Hypomagnetic Conditions and Their Biological Action (Review)
Abstract
Simple Summary
Abstract
1. Introduction
2. Experimental Approaches
| № | Biological Object | Characteristics | Effect, % | Magnetic Flux Density | Time | N | Statistic | Validation | Experimental Setup | Size or Volume | SJR | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Human, men and women, average age of 45 ± 18 years | Heartbeat rate (≥40 years) Heartbeat rate (<40 years) Diastolic blood pressure (under 40 years) Capillary blood flow rate | −20% +10–15% −4–5% +22–23% | 10 nT >> >> >> | 120 min >> >> >> | 32 >> >> >> | ANOVA, F-test | Magnetometer, 3-axis, spatial distribution, HMF variation < 10 nT GMF: ~48 μT, meteorological data were used to choose assay days | Helmholtz coils (3-axis) | 2.6 m3 | 0.42 (Q2) | [44] |
| 2 | Rat Rattus norvegicus adult | Number of erythrocytes (RBC), Hematocrit (EPV), Erythrocyte volume (MCV), Hemolysis | +12% +7% −10% −85% | 0.192 μT >> >> >> | 1–4 days >> >> >> | 3 >> >> >> | Student’s paired t-test | Magnetometer, 3-axis, 1 point | Shielding chamber from amorphous magnetic material AMAG-172 | - | - | [37] |
| 3 | Zebrafish Danio renio wild type (AB strain), embryos | Viability Heartbeat rate | −10% +5% | <300 nT >> | 120 h >> | 200 >> | Shapiro–Wilk W-test or Kolmogorov–Smirnov test, Levene’s test, t-test, Cosinor analysis (for circadian rhythms) | Magnetometer 3-axis spatial distribution GMF: 51.7 μT AFM: 50 Hz, < 15 nT without harmonics | Helmholtz coils (3-axis) | Ø 50 cm | 1.03 (Q1) | [48] |
| 4 | Human men (24–53 years) and women (26–49 years) | Higher nervous activity: test for matching the meaning of a word and its color: lead time error rate Letter recognition test: lead time error rate | +10% +15% +5% +150% | <0.4 μT >> >> >> | 1 h 17 min >> >> >> | 40 >> >> >> | Multivariate analysis of variance (MANOVA) | Magnetometer, 3-axis, spatial distribution, variation < 0.2 μT GMF: ~41 μT AMF variations complicated | Helmholtz coils | ~3 m3 | 0.4 (Q3) | [42] |
| 5 | Rat, Rattus norvegicus line Wistar | Open field testing: horizontal component, vertical component, general physical activity Power of EEG rhythms: Theta Alpha Beta Gamma | −20% −30% −50% −50% −50% −50% −50% | 50 nT >> >> >> >> >> >> | 21 days >> >> >> >> >> >> | 12 >> >> >> >> >> >> | Wilcoxon signed-rank test, Kolmogorov–Smirnov test | Magnetometer, 3-axis, 1 point, HMF variation: < 50 nT | Helmholtz coils (2-axis) | Ø 50 cm | - | [57] |
| 6 | Rat, Rattus norvegicus line Wistar | Number of aggression acts (day) Number of aggression acts (night) | +130% +17 times | 50–150 nT >> | 21 days >> | 12 >> | Wilcoxon signed-rank test, Kolmogorov–Smirnov test | Magnetometer, 3-axis, 1 point, HMF variation: < 50 nT | Helmholtz coils | Ø 50 cm | - | [58] |
| 7 | Golden hamster Ochrotomys nuttalli adults | Proportion of noradrenergic neurons in areas A3 and A7 of the brainstem | −29% −35% | 22 nT >> | 60 180 days | 5 >> | One-way ANOVA or Student’s t-test | Magnetometer 3-axis spatial distribution: 0.022–2.8 μT | Permalloy chamber | 70 cm × 70 cm × 90 cm | 0.42 (Q3) | [59] |
| 8 | Mice (M. musculus) C57BL/6 J adults, 8–10 weeks | Behavioral tests: Freezing in context test Freezing in cue test | −15% −12% | 170 nT >> | 8 weeks >> | 10 >> | One-way or two-way ANOVA or Student’s t-test | Magnetometer, 3-axis, spatial distribution, ambient magnetic fields, noise and light were measured. SMF in incubator: 39.4 ± 3.6 μT. AMF: 50 Hz Bt PSD1/2 2.37 nT/√Hz | Helmholtz coils (3-axis) | Ø 50 cm | 5.12 (Q1) | [60] |
| 9 | Mice, C57BL/6J, 7 weeks old | Open field behavior test: percent time spent in the center, total traveled distances, time spent exploring the novel location, time spent exploring a novel object | −80% 0% −30% −30% | 31.9 nT >> >> >> | 8 weeks >> >> >> | 10 >> >> >> | Double-blind study, unpaired Student’s t-test | Magnetometer 3-axis 1 point, time distribution, HMF variation: < 4.5 nT GMF: ~55 μT temperature, illumination, and relative humidity equal in all conditions | Helmholtz coils (3-axis) | 2 m × 2 m × 2 m | 1.15 (Q1) | [61] |
| 10 | Chicken Gallus gallus domesticus incubated in hypomagnetic conditions, eggs and chicks hatched from them | Retained curve in one-trial passive avoidance task (OTPAT) Temporary mean memory test Long-term memory test | −68.4% −74.8% | 354 nT >> | 21 days >> | 10 >> | One-way ANOVA | Magnetometer, 1-axis, 1 point HMF variation: < 254 nT | Helmholtz coils (3-axis) | Ø 120 cm | 1.45 | [62] |
| 11 | Fruit fly Drosophila melanogaster imago, females 3–4-diurnal Prussian wild type (10–19 successive generations) | Performance index (PI) of operant visual learning and memory (L/M) formation of flies | −65% | 100–680 nT | 40–80 days | 445 | One-way ANOVA | Magnetometer, 1-axis, spatial distribution, GMF: 52.21 μT | Helmholtz coils (3-axis) | 50 cm × 50 cm × 50 cm | 0.8 (Q2) | [63] |
| 12 | Brown planthopper, Nilaparvata lugens males and females, imago | Direction of movement in food (decrease transition to random movement) | −100% | ~500 nT | 24–48 h | 500 | Student’s t-test | Magnetometer, 3-axis, spatial distribution (homogeneity HMF at Ø 150 mm) GMF: 52.5 ± 0.8 μT | Helmholtz coils (3-axis) | Ø 15 cm | 0.7 (Q1) | [64] |
| 13 | Oriental armyworm, Mythimna separata, adults, males and females | Flight spatial orientation | −100% | 500 nT | 20 s | 9 | Rayleigh’s test, Watson–Williams test | Magnetometer, 3-axis, 3D map, HMF variation: < 4% | Helmholtz coils | Ø 120 cm | 0.82 (Q1) | [65] |
| 14 | Black Garden Ant (Lasius niger) | Behavior: Time to reach food, Time to return to the nest, Mistakes to reach food | +200% +40% +300% | ~40 nT >> >> | 14 days >> >> | 1000 >> >> | Kolmogorov–Smirnov test, one-way ANOVA, Tukey’s post hoc test | Magnetometer, 3-axis, spatial distribution, HMF variation: < 6 µT GMF: ~42 µT GMF variation: <20 nT | Helmholtz coils (3-axis) | Ø 128 cm | 1.15 (Q1) | [66] |
| 15 | Brown planthopper, S. furcifera, males and females, imago | Positive phototaxis Speed, duration, and range of flight Body weight | −20% −40% −8% | ~477 nT >> >> | 1–5 days >> >> | 40 >> >> | One-way or two-way ANOVA | Magnetometer, 1-axis, spatial distribution (0–1.06 μT) GMF: ~50 μT | Helmholtz coils (3-axis) | Ø 30 cm | 0.74 (Q1) | [45] |
| 16 | Rat (Rattus norvegicus) Wistar line, females and males | Concentration of Fe, Mn, Co, Ni, Cr, Cu in hair | −5–40% (depending on the element and sex of the animal) | <20 nT | 7 months | 8 | One-way ANOVA | Magnetometer 1-axis 1 point | Chamber from steel type S235JRG2 | ~1 m × 1 m × 1 m | 0.94 (Q1) | [29] |
| 17 | Fishes, 0–1 year, Carassius carassius, Rutilus rutilus, Cyprinus carpio, snail Limnaea stagnalis, planktonic crustaceans, Daphnia magna (imago) | The concentration of Fe, Mn, Co, Ni, Cr, Cu in the brain, skeletal muscles (fishes), or all organisms (daphnia) | −50 % (depending on the element, organ, and species) | <10 nT | 1 h | 7 | Mann–Whitney test | Magnetometer 1-axis 1 point GMF: 51.7 μT | Helmholtz coils (3-, 1-axis) | Ø 50 cm | 0.31 (Q3) | [67] |
| 18 | Brown planthopper, Nilaparvata lugens migrating adults, eggs | Body weight of hatched insects Body weight of 5th instar nymphs Feeding of 5th instar nymphs Glucose content in 5th instar nymphs | 15% −35% −35% +20% −15% | 480 nT >> >> >> >> | 48 h >> >> >> >> | 20 >> >> >> >> | Shapiro–Wilk test, Levene’s test, one-way ANOVA, or Mann–Witney U-test | Magnetometer, 1-axis, spatial distribution, HMF variation: < 5% GMF: ~50 μT | Shielding chamber from μ-metal alloy and Helmholtz coils (3-axis) | Ø 30 cm | 0.94 (Q1) | [68] |
| 19 | Rat Rattus norvegicus line Sprague Dawley 250–270 g | Body weight Strength characteristics of bones: Ultimate Power Hardness factor Elastic modulus Density Weight Number of trabeculae Degree of bone anisotropy concentration of receptor activator of nuclear factor-kB ligand (RANKL) in bone tissue Serum: Concentrations of bALP, DPD, and GCs | −17% −18% +18% +17% −18% −15% +50% −25% −75% +35% | <300 nT >> >> >> >> >> >> >> >> >> | 28 days >> >> >> >> >> >> >> >> >> | 30 >> >> >> >> >> >> >> >> >> | One-way or two-way ANOVA | Magnetometer, 1-axis, 1 point GMF: ~50 µT, illumination and ventilation conditions as HMF and GMF were equal | Shielding chamber (aluminum/permalloy/silicone/iron) | 1.86 m × 1.66 m × 1.5 m | Rat (Rattus norvegicus) line Sprague Dawley, 250–270 g | [69] |
| 20 | Mice, males C57BL/6 hindlimb suspension model | Bone mineral content, Ultimate bending moment, Ultimate stress, Bone volume fraction, Trabecular separation, Connectivity density, Osteoblast number, Osteoclast number, Osteoclast surface, Bone eroded surface, Serum levels of tartrate- resistant acid phosphatase (bone resorption marker) Serum iron, Ferritin level Total iron content: liver, spleen Bone iron, Bone marrow iron | −20% −15% −15% −40% +15% −40% −40% +30% +15% +30% +20% +30% +20% +20% +35% +20% +20% | <300 nT >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | 4 weeks >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | 6 >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | Two-way ANOVA, Sidak’s post hoc test | Magnetometer, spatial distribution, AMF in control incubator 50 Hz ~1 μT AMF in an experimental incubator 50 Hz, < 12 nT GMF: ~45 μT | Permalloy chamber | 550 m × 420 m × 420 m | 1.13 (Q1) | [70] |
| 21 | Mouse M. musculus line NMRIz, pregnant females, embryos 3 days after fertilization | Birth rate, Number of implanted embryos, Histological abnormalities, resorption | −30% −30% +Qualitatively | <200 nT >> >> | 12 days >> >> | 5 >> >> | Student’s t-test | Magnetometer, 1-axis, 1 point, GMF: ~40 μT | Permalloy chamber | - | 0.4 (Q3) | [71] |
| 22 | Brown planthopper S. furcifera eggs and nymphs | Body weight (2 days old): Female, Male Positive chemotaxis: Females (5 days old), Males (2 days old), Males (5 days old) Flight speed (2 days old): Females, Males Flight duration: Female, Male Flight distance: Female, Male | −5% −10% +40% +30% +30% +30% −20% −80% +40% −60% N/A | 477 nT >> >> >> >> >> >> >> >> >> >> | 2000 h >> >> >> >> >> >> >> >> >> >> | 40 >> 115 >> >> 23 46 23 46 23 46 | Two-way ANOVA, MANOVA, Shapiro–Wilk test (normality), chi-square test (two-tailed) with Yates’s correction, Student’s t-test | Magnetometer, 3-axis, one point, HMF variation: < 25 nT GMF: ~52 μT, temperature variation: < 0.1 °C | Helmholtz coils | Ø 120 cm | 1.04 (Q1) | [72] |
| 23 | Oriental armyworm; Mythimna separata eggs, larvae, pupae, and imago (females and males) | Duration of development stages: larval doll imago (males) Pupa mass Number of eggs laid by one female | +5% +2% +5% −20% −5% −45% | <500 nT >> >> >> >> >> | 12 h >> >> >> >> >> | 300 >> >> >> >> >> | One-way or two-way ANOVA | Magnetometer, 1-axis, 1 point, time distribution, HMF variation: < 500 nT | Helmholtz coils | Ø 50 mm | 0.94 (Q1) | [73] |
| 24 | Crustaceans, Daphnia magna Daphnia carinata newborns and adults | Newborn sizes Adult sizes Life length | −15% −5% −5% | <15 nT >> >> | 24 h >> >> | 30 >> >> | Kolmogorov–Smirnov test, Levene’s test (homoscedasticity), one-way analysis of variance (ANOVA), Dunnett’s post hoc test | Magnetometer, 3-axis, spatial distribution AFM: 50 Hz < 12 nT GMF 51.7 mT | Helmholtz coils (3-axis) | Ø 50 cm | 0.4 (Q3) | [74] |
