. 1990, 269, 61–64. [Google Scholar] [CrossRef]
- Frandemiche, M.L.; De Seranno, S.; Rush, T.; Borel, E.; Elie, A.; Arnal, I.; Lanté, F.; Buisson, A. Activity-Dependent Tau Protein Translocation to Excitatory Synapse Is Disrupted by Exposure to Amyloid-Beta Oligomers. J. Neurosci. 2014, 34, 6084–6097. [Google Scholar] [CrossRef]
- Tracy, T.E.; Sohn, P.D.; Minami, S.S.; Wang, C.; Min, S.-W.; Li, Y.; Zhou, Y.; Le, D.; Lo, I.; Ponnusamy, R.; et al. Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron 2016, 90, 245–260. [Google Scholar] [CrossRef]
- Cabrales Fontela, Y.; Kadavath, H.; Biernat, J.; Riedel, D.; Mandelkow, E.; Zweckstetter, M. Multivalent Cross-Linking of Actin Filaments and Microtubules through the Microtubule-Associated Protein Tau. Nat. Commun. 2017, 8, 1981. [Google Scholar] [CrossRef]
- Orr, M.E.; Sullivan, A.; Frost, B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.R.; Berger, F.; Berger, C.L.; Hendricks, A.G. Tau Directs Intracellular Trafficking by Regulating the Forces Exerted by Kinesin and Dynein Teams. Traffic 2018, 19, 111–121. [Google Scholar] [CrossRef]
- Cuchillo-Ibanez, I.; Seereeram, A.; Byers, H.L.; Leung, K.-Y.; Ward, M.A.; Anderton, B.H.; Hanger, D.P. Phosphorylation of Tau Regulates Its Axonal Transport by Controlling Its Binding to Kinesin. FASEB J. 2008, 22, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, M.H.; Jalal, A.; Brenner, B.; Mandelkow, E.; Kumar, S.; Scholz, T. Tau Protein Diffuses along the Microtubule Lattice. J. Biol. Chem. 2012, 287, 38559–38568. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Delerue, F.; Bi, M.; Gladbach, A.; van Eersel, J.; Wölfing, H.; Chieng, B.C.; Christie, M.J.; Napier, I.A.; et al. Dendritic Function of Tau Mediates Amyloid-β Toxicity in Alzheimer’s Disease Mouse Models. Cell 2010, 142, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Kramer, E.M.; Cardine, A.M.; Schraven, B.; Brandt, R.; Trotter, J. Process Outgrowth of Oligodendrocytes Is Promoted by Interaction of Fyn Kinase with the Cytoskeletal Protein Tau. J. Neurosci. 2002, 22, 698–707. [Google Scholar] [CrossRef]
- Sjöberg, M.K.; Shestakova, E.; Mansuroglu, Z.; Maccioni, R.B.; Bonnefoy, E. Tau Protein Binds to Pericentromeric DNA: A Putative Role for Nuclear Tau in Nucleolar Organization. J. Cell Sci. 2006, 119, 2025–2034. [Google Scholar] [CrossRef]
- Maina, M.B.; Bailey, L.J.; Wagih, S.; Biasetti, L.; Pollack, S.J.; Quinn, J.P.; Thorpe, J.R.; Doherty, A.J.; Serpell, L.C. The Involvement of Tau in Nucleolar Transcription and the Stress Response. Acta Neuropathol. Commun. 2018, 6, 70. [Google Scholar] [CrossRef]
- Mansuroglu, Z.; Benhelli-Mokrani, H.; Marcato, V.; Sultan, A.; Violet, M.; Chauderlier, A.; Delattre, L.; Loyens, A.; Talahari, S.; Bégard, S.; et al. Loss of Tau Protein Affects the Structure, Transcription and Repair of Neuronal Pericentromeric Heterochromatin. Sci. Rep. 2016, 6, 33047. [Google Scholar] [CrossRef]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English Translation of Alzheimer’s 1907 Paper, “Uber Eine Eigenartige Erkankung Der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef]
- Terry, R.D. The Fine Structure of Neurofibrillary Tangles in Alzheimer’s Disease. J. Neuropathol. Exp. Neurol. 1963, 22, 629–642. [Google Scholar] [CrossRef]
- Brion, J.P.; Passareiro, H.; Nunez, J.; Flamentdurand, J. Immunological Detection of Tau Protein in Neurofibrillary Tangles of Alzheimers-Disease. Arch. Biol. 1985, 96, 229–235. [Google Scholar]
