The Oxygen–Ozone Adjunct Medical Treatment According to the Protocols from the Italian Scientific Society of Oxygen–Ozone Therapy: How Ozone Applications in the Blood Can Influence Clinical Therapy Success via the Modulation of Cell Biology and Immunity
Abstract
:Simple Summary
Abstract
1. Introduction
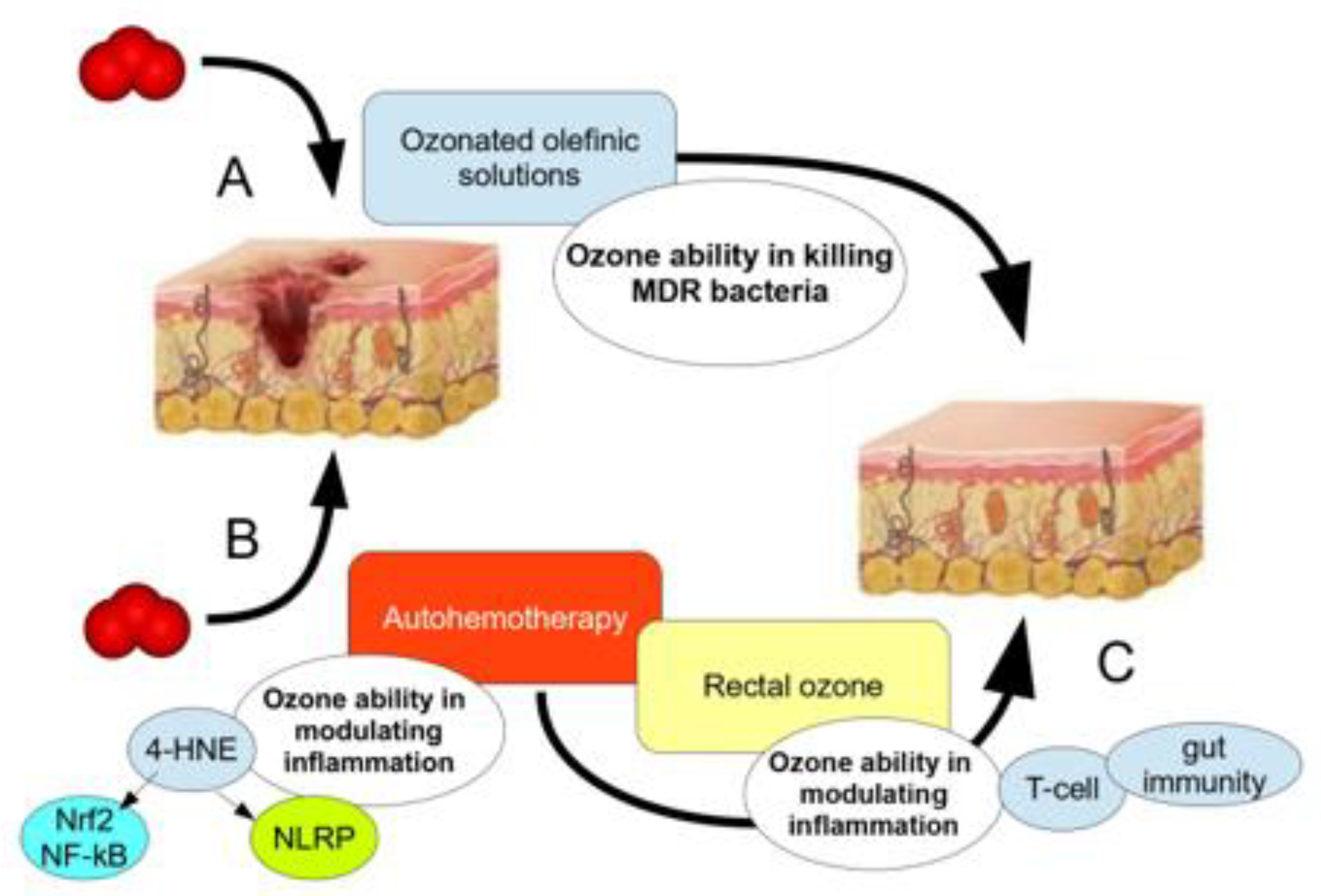
2. Ozone in the Treatment of Infected Wounds and in Antibiotic Resistance
Use of Topical Ozone and O2-O3 Major Autohemotherapy (O2-O3-MAHT)
3. Ozone in the Treatment of Painful Inflammation, Disabilities and Fatigue—The Knee Osteoarthritis Model
Use of Ozonated Blood via O2-O3 Minor Autohemotherapy (O2-O3-mAHT)
4. Materials and Methods
4.1. Patients’ Recruitment in the Ozone-MDR Study
4.2. Protocols of Ozone Therapy
4.3. Evaluating Patients’ Outcomes: Inflammation and Antimicrobial Biomarkers
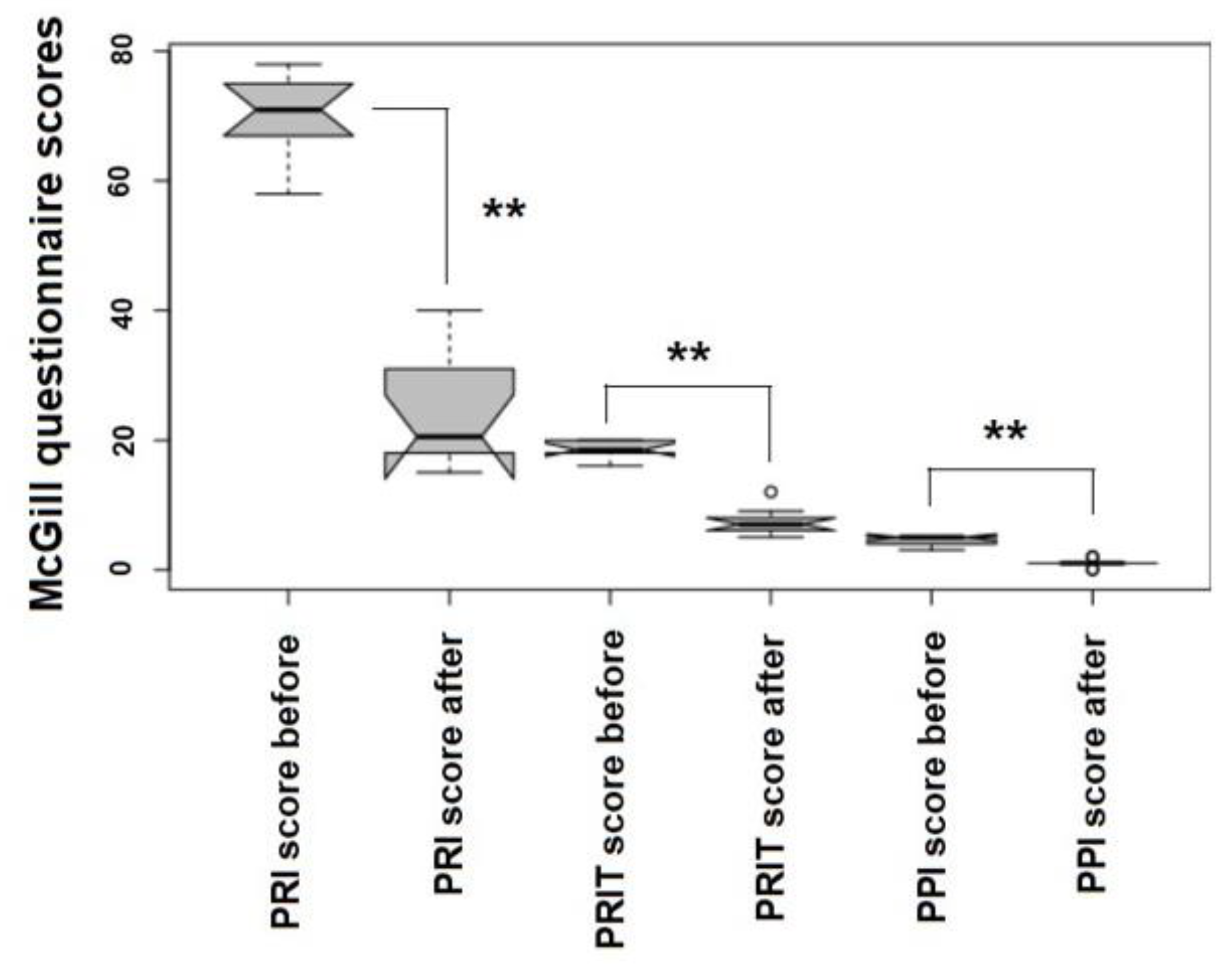
4.4. Evaluating Patients’ Outcomes: The McGill Quality of Life (QoL) Questionnaire
4.5. Statistics
4.6. Patients: Study on Knee OA
4.7. Whole Blood Ozonation in Knee OA
4.8. Patient’s Knee Infiltrations
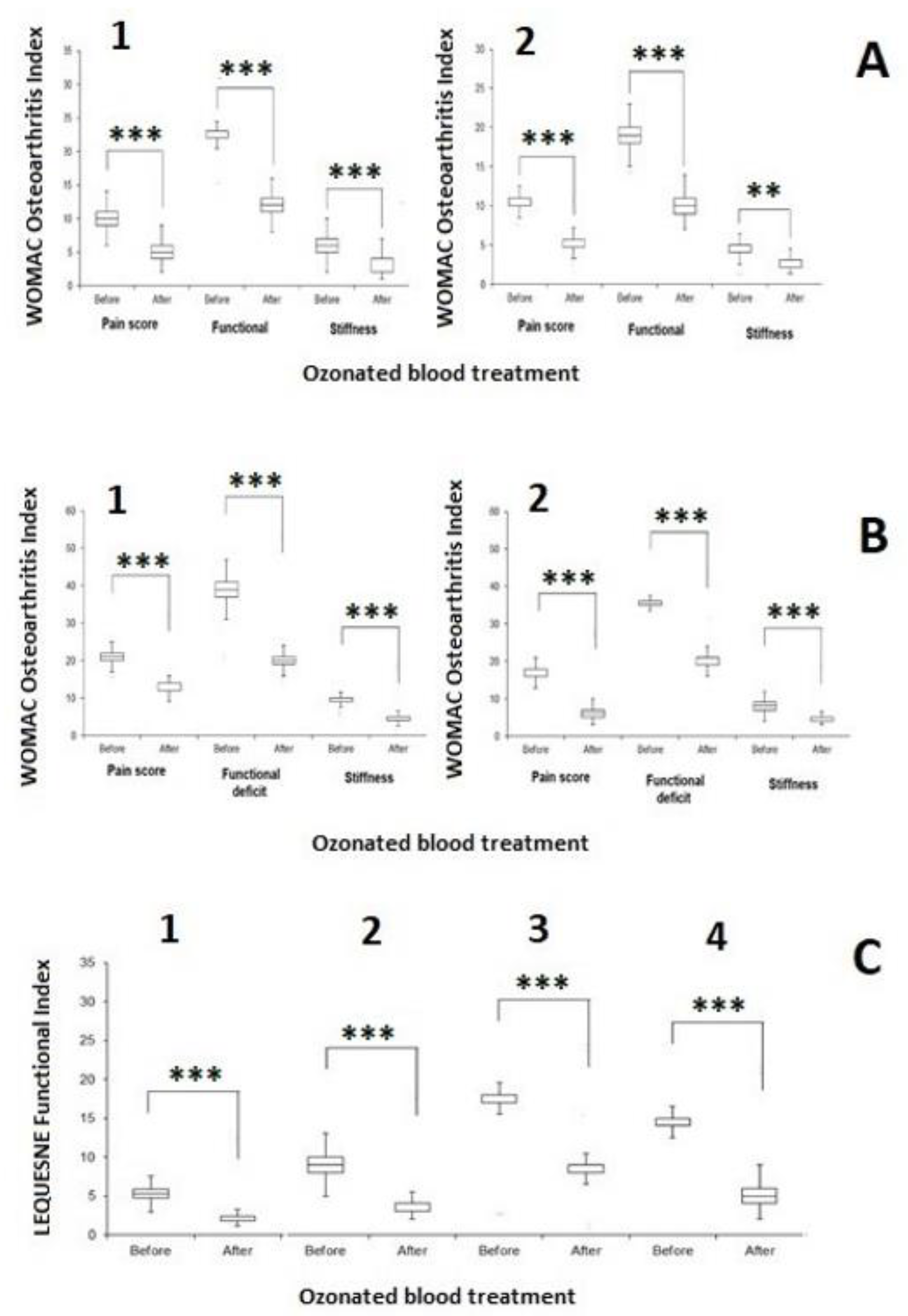
4.9. WOMAC Index and Lequesne Algofunctional Index
4.10. Statistics
5. Results
5.1. Major Ozone Autohemotherapy along with Topical Ozone Reduces Infection and Inflammation in Post-Surgical Wounds
5.2. The Effect of the Ozonated Blood in the Minor Autohemotherapy on Knee OA
6. Discussion
6.1. Ozone in Major Autohemotherapy against MDR Bacteria
6.2. Minor Autohemotherapy with Ozonated Blood Reduces Discomfort and Disability Markers in Knee OA
6.3. The SIOOT Protocol of Ozonated Blood
7. Conclusions
8. Acronyms
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coffaro, B.; Weisel, C.P. Reactions and Products of Squalene and Ozone: A Review. Environ. Sci. Technol. 2022, 56, 7396–7411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Misztal, P.K.; Arata, C.; Weschler, C.J.; Nazaroff, W.W.; Goldstein, A.H. Observing ozone chemistry in an occupied residence. Proc. Natl. Acad. Sci. USA 2021, 118, e2018140118. [Google Scholar] [CrossRef]
- Wentworth, P., Jr.; Nieva, J.; Takeuchi, C.; Galve, R.; Wentworth, A.D.; Dilley, R.B.; DeLaria, G.A.; Saven, A.; Babior, B.M.; Janda, K.D.; et al. Evidence for ozone formation in human atherosclerotic arteries. Science 2003, 302, 1053–1056. [Google Scholar] [CrossRef]
- Lerner, R.A.; Eschenmoser, A. Ozone in biology. Proc. Natl. Acad. Sci. USA 2003, 100, 3013–3015. [Google Scholar] [CrossRef]
- Wentworth, P., Jr.; Wentworth, A.D.; Zhu, X.; Wilson, I.A.; Janda, K.D.; Eschenmoser, A.; Lerner, R.A. Evidence for the production of trioxygen species during antibody-catalyzed chemical modification of antigens. Proc. Natl. Acad. Sci. USA 2003, 100, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, P., Jr.; McDunn, J.E.; Wentworth, A.D.; Takeuchi, C.; Nieva, J.; Jones, T.; Bautista, C.; Ruedi, J.M.; Gutierrez, A.; Janda, K.D.; et al. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science 2002, 298, 2195–2199. [Google Scholar] [CrossRef]
- Babior, B.M.; Takeuchi, C.; Ruedi, J.; Gutierrez, A.; Wentworth, P., Jr. Investigating antibody-catalyzed ozone generation by human neutrophils. Proc. Natl. Acad. Sci. USA 2003, 100, 3031–3034. [Google Scholar] [CrossRef]
- Glaze, W.H. Reaction products of ozone: A review. Environ. Health Perspect. 1986, 69, 151–157. [Google Scholar] [CrossRef]
- Tricarico, G.; Travagli, V. The Relationship between Ozone and Human Blood in the Course of a Well-Controlled, Mild, and Transitory Oxidative Eustress. Antioxidants 2021, 10, 1946. [Google Scholar] [CrossRef]
- Travagli, V.; Iorio, E.L. The Biological and Molecular Action of Ozone and Its Derivatives: State-of-the-Art, Enhanced Scenarios, and Quality Insights. Int. J. Mol. Sci. 2023, 24, 8465. [Google Scholar] [CrossRef] [PubMed]
- Weschler, C.J. Ozone in indoor environments: Concentration and chemistry. Indoor Air 2000, 10, 269–288. [Google Scholar] [CrossRef]
- Hirahara, Y.; Iwata, K.; Nakamuro, K. Effect of Citric Acid on Prolonging the Half-life of Dissolved Ozone in Water. Food Saf. 2019, 7, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.A.; Zanardi, I.; Travagli, V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.M.; Gow, A.J.; Massa, C.B.; Laskin, J.D.; Laskin, D.L. Prolonged injury and altered lung function after ozone inhalation in mice with chronic lung inflammation. Am. J. Respir. Cell Mol. Biol. 2012, 47, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, E.; Kim, W.J. Health Effects of Ozone on Respiratory Diseases. Tuberc. Respir. Dis. 2020, 83 (Suppl. 1), S6–S11. [Google Scholar] [CrossRef]