| 25 | Human men and women (<40 years) | Pupil diameter | +1.6% | 300–600 nT | 40 min | 40 | One-way ANOVA | Magnetometer, 3-axis, spatial distribution, variation: < 0.4 μT GMF: ~41 μT AMF variations complicated | Helmholtz coils | 1 m × 1 m × 1.5 m | - | [75] |
| 26 | Tardigrades (Paramacrobiotus experimentalis) females and males of different age | Proportions of active animals | −10% | <250 nT | 7 days | 45 | two-way ANOVA, Tukey post hoc test | Magnetometer 1-axis 1 point GMF: ~50 μT | μ-Metal shielding chamber (approximately 77% nickel, 16% iron, 5% copper, and 2% molybdenum) | 18.5 cm × 12 cm × 33 cm | 1.03 (Q1) | [76] |
| 27 | Helix albescens large common snail | Duration of circadian rhythms | −17% +19% | 0.5–2 µT >> | 3 days 21 days | 20 >> | Fourier transformation, Student’s t-test (normality tested) | Magnetometer, 1-axis, 1 point, spectral density of magnetic noise: < 10 nT/Hz | Room covered with Dynamo iron leaves | 2 m × 3 m × 2 m | 1.07 (Q1) | [77] |
| 28 | Tardigrades Echiniscus testudo and Milnesium inceptum | Mortality rate: (1) dehydrated (2) during dehydration (3) returning to active life after dehydration | +45% +80% +200% | <25 nT >> >> | 21 days >> >> | 100 >> >> | One-way ANOVA, Tukey test as a post hoc test, or Student’s t-test with the Cochran–Cox adjustment | Magnetometer, 1-axis, 1 point GMF: ~50 GMF | Shielding chamber amorphous magnet (μ-metal) | 18.5 cm × 12 cm × 33 cm | 0.7 (Q1) | [38] |
3. Effects of HMC on Living Objects
3.1. Effects of HMC on Animals (Organ and Organism Level)
3.1.1. Nervous System
3.1.2. Cardiovascular System and Immunity
3.1.3. Musculoskeletal System, Metabolism, and Other Effects
3.2. Effects of HMC on Plants
| № | Biological Object | Characteristics | Effect, % | Magnetic Flux Density | Time | N | Statistic | Validation | Experimental Setup | Size or Volume | SJR | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Arabidopsis thaliana seedlings, WT and spl7-KO | Fe uptake by roots, Zn uptake by roots, Expression of Fe-deficiency-induced genes in roots: IRT1, AHA2, FIT, ILR, bHLH38, bHLH39, 3, FRO2, Spl7 knockout or Fe supplementation alters hypomagnetic condition effects | −2 times −2 times +2–10 times −2–3 times | ~40 μT >> >> >> | 96 h >> >> >> | 4 >> 3 >> | One-way ANOVA | Magnetometer, 3-axis, spatial distribution, GMF: 41–43 μT | Helmholtz coils (1-axis) | - | 1.23 (Q1) | [91] |
| 2 | Arabidopsis thaliana Landsberg erecta, wild type, seedlings | Hypocotyl lengths: blue light, darkness | −3% +6% | ~10 nT >> | 72 h >> | 30 >> | Paired t-test | Magnetometer, 1-axis, 1 point SMD variation: < 10 μT GMF: ~50 mT | Helmholtz coils (1-axis) | Ø 22 cm | 1.2 (Q1) | [98] |
| 3 | Arabidopsis thaliana, wild type and spl7, amiFRO5, and amiFRO4/5 mutant lines | Fe concentration: control S index: S deficit Shoot area: control, Fe deficit Root length: control, Fe deficit, S deficit, Fe and S deficit Gene expression (part): AHA (Fe deficit), FRO (Fe and S deficit), PYE (Fe deficit), bHLH38 (Fe and S deficit) | −25% −20% −5% −5% −10% −10% −10% −10% −55% +45% −50% +50% | 42 nT >> >> >> >> >> >> >> >> >> >> >> | 7 days >> >> >> >> >> >> >> >> >> >> >> | 3 >> >> >> >> >> >> >> >> >> >> >> | Two-way ANOVA, Tukey’s post hoc test | Magnetometer, 3-axis, time distribution, variation: < 2nT, GMF: 41–43 μT | Helmholtz coil (3-axis) | Ø 128 cm | 1.15 (Q1) | [99] |
| 4 | Lima bean (Phaseolus lunatus) seeds and seedlings | Tomato leaf density, leaf area, relative water content, the major axis of chloroplast length, total carbohydrate content, total protein content, percentage of leaf carbon, carbon isotope discrimination (δ13C) Concentrations: Chlorophyll a, Chlorophyll b, Chlorophyll a’, Chlorophyll b’, Pheophytin a, Lutein, Trans-α-carotene, cis-α-carotene, Trans-β-carotene, 9-cis-β-carotene Protein expression: catalase, ascorbate peroxidase, peroxidase, glutathione reductase, glutathione peroxidase ROS production: peroxide, H2O2 | +50% −30% N/A +20% −20% +10% +5% +30% −20% −20% +250% +100% +100% −40% −30% −25% −75% −40% −25% −2500% +10% −200% +200% +500% −75% −10% | ~40 nT >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | 96 h >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | 3 >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | Paired Student’s t-test and Bonferroni post hoc test | Magnetometer, 3-axis, time distribution, variation: < 2 nT GMF: 41.94 μT | Helmholtz coil (3-axis) | Ø 128 cm | 1.15 (Q1) | [97] |
| 5 | Arabidopsis thaliana ecotype Landsberg erecta, WT or cry1cry2 mutants | Photosynthesis gene expression: rbcl (ribulose 1,5 bisphosphate), cab4 (chlorophyll a,b binding protein), pal4 (phenylalanine ammonia lyase), ef1 (elongation factor-1) | −20% −60% −20% −5% | 0.2 μT >> >> >> | 120 h >> >> >> | 4 >> >> >> | Student’s t-test | Magnetometer, 3-axis, spatial distribution, power supplies were separated from the μ-metal cylinder GMF: ~38 μT | Faraday-cage room, Helmholtz coils (2-axis) | 5.04 × 2.04 × 2.1 m Ø 18 cm | 0.88 (Q1) | [100] |
| 6 | Arabidopsis thaliana ecotype Col0, seedlings | Expression of circadian rhythm regulator genes: LHY, PRR7, GI | −80% −80% +60% | ~40 nT >> >> | 7 days >> >> | 3 >> >> | Two-way ANOVA, Tukey post hoc test | Magnetometer, 3-axis, spatial distribution, GMF: 40–45 μT | Helmholtz coil (3-axis) | - | 0.88 (Q1) >> | [101] |
| 7 | Arabidopsis thaliana (Col-0), Wt and cry1cry2-, phot1-, phyA-, and phyAphyB-deficient mutants, seedlings (1 week) | Changes in cryptochrome expression in response to blue light: Wt, phyA mutant Changes in phyA (phytochrome A) expression in response to red light Changes in cryptochrome expression in response to red light | +100% −100% −100% | ~40 nT >> >> | 96 h >> >> | 3 >> >> | Kolmogorov–Smirnov test (normality), one-way ANOVA, Tukey, and Bonferroni post hoc tests | Magnetometer, 3-axis, spatial distribution, sample rate: 10 s | Helmholtz coil (3-axis) | - | 0.87 (Q1) | [94] |
| 8 | Soy Glycine max seeds and seedlings | Gravitropism angle, Radicle weight ratio, Germination percentage, Germination rate, A ratio of root length to seed length | −50% +18% N/A −10% +12% | <111 nT >> >> >> >> | 1 h >> >> >> >> | 10 >> >> >> >> | Two-way ANOVA | Magnetometer 3-axis 1 point Temperature and relative humidity equal in both conditions | Chamber from 12 layers of permalloy sheets, enclosed within an outer aluminum layer | ~10 cm × 10 cm × 10 cm | 0.6 (Q2) | [88] |
| 9 | Arabidopsis thaliana ecotype Columbia | Epicotyl length, Adult habitus-acquisition of rosette morphology, Expression of phytochrome B signaling pathway genes: PHYB, CO, FT | +30% qualitatively −40% −40% −50% | <50 nT >> >> >> >> | 36 days >> >> >> >> | 20 3 >> >> | Student’s t-test | Magnetometer, 3-axis, spatial distribution, GMF: ~45 μT | Helmholtz coil (axis) | Ø 88 cm | 0.6 (Q2) | [96] |
| 10 | Arabidopsis thaliana Adult | Biomass (total) Biomass (dry) Flowering time Number of fruits per plant Seed weight per plant Harvest index (ratio between seed weight and total biomass) | −30% −40% +5% −20% −20% −20% | <1 μT >> >> >> >> >> | 35 days >> >> >> >> >> | 20 >> >> >> >> >> | One-way ANOVA | Magnetometer, 3-axis, 3D map GMF: ~42 μT HMF variation: < 50 nT | Helmholtz coil (3-axis) | Ø 80 cm | 0.43 (Q3) | [89] |
| 11 | Arabidopsis thaliana (Col-0), Wt | Time from germination to flowering, Time from germination to fruiting, Restoration of characteristics above after change in hypomagnetic condition to geomagnetic Leaf area index, Stem length, Expression of clock genes and photoperiod pathway genes, Expression of floral meristem genes, Expression of GA20ox2 | +20% +15% +100% −15% −30% −1.5–2.2 times −3–5 times −50 times | 41 nT >> >> >> >> >> >> >> | 15 min >> >> >> >> >> >> >> | 15 >> >> >> >> >> >> >> | Kolmogorov–Smirnov test, one-way ANOVA | Magnetometer, 3-axis, time distribution, variation: < 2 nT GMF: 41.94 μT | Helmholtz coil (3-axis) | Ø 128 cm | 0.43 (Q3) | [90] |
| 12 | Arabidopsis thaliana ecotype Columbia Col-4 Adult WT cry1-/cry2-mutants | WT: Expression of GA3ox1, Expression of GA3ox2, Expression of GA3ox3, LFY, SOC1, Gibberrilin concentration cry1-/cry2-mutants: Expression of GA3ox1, Expression of GA3ox2, Expression of GA3ox3, Gibberrilin concentration | −45% −55% −55% −35% −30% ~50% - 0 0 0 | <1 μT >> >> >> >> >> >> >> >> | 33 days >> >> >> >> >> >> >> | 3 >> >> >> >> >> >> >> | One-way ANOVA | Magnetometer, 3-axis, spatial distribution, GMF: ~45 μT | Helmholtz coil (3-axis) | Ø 88 cm | 0.42 (Q3) | [31] |
| 13 | Arabidopsis thaliana ecotype Columbia Col-4 Adult WT cry1-/cry2-mutants | WT: Auxin Levels in leaves, Auxin Levels in roots, Expressions of Auxin Transporter Genes, Expressions of Auxin Signaling Genes cry1-/cry2-mutants: Inhibition of the hypomagnetic field effects | −25% +40% +20% +30% 0 | <1 μT >> >> >> >> | 33 days >> >> >> >> | 3 >> >> >> >> | One-way ANOVA | Magnetometer, 3-axis, spatial distribution, GMF: ~45 μT | Helmholtz coil | Ø 20 cm | 0.42 (Q3) | [95] |
| 14 | Arabidopsis thaliana adult, wild type | Cation content in roots: NH3+, K+, Ca2+, Mg2+ Gene expression: Ca2+-transporting ATPase 11, Mg2+ transporter CorA-like protein-related Aniom content in roots: Cl−, SO42−, NO3−, PO4−3, Gene expression: Cl− channel protein (CLC-A), Cl− channel protein (CLC-C), Cl− channel protein (CLC-G), SO4− transporter (Sultr3;1), NO3− transporter (NRT1.6), NO3− transporter (NRT2.4), PO43- transporter (PHT1;8) | +25% +5% −15% +5% +50% −5% −15% −10% −50% +80% −10% +40% −90% +30% +8% −15% +10% −30% +2% +15% +60% −5% −40% +90% −80% +30% −30% +5% +8% −40% +5% +5% −50% −10% +5% −50% +15% −12% −25% +10% −15% −25% −10% −15% −3% −11% −3% +43% +46% +46% | <33 nT >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | 1 h 4 h 24 h 48 h 10 min 1 h 4 h 48 h 96 h 10 min 4 h 48 h 96 h 10 min 1 h 4 h 24 h 96 h 4 h 48 h 10 min 1 h 4 h 48 h 96 h 10 min 4 h 24 h 48 h 96 h 10 min 1 h 4 h 24 h 48 h 96 h 10 min 1 h 4 h 24 h 48 h 96 h 1 h 4 h 1 h 96 h 1 h 48 h 48 h 48 h | 3 >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> >> | Paired Student’s t-test, and Bonferroni post hoc test, and Hochberg (BH) multiple testing correction | Magnetometer, 3-axis, spatial distribution, GMF: 41.94 μT | Helmholtz coils (3-axis) | Ø 128 cm | 0.41 (Q2) | [92] |
| 15 | Arabidopsis thaliana Columbia ecotype Col-4, seedlings | cry2 phosphorylation rate, cry2 dephosphorylation rate | −20% −15% −10% −20% −20% −10% | <50 nT >> >> >> >> >> | 30 60 90 30 60 90 min | 3 >> >> >> >> >> | Student’s t-test | Magnetometer, 3-axis, spatial distribution, GMF: ~45 μT | Helmholtz coils (axis) | Ø 88 cm | 0.6 (Q2) | [102] |
| 16 | Arabidopsis thaliana, seedlings, wild type or cry1cry2 mutants, phyAB mutants | Seed germination: Wt Blue light: 50, 60, 70 h Darkness: 50 h cry1cry2 mutants Blue light: 50, 60, 70 h, darkness Hypocotyl length Wt Blue light Darkness cry1cry2 mutants Blue light Darkness phyAB mutants Blue light, Darkness | −50% −60% −45% −50% −80% −50% −40% N/A N/A −50% −30% −40% −40% N/A | <200 nT >> >> >> >> >> >> >> >> >> >> >> >> >> | 96 h >> >> >> >> >> >> >> >> >> >> >> >> >> | 50 >> >> >> >> >> >> >> >> >> >> >> >> >> | Student’s t-test | Magnetometer, 1-axis, spatial distribution, GMF: ~50 μT | μ-Metal chamber and Helmholtz coils (1-axis) | 25 cm × 40 cm Ø 18 cm | 0.68 (Q1) | [103] |
3.3. Effects of HMC on Cell Level
3.4. Effects of HMC at the Molecular Level In Vivo