- Schweighauser, M.; Shi, Y.; Tarutani, A.; Kametani, F.; Murzin, A.G.; Ghetti, B.; Matsubara, T.; Tomita, T.; Ando, T.; Hasegawa, K.; et al. Structures of α-Synuclein Filaments from Multiple System Atrophy. Nature 2020, 585, 464–469. [Google Scholar] [CrossRef]
- Zhang, W.; Falcon, B.; Murzin, A.G.; Fan, J.; Crowther, R.A.; Goedert, M.; Scheres, S.H. Heparin-Induced Tau Filaments Are Polymorphic and Differ from Those in Alzheimer’s and Pick’s Diseases. Elife 2019, 8, e43584. [Google Scholar] [CrossRef]
- Rösler, T.W.; Tayaranian Marvian, A.; Brendel, M.; Nykänen, N.P.; Höllerhage, M.; Schwarz, S.C.; Hopfner, F.; Koeglsperger, T.; Respondek, G.; Schweyer, K.; et al. Four-Repeat Tauopathies. Prog. Neurobiol. 2019, 180, 101644. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, J.; Jog, M. Tauopathy and Movement Disorders—Unveiling the Chameleons and Mimics. Front. Neurol. 2020, 11, 1359. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Ríos, K.L.L. Tau Oligomers and Aggregation in Alzheimer’s Disease. Wiley Online Libr. 2010, 112, 1353–1367. [Google Scholar] [CrossRef]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau Protein Isoforms, Phosphorylation and Role in Neurodegenerative Disorders. Brain Res. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Cho, J.H.; Johnson, G.V. Glycogen Synthase Kinase 3β Phosphorylates Tau at Both Primed and Unprimed Sites: Differential Impact on Microtubule Binding. J. Biol. 2003, 278, 187–193. [Google Scholar]
- Shukla, V.; Skuntz, S.; Pant, H.C. Deregulated Cdk5 Activity Is Involved in Inducing Alzheimer’s Disease. Arch. Med. Res. 2012, 43, 655–662. [Google Scholar] [CrossRef]
- Tell, V.; Hilgeroth, A. Recent Developments of Protein Kinase Inhibitors as Potential AD Therapeutics. Front. Cell. Neurosci. 2013, 6, 189. [Google Scholar] [CrossRef]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. C-Jun N-Terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front. Pharmacol. 2016, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Sontag, J.M.; Sontag, E. Protein Phosphatase 2A Dysfunction in Alzheimer’s Disease. Front. Mol. Neurosci. 2014, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Ercan-Herbst, E.; Ehrig, J.; Schöndorf, D.C.; Behrendt, A.; Klaus, B.; Gomez Ramos, B.; Prat Oriol, N.; Weber, C.; Ehrnhoefer, D.E. A Post-Translational Modification Signature Defines Changes in Soluble Tau Correlating with Oligomerization in Early Stage Alzheimer’s Disease Brain. Acta Neuropathol. Commun. 2019, 7, 192. [Google Scholar] [CrossRef]
- Min, S.-W.; Cho, S.-H.; Zhou, Y.; Schroeder, S.; Haroutunian, V.; Seeley, W.W.; Huang, E.J.; Shen, Y.; Masliah, E.; Mukherjee, C.; et al. Acetylation of Tau Inhibits Its Degradation and Contributes to Tauopathy. Neuron 2010, 67, 953–966. [Google Scholar] [CrossRef]
- Maina, M.B.; Al-Hilaly, Y.K.; Burra, G.; Rickard, J.E.; Harrington, C.R.; Wischik, C.M.; Serpell, L.C. Oxidative Stress Conditions Result in Trapping of PHF-Core Tau (297–391) Intermediates. Cells 2021, 10, 703. [Google Scholar] [CrossRef]
- Maina, M.B.; Al-Hilaly, Y.K.; Oakley, S.; Burra, G.; Khanom, T.; Biasetti, L.; Mengham, K.; Marshall, K.; Harrington, C.R.; Wischik, C.M.; et al. Dityrosine Cross-Links Are Present in Alzheimer’s Disease-Derived Tau Oligomers and Paired Helical Filaments (PHF) Which Promotes the Stability of the PHF-Core Tau (297–391) In Vitro. J. Mol. Biol. 2022, 434, 167785. [Google Scholar] [CrossRef]
