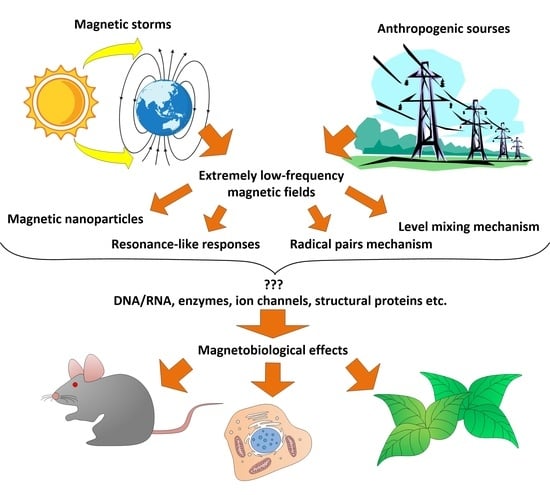
Biological Effects of Magnetic Storms and ELF Magnetic Fields
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- The use of adequate methods of statistical analysis (ANOVA, ranks, or parametric tests after checking their applicability);
- (2)
- A detailed description of the ELF-MF’s characteristics and an assessment of its homogeneity within the experimental setup (preferably, the presence of a 3D map of the spatial distribution of induction during the experiment);
- (3)
- The availability of instrumental verification of the parameters of the surrounding MF, measures to compensate (if necessary) for the installation for generating the MF, and possible sources of artifacts (background fields, field inhomogeneity in the installation);
- (4)
- The SJR rating of the journal in which the work was published, as a measure of the relevance of the work as a whole (we chose the threshold SJR > 0.4).
2. Biological Effects of Magnetic Storms
2.1. Approaches to Research
- (1)
- Analysis of a large array of data: physiological, usually clinical, and data on geomagnetic activity [29].
- (2)
2.2. Biological Effects
| No | Object (Species) | Estimated Parameter | Effect, % | f, Hz | TVMF Induction (b) | Duration | n | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | Human Adults, healthy, living above 70° north latitude | Amplitude of fluctuations in melatonin concentration in saliva | −20% | 10−5 | >80 nT | year | 20 | [60] |
| 2 | Human Adults, healthy, males, 23.9 ± 5.5 years (laboratory simulation) | The rate of blood movement through the capillaries | +30% | ~7 × 10−5 | ~150 nT | 18–24 h | 8 | [28] |
| Systolic pressure | -N/A | — | — | — | — | |||
| Heart rate variability: HF LF VLF | +25% +25% +25% | — — — | — — — | — — — | — — — | |||
| 3 | Human Adults, healthy, 26.1 ± 5.5 years Body mass index 23.9 ± 3.9 kg/m2 Heart rate 80.4 ± 5.4 bmp Systolic and diastolic pressure 114.5 ± 9.1 and 72.0 ± 8.1 mmHg. (laboratory simulation) | Heart rate variability: LF (incline 9.6°) HF (horizontal position) | −20% +40% | ~7 × 10−5 — | ~150 nT — | 5–24 h — | 8 — | [58,71] |
| Correlation between changes in parameters of the cardiovascular system (HRV and capillary blood flow velocity) and the characteristics of the TVMF (Bx, By) | <0.05 | — | — | — | — | |||
| 4 | Human Adults, healthy, women, 24–49 years | Length of the RR interval with increasing oscillations of MF induction | +50% | 0.01–3 Hz | 20 (2–90) nT | 2 days | 17 | [114] |
| 5 | Human Adults, healthy, women, 24–49 years | Regression coefficients of HRV signals with Ap index: HF LF VLF | 200% 200% 200% | 0.002–3.5 Hz (resonant 7.83 and ~14, 20, 26, 33, 39, 45) | 20 (2–90) nT — — | 2 days — — | 17 — — | [29] |
| Ratio LF/HF | −50% | — | — | — | — | |||
| Regression coefficients of HRV with induction of GMF: HF LF VLF | 400% 150% 200% | — — — | — — — | — — — | — — — | |||
| 6 | Human Population of 263 cities, data of National Center for Health Statistics (NCHS), USA | Risk of death from diseases: General | +50% | 0.002–3.5 | 2–60 nT | 2 days | >44 220 000 | [59] |
| Stroke | +50% | — | — | — | — | |||
| Myocardial infarction | +100% | — | — | — | — | |||
| Other cardiovascular diseases | +40% | — | — | — | — | |||
