Transcriptional Analysis of the Endostyle Reveals Pharyngeal Organ Functions in Ascidian
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Preparation
2.2. Preparation of Cryosection and HE Staining
2.3. RNA Extraction and Transcriptome Sequencing
2.4. Transcriptome Analysis
2.5. Quantitative Reverse-Transcription PCR (qRT-PCR)
2.6. OSG Identification
2.7. Endostyle OSG Projection on Zebrafish Organ Transcriptome Profile
2.8. Transcriptional Profile Comparison between the Endostyle and the Potentially Similar Organs
2.9. Similarity of the Endostyle Transcriptome Profile to Human Organs/Tissues Enriched Gene Set
3. Results
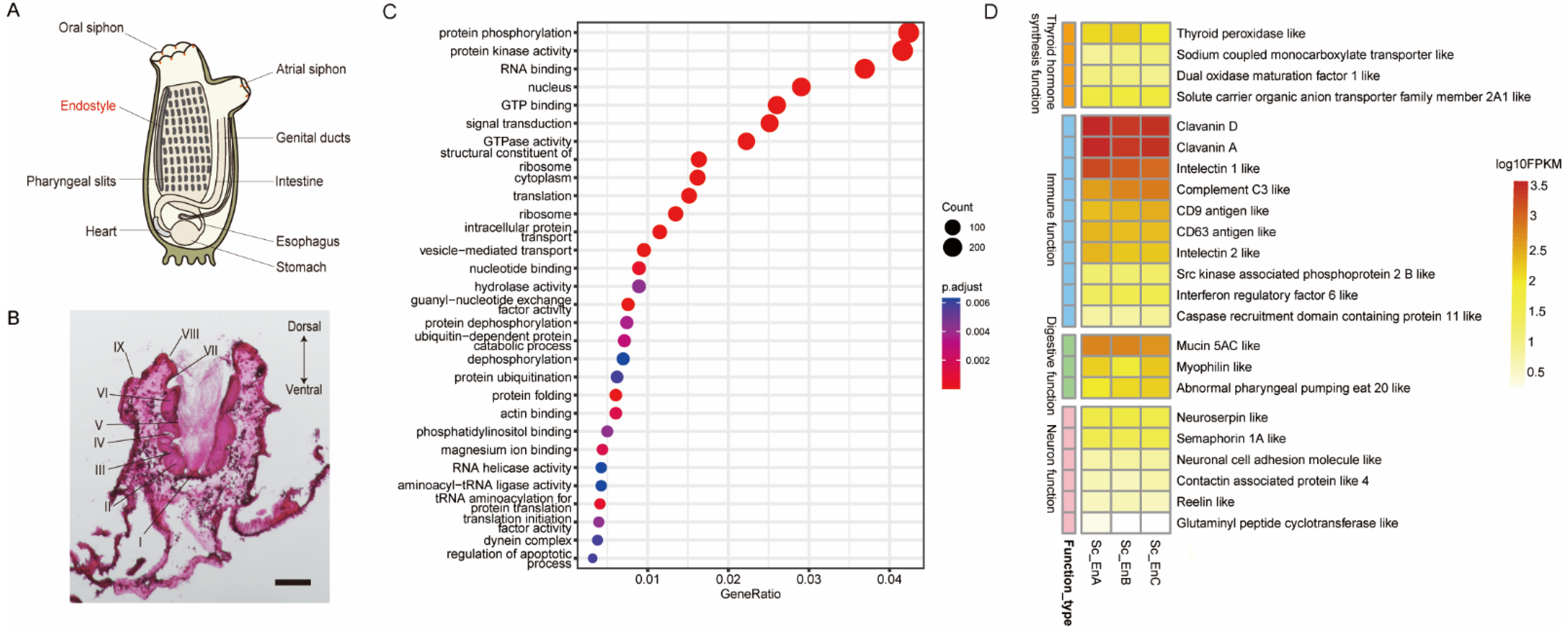
3.1. Morphological Observation and Expression Profile of the Endostyle
3.2. Longitudinal Distinct Genes of the Endostyle
3.3. OSG-Based Expression Similarity Analysis
3.4. Transcriptomic Profile Comparison Reveals Features of the Endostyle
3.5. The Expression Similarity of Human OSGs Homolog in the Endostyle
4. Discussion
4.1. Morphological and Functional Definition of the Endostyle
4.2. Longitudinal Heterogeneity within the Endostyle
4.3. OSG of the Endostyle Showed Distinct Expression Profiles Compared with Non-Endostyle Adult Organs in Ascidian
4.4. Cross-Species Comparison Based on the OSGs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujita, H.; Nanba, H. Fine structure and its functional properties of the endostyle of ascidians, Ciona intestinalis. A part of phylogenetic studies of the thyroid gland. Z. Fur Zellforsch. Und Mikrosk. Anat. 1971, 121, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Miyamoto, Y.; Satou, Y.; Satoh, N.; Ogasawara, M. Novel endostyle-specific genes in the ascidian Ciona intestinalis. Zool. Sci. 2003, 20, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D.; Chen, J. Origin and early evolution of the vertebrates: New insights from advances in molecular biology, anatomy, and palaeontology. Bioessays 2001, 23, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Barrington, E.J.; Thorpe, A. The identification of monoiodotyrosine, diiodotyrosine and thyroxine in extracts of the endostyle of the ascidian, Ciona Intestinalis L. Proc. R. Soc. Lond. B Biol. Sci. 1965, 163, 136–149. [Google Scholar] [CrossRef]
- Barrington, E.J.W. The Distribution and significance of organically bound iodine in the ascidian Ciona intestinalis Linnaeus. J. Mar. Biol. Assoc. U. K. 1957, 36, 1–16. [Google Scholar] [CrossRef]
- Barrington, E.J.W. The localization of organically bound iodine in the endostyle of amphioxus. J. Mar. Biol. Assoc. U. K. 1958, 37, 117–125. [Google Scholar] [CrossRef]
- Thomas, I.M. The accumulation of radioactive iodine by Amphioxus. J. Mar. Biol. Assoc. U. K. 1956, 35, 203–210. [Google Scholar] [CrossRef]
- Fredriksson, G.; Öfverholm, T.; Ericson, L.E. Electron-microscopic studies of iodine-binding and peroxidase activity in the endostyle of the larval amphioxus (Branchiostoma lanceolatum). Cell Tissue Res. 1985, 241, 257–266. [Google Scholar] [CrossRef]
- Hiruta, J.; Mazet, F.; Yasui, K.; Zhang, P.; Ogasawara, M. Comparative expression analysis of transcription factor genes in the endostyle of invertebrate chordates. Dev. Dyn. 2005, 233, 1031–1037. [Google Scholar] [CrossRef]