| № | Biological Object | Characteristics | Effect, % | Magnetic Flux Density | Time | N | Statistic | Validation | Experimental Setup | Size or Volume | SJR | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mice (M. musculus) C57BL/6 J adults, 8–10 weeks | Proportions of hippocampal neuron types: BrdU+ cells BrdU+ Grap+ SOD2+ type1 cells BrdU+ Grap+ SOD2+ type1 cells Expression of negative regulation of proliferation genes Expression of oxidative stress response genes | −12% −12% −25% +102–105 times −102–105 times −8% | 170 nT >> >> >> >> >> | 8 weeks >> >> >> >> >> | 10 >> >> >> >> >> | One-way or two-way ANOVA or Student’s t-test | Magnetometer, 3-axis, spatial distribution, ambient magnetic fields, noise, and light were measured. SMF in incubator: 39.4 ± 3.6 μT. AMF: 50 Hz Bt PSD1/2 2.37 nT/√Hz | Helmholtz coils (3-axis) | Ø 50 cm | 5.12 (Q1) | [60] |
| 2 | Mice M. musculus line C57BL/6 J newborns | Proportions of hippocampal neuron types: BrdU+ cells BrdU+ GFAP+ S100β- cells BrdU+ Ki67+ DCX- cells BrdU+ Ki67+ DCX- cells BrdU+ DCX+ NeuN+ cells BrdU+ DCX- NeuN+ cells Dendrite length | −15–25 −50% −60–99% −60–80% −5–30% −40–50% −5% | 0.17 μT >> >> >> >> >> >> | 4 weeks >> >> >> >> >> >> | 6 >> >> >> >> >> >> | One-way or two-way ANOVA or Student’s t-test | Magnetometer, 3-axis, spatial distribution, ambient magnetic fields, noise, and light were measured. SMF in incubator: 39.4 ± 3.6 μT. AMF: 50 Hz Bt PSD1/2 2.37 nT/√Hz | Helmholtz coils (3-axis) | Ø 50 cm | 5.12 (Q1) | [60] |
| 3 | Mice, Mus musculus line C57BL/6 neonatal, young (P15), adult (2 months) | Primary brain culture from a region of the brain, hippocampus: Cell diameter, proliferation rate The expression of proteins Nestin, Sox2, Neurod1, GFAP, βIII-tubuline | +50% +30% −50% | <85 nT >> >> | 7 days >> >> | 24 >> | One-way ANOVA and χ2 test | Magnetometer, spatial distribution Local MF for cells (incubator): 15.1 ± 2.2 μT GMF for animals: 49.88 ± 1.82 μT | Magnetic shielding chamber and Helmholtz coils (3-axis) | Ø 40 cm | 3.37 (Q1) | [30] |
| 4 | Human neuroblastoma cell line SH-SY5Y | H2O2 production Superoxide dismutase activity Cell cycle phase ratio: proportion of S phase in the cell cycle | −50% −60% +200% | <500 nT >> >> | 16 h >> >> | 3 >> >> | Shapiro–Wilk test, one-way ANOVA, Bonferroni post hoc test | Magnetometer 3-axis 3D map GMF: ~45 μT | Permalloy chamber | 10 cm × 10 cm × 10 cm | 3.37 (Q1) | [39] |
| 5 | Human neuroblastoma cell line SH-SY5Y | Expression of genes regulating survival, cell division, adhesion, apoptosis, functions (a total of 2464 analyzed) | +216 genes −2248 genes | <200 nT >> | 1–4 days >> | 6 >> | One-way ANOVA | Magnetometer, 3-axis, spatial distribution, AFM (control): 50 Hz, 575.7 ± 29.1 nT AFM (experiment): 50 Hz, <12.0 nT | Permalloy chamber | 0.24 m3 | 1.45 (Q1) | [49] |
| 6 | Ansell’s mole-rats (F. anselli), adult | Number of c-Fos-IR+ cells Subcortical nuclei, cortical regions, hippocampus, striatum, and primary motor and primary somatosensory cortices | −50% −40% +60% | ~300 nT >> >> | 1 h >> >> | 22 >> >> | One-way ANOVA, Tukey post hoc test | Magnetometer, 3-axis, spatial distribution, HMF variation: <1% GMF: ~46 μT | Helmholtz coils (1-axis) and μ-metal chamber | Ø 170 cm 2 m × 2 m × 2 m | 1.2 (Q1) | [78] |
| 7 | Mice, C57BL/6J, 7 weeks old | ROS levels in hippocampus: DG region, CA region Gene expressions: NADPH oxidase 4, eosinophil peroxidase, keratin 1, nitric oxide synthase 2, glutathione peroxidase 3, heat shock protein 1A | +30% +30% +155% +85% +86% +60% −70% −64% | 31.9 nT >> >> >> >> >> >> >> >> | 8 weeks >> >> >> >> >> >> >> >> | 4 >> >> >> >> >> >> >> >> | Double-blind study, unpaired Student’s t-test | Magnetometer 3-axis 1 point, time distribution, HMF variation: < 4.5 nT GMF: ~55 μT Temperature, illumination, and relative humidity equal in all conditions | Helmholtz coils (3-axis) | 2 m × 2 m × 2 m | 1.15 (Q1) | [61] |
| 8 | Drosophila melanogaster sperm | Cell mobility Oxygen consumption by cells (pmolO2/mL/min/test) Protein expressions: blw (the catalytic subunit F1 ATP synthase), c1 cytochrome, cyt c1 oxidase | −30% −25% N/A | <1 nT >> >> | 6 h >> >> | 200 >> >> | One-way ANOVA, Student’s t-test | Magnetometer, 1-axis, 1 point, GMF: 48 μT | Helmholtz coils | - | 1.15 (Q1) | [51] |
| 9 | Black Garden Ant (Lasius niger) | Gene expression: MagR cry Protein content: SOD GSR H2O2 content Endogenous amine concentrations: tyramine (TA), octopamine (OA), L-DOPA, dopamine (DA), serotonin (Ser), melatonin (Mel) | +20% −18% +38% −20% −60% −20% −80% −80% −75% −80% +10% | ~40 nT >> >> >> >> >> >> >> >> >> >> | 14 days >> >> >> >> >> >> >> >> >> >> | 30 >> >> >> >> >> >> >> >> >> >> | Kolmogorov–Smirnov test, one-way ANOVA, Tukey’s post hoc test | Magnetometer, 3-axis, spatial distribution, HMF variation: < 6 µT GMF: ~42 µT GMF variation: < 20 nT | Helmholtz coils (3-axis) | Ø 128 cm | 1.15 (Q1) | [66] |
| 10 | Tardigrades (Paramacrobiotus experimentalis) females and males of different age | Mitochondrial potential | −6% | <250 nT | 15 days | 45 | Two-way ANOVA, Tukey post hoc test | Magnetometer 1-axis 1 point GMF: ~50 μT | μ-Metal shielding chamber (approximately 77% nickel, 16% iron, 5% copper, and 2% molybdenum) | 18.5 cm × 12 cm × 33 cm | 1.03 (Q1) | [76] |
| 11 | Human neuroblastoma SH-SY5Y | Migration and adhesion (rate, distance, cell count) Morphology (outgrowth width) Actin assembly in vitro | −40% - −50% −10% | <200 nT >> <500 nT | 4 days >> 48 h | 4 >> 6 | One-way ANOVA, Chi-square test, Kolmogorov–Smirnov test | Magnetometer, 3-axis, spatial distribution AMF: 12.0 ± 0.0 nT at 50 Hz (in permalloy chamber) SMF: 15.1 ± 2.2 μT; AMF: 575.7 ± 29.1 nT at 50 Hz (incubator) SMF: 52.5 ± 0.4 μT; AMF: 14.0 ± 1.0 nT at 50 Hz (control animals) | Permalloy chamber Helmholtz coils (3-axis) | 50 cm × 50 cm × 50 cm Ø 40 cm | 0.97 (Q1) | [47] |
| 12 | Mouse embryonic stem cells (mESCs) differentiate into neuronal cells | Expression of neuronal differentiation markers: Huj1 Map2 Proportion of differentiated cells Brachyury expression | −90% −75% −80% −80% | <10 nT >> >> >> | 12 days >> >> >> | 3 >> >> >> | Shapiro–Wilk test, one-way ANOVA, Bonferroni post hoc test, Student’s t-test (normal distribution) | Magnetometer, 3-axis, 1 point | Helmholtz coils (3-axis) | - | 0.97 (Q1) | [107] |
| 13 | Oriental armyworm; Mythimna separata eggs, larvae, pupae, and imago (females and males) | Vitellogenin Vg gene expression | −50% | <500 nT | 12 h | 300 | One-way or two-way ANOVA | Magnetometer, 1-axis, 1 point, time distribution, HMF variation: < 500 nT | Helmholtz coils | Ø 50 mm | 0.94 (Q1) | [73] |
| 14 | Human neuroblastoma cell line SH-SY5Y | Number of cells in a culture Proliferation rate Number of cells in G0 phase Number of cells in G1 phase Number of cells in G2/M phase | +8% +8% +7% −7% −5% | <150 nT >> >> >> >> | 2 days >> >> >> >> | 3 >> >> >> >> | One-way ANOVA | Magnetometer, 3-axis, spatial distribution, temperature and relative humidity equal in all conditions, GMF (incubator): < 11 μT GMF (laboratory): ~56 μT | Permalloy chamber | 0.24 m3 | 0.89 (Q1) | [108] |
| 15 | Fibrosarcoma HT1080 and pancreatic AsPC-1 cancer cells | H2O2 production | −12% | 500 nT | 24 h | 3 | One-way ANOVA | Magnetometer, 3-axis, spatial distribution, HMF variation: 0.5–2 µT Temperature variation: < 0.1 °C GMF: ~45 μT | μ-Metal cylinder and Helmholtz coils (3-axis) | Ø 12.5 cm | 0.89 (Q1) | [112] |
| 16 | Cow (Bos taurus) and human (Homo sapiens) | Self-assembly rate of tubulin from α/β-subunits: no tau protein in the presence of tau (recombinant human tau23) protein | −40% −90% | 10–100 nT >> | 20 min >> | 7 >> | Tsou’s method | Magnetometer 1-axis 1 point GMF: ~50 μT | Helmholtz coils (1-axis) | Ø 40 cm | 0.79 (Q1) | [35] |
| 17 | Human neuroblastoma cell line SH- SY5Y | Proliferation rate Glucose consumption Lactic acid concentration Lactate dehydrogenase activity ATP concentration ADP/ATP ratio Mitochondrial potential | +12% +22% +18% +7% +13% −9% −10% | <200 nT; >> >> >> >> >> >> | 72 h >> >> >> >> >> >> | 3 >> >> >> >> >> >> | Two-way ANOVA, Tukey’s post hoc test (multiple comparisons, Student’s two-tailed t-test (two groups) | Magnetometer, 3-axis, spatial distribution, AMF: 50 Hz, <12.0 nT SMF (control incubator) 15.1 ± 2.2 μT; AMF: 50 Hz (control incubator), 575.7 ± 29.1 nT | Permalloy chamber | 50 cm × 50 cm × 50 cm | 0.79 (Q1) | [50] |
| 18 | Brown planthopper, S. furcifera males and females, imago | Gene expression cry1 cry2 Adipokinetic hormone concentration Expression Adipokinetic hormone receptor | −20% +10% +10% −17% +25% | ~477 nT >> >> >> >> | 1–5 days >> >> >> >> >> | 40 >> >> >> >> >> | One-way or two-way ANOVA | Magnetometer, 1-axis, spatial distribution (0–1.06 μT) GMF: ~50 μT | Helmholtz coils (3-axis) | Ø 30 cm | 0.74 (Q1) | [45] |
| 19 | Human bronchial epithelial cell line BEAS-2B after X-ray exposition (1 Gy/min) | Survival, DNA fragmentation, γH2AX expression, Colocalization coefficient of γH2AX and p53BP1 | +6% 0% −40% +40% | 50 nT. >> >> >> | 30–320 min >> >> >> | 3 >> >> >> | One-way ANOVA | Magnetometer, 1-axis, spatial distribution, SMF (incubator): 6–13 μT GMF: ~47 μT | Permalloy chamber Helmholtz coils (3-axis) | Ø 40 cm | 0.43 (Q3) | [118] |
| 20 | Human fibrosarcoma cell line HT1080 and human colorectal cancer cell line HCT116 | Proliferation | −19% | 200 nT | 1–3 days | 9 | One-way ANOVA | Magnetometer, 1-axis, spatial distribution, SMF (incubator): 6–13 μT GMF: ~43 μT | Helmholtz coils (3-axis) | Ø 50 cm | 0.43 (Q3) | [104] |
| 21 | Jurkat cells | Anti-CD3-antibody-induced Ca2+ influx characteristics: Basal slope: G0/G1 phase cells, S phase cells Reak: G0/G1 phase cells, G2-M phase cells Active intercept: G0/G1 phase cells, S phase cells, G2-M phase cells Active average: G0/G1 phase cells, G2-M phase cells | +20% −10% +4% −12% +104% +83% +81% +82% +65% | <300 nT >> >> >> >> >> >> >> >> | 20 min >> >> >> >> >> >> >> >> | 10 >> >> >> >> >> >> >> >> | MANOVA or paired Student’s t-test | Magnetometer, 1-axis, 1 point AMF variation: <1 nT | μ-Metal chamber | 33 cm × 38 cm × 20 cm | 0.43 (Q3) | [119] |
| 22 | Human umbilical vein endothelial cells (HUVECs) | Proliferation eNOS expression VEGF gene expression | N/A N/A N/A | 300–500 nT | 24 h | 3 | Student’s t-test | Magnetometer, 3-axis, spatial distribution, SMF (incubator): 6–12 μT | Helmholtz coils and μ-metal chamber | 8.5 cm × 12.5 cm × 6.5 cm | 0.43 (Q3) | [34] |
| 23 | Mice M. musculus line C57BL/6 newborns (E18) | Viability of femoral muscle myocytes Proportion of cells in apoptosis and necrosis Myosin packaging quality Residual glucose, mM Glycogen, μmool/g protein ATP, μmool/g protein ADP/ATP ratio | −5–10 N/A qualitatively +10% +10% −60 +60–80 | <1 μT >> >> >> >> >> >> | 3 days >> >> >> >> >> >> | 11 6 12 >> | One-way ANOVA or Student’s t-test | Magnetometer, 3-axis, 3D map SMF (control incubator): 38–55 μT AMF: 55–62 Hz, 105. ± 19.2 nT | Helmholtz coils (3-axis) | Ø 40 cm | 0.42 (Q3) | [86] |
| 24 | Human adults, healthy blood cells | Activity of aspartate aminotransferase Activity of alanine aminotransferase Hemolysis | −12% −28% +9.5 times | 100 nT >> >> | 72 h >> >> | 10 >> >> | Student’s t-test | Magnetometer, 1-axis, 1 point GMF: ~50 μT | Helmholtz coils | - | 0.4 (Q3) | [81] |