| 7 | Human Patients of Nizhnekolomsk hospital, Penza region, Russia | Risk of heart attack Stroke risk | +50% +50% | 0.002–3.5 — | 200 nT — | 2 days — | 927 и 942 | [106] |
| 8 | Human Analysis of archival data, men, women | Suicide rate | +70% | 0.002–3.5 | 300 nT | 2 days | 1487 | [115] |
| 9 | Human Patients of the Hospital of Kaunas University of Medicine, Lithuania | Risk of developing myocardial infarction without changes in the ST fragment on the ECG | +39% | 0.002–3.5 | >71 nT | 1 day | 2008 | [68] |
| Risk of developing myocardial infarction with changes in the ST fragment on the ECG | +54% | 0.002–3.5 | >71 nT | 2 days | ||||
| 10 | Human Healthy volunteers of both sexes, 34–52 years old | Correlations (log(ρ)) of microcirculation oscillations with advising frequencies during geomagnetic disturbances 1: Endothelial Neurogenic Myogenic Respiratory Cardiac rhythm | 2.0 2.0 2.5 1.0 0.5 | 0.01 0.03 0.1 0.3 1.0 | >50 nT — — — — | 2 days — — — — | 9 — — — — | [62] |
| 11 | Human Men, women, age 25–65+ years, patients of Kaunas city hospital (geomagnetic latitude 52.38 N) | Risk of acute myocardial infarction | +10% | 0.0016–5 | >140 nT | 1–4 days | 13,629 | [108] |
| Risk of myocardial infarction | +63% | — | — | 3 h | 10,000 | [107] | ||
| 12 | Human Men and women with myocardial infarction | Correlation between GMF induction and the risk of myocardial infarction (Women) 1 | −0.5 −0.5 −0.5 N/A | 3.5 7 15 32 | >80 nT — — — | 1 day — — — | 435 — — — | [61] |
| Correlation between GMF induction and the risk of myocardial infarction (Men) | −0.35 −0.35 −0.35 −0.35 | 3.5 7 15 32 | — — — — | — — — — | 268 — — — | |||
| 13 | Human Men and women, 21–85 years | Systolic blood pressure, Diastolic blood pressure Average daily heart rate | +10% +10% +10% | 0.0016–5 — — | >120 nT — — | 24 h — — | 447 — — | [98] |
| 14 | Human Men and women, 21–35 years (simulation in the laboratory) | Systolic blood pressure | +5% | 0.0016 | 50 nT | 24 h | 3 | [78] |
| Heart rate | −5% | — | — | — | — | |||
| Heart rate variability: ULF (0.001–0.003 Hz) VLF (0.003–0.04 Hz) LF (0.04–0.15 Hz) HF (0.15–0.4 Hz) | +15% −10% −25% −25% −10% | — — — — — | — — — — — | — — — — — | — — — — — | |||
| 15 | Human Pregnant women (healthy and pregnancy hypertension) | Risk of developing hypertension during pregnancy | +40% | 0.0016–5 | >200 nT | 4 days | 19,843 | [109] |
| 16 | Human Men and women | Risk of ventricular tachycardia | −60% | 0.0016–5 | >120 nT | 24 h | 233 | [109] |
| 17 | Human Men and women | Paroxysmal atrial fibrillation | −45% | 0.0016–5 | >130 nT | 24 h | 653 | [116] |
| 18 | Human Men and women | Growth hormone Prolactin | +20% +30% | 0.0016–5 — | >70 nT — | 24 h | 1752 | [86] |
| 19 | Human Men and women, patients with atherosclerosis and healthy volunteers | Blood cholesterol concentration in atherosclerosis Triglyceride concentration in the blood of healthy people | −5% −7% | 0.0016–5 — | >120 nT — | 24 h — | 1200 — | [85] |
| 20 | Human Men and women | Platelet count | +7% +5% | 0.0016–5 — | >41 >70 nT | 48 h — | 1053 — | [82] |
| 21 | Human Men and women | Prothrombin time | +4% +8% | 0.0016–5 — | >41 >70 nT | 48 h — | 1331 — | [83] |
| 22 | Human Men and women | ADP platelet aggregation | +25% | 0.0016–5 | >41 nT | 24 h | 162 | [83] |
