The Course of Mechanical Stress: Types, Perception, and Plant Response
Abstract
Simple Summary
Abstract
1. Introduction
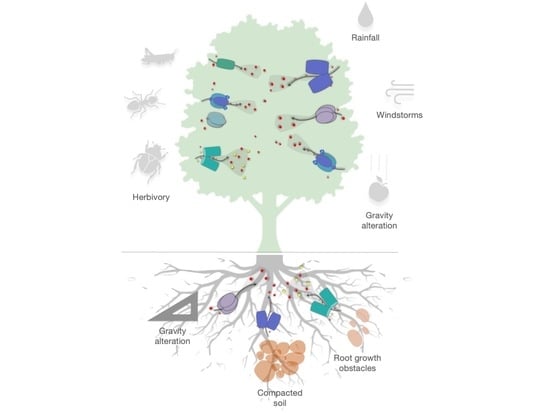
2. Different Typologies of Mechanical Stress
2.1. Wind
2.2. Rain and Herbivory
2.3. Gravity
2.4. Bending, Slope, and Touch
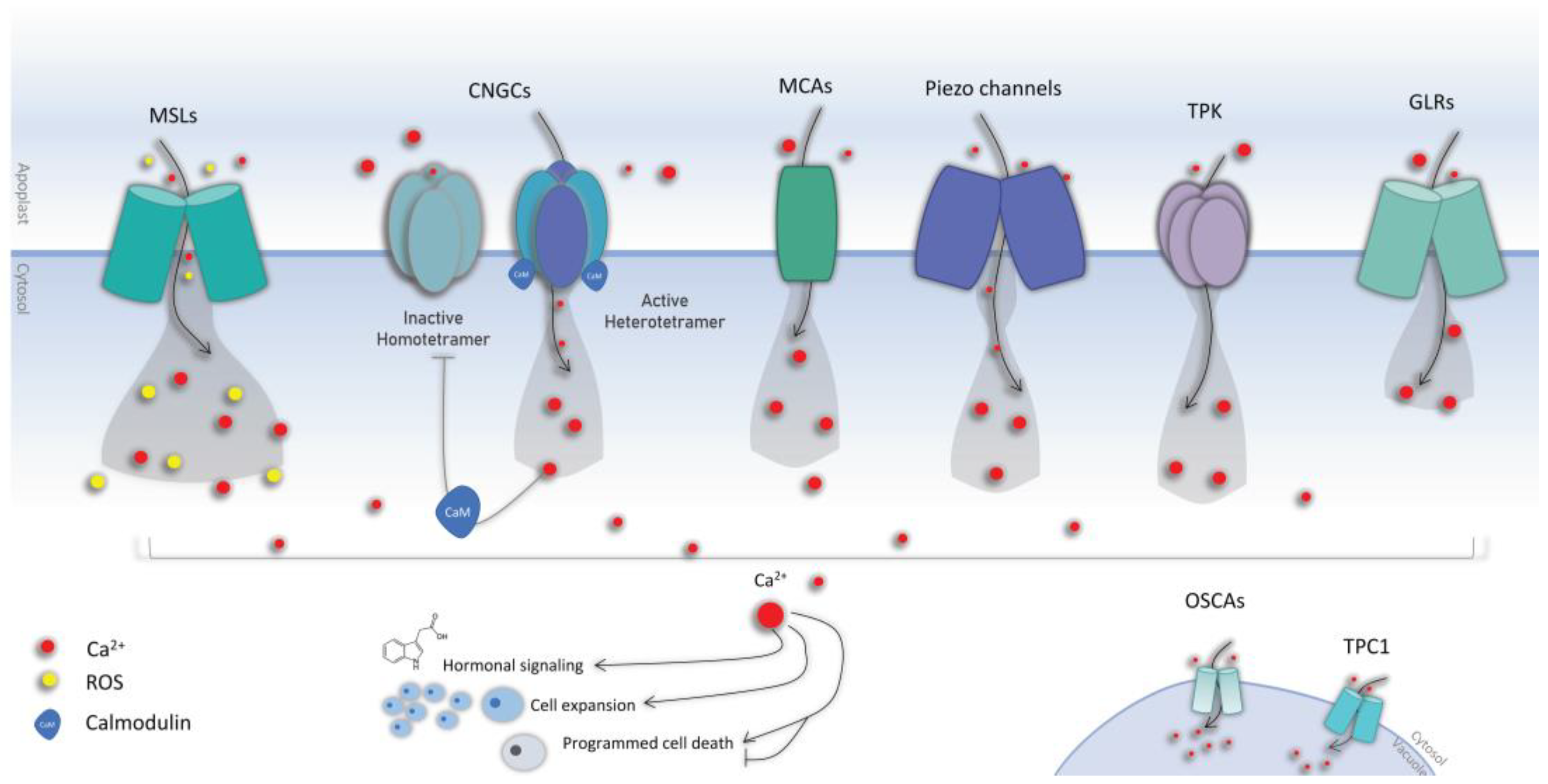
3. Mechanical Stress Perception
3.1. Calcium Signaling
3.2. Calcium Channels
3.3. Mechanosensitive Channels
3.3.1. The Mid1-Complementing Activity (MCA) Channels
3.3.2. The Mechanosensitive Channel of Small Conductance-like (MSL)
3.3.3. Piezo
3.3.4. Other Channels
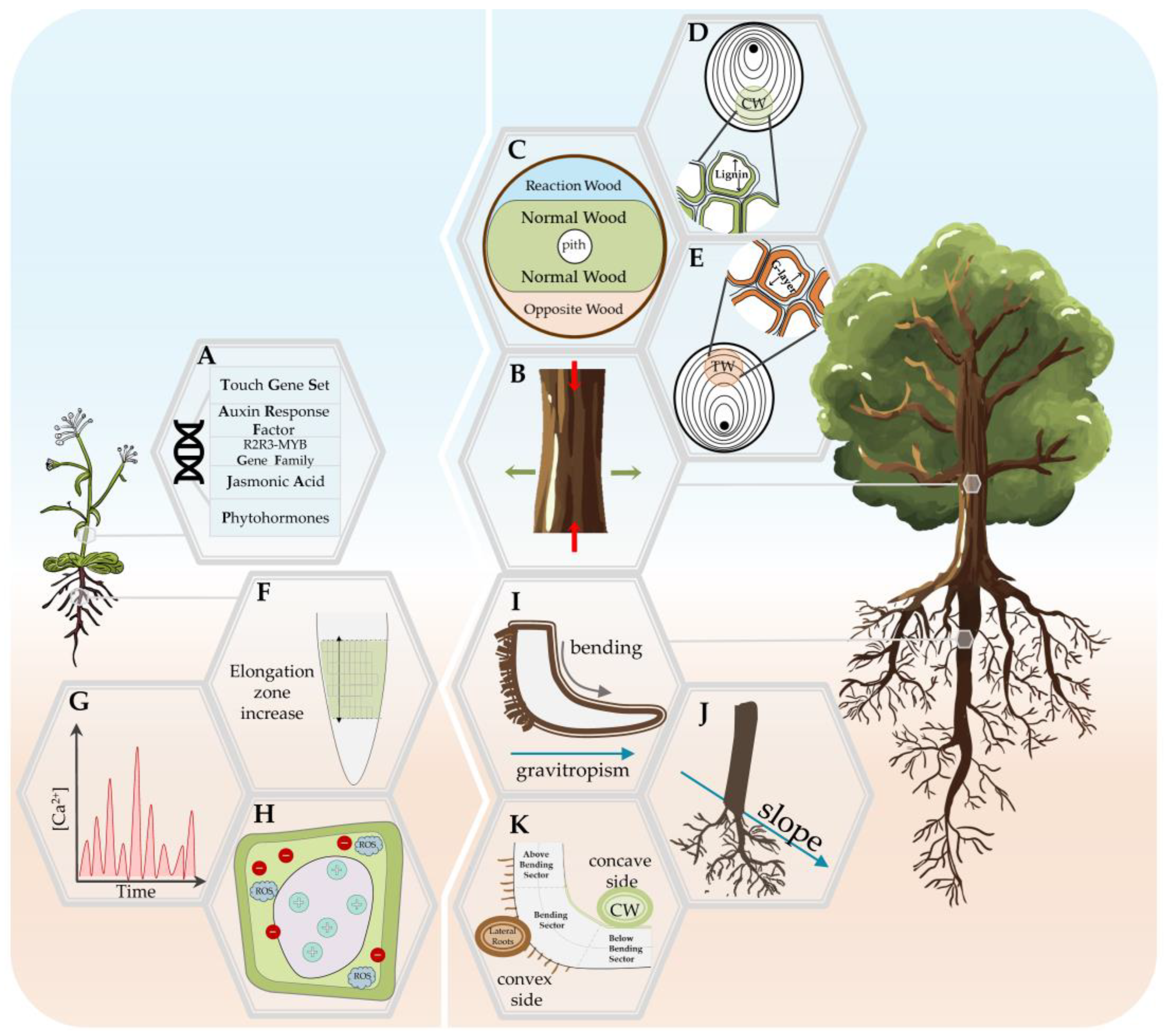
4. The Plant Response to Mechanical Stress
4.1. Thigmomorphogenesis in Stem
4.1.1. Mechanical Stress Response in Annual Plants: Arabidopsis Model
4.1.2. Woody Plants’ Stem Response—The Role of Reaction Wood
4.2. Thigmomorphogenesis in the Roots
4.2.1. Young Roots Response to Mechanical Stress
4.2.2. Woody Roots Response
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamers, J.; Van Der Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef]
- Elhakeem, A.; Markovic, D.; Broberg, A.; Anten, N.P.R.; Ninkovic, V. Aboveground Mechanical Stimuli Affect Belowground Plant-Plant Communication. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Coutand, C. The Effect of Mechanical Stress on Plant Susceptibility to Pests: A Mini Opinion Review. Plants 2020, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Brenya, E.; Pervin, M.; Chen, Z.H.; Tissue, D.T.; Johnson, S.; Braam, J.; Cazzonelli, C.I. Mechanical Stress Acclimation in Plants: Linking Hormones and Somatic Memory to Thigmomorphogenesis. Plant Cell Environ. 2022, 45, 989–1010. [Google Scholar] [CrossRef]
- Sparke, M.A.; Wünsche, J.N. Mechanosensing of Plants. In Horticultural Reviews; Warrington, I., Ed.; John Wiley & Sons, Inc.: Wiley Online Library, 2019; Volume 47, pp. 43–83. ISBN 9781119625407. [Google Scholar]
- Trinh, D.-C.; Alonso-Serra, J.; Asaoka, M.; Colin, L.; Cortes, M.; Malivert, A.; Takatani, S.; Zhao, F.; Traas, J.; Trehin, C. How Mechanical Forces Shape Plant Organs. Curr. Biol. 2021, 31, R143–R159. [Google Scholar] [CrossRef] [PubMed]
- Pilate, G.; Déjardin, A.; Laurans, F.; Leplé, J.C. Tension Wood as a Model for Functional Genomics of Wood Formation. New Phytol. 2004, 164, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, B.; Berry, P.; Moulia, B. Review: Wind Impacts on Plant Growth, Mechanics and Damage. Plant Sci. 2016, 245, 94–118. [Google Scholar] [CrossRef]
- Jonsson, K.; Ma, Y.; Routier-Kierzkowska, A.-L.; Bhalerao, R.P. Multiple Mechanisms behind Plant Bending. Nat. Plants 2022, 9, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Gong, M. Mechanical Stimulation-Induced Cross-Adaptation in Plants: An Overview. J. Plant Biol. 2011, 54, 358–364. [Google Scholar] [CrossRef]
- Coutand, C.; Mitchell, S.J. Editorial: Mechanical Signaling in Plants: From Perception to Consequences for Growth and Morphogenesis (Thigmomorphogenesis) and Ecological Significance. Front. Plant Sci. 2016, 7, 6–7. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Hamant, O. Widespread Mechanosensing Controls the Structure behind the Architecture in Plants. Curr. Opin. Plant Biol. 2013, 16, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A. Mechanical Feedback-Loop Regulation of Morphogenesis in Plants. Development 2020, 147, dev177964. [Google Scholar] [CrossRef]
- Landrein, B.; Hamant, O. How Mechanical Stress Controls Microtubule Behavior and Morphogenesis in Plants: History, Experiments and Revisited Theories. Plant J. 2013, 75, 324–338. [Google Scholar] [CrossRef]
- Du, F.; Jiao, Y. Mechanical Control of Plant Morphogenesis: Concepts and Progress. Curr. Opin. Plant Biol. 2020, 57, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R. Coordination of Plant Cell Growth and Division: Collective Control or Mutual Agreement? Curr. Opin. Plant Biol. 2016, 34, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Krupinski, P.; Wightman, R.; Milani, P.; Berquand, A.; Boudaoud, A.; Hamant, O.; Jönsson, H.; Meyerowitz, E.M. Subcellular and Supracellular Mechanical Stress Prescribes Cytoskeleton Behavior in Arabidopsis Cotyledon Pavement Cells. eLife 2014, 3, e01967. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortty, B.; Willemsen, V.; de Zeeuw, T.; Liao, C.-Y.; Weijers, D.; Mulder, B.; Scheres, B. A Plausible Microtubule-Based Mechanism for Cell Division Orientation in Plant Embryogenesis. Curr. Biol. 2018, 28, 3031–3043. [Google Scholar] [CrossRef]
- Toyota, M.; Gilroy, S. Gravitropism and Mechanical Signaling in Plants. Am. J. Bot. 2013, 100, 111–125. [Google Scholar] [CrossRef]
- Robinson, S. Mechanobiology of Cell Division in Plant Growth. New Phytol. 2021, 231, 559–564. [Google Scholar] [CrossRef]
- Zeng, J.; Li, X.; Ge, Q.; Dong, Z.; Luo, L.; Tian, Z.; Zhao, Z. Endogenous Stress-Related Signal Directs Shoot Stem Cell Fate in Arabidopsis thaliana. Nat. Plants 2021, 7, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Love, J.; Björklund, S.; Vahala, J.; Hertzberg, M.; Kangasjärvi, J.; Sundberg, B. Ethylene Is an Endogenous Stimulator of Cell Division in the Cambial Meristem of Populus. Proc. Natl. Acad. Sci. USA 2009, 106, 5984–5989. [Google Scholar] [CrossRef] [PubMed]
- Telewski, F.W. Thigmomorphogenesis: The Response of Plants to Mechanical Perturbation. Italus Hortus 2016, 23, 1–16. [Google Scholar]
- Moulia, B.; Coutand, C.; Julien, J.L. Mechanosensitive Control of Plant Growth: Bearing the Load, Sensing, Transducing, and Responding. Front. Plant Sci. 2015, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Tasaka, M.; Morita, M.T. Mechanism of Higher Plant Gravity Sensing. Am. J. Bot. 2013, 100, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Franchel, J.; Venisse, J.-S.; Drevet, J.R.; Label, P.; Coutand, C.; Roeckel-Drevet, P. Early Transcriptional Response to Gravistimulation in Poplar without Phototropic Confounding Factors. AoB Plants 2021, 13, plaa071. [Google Scholar] [CrossRef] [PubMed]
- De Zio, E.; Montagnoli, A.; Karady, M.; Terzaghi, M.; Sferra, G.; Antoniadi, I.; Scippa, G.S.; Ljung, K.; Chiatante, D.; Trupiano, D. Reaction Wood Anatomical Traits and Hormonal Profiles in Poplar Bent Stem and Root. Front. Plant Sci. 2020, 11, 590985. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, M.J. Wind and Other Mechanical Effects in the Development and Behavior of Plants, with Special Emphasis on the Role of Hormones. In Hormonal Regulation of Development III; Springer: Berlin, Germany, 1985; pp. 444–484. [Google Scholar]