- Holm, S.M.; Balmes, J.R. Systematic Review of Ozone Effects on Human Lung Function, 2013 Through 2020. Chest 2022, 161, 190–201. [Google Scholar] [CrossRef]
- Hemming, J.M.; Hughes, B.R.; Rennie, A.R.; Tomas, S.; Campbell, R.A.; Hughes, A.V.; Arnold, T.; Botchway, S.W.; Thompson, K.C. Environmental Pollutant Ozone Causes Damage to Lung Surfactant Protein B (SP-B). Biochemistry 2015, 54, 5185–5197. [Google Scholar] [CrossRef]
- Dengiz, E.; Özcan, Ç.; Güven, Y.İ.; Uçar, S.; Ener, B.K.; Sözen, S.; Yağcı, B.; Güzel, İ.A.; Yiğit, B.; Andaç, A.; et al. Ozone gas applied through nebulization as adjuvant treatment for lung respiratory diseases due to COVID-19 infections: A prospective randomized trial. Med. Gas Res. 2022, 12, 55–59. [Google Scholar]
- Travagli, V.; Zanardi, I.; Bernini, P.; Nepi, S.; Tenori, L.; Bocci, V. Effects of ozone blood treatment on the metabolite profile of human blood. Int. J. Toxicol. 2010, 29, 165–174. [Google Scholar] [CrossRef]
- Franzini, M.; Valdenassi, L.; Chirumbolo, S. The immune mito-hormetic landscape in the oxygen-ozone therapy. Towards a novel medical approach. Int. Immunopharmacol. 2023, 121, 110598. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, D.; Wang, X.; Zhang, Y.; Fang, S.; Qiu, X.; Chen, Q. Effects of ozone for treating chronically refractory wounds and ulcers: A protocol for systematic review and meta-analysis of randomized clinical trials. Medicine 2020, 99, e20457. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Franzini, M.; Pandolfi, S.; Valdenassi, L. Ozone therapy in the huge concern of multidrug resistance (MDR) bacteria. The worldwide perspective. Occup. Med. Health Aff. 2023, 11, 478. [Google Scholar]
- Chirumbolo, S.; Valdenassi, L.; Franzini, M. Ozone adjunct treatment in facing multidrug resistant bacteria? Clin. Case Rep. 2023, in press. [Google Scholar] [CrossRef]
- Czekalski, N.; Imminger, S.; Salhi, E.; Veljkovic, M.; Kleffel, K.; Drissner, D.; Hammes, F.; Bürgmann, H.; von Gunten, U. Inactivation of Antibiotic Resistant Bacteria and Resistance Genes by Ozone: From Laboratory Experiments to Full-Scale Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11862–11871. [Google Scholar] [CrossRef]
- Lüddeke, F.; Heß, S.; Gallert, C.; Winter, J.; Güde, H.; Löffler, H. Removal of total and antibiotic resistant bacteria in advanced wastewater treatment by ozonation in combination with different filtering techniques. Water Res. 2015, 69, 243–251. [Google Scholar] [CrossRef]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Potent Activity of a High Concentration of Chemical Ozone against Antibiotic-Resistant Bacteria. Molecules 2022, 27, 3998. [Google Scholar] [CrossRef]
- Roth, A.; Maruthamuthu, M.K.; Nejati, S.; Krishnakumar, A.; Selvamani, V.; Sedaghat, S.; Nguyen, J.; Seleem, M.N.; Rahimi, R. Wearable adjunct ozone and antibiotic therapy system for treatment of Gram-negative dermal bacterial infection. Sci. Rep. 2022, 12, 13927. [Google Scholar] [CrossRef]
- Yousefi, B.; Banihashemian, S.Z.; Feyzabadi, Z.K.; Hasanpour, S.; Kokhaei, P.; Abdolshahi, A.; Emadi, A.; Eslami, M. Potential therapeutic effect of oxygen-ozone in controlling of COVID-19 disease. Med. Gas Res. 2022, 12, 33–40. [Google Scholar] [CrossRef]
- Rowen, R.J. Ozone therapy as a primary and sole treatment for acute bacterial infection: Case report. Med. Gas Res. 2018, 8, 121–124. [Google Scholar] [CrossRef]
- Spadea, L.; Zanotto, E.; Cavallo, R.; Campagna, G.; Giannico, M.I.; Costagliola, C.; ELOOM Study Investigators. Effectiveness of liposomal ozonized oil in reducing ocular microbial flora in patients undergoing cataract surgery. J. Cataract Refract. Surg. 2021, 47, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Rowen, R.J. Ozone therapy in conjunction with oral antibiotics as a successful primary and sole treatment for chronic septic prosthetic joint: Review and case report. Med. Gas Res. 2018, 8, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Chirumbolo, S.; Ricevuti, G. Oxygen-ozone treatment and COVID-19: Antioxidants targeting endothelia lead the scenery. Intern. Emerg. Med. 2022, 17, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Franzini, M.; Valdenassi, L.; Ricevuti, G.; Chirumbolo, S.; Depfenhart, M.; Bertossi, D.; Tirelli, U. Oxygen-ozone (O2-O3) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int. Immunopharmacol. 2020, 88, 106879. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Miyoshi, T.; Arai, T.; Endo, N.; Itoh, H.; Makino, K.; Mizugishi, K.; Uchiyama, T.; Sasada, M. Ozone production by amino acids contributes to killing of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 16912–16917. [Google Scholar] [CrossRef] [PubMed]