- Ogasawara, M. Overlapping expression of amphioxus homologs of the thyroid transcription factor-1 gene and thyroid peroxidase gene in the endostyle: Insight into evolution of the thyroid gland. Dev. Genes Evol. 2000, 210, 231–242. [Google Scholar] [CrossRef]
- Kluge, B.; Renault, N.; Rohr, K.B. Anatomical and molecular reinvestigation of lamprey endostyle development provides new insight into thyroid gland evolution. Dev. Genes Evol. 2005, 215, 32–40. [Google Scholar] [CrossRef]
- Yoshida, K.; Nakahata, A.; Treen, N.; Sakuma, T.; Yamamoto, T.; Sasakura, Y. Hox-mediated endodermal identity patterns pharyngeal muscle formation in the chordate pharynx. Development 2017, 144, 1629–1634. [Google Scholar] [CrossRef]
- Canestro, C.; Bassham, S.; Postlethwait, J.H. Evolution of the thyroid: Anterior-posterior regionalization of the Oikopleura endostyle revealed by Otx, Pax2/5/8, and Hox1 expression. Dev. Dyn. 2008, 237, 1490–1499. [Google Scholar] [CrossRef]
- Onuma, T.A.; Nakanishi, R.; Sasakura, Y.; Ogasawara, M. Nkx2-1 and FoxE regionalize glandular (mucus-producing) and thyroid-equivalent traits in the endostyle of the chordate Oikopleura dioica. Dev. Biol. 2021, 477, 219–231. [Google Scholar] [CrossRef]
- Yamagishi, M.; Huang, T.; Hozumi, A.; Onuma, T.A.; Sasakura, Y.; Ogasawara, M. Differentiation of endostyle cells by Nkx2-1 and FoxE in the ascidian Ciona intestinalis type A: Insights into shared gene regulation in glandular- and thyroid-equivalent elements of the chordate endostyle. Cell Tissue Res. 2022, 390, 189–205. [Google Scholar] [CrossRef]
- Nilsson, M.; Fagman, H. Development of the thyroid gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef]
- Takagi, W.; Sugahara, F.; Higuchi, S.; Kusakabe, R.; Pascual-Anaya, J.; Sato, I.; Oisi, Y.; Ogawa, N.; Miyanishi, H.; Adachi, N.; et al. Thyroid and endostyle development in cyclostomes provides new insights into the evolutionary history of vertebrates. BMC Biol. 2022, 20, 76. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Capillo, G.; Lauriano, E.R. Localization of vasoactive intestinal peptide and toll-like receptor 2 immunoreactive cells in endostyle of urochordate Styela plicata (Lesueur, 1823). Microsc. Res. Tech. 2022, 85, 2651–2658. [Google Scholar] [CrossRef]
- Parrinello, D.; Sanfratello, M.A.; Vizzini, A.; Parrinello, N.; Cammarata, M. Ciona intestinalis galectin (CiLgals-a and CiLgals-b) genes are differentially expressed in endostyle zones and challenged by LPS. Fish Shellfish. Immunol. 2015, 42, 171–176. [Google Scholar] [CrossRef]
- Voskoboynik, A.; Soen, Y.; Rinkevich, Y.; Rosner, A.; Ueno, H.; Reshef, R.; Ishizuka, K.J.; Palmeri, K.J.; Moiseeva, E.; Rinkevich, B.; et al. Identification of the endostyle as a stem cell niche in a colonial chordate. Cell Stem Cell 2008, 3, 456–464. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Voskoboynik, A.; Rosner, A.; Rabinowitz, C.; Paz, G.; Oren, M.; Douek, J.; Alfassi, G.; Moiseeva, E.; Ishizuka, K.J.; et al. Repeated, long-term cycling of putative stem cells between niches in a basal chordate. Dev. Cell 2013, 24, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Rosental, B.; Kowarsky, M.; Seita, J.; Corey, D.M.; Ishizuka, K.J.; Palmeri, K.J.; Chen, S.Y.; Sinha, R.; Okamoto, J.; Mantalas, G.; et al. Complex mammalian-like haematopoietic system found in a colonial chordate. Nature 2018, 564, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H.J.N. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Osugi, T.; Shiraishi, A.; Wada, A.; Satake, H. Comparative analysis of transcriptomic profiles among ascidians, zebrafish, and mice: Insights from tissue-specific gene expression. PLoS ONE 2021, 16, e0254308. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, J.; Lu, Q.; Ren, P.; Guo, X.; Wang, J.; Li, X.; Chang, Y.; Duan, S.; Wang, S.; et al. Genomic basis of environmental adaptation in the leathery sea squirt (Styela clava). Mol. Ecol. Resour. 2020, 20, 1414–1431. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Yu, H.; Dong, B. Genome-Wide Identification, Comparison, and Expression Analysis of Transcription Factors in Ascidian Styela clava. Int. J. Mol. Sci. 2021, 22, 4317. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wilkinson, L. ggplot2: Elegant Graphics for Data Analysis by H. WICKHAM. Biometrics 2011, 67, 678–679. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Fujihara, Y.; Herberg, S.; Blaha, A.; Panser, K.; Kobayashi, K.; Larasati, T.; Novatchkova, M.; Theussl, H.C.; Olszanska, O.; Ikawa, M.; et al. The conserved fertility factor SPACA4/Bouncer has divergent modes of action in vertebrate fertilization. Proc. Natl. Acad. Sci. USA 2021, 118, e2108777118. [Google Scholar] [CrossRef]
- Pasquier, J.; Cabau, C.; Nguyen, T.; Jouanno, E.; Severac, D.; Braasch, I.; Journot, L.; Pontarotti, P.; Klopp, C.; Postlethwait, J.H.; et al. Gene evolution and gene expression after whole genome duplication in fish: The PhyloFish database. BMC Genom. 2016, 17, 368. [Google Scholar] [CrossRef]
- Rubin, S.A.; Baron, C.S.; Pessoa Rodrigues, C.; Duran, M.; Corbin, A.F.; Yang, S.P.; Trapnell, C.; Zon, L.I. Single-cell analyses reveal early thymic progenitors and pre-B cells in zebrafish. J. Exp. Med. 2022, 219, e20220038. [Google Scholar] [CrossRef]
- Gillotay, P.; Shankar, M.; Haerlingen, B.; Sema Elif, E.; Pozo-Morales, M.; Garteizgogeascoa, I.; Reinhardt, S.; Kränkel, A.; Bläsche, J.; Petzold, A.; et al. Single-cell transcriptome analysis reveals thyrocyte diversity in the zebrafish thyroid gland. EMBO Rep. 2020, 21, e50612. [Google Scholar] [CrossRef]
- Maag, J.L.V. gganatogram: An R package for modular visualisation of anatograms and tissues based on ggplot2. F1000Res 2018, 7, 1576. [Google Scholar] [CrossRef]
- Holley, M.C. Cell shape, spatial patterns of cilia, and mucus-net construction in the ascidian endostyle. Tissue Cell 1986, 18, 667–684. [Google Scholar] [CrossRef]
- Thorndyke, M.C. Evidence for a ‘mammalian’ thyroglobulin in endostyle of the ascidian Styela clava. Nature 1978, 271, 61–62. [Google Scholar] [CrossRef]
- Fong, P. Thyroid iodide efflux: A team effort? J. Physiol. 2011, 589, 5929–5939. [Google Scholar] [CrossRef]
- Nonaka, M.; Satake, H. Urochordate immunity. Adv. Exp. Med. Biol. 2010, 708, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Yang, G. A comparative review of intelectins. Scand. J. Immunol. 2020, 92, e12882. [Google Scholar] [CrossRef] [PubMed]