- Vogel, S. Life in Moving Fluids: The Physical Biology of Flow—Revised and Expanded Second Edition; NED-New edition; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Telewski, F.W. Wind induced physiological and developmental responses in trees. In Wind and Trees; Coutts, M.P., Grace, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 237–263. [Google Scholar]
- Puijalon, S.; Bornette, G.; Sagnes, P. Adaptations to Increasing Hydraulic Stress: Morphology, Hydrodynamics and Fitness of Two Higher Aquatic Plant Species. J. Exp. Bot. 2005, 56, 777–786. [Google Scholar] [CrossRef]
- Du, S.; Yamamoto, F. An Overview of the Biology of Reaction Wood Formation. J. Integr. Plant Biol. 2007, 49, 131–143. [Google Scholar] [CrossRef]
- Van Moerkercke, A.; Duncan, O.; Zander, M.; Šimura, J.; Broda, M.; Vanden Bossche, R.; Lewsey, M.G.; Lama, S.; Singh, K.B.; Ljung, K. A MYC2/MYC3/MYC4-Dependent Transcription Factor Network Regulates Water Spray-Responsive Gene Expression and Jasmonate Levels. Proc. Natl. Acad. Sci. USA 2019, 116, 23345–23356. [Google Scholar] [CrossRef]
- Braam, J.; Davis, R.W. Rain-, Wind-, and Touch-Induced Expression of Calmodulin and Calmodulin-Related Genes in Arabidopsis. Cell 1990, 60, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Nomoto, M.; Itaya, T.; Aratani, Y.; Iwamoto, M.; Matsuura, T.; Hayashi, Y.; Mori, T.; Skelly, M.J.; Yamamoto, Y.Y. Mechanosensory Trichome Cells Evoke a Mechanical Stimuli–Induced Immune Response in Arabidopsis thaliana. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Waterman, J.M.; Cazzonelli, C.I.; Hartley, S.E.; Johnson, S.N. Simulated Herbivory: The Key to Disentangling Plant Defence Responses. Trends Ecol. Evol. 2019, 34, 447–458. [Google Scholar] [CrossRef]
- Choi, W.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating Rapid Long-distance Signaling in Plants with Ca2+, ROS and Electrical Signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef]
- Kloth, K.J.; Dicke, M. Rapid Systemic Responses to Herbivory. Curr. Opin. Plant Biol. 2022, 68, 102242. [Google Scholar] [CrossRef]
- Walling, L.L. The Myriad Plant Responses to Herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Johnson, S.N.; Erb, M.; Hartley, S.E. Roots under Attack: Contrasting Plant Responses to Below-and Aboveground Insect Herbivory. New Phytol. 2016, 210, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Tocquard, K.; Venisse, J.S.; Legué, V.; Roeckel-Drevet, P. Gravity Sensing, a Largely Misunderstood Trigger of Plant Orientated Growth. Front. Plant Sci. 2014, 5, 610. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, N.; Morita, M.T. Gravity Sensing and Responses in the Coordination of the Shoot Gravitropic Setpoint Angle. New Phytol. 2022, 236, 1637–1654. [Google Scholar] [CrossRef]
- Barlow, P.W.; Parker, J.S.; Butler, R.; Brain, P. Gravitropism of Primary Roots of Zea mays L. at Different Displacement Angles. Ann. Bot. 1993, 71, 783–788. [Google Scholar] [CrossRef]
- Chiatante, D.; Baraldi, A.; Di Iorio, A.; Sarnataro, M.; Scippa, G.S. Root Response to Mechanical Stress in Plants Growing on Slopes: An Experimental System for Morphological, Biochemical and Molecular Analysis. In Roots: The Dynamic Interface between Plants and the Earth; Springer: Berlin, Germany, 2003; pp. 427–437. [Google Scholar]
- Wang, J.-W.; Wang, L.-J.; Mao, Y.-B.; Cai, W.-J.; Xue, H.-W.; Chen, X.-Y. Control of Root Cap Formation by MicroRNA-Targeted Auxin Response Factors in Arabidopsis. Plant Cell 2005, 17, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrink, J.P.; Kiss, J.Z. Plant Responses to Gravity. Semin. Cell Dev. Biol. 2019, 92, 122–125. [Google Scholar]
- Su, S.-H.; Keith, M.A.; Masson, P.H. Gravity Signaling in Flowering Plant Roots. Plants 2020, 9, 1290. [Google Scholar] [CrossRef] [PubMed]
- Hosamani, R.; Swamy, B.K.; Dsouza, A.; Sathasivam, M. Plant Responses to Hypergravity: A Comprehensive Review. Planta 2023, 257, 17. [Google Scholar] [CrossRef] [PubMed]
- Soga, K. Resistance of Plants to Gravitational Force. J. Plant Res. 2013, 126, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.K.; Hosamani, R.; Sathasivam, M.; Chandrashekhar, S.S.; Reddy, U.G.; Moger, N. Novel Hypergravity Treatment Enhances Root Phenotype and Positively Influences Physio-Biochemical Parameters in Bread Wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 1–16. [Google Scholar]
- Sathasivam, M.; Swamy, B.K.; Krishnan, K.; Sharma, R.; Nayak, S.N.; Uppar, D.S.; Hosamani, R. Insights into the Molecular Basis of Hypergravity-Induced Root Growth Phenotype in Bread Wheat (Triticum aestivum L.). Genomics 2022, 114, 110307. [Google Scholar] [CrossRef]
- Soga, K.; Wakabayashi, K.; Hoson, T. Growth and Cortical Microtubule Dynamics in Shoot Organs under Microgravity and Hypergravity Conditions. Plant Signal. Behav. 2018, 13, 135–144. [Google Scholar] [CrossRef]
- Toyota, M.; Ikeda, N.; Sawai-Toyota, S.; Kato, T.; Gilroy, S.; Tasaka, M.; Morita, M.T. Amyloplast Displacement Is Necessary for Gravisensing in Arabidopsis Shoots as Revealed by a Centrifuge Microscope. Plant J. 2013, 76, 648–660. [Google Scholar] [CrossRef]
- Scippa, G.S.; Di Michele, M.; Di Iorio, A.; Costa, A.; Lasserre, B.; Chiatante, D. The Response of Spartium junceum Roots to Slope: Anchorage and Gene Factors. Ann. Bot. 2006, 97, 857–866. [Google Scholar] [CrossRef]
- Chiatante, D.; Scippa, G.S.; Di Iorio, A.; Sarnataro, M. The Stability of Trees Growing on Slope Depends upon a Particular Conformational Structure Imposed by Mechanical Stress in Their Root System. In Proceedings of the International Conference: Forest Research: A Challenge for an Integrated European Approach, Thessaloniki, Greece, 27 August 2001; pp. 477–482. [Google Scholar]
- Chiatante, D.; Sarnataro, M.; Fusco, S.; Di Iorio, A.; Scippa, G.S. Modification of Root Morphological Parameters and Root Architecture in Seedlings of Fraxinus ornus L. and Spartium junceum L. Growing on Slopes. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2003, 137, 47–55. [Google Scholar]
- Lombardi, F.; Scippa, G.S.; Lasserre, B.; Montagnoli, A.; Tognetti, R.; Marchetti, M.; Chiatante, D. The Influence of Slope on Spartium junceum Root System: Morphological, Anatomical and Biomechanical Adaptation. J. Plant Res. 2017, 130, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Pigliucci, M. Touchy and Bushy: Phenotypic Plasticity and Integration in Response to Wind Stimulation in Arabidopsis thaliana. Int. J. Plant Sci. 2002, 163, 399–408. [Google Scholar] [CrossRef]
- Liu, Y.; Schieving, F.; Stuefer, J.F.; Anten, N.P.R. The Effects of Mechanical Stress and Spectral Shading on the Growth and Allocation of Ten Genotypes of a Stoloniferous Plant. Ann. Bot. 2007, 99, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.L.; Monshausen, G.B.; Krol, A.; Gilroy, S. Mechanical Stimuli Modulate Lateral Root Organogenesis. Plant Physiol. 2009, 151, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.L.; Strohm, A.K.; Masson, P.H. Gravity Sensing and Signal Transduction in Vascular Plant Primary Roots. Am. J. Bot. 2013, 100, 126–142. [Google Scholar] [CrossRef]