| 23 | Human Men and women | Fibrinogen concentration in blood | +11% | 0.0016–5 | >110 nT | 24 h | 100 | [84] |
| 24 | Human Men and women | Average capillary closure time | +7% | 0.0016–5 | 70 nT | 24 h | 120 | [84] |
| 25 | Human Men and women | Basophils count Leucocyte count | −60% −40% | 0.0016–5 — | 70–120 nT — | 24 h — | 400 — | [67] |
| 26 | Human Men and women with migraine | Frequency of severe and moderate migraine episodes | +10% +32% +68% | 0.0016–5 — — | 40 70 120 nT | 2 day — — | 486 — — | [66] |
| 27 | Human Healthy ~41 years | Heart rate | −4% | 0.0016–5 | 69 nT | 24 h | 14 | [99] |
| Heart rate variability (LF/HF ratio) | −15% | — | — | — | — | |||
| Well-being (survey) | −30% | — | — | 48 h | — | |||
| 28 | Human Men and women (21–35 years old) | Systolic pressure | +5% | 0.0016 | 50 nT | 24 h | 3 | [78] |
| Heart rate | −5% | — | — | — | — | |||
| Heart rate variability: ULF (0.001–0.003) VLF (0.003–0.04) LF (0.04–0.15) HF (0.15–0.4) | +15% −10% −25% −25% | — — — — | — — — — | — — — — | — — — — | |||
| 29 | Human Men and women (24–73 years old) | Systolic blood pressure relative value Sensitive people proportion | 3% −32% | 7.5–8.5 — | >1.97 pT — | 24 h — | 112 — | [117] |
| Diastolic blood pressure relative value, sensitive people proportion | −3% −27% | — — | — — | — — | — — | |||
| Mean arterial pressure, relative value, Sensitive people proportion | −2% −30% | — — | — — | — — | — — | |||
| Heart rate | N/A | — | — | — | — | |||
| Depression score relative value Sensitive people proportion | −3% −20% | — — | — — | — — | — — |
3. Magnetobiological Effects of Anthropogenic ELF-MFs
3.1. Effects on the Whole Organism (Laboratory Studies)
3.2. Effects at the Molecular–Cellular Level (Laboratory Studies)
| No | Object (Species) | Characteristics | Effect, % | f, Hz | Induction | Duration | n | Statistic | Installation Type | Installation Size | Verification | JSR | Refs. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b (TVMF) | B (SMF) | |||||||||||||
| 1 | Human cord blood lymphocytes | Viability | −15% −20% −26% | 7.8 — — | 6, 17, 24 μT | 4.1 µT — — | 72 h — — | 6 — — | One-way ANOVA, post hoc Fisher LSD | System of perpendicular coils (2 axes) | 10 × 10 cm | Magnetometer, 3D-map variation <5% The external field was reduced by a μ-metal chamber to 3.7 μT. | 0.42 | [124] |
| 2 | Human pluripotent cell line iPS (7F3955- pMXs#1) | Proportion of CD34 + CD38—cells (differentiated) | N/A N/A N/A N/A | 50 — — — | 0 100 200 300 mT | 4.1 µT — — — | 7 day — — — | 5 — — — | One-way ANOVA, post hoc Fisher LSD | Helmholtz coils (1 axis) | Ø 20 cm | Magnetometer, one point, variation <5%. The external field was reduced by a μ-metal chamber to 3.7 μT. | 0.42 | [172] |
| 3 | Fire ants Solenopsis sp. Imago | Time to escape the trap | −20% +30% −50% | 60 — — | 21 40 57 | 26 29 26 | 200 s — — | 30 — — | Rayleigh test, Watson U2 test | Helmholtz coils (1 axis) | 18 × 18 cm | Magnetometer, time profile of ELF-MF was shown GMF 21 μT | 0.3 | [133] |
| Proportion of insects moving along the line GMF | −8% −8% | — — | 57 40 μT | 10 26 μT | — — | — — | ||||||||