- Godeaux, J. Functions of the endostyle in the tunicates. Funct. Endostyle Tunicates 1989, 45, 228–242. [Google Scholar]
- Osugi, T.; Sasakura, Y.; Satake, H. The ventral peptidergic system of the adult ascidian Ciona robusta (Ciona intestinalis Type A) insights from a transgenic animal model. Sci. Rep. 2020, 10, 1892. [Google Scholar] [CrossRef]
- Paululat, A.; Goubeaud, A.; Damm, C.; Knirr, S.; Burchard, S.; Renkawitz-Pohl, R. The mesodermal expression of rolling stone (rost) is essential for myoblast fusion in Drosophila and encodes a potential transmembrane protein. J. Cell Biol. 1997, 138, 337–348. [Google Scholar] [CrossRef]
- Welcker, D.; Stein, C.; Feitosa, N.M.; Armistead, J.; Zhang, J.L.; Lütke, S.; Kleinridders, A.; Brüning, J.C.; Eming, S.A.; Sengle, G.; et al. Hemicentin-1 is an essential extracellular matrix component of the dermal-epidermal and myotendinous junctions. Sci. Rep. 2021, 11, 17926. [Google Scholar] [CrossRef]
- Cleator, J.H.; Zhu, W.Q.; Vaughan, D.E.; Hamm, H.E. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood 2006, 107, 2736–2744. [Google Scholar] [CrossRef]
- Wang, W.; Sumiyoshi, H.; Yoshioka, H.; Fujiwara, S. Interactions between epiplakin and intermediate filaments. J. Dermatol. 2006, 33, 518–527. [Google Scholar] [CrossRef]
- Mátés, L.; Korpos, E.; Déak, F.; Liu, Z.; Beier, D.R.; Aszódi, A.; Kiss, I. Comparative analysis of the mouse and human genes (Matn2 and MATN2) for matrilin-2, a filament-forming protein widely distributed in extracellular matrices. Matrix. Biol. 2002, 21, 163–174. [Google Scholar] [CrossRef]
- Nakayama, S.; Satou, K.; Orito, W.; Ogasawara, M. Ordered expression pattern of Hox and ParaHox genes along the alimentary canal in the ascidian juvenile. Cell Tissue Res. 2016, 365, 65–75. [Google Scholar] [CrossRef]
- Sakashita, C.; Fukuda, T.; Okabe, S.; Kobayashi, H.; Hirosawa, S.; Tokuhisa, T.; Miyasaka, N.; Miura, O.; Miki, T. Cloning and characterization of the human BAZF gene, a homologue of the BCL6 oncogene. Biochem. Biophys. Res. Commun. 2002, 291, 567–573. [Google Scholar] [CrossRef]
- Davidson, A.J.; Zon, L.I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef]
- Vanni, V.; Anselmi, C.; Ballarin, L.; Drago, L.; Gasparini, F.; Gordon, T.; Peronato, A.; Rosental, B.; Rosner, A.; Rinkevich, B.; et al. Current Knowledge on Stem Cells in Ascidians. In Advances in Aquatic Invertebrate Stem Cell Research; MDPI: Basel, Switzerland, 2022. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Salvatore, G. Thyroid hormone biosynthesis in agnatha and protochordata. Gen. Comp. Endocrinol. 1969, 2, 535–551. [Google Scholar] [CrossRef]
- Alu, K.; Kazuo, I. Region-Specific Loss of Two-Headed Ciliary Dyneins in Ascidian Endostyle. Zool. Sci. 2020, 37, 512–518. [Google Scholar] [CrossRef]
- Petersen, J.K. Ascidian suspension feeding. J. Exp. Mar. Biol. Ecol. 2007, 342, 127–137. [Google Scholar] [CrossRef]
- Azumi, K.; De Santis, R.; De Tomaso, A.; Rigoutsos, I.; Yoshizaki, F.; Pinto, M.R.; Marino, R.; Shida, K.; Ikeda, M.; Ikeda, M.; et al. Genomic analysis of immunity in a Urochordate and the emergence of the vertebrate immune system: “waiting for Godot”. Immunogenetics 2003, 55, 570–581. [Google Scholar] [CrossRef]
- Galliciotti, G.; Sonderegger, P. Neuroserpin. Front. Biosci. 2006, 11, 33–45. [Google Scholar] [CrossRef]