- Jacobsen, A.G.R.; Jervis, G.; Xu, J.; Topping, J.F.; Lindsey, K. Root Growth Responses to Mechanical Impedance Are Regulated by a Network of ROS, Ethylene and Auxin Signalling in Arabidopsis. New Phytol. 2021, 231, 225–242. [Google Scholar] [CrossRef]
- Börnke, F.; Rocksch, T. Thigmomorphogenesis—Control of Plant Growth by Mechanical Stimulation. Sci. Hortic. (Amsterdam). 2018, 234, 344–353. [Google Scholar] [CrossRef]
- Andersson-Gunnerås, S.; Mellerowicz, E.J.; Love, J.; Segerman, B.; Ohmiya, Y.; Coutinho, P.M.; Nilsson, P.; Henrissat, B.; Moritz, T.; Sundberg, B. Biosynthesis of Cellulose-Enriched Tension Wood in Populus: Global Analysis of Transcripts and Metabolites Identifies Biochemical and Developmental Regulators in Secondary Wall Biosynthesis. Plant J. 2006, 45, 144–165. [Google Scholar] [CrossRef]
- Coutand, C.; Martin, L.; Leblanc-Fournier, N.; Decourteix, M.; Julien, J.L.; Moulia, B. Strain Mechanosensing Quantitatively Controls Diameter Growth and PtaZFP2 Gene Expression in Poplar. Plant Physiol. 2009, 151, 223–232. [Google Scholar] [CrossRef]
- Vahala, J.; Felten, J.; Love, J.; Gorzsás, A.; Gerber, L.; Lamminmäki, A.; Kangasjärvi, J.; Sundberg, B. A Genome-Wide Screen for Ethylene-Induced Ethylene Response Factors (ERFs) in Hybrid Aspen Stem Identifies ERF Genes That Modify Stem Growth and Wood Properties. New Phytol. 2013, 200, 511–522. [Google Scholar] [CrossRef]
- Martin, L.; Decourteix, M.; Badel, E.; Huguet, S.; Moulia, B.; Julien, J.L.; Leblanc-Fournier, N. The Zinc Finger Protein PtaZFP2 Negatively Controls Stem Growth and Gene Expression Responsiveness to External Mechanical Loads in Poplar. New Phytol. 2014, 203, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Pomiès, L.; Decourteix, M.; Franchel, J.; Moulia, B.; Leblanc-Fournier, N. Poplar Stem Transcriptome Is Massively Remodelled in Response to Single or Repeated Mechanical Stimuli. BMC Genomics 2017, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Niez, B.; Dlouha, J.; Moulia, B.; Badel, E. Water-Stressed or Not, the Mechanical Acclimation Is a Priority Requirement for Trees. Trees Struct. Funct. 2019, 33, 279–291. [Google Scholar] [CrossRef]
- Ma, Y.; MacMillan, C.P.; de Vries, L.; Mansfield, S.D.; Hao, P.; Ratcliffe, J.; Bacic, A.; Jonhson, K.L. FLA11 and FLA12 Glycoproteins Fine-tune Stem Secondary Wall Properties in Response to Mechanical Stress. New Phytol. 2021, 223.4, 1750–1767. [Google Scholar] [CrossRef]
- Wu, L.; Joshi, C.P.; Chiang, V.L. A Xylem-specific Cellulose Synthase Gene from Aspen (Populus tremuloides) Is Responsive to Mechanical Stress. Plant J. 2000, 22, 495–502. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Dong, M.; Yu, F.-H.; Jiang, H.; Yu, S.-Q.; Lin, X.-Q.; He, W.-M. Mechanical Shaking and Soil Water Affect the Growth of Psammochloa villosa in the Mu Us Sandland. J. Arid Environ. 2011, 75, 974–977. [Google Scholar] [CrossRef]
- Coutand, C.; Chevolot, M.; Lacointe, A.; Rowe, N.; Scotti, I. Mechanosensing of Stem Bending and Its Interspecific Variability in Five Neotropical Rainforest Species. Ann. Bot. 2010, 105, 341–347. [Google Scholar] [CrossRef]
- Ishihara, K.; Lee, E.; Borthakur, D. Thigmomorphogenesis: Morphological, Biochemical Changes, and Transcriptional Level Changes in Response to Mechanical Stress in Acacia Koa A. Gray. Can. J. For. Res. 2016, 47, 1–299. [Google Scholar]
- Tixier, A.; Badel, E.; Franchel, J.; Lakhal, W.; Leblanc-Fournier, N.; Moulia, B.; Julien, J.L. Growth and Molecular Responses to Long-Distance Stimuli in Poplars: Bending vs Flame Wounding. Physiol. Plant. 2014, 150, 225–237. [Google Scholar] [CrossRef]
- Tinturier, E.; Badel, É.; Leblanc-Fournier, N.; Julien, J.L. Stem Bending Generates Electrical Response in Poplar. Physiol. Plant. 2021, 173, 954–960. [Google Scholar] [CrossRef]
- Chaki, M.; Valderrama, R.; Fernández-Ocaña, A.M.; Carreras, A.; Gómez-Rodríguez, M.V.; Pedrajas, J.R.; Begara-Morales, J.C.; Sánchez-Calvo, B.; Luque, F.; Leterrier, M.; et al. Mechanical Wounding Induces a Nitrosative Stress by Down-Regulation of GSNO Reductase and an Increase in S-Nitrosothiols in Sunflower (Helianthus annuus) Seedlings. J. Exp. Bot. 2011, 62, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, U.; Singh, A.; Frankenstein, C.; Möller, R. Cell Wall Modifications in Woody Stems Induced by Mechanical Stress. New Zeal. J. For. Sci. 2006, 36, 72–86. [Google Scholar]
- Walley, J.W.; Coughlan, S.; Hudson, M.E.; Covington, M.F.; Kaspi, R.; Banu, G.; Harmer, S.L.; Dehesh, K. Mechanical Stress Induces Biotic and Abiotic Stress Responses via a Novel Cis-Element. PLoS Genet. 2007, 3, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, G.V.; Krutovsky, K.V. Mechanical Stress Effects on Transcriptional Regulation of Genes Encoding Microtubule- and Actin-Associated Proteins. Physiol. Mol. Biol. Plants 2022, 28, 17–30. [Google Scholar] [CrossRef]
- Van Gaal, T.; Erwin, J.E. Diurnal Variation in Thigmotropic Inhibition of Stem Elongation. Horttechnology 2005, 15, 291–294. [Google Scholar] [CrossRef]
- Bossdorf, O.; Pigliucci, M. Plasticity to Wind Is Modular and Genetically Variable in Arabidopsis thaliana. Evol. Ecol. 2009, 23, 669–685. [Google Scholar] [CrossRef]
- Puijalon, S.; Bouma, T.J.; Douady, C.J.; van Groenendael, J.; Anten, N.P.R.; Martel, E.; Bornette, G. Plant Resistance to Mechanical Stress: Evidence of an Avoidance–Tolerance Trade-off. New Phytol. 2011, 191, 1141–1149. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Casado-Garcia, R.; Pierik, R.; Pons, T.L. Ethylene Sensitivity Affects Changes in Growth Patterns, but Not Stem Properties, in Response to Mechanical Stress in Tobacco. Physiol. Plant. 2006, 128, 274–282. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Casado-Garcia, R.; Nagashima, H. Effects of Mechanical Stress and Plant Density on Mechanical Characteristics, Growth, and Lifetime Reproduction of Tobacco Plants. Am. Nat. 2005, 166, 650–660. [Google Scholar] [CrossRef]
- Garner, L.C.; Björkman, T. Mechanical Conditioning of Tomato Seedlings Improves Transplant Quality without Deleterious Effects on Field Performance. HortScience 1999, 34, 848–851. [Google Scholar] [CrossRef]
- Mickovski, S.B.; Ennos, A.R. The Effect of Unidirectional Stem Flexing on Shoot and Root Morphology and Architecture in Young Pinus sylvestris Trees. Can. J. For. Res. 2003, 33, 2202–2209. [Google Scholar] [CrossRef]
- Niklas, K.J. Effects of Vibration on Mechanical Properties and Biomass Allocation Pattern of Capsella bursa-pastoris (Cruciferae). Ann. Bot. 1998, 82, 147–156. [Google Scholar] [CrossRef]