- Boyko, S.; Qi, X.U.; Chen, T.H.; Surewicz, K.; Surewicz, W.K. Liquid–Liquid Phase Separation of Tau Protein: The Crucial Role of Electrostatic Interactions. J. Biol. Chem. 2019, 294, 11054–11059. [Google Scholar] [CrossRef]
- Boyko, S.; Surewicz, W.K. Tau Liquid–Liquid Phase Separation in Neurodegenerative Diseases. Trends Cell Biol. 2022, 32, 611–623. [Google Scholar] [CrossRef]
- Forrest, S.L.; Kril, J.J.; Stevens, C.H.; Kwok, J.B.; Hallupp, M.; Kim, W.S.; Huang, Y.; McGinley, C.V.; Werka, H.; Kiernan, M.C.; et al. Retiring the Term FTDP-17 as MAPT Mutations Are Genetic Forms of Sporadic Frontotemporal Tauopathies. Brain 2018, 141, 521–534. [Google Scholar] [CrossRef]
- McCarthy, A.; Lonergan, R.; Olszewska, D.A.; O’Dowd, S.; Cummins, G.; Magennis, B.; Fallon, E.M.; Pender, N.; Huey, E.D.; Cosentino, S.; et al. Closing the Tau Loop: The Missing Tau Mutation. Brain 2015, 138, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, H.; Xie, H.; Jia, Z.; Chen, Q. Molecular Imaging Biomarkers in Familial Frontotemporal Lobar Degeneration: Progress and Prospects. Front. Neurol. 2022, 13, 933217. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Falcon, B.; Zhang, W.; Ghetti, B.; Scheres, S.H.W. Distinct Conformers of Assembled Tau in Alzheimer’s and Pick’s Diseases. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 163–171. [Google Scholar] [CrossRef]
- Crary, J.F. Primary Age-Related Tauopathy and the Amyloid Cascade Hypothesis: The Exception That Proves the Rule? J. Neurol. Neuromed. 2016, 1, 53–57. [Google Scholar] [CrossRef]
- Kim, B.; Sullivan, K.A.; Backus, C.; Feldman, E.L. Cortical Neurons Develop Insulin Resistance and Blunted Akt Signaling: A Potential Mechanism Contributing to Enhanced Ischemic Injury in Diabetes. Antioxid. Redox Signal. 2011, 14, 1829–1839. [Google Scholar] [CrossRef]
- Liu, L.; Drouet, V.; Wu, J.W.; Witter, M.P.; Small, S.A.; Clelland, C.; Duff, K. Trans-Synaptic Spread of Tau Pathology In Vivo. PLoS ONE 2012, 7, e31302. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.; Dujardin, S.; Schraen-Maschke, S.; Meno-Tetang, G.; Duyckaerts, C.; Courade, J.-P.; Buée, L. From the Prion-like Propagation Hypothesis to Therapeutic Strategies of Anti-Tau Immunotherapy. Acta Neuropathol. 2020, 139, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of Microglia and Inhibition of Exosome Synthesis Halt Tau Propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Bégard, S.; Caillierez, R.; Lachaud, C.; Delattre, L.; Carrier, S.; Loyens, A.; Galas, M.-C.; Bousset, L.; Melki, R.; et al. Ectosomes: A New Mechanism for Non-Exosomal Secretion of Tau Protein. PLoS ONE 2014, 9, e100760. [Google Scholar] [CrossRef]
- Switon, K.; Kotulska, K.; Janusz-Kaminska, A.; Zmorzynska, J.; Jaworski, J. Molecular Neurobiology of MTOR. Neuroscience 2017, 341, 112–153. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Magrì, A.; Medina, D.X.; Wisely, E.V.; López-Aranda, M.F.; Silva, A.J.; Oddo, S. MTOR Regulates Tau Phosphorylation and Degradation: Implications for Alzheimer’s Disease and Other Tauopathies. Aging Cell 2013, 12, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Zempel, H.; Luedtke, J.; Kumar, Y.; Biernat, J.; Dawson, H.; Mandelkow, E.; Mandelkow, E.M. Amyloid-β Oligomers Induce Synaptic Damage via Tau-Dependent Microtubule Severing by TTLL6 and Spastin. EMBO J. 2013, 32, 2920–2937. [Google Scholar] [CrossRef] [PubMed]