- Nyffele PTBoyle, N.A.; Eltepu LWong, C.H.; Eschenmoser, A.; Lerner, R.A.; Wentworth, P., Jr. Dihydrogen Trioxide (HOOOH) Is Generated during the Thermal Reaction between Hydrogen Peroxide and Ozone. Angew. Chem. Int. Ed. 2004, 43, 4656–4659. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, L. Could ozone be an effective disinfection measure against the novel coronavirus (SARS-CoV-2)? J. Prev. Med. Hyg. 2020, 61, E301–E303. [Google Scholar]
- Sharma, M.; Hudson, J.B. Ozone gas is an effective and practical antibacterial agent. Am. J. Infect. Control 2008, 36, 559–563. [Google Scholar] [CrossRef]
- Bocci, V. Ozone as Janus: This controversial gas can be either toxic or medically useful. Mediat. Inflamm. 2004, 13, 3–11. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Wang, K.; Chen, M.; Wang, Y.; Chen, Z.; Zhao, W.; Ou, S. Elucidating the molecular mechanisms of ozone therapy for neuropathic pain management by integrated transcriptomic and metabolomic approach. Front. Genet. 2023, 14, 1231682. [Google Scholar] [CrossRef]
- Tirelli, U.; Franzini, M.; Valdenassi, L.; Pandolfi, S.; Taibi, R.; Chirumbolo, S. Fibromyalgia treated with oxygen-ozone auto-haemotherapy (O2-O3-AHT): A case study on 200 patients with a modified 10-PI-NRS evaluation. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7974–7979. [Google Scholar] [PubMed]
- Tirelli, U.; Franzini, M.; Valdenassi, L.; Pandolfi, S.; Berretta, M.; Ricevuti, G.; Chirumbolo, S. Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Greatly Improved Fatigue Symptoms When Treated with Oxygen-Ozone Autohemotherapy. J. Clin. Med. 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Tirelli, U.; Franzini, M.; Valdenassi, L.; Pisconti, S.; Taibi, R.; Torrisi, C.; Pandolfi, S.; Chirumbolo, S. Fatigue in post-acute sequelae of SARS-CoV2 (PASC) treated with oxygen-ozone autohemotherapy-preliminary results on 100 patients. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5871–5875. [Google Scholar] [PubMed]
- Li, X.R.; Cui, J.J.; Ge, W.P.; Wang, Z.W.; Chu, Y.C.; Zheng, G.R. Ozonated autohemotherapy combined with pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia in older adults: A retrospective study. Med. Gas Res. 2024, 14, 12–18. [Google Scholar] [PubMed]
- Latini, E.; Nusca, S.M.; Curci, E.R.; Boaretto, D.; Santoboni, F.; Trischitta, D.; Vetrano, M.; Vulpiani, M.C. Intramuscular paravertebral oxygen-ozone therapy for chronic neck pain and low back pain: Evaluation of 6-month clinical outcomes. Med. Gas Res. 2024, 14, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.T.; Zong, L.J.; Jia, R.M.; Qin, X.M.; Ruan, S.R.; Lu, L.L.; Wang, P.; Hu, L.; Liu, W.T.; Yang, Y.; et al. Ozone attenuates chemotherapy-induced peripheral neuropathy via upregulating the AMPK-SOCS3 axis. J. Cancer Res. Ther. 2023, 19, 1031–1039. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Van Manen, M.D.; Nace, J.; Mont, M.A. Management of primary knee osteoarthritis and indications for total knee arthroplasty for general practitioners. J. Am. Osteopath. Assoc. 2012, 112, 709–715. [Google Scholar]
- Figueiredo, C.P.; Simon, D.; Englbrecht, M.; Haschka, J.; Kleyer, A.; Bayat, S.; Hueber, A.; Pereira, R.M.; Rech, J.; Schett, G. Quantification and Impact of Secondary Osteoarthritis in Patients With Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 2114–2121. [Google Scholar] [CrossRef]
- Samson, D.J.; Grant, M.D.; Ratko, T.A.; Bonnell, C.J.; Ziegler, K.M.; Aronson, N. Treatment of primary and secondary osteoarthritis of the knee. Evid. Rep. Technol. Assess. (Full Rep.) 2007, 157, 1–157. [Google Scholar]