| 25 | Mice M. musculus line CD-1 adults 24–26 g, males | fMLF or PMA induces ROS production by peritoneal granulocytes | −25% | 20 nT | 1.5 h | 10 | Student’s t-test | Magnetometer, 1-axis, spatial distribution Ambient GMF: ~42 μT AMF: 50 Hz, 15-50 nT | Permalloy chamber | - | 0.18 (Q4) | [82] |
| 26 | Rat (Rattus norvegicus) newborns | Cytosolic Ca2+ concentration | −8% | ~300 nT | 7 days | 3 | Student’s t-test | Magnetometer, 1-axis, 1 point GMF: ~48 μT | Nanomaterial-based ASM AMAG 172 chamber | - | 0.18 (Q4) | [109] |
| 27 | Mice M. musculus C57BL/6 (4–6 weeks old), male | Condition of skeletal muscle cells Citric acid concentration in muscles Number of SS mitochondria Mitochondrial length | qualitatively −30% −20% +15% | 1.12 μT >> >> >> | 30 days >> >> >> | 10 >> >> >> | Kolmogorov–Smirnov test, one-way ANOVA, Student’s t-test, or Mann–Whitney U-test | Magnetometer 3-axis 3D map SMF variation: < 430 nT AMF: 120 Hz, <230 nT | Helmholtz coil (3-axis) | Ø 40 cm | 0.13 (Q4) | [85] |
| 28 | Rat, Rattus norvegicus line Wistar | Proportion of c-fos+ neurons in the thalamus Proportion of active MOROP3+ neurons in the thalamus and periaqueductal area Proportion of active MOROP3+ neurons in the frontal cortex and superior colliculus | −20% −80% −2% | 50–150 nT >> >> | 21 days >> >> | 12 >> >> | Wilcoxon signed-rank test, Kolmogorov–Smirnov test | Magnetometer, 3-axis, 1 point, HMF variation: < 50 nT | Helmholtz coils | Ø 50 cm | - | [58] |
| 29 | Brown planthopper Nilaparvata lugens adults, macropterous and brachyppterous | Stability of expression of AK and α-Tub1 | −75% | 523 nT | 2000 h | One-way ANOVA, benchmarks of Cohen for small effects | Magnetometer, 3-axis, one point, HMF variation: < 2% GMF: 50 μT | Helmholtz coils | Ø 120 cm | 1.03 (Q1) | [116] | |
| 30 | Murine osteoblastic cell line MC3T3-E1 | Cell proliferation, Cell area Cell cycle phase duration: S, G2/M, Fe concentration in medium, Ca concentration, Nodule area, Total protein Gene expression: ALP, BSP, CoI, DMP1, OC, TfR1 | N/A +20% −20% +20% −10% −20% −60% +10% +20% −15% +40% −30% +80% +80% | 500 nT >> >> >> >> >> >> >> >> >> >> >> >> >> | 48 h >> 24 h >> 8 days 8 days >> >> 8 days >> >> >> >> >> | 3 >> >> >> >> >> >> >> >> >> >> >> >> >> | One-way ANOVA, Newman–Keuls test | Magnetometer, spatial distribution, AMF in control incubator 50 Hz ~1 μT AMF in an experimental incubator 50 Hz, < 12 nT GMF: ~45 μT | Permalloy chamber | 550 × 420 × 420 m | 0.73 (Q1) | [110] |
3.5. Effects of HMC on Bacteria
3.6. Effects of HMC on Solutions
| № | Biological Object | Characteristics | Effect, % | Magnetic Flux Density | Time | N | Statistic | Validation | Experimental Setup | Size or Volume | SJR | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pseudomonas (strain P3) Enterobacter (strain E1) | MIC for antibiotics: ampicillin, kanamy, tetracycline, ofloxacin, ceftazidime, tetracycline, ofloxacin | +80% −90% +30% −30% −50% −80% −60% | <500 nT >> >> >> >> >> >> >> | 6 days >> >> >> >> >> >> >> | 6 >> >> >> >> >> >> >> | Student’s t-test | Magnetometer, 1-axis, 1 point | Helmholtz coils | - | 0.55 (Q2) | [122] |
| 2 | Magnetospirillum magneticum | Magnetosome size Gene expression: mms13, mms6, magA | −9% +70% −10% N/A | <500 nT >> >> >> | 16 h >> >> >> | >300 >> >> >> | Two-way ANOVA, two-tailed Student’s t-test, Mann–Whitney U-test | Magnetometer, 3-axis, spatial distribution, stability HMF area: 200 mm × 200 mm × 200 mm | Helmholtz coil | Ø 1050 mm | 0.53 (Q2) | [32] |
| 3 | E. coli | MIC for antibiotic (proportions of analyzed strains): ofloxacin, kanamycin, tetracycline, ceftazidime, ampicillin | −9% +12% −19% +12% −10% | ~40 nT >> >> >> >> | 6 days >> >> >> >> | 6 >> >> >> >> | Two-tailed Student’s t-test | Magnetometer, 1-axis, 1 point | Helmholtz coils | Ø 40 cm | 0.4 (Q3) | [121] |
| 4 | Escherichia coli strain K12 AB1157 in stationary growth phase | Maximum relative viscosity | −18% +18% | 30, 60, or 80 nT 45, 70, or 95 nT | 15 min >> | 15 >> | Student’s t-test | Magnetometer, 3-axis, spatial distribution, AFM: 50 Hz, <30 nT | Helmholtz coils (2-axis) | Ø 19.6 cm | 0.43 (Q3) | [140] |
4. Potential Effects of HMC on Organisms Depending on Induction
5. Mechanisms of Action of Hypomagnetic Conditions on Living Systems
5.1. Probable Mechanisms of Static Magnetic Field Effects
- (1)
- action of the Lorentz force on charged particles;
- (2)
- participation of stable magnetic nanoparticles;
- (3)
- radical pair mechanism;
- (4)
- level mixing mechanism.
5.1.1. The Action of the Lorentz Force on Charged Particles
5.1.2. Nanoparticles with Magnetic Properties
5.1.3. Radical Pair Mechanism
5.1.4. Level Mixing Mechanism
5.2. Specific Responses
6. Dependence of Biological Effect Magnitude on Quantitative Characteristics of HMC
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panovska, S.; Korte, M.; Constable, C.G. One Hundred Thousand Years of Geomagnetic Field Evolution. Rev. Geophys. 2019, 57, 1289–1337. [Google Scholar] [CrossRef]
- Buddhi, D.; Singh, R.; Gehlot, A. Magnetic Field in the Solar System—A Brief Review. In Global Emerging Innovation Summit (GEIS-2021); Bentham Books: Potomac, MD, USA, 2021. [Google Scholar]
- Erdmann, W.; Kmita, H.; Kosicki, J.Z.; Kaczmarek, Ł. How the Geomagnetic Field Influences Life on Earth—An Integrated Approach to Geomagnetobiology. Orig. Life Evol. Biosph. 2021, 51, 231–257. [Google Scholar] [CrossRef]
- Finlay, C.C.; Maus, S.; Beggan, C.D.; Bondar, T.N.; Chambodut, A.; Chernova, T.A.; Chulliat, A.; Golovkov, V.P.; Hamilton, B.; Hamoudi, M.; et al. International Geomagnetic Reference Field: The eleventh generation. Geophys. J. Int. 2010, 183, 1216–1230. [Google Scholar] [CrossRef]
- Curto, J.J. Geomagnetic solar flare effects: A review. J. Space Weather Space Clim. 2020, 10, 27. [Google Scholar] [CrossRef]
- Herrmann, F.; Vorbach, T. The geodynamo for non-geophysicists. Eur. J. Phys. 2020, 41, 045803. [Google Scholar] [CrossRef]
- Yamazaki, Y. Solar and lunar daily geomagnetic variations and their equivalent current systems observed by Swarm. Earth Planets Space 2022, 74, 99. [Google Scholar] [CrossRef]
- Morozova, A.; Rebbah, R. Comparison of the solar variations of the geomagnetic field at the Coimbra Magnetic Observatory (COI) obtained by different methods: Effect of the solar and geomagnetic activity. arXiv 2021, arXiv:2104.00391. [Google Scholar] [CrossRef]
- Steinhilber, F.; Abreu, J.A.; Beer, J.; McCracken, K.G. Interplanetary magnetic field during the past 9300 years inferred from cosmogenic radionuclides. J. Geophys. Res. Space Phys. 2010, 115, 1–14. [Google Scholar] [CrossRef]
- Mo, W.-c.; Liu, Y.; He, R. A Biological Perspective of The Hypomagnetic Field: From Definition Towards Mechanism. Prog. Biochem. Biophys. 2012, 39, 835–842. [Google Scholar]
- Berguig, M.S.; Hamoudi, M.; Lemouël, J.L.; Cohen, Y. Validate global mapping of internal lunar magnetic field. Arab. J. Geosci. 2011, 6, 1063–1072. [Google Scholar] [CrossRef]
- Cain, J.C.; Ferguson, B.B.; Mozzoni, D. An n = 90 internal potential function of the Martian crustal magnetic field. J. Geophys. Res. Planets 2003, 108, 1–19. [Google Scholar] [CrossRef]
- Sinčák, M.; Sedlakova-Kadukova, J. Hypomagnetic Fields and Their Multilevel Effects on Living Organisms. Processes 2023, 11, 282. [Google Scholar] [CrossRef]
- Vidotto, A.A. The evolution of the solar wind. Living Rev. Sol. Phys. 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Lammer, H.; Bredehöft, J.H.; Coustenis, A.; Khodachenko, M.L.; Kaltenegger, L.; Grasset, O.; Prieur, D.; Raulin, F.; Ehrenfreund, P.; Yamauchi, M.; et al. What makes a planet habitable? Astron. Astrophys. Rev. 2009, 17, 181–249. [Google Scholar] [CrossRef]
- Tarduno, J.A.; Cottrell, R.D.; Watkeys, M.K.; Hofmann, A.; Doubrovine, P.V.; Mamajek, E.E.; Liu, D.; Sibeck, D.G.; Neukirch, L.P.; Usui, Y. Geodynamo, Solar Wind, and Magnetopause 3.4 to 3.45 Billion Years Ago. Science 2010, 327, 1238–1240. [Google Scholar] [CrossRef]
- Michalski, G.; Bhattacharya, S.K.; Girsch, G. NOx cycle and the tropospheric ozone isotope anomaly: An experimental investigation. Atmos. Chem. Phys. 2014, 14, 4935–4953. [Google Scholar] [CrossRef]
- Tarduno, J.A.; Blackman, E.G.; Mamajek, E.E. Detecting the oldest geodynamo and attendant shielding from the solar wind: Implications for habitability. Phys. Earth Planet. Inter. 2014, 233, 68–87. [Google Scholar] [CrossRef]
- Wiltschko, R.; Nießner, C.; Wiltschko, W. The Magnetic Compass of Birds: The Role of Cryptochrome. Front. Physiol. 2021, 12, 667000. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Jones, D.S.; MacFadden, B.J. Magnetite Biomineralization and Magnetoreception in Organisms: A New Biomagnetism; Springer Science & Business Media: Berlin, Germany, 2013; Volume 5. [Google Scholar]
- Obleser, P.; Hart, V.; Malkemper, E.P.; Begall, S.; Holá, M.; Painter, M.S.; Červený, J.; Burda, H. Compass-controlled escape behavior in roe deer. Behav. Ecol. Sociobiol. 2016, 70, 1345–1355. [Google Scholar] [CrossRef]
- Hart, V.; Malkemper, E.P.; Kušta, T.; Begall, S.; Nováková, P.; Hanzal, V.; Pleskač, L.; Ježek, M.; Policht, R.; Husinec, V.; et al. Directional compass preference for landing in water birds. Front. Zool. 2013, 10, 38. [Google Scholar] [CrossRef]
- Bianco, G.; Köhler, R.C.; Ilieva, M.; Åkesson, S. Magnetic body alignment in migratory songbirds: A computer vision approach. J. Exp. Biol. 2019, 222, jeb196469. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Sheehan, P.E.; Perry, L.L.; O’Connor, K.; Csonka, L.N.; Applegate, B.M.; Whitman, L.J. Quantifying the Magnetic Advantage in Magnetotaxis. Biophys. J. 2006, 91, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Uebe, R.; Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Begall, S.; Červený, J.; Neef, J.; Vojtěch, O.; Burda, H. Magnetic alignment in grazing and resting cattle and deer. Proc. Natl. Acad. Sci. USA 2008, 105, 13451–13455. [Google Scholar] [CrossRef]
- Davies, E. The decrease in diurnal oxygen production in Elodea under the influence of high geomagnetic variability: The role of light, temperature and atmospheric pressure. Int. J. Biometeorol. 2023, 67, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xue, Y.; Yang, J.; Shang, P.; Yuan, X. Biological Effects of Hypomagnetic Field: Ground-Based Data for Space Exploration. Bioelectromagnetics 2021, 42, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Tombarkiewicz, B. Effect of long-term geomagnetic field deprivation on the concentration of some elements in the hair of laboratory rats. Environ. Toxicol. Pharmacol. 2008, 26, 75–79. [Google Scholar] [CrossRef]
- Fu, J.-P.; Mo, W.-C.; Liu, Y.; Bartlett, P.F.; He, R.-Q. Elimination of the geomagnetic field stimulates the proliferation of mouse neural progenitor and stem cells. Protein Cell 2016, 7, 624–637. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Y.; Zhang, Y.; Li, Y.; Wei, S. Gibberellins are involved in effect of near-null magnetic field on Arabidopsis flowering. Bioelectromagnetics 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Wang, X.K.; Ma, Q.F.; Jiang, W.; Lv, J.; Pan, W.D.; Song, T.; Wu, L.-F. Effects of Hypomagnetic Field on Magnetosome Formation ofMagnetospirillum MagneticumAMB-1. Geomicrobiol. J. 2008, 25, 296–303. [Google Scholar] [CrossRef]