| 4 | Planaria Girardia tigrina Asexual laboratory race, length 7–8 mm | Regeneration index (amputation of 1/5 body part) | +20% +30% +15% +2% +28% +2% +12% +0% +11% −18% | 60 — — — — — — — — — | 29 55 88 105 164 227 265 311 361 412 | 42 μT — — — — — — — — — | 3 days — — — — — — — — — | 30 — — — — — — — — — | Student’s t-test | Helmholtz coils | Ø 30 cm | Magnetometer, one point, TVMF ambient 50 Hz 5 nT | 0.18 | [148] |
| Flax Linum bienne upper segments of stems without leaves 2.5 cm long | Deviation of the apical end of a segment from the horizontal plane (gravitropism) | +3.5% +2% +3% +2% | — — — — | 55 105 164 227 μT | — — — — | 2 h — — — | 20 — — — | |||||||
| 5 | Planaria Schmidtea mediterranea, Asexual laboratory race, length 10 mm | Rate of growth of the planarian head blastema | −10% −24% +3% +25% +5% | 13 16 27 30 33 | 74 μT — — — — | 41 μT — — — — | 24 h — — — — | 5 — — — — | ANOVA | Helmholtz coils | Ø 30 cm | Magnetometer, one point, TVMF ambient 50 Hz <6 nT | 0.79 | [54] |
| 6 | Cows Bos taurus Males and females, adults | Orientation in space in the north-south direction (Satellite observation, image analysis) | −99% | 50- 60 | 5–15 μT | ~40 μT 1 | 24 h | 1699 | Rayleigh test, Watson–Williams F test, Mardia–Watson–Wheeler test | High-voltage power lines | 50 × 150 cm | Not applicable | 4.03 | [64] |
| Roe deer Capreolus capreolus Males and females, adults | −99% | — | — | — | — | 653 | ||||||||
| 7 | Honey bee Apis cerana Larvae (2 days) | Survival | −60% | 50 | 3 mT | ~50 µT | 20 days | 72 | Duncan’s post hoc test, Dunnett’s post hoc test, Log-rank (Mantel–Cox) test | Commercially available ELF-EMF generator (Litian magnetic and electric Science and Technology Co., Ltd., Mianyang, China) | 15 × 10 × 10 cm | ELF-FM ≫ GMF | 0.68 | [154] |
| Body mass | −10% | — | — | — | — | — | ||||||||
| Duration of development | +5% | — | — | — | — | — | ||||||||
| Gene expression: increasing | +153 genes | — | — | — | — | — | ||||||||
| decreasing | −269 genes | — | — | — | — | — | ||||||||
| 8 | Human Adults, healthy, 26.1 ± 5.5 years, body mass index 23.9 ± 3.9 kg/m2, heart rate 80.4 ± 5.4 beats/min Systolic pressure 114.5 ± 9.1 mmHg Diastolic pressure 72.0 ± 8.1 mmHg. | The rate of blood movement through the capillaries | +30% | 7 × 10−5 | 205 nT — | 49 µT — | 18–24 h — | 8 — | F test (CBV and BP), factorial ANOVA (RR intervals) | Helmholtz coils (3 axes) imitation of a magnetic storm k = 7 | 2.5 × 2.5 × 2.5 m | Magnetometer, one point, variation <0.03%. Noise and GMF were compensated | 0.65 | [28,71] |
| Systolic pressure | N/A | — | — | — | — | — | ||||||||
| Heart rate variability: HF LF VLF | +25% +25% +25% | — — — | — — — | — — — | — — — | — — — | ||||||||
| Correlation between changes in parameters of the cardiovascular system (HRV, capillary blood flow velocity) and characteristics of TVMF (Bx, By) | <0.05 | — | — | — | — | — | ||||||||
| 9 | Human Adults, healthy, 26.1 ± 5.5 years, body mass index 23.9 ± 3.9 kg/m2 | Heart rate variability: LF (tilt 9.6°) HF (horizontal position) | −20% +40% | 7 × 10−5 | 205 nT — | 49 µT — | 5–24 h — | 8 — | Factorial ANOVA | Helmholtz coils (3 axes) imitation of a magnetic storm k = 7 and microgravity | 2.5 × 2.5 × 2.5 m | Magnetometer, one point, variation <0.03%. Noise and GMF were compensated | 1.03 | [58] |