- Mangone, G.; Orioli, A.; Pinna, A.; Pasquetti, P. Infiltrative treatment with Platelet Rich Plasma (PRP) in gonarthrosis. Clin. Cases Miner. Bone Metab. 2014, 11, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Palco, M.; Fenga, D.; Basile, G.C.; Rizzo, P.; Cavalieri, B.; Leonetti, D.; Alito, A.; Bruschetta, A.; Traina, F. Platelet-Rich Plasma Combined with Hyaluronic Acid versus Leucocyte and Platelet-Rich Plasma in the Conservative Treatment of Knee Osteoarthritis. A Retrospective Study. Medicina 2021, 57, 232. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Z.; Nie, M.J.; Zhao, J.Z.; Zhang, G.C.; Zhang, Q.; Wang, B. Platelet-rich plasma versus hyaluronic acid in the treatment of knee osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Dallari, D.; Stagni, C.; Rani, N.; Sabbioni, G.; Pelotti, P.; Torricelli, P.; Tschon, M.; Giavaresi, G. Ultrasound-Guided Injection of Platelet-Rich Plasma and Hyaluronic Acid, Separately and in Combination, for Hip Osteoarthritis: A Randomized Controlled Study. Am. J. Sports Med. 2016, 44, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, Y.B.; Ha, C.W.; Roh, Y.J.; Park, J.G. Adverse Reactions and Clinical Outcomes for Leukocyte-Poor Versus Leukocyte-Rich Platelet-Rich Plasma in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2021, 9, 23259671211011948. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, A.; Maleki, A.; Dolati, S.; Aghebati-Maleki, A.; Aghebati-Maleki, L. Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed. Pharmacother. 2018, 104, 652–660. [Google Scholar] [CrossRef]
- Civinini, R.; Nistri, L.; Martini, C.; Redl, B.; Ristori, G.; Innocenti, M. Growth factors in the treatment of early osteoarthritis. Clin. Cases Miner. Bone Metab. 2013, 10, 26–29. [Google Scholar] [CrossRef]
- Napolitano, M.; Matera, S.; Bossio, M.; Crescibene, A.; Costabile, E.; Almolla, J.; Almolla, H.; Togo, F.; Giannuzzi, C.; Guido, G. Autologous platelet gel for tissue regeneration in degenerative disorders of the knee. Blood Transfus. 2012, 10, 72–77. [Google Scholar]
- Chen, Y.R.; Yan, X.; Yuan, F.Z.; Ye, J.; Xu, B.B.; Zhou, Z.X.; Mao, Z.M.; Guan, J.; Song, Y.F.; Sun, Z.W.; et al. The Use of Peripheral Blood-Derived Stem Cells for Cartilage Repair and Regeneration In Vivo: A Review. Front. Pharmacol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Papadopoulos, K.I.; Turajane, T. Erratum: Commentary: Autologous Peripheral Blood Stem Cells (PBSC) are Safe and Effective in Knee Osteoarthritis. Front. Pharmacol. 2021, 12, 652738. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Yin, M.H.; Jiang, D.; Zhang, Z.Z.; Qi, Y.S.; Wang, H.J.; Yu, J.K. The Chondrogenic Potential of Progenitor Cells Derived from Peripheral Blood: A Systematic Review. Stem Cells Dev. 2016, 25, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, H.S.; Yurtbay, A.; Say, F. Platelet Rich Plasma Versus Autologous Conditioned Serum in Osteoarthritis of the Knee: Clinical Results of a Five-Year Retrospective Study. Cureus 2022, 14, e24500. [Google Scholar] [CrossRef] [PubMed]
- Manoto, S.L.; Maepa, M.J.; Motaung, S.K. Medical ozone therapy as a potential treatment modality for regeneration of damaged articular cartilage in osteoarthritis. Saudi J. Biol. Sci. 2018, 25, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Valacchi, G.; Corradeschi, F.; Fanetti, G. Studies on the biological effects of ozone: 8. Effects on the total antioxidant status and on interleukin-8 production. Mediat. Inflamm. 1998, 7, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Zanardi, I.; Bocci, V. A realistic evaluation of the action of ozone on whole human blood. Int. J. Biol. Macromol. 2006, 39, 317–320. [Google Scholar] [CrossRef]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef]
- Tricarico, G.; Isakovic, J.; Song, M.S.; Rustichelli, F.; Travagli, V.; Mitrecic, D. Ozone influences migration and proliferation of neural stem cells in vitro. Neurosci. Lett. 2020, 739, 135390. [Google Scholar] [CrossRef]
- Costanzo, M.; Boschi, F.; Carton, F.; Conti, G.; Covi, V.; Tabaracci, G.; Sbarbati, A.; Malatesta, M. Low ozone concentrations promote adipogenesis in human adipose-derived adult stem cells. Eur. J. Histochem. 2018, 62, 2969. [Google Scholar] [CrossRef]
- Niu, M.; Chen, P. Crosstalk between gut microbiota and sepsis. Burns Trauma. 2021, 9, tkab036. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Luis, J.; Menéndez-Cepero, S.; Macías-Abraham, C.; Fariñas-Rodríguez, L. Systemic Ozone Therapy by Rectal Insufflation for Immunoglobulin A Deficiency. MEDICC Rev. 2018, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.R.; Russell, L.B.; Leis, A.; Shahidi, J.; Porterfield, P.; Kuhl, D.R.; Gadermann, A.M.; Sawatzky, R. More comprehensively measuring quality of life in life-threatening illness: The McGill Quality of Life Questionnaire—Expanded. BMC Palliat. Care 2019, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Serrano, P.V.; Serrano, G.B.; Torres, I.L.S.; Graudner, R.R.; Caumo, W. The McGill Quality of Life Questionnaire-Revised (MQOL-R). Psychometric properties and validation of a Brazilian version on palliative care patients: A cross-sectional study. Health Qual. Life Outcomes 2020, 18, 368. [Google Scholar] [CrossRef] [PubMed]