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.L.; et al. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 2010, 68, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Huntley, M.A.; De Mazière, A.; Meilandt, W.J.; Wu, T.; Srinivasan, K.; Jiang, Z.; Gandham, V.; Friedman, B.A.; Ngu, H.; et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 2018, 100, 1322–1336.e7. [Google Scholar] [CrossRef]
- Xu, J.; King, S.J.; Lapierre-Landry, M.; Nemec, B. Interplay between Velocity and Travel Distance of Kinesin-Based Transport in the Presence of Tau. Biophys. J. 2013, 105, L23–L25. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.D.; Götz, J. Phosphorylated Tau Interacts with C-Jun N-Terminal Kinase-Interacting Protein 1 (JIP1) in Alzheimer Disease. J. Biol. Chem. 2009, 284, 20909–20916. [Google Scholar] [CrossRef]
- Brelstaff, J.; Tolkovsky, A.M.; Ghetti, B.; Goedert, M.; Spillantini, M.G. Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep. 2018, 24, 1939–1948.e4. [Google Scholar] [CrossRef]
- Frost, B.; Hemberg, M.; Lewis, J.; Feany, M.B. Tau Promotes Neurodegeneration through Global Chromatin Relaxation. Nat. Neurosci. 2014, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Min, W. Thioredoxin Promotes ASK1 Ubiquitination and Degradation to Inhibit ASK1-Mediated Apoptosis in a Redox Activity-Independent Manner. Circ. Res. 2002, 90, 1259–1266. [Google Scholar] [CrossRef]
- Bishopric, N.H.; Webster, K.A. Preventing Apoptosis with Thioredoxin: ASK Me How. Circ. Res. 2002, 90, 1237–1239. [Google Scholar] [CrossRef]
- Paonessa, F.; Evans, L.D.; Solanki, R.; Larrieu, D.; Wray, S.; Hardy, J.; Jackson, S.P.; Livesey, F.J. Microtubules Deform the Nuclear Membrane and Disrupt Nucleocytoplasmic Transport in Tau-Mediated Frontotemporal Dementia. Cell Rep. 2019, 26, 582–593.e5. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Daigle, J.G.; Kapinos, L.E.; Coyne, A.; Schiantarelli, J.; Carlomagno, Y.; Cook, C.; Miller, S.J.; Dujardin, S.; Amaral, A.S.; et al. Tau Protein Disrupts Nucleocytoplasmic Transport in Alzheimer’s Disease. Neuron 2018, 99, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Munnamalai, V.; Suter, D.M. Reactive Oxygen Species Regulate F-Actin Dynamics in Neuronal Growth Cones and Neurite Outgrowth. J. Neurochem. 2009, 108, 644. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Götz, J.; Feany, M.B. Connecting the Dots between Tau Dysfunction and Neurodegeneration. Trends Cell Biol. 2015, 25, 46–53. [Google Scholar] [CrossRef]
- Ramirez, P.; Zuniga, G.; Sun, W.; Beckmann, A.; Ochoa, E.; DeVos, S.L.; Hyman, B.; Chiu, G.; Roy, E.R.; Cao, W.; et al. Pathogenic Tau Accelerates Aging-Associated Activation of Transposable Elements in the Mouse Central Nervous System. Prog. Neurobiol. 2022, 208, 102181. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Jeong, H.-H.; Hsieh, Y.-C.; Klein, H.-U.; Bennett, D.A.; De Jager, P.L.; Liu, Z.; Shulman, J.M. Tau Activates Transposable Elements in Alzheimer’s Disease. Cell Rep. 2018, 23, 2874–2880. [Google Scholar] [CrossRef]
- Sun, W.; Samimi, H.; Gamez, M.; Zare, H.; Frost, B. Pathogenic Tau-Induced PiRNA Depletion Promotes Neuronal Death through Transposable Element Dysregulation in Neurodegenerative Tauopathies. Nat. Neurosci. 2018, 21, 1038–1048. [Google Scholar] [CrossRef]