| 10 | Human leukemia cells K562 | HSP70 protein concentration | +100% +50% | 50 — | 25 100 μT | 41.8 μT — | 1 h — | 3 — | Non-parametric Chi-square test, Kruskal–Wallis test, ANOVA, Dunnett’s post hoc test | Helmholtz coils | - | Magnetometer, one point, variation <0.5 μT | 0.45 | [219] |
| 11 | Mice Males and females, 10 and 15 days, respectively | Protein expression: c-Jun c-Fos (markers of neuronal differentiation) | −15% N/A | 50 — | 2 mT — | 40 μT — | 4 days — | 3 — | Student’s t-test | Solenoid | - | Temperature variation <0.1 °C | 0.4 | [186] |
| 12 | Escherichia coli strains K12 AB1157 K12 EMG2 K12 GE499 K12 GE500 Human lymphocytes (men, ~30 years old, non-smokers) | Chromatin conformation measured by anomalous viscosity time dependencies (AVTD): | +25% +20% +5% +30% N/A −20% −20% N/A N/A N/A N/A −10% | 9 12 16 18 25 60 9 12 16 18 25 60 | 30 μT — — — — — — — — — — — | 43 μT — — — — — — — — — — — | 15 h — — — — — — — — — — — | 8 — — — — — — — — — — — | Student’s t-test | Helmholtz coils | - | Magnetometer, one point, variation, temperature variation <0.1 °C, GMF 43 μT (collinear) 19 μT (perpendicular) | 0.86 | [187] |
| 13 | Human breast cancer cells MCF-7 | Cell survival | N/A | 50 | 500 μT | 37 μT | 30 min | 8 | ANOVA, Bonferroni post hoc test | Solenoids system | 44 × 14 cm | Magnetometer, one point | 0.4 | [189] |
| Expression of genes of the antioxidant system: SOD2 | −40% | 50 | 250 μT | — | 30 min | — | ||||||||
| MSGT3 | +36% +20% | — — | — — | — — | 15 min 5 min | — — | ||||||||
| GSTO1 | −40% −14% −23% | — — — | — — — | — — — | 5 min 15 min 30 min | — — — | ||||||||
| GSTM3 | −31% −33% +33% | — — — | — — — | — — — | 5 min 15 min 30 min | — — — | ||||||||
| MGST1 | +36% −37% | — — | — — | — — | 30 min 15 min | — — | ||||||||
| 14 | Gallus gallus spp. domesticus chicks 5 days after hatching | Release of Ca2+ from brain tissue | +13% | 315 | 61 nT | 38 μT | 20 min | 32 | One-way ANOVA | Helmholtz coils (1 axis) | Ø 47 cm | Magnetometer, one point, GMF ~38 μT | 0.42 | [166] |
| 15 | Gallus gallus spp. domesticus chicks 5 days after hatching | Release of Ca2+ from brain tissue | +11% +13% +14% +11% +18% +14% +15% +9% +14% | 45 50 60 15 45 60 75 90 105 | 61 nT — — 61 nT — — — — — | 38 μT | 20 min — — 20 min — — — — — | 32 | Two-way ANOVA | Helmholtz coils (1 axis) | Ø 47 cm | Magnetometer, one point, GMF ~38 μT | 0.42 | [167] |
| 16 | Neuronal cell line PC-12 | Neurite growth rate | −5% −25% −75% −75% −40% −20% | 45 — — — — — | 7.0 14, 20 25 37 46 μT | 36.6 μT — — — — — | 23 h — — — — — | 3 — — — — — | Bessel function | Helmholtz coils (2 axes) | Ø 20 cm | Magnetometer, one point, variation SMF <0.2 μT. Ambient TVFM 60 Hz, <0.9 μT | 0.42 | [168] |
| 17 | Neuronal cell line PC-12 | Percentage of cells with neurites | +20% −30% −60% | 45 — — | 20 ↔ 30 ↔↕ 30 ↕ μT | 36.6 μT — — | 23 h — — | 3 — — | Student’s t-test | Helmholtz coils (2 axes) | Ø 20 cm | Magnetometer, one point, variation SMF <0.2 μT. Ambient AFM 60 Hz, <0.9 μT | 0.79 | [169] |
| 18 | Neuronal cell line PC-12 | Percentage of cells with neurites (double-blind experiment) | −70% | 45 | 23.8 μT | 36.6 μT | 23 h | 3 | Double-blind test, binomial test | Helmholtz coils (2 axes) | Ø 20 cm | TVMF 50 Hz <0.08 μT SMF <0.36 μT. The external field was reduced by a μ-metal chamber | 0.42 | [170] |