- Szende, A.; Leidy, N.K.; Revicki, D. Health-related quality of life and other patient-reported outcomes in the European centralized drug regulatory process: A review of guidance documents and performed authorizations of medicinal products 1995 to 2003. Value Health 2005, 8, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Weidow, J.; Cederlund, C.G.; Ranstam, J.; Kärrholm, J. Ahlbäck grading of osteoarthritis of the knee: Poor reproducibility and validity based on visual inspection of the joint. Acta Orthop. 2006, 77, 262–266. [Google Scholar] [CrossRef]
- Lequesne, M.G. The algofunctional indices for hip and knee osteoarthritis. J. Rheumatol. 1997, 24, 779–781. [Google Scholar]
- Egbewale, B.E.; Lewis, M.; Sim, J. Bias, precision and statistical power of analysis of covariance in the analysis of randomized trials with baseline imbalance: A simulation study. BMC Med. Res. Methodol. 2014, 14, 49. [Google Scholar] [CrossRef]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms 2021, 10, 40. [Google Scholar] [CrossRef]
- Pietrocola, G.; Ceci, M.; Preda, F.; Poggio, C.; Colombo, M. Evaluation of the antibacterial activity of a new ozonized olive oil against oral and periodontal pathogens. J. Clin. Exp. Dent. 2018, 10, e1103–e1108. [Google Scholar] [CrossRef]
- Higa, B.; Cintra, B.S.; Álvarez, C.M.; Ribeiro, A.B.; Ferreira, J.C.; Tavares, D.C.; Enriquez, V.; Martinez, L.R.; Pires, R.H. Ozonated oil is effective at killing Candida species and Streptococcus mutans biofilm-derived cells under aerobic and microaerobic conditions. Med. Mycol. 2022, 60, myac055. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef]
- Cenci, A.; Macchia, I.; La Sorsa, V.; Sbarigia, C.; Di Donna, V.; Pietraforte, D. Mechanisms of Action of Ozone Therapy in Emerging Viral Diseases: Immunomodulatory Effects and Therapeutic Advantages with Reference to SARS-CoV-2. Front. Microbiol. 2022, 13, 871645. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; Valdenassi, L.; Simonetti, V.; Bertossi, D.; Ricevuti, G.; Franzini, M.; Pandolfi, S. Insights on the mechanisms of action of ozone in the medical therapy against COVID-19. Int. Immunopharmacol. 2021, 96, 107777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Vicente, A.M.; Araico, A.; Dominguez, J.N.; Terencio, M.C.; Ferrándiz, M.L. Role of nuclear factor-kappaB and heme oxygenase-1 in the mechanism of action of an anti-inflammatory chalcone derivative in RAW 264.7 cells. Br. J. Pharmacol. 2004, 142, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Gao, Y.; Shou, S.; Chai, Y. The roles of macrophage polarization in the host immune response to sepsis. Int. Immunopharmacol. 2021, 96, 107791. [Google Scholar] [CrossRef]
- Wegiel, B.; Larsen, R.; Gallo, D.; Chin, B.Y.; Harris, C.; Mannam, P.; Kaczmarek, E.; Lee, P.J.; Zuckerbraun, B.S.; Flavell, R.; et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J. Clin. Investig. 2014, 124, 4926–4940. [Google Scholar] [CrossRef]
- Kang, I.S.; Kim, R.I.; Kim, C. Carbon Monoxide Regulates Macrophage Differentiation and Polarization toward the M2 Phenotype through Upregulation of Heme Oxygenase 1. Cells 2021, 10, 3444. [Google Scholar] [CrossRef]
- Harvey, C.J.; Thimmulappa, R.K.; Sethi, S.; Kong, X.; Yarmus, L.; Brown, R.H.; Feller-Kopman, D.; Wise, R.; Biswal, S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Transl. Med. 2011, 3, 78ra32. [Google Scholar] [CrossRef]