- Grinberg, M.A.; Vodeneev, V.A.; Il’in, N.V.; Mareev, E.A. Laboratory Simulation of Photosynthesis in a Wide Range of Electromagnetic and Radiation Environment Parameters. Astron. Rep. 2023, 67, 71–77. [Google Scholar] [CrossRef]
- Martino, C.F.; Perea, H.; Hopfner, U.; Ferguson, V.L.; Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics 2010, 31, 296–301. [Google Scholar] [CrossRef]
- Wang, D.L.; Wang, X.S.; Xiao, R.; Liu, Y.; He, R.Q. Tubulin assembly is disordered in a hypogeomagnetic field. Biochem. Biophys. Res. Commun. 2008, 376, 363–368. [Google Scholar] [CrossRef]
- Beischer, D.E. The null magnetic field as reference for the study of geomagnetic directional effects in animals and man. Ann. N. Y. Acad. Sci. 1971, 188, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Katiukhin, L.N. Rheological properties of the erythrocytes in weakened static magnetic field of the earth in vitro study. J. Sci. Res. Rep. 2019, 22, 1–12. [Google Scholar] [CrossRef]
- Erdmann, W.; Idzikowski, B.; Kowalski, W.; Kosicki, J.Z.; Kaczmarek, Ł. Tolerance of two anhydrobiotic tardigrades Echiniscus testudo and Milnesium inceptum to hypomagnetic conditions. PeerJ 2021, 9, e10630. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhang, Z.-J.; Mo, W.-C.; Hu, P.-D.; Ding, H.-M.; Liu, Y.; Hua, Q.; He, R.-Q. Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase. Protein Cell 2017, 8, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.-C.; Liu, Y.; Cooper, H.M.; He, R.-Q. Altered development of Xenopus embryos in a hypogeomagnetic field. Bioelectromagnetics 2012, 33, 238–246. [Google Scholar] [CrossRef]
- Beischer, D.E.; Miller II, E.F.; Knepton, J.C. Exposure of Man to Low Intensity Magnetic Fields in a Coil System; Naval Aerospace Medical Institute, Naval Aviation Medical Center: Falls Church, VA, USA, 1967; Volume 1018. [Google Scholar]
- Binhi, V.N.; Sarimov, R.M. Zero magnetic field effect observed in human cognitive processes. Electromagn. Biol. Med. 2009, 28, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.M.; Binhi, V.N.; Milyaev, V.A. The influence of geomagnetic field compensation on human cognitive processes. Biophysics 2008, 53, 433–441. [Google Scholar] [CrossRef]
- Gurfinkel, Y.I.; At’kov, O.Y.; Vasin, A.L.; Breus, T.K.; Sasonko, M.L.; Pishchalnikov, R.Y. Effect of zero magnetic field on cardiovascular system and microcirculation. Life Sci. Space Res. 2016, 8, 1–7. [Google Scholar] [CrossRef]
- Wan, G.-J.; Jiang, S.-L.; Zhao, Z.-C.; Xu, J.-J.; Tao, X.-R.; Sword, G.A.; Gao, Y.-B.; Pan, W.-D.; Chen, F.-J. Bio-effects of near-zero magnetic fields on the growth, development and reproduction of small brown planthopper, Laodelphax striatellus and brown planthopper, Nilaparvata lugens. J. Insect Physiol. 2014, 68, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.; Binhi, V. Low-Frequency Magnetic Fields in Cars and Office Premises and the Geomagnetic Field Variations. Bioelectromagnetics 2020, 41, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.-C.; Zhang, Z.-J.; Wang, D.-L.; Liu, Y.; Bartlett, P.F.; He, R.-Q. Shielding of the Geomagnetic Field Alters Actin Assembly and Inhibits Cell Motility in Human Neuroblastoma Cells. Sci. Rep. 2016, 6, 22624. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.; Machikhin, A.; Sizov, D.; Guryleva, A.; Sizova, A.; Zhdanova, S.; Tchougounov, V.; Burlakov, A. Influence of hypomagnetic field on the heartbeat in zebrafish embryos. Front. Physiol. 2022, 13, 1040083. [Google Scholar] [CrossRef]
- Mo, W.; Liu, Y.; Bartlett, P.F.; He, R. Transcriptome profile of human neuroblastoma cells in the hypomagnetic field. Sci. China Life Sci. 2014, 57, 448–461. [Google Scholar] [CrossRef]
- Wang, G.-M.; Fu, J.-P.; Mo, W.-C.; Zhang, H.-T.; Liu, Y.; He, R.-Q. Shielded geomagnetic field accelerates glucose consumption in human neuroblastoma cells by promoting anaerobic glycolysis. Biochem. Biophys. Res. Commun. 2022, 601, 101–108. [Google Scholar] [CrossRef]
- Ogneva, I.V.; Usik, M.A.; Burtseva, M.V.; Biryukov, N.S.; Zhdankina, Y.S.; Sychev, V.N.; Orlov, O.I. Drosophila melanogaster Sperm under Simulated Microgravity and a Hypomagnetic Field: Motility and Cell Respiration. Int. J. Mol. Sci. 2020, 21, 5985. [Google Scholar] [CrossRef]
- Gurfinkel, Y.I.; Vasin, A.L.; Pishchalnikov, R.Y.; Sarimov, R.M.; Sasonko, M.L.; Matveeva, T.A. Geomagnetic storm under laboratory conditions: Randomized experiment. Int. J. Biometeorol. 2018, 62, 501–512. [Google Scholar] [CrossRef]
- Pishchalnikov, R.Y.; Gurfinkel, Y.I.; Sarimov, R.M.; Vasin, A.L.; Sasonko, M.L.; Matveeva, T.A.; Binhi, V.N.; Baranov, M.V. Cardiovascular response as a marker of environmental stress caused by variations in geomagnetic field and local weather. Biomed. Signal Process. Control 2019, 51, 401–410. [Google Scholar] [CrossRef]
- Belova, N.A.; Lednev, V.V. Dependence of gravitotropic reaction in segments of flax stems on frequency and amplitude of variable components of a weak combined magnetic field. Biophysics 2000, 45, 1108–1111. [Google Scholar]
- Belova, N.A.; Ermakov, A.M.; Znobishcheva, A.V.; Srebnitskaya, L.K.; Lednev, V.V. The influence of extremely weak alternating magnetic fields on the regeneration of planarians and the gravitropic response of plants. Biophysics 2010, 55, 704–709. [Google Scholar] [CrossRef]
- Canova, A.; del Pino López, J.; Giaccone, L.; Manca, M. Active Shielding System for ELF Magnetic Fields. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Krivova, N.A.; Gull, Y.V.; Zelenskaya, A.Y.; Bondartseva, N.V. Effects of long-term geomagnetic field deprivation on bioelectrical activity of brain of laboratory rats. Tomsk. State Univ. J. 2011, 348, 155–160. [Google Scholar]
- Khodanovich, M.Y.; Gul, E.V.; Zelenskaja, A.E.; Pan, E.S.; Krivova, N.A. Effect of long-term geomagnetic field weakening on aggressiveness of rats and opioidergic neurons activation. Tomsk. State Univ. J. Biol. 2013, 1, 146–160. (In Russian) [Google Scholar]
- Zhang, X.; Li, J.-F.; Wu, Q.-J.; Li, B.; Jiang, J.-C. Effects of hypomagnetic field on noradrenergic activities in the brainstem of golden hamster. Bioelectromagnetics 2007, 28, 155–158. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhan, A.; Wang, M.; Tian, L.; Guo, W.; Pan, Y. Long-term exposure to a hypomagnetic field attenuates adult hippocampal neurogenesis and cognition. Nat. Commun. 2021, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Luo, Y.; Zhan, A.; Ren, J.; Qin, H.; Pan, Y. Hypomagnetic Field Induces the Production of Reactive Oxygen Species and Cognitive Deficits in Mice Hippocampus. Int. J. Mol. Sci. 2022, 23, 3622. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Li, B.; Li, D.; Jiang, J. Long-term memory was impaired in one-trial passive avoidance task of day-old chicks hatching from hypomagnetic field space. Chin. Sci. Bull. 2003, 48, 2454–2457. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, H.; Xi, W.; Zhou, X.; Xu, S.; Zhang, K.; Jiang, J.; Li, Y.; Guo, A. Exposure to hypomagnetic field space for multiple generations causes amnesia in Drosophila melanogaster. Neurosci. Lett. 2004, 371, 190–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, W. Removal or component reversal of local geomagnetic field affects foraging orientation preference in migratory insect brown planthopper Nilaparvata lugens. PeerJ 2021, 9, e12351. [Google Scholar] [CrossRef]
- Xu, J.; Pan, W.; Zhang, Y.; Li, Y.; Wan, G.; Chen, F.; Sword, G.A.; Pan, W. Behavioral evidence for a magnetic sense in the oriental armyworm, Mythimna separata. Biol. Open 2017, 6, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Casacci, L.P.; Bianco Dolino, G.; Badolato, G.; Maffei, M.E.; Barbero, F. The Geomagnetic Field (GMF) Is Necessary for Black Garden Ant (Lasius niger L.) Foraging and Modulates Orientation Potentially through Aminergic Regulation and MagR Expression. Int. J. Mol. Sci. 2023, 24, 4387. [Google Scholar] [CrossRef] [PubMed]
- Kantserova, N.P.; Krylov, V.V.; Lysenko, L.A.; Ushakova, N.V.; Nemova, N.N. Effects of Hypomagnetic Conditions and Reversed Geomagnetic Field on Calcium-Dependent Proteases of Invertebrates and Fish. Izv. Atmos. Ocean. Phys. 2018, 53, 719–723. [Google Scholar] [CrossRef]
- Wan, G.J.; Jiang, S.L.; Zhang, M.; Zhao, J.Y.; Zhang, Y.C.; Pan, W.D.; Sword, G.A.; Chen, F.J. Geomagnetic field absence reduces adult body weight of a migratory insect by disrupting feeding behavior and appetite regulation. Insect Sci. 2020, 28, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Xie, L.; Zheng, Q.; Yang, P.F.; Zhang, W.J.; Ding, C.; Qian, A.R.; Shang, P.A. A Hypomagnetic Field Aggravates Bone Loss Induced by Hindlimb Unloading in Rat Femurs. PLoS ONE 2014, 9, e105604. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Meng, X.; Dong, D.; Xue, Y.; Chen, X.; Wang, S.; Shen, Y.; Zhang, G.; Shang, P. Iron overload involved in the enhancement of unloading-induced bone loss by hypomagnetic field. Bone 2018, 114, 235–245. [Google Scholar] [CrossRef]
- Fesenko, E.E.; Mezhevikina, L.M.; Osipenko, M.A.; Gordon, R.Y.; Khutzian, S.S. Effect of the “zero” Magnetic Field on Early Embryogenesis in Mice. Electromagn. Biol. Med. 2010, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.-J.; Yuan, R.; Wang, W.-J.; Fu, K.-Y.; Zhao, J.-Y.; Jiang, S.-L.; Pan, W.-D.; Sword, G.A.; Chen, F.-J. Reduced geomagnetic field may affect positive phototaxis and flight capacity of a migratory rice planthopper. Anim. Behav. 2016, 121, 107–116. [Google Scholar] [CrossRef]
- Yan, M.-m.; Zhang, L.; Cheng, Y.-x.; Sappington, T.W.; Pan, W.-d.; Jiang, X.-f. Effect of a near-zero magnetic field on development and flight of oriental armyworm (Mythimna separata). J. Integr. Agric. 2021, 20, 1336–1345. [Google Scholar] [CrossRef]
- Krylov, V.V.; Bolotovskaya, I.V.; Osipova, E.A. The response of european Daphnia magna straus and australian Daphnia carinata king to changes in geomagnetic field. Electromagn. Biol. Med. 2013, 32, 30–39. [Google Scholar] [CrossRef]
- Binhi, V.N.; Sarimov, R.M. Effect of the hypomagnetic field on the size of the eye pupil. arXiv 2013, arXiv:1302.2741. [Google Scholar] [CrossRef]
- Moissl-Eichinger, C.; Erdmann, W.; Idzikowski, B.; Kowalski, W.; Szymański, B.; Kosicki, J.Z.; Kaczmarek, Ł. Can the tardigrade Hypsibius dujardini survive in the absence of the geomagnetic field? PLoS ONE 2017, 12, e0183380. [Google Scholar] [CrossRef][Green Version]
- Temour’yants, N.A.; Kostyuk, A.S.; Tumanyants, K.N. Dynamics and Infradian Rhythmics of Thermal/Pain Sensitivity of the Helix Mollusc under the Action of Electromagnetic Fields. Neurophysiology 2011, 42, 276–285. [Google Scholar] [CrossRef]
- Burger, T.; Lucová, M.; Moritz, R.E.; Oelschläger, H.H.A.; Druga, R.; Burda, H.; Wiltschko, W.; Wiltschko, R.; Němec, P. Changing and shielded magnetic fields suppress c-Fos expression in the navigation circuit: Input from the magnetosensory system contributes to the internal representation of space in a subterranean rodent. J. R. Soc. Interface 2010, 7, 1275–1292. [Google Scholar] [CrossRef]
- Gurfinkel, Y.I.; Vasin, A.L.; Matveeva, T.A.; Sasonko, M.L. Evaluation of the hypomagnetic environment effects on capillary blood circulation, blood pressure and heart rate. Aviakosmicheskaia I Ekol. Meditsina Aerosp. Environ. 2014, 48, 24–30. [Google Scholar] [CrossRef]