| 19 | Gallus gallus spp. domesticus chicks 5 days after hatching | Release of Ca2+ from brain tissue | +12% +13% +15% +14% +12% +11% | 16 — — — — — | 1.75 3.85 5.57 6.82 7.65 7.77 μT | <0.1 μT | 20 min | 32 | Two-way ANOVA | Helmholtz coils (1 axis) | Ø 47 cm | Magnetometer, one point GMF 38 μT | 0.42 | [165] |
| 20 | Rabbit kidney Na/K-ATPase Oryctolagus cuniculus domesticus | Enzyme activity | +10% | 60 | 310 нT | <0.1 μT | 15 min | 3 | Enzyme kinetics analysis methods | Specially designed and verified installation | - | Magnetometer, 3D map, variation < 3% MF in the thermostat < 0.1 μT | 0.72 | [190] |
| 21 | Cytochrome oxidase, rat liver of Rattus norvegicus Sprague–Dawley | Enzyme activity | +5% +15% +20% +40% | 60 — — — | 2 5 7 10 мкT | <0.1 μT — — — | 8 min — — — | 3 — — — | Enzyme kinetics analysis methods | Specially designed and verified installation | - | Magnetometer, background MF < 0.1 μT | 0.72 | [191] |
| 22 | Fibroblast line L929 | Ornithine carboxylase activity | +40% +80% +80% +110% +80% +100% | 60 — — — — — | 4 5 6 8 9 20 μT | 0 μT — — — — — | 4 h — — — — — | 5–10 — — — — — | Two-tailed Student’s t-test | Helmholtz coils | Ø 10.5 cm | Magnetometer, one point, variation <15% | 0.72 | [192] |
| 23 | Belousov–Zhabotinski (BZ) reaction Starting frequency 0.03 | Frequency of cycles of changes in the redox potential Fe2+/Fe3+ at a temperature of 15–19 °C | +5% | 60 | 28 μT | 0.1 μT | 20 min | 8 | Regression analysis methods | Helmholtz coils | 13 × 14 cm | Magnetometer, one point, SMF variation < 0.1 μT. GMF shielded with μ-metal | 0.78 | [212] |
| 24 | Hela cell line after heating 43 °C for 20 min | SHP70 expression | +15% +60% | 60 — | 8 80 μT | 20 μT — | 20 min — | 3 — | Tukey test, normality Kolmogorov–Smirnov test | Solenoid | 5.27 × 25.0 cm | Magnetometer, one point. GMF 20 μT | 0.88 | [220] |
| 25 | Endothelial cells: SPAE | Inducible (heating 44 °C 30 min) HSP70 protein level | N/A +46% +45% +71% +78% +79% | 50 — — — — — | 150 300 680 μT — — — | 12 μT | 24 h — 8 16 24 48 | 3 | Student’s t-test | Solenoid | Not discribed | 1–12 μT (without experiment) 2–16 μT (during experiments) Magnetometer, 3D map, accuracy < 2 μT | 0.79 | [221] |
| HUVECs | +40% | — | — | — | 24 h | — | ||||||||
| Human leukemia and lymphoma cells: CEM | +60% | — | — | — | — | — | ||||||||
| HL-60 | +65% | — | — | — | — | — | ||||||||
| U937 | +61% | — | — | — | — | — | ||||||||
| 26 | Human promyelocytic lineage cells HL-60 (lymphoblasts) | Chloramphenicol acetyltransferase (CAT) activity | +150% | 60 | 8 μT | <0.1 μT | 20 min | 3 | Student’s t-test | Helmholtz coils (1 axis) in a μ-metal container | 13 × 14 cm | Magnetometer, one point, SMF variation <0.1 μT. GMF shielded with μ-metal (90 times reduction) | 0.78 | [193] |
| HSP70 mRNA expression | +80% | — | — | — | — | — | ||||||||
| HSP70 protein concentration | +210% | — | — | — | — | — | ||||||||
| 27 | Chicken Gallus gallus spp. domesticus White Leghorn, fertilized eggs | Embryo survival after 1 h of hypoxia | N/A +100% +200% +200% N/A +50% +100% +150% | 60 — — — — — — — | 2↕ 4 8 10 μT 2↔ 4 8 10 μT | 40–50 μT — — — — — — — | 20 min — — — — — — — | 40 — — — — — — — | x2 analysis | Helmholtz coils (1 axis) | Ø 2 m | Magnetometer, one point, SMF <0.5 μT. GMF 40–50 μT | 0.72 | [155] |