- Elomaa, O.; Sankala, M.; Pikkarainen, T.; Bergmann, U.; Tuuttila, A.; Raatikainen-Ahokas, A.; Sariola, H.; Tryggvason, K. Structure of the human macrophage MARCO receptor and characterization of its bacteria-binding region. J. Biol. Chem. 1998, 273, 4530–4538. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.G.; Chávez, C.L.; Zhang, C.; Sowden, M.; Yan, C.; Berk, B.C. The lipid peroxidation product 4-hydroxynonenal inhibits NLRP3 inflammasome activation and macrophage pyroptosis. Cell Death Differ. 2022, 29, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zeng, Q.; Xiang, Y.; Gao, L.; Huang, J.; Huang, J.; Wu, K.; Lu, J. The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol. Med. Rep. 2018, 17, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Peirone, C.; Amaral, J.S.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Martins, Â.; Carvalho, Á.; Maltez, L.; Pereira, J.E.; et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules 2020, 25, 3601. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Peirone, C.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Pires, I.; Maltez, L.; Pereira, J.E.; Igrejas, G.; Poeta, P. Topical Application of Ozonated Oils for the Treatment of MRSA Skin Infection in an Animal Model of Infected Ulcer. Biology 2021, 10, 372. [Google Scholar] [CrossRef]
- Grandi, G.; Cavallo, R.; Zanotto, E.; Cipriani, R.; Panico, C.; Protti, R.; Scapagnini, G.; Davinelli, S.; Costagliola, C. In vitro antimicrobial activity of ozonated oil in liposome eyedrop against multidrug-resistant bacteria. Open Med. 2022, 17, 1057–1063. [Google Scholar] [CrossRef]
- Gulmen, S.; Kurtoglu, T.; Meteoglu, I.; Kaya, S.; Okutan, H. Ozone therapy as an adjunct to vancomycin enhances bacterial elimination in methicillin resistant Staphylococcus aureus mediastinitis. J. Surg. Res. 2013, 185, 64–69. [Google Scholar] [CrossRef]
- Franzini, M.; Valdenassi, L.; Pandolfi, S.; Tirelli, U.; Ricevuti, G.; Simonetti, V.; Berretta, M.; Vaiano, F.; Chirumbolo, S. The biological activity of medical zone in the hormetic range and the role of full expertise professionals. Front. Public Health 2022, in press. [Google Scholar] [CrossRef]
- Hamilton, R.F., Jr.; Hazbun, M.E.; Jumper, C.A.; Eschenbacher, W.L.; Holian, A. 4-Hydroxynonenal mimics ozone-induced modulation of macrophage function ex vivo. Am. J. Respir. Cell Mol. Biol. 1996, 15, 275–282. [Google Scholar] [CrossRef]
- Timblin, G.A.; Tharp, K.M.; Ford, B.; Winchester, J.M.; Wang, J.; Zhu, S.; Khan, R.I.; Louie, S.K.; Iavarone, A.T.; Ten Hoeve, J.; et al. Mitohormesis reprogrammes macrophage metabolism to enforce tolerance. Nat. Metab. 2021, 3, 618–635. [Google Scholar] [CrossRef]
- Spassim, M.R.; Dos Santos, R.T.; Rossato-Grando, L.G.; Cardoso, L.; da Silva, J.S.; de Souza, S.O.; Wibelinger, L.M.; Bertol, C.D. Intra-articular ozone slows down the process of degeneration of articular cartilage in the knees of rats with osteoarthritis. Knee 2022, 35, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Jesus, C.C.; Dos Santos, F.C.; de Jesus, L.M.O.B.; Monteiro, I.; Sant’Ana, M.S.S.C.; Trevisani, V.F.M. Comparison between intra-articular ozone and placebo in the treatment of knee osteoarthritis: A randomized, double-blinded, placebo-controlled study. PLoS ONE 2017, 12, e0179185. [Google Scholar] [CrossRef]
- Giombini, A.; Menotti, F.; Di Cesare, A.; Giovannangeli, F.; Rizzo, M.; Moffa, S.; Martinelli, F. Comparison between intrarticular injection of hyaluronic acid, oxygen ozone, and the combination of both in the treatment of knee osteoarthrosis. J. Biol. Regul. Homeost. Agents 2016, 30, 621–625. [Google Scholar] [PubMed]
- Qing, L.; Lei, P.; Liu, H.; Xie, J.; Wang, L.; Wen, T.; Hu, Y. Expression of hypoxia-inducible factor-1α in synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis. Exp. Ther. Med. 2017, 13, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Seyam, O.; Smith, N.L.; Reid, I.; Gandhi, J.; Jiang, W.; Khan, S.A. Clinical utility of ozone therapy for musculoskeletal disorders. Med. Gas Res. 2018, 8, 103–110. [Google Scholar] [PubMed]
- Oliveira, M.F.; Geihs, M.A.; França, T.F.A.; Moreira, D.C.; Hermes-Lima, M. Is “Preparation for Oxidative Stress” a Case of Physiological Conditioning Hormesis? Front. Physiol. 2018, 9, 945. [Google Scholar] [CrossRef]
- Onyango, A.N. Endogenous Generation of Singlet Oxygen and Ozone in Human and Animal Tissues: Mechanisms, Biological Significance, and Influence of Dietary Components. Oxid. Med. Cell Longev. 2016, 2016, 2398573. [Google Scholar] [CrossRef]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef]
- Serra, M.E.G.; Baeza-Noci, J.; Mendes Abdala, C.V.; Luvisotto, M.M.; Bertol, C.D.; Anzolin, A.P. The role of ozone treatment as integrative medicine. An evidence and gap map. Front. Public Health. 2023, 10, 1112296. [Google Scholar] [CrossRef]
- Costa, T.; Linhares, D.; Ribeiro da Silva, M.; Neves, N. Ozone therapy for low back pain. A systematic review. Acta Reumatol. Port. 2018, 43, 172–181. [Google Scholar]