- Saint-Aubert, L.; Lemoine, L.; Chiotis, K.; Leuzy, A.; Rodriguez-Vieitez, E.; Nordberg, A. Tau PET Imaging: Present and Future Directions. Mol. Neurodegener. 2017, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Manser, P.T.; Sanabria Bohorquez, S.; Wildsmith, K.R.; Pickthorn, K.; Baker, S.L.; Ward, M.; Kerchner, G.A.; Weimer, R.M. Baseline [18F]GTP1 Tau PET Imaging Is Associated with Subsequent Cognitive Decline in Alzheimer’s Disease. Alzheimers. Res. Ther. 2021, 13, 196. [Google Scholar] [CrossRef]
- Lantero-Rodriguez, J.; Snellman, A.; Benedet, A.L.; Milà-Alomà, M.; Camporesi, E.; Montoliu-Gaya, L.; Ashton, N.J.; Vrillon, A.; Karikari, T.K.; Gispert, J.D.; et al. P-tau235: A Novel Biomarker for Staging Preclinical Alzheimer’s Disease. EMBO Mol. Med. 2021, 13, e15098. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Bateman, R.J.; Hirtz, C.; Marin, P.; Becher, F.; Sato, C.; Gabelle, A.; Lehmann, S. Cerebrospinal Fluid Phospho-Tau T217 Outperforms T181 as a Biomarker for the Differential Diagnosis of Alzheimer’s Disease and PET Amyloid-Positive Patient Identification. Alzheimer’s Res. Ther. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Cousins, K.A.Q.; Phillips, J.S.; Irwin, D.J.; Lee, E.B.; Wolk, D.A.; Shaw, L.M.; Zetterberg, H.; Blennow, K.; Burke, S.E.; Kinney, N.G.; et al. ATN Incorporating Cerebrospinal Fluid Neurofilament Light Chain Detects Frontotemporal Lobar Degeneration. Alzheimer’s Dement. 2021, 17, 822–830. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, K.M.; Yang, L.; Dong, Q.; Yu, J.T. Tauopathies: New Perspectives and Challenges. Mol. Neurodegener. 2022, 17, 28. [Google Scholar] [CrossRef]
- Moscoso, A.; Karikari, T.K.; Grothe, M.J.; Ashton, N.J.; Lantero-Rodriguez, J.; Snellman, A.; Zetterberg, H.; Blennow, K.; Schöll, M. CSF Biomarkers and Plasma P-Tau181 as Predictors of Longitudinal Tau Accumulation: Implications for Clinical Trial Design. Alzheimer’s Dement. 2022, 18, 2614–2626. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal Fluid P-Tau217 Performs Better than p-Tau181 as a Biomarker of Alzheimer’s Disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Thijssen, E.H.; La Joie, R.; Strom, A.; Fonseca, C.; Iaccarino, L.; Wolf, A.; Spina, S.; Allen, I.E.; Cobigo, Y.; Heuer, H.; et al. Plasma Phosphorylated Tau 217 and Phosphorylated Tau 181 as Biomarkers in Alzheimer’s Disease and Frontotemporal Lobar Degeneration: A Retrospective Diagnostic. Lancet Neurol. 2021, 20, 739–752. [Google Scholar] [CrossRef]
- Congdon, E.; Sigurdsson, E. Tau-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Bittar, A.; Bhatt, N.; Kayed, R. Advances and Considerations in AD Tau-Targeted Immunotherapy. Neurobiol. Dis. 2020, 134, 104707. [Google Scholar] [CrossRef]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N.; et al. ADAMANT: A Placebo-Controlled Randomized Phase 2 Study of AADvac1, an Active Immunotherapy against Pathological Tau in Alzheimer’s Disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef]
- 18-Months Safety Follow-Up Study of AADvac1, an Active Tau Vaccine for Alzheimer’s Disease (FUNDAMANT). Available online: https://www.clinicaltrials.gov/ct2/show/NCT02031198 (accessed on 4 January 2023).
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s Disease: Targeting β-Amyloid and Beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate the Safety, Tolerability and Immunogenicity of Tau Targeted Vaccines in Participants with Early Alzheimer’s Disease. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04445831 (accessed on 4 January 2023).