- Demin, A.V.; Suvorov, A.V.; Orlov, O.I. Features of hemodynamics in healthy men under hypomagnetic conditions. Aviakosm. Ekolog. Med. 2021, 55, 63–68. (In Russian) [Google Scholar]
- Ciorba, D.; Morariu, V.V. Life in zero magnetic field. Iii. Activity of aspartate aminotransferase and alanine aminotransferase during in vitro aging of human blood. Electro-Magnetobiology 2009, 20, 313–321. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. The Effect of a “Zero” Magnetic Field on the Production of Reactive Oxygen Species in Neutrophils. Biophysics 2018, 63, 365–368. [Google Scholar] [CrossRef]
- Mo, W.-C.; Fu, J.-P.; Ding, H.-M.; Liu, Y.; Hua, Q.; He, R.-Q. Hypomagnetic Field Alters Circadian Rhythm and Increases Algesia in Adult Male Mice. Prog. Biochem. Biophys. 2015, 42, 639–646. [Google Scholar] [CrossRef]
- Nepomnyashchikh, L.M.; Lushnikova, E.L.; Klinnikova, M.G.; Molodykh, O.P.; Ashcheulova, N.V. Effect of hypogeomagnetic field on tissue and intracellular reorganization of mouse myocardium. Bull. Exp. Biol. Med. 1997, 124, 1021–1024. [Google Scholar] [CrossRef]
- Hu, P.; Mo, W.-C.; Fu, J.-P.; Liu, Y.; He, R.Q. Long-term hypogeomagnetic field exposure reduces muscular mitochondrial function and exercise capacity in adult male mice. Prog. Biochem. Biophys. 2020, 47, 426–438. [Google Scholar] [CrossRef]
- Fu, J.-P.; Mo, W.-C.; Liu, Y.; He, R.-Q. Decline of cell viability and mitochondrial activity in mouse skeletal muscle cell in a hypomagnetic field. Bioelectromagnetics 2016, 37, 212–222. [Google Scholar] [CrossRef]
- Tsetlin, V.V.; Zotin, A.A.; Moisa, S.S. Effect of altered magnetic field on the development of great ramshorn Planorbarius corneus (gastropoda, planorbidae). Aviakosm. Ekolog. Med. 2014, 48, 36–44. [Google Scholar] [PubMed]
- Mo, W.-C.; Zhang, Z.-J.; Liu, Y.; Zhai, G.-J.; Jiang, Y.-D.; He, R.-Q. Effects of a hypogeomagnetic field on gravitropism and germination in soybean. Adv. Space Res. 2011, 47, 1616–1621. [Google Scholar] [CrossRef]
- Xu, C.; Wei, S.; Lu, Y.; Zhang, Y.; Chen, C.; Song, T. Removal of the local geomagnetic field affects reproductive growth inArabidopsis. Bioelectromagnetics 2013, 34, 437–442. [Google Scholar] [CrossRef]
- Agliassa, C.; Narayana, R.; Bertea, C.M.; Rodgers, C.T.; Maffei, M.E. Reduction of the geomagnetic field delays Arabidopsis thaliana flowering time through downregulation of flowering-related genes. Bioelectromagnetics 2018, 39, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Maffei, M.E.; Vigani, G. The Geomagnetic Field Is a Contributing Factor for an Efficient Iron Uptake in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 325. [Google Scholar] [CrossRef]
- Narayana, R.; Fliegmann, J.; Paponov, I.; Maffei, M.E. Reduction of geomagnetic field (GMF) to near null magnetic field (NNMF) affects Arabidopsis thaliana root mineral nutrition. Life Sci. Space Res. 2018, 19, 43–50. [Google Scholar] [CrossRef]
- Negishi, Y.; Hashimoto, A.; Tsushima, M.; Dobrota, C.; Yamashita, M.; Nakamura, T. Growth of pea epicotyl in low magnetic field implication for space research. Adv. Space Res. 1999, 23, 2029–2032. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Narayana, R.; Christie, J.M.; Maffei, M.E. Geomagnetic field impacts on cryptochrome and phytochrome signaling. J. Photochem. Photobiol. B Biol. 2018, 185, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Yu, Y.; Li, Y.; Wei, S. Suppression of Arabidopsis flowering by near-null magnetic field is mediated by auxin. Bioelectromagnetics 2018, 39, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yin, X.; Lv, Y.; Wu, C.; Zhang, Y.; Song, T. A near-null magnetic field affects cryptochrome-related hypocotyl growth and flowering in Arabidopsis. Adv. Space Res. 2012, 49, 834–840. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; Betterle, N.; Mannino, G.; D’Alessandro, S.; Nocito, F.F.; Ljumovic, K.; Vigani, G.; Ballottari, M.; Maffei, M.E. The Geomagnetic Field (GMF) Is Required for Lima Bean Photosynthesis and Reactive Oxygen Species Production. Int. J. Mol. Sci. 2023, 24, 2896. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.-R.; Henbest, K.B.; Maeda, K.; Pannell, J.R.; Timmel, C.R.; Hore, P.J.; Okamoto, H. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 2009, 6, 1193–1205. [Google Scholar] [CrossRef]
- Vigani, G.; Islam, M.; Cavallaro, V.; Nocito, F.F.; Maffei, M.E. Geomagnetic Field (GMF)-Dependent Modulation of Iron-Sulfur Interplay in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 10166. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.K.; Galland, P. Effects of weak static magnetic fields on the gene expression of seedlings of Arabidopsis thaliana. J. Plant Physiol. 2018, 231, 9–18. [Google Scholar] [CrossRef]
- Agliassa, C.; Maffei, M.E. Reduction of geomagnetic field (GMF) to near null magnetic field (NNMF) affects some Arabidopsis thaliana clock genes amplitude in a light independent manner. J. Plant Physiol. 2019, 232, 23–26. [Google Scholar] [CrossRef]
- Xu, C.; Lv, Y.; Chen, C.; Zhang, Y.; Wei, S. Blue light-dependent phosphorylations of cryptochromes are affected by magnetic fields in Arabidopsis. Adv. Space Res. 2014, 53, 1118–1124. [Google Scholar] [CrossRef]
- Dhiman, S.K.; Wu, F.; Galland, P. Effects of weak static magnetic fields on the development of seedlings of Arabidopsis thaliana. Protoplasma 2023, 260, 767–786. [Google Scholar] [CrossRef]
- Martino, C.F.; Portelli, L.; McCabe, K.; Hernandez, M.; Barnes, F. Reduction of the Earth’s magnetic field inhibits growth rates of model cancer cell lines. Bioelectromagnetics 2010, 31, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. Magnetobiology: Experiments and Models; MILTA: Moscow, Russia, 2002; p. 592. [Google Scholar]
- Li, J.F.; Wu, Q.J.; Wang, Q.; Jiang, J.C.; Jin, H.Q.; Lin, Y.F. Effect of magnetic free field space (MFFS) on GABA, glycine andtaurine of cortex, cerebellum and basilar nucleus in hamster. Prog. Biochem. Biophys. 2001, 28, 358–361. [Google Scholar]
- Baek, S.; Choi, H.; Park, H.; Cho, B.; Kim, S.; Kim, J. Effects of a hypomagnetic field on DNA methylation during the differentiation of embryonic stem cells. Sci. Rep. 2019, 9, 1333. [Google Scholar] [CrossRef] [PubMed]
- Roemer, K.; Mo, W.-c.; Zhang, Z.-j.; Liu, Y.; Bartlett, P.F.; He, R.-Q. Magnetic Shielding Accelerates the Proliferation of Human Neuroblastoma Cell by Promoting G1-Phase Progression. PLoS ONE 2013, 8, e54775. [Google Scholar] [CrossRef]
- Eldashev, I.S.; Shchegolev, B.F.; Surma, S.V.; Belostotskaya, G.B. Influence of low-intensity magnetic fields on the development of satellite muscle cells of a newborn rat in primary culture. Biophysics 2011, 55, 765–770. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Ding, C.; Dong, D.; Shang, P. Regulation of Osteoblast Differentiation and Iron Content in MC3T3-E1 Cells by Static Magnetic Field with Different Intensities. Biol. Trace Elem. Res. 2018, 184, 214–225. [Google Scholar] [CrossRef]
- Juutilainen, J.; Herrala, M.; Luukkonen, J.; Naarala, J.; Hore, P.J. Magnetocarcinogenesis: Is there a mechanism for carcinogenic effects of weak magnetic fields? Proc. R. Soc. B Biol. Sci. 2018, 285, 20180590. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Martino, C.F.; Castello, P.R. Modulation of Hydrogen Peroxide Production in Cellular Systems by Low Level Magnetic Fields. PLoS ONE 2011, 6, e22753. [Google Scholar] [CrossRef]
- Robison, J.G.; Pendleton, A.R.; Monson, K.O.; Murray, B.K.; O’Neill, K.L. Decreased DNA repair rates and protection from heat induced apoptosis mediated by electromagnetic field exposure. Bioelectromagnetics 2002, 23, 106–112. [Google Scholar] [CrossRef]
- Babych, V.I. The characteristics of tissue lipid peroxidation in the internal organs and the lipid metabolic indices of the blood plasma in a low geomagnetic field. Fiziolohichnyi Zhurnal 1995, 41, 44–49. [Google Scholar]
- Babych, V.I. The characteristics of tissue lipid peroxidation of the internal organs in anaphylaxis under the action of a hypo- or hypermagnetic field. Fiziolohichnyi Zhurnal 1996, 42, 66–71. [Google Scholar] [PubMed]
- Zhang, Y.; Zeng, L.; Wei, Y.; Zhang, M.; Pan, W.; Sword, G.A.; Yang, F.; Chen, F.; Wan, G. Reliable reference genes for gene expression analyses under the hypomagnetic field in a migratory insect. Front. Physiol. 2022, 13, 954228. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Alipov, Y.D.; Harms-Ringdahl, M. Effects of zero magnetic field on the conformation of chromatin in human cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1997, 1336, 465–473. [Google Scholar] [CrossRef]
- Xue, X.; Ali, Y.F.; Liu, C.; Hong, Z.; Luo, W.; Nie, J.; Li, B.; Jiao, Y.; Liu, N.-A. Geomagnetic Shielding Enhances Radiation Resistance by Promoting DNA Repair Process in Human Bronchial Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 9304. [Google Scholar] [CrossRef] [PubMed]
- McCreary, C.R.; Dixon, S.J.; Fraher, L.J.; Carson, J.J.L.; Prato, F.S. Real-time measurement of cytosolic free calcium concentration in Jurkat cells during ELF magnetic field exposure and evaluation of the role of cell cycle. Bioelectromagnetics 2006, 27, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-D.; Petersen, N.; Zhang, W.-J.; Cargou, S.; Ruan, J.; Murat, D.; Santini, C.-L.; Song, T.; Kato, T.; Notareschi, P.; et al. Swimming behaviour and magnetotaxis function of the marine bacterium strain MO-1. Environ. Microbiol. Rep. 2014, 6, 14–20. [Google Scholar] [CrossRef]
- Poiata, A.; Creanga, D.E.; Morariu, V.V. Life in zero magnetic field. V. E. coli resistance to antibiotics. Electromagn. Biol. Med. 2009, 22, 171–182. [Google Scholar] [CrossRef]
- Creanga, D.E.; Poiata, A.; Morariu, V.V.; Tupu, P. Zero-magnetic field effect in pathogen bacteria. J. Magn. Magn. Mater. 2004, 272–276, 2442–2444. [Google Scholar] [CrossRef]
- Ilyin, V.K.; Orlov, O.I.; Morozova, Y.A.; Skedina, M.A.; Vladimirov, S.K.; Plotnikov, E.V.; Artamonov, A.A. Prognostic model for bacterial drug resistance genes horizontal spread in space-crews. Acta Astronaut. 2022, 190, 388–394. [Google Scholar] [CrossRef]
- Lutz, K.; Cadiou, H.; Trevino, T.; Cinelli, I. Electromagnetic fields to sustain life on earth, in apace, and planets. In Proceedings of the 72nd International Astronautical Congress (IAC), Dubai, United Arab Emirates, 25–29 October 2021; p. IAC-21-A1.19 1-28. [Google Scholar]
- Blagau, A.; Ersen, A.; Dobrescu, C.; Marghitu, O. Astro Pi sensor onboard the International Space Station as magnetic field surveyor. Acta Astronaut. 2022, 195, 456–464. [Google Scholar] [CrossRef]
- Pandey, S.; Garg, T.; Singh, K.; Rai, S. Effect of magnetically induced water structure on the oestrous cycles of albino female mice Musmusculus. Electro-Magnetobiology 1996, 15, 133–140. [Google Scholar] [CrossRef]
- Rai, S.; Garg, T.; Vashistha, H. Possible Effect of Magnetically Induced Water Structures on Photosynthetic Electron Transport Chains of a Green Alga Chlorella Vulgarts. Electro-Magnetobiology 1996, 15, 49–55. [Google Scholar] [CrossRef]
- Devyatkov, N.D.; Kislov, V.Y.; Kislov, V.V.; Kolesov, V.V.; Smirnov, V.F.; Chigin, E.P. Detection of the effect of normalisation of the functional state of human internal organs under the influence of water activated by millimetre radiation. Millimetre Waves Biol. Med. 1996, 8, 65–68. [Google Scholar]