- Barbosa, L.T.; Rodrigues, C.F.S.; Andrade, R.R.; Barbosa, F.T. The effectiveness of percutaneous injections of ozonotherapy in low back pain. Rev. Assoc. Med. Bras. 2020, 66, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Paoloni, M.; Di Sante, L.; Cacchio, A.; Apuzzo, D.; Marotta, S.; Razzano, M.; Franzini, M.; Santilli, V. Intramuscular oxygen-ozone therapy in the treatment of acute back pain with lumbar disc herniation: A multicenter, randomized, double-blind, clinical trial of active and simulated lumbar paravertebral injection. Spine 2009, 34, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, F.N.; Dotta, L.; Sasse, A.; Teixera, M.J.; Fonoff, E.T. Ozone therapy as a treatment for low back pain secondary to herniated disc: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2012, 15, E115–E129. [Google Scholar] [PubMed]
- Chirumbolo, S.; Simonetti, V.; Valdenassi, L.; Pandolfi, S.; Vaiano, F.; Franzini, M. Editorial—A practical assessment to prevent serious complications in the use of a gaseous mixture of oxygen-ozone injected by needle-mediated infiltration. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2224–2226. [Google Scholar] [PubMed]
- Rossino, M.G.; Lulli, M.; Amato, R.; Cammalleri, M.; Monte, M.D.; Casini, G. Oxidative Stress Induces a VEGF Autocrine Loop in the Retina: Relevance for Diabetic Retinopathy. Cells 2020, 9, 1452. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, H.; Wang, H.; Li, X.; Bu, X.; Wang, Q.; Gao, Y.; Wen, G.; Zhou, Y.; Cong, Z.; et al. Interplay between VEGF and Nrf2 regulates angiogenesis due to intracranial venous hypertension. Sci. Rep. 2016, 6, 37338. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Che, Y.; Gao, C.; Zhu, L.; Gao, J.; Vo, N.V. Immune exposure: How macrophages interact with the nucleus pulposus. Front. Immunol. 2023, 14, 1155746. [Google Scholar] [CrossRef]
- Xiao, W.; Tang, H.; Wu, M.; Liao, Y.; Li, K.; Li, L.; Xu, X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2017, 37, BSR20170658. [Google Scholar] [CrossRef]
- Makita, Y.; Imamura, Y.; Masumo, K.; Tamura, I.; Fujiwara, S.; Shiota, G.; Shiba, A.; Wang, P. the effect of ozone on collagen type-I and inflammatory cytokine production in human gingival fibroblasts. Dentistry 2015, 5, 10. [Google Scholar]
- Franzini, M.; Valdenassi, L.; Pandolfi, S.; Ricevuti, G.; Tirelli, U.; Vaiano, F.; Chirumbolo, S. Comments on the optimal use of medical ozone in clinics versus the Ozone High Dose Therapy (OHT) approach. Transl. Med. Commun. 2022, 7, 26. [Google Scholar] [CrossRef]
- Masan, J.; Sramka, M.; Rabarova, D. The possibilities of using the effects of ozone therapy in neurology. Neuro Endocrinol. Lett. 2021, 42, 13–21. [Google Scholar] [PubMed]
- Molinari, F.; Rimini, D.; Liboni, W.; Acharya, U.R.; Franzini, M.; Pandolfi, S.; Ricevuti, G.; Vaiano, F.; Valdenassi, L.; Simonetti, V. Cerebrovascular pattern improved by ozone autohemotherapy: An entropy-based study on multiple sclerosis patients. Med. Biol. Eng. Comput. 2017, 55, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Valdenassi, L.; Rossi, E.; Corsetti, M.T.; Sarli, R.; Bellardi, D.; Chirumbolo, S.; Simonetti, V.; Pandolfi, S.; Franzini, M. Sjögren syndrome successfully treated with oxygen-ozone auto-hemotherapy (O2-O3-AHT). A case report. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5911–5917. [Google Scholar] [PubMed]
- Rai, P.; Janardhan, K.S.; Meacham, J.; Madenspacher, J.H.; Lin, W.C.; Karmaus, P.W.F.; Martinez, J.; Li, Q.Z.; Yan, M.; Zeng, J.; et al. IRGM1 links mitochondrial quality control to autoimmunity. Nat. Immunol. 2021, 22, 312–321. [Google Scholar] [CrossRef]
- Barrera, M.J.; Aguilera, S.; Castro, I.; Carvajal, P.; Jara, D.; Molina, C.; González, S.; González, M.J. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjögren’s syndrome. Autoimmun. Rev. 2021, 20, 102867. [Google Scholar] [CrossRef]
- Allegra, M.; Restivo, I.; Fucarino, A.; Pitruzzella, A.; Vasto, S.; Livrea, M.A.; Tesoriere, L.; Attanzio, A. Proeryptotic Activity of 4-Hydroxynonenal: A New Potential Physiopathological Role for Lipid Peroxidation Products. Biomolecules 2020, 10, 770. [Google Scholar] [CrossRef]
- Bocci, V. Autohaemotherapy after treatment of blood with ozone. A reappraisal. J. Int. Med. Res. 1994, 22, 131–144. [Google Scholar] [CrossRef]