- Jadhav, S.; Avila, J.; Schöll, M.; Kovacs, G.G.; Kövari, E.; Skrabana, R.; Evans, L.D.; Kontsekova, E.; Malawska, B.; de Silva, R.; et al. A Walk through Tau Therapeutic Strategies. Acta Neuropathol. Commun. 2019, 7, 22. [Google Scholar] [CrossRef]
- Mignon, L.; Kordasiewicz, H.; Lane, R.; Smith, A.; Miller, T.; Narayanan, P.; Swayze, E.; Norris, D.; Fitzsimmons, B.; Bennett, F. Design of the First-in-Human Study of IONIS-MAPTRx, a Tau-Lowering Antisense Oligonucleotide, in Patients with Alzheimer Disease (S2.006). Neurology 2018, 90 (Suppl. 15), S2.006. [Google Scholar]
- Wischik, C.M.; Bentham, P.; Gauthier, S.; Miller, S.; Kook, K.; Schelter, B.O. Oral Tau Aggregation Inhibitor for Alzheimer’s Disease: Design, Progress and Basis for Selection of the 16 Mg/Day Dose in a Phase 3, Randomized, Placebo-Controlled Trial of Hydromethylthionine Mesylate. J. Prev. Alzheimer’s Dis. 2022, 9, 780–790. [Google Scholar] [CrossRef]
- Alvarez, X.A.; Winston, C.N.; Barlow, J.W.; Sarsoza, F.M.; Alvarez, I.; Aleixandre, M.; Linares, C.; García-Fantini, M.; Kastberger, B.; Winter, S.; et al. Modulation of Amyloid-β and Tau in Alzheimer’s Disease Plasma Neuronal-Derived Extracellular Vesicles by Cerebrolysin® and Donepezil. J. Alzheimers. Dis. 2022, 90, 705–717. [Google Scholar] [CrossRef]
- Teng, E.; Manser, P.T.; Pickthorn, K.; Brunstein, F.; Blendstrup, M.; Bohorquez, S.S.; Wildsmith, K.R.; Toth, B.; Dolton, M.; Ramakrishnan, V.; et al. Safety and Efficacy of Semorinemab in Individuals with Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.; Jensen, M.L.; Wang, C.; Hagedorn, P.H.; Li, Y.; Weed, M.; Meredith, J.E.; Guss, V.; Jones, K.; Gill, M.; et al. Identification and Characterization of a MAPT-Targeting Locked Nucleic Acid Antisense Oligonucleotide Therapeutic for Tauopathies. Mol. Ther.-Nucleic Acids 2022, 29, 625–642. [Google Scholar] [CrossRef] [PubMed]
- Polis, B.; Squillario, M.; Gurevich, V.; Srikanth, K.D.; Assa, M.; Samson, A.O. Effects of Chronic Arginase Inhibition with Norvaline on Tau Pathology and Brain Glucose Metabolism in Alzheimer’s Disease Mice. Neurochem. Res. 2022, 47, 1255–1268. [Google Scholar] [CrossRef]
- Singer, K.E.; McGlone, E.D.; Collins, S.M.; Wallen, T.E.; Morris, M.C.; Schuster, R.M.; England, L.G.; Robson, M.J.; Goodman, M.D. Propranolol Reduces P-Tau Accumulation and Improves Behavior Outcomes in a Polytrauma Murine Model. J. Surg. Res. 2022, 282, 183–190. [Google Scholar] [CrossRef]
- Tong, B.C.-K.; Huang, A.S.; Wu, A.J.; Iyaswamy, A.; Ho, O.K.-Y.; Kong, A.H.-Y.; Sreenivasmurthy, S.G.; Zhu, Z.; Su, C.; Liu, J.; et al. Tetrandrine Ameliorates Cognitive Deficits and Mitigates Tau Aggregation in Cell and Animal Models of Tauopathies. J. Biomed. Sci. 2022, 29, 85. [Google Scholar] [CrossRef]
- Bittar, A.; Al-Lahham, R.; Bhatt, N.; Moore, K.; Montalbano, M.; Jerez, C.; Fung, L.; McAllen, S.; Ellsworth, A.; Kayed, R. Passive Immunotherapy Targeting Tau Oligomeric Strains Reverses Tauopathy Phenotypes in Aged Human-Tau Mice in a Mouse Model-Specific Manner. J. Alzheimer’s Dis. 2022, 90, 1103–1122. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.O.; El-Desouky, S.; Elsherbiny, D.A.; Salama, M.; Azab, S.S. Glimepiride Mitigates Tauopathy and Neuroinflammation in P301S Transgenic Mice: Role of AKT/GSK3β Signaling. Inflammopharmacology 2022, 30, 1871–1890. [Google Scholar] [CrossRef]
- Shcherbinin, S.; Evans, C.D.; Lu, M.; Andersen, S.W.; Pontecorvo, M.J.; Willis, B.A.; Gueorguieva, I.; Hauck, P.M.; Brooks, D.A.; Mintun, M.A.; et al. Association of Amyloid Reduction after Donanemab Treatment with Tau Pathology and Clinical Outcomes: The TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Riederer, F. Donanemab in Early Alzheimer’s Disease. J. Neurol. Neurochir. Psychiatr. 2021, 22, 142–143. [Google Scholar] [CrossRef]