- Takano, M.; Takahashi, H.; Suge, H. Mechanical Stress and Gibberellin: Regulation of Hollowing Induction in the Stem of a Bean Plant, Phaseolus vulgaris L. Plant Cell Physiol. 1995, 36, 101–108. [Google Scholar] [CrossRef]
- Saidi, I.; Ammar, S.; Demont-Caulet, N.; Thévenin, J.; Lapierre, C.; Bouzid, S.; Jouanin, L. Thigmomorphogenesis in Solanum lycopersicum: Morphological and Biochemical Responses in Stem after Mechanical Stimulation. Plant Sci. 2009, 177, 1–6. [Google Scholar] [CrossRef]
- Braam, J.; Sistrunk, M.L.; Polisensky, D.H.; Xu, W.; Purugganan, M.M.; Antosiewicz, D.M.; Campbell, P.; Johnson, K.A. Plant Responses to Environmental Stress: Regulation and Functions of the Arabidopsis TCH Genes. Planta 1997, 203, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Fal, K.; Hamant, O.; Haswell, E.S. The RNA Polymerase-Associated Factor 1 Complex Is Required for Plant Touch Responses. J. Exp. Bot. 2017, 68, 499–511. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Chen, J.; Knox, J.P. Cell Wall Pectic Arabinans Influence the Mechanical Properties of Arabidopsis thaliana Inflorescence Stems and Their Response to Mechanical Stress. Plant cell Physiol. 2013, 54, 1278–1288. [Google Scholar] [CrossRef]
- Scippa, G.S.; Trupiano, D.; Rocco, M.; Di Iorio, A.; Chiatante, D. Unravelling the Response of Poplar (Populus nigra) Roots to Mechanical Stress Imposed by Bending. Plant Biosyst. 2008, 142, 401–413. [Google Scholar] [CrossRef]
- Baral, A.; Aryal, B.; Jonsson, K.; Morris, E.; Demes, E.; Takatani, S.; Verger, S.; Xu, T.; Bennett, M.; Hamant, O.; et al. External Mechanical Cues Reveal a Katanin-Independent Mechanism behind Auxin-Mediated Tissue Bending in Plants. Dev. Cell 2021, 56, 67–80.e3. [Google Scholar] [CrossRef]
- Jonsson, K.; Lathe, R.S.; Kierzkowski, D.; Routier-Kierzkowska, A.-L.; Hamant, O.; Bhalerao, R.P. Mechanochemical Feedback Mediates Tissue Bending Required for Seedling Emergence. Curr. Biol. 2021, 31, 1154–1164. [Google Scholar] [CrossRef]
- Ditengou, F.A.; Teale, W.D.; Kochersperger, P.; Flittner, K.A.; Kneuper, I.; Van Der Graaff, E.; Nziengui, H.; Pinosa, F.; Li, X.; Nitschke, R.; et al. Mechanical Induction of Lateral Root Initiation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 18818–18823. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Park, J.M.; Cho, H.S.; Jeon, J.H. PIN-mediated Polar Auxin Transport Facilitates Root−Obstacle Avoidance. New Phytol. 2020, 225, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Colombi, T.; Braun, S.; Keller, T.; Walter, A. Artificial Macropores Attract Crop Roots and Enhance Plant Productivity on Compacted Soils. Sci. Total Environ. 2017, 574, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, J.; Horn, R.; Pietrusiewicz, J.; Siczek, A. Effects of Soil Compaction on Root Elongation and Anatomy of Different Cereal Plant Species. Soil Tillage Res. 2012, 121, 74–81. [Google Scholar] [CrossRef]
- Bingham, I.J.; Bengough, A.G.; Rees, R.M. Soil Compaction–N Interactions in Barley: Root Growth and Tissue Composition. Soil Tillage Res. 2010, 106, 241–246. [Google Scholar] [CrossRef]
- Iijima, M.; Kato, J.; Taniguchi, A. Combined Soil Physical Stress of Soil Drying, Anaerobiosis and Mechanical Impedance to Seedling Root Growth of Four Crop Species. Plant Prod. Sci. 2007, 10, 451–459. [Google Scholar] [CrossRef]
- Kolb, E.; Hartmann, C.; Genet, P. Radial Force Development during Root Growth Measured by Photoelasticity. Plant Soil 2012, 360, 19–35. [Google Scholar] [CrossRef]
- Potocka, I.; Szymanowska-Pułka, J.; Karczewski, J.; Nakielski, J. Effect of Mechanical Stress on Zea Root Apex. I. Mechanical Stress Leads to the Switch from Closed to Open Meristem Organization. J. Exp. Bot. 2011, 62, 4583–4593. [Google Scholar] [CrossRef]
- Demidchik, V.; Shabala, S.; Isayenkov, S.; Cuin, T.A.; Pottosin, I. Calcium Transport across Plant Membranes: Mechanisms and Functions. New Phytol. 2018, 220, 49–69. [Google Scholar] [CrossRef]
- Dodd, A.N.; Kudla, J.; Sanders, D. The Language of Calcium Signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium Signaling Network in Plants: An Overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Leitão, N.; Dangeville, P.; Carter, R.; Charpentier, M. Nuclear Calcium Signatures Are Associated with Root Development. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johns, S.; Hagihara, T.; Toyota, M.; Gilroy, S. The Fast and the Furious: Rapid Long-Range Signaling in Plants. Plant Physiol. 2021, 185, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Köster, P.; DeFalco, T.A.; Zipfel, C. Ca2+ Signals in Plant Immunity. EMBO J. 2022, 41, e110741. [Google Scholar] [CrossRef] [PubMed]
- Suda, H.; Toyota, M. Integration of Long-Range Signals in Plants: A Model for Wound-Induced Ca2+, Electrical, ROS, and Glutamate Waves. Curr. Opin. Plant Biol. 2022, 69, 102270. [Google Scholar] [CrossRef]
- Hander, T.; Fernández-Fernández, Á.D.; Kumpf, R.P.; Willems, P.; Schatowitz, H.; Rombaut, D.; Staes, A.; Nolf, J.; Pottie, R.; Yao, P. Damage on Plants Activates Ca2+-Dependent Metacaspases for Release of Immunomodulatory Peptides. Science 2019, 363, eaar7486. [Google Scholar] [CrossRef]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.; Zhang, W.; Su, Y.; Xiao, L.; Deng, H.; Xie, D. Injury Activates Ca2+/Calmodulin-Dependent Phosphorylation of JAV1-JAZ8-WRKY51 Complex for Jasmonate Biosynthesis. Mol. Cell 2018, 70, 136–149.e7. [Google Scholar] [CrossRef]
- Tsugama, D.; Liu, S.; Fujino, K.; Takano, T. Calcium Signalling Regulates the Functions of the BZIP Protein VIP1 in Touch Responses in Arabidopsis thaliana. Ann. Bot. 2018, 122, 1219–1229. [Google Scholar] [CrossRef]
- Bellandi, A.; Papp, D.; Breakspear, A.; Joyce, J.; Johnston, M.G.; de Keijzer, J.; Raven, E.C.; Ohtsu, M.; Vincent, T.R.; Miller, A.J. Diffusion and Bulk Flow of Amino Acids Mediate Calcium Waves in Plants. Sci. Adv. 2022, 8, eabo6693. [Google Scholar] [CrossRef]
- Waadt, R.; Köster, P.; Andrés, Z.; Waadt, C.; Bradamante, G.; Lampou, K.; Kudla, J.; Schumacher, K. Dual-Reporting Transcriptionally Linked Genetically Encoded Fluorescent Indicators Resolve the Spatiotemporal Coordination of Cytosolic Abscisic Acid and Second Messenger Dynamics in Arabidopsis. Plant Cell 2020, 32, 2582–2601. [Google Scholar] [CrossRef]
- Suda, H.; Mano, H.; Toyota, M.; Fukushima, K.; Mimura, T.; Tsutsui, I.; Hedrich, R.; Tamada, Y.; Hasebe, M. Calcium Dynamics during Trap Closure Visualized in Transgenic Venus Flytrap. Nat. Plants 2020, 6, 1219–1224. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, P.J. Ca2+talyzing Initial Responses to Environmental Stresses. Trends Plant Sci. 2021, 26, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J.M. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef] [PubMed]