| 28 | Human breast cancer cell line MCF-7 | Melatonin-induced proliferation inhibition 10−9 M | 100% 100% | 60 — | 0.2 1.2 μT | 0 μT | 7 days — | 5 | ANOVA | Merritt’s coils (2 axis) | 16 × 16 × 16 cm | Magnetometer, one point, variation, SMF <5%, GMF and 60 Hz, 1.4 μT, TVMF <2% | 2.16 | [184] |
| 29 | Children, boys and girls, healthy or with leukemia | Risk of developing leukemia | ×1.27–3.13 | 50– 60 | ≥0.4 μT | ~45 μT | >1 year | 10,338 3203 | x2 analysis | Meta-analysis of the assessment of the magnetic situation in cities | Not applicable | Not applicable | 2.78 | [70] |
| 30 | Children, boys and girls, healthy or with leukemia | Risk of developing leukemia | ×1.2–2.13 | 50– 60 | ≥0.3 μT | 35–45 μT | >1 year | meta-analysis | Inverse-variance weighted (Woolf), Mantel–Haenszel, and maximum-likelihood (ML) tabular methods, and using ML logistic regression | Meta-analysis of the assessment of the magnetic situation in cities | Not applicable | Not applicable | 1.96 | [69] |
| 31 | Chinese hamster lung cells (CHL) | Epidermal growth factor receptor (EGFR) clustering, qualitatively: sinusoidal field, sine + noise | ++ + | 50 — | 400 μT — | 18.5 μT — | 30 min — | 3 | ANOVA and least significant difference (LSD) test | Helmholtz coils (3 axes) | Ø 36 cm | Magnetometer, oscilloscope SMF <18.5 μT TVMF 50 Hz, <1–2 μT | 0.62 | [174] |
| Phosphorylation of signaling protein Ras: sinusoidal field sine + noise | +90% +5% | — — | — — | — — | — — | — — | ||||||||
| 32 | Diatom Amphora coffeaeformis | Mobility at a frequency of 16 Hz at different Ca2+ concentrations: 0.1 мM 0.25 мM 0.5 мM | +200% +900% +300% | 16 16 16 | 20.9 μT — — | 52 μT — — | 2 days — — | 12 — — | x2 analysis and ANOVA | Helmholtz coils (3 axes) | Ø 23 cm | Magnetometer, one point, variation <30 nT. GMF 52 μT TVMF ambient 60 Hz, <0.1 μT | 0.42 | [149] |
| Mobility at Ca2+ concentration 0.25 mM and frequencies | +200% +500% +600% N/A | 14 16 18 32 | — — — — | — — — — | — — — — | — — — — | ||||||||
| 33 | Human bone marrow cell line TE-85 | Ca2+ release | +120% | 16.3 | 40 μT | 20 μT | 35 min | 6 | Student’s t-test | Helmholtz coils (3 axes) | Ø 30 cm | Magnetometer, one point. GMF 40 μT | 0.97 | [171] |
| 34 | Rats Wistar, males, adult | Concentration of 6-sulfatoxymelatonin in urine at night | +15% | 50 | 100 μT | 1 μT | 24 h | 5 | Student’s t-test | Helmholtz coils (1 axis) | Ø 42 cm | Magnetometer, one point | 0.42 | [195] |
| 35 | Rats Wistar, males, adult | Serotonin-N-acetyltransferase activity | −10% | 50 | 1 mT | 38 μT | 1 h | 48 | ANOVA followed by the Student–Newman–Keuls test | Solenoid (1 axis) | 20 × 20 cm | Magnetometer, one point | 0.4 | [199] |
| 36 | Rats Wistar–King, males 11–18 weeks, 300–370 g. | Melatonin concentration at midnight in the pineal gland | 20% −40% | 50 — | 5 250 | 26 μT — | 6 weeks | 400 — | Student’s t-test | Helmholtz coils | - | Magnetometer, one point, variation, TVMF 50 Hz <16 nT SMF <2% GMF 40 μT (total) 26 μT (horizontal) | 0.42 | [196] |
| Melatonin concentration at midnight in the blood plasma | −20% −25% | — — | 5 250 μT | — — | — — | — — | ||||||||
| 37 | Human Men and women (21–35 years old) | Systolic pressure | +5% | 0.0016 | 50 nT | 40 nT | 24 h | 3 | Student’s t-test at a significance level of 0.001 | Helmholtz coils (magnetic storm simulation) | 3 × 3 × 3 m | Magnetometer, one point | 1.37 | [78] |