- Cunningham, B.A.; Hemperly, J.J.; Murray, B.A.; Prediger, E.A.; Brackenbury, R.; Edelman, G.M. Neural cell adhesion molecule: Structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science 1987, 236, 799–806. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Z.; Ha, P.; Chen, X.; Jiang, W.; Sun, S.; Chen, F.; Asatrian, G.; Berthiaume, E.A.; Kim, J.K.; et al. Neurexin superfamily cell membrane receptor contactin-associated protein like-4 (Cntnap4) is involved in neural EGFL-like 1 (Nell-1)-responsive osteogenesis. J. Bone Miner. Res. 2018, 33, 1813–1825. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin functions, mechanisms of action and signaling pathways during brain development and maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Pesce, J.T.; Ramalingam, T.; Wilson, M.S.; White, S.; Cheever, A.W.; Ricklefs, S.M.; Porcella, S.F.; Li, L.; Ellies, L.G.; et al. Cationic amino acid transporter-2 regulates immunity by modulating arginase activity. PLoS Pathog. 2008, 4, e1000023. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, X.; Wang, X.; Feng, H.; Gou, M.; Jin, W.; Wang, X.; Liu, X.; Dong, C. The transcription factor Tox2 drives T follicular helper cell development via regulating chromatin accessibility. Immunity 2019, 51, 826–839.e825. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, E. Structural insight into Slit-Robo signalling. Biochem. Soc. Trans. 2008, 36, 251–256. [Google Scholar] [CrossRef]
- Arendt, D. The evolution of cell types in animals: Emerging principles from molecular studies. Nat. Rev. Genet. 2008, 9, 868–882. [Google Scholar] [CrossRef]
- Arendt, D.; Musser, J.M.; Baker, C.V.H.; Bergman, A.; Cepko, C.; Erwin, D.H.; Pavlicev, M.; Schlosser, G.; Widder, S.; Laubichler, M.D.; et al. The origin and evolution of cell types. Nat. Rev. Genet. 2016, 17, 744–757. [Google Scholar] [CrossRef]




| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Purpose |
|---|---|---|---|
| Homeobox protein Hox A3 | ATGTGTAGCTATCAATATATGTATGATG | CTTTGCTAATACCGTCAGACCC | RT-PCR |
| Homeobox protein Hox A4 | ATGGCTGCGTTACATGATG | AGATTCGGGAGAGCCCC | RT-PCR |
| Proto-oncogene c-Fos-like | GCTGCCAAAGGATTGCCTTC | TCTGTCCCTTCGTCTTTGCC | RT-PCR |
| Sc-18s | CTGAGTGAAGCAGCGAGTGTCTAACCTA | GCAAGTCCCTATCCCAATCACGAA | RT-PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, A.; Zhang, W.; Wei, J.; Liu, P.; Dong, B. Transcriptional Analysis of the Endostyle Reveals Pharyngeal Organ Functions in Ascidian. Biology 2023, 12, 245. https://doi.org/10.3390/biology12020245
Jiang A, Zhang W, Wei J, Liu P, Dong B. Transcriptional Analysis of the Endostyle Reveals Pharyngeal Organ Functions in Ascidian. Biology. 2023; 12(2):245. https://doi.org/10.3390/biology12020245
Chicago/Turabian StyleJiang, An, Wei Zhang, Jiankai Wei, Penghui Liu, and Bo Dong. 2023. "Transcriptional Analysis of the Endostyle Reveals Pharyngeal Organ Functions in Ascidian" Biology 12, no. 2: 245. https://doi.org/10.3390/biology12020245
APA StyleJiang, A., Zhang, W., Wei, J., Liu, P., & Dong, B. (2023). Transcriptional Analysis of the Endostyle Reveals Pharyngeal Organ Functions in Ascidian. Biology, 12(2), 245. https://doi.org/10.3390/biology12020245