- Ou, W.; Yang, J.; Simanauskaite, J.; Choi, M.; Castellanos, D.M.; Chang, R.; Sun, J.; Jagadeesan, N.; Parfitt, K.D.; Cribbs, D.H.; et al. Biologic TNF-α Inhibitors Reduce Microgliosis, Neuronal Loss, and Tau Phosphorylation in a Transgenic Mouse Model of Tauopathy. J. Neuroinflamm. 2021, 18, 312. [Google Scholar] [CrossRef]
- Xia, L.; Pang, Y.; Li, J.; Wu, B.; Du, Y.; Chen, Y.; Luo, M.; Wang, Y.; Dong, Z.; Zhu, L.Q. Dihydroartemisinin Induces O-GlcNAcylation and Improves Cognitive Function in a Mouse Model of Tauopathy. J. Alzheimer’s Dis. 2021, 84, 239–248. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Li, L.-J.; Dong, Q.-X.; Zhu, J.; Huang, Y.-R.; Hou, S.-J.; Yu, X.-L.; Liu, R.-T. Rutin Prevents Tau Pathology and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef]
- Anglada-Huguet, M.; Rodrigues, S.; Hochgräfe, K.; Mandelkow, E.; Mandelkow, E.-M. Inhibition of Tau Aggregation with BSc3094 Reduces Tau and Decreases Cognitive Deficits in RTg4510 Mice. Alzheimer’s Dement. 2021, 7, e12170. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Chinnathambi, S. Epigallocatechin-3-Gallate Modulates Tau Post-Translational Modifications and Cytoskeletal Network. Oncotarget 2021, 12, 1083–1099. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Jiang, T.; Li, S.; Ye, J.; Zheng, J.; Wang, X.; Liu, Y.; Deng, M.; Ke, D.; et al. A Novel Small-Molecule PROTAC Selectively Promotes Tau Clearance to Improve Cognitive Functions in Alzheimer-like Models. Theranostics 2021, 11, 5279–5295. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.F.; Camargo, C.; Premer, C.; Hare, J.M.; Baumel, B.S.; Pinto, M. Intravenous Administration of Mesenchymal Stem Cells Reduces Tau Phosphorylation and Inflammation in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Exp. Neurol. 2021, 341, 113706. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.; Boxer, A.L.; Golbe, L.I.; Höglinger, G.U.; Morris, H.R.; Litvan, I.; Lang, A.E.; Corvol, J.-C.; Aiba, I.; Grundman, M.; et al. Safety and Efficacy of Anti-Tau Monoclonal Antibody Gosuranemab in Progressive Supranuclear Palsy: A Phase 2, Randomized, Placebo-Controlled Trial. Nat. Med. 2021, 27, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Rühlmann, C.; Wölk, T.; Blümel, T.; Stahn, L.; Vollmar, B.; Kuhla, A. Long-Term Caloric Restriction in ApoE-Deficient Mice Results in Neuroprotection via Fgf21-Induced AMPK/MTOR Pathway. Aging (Albany NY) 2016, 8, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, M.L.; Joly-Amado, A.; Azam, S.; Elza, M.; Selenica, M.L.; Pappas, C.; Small, B.; Engelman, R.; Gordon, M.N.; Morgan, D. Partial Rescue of Memory Deficits Induced by Calorie Restriction in a Mouse Model of Tau Deposition. Behav. Brain Res. 2014, 271, 79–88. [Google Scholar] [CrossRef] [PubMed]



| Model/Cell Line | Therapeutic Intervention | Intended Mechanism | Potential Target | Reference |
|---|---|---|---|---|
| Human patients with probable AD or MCI-AD | Hydromethylthionine mesylate | Inhibiting tau aggregation by targeting pathological tau oligomers and filaments In the Phase III clinical trial. | Tau aggregation | [98] |
| Human patients with mild to advanced AD | Cerebrolysin® & Donepezil | Unknown | Tau expression and tau phosphorylation | [99] |
| Prodromal to mild AD patients | Semorinemab | Not slowing tau accumulation pathology (not effective), and no change in clinical AD progression. | Oligomeric tau | [100] |