- Zelman, A.K.; Dawe, A.; Gehring, C.; Berkowitz, G.A. Evolutionary and Structural Perspectives of Plant Cyclic Nucleotide-Gated Cation Channels. Front. Plant Sci. 2012, 3, 95. [Google Scholar] [CrossRef]
- Duszyn, M.; Świeżawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic Nucleotide Gated Channels (CNGCs) in Plant Signalling—Current Knowledge and Perspectives. J. Plant Physiol. 2019, 241, 153035. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Toyota, M.; Moeder, W.; Chin, K.; Fortuna, A.; Champigny, M.; Vanneste, S.; Gilroy, S.; Beeckman, T.; Nambara, E. Cyclic Nucleotide-Gated Ion Channel 2 Modulates Auxin Homeostasis and Signaling. Plant Physiol. 2021, 187, 1690–1703. [Google Scholar] [CrossRef]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.I.; Li, L. A Calmodulin-Gated Calci-um Channel Links Pathogen Patterns to Plant Immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Wang, L.; Ning, Y.; Sun, J.; Wilkins, K.A.; Matthus, E.; McNelly, R.E.; Dark, A.; Rubio, L.; Moeder, W.; Yoshioka, K. Arabidopsis thaliana CYCLIC NUCLEOTIDE-GATED CHANNEL2 Mediates Extracellular ATP Signal Transduction in Root Epidermis. New Phytol. 2022, 234, 412–421. [Google Scholar] [CrossRef]
- Tan, Y.-Q.; Yang, Y.; Shen, X.; Zhu, M.; Shen, J.; Zhang, W.; Hu, H.; Wang, Y.-F. Multiple Cyclic Nucleotide-Gated Channels Function as ABA-Activated Ca2+ Channels Required for ABA-Induced Stomatal Closure in Arabidopsis. Plant Cell 2022 35, 239–259. [CrossRef]
- Tan, Y.-Q.; Yang, Y.; Zhang, A.; Fei, C.-F.; Gu, L.-L.; Sun, S.-J.; Xu, W.; Wang, L.; Liu, H.; Wang, Y.-F. Three CNGC Family Members, CNGC5, CNGC6, and CNGC9, Are Required for Constitutive Growth of Arabidopsis Root Hairs as Ca2+-Permeable Channels. Plant Commun. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Shih, H.-W.; Miller, N.D.; Dai, C.; Spalding, E.P.; Monshausen, G.B. The Receptor-like Kinase FERONIA Is Required for Mechanical Signal Transduction in Arabidopsis Seedlings. Curr. Biol. 2014, 24, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; DeFalco, T.A.; Karia, P.; Snedden, W.A.; Moeder, W.; Yoshioka, K.; Dietrich, P. Calmodulin as a Ca2+-Sensing Subunit of Arabidopsis Cyclic Nucleotide-Gated Channel Complexes. Plant Cell Physiol. 2017, 58, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Moeder, W.; Yoshioka, K. Opening the Gates: Insights into Cyclic Nucleotide-Gated Channel-Mediated Signaling. Trends Plant Sci. 2016, 21, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Dutta, S.; Pal, A.; Sengupta, M.; Chattopadhyay, S. Calmodulin7: Recent Insights into Emerging Roles in Plant Development and Stress. Plant Mol. Biol. 2021, 107, 1–20. [Google Scholar] [CrossRef]
- Zeb, Q.; Wang, X.; Hou, C.; Zhang, X.; Dong, M.; Zhang, S.; Zhang, Q.; Ren, Z.; Tian, W.; Zhu, H. The Interaction of CaM7 and CNGC14 Regulates Root Hair Growth in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 887–896. [Google Scholar] [CrossRef]
- Brost, C.; Studtrucker, T.; Reimann, R.; Denninger, P.; Czekalla, J.; Krebs, M.; Fabry, B.; Schumacher, K.; Grossmann, G.; Dietrich, P. Multiple Cyclic Nucleotide-gated Channels Coordinate Calcium Oscillations and Polar Growth of Root Hairs. Plant J. 2019, 99, 910–923. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, C.; Tian, W.; Li, L.; Zhu, H. Electrophysiological Studies Revealed CaM1-Mediated Regulation of the Arabidopsis Calcium Channel CNGC12. Front. Plant Sci. 2019, 10, 1090. [Google Scholar] [CrossRef]
- Świeżawska-Boniecka, B.; Duszyn, M.; Kwiatkowski, M.; Szmidt-Jaworska, A.; Jaworski, K. Cross Talk between Cyclic Nucleotides and Calcium Signaling Pathways in Plants–Achievements and Prospects. Front. Plant Sci. 2021, 12, 643560. [Google Scholar] [CrossRef]
- Meena, M.K.; Prajapati, R.; Krishna, D.; Divakaran, K.; Pandey, Y.; Reichelt, M.; Mathew, M.K.; Boland, W.; Mithöfer, A.; Vadassery, J. The Ca2+ Channel CNGC19 Regulates Arabidopsis Defense against Spodoptera Herbivory. Plant Cell 2019, 31, 1539–1562. [Google Scholar] [CrossRef]
- Pan, Y.; Chai, X.; Gao, Q.; Zhou, L.; Zhang, S.; Li, L.; Luan, S. Dynamic Interactions of Plant CNGC Subunits and Calmodulins Drive Oscillatory Ca2+ Channel Activities. Dev. Cell 2019, 48, 710–725. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, F.; Wen, Z.; Li, Y.; Wang, F.; Zhu, T.; Zhuo, W.; Jin, X.; Wang, Y.; Zhao, H. Genome-Wide Survey and Expression Analysis of the OSCA Gene Family in Rice. BMC Plant Biol. 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.E.; Dubin, A.E.; Whitwam, T.; Jojoa-Cruz, S.; Cahalan, S.M.; Mousavi, S.A.R.; Ward, A.B.; Patapoutian, A. OSCA/TMEM63 Are an Evolutionarily Conserved Family of Mechanically Activated Ion Channels. eLife 2018, 7, e41844. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, P.; Liu, Z.; Wang, L.; Huang, Z.; Zhang, S.; Wu, J. Genome-Wide Identification and Expression Analysis of the OSCA Gene Family in Pyrus bretschneideri. Can. J. Plant Sci. 2018, 98, 918–929. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Gilroy, S. Feeling Green: Mechanosensing in Plants. Trends Cell Biol. 2009, 19, 228–235. [Google Scholar] [CrossRef]
- Basu, D.; Haswell, E.S. Plant Mechanosensitive Ion Channels: An Ocean of Possibilities. Curr. Opin. Plant Biol. 2017, 40, 43–48. [Google Scholar] [CrossRef]
- Hamant, O.; Haswell, E.S. Life behind the Wall: Sensing Mechanical Cues in Plants. BMC Biol. 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S. Arabidopsis Plasma Membrane Protein Crucial for Ca2+ Influx and Touch Sensing in Roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef]
- Kurusu, T.; Yamanaka, T.; Nakano, M.; Takiguchi, A.; Ogasawara, Y.; Hayashi, T.; Iida, K.; Hanamata, S.; Shinozaki, K.; Iida, H. Involvement of the Putative Ca2+-Permeable Mechanosensitive Channels, NtMCA1 and NtMCA2, in Ca2+ Uptake, Ca2+-Dependent Cell Proliferation and Mechanical Stress-Induced Gene Expression in Tobacco (Nicotiana tabacum) BY-2 Cells. J. Plant Res. 2012, 125, 555–568. [Google Scholar] [CrossRef]
- Basu, D.; Haswell, E.S. The Mechanosensitive Ion Channel MSL10 Potentiates Responses to Cell Swelling in Arabidopsis Seedlings. Curr. Biol. 2020, 30, 2716–2728. [Google Scholar] [CrossRef]
- Engelsdorf, T.; Gigli-Bisceglia, N.; Veerabagu, M.; McKenna, J.F.; Vaahtera, L.; Augstein, F.; Van der Does, D.; Zipfel, C.; Hamann, T. The Plant Cell Wall Integrity Maintenance and Immune Signaling Systems Cooperate to Control Stress Responses in Arabidopsis thaliana. Sci. Signal. 2018, 11, eaao3070. [Google Scholar] [CrossRef]