- Ružič, R.; Jerman, I. Influence of Ca2+ in biological effects of direct and indirect ELF magnetic field stimulation. Electro-Magnetobiology 1998, 17, 205–216. [Google Scholar]
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. The Role of Water in the Effect of Weak Combined Magnetic Fields on Production of Reactive Oxygen Species (ROS) by Neutrophils. Appl. Sci. 2020, 10, 3326. [Google Scholar] [CrossRef]
- Colic, M.; Morse, D. Mechanism of the long-term effects of electromagnetic radiation on solutions and suspended colloids. Langmuir 1998, 14, 783–787. [Google Scholar] [CrossRef]
- Lobyshev, V.I. Evolution of High-Frequency Conductivity of Pure Water Samples Subjected to Mechanical Action: Effect of a Hypomagnetic Filed. Phys. Wave Phenom. 2021, 29, 98–101. [Google Scholar] [CrossRef]
- Penkov, N. Temporal dynamics of the scattering properties of deionized water. Phys. Wave Phenom. 2020, 28, 135–139. [Google Scholar] [CrossRef]
- Konovalov, A.; Ryzhkina, I.; Maltzeva, E.; Murtazina, L.; Kiseleva, Y.; Kasparov, V.; Palmina, N. Nanoassociate formation in highly diluted water solutions of potassium phenosan with and without permalloy shielding. Electromagn. Biol. Med. 2015, 34, 141–146. [Google Scholar] [CrossRef]
- Xiao-Feng, P.; Gui-Fa, S. The Changes of Physical Properties of Water Arising from the Magnetic Field and its Mechanism. Mod. Phys. Lett. B 2013, 27, 1350228. [Google Scholar] [CrossRef]
- Shen, X. Increased dielectric constant in the water treated by extremely low frequency electromagnetic field and its possible biological implication. J. Phys. Conf. Ser. 2011, 329, 012019. [Google Scholar] [CrossRef]
- Belov, A.A.; Konyukhov, V.K.; Stepanov, A.V. Fluctuations of water dielectric permittivity under thermal and mechanical effects on water. Short Commun. Phys. FIAN 1997, 7, 8. [Google Scholar]
- Astashev, M.; Serov, D.; Sarimov, R.; Gudkov, S. Influence of the Vibration Impact Mode on the Spontaneous Chemiluminescence of Aqueous Protein Solutions. Phys. Wave Phenom. 2023, 31, 189–199. [Google Scholar] [CrossRef]
- Novikov, V.V.; Kuvichkin, V.V.; Fesenko, E.E. Effect of weak combined low frequency constant and alternative magnetic fields on intrinsic fluorescence of proteins in aqueous solutions. Biophysics 1999, 44, 224–230. [Google Scholar]
- Binhi, V.N.; Alipov, Y.D.; Belyaev, I.Y. Effect of static magnetic field on E. coli cells and individual rotations of ion-protein complexes. Bioelectromagnetics 2001, 22, 79–86. [Google Scholar] [CrossRef]
- Demin, A.V. Characteristics of healthy men hemodynamics in model martian hypomagnetic environment. Biomed. Radioelectron. 2022, 25, 22–30. [Google Scholar] [CrossRef]
- Ferrone, K.; Willis, C.; Guan, F.; Ma, J.; Peterson, L.; Kry, S. A Review of Magnetic Shielding Technology for Space Radiation. Radiation 2023, 3, 46–57. [Google Scholar] [CrossRef]
- Bhattacharjie, A.; Michael, I. Mass and magnetic dipole shielding against electrons of the artificial radiation belt. AIAA J. 1964, 2, 2198–2201. [Google Scholar] [CrossRef]
- Trukhanov, K.; Morozov, D. Optimization of a Magnetic Radiation Shield. Sov. Phys. Tech. Phys. 1970, 15, 979. [Google Scholar]
- Barthel, J.; Sarigul-Klijn, N. A review of radiation shielding needs and concepts for space voyages beyond Earth’s magnetic influence. Prog. Aerosp. Sci. 2019, 110, 100553. [Google Scholar] [CrossRef]
- Musenich, R.; Calvelli, V.; Giraudo, M.; Vuolo, M.; Ambroglini, F.; Battiston, R. The Limits of Space Radiation Magnetic Shielding: An Updated Analysis. IEEE Trans. Appl. Supercond. 2018, 28, 0500105. [Google Scholar] [CrossRef]
- Spillantini, P.; Casolino, M.; Durante, M.; Mueller-Mellin, R.; Reitz, G.; Rossi, L.; Shurshakov, V.; Sorbi, M. Shielding from cosmic radiation for interplanetary missions: Active and passive methods. Radiat. Meas. 2007, 42, 14–23. [Google Scholar] [CrossRef]
- Bamford, R.A.; Kellett, B.J.; Green, J.L.; Dong, C.; Airapetian, V.; Bingham, R. How to create an artificial magnetosphere for Mars. Acta Astronaut. 2022, 190, 323–333. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytical review of experiments and theories. PLoS ONE 2017, 12, e0179340. [Google Scholar] [CrossRef] [PubMed]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Blackman, C.F.; Benane, S.G.; House, D.E. Evidence for direct effect of magnetic fields on neurite outgrowth. FASEB J. 1993, 7, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Dejean, V.; Sowood, D.J.C.; et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef]
- Binhi, V.N. Random Effects in Magnetobiology and a Way to Summarize Them. Bioelectromagnetics 2021, 42, 501–515. [Google Scholar] [CrossRef]
- Hoff, A.J.; Rademaker, H.; Van Grondelle, R.; Duysens, L.N.M. On the magnetic field dependence of the yield of the triplet state in reaction centers of photosynthetic bacteria. Biochim. Biophys. Acta BBA Bioenerg. 1977, 460, 547–554. [Google Scholar] [CrossRef]
- Schulten, K.; Swenberg, C.E.; Weller, A. A Biomagnetic Sensory Mechanism Based on Magnetic Field Modulated Coherent Electron Spin Motion. Z. Für Phys. Chem. 1978, 111, 1–5. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. Rotations of macromolecules affect nonspecific biological responses to magnetic fields. Sci. Rep. 2018, 8, 13495. [Google Scholar] [CrossRef]
- Binhi, V.N. Nonspecific magnetic biological effects: A model assuming the spin-orbit coupling. J. Chem. Phys. 2019, 151, 204101. [Google Scholar] [CrossRef] [PubMed]
- Kholodov, Y.A. Bypassing the Sensory Organs? Znanie: Moscow, Russia, 1991; Volume 11. [Google Scholar]
- Sarimov, R.; Alipov, E.D.; Belyaev, I.Y. Fifty hertz magnetic fields individually affect chromatin conformation in human lymphocytes: Dependence on amplitude, temperature, and initial chromatin state. Bioelectromagnetics 2011, 32, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Belyavskaya, N.A. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Novitsky, Y.I. The Effect of a Constant Magnetic Field on Plants; Nauka: Moscow, Russia, 2016. (In Russian) [Google Scholar]
- Breus, T.K.; Binhi, V.N.; Petrukovich, A.A. Magnetic factor in solar-terrestrial relations and its impact on the human body: Physical problems and prospects for research. Phys. Uspekhi 2016, 59, 502–510. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Serov, D.A.; Gudkov, S.V. Biological effects of alternating magnetic fields. Bioelogy 2023, in press. [Google Scholar]
- Binhi, V.N.; Prato, F.S. A physical mechanism of magnetoreception: Extension and analysis. Bioelectromagnetics 2017, 38, 41–52. [Google Scholar] [CrossRef]
- Binhi, V.N. Nuclear spins in the primary mechanisms of biological action of magnetic fields. Biophysics 1995, 40, 677–691. [Google Scholar]
- Buchachenko, A.L. Magnetic field-dependent molecular and chemical processes in biochemistry, genetics and medicine. Russ. Chem. Rev. 2014, 83, 1–12. [Google Scholar] [CrossRef]
- Binhi, V.N. Principles of Electromagnetic Biophysics; Fizmatlit: Moscow, Russia, 2011. [Google Scholar]
- Binhi, V.N. Magnetobiology: Underlying Physical Problems; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Yang, X.; Li, Z.; Polyakova, T.; Dejneka, A.; Zablotskii, V.; Zhang, X. Effect of static magnetic field on DNA synthesis: The interplay between DNA chirality and magnetic field left-right asymmetry. FASEB Bioadv. 2020, 2, 254–263. [Google Scholar] [CrossRef]
- Famiano, M.A.; Boyd, R.N.; Kajino, T.; Onaka, T. Selection of amino acid chirality via neutrino interactions with 14N in crossed electric and magnetic fields. Astrobiology 2018, 18, 190–206. [Google Scholar] [CrossRef]
- Herd, C.D.K.; Blinova, A.; Simkus, D.N.; Huang, Y.; Tarozo, R.; Alexander, C.M.O.D.; Gyngard, F.; Nittler, L.R.; Cody, G.D.; Fogel, M.L.; et al. Origin and Evolution of Prebiotic Organic Matter as Inferred from the Tagish Lake Meteorite. Science 2011, 332, 1304–1307. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for Extraterrestrial Amino-acids and Hydrocarbons in the Murchison Meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef]
- Binhi, V.N.; Chernavsi, D.S. The stochastic resonance of magnetosomes fixed in the cytoskeleton. Biofizika 2005, 50, 684–688. (In Russian) [Google Scholar]
- Kirschvink, J.L.; Winklhofer, M.; Walker, M.M. Biophysics of magnetic orientation: Strengthening the interface between theory and experimental design. J. R. Soc. Interface 2010, 7, S179–S191. [Google Scholar] [CrossRef] [PubMed]
- Milyaev, V.A.; Binhi, V.N. On the physical nature of magnetobiological effects. Quantum Electron. 2006, 36, 691–701. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. Magnetobiology: The kT paradox and possible solutions. Electromagn. Biol. Med. 2007, 26, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Savin, A.V. Effects of weak magnetic fields on biological systems: Physical aspects. Phys. Uspekhi 2003, 46, 259–291. [Google Scholar] [CrossRef]
- Binhi, V.N. Mechanism of magnetosensitive binding of ions by certain proteins. Biophysics 1997, 42, 338–342. [Google Scholar]
- Steiner, U.E.; Ulrich, T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 2002, 89, 51–147. [Google Scholar] [CrossRef]
- Afanasyeva, M.S.; Taraban, M.B.; Purtov, P.A.; Leshina, T.V.; Grissom, C.B. Magnetic Spin Effects in Enzymatic Reactions: Radical Oxidation of NADH by Horseradish Peroxidase. J. Am. Chem. Soc. 2006, 128, 8651–8658. [Google Scholar] [CrossRef]
- Shcherbakov, I.A. Current Trends in the Studies of Aqueous Solutions. Phys. Wave Phenom. 2022, 30, 129–134. [Google Scholar] [CrossRef]
- Lyakhov, G.A.; Man’ko, V.I.; Suyazov, N.V.; Shcherbakov, I.A.; Shermeneva, M.A. Physical mechanisms of activation of radical reactions in aqueous solutions under mechanical and magnetic effect: Problem of singlet oxygen. Phys. Wave Phenom. 2022, 30, 174–181. [Google Scholar] [CrossRef]
- Kattnig, D.R.; Solov’yov, I.A.; Hore, P.J. Electron spin relaxation in cryptochrome-based magnetoreception. Phys. Chem. Chem. Phys. 2016, 18, 12443–12456. [Google Scholar] [CrossRef]
- Gauger, E.M.; Rieper, E.; Morton, J.J.L.; Benjamin, S.C.; Vedral, V. Sustained Quantum Coherence and Entanglement in the Avian Compass. Phys. Rev. Lett. 2011, 106, 040503. [Google Scholar] [CrossRef]
- Maeda, K.; Robinson, A.J.; Henbest, K.B.; Hogben, H.J.; Biskup, T.; Ahmad, M.; Schleicher, E.; Weber, S.; Timmel, C.R.; Hore, P.J. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl. Acad. Sci. USA 2012, 109, 4774–4779. [Google Scholar] [CrossRef]
- Binhi, V.N. Amplitude and frequency dissociation spectra of ion-protein complexes rotating in magnetic fields. Bioelectromagnetics 2000, 21, 34–45. [Google Scholar] [CrossRef]
- Binhi, V.N.; Rubin, A.B. Theoretical Concepts in Magnetobiology after 40 Years of Research. Cells 2022, 11, 274. [Google Scholar] [CrossRef]
- Lambert, C.J. Basic concepts of quantum interference and electron transport in single-molecule electronics. Chem. Soc. Rev. 2015, 44, 875–888. [Google Scholar] [CrossRef]
- Wan, G.; Hayden, A.N.; Iiams, S.E.; Merlin, C. Cryptochrome 1 mediates light-dependent inclination magnetosensing in monarch butterflies. Nat. Commun. 2021, 12, 771. [Google Scholar] [CrossRef]