| Patient | Date of Birth | Clinical Condition in the Entrance | Microbiology Before After | Antibiotic Resistance | O2-O3-Maht | Outcome | |
|---|---|---|---|---|---|---|---|
| 1 | JT | 28 August 1964 | 14 February 2018. Pneumonia with chronic foci; left lung resistant to antimicrobial therapy; CRP = 13.50 mg/dL, ESR = 38 mm, WBC = 12.40 × 103/μL | S. aureus 3.0 × 106 CFU/mL 0.5 × 102 CFU/mL | MRSA Failure to levofloxacin 500 mg + 250 mg 7 days | Five sessions of O2-O3-MAHT (1) | 15 May 2019. Complete eradication of parenchymal foci. |
| 2 | GA | 24 February 1969 | 4 March 2019. Bacterial infection of shoulder prosthesis. | S. aureus 4.7 × 107 CFU/mL 3.2 × 101 CFU/mL | MRSA | Five sessions of O2-O3-MAHT (1) | 6 May 2019. Inflammation and infection disappeared. |
| 3 | AC | 27 July 1975 | 8 April 2019. Osteomyelitis process following a tibial malleolar fracture with joint effusion; oedema in neighboring soft tissues; inflammation and development of pseudoarthrosis. | S. aureus 2.3 × 106 CFU/mL 4.3 × 102 CFU/mL | MRSA Amoxicillin/clavulanate CiprofloxacinColistin Trimethoprim/sulfamethoxazole | Five sessions of O2-O3-MAHT and ozone microinjections (3,2) | 25 June 2019. Reduction in the oedema, joint effusion and pseudo-arthrosis symptoms. Reduction in inflammatory biomarkers. |
| 4 | RB | 4 November 1963 | 13 June 2022. Mycobacterium infection, with BAL fluid positive for alveolar macrophages and other innate cells. Grocott Ziehl–Neelsen test for cytomegalovirus was negative. The patient suffered from a cough and hot chest | S. aureus 6.9 × 105 CFU/mL 1.1 × 102 CFU/mL | MRSA Amikacin Linezolid Moxifloxacin | Five sessions of O2-O3-MAHT (1) | 5 September 2022. Reduction in inflammatory foci. |
| 5 | ZB | 16 September 1954 | 18 February 2022. Post-surgery retro-peritoneum, para-aortic effusion with Gram-positive cocci due to septic infection of aortic prosthesis; CRP = 111 mg/dl. | S. aureus 7.3 × 106 CFU/mL 2.0 × 103 CFU/mL | MRSA Penicillin G Amoxicillin/clavulanate | Five sessions of O2-O3-MAHT (1,4) | 19 May 2022. Reduction in sepsis via PET (SUVmax = 6.93 vs. 8.48). |
| 6 | AC | 18 October 1972 | 24 August 2020. Surgery on hip arthroprosthesis. MRSA infection; CRP = 98 mg/dL, α1-globulin = 3.2, β1-globulin = 10.2 | S. aureus 4.8 × 108 CFU/mL 3.0 × 103 CFU/mL | MRSA | Five sessions of O2-O3-MAHT (1) | 11 November 2020. Strong reduction in inflammation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirumbolo, S.; Valdenassi, L.; Tirelli, U.; Ricevuti, G.; Pandolfi, S.; Vaiano, F.; Galoforo, A.; Loprete, F.; Simonetti, V.; Chierchia, M.; et al. The Oxygen–Ozone Adjunct Medical Treatment According to the Protocols from the Italian Scientific Society of Oxygen–Ozone Therapy: How Ozone Applications in the Blood Can Influence Clinical Therapy Success via the Modulation of Cell Biology and Immunity. Biology 2023, 12, 1512. https://doi.org/10.3390/biology12121512
Chirumbolo S, Valdenassi L, Tirelli U, Ricevuti G, Pandolfi S, Vaiano F, Galoforo A, Loprete F, Simonetti V, Chierchia M, et al. The Oxygen–Ozone Adjunct Medical Treatment According to the Protocols from the Italian Scientific Society of Oxygen–Ozone Therapy: How Ozone Applications in the Blood Can Influence Clinical Therapy Success via the Modulation of Cell Biology and Immunity. Biology. 2023; 12(12):1512. https://doi.org/10.3390/biology12121512
Chicago/Turabian StyleChirumbolo, Salvatore, Luigi Valdenassi, Umberto Tirelli, Giovanni Ricevuti, Sergio Pandolfi, Francesco Vaiano, Antonio Galoforo, Fortunato Loprete, Vincenzo Simonetti, Marianna Chierchia, and et al. 2023. "The Oxygen–Ozone Adjunct Medical Treatment According to the Protocols from the Italian Scientific Society of Oxygen–Ozone Therapy: How Ozone Applications in the Blood Can Influence Clinical Therapy Success via the Modulation of Cell Biology and Immunity" Biology 12, no. 12: 1512. https://doi.org/10.3390/biology12121512
APA StyleChirumbolo, S., Valdenassi, L., Tirelli, U., Ricevuti, G., Pandolfi, S., Vaiano, F., Galoforo, A., Loprete, F., Simonetti, V., Chierchia, M., Bellardi, D., Richelmi, T., & Franzini, M. (2023). The Oxygen–Ozone Adjunct Medical Treatment According to the Protocols from the Italian Scientific Society of Oxygen–Ozone Therapy: How Ozone Applications in the Blood Can Influence Clinical Therapy Success via the Modulation of Cell Biology and Immunity. Biology, 12(12), 1512. https://doi.org/10.3390/biology12121512