- Okamoto, T.; Takatani, S.; Motose, H.; Iida, H.; Takahashi, T. The Root Growth Reduction in Response to Mechanical Stress Involves Ethylene-Mediated Microtubule Reorganization and Transmembrane Receptor-Mediated Signal Transduction in Arabidopsis. Plant Cell Rep. 2021, 40, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-Permeable Mechanosensitive Channels MCA1 and MCA2 Mediate Cold-Induced Cytosolic Ca2+ Increase and Cold Tolerance in Arabidopsis. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Otomi, Y.; Nakajima, Y.; Soga, K.; Wakabayashi, K.; Iida, H.; Hoson, T. MCA1 and MCA2 Are Involved in the Response to Hypergravity in Arabidopsis Hypocotyls. Plants 2020, 9, 590. [Google Scholar] [CrossRef]
- Haswell, E.S. MscS-like Proteins in Plants. Curr. Top. Membr. 2007, 58, 329–359. [Google Scholar]
- Haswell, E.S.; Peyronnet, R.; Barbier-Brygoo, H.; Meyerowitz, E.M.; Frachisse, J.-M. Two MscS Homologs Provide Mechanosensitive Channel Activities in the Arabidopsis Root. Curr. Biol. 2008, 18, 730–734. [Google Scholar] [CrossRef]
- Veley, K.M.; Maksaev, G.; Frick, E.M.; January, E.; Kloepper, S.C.; Haswell, E.S. Arabidopsis MSL10 Has a Regulated Cell Death Signaling Activity That Is Separable from Its Mechanosensitive Ion Channel Activity. Plant Cell 2014, 26, 3115–3131. [Google Scholar] [CrossRef]
- Moe-Lange, J.; Gappel, N.M.; Machado, M.; Wudick, M.M.; Sies, C.S.A.; Schott-Verdugo, S.N.; Bonus, M.; Mishra, S.; Hartwig, T.; Bezrutczyk, M. Interdependence of a Mechanosensitive Anion Channel and Glutamate Receptors in Distal Wound Signaling. Sci. Adv. 2021, 7, eabg4298. [Google Scholar] [CrossRef]
- Tran, D.; Girault, T.; Guichard, M.; Thomine, S.; Leblanc-Fournier, N.; Moulia, B.; De Langre, E.; Allain, J.-M.; Frachisse, J.-M. Cellular Transduction of Mechanical Oscillations in Plants by the Plasma-Membrane Mechanosensitive Channel MSL10. Proc. Natl. Acad. Sci. USA 2021, 118, e1919402118. [Google Scholar] [CrossRef]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate Triggers Long-Distance, Calcium-Based Plant Defense Signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, X.; Liu, S.-Y.; Chai, L.-X.; Zhu, F.-F.; Zhang, X.-P.; Zou, J.-Z.; Wang, X.-B. Genetic Analysis of a Piezo-like Protein Suppressing Systemic Movement of Plant Viruses in Arabidopsis thaliana. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Fang, X.; Liu, B.; Shao, Q.; Huang, X.; Li, J.; Luan, S.; He, K. AtPiezo Plays an Important Role in Root Cap Mechanotransduction. Int. J. Mol. Sci. 2021, 22, 467. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.R.; Dubin, A.E.; Zeng, W.-Z.; Coombs, A.M.; Do, K.; Ghadiri, D.A.; Keenan, W.T.; Ge, C.; Zhao, Y.; Patapoutian, A. PIEZO Ion Channel Is Required for Root Mechanotransduction in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2102188118. [Google Scholar] [CrossRef] [PubMed]
- Radin, I.; Richardson, R.A.; Coomey, J.H.; Weiner, E.R.; Bascom, C.S.; Li, T.; Bezanilla, M.; Haswell, E.S. Plant PIEZO Homologs Modulate Vacuole Morphology during Tip Growth. Science 2021, 373, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, Z.; Qing, D.; Ren, F.; Liu, S.; Zheng, Q.; Liu, J.; Zhang, W.; Dai, C.; Wu, M. Quantitative and Functional Posttranslational Modification Proteomics Reveals That TREPH1 Plays a Role in Plant Touch-Delayed Bolting. Proc. Natl. Acad. Sci. USA 2018, 115, E10265–E10274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, Y.; Lu, P.; Feng, C.; Niu, Q.; Lin, G.; Kong, D.; Liu, L.; Luan, S.; Li, L. Arabidopsis MLO4 Functions as a Ca2+ Channel Essential for Mechanosensing in Root Tips. bioRxiv 2022. bioRxiv2022-06. [Google Scholar]
- Malabarba, J.; Meents, A.K.; Reichelt, M.; Scholz, S.S.; Peiter, E.; Rachowka, J.; Konopka-Postupolska, D.; Wilkins, K.A.; Davies, J.M.; Oelmüller, R.; et al. ANNEXIN1 Mediates Calcium-Dependent Systemic Defense in Arabidopsis Plants upon Herbivory and Wounding. New Phytol. 2021, 231, 243–254. [Google Scholar] [CrossRef]
- Braam, J. In Touch: Plant Responses to Mechanical Stimuli. New Phytol. 2004, 165, 373–389. [Google Scholar] [CrossRef]
- Hagihara, T.; Toyota, M. Mechanical Signaling in the Sensitive Plant Mimosa pudica L. Plants 2020, 9, 587. [Google Scholar] [CrossRef]
- Hagihara, T.; Mano, H.; Miura, T.; Hasebe, M.; Toyota, M. Calcium-Mediated Rapid Movements Defend against Herbivorous Insects in Mimosa pudica. Nat. Commun. 2022, 13, 6412. [Google Scholar] [CrossRef]
- Mitchell, C.A. Recent Advances in Plant Response to Mechanical Stress: Theory and Application. In Proceedings of the Recent Advances in Plant Response to Stress: Bridging the Gap between Science and Technology, Corvallis, OR, USA, 7 August 1994; Volume 31, pp. 31–35. [Google Scholar]
- Ennos, A.R. Wind as an Ecological Factor. Trends Ecol. Evol. 1997, 12, 108–111. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.H.; Shi, R.; Clark, C.; Li, L.; Chiang, V.L. Novel and and Mechanical Stress-Responsive MicroRNAs in Populus trichocarpa That Are Absent from Arabidopsis. Plant Cell 2005, 17, 2186–2203. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Sun, Y.H.; Chiang, V.L. Stress-Responsive MicroRNAs in Populus. Plant J. 2008, 55, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Han, K.H.; Park, S.; Yang, J. Plant Body Weight-Induced Secondary Growth in Arabidopsis and Its Transcription Phenotype Revealed by Whole-Transcriptome Profiling. Plant Physiol. 2004, 135, 1069–1083. [Google Scholar] [CrossRef]
- Sehr, E.M.; Agusti, J.; Lehner, R.; Farmer, E.E.; Schwarz, M.; Greb, T. Analysis of Secondary Growth in the Arabidopsis Shoot Reveals a Positive Role of Jasmonate Signalling in Cambium Formation. Plant J. 2010, 63, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Yao, C.; Henderson, Z.; Kim, S.; Braam, J. Arabidopsis Touch-Induced Morphogenesis Is Jasmonate Mediated and Protects against Pests. Curr. Biol. 2012, 22, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Uyttewaal, M.; Burian, A.; Alim, K.; Landrein, B.; Borowska-Wykrt, D.; Dedieu, A.; Peaucelle, A.; Ludynia, M.; Traas, J.; Boudaoud, A.; et al. Mechanical Stress Acts via Katanin to Amplify Differences in Growth Rate between Adjacent Cells in Arabidopsis. Cell 2012, 149, 439–451. [Google Scholar] [CrossRef]
- Ragni, L.; Hardtke, C.S. Small but Thick Enough—The Arabidopsis Hypocotyl as a Model to Study Secondary Growth. Physiol. Plant. 2014, 151, 164–171. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Des Marais, D.L. Life History Variation as a Model for Understanding Trade-Offs in Plant–Environment Interactions. Curr. Biol. 2020, 30, R180–R189. [Google Scholar] [CrossRef]
- Felten, J.; Sundberg, B. Biology, Chemistry and Structure of Tension Wood. In Cellular Aspects of Wood Formation; Springer: Berlin, Germany, 2013; pp. 203–224. [Google Scholar]