- Gunkel, M.; Schoneberg, J.; Alkhaldi, W.; Irsen, S.; Noe, F.; Kaupp, U.B.; Al-Amoudi, A. Higher-order architecture of rhodopsin in intact photoreceptors and its implication for phototransduction kinetics. Structure 2015, 23, 628–638. [Google Scholar] [CrossRef]
- Marshak, D.W.; Mills, S.L. Short-wavelength cone-opponent retinal ganglion cells in mammals. Vis. Neurosci. 2014, 31, 165–175. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, B.; Su, J.; Liao, J.; Lin, C.; Oka, Y. Cryptochromes Orchestrate Transcription Regulation of Diverse Blue Light Responses in Plants. Photochem. Photobiol. 2017, 93, 112–127. [Google Scholar] [CrossRef]
- Gegear, R.J.; Foley, L.E.; Casselman, A.; Reppert, S.M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 2010, 463, 804–807. [Google Scholar] [CrossRef]
- Iwaniuk, A.; Heyers, D.; Manns, M.; Luksch, H.; Güntürkün, O.; Mouritsen, H. A Visual Pathway Links Brain Structures Active during Magnetic Compass Orientation in Migratory Birds. PLoS ONE 2007, 2, e937. [Google Scholar] [CrossRef]
- Ozturk, N. Phylogenetic and Functional Classification of the Photolyase/Cryptochrome Family. Photochem. Photobiol. 2017, 93, 104–111. [Google Scholar] [CrossRef]
- Pooam, M.; Arthaut, L.-D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2018, 249, 319–332. [Google Scholar] [CrossRef]
- Hammad, M.; Albaqami, M.; Pooam, M.; Kernevez, E.; Witczak, J.; Ritz, T.; Martino, C.; Ahmad, M. Cryptochrome mediated magnetic sensitivity in Arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem. Photobiol. Sci. 2020, 19, 341–352. [Google Scholar] [CrossRef]
- Ahmad, M.; Cashmore, A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.-O.; van der Horst, G.T.J.; Batschauer, A.; Ahmad, M. The Cryptochromes: Blue Light Photoreceptors in Plants and Animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef]
- Deviers, J.; Cailliez, F.; de la Lande, A.; Kattnig, D.R. Anisotropic magnetic field effects in the re-oxidation of cryptochrome in the presence of scavenger radicals. J. Chem. Phys. 2022, 156, 025101. [Google Scholar] [CrossRef]
- Thoradit, T.; Thongyoo, K.; Kamoltheptawin, K.; Tunprasert, L.; El-Esawi, M.A.; Aguida, B.; Jourdan, N.; Buddhachat, K.; Pooam, M. Cryptochrome and quantum biology: Unraveling the mysteries of plant magnetoreception. Front. Plant Sci. 2023, 14, 1266357. [Google Scholar] [CrossRef]
- Richards, O.W.; Davies, R.G. Imms’ General Textbook of Entomology: Structure, Physiology and Development, 10th ed.; Chapman and Hall: London, UK, 1977. [Google Scholar]
- Binhi, V.N. A limit in the dynamic increase in the accuracy of group migration. BioSystems 2018, 166, 19–25. [Google Scholar] [CrossRef]
- Xu, P.; Xiang, Y.; Zhu, H.; Xu, H.; Zhang, Z.; Zhang, C.; Zhang, L.; Ma, Z. Wheat Cryptochromes: Subcellular Localization and Involvement in Photomorphogenesis and Osmotic Stress Responses. Plant Physiol. 2009, 149, 760–774. [Google Scholar] [CrossRef]
- Stenkamp, D.L. Development of the Vertebrate Eye and Retina. Prog. Mol. Biol. Transl. Sci. 2015, 134, 397–414. [Google Scholar]
- Cochran, W.W.; Mouritsen, H.; Wikelski, M. Migrating Songbirds Recalibrate Their Magnetic Compass Daily from Twilight Cues. Science 2004, 304, 405–408. [Google Scholar] [CrossRef]
- Moore, F.R. Integration of environmental stimuli in the migratory orientation of the savannah sparrow (Passerculus sandwichensis). Anim. Behav. 1985, 33, 657–663. [Google Scholar] [CrossRef]
- Güler, A.D.; Ecker, J.L.; Lall, G.S.; Haq, S.; Altimus, C.M.; Liao, H.-W.; Barnard, A.R.; Cahill, H.; Badea, T.C.; Zhao, H.; et al. Melanopsin cells are the principal conduits for rod–cone input to non-image-forming vision. Nature 2008, 453, 102–105. [Google Scholar] [CrossRef]
- Solov’yov, I.A.; Mouritsen, H.; Schulten, K. Acuity of a cryptochrome and vision-based magnetoreception system in birds. Biophys. J. 2010, 99, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Yamamoto, J.; Martin, R.; Iwai, S.; Brettel, K. Discovery and functional analysis of a 4th electron-transferring tryptophan conserved exclusively in animal cryptochromes and (6-4) photolyases. Chem. Commun. 2015, 51, 15502–15505. [Google Scholar] [CrossRef]
- Cailliez, F.; Müller, P.; Firmino, T.; Pernot, P.; de la Lande, A. Energetics of Photoinduced Charge Migration within the Tryptophan Tetrad of an Animal (6–4) Photolyase. J. Am. Chem. Soc. 2016, 138, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Romero, E.; Augulis, R.; Novoderezhkin, V.I.; Ferretti, M.; Thieme, J.; Zigmantas, D.; van Grondelle, R. Quantum coherence in photosynthesis for efficient solar-energy conversion. Nat. Phys. 2014, 10, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V. Do naturally occurring magnetic nanoparticles in the human body mediate increased risk of childhood leukaemia with EMF exposure? Int. J. Radiat. Biol. 2008, 84, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, R. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Kobayashi-Kirschvink, A.; Woodford, B.J. Magnetite biomineralization in the human brain. Proc. Natl. Acad. Sci. USA 1992, 89, 7683–7687. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Lefèvre, C.T.; Schüler, D. Magnetotactic Bacteria. In The Prokaryotes: Prokaryotic Physiology and Biochemistry; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 453–494. [Google Scholar]
- Bazylinski, D.A.; Schübbe, S. Controlled biomineralization by and applications of magnetotactic bacteria. Adv. Appl. Microbiol. 2007, 62, 21–62. [Google Scholar] [PubMed]
- Zeytuni, N.; Ozyamak, E.; Ben-Harush, K.; Davidov, G.; Levin, M.; Gat, Y.; Moyal, T.; Brik, A.; Komeili, A.; Zarivach, R. Self-recognition mechanism of MamA, a magnetosome-associated TPR-containing protein, promotes complex assembly. Proc. Natl. Acad. Sci. USA 2011, 108, E480–E487. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhao, J.; Yao, H.; Chu, S.; Song, Z.; He, Z.; Zhang, W. Magnetotactic bacteria: Characteristics and environmental applications. Front. Environ. Sci. Eng. 2020, 14, 56. [Google Scholar] [CrossRef]
- Riese, C.N.; Wittchen, M.; Jérôme, V.; Freitag, R.; Busche, T.; Kalinowski, J.; Schüler, D. The transcriptomic landscape of Magnetospirillum gryphiswaldense during magnetosome biomineralization. BMC Genom. 2022, 23, 699. [Google Scholar] [CrossRef]
- Grassi-Schultheiss, P.P.; Heller, F.; Dobson, J. Analysis of magnetic material in the human heart, spleen and liver. BioMetals 1997, 10, 351–355. [Google Scholar] [CrossRef]
- Schultheiss-Grassi, P.P.; Dobson, J. Magnetic analysis of human brain tissue. Biometals 1999, 12, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, A.; Davidson, M.; Toastmann, H.; Channell, J.E.T.; Guyodo, Y.; Batich, C.; Dobson, J. Detection, identification and mapping of iron anomalies in brain tissue using X-ray absorption spectroscopy. J. R. Soc. Interface 2005, 2, 33–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stovbun, S.V.; Zlenko, D.V.; Bukhvostov, A.A.; Vedenkin, A.S.; Skoblin, A.A.; Kuznetsov, D.A.; Buchachenko, A.L. Magnetic field and nuclear spin influence on the DNA synthesis rate. Sci. Rep. 2023, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. Stochastic dynamics of magnetic nanoparticles and a mechanism of biological orientation in the geomagnetic field. Physics 2005, 6, 23–27. [Google Scholar] [CrossRef]
- Walker, M.M.; Dennis, T.E.; Kirschvink, J.L. The magnetic sense and its use in long-distance navigation by animals. Curr. Opin. Neurobiol. 2002, 12, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Wiltschko, W.; Munro, U.; Wiltschko, R.; Kirschvink, J.L. Magnetite-based magnetoreception in birds: The effect of a biasing field and a pulse on migratory behavior. J. Exp. Biol. 2002, 205, 3031–3037. [Google Scholar] [CrossRef]
- Davila, A.F.; Fleissner, G.; Winklhofer, M.; Petersen, N. A new model for a magnetoreceptor in homing pigeons based on interacting clusters of superparamagnetic magnetite. Phys. Chem. Earth 2003, 28, 647–652. [Google Scholar] [CrossRef]
- Hanzlik, M.; Heunemann, C.; Holtkamp-Rotzler, E.; Winklhofer, M.; Petersen, N.; Fleissner, G. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. BioMetals 2000, 13, 325–331. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J.; et al. A magnetic protein biocompass. Nat. Mater. 2015, 15, 217–226. [Google Scholar] [CrossRef]
- Mandilaras, K.; Missirlis, F. Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics 2012, 4, 928–936. [Google Scholar] [CrossRef]
- Meister, M. Physical limits to magnetogenetics. eLife 2016, 5, e17210. [Google Scholar] [CrossRef]
- Dunlop, D.J. Magnetite: Behavior near the Single-Domain Threshold. Science 1972, 176, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.L.; Kattnig, D.R. Magnetoreception in cryptochrome enabled by one-dimensional radical motion. AVS Quantum Sci. 2023, 5, 022601. [Google Scholar] [CrossRef]
- McCraty, R.; Zayas, M.A. Cardiac coherence, self-regulation, autonomic stability, and psychosocial well-being. Front. Psychol. 2014, 5, 1090. [Google Scholar] [CrossRef] [PubMed]
- Safronova, V.G.; Vulfius, C.A.; Astashev, M.E.; Tikhonova, I.V.; Serov, D.A.; Jirova, E.A.; Pershina, E.V.; Senko, D.A.; Zhmak, M.N.; Kasheverov, I.E.; et al. α9α10 nicotinic acetylcholine receptors regulate murine bone marrow granulocyte functions. Immunobiology 2021, 226, 152047. [Google Scholar] [CrossRef] [PubMed]
- Siniavin, A.; Streltsova, M.; Kudryavtsev, D.; Shelukhina, I.; Utkin, Y.; Tsetlin, V. Activation of α7 Nicotinic Acetylcholine Receptor Upregulates HLA-DR and Macrophage Receptors: Potential Role in Adaptive Immunity and in Preventing Immunosuppression. Biomolecules 2020, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Barberà, M.; Collantes-Alegre, J.M.; Martínez-Torres, D. Mapping and quantification of cryptochrome expression in the brain of the pea aphid Acyrthosiphon pisum. Insect Mol. Biol. 2021, 31, 159–169. [Google Scholar] [CrossRef]
- Vechtomova, Y.; Telegina, T.; Buglak, A.; Kritsky, M. UV Radiation in DNA Damage and Repair Involving DNA-Photolyases and Cryptochromes. Biomedicines 2021, 9, 1564. [Google Scholar] [CrossRef]
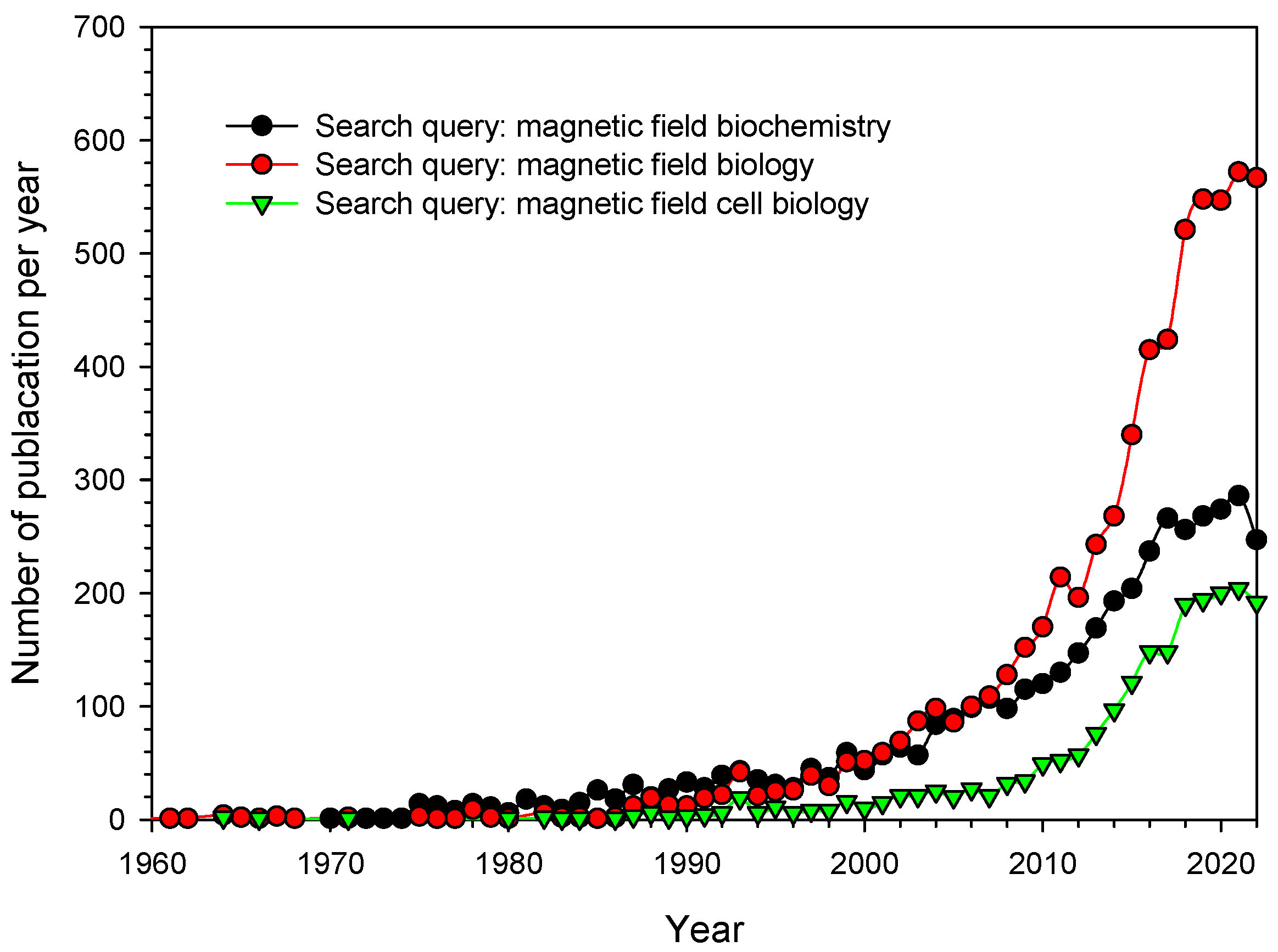
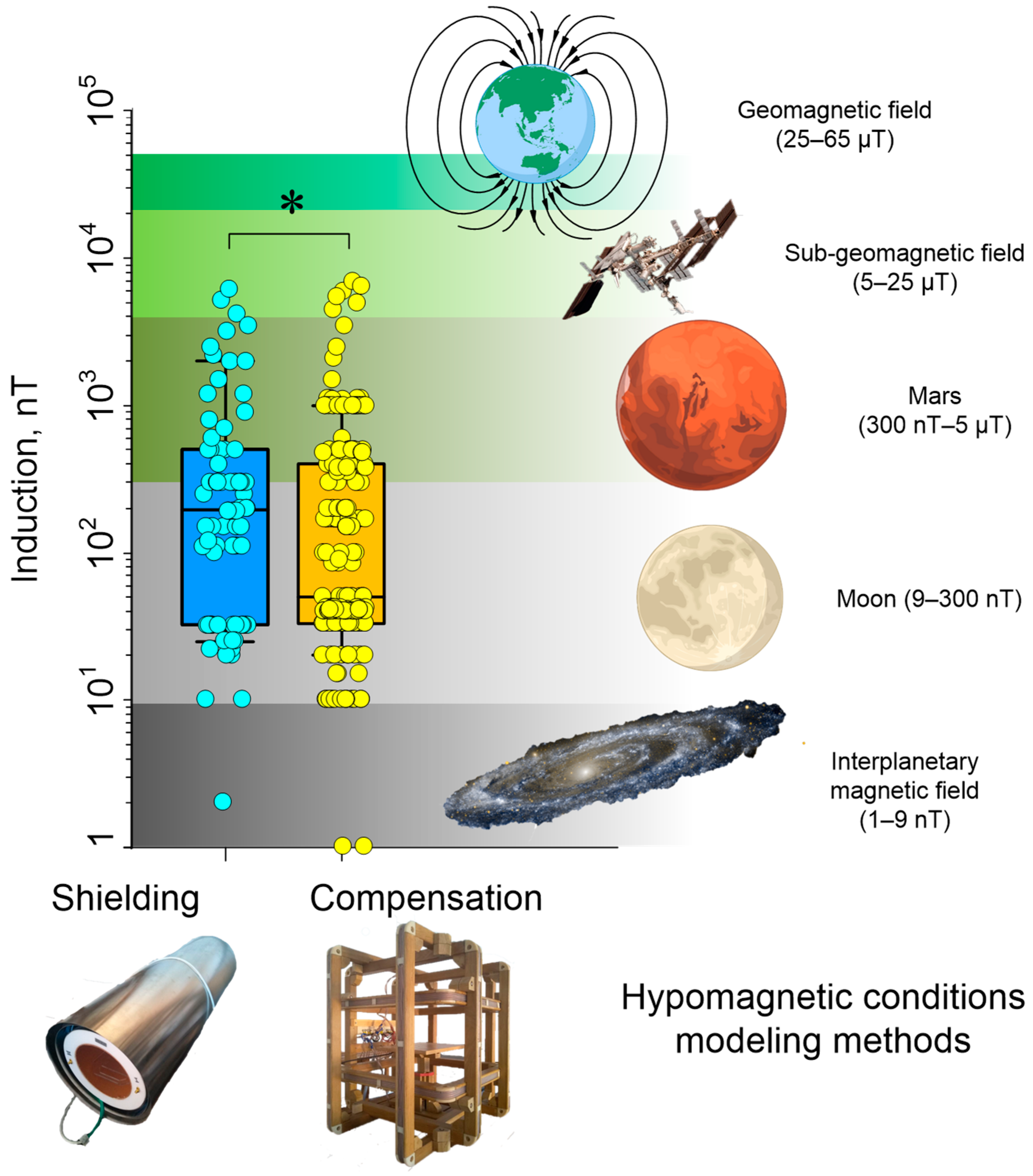
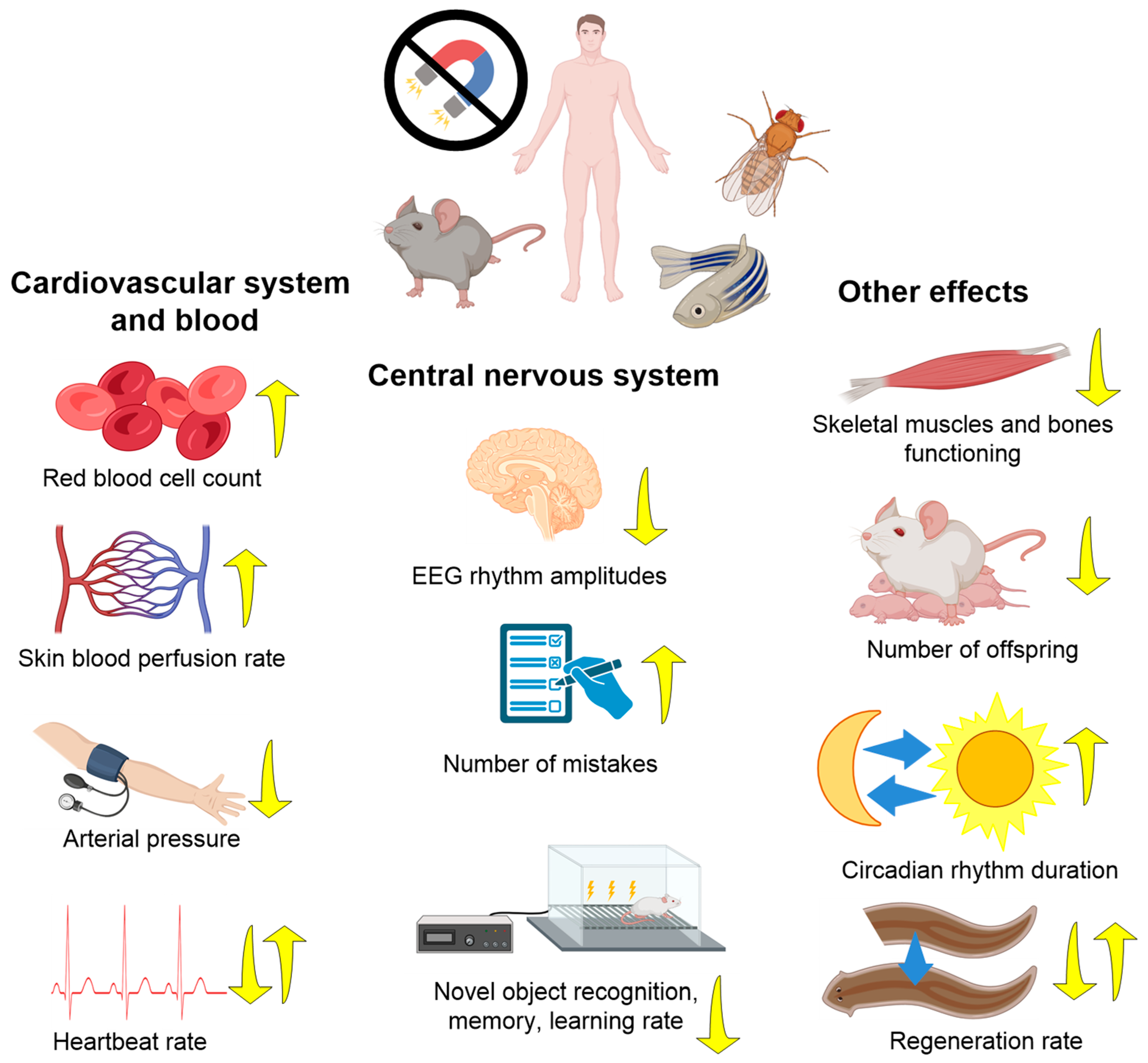
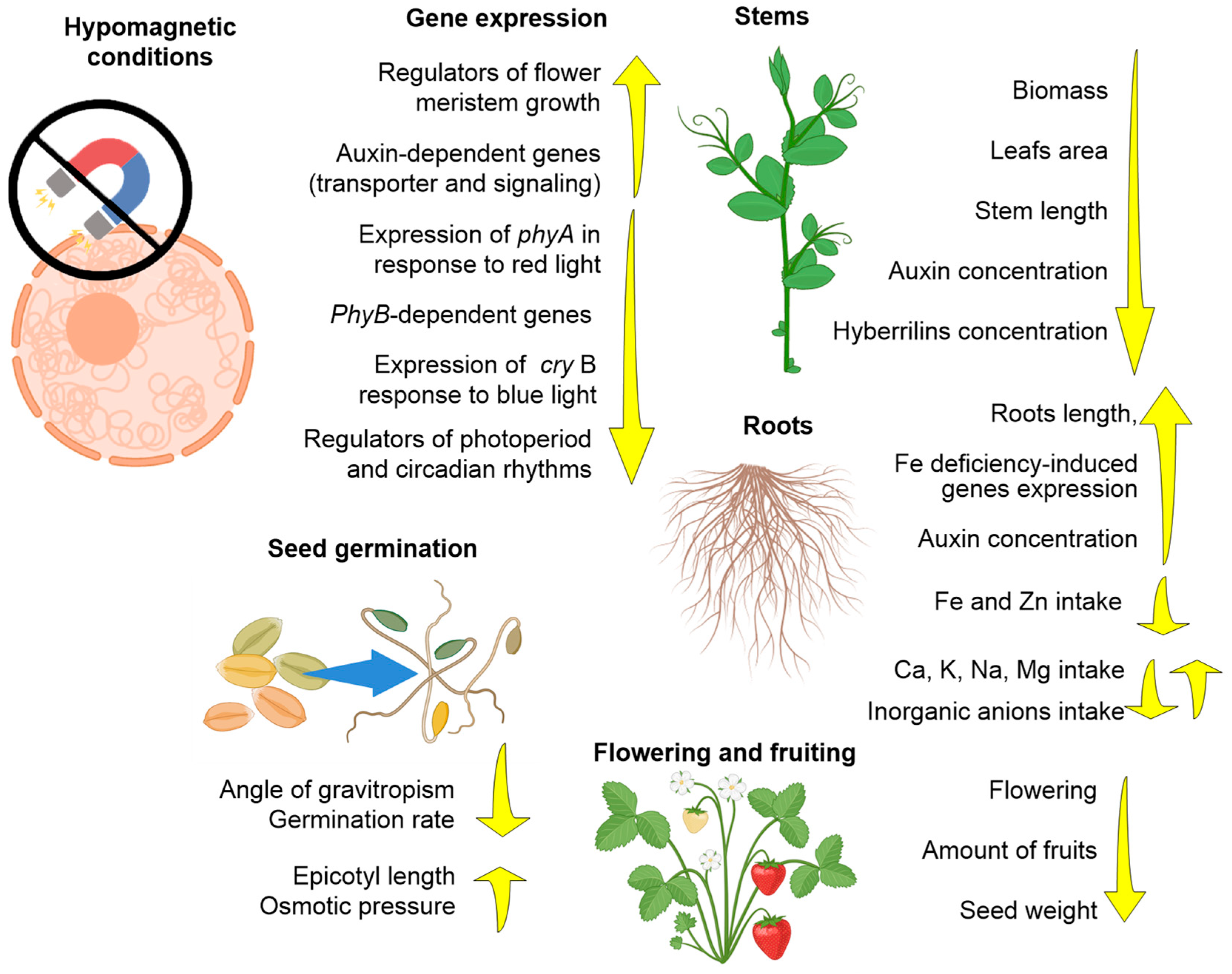
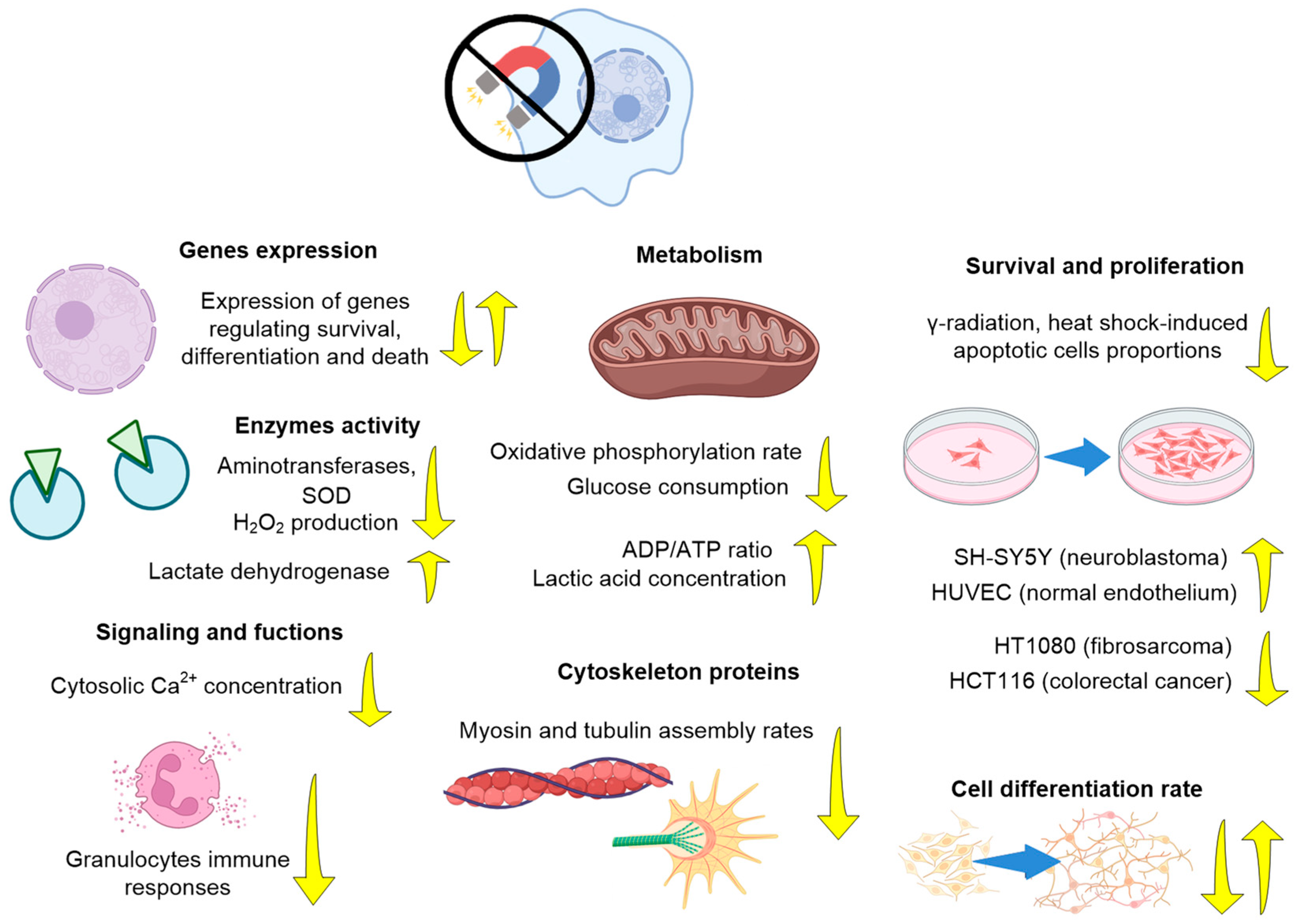
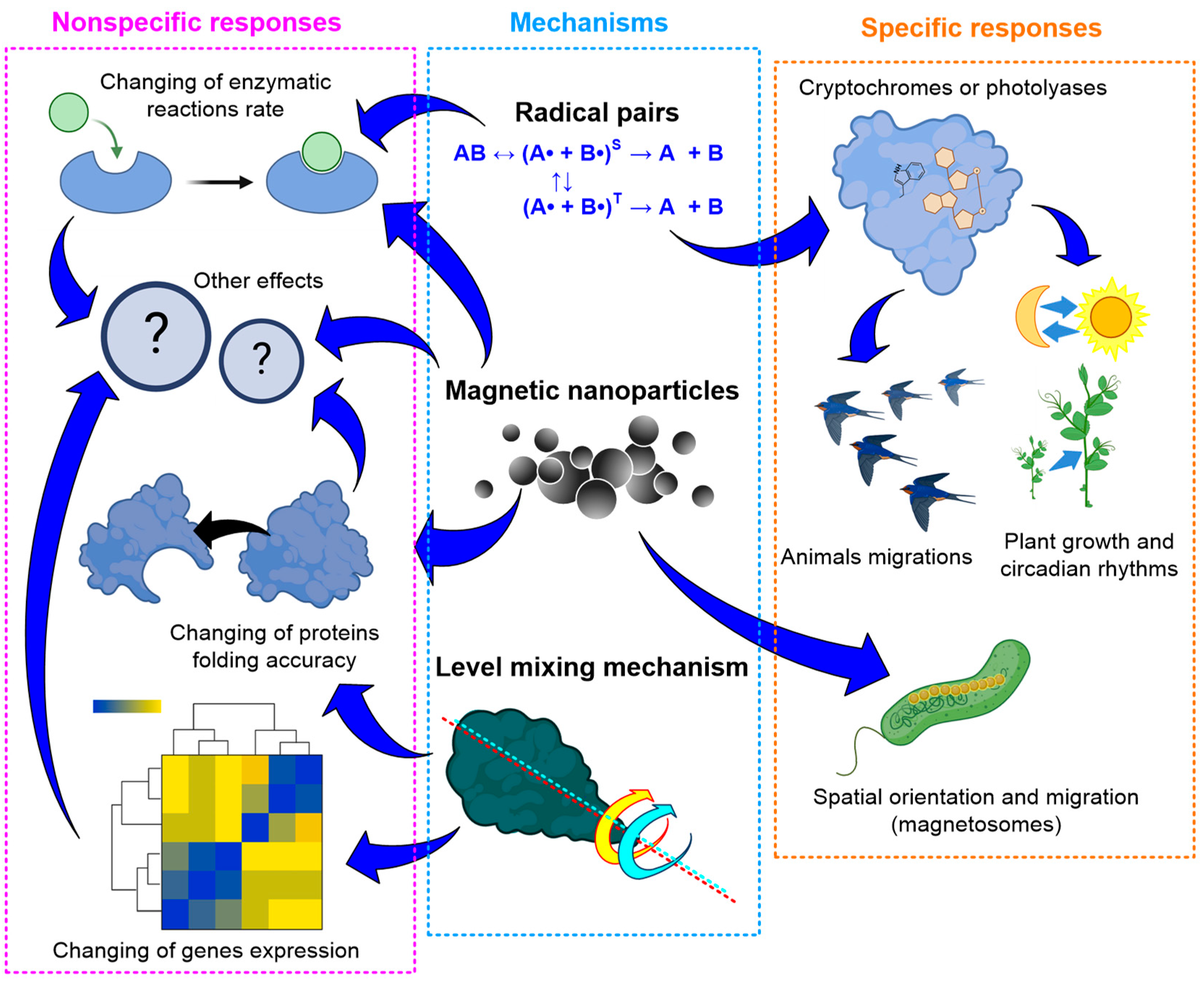
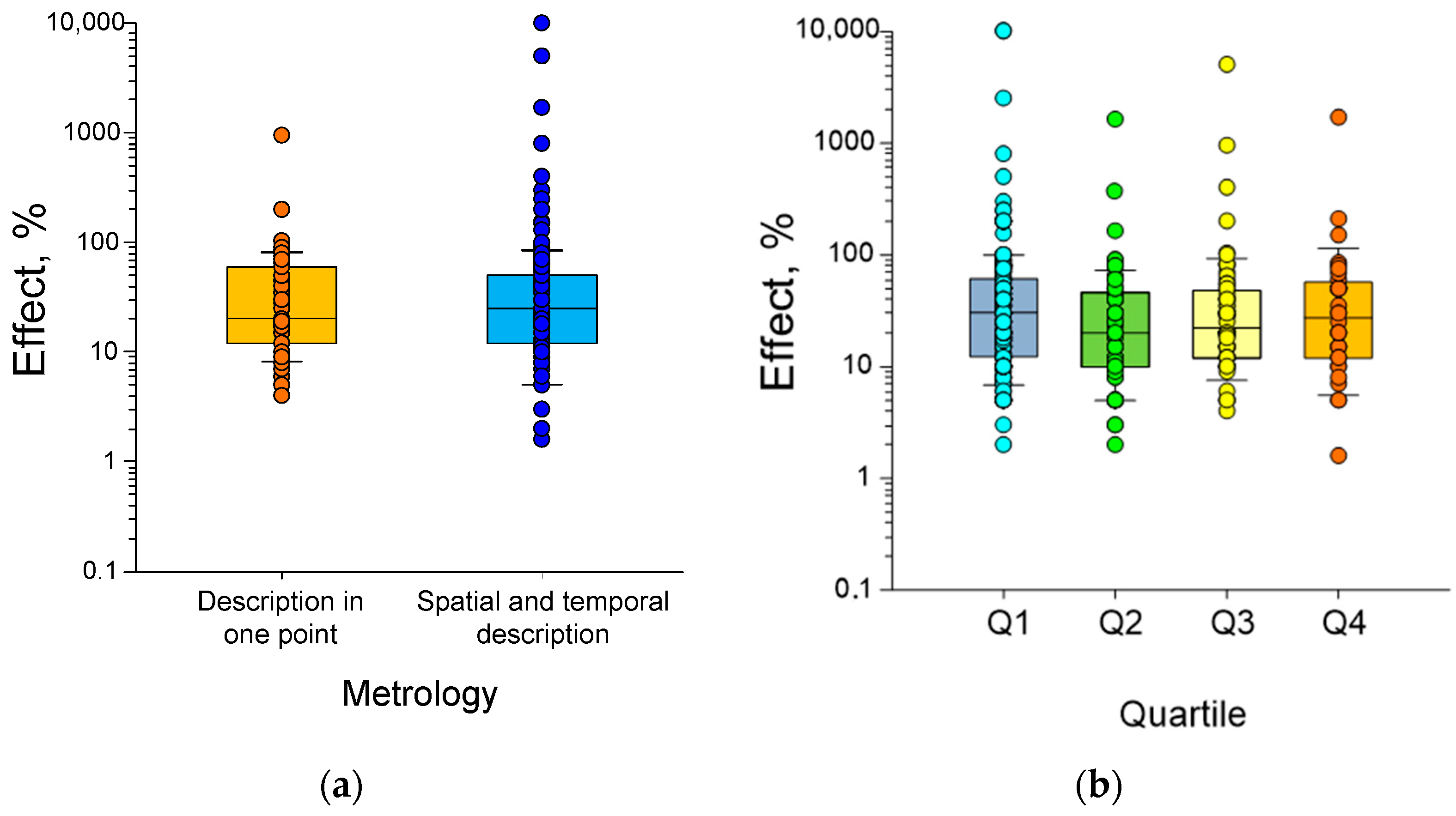
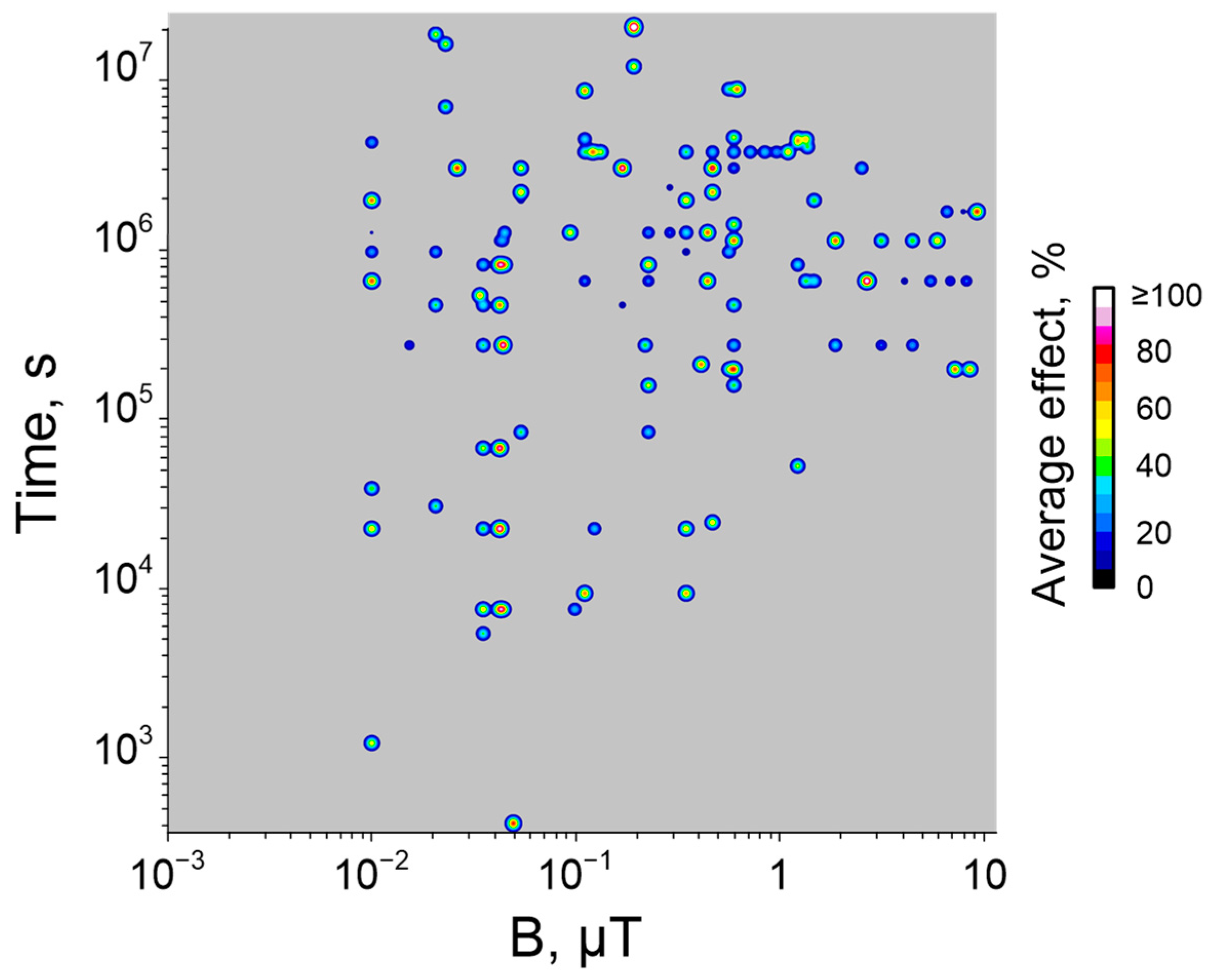

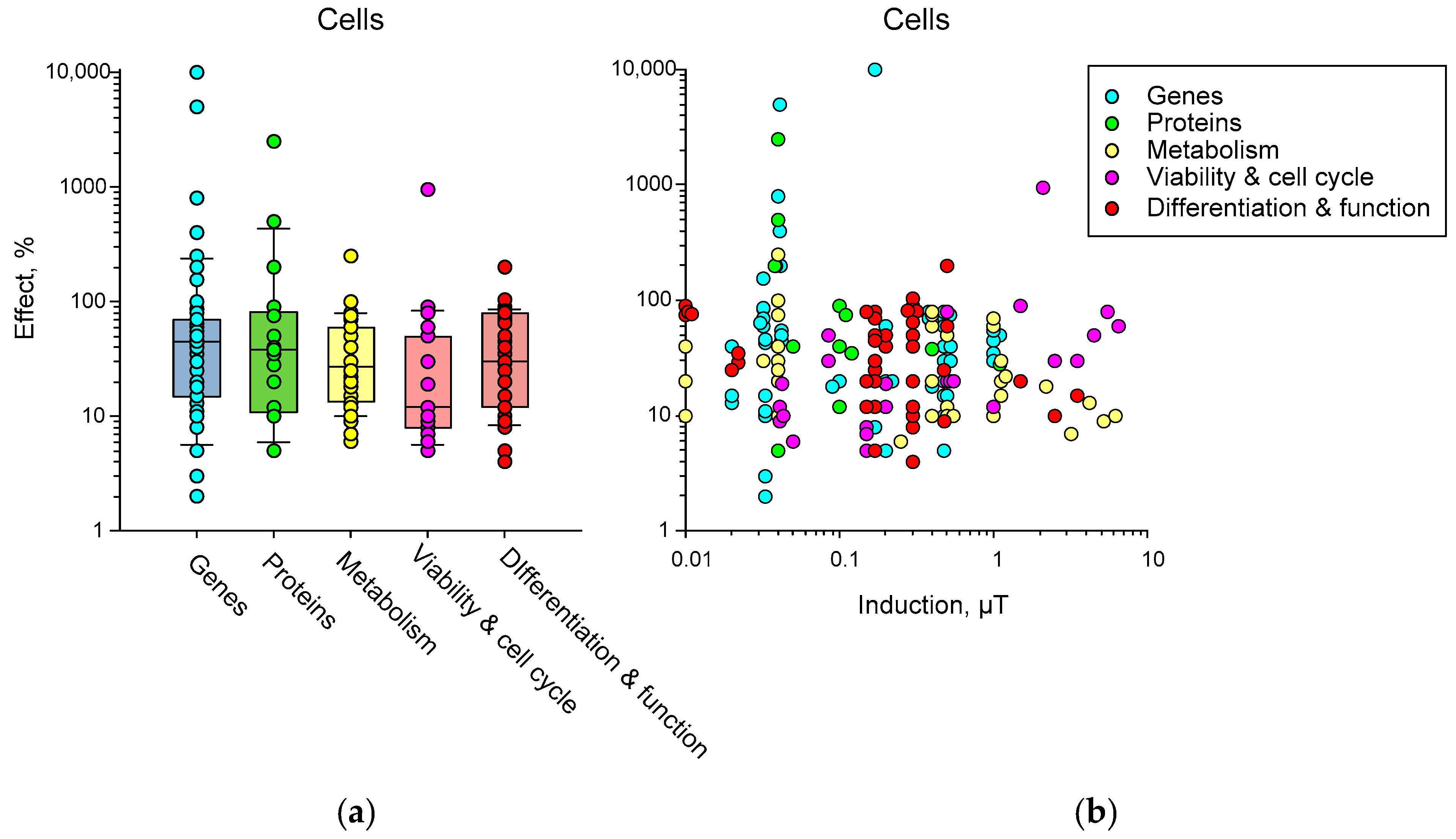
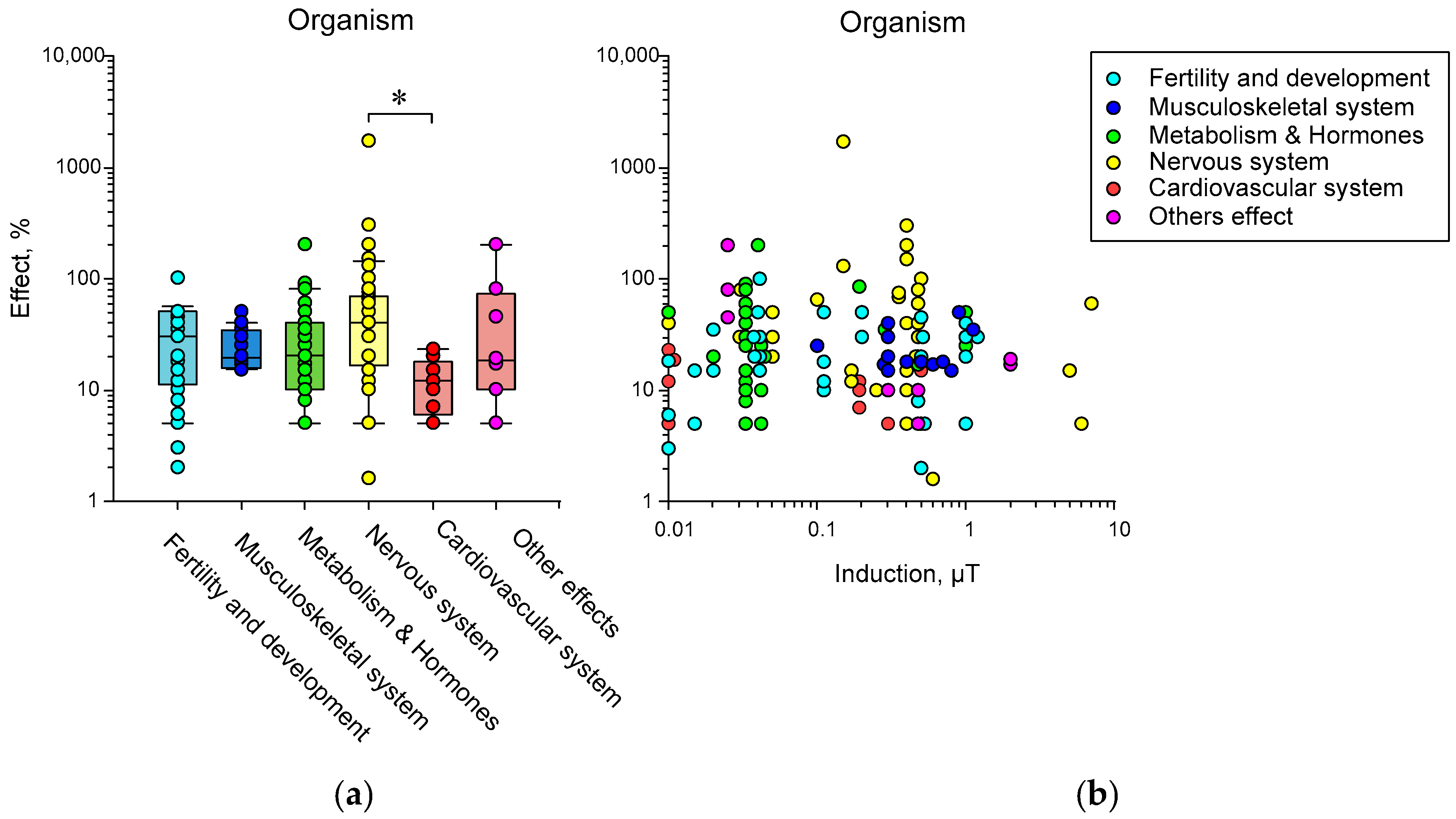
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarimov, R.M.; Serov, D.A.; Gudkov, S.V. Hypomagnetic Conditions and Their Biological Action (Review). Biology 2023, 12, 1513. https://doi.org/10.3390/biology12121513
Sarimov RM, Serov DA, Gudkov SV. Hypomagnetic Conditions and Their Biological Action (Review). Biology. 2023; 12(12):1513. https://doi.org/10.3390/biology12121513
Chicago/Turabian StyleSarimov, Ruslan M., Dmitriy A. Serov, and Sergey V. Gudkov. 2023. "Hypomagnetic Conditions and Their Biological Action (Review)" Biology 12, no. 12: 1513. https://doi.org/10.3390/biology12121513
APA StyleSarimov, R. M., Serov, D. A., & Gudkov, S. V. (2023). Hypomagnetic Conditions and Their Biological Action (Review). Biology, 12(12), 1513. https://doi.org/10.3390/biology12121513