| Cyno monkeys, C57Bl/6J mice model, PAC transgenic mice, hESC line SA001 cell line | ASO-001933 | Selective and long-lasting reduction in tau levels by locked nucleic acid (LNA)- modified ASOs. | Tau aggregation | [101] |
| 3 × Tg mice model | Norvaline | Diminishing tau phosphorylation levels. | Phosphorylated tau | [102] |
| Murine polytrauma mouse model | Propranolol | Decreasing hippocampal p-tau accumulation. | Accumulation of phosphorylated tau | [103] |
| Ty1-hTau.P301S mice and SH-SY5Y cell line | Tetrandrine | Promoting tau clearance and degradation via autophagy, rescuing lysosomal Ca2+ homeostasis, and diminishing NFT development. | Lysosomal two-pore channel 2 (TPC2) | [104] |
| Htau and JNPL3 mouse models | Tau oligomer monoclonal antibodies (TOMAs) | Reducing tau oligomer levels by tau passive immunotherapy. | Tau oligomeric strains | [105] |
| P301S mouse model | Glimepiride | Decreasing GSK3β, increasing phosphorylated-AKT/total-AKT, increasing PP2A, and normalizing CDK5 levels. Decreasing neuroinflammation and apoptosis by reducing NF-kB, TNF-α and caspase 3 levels | Phosphorylated tau | [106] |
| Human AD patients | Donanemab | Slowing tau accumulation | Tau accumulation | [107,108] |
| PS19 mouse model | Etanercept and TfRMAb-TNFR | Reducing phosphorylated tau and microgliosis, increasing PSD95 expression and attenuating hippocampal neuron loss. | TNF-α (Inhibitors) | [109] |
| AAVhTau mouse model | Dihydroartemisinin (DHA) | Inducing tau O-Glc-N-Acylation modification, reducing tau phosphorylation, improving learning and memory and increasing hippocampal CA1 long-term potentiation (LTP). | PTMs on tau protein | [110] |
| Tau-P301S mouse model | Rutin | Inhibiting tau aggregation and its oligomer-induced cytotoxicity, reducing the production of proinflammatory cytokines and preserving neurons. | Tau aggregation | [111] |
| rTg4510 mouse model | BSc3094 | Reducing tau phosphorylation, improving cognition and reducing anxiety-like behavior. | Tau aggregation | [112] |
| Neuroblastoma cell model (with methyl glyoxal (MG)-induced Tau glycation) | Epigallocatechin-3-gallate (EGCG) | Inhibiting glycation, modulating tau phosphorylation, enhancing actin-rich neuritic extensions and preserving the actin and tubulin cytoskeleton. | Cytoskeleton stabilizer | [113] |
| SH-SY5Y and HEK293 cell lines hTau-transgenic, tauP301L and 3 × Tg-AD mouse models | C004019 | Promoting tau ubiquitination-proteasome-dependent proteolysis Improving synaptic and cognitive functions in animal models. | Tau clearance | [114] |
| 3 × Tg-AD mouse model | Intravenous administration of mesenchymal stem cells | Decreasing pathological tau phosphorylation at T205, S214, T231 and S396 but not levels of Aβ-42. | Phosphorylated tau | [115] |
| Human PSP patients | Gosuranemab | Decreasing unbound N-terminal tau in CSF. In spite of this effect, gosuranemab did not show clinical efficacy in PSP patients. Phase II clinical trial is completed. | N-terminal tau | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabeshmehr, P.; Eftekharpour, E. Tau; One Protein, So Many Diseases. Biology 2023, 12, 244. https://doi.org/10.3390/biology12020244
Tabeshmehr P, Eftekharpour E. Tau; One Protein, So Many Diseases. Biology. 2023; 12(2):244. https://doi.org/10.3390/biology12020244
Chicago/Turabian StyleTabeshmehr, Parisa, and Eftekhar Eftekharpour. 2023. "Tau; One Protein, So Many Diseases" Biology 12, no. 2: 244. https://doi.org/10.3390/biology12020244
APA StyleTabeshmehr, P., & Eftekharpour, E. (2023). Tau; One Protein, So Many Diseases. Biology, 12(2), 244. https://doi.org/10.3390/biology12020244