- Pilate, G.; Chabbert, B.; Cathala, B.; Yoshinaga, A.; Leplé, J.-C.; Laurans, F.; Lapierre, C.; Ruel, K. Lignification and Tension Wood. C. R. Biol. 2004, 327, 889–901. [Google Scholar] [CrossRef]
- Ruelle, J. Morphology, Anatomy and Ultrastructure of Reaction Wood. In The Biology of Reaction Wood; Springer: Berlin, Germany, 2014; pp. 13–35. [Google Scholar]
- Badel, E.; Ewers, F.W.; Cochard, H.; Telewski, F.W. Acclimation of Mechanical and Hydraulic Functions in Trees: Impact of the Thigmomorphogenetic Process. Front. Plant Sci. 2015, 6, 266. [Google Scholar] [CrossRef]
- Peng, H.; Salmén, L.; Jiang, J.; Lu, J. Creep Properties of Compression Wood Fibers. Wood Sci. Technol. 2020, 54, 1497–1510. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Singh, A.P. Formation and Structure of Compression Wood. In Cellular Aspects of Wood Formation; Springer: Berlin, Germany, 2013; pp. 225–256. [Google Scholar]
- Miodek, A.; Gizińska, A.; Włoch, W.; Kojs, P. What Do We Know about Growth of Vessel Elements of Secondary Xylem in Woody Plants? Biol. Rev. 2021, 96, 2911–2924. [Google Scholar] [CrossRef] [PubMed]
- Purusatama, B.D.; Kim, N.H. Quantitative Anatomical Characteristics of Compression Wood, Lateral Wood, and Opposite Wood in the Stem Wood of Ginkgo biloba L. BioResources 2018, 13, 8076–8088. [Google Scholar] [CrossRef]
- Plomion, C.; Pionneau, C.; Brach, J.; Costa, P.; Baillères, H. Compression Wood-Responsive Proteins in Developing Xylem of Maritime Pine (Pinus pinaster Ait.). Plant Physiol. 2000, 123, 959–969. [Google Scholar] [CrossRef]
- Wang, D.; Lin, L.; Fu, F. Deformation Mechanisms of Wood Cell Walls under Tensile Loading: A Comparative Study of Compression Wood (CW) and Normal Wood (NW). Cellulose 2020, 27, 4161–4172. [Google Scholar] [CrossRef]
- De Zio, E.; Trupiano, D.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Grosso, A.; Marra, M.; Scaloni, A.; Scippa, G.S. Poplar Woody Taproot under Bending Stress: The Asymmetric Response of the Convex and Concave Sides. Ann. Bot. 2016, 118, 865–883. [Google Scholar] [CrossRef]
- Ghislain, B.; Clair, B. Diversity in the Organisation and Lignification of Tension Wood Fibre Walls—A Review. IAWA J. 2017, 38, 245–265. [Google Scholar] [CrossRef]
- Funada, R.; Miura, T.; Shimizu, Y.; Kinase, T.; Nakaba, S.; Kubo, T.; Sano, Y. Gibberellin-Induced Formation of Tension Wood in Angiosperm Trees. Planta 2008, 227, 1409–1414. [Google Scholar] [CrossRef]
- Bedon, F.; Grima-Pettenati, J.; Mackay, J. Conifer R2R3-MYB Transcription Factors: Sequence Analyses and Gene Expression in Wood-Forming Tissues of White Spruce (Picea glauca). BMC Plant Biol. 2007, 7, 1–17. [Google Scholar] [CrossRef]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardising Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef]
- De Smet, I.; Tetsumura, T.; De Rybel, B.; dit Frey, N.F.; Laplaze, L.; Casimiro, I.; Swarup, R.; Naudts, M.; Vanneste, S.; Audenaert, D. Auxin-Dependent Regulation of Lateral Root Positioning in the Basal Meristem of Arabidopsis. Development 2007, 134, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Grieneisen, V.A.; Hofhuis, H.; Ten Hove, C.A.; Hogeweg, P.; Marée, A.F.M.; Scheres, B. Root System Architecture from Coupling Cell Shape to Auxin Transport. PLoS Biol. 2008, 6, e307. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Godin, C.; Jay-Allemand, C.; Laplaze, L. Auxin Fluxes in the Root Apex Co-Regulate Gravitropism and Lateral Root Initiation. J. Exp. Bot. 2008, 59, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Guédon, Y.; Jay-Allemand, C.; Godin, C.; Laplaze, L. An Auxin Transport-Based Model of Root Branching in Arabidopsis thaliana. PLoS ONE 2008, 3, e3673. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Geldner, N.; Richter, S.; Vieten, A.; Marquardt, S.; Torres-Ruiz, R.A.; Mayer, U.; Jürgens, G. Partial Loss-of-Function Alleles Reveal a Role for GNOM in Auxin Transport-Related, Post-Embryonic Development of Arabidopsis. Development 2004, 131, 389–400. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Gilroy, S. The Exploring Root—Root Growth Responses to Local Environmental Conditions. Curr. Opin. Plant Biol. 2009, 12, 766–772. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Weisenseel, M.H.; Gilroy, S. Ca2+ Regulates Reactive Oxygen Species Production and PH during Mechanosensing in Arabidopsis Roots. Plant Cell 2009, 21, 2341–2356. [Google Scholar] [CrossRef]
- Díaz-Sala, C. A Perspective on Adventitious Root Formation in Tree Species. Plants 2020, 9, 1789. [Google Scholar] [CrossRef]
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Viscosi, V.; Chiatante, D.; Scippa, G.S. The Proteome of Populus nigra Woody Root: Response to Bending. Ann. Bot. 2012, 110, 415–432. [Google Scholar] [CrossRef]
- Hellgren, J.M.; Olofsson, K.; Sundberg, B. Patterns of Auxin Distribution during Gravitational Induction of Reaction Wood in Poplar and Pine. Plant Physiol. 2004, 135, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Funada, R.; Mizukami, E.; Kubo, T.; Fushitani, M.; Sugiyama, T. Distribution of Indole-3-Acetic Acid and Compression Wood Formation in the Stems of Inclined Cryptomeria Japonica. IAA Inclin. Cryptomeria Jpn. 1990, 44, 331–334. [Google Scholar]
- Du, S.; Uno, H.; Yamamoto, F. Roles of Auxin and Gibberellin in Gravity-Induced Tension Wood Formation in Aesculus turbinata Seedlings. IAWA J. 2004, 25, 337–347. [Google Scholar] [CrossRef]
- Trupiano, D.; Di Iorio, A.; Montagnoli, A.; Lasserre, B.; Rocco, M.; Grosso, A.; Scaloni, A.; Marra, M.; Chiatante, D.; Scippa, G.S. Involvement of Lignin and Hormones in the Response of Woody Poplar Taproots to Mechanical Stress. Physiol. Plant. 2012, 146, 39–52. [Google Scholar] [CrossRef]
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Chiatante, D.; Scippa, G.S. Poplar Woody Root Proteome during the Transition Dormancy-Active Growth. Plant Biosyst. 2013, 147, 1095–1100. [Google Scholar] [CrossRef]
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Rossi, M.; Viscosi, V.; Chiatante, D.; Scippa, G.S. Temporal Analysis of Poplar Woody Root Response to Bending Stress. Physiol. Plant. 2014, 150, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Trupiano, D.; Tamburro, M.; Ripabelli, G.; Montagnoli, A.; Chiatante, D.; Scippa, G.S. MicroRNAs Expression Patterns in the Response of Poplar Woody Root to Bending Stress. Planta 2015, 242, 339–351. [Google Scholar] [CrossRef]
- De Zio, E.; Trupiano, D.; Karady, M.; Antoniadi, I.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Ljung, K.; Scippa, G.S. Tissue-Specific Hormone Profiles from Woody Poplar Roots under Bending Stress. Physiol. Plant. 2019, 165, 101–113. [Google Scholar] [CrossRef]
- Dimitrova, A.; Sferra, G.; Scippa, G.S.; Trupiano, D. Network-Based Analysis to Identify Hub Genes Involved in Spatial Root Response to Mechanical Constrains. Cells 2022, 11, 3121. [Google Scholar] [CrossRef]
- Chiatante, D.; Montagnoli, A.; Trupiano, D.; Sferra, G.; Bryant, J.; Rost, T.L.; Scippa, G.S. Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals? Cells 2021, 10, 2544. [Google Scholar] [CrossRef]
| MS | Method/Duration | Species | Organ | Observations | Reference |
|---|---|---|---|---|---|
| Bending | Bending device/ Transient—5 months | Populus sp. | Stem | RW formation on the convex side | [28,65,66,67,68,69,70] |
| Lead sheet compression/2 or 7 days | Arabidopsis thaliana | FLA11 and FLA12 are possible MS-responsive cell surface sensors regulating stem secondary wall development | [71] | ||
| N/A/4–40 h | Populus tremuloides | Understanding of CesA cDNA (PtCesA) regulation in RW formation | [72] | ||
| Paper-mediated/Daily, 4 months | Psammochloa villosa | Decreased plant height, total biomass, and root/shoot ratio | [73] | ||
| Plastic tube pressed on the stem base/5 days | Caesalpiniaceae/ Clusiaceae | Variable responses between five examined species | [74] | ||
| Manual bending/1 week | Acacia koa | Reduced stem elongation, increased stem diameter, increase of anthocyanin and lignin. | [75] | ||
| Bending/flame | Manual/8 s | Populus tremula x alba | Inhibited primary growth, JA-mediated response | [76] | |
| Bending | Clamping rings/transient | Extracellular electrical signaling | [77] | ||
| Gravistimulation | Tilting/24 h | Identification of key genes regulated in the early gravitropic response | [27] | ||
| Wounding | Forceps/transient | Helianthus annus | Hypocotyl | identification of GSNOT and SNO as key new elements in the wound signaling pathway | [78] |
| Bark removal with saw and chisel | Populus sp. | Stem | Increased wall thickness, modified lignin topochemistry | [79] | |
| With hemostat/transient | Arabidopsis thaliana | Leaves | Identification of rapid wound-responsive genes | [80] | |
| Raindrop | Droplets/15 min | Arabidopsis thaliana | Leaves | Intercellular calcium waves, induction of defence-related genes | [36] |
| Brush | Brush/≤60 min | ||||
| Clinorotation | Clinorotation/2 days | Arabidopsis thaliana | Stem and root | Transcriptional regulation of genes encoding microtubule- and actin-associated proteins | [81] |
| Wind | Fan-mediated/various exposure | Solanum lycopersicum | Stem | Restricted stem elongation | [82] |
| Fan-mediated/6 h per day | Arabidopsis thaliana | Stem | Affected plant growth and phenology | [83] | |
| Fan-mediated/6–16 h per day | Stem | Impacted branching degree and fecundity | [59] | ||
| Waves | Flow flume system/20 s | Aquatic species | Stem/leaf/petioles | Negative correlation between avoidance and tolerance | [84] |
| Flexure | Various manual flexions/26 days | Nicotiana tabacum | Stem and leaves | Shorter, thicker stems with a lower Young’s modulus | [85] |
| Daily manual flexure/90 s, for 72 days | Stem | Higher mass allocation to roots | [86] | ||
| Stick-mediated strokes/daily, for 20 days | Solanum lycopersicum | Stem | Increase in root/shoot dry weight ratios | [87] | |
| Stick-mediated flexing/1 min, for 6 months | Pinus sylvestris | Stem and root | Reduced shoot height, higher root cross-sectional area and more lateral roots | [88] | |
| Vibrations | Toothbrush/one minute per day, for 49 days | Capsella bursa-pastoris | Entire shoot | Increase in root/shoot biomass, accelerated senescence | [89] |
| Rubbing | Finger rubbing/once daily, for 5 days | Phaseolus vulgaris | Stem | Reduced first internodes length, thicker stems, reduced hollowing of the first internodes | [90] |
| Finger rubbing/10 s | Solanum lycopersicum | Lignification-driven inhibited internode elongation | [91] | ||
| Touch | Water spray/Seconds | Arabidopsis thaliana | TOUCH genes-driven cell expansion | [92] | |
| Touch and brushing | Hand touching, paint brushing/8–10 days | Reduced stem height, pivotal role of the RNA Polymerase-Associated Factor 1 Complex | [93] | ||
| Brushing | Paint brushing/10–20 s, for 7 days | Reduced inflorescence stem height, pivotal role for the pectic cell wall Arabinans | [94] | ||
| Bending | Tying around 90° mesh/5–6 months | Populus nigra | Woody taproot | Lateral root formation toward convex stretched side, lignification of concave compressed side (RW formation), root sector/side-specific hormonal profiles | [28,95] |
| Bending | Hook development model/N/A | Arabidopsis thaliana | Hypocotyl | Cellulose and PIN are essential for hook formation, auxin and pectin methylesterification crosstalk | [96,97] |
| Gravity/bending | Manual bending/Transient | Root | Lateral root initiation | [61,98] | |
| Barrier exposure | Barrier, waving assay/N/A | Rapid and transient increases in cytosolic Ca2+, ROS production | [61] | ||
| In vitro barrier | Barrier exposure/6–30 h | Rapid obstacle avoidance forming a ‘step-like’ growth pattern | [63] | ||
| Obstacle exposure | Blades/200 min | PIN-mediated polar auxin transport facilitates root bending during obstacle avoidance | [99] | ||
| Compacted soil | Artificial macropores/4 months | Triticum aestivum | Root tip | Growth towards favorable soil conditions | [100] |
| Agricultural machinery/7 days | Root | Invaginations and cortex cell deformation | [101] | ||
| Dense containers/14 days | Hordeum vulgare | Root | Reduced total root length and leaf area, and altered biomass partitioning | [102] | |
| Drying/48 h | Zea mays | Root | Highly decreased root elongation and diameter | [103] | |
| Rigid pores | Photoelastic disks/5 days | Cicer arietinum | Root | No significant growth reduction | [104] |
| Rigid tubes | Growth through narrow gap/24 h | Zea mays | Root apex | Atypical oblique divisions of the root cap cells | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouhen, M.; Dimitrova, A.; Scippa, G.S.; Trupiano, D. The Course of Mechanical Stress: Types, Perception, and Plant Response. Biology 2023, 12, 217. https://doi.org/10.3390/biology12020217
Kouhen M, Dimitrova A, Scippa GS, Trupiano D. The Course of Mechanical Stress: Types, Perception, and Plant Response. Biology. 2023; 12(2):217. https://doi.org/10.3390/biology12020217
Chicago/Turabian StyleKouhen, Mohamed, Anastazija Dimitrova, Gabriella Stefania Scippa, and Dalila Trupiano. 2023. "The Course of Mechanical Stress: Types, Perception, and Plant Response" Biology 12, no. 2: 217. https://doi.org/10.3390/biology12020217
APA StyleKouhen, M., Dimitrova, A., Scippa, G. S., & Trupiano, D. (2023). The Course of Mechanical Stress: Types, Perception, and Plant Response. Biology, 12(2), 217. https://doi.org/10.3390/biology12020217