| Heart rate | −5% | — | — | — | — | — | ||||||||
| Heart rate variability ULF (0.001–0.003) VLF (0.003–0.04) LF (0.04–0.15) HF (0.15–0.4) | +15% −10% −25% −25% −10% | — — — — — | — — — — — | — — — — — | — — — — — | — — — — — | ||||||||
| 38 | Human Human peripheral blood lymphocytes | Proportion of apoptotic cells | −45% −36% | 50 — | 80 800 μT | 40 μT — | 44 h — | 3 — | Two-way ANCOVA, and the Tukey honest significant difference (HSD) test | Helmholtz coils (1 axis) | 42 cm Ø 20 cm | Magnetometer, one point, variation <1% | 0.42 | [162] |
| Nuclear division index (NDI) | +5% +25% | — — | 80 800 μT | — — | — — | — — | ||||||||
| Proportion of cells with micronuclei | +15% −40% | — — | 80 800 μT | — — | — — | — — | ||||||||
| 39 | Human neuroblastoma cell line SH-SY5Y | Survival cells | −15% | 60 | 2 mT | 38 μT | 3 h | 10 | Student’s t-test for extremely low samples | Rodin’s star-coil | Ø 30 cm | Magnetometer, 3D map, ELF-MF≫ GMF | 0.42 | [200] |
| Number of cells | −60% | — | — | — | — | — | ||||||||
| Cell proteome analysis: increase in expression, decreased expression | +7% +5% | — — | — — | — — | — — | — — | ||||||||
| Expression of individual proteins: prohibitin | +90% | — | — | — | — | — | ||||||||
| 4-HNE | −90% | — | — | — | — | — | ||||||||
| F-actin | qualitatively | — | — | — | — | — | ||||||||
| Guanine nucleotide-binding protein subunit beta-5, | +30% | — | — | — | — | — | ||||||||
| Alpha-tubulin | +39% | — | — | — | — | — | ||||||||
| Prohibitin | +13% | — | — | — | — | — | ||||||||
| Alpha-ketoglutarate-dependent dioxygenase FTO | 1/2.3 | — | — | — | — | — | ||||||||
| Serine/threonine-protein kinase 32C | ×12.07 | — | — | — | — | — | ||||||||
| T-complex protein 1 subunit alpha | −41% | — | — | — | — | — | ||||||||
| ATP synthase subunit beta, mitochondrial | +41% | — | — | — | — | — | ||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP4 | +48% | — | — | — | — | — | ||||||||
| PDZ and LIM domain protein 3 | +72% | — | — | — | — | — | ||||||||
| Sin3 histone deacetylase corepressor complex component SDS3 | +31% | — | — | — | — | — | ||||||||
| Nuclear fragmentation | +35% | — | — | — | — | — | ||||||||
| Actin filament disruption | +35% | — | — | — | — | — | ||||||||
| Disruption of β-tubulin strands | +35% | — | — | — | — | — | ||||||||
| 40 | Meta-analysis of articles on the relationship between the risk of developing amyotrophic lateral sclerosis Data from 62 articles. Women, >18 years old. USA, Denmark, Sweden, Switzerland | Development risk Mortality | +14% | 50– 60 | 0.3–2.5 μT | ~36 μT | >1 year | ~20,000 | Pooled analysis of the large sample size | Industrial fields | Not applicable | Not applicable | 0.42 | [65] |
| 41 | Human Men, healthy, 18–27 years old, body mass index 24 ± 2 | Heart rate (HR) HR variability (HRV) VLF LF HF | −5% +10% +300% +200% +50% | 50 — — — — | 100 nT — — — — | 28 µT — — — — | 15 min — — — — | 17 — — — — | ANOVA, corrected degrees of freedom via Greenhouse–Geisser estimates of sphericity if the assumption of sphericity was violated. t-tests with Bonferroni correction | Helmholtz coil (1 axis) | Ø 70 cm | Magnetometer, one point, variation SMF < 2 µT (26–30 µT), GMF 44 µT, TVMF 50 Hz 0.01 µT | 0.42 | [122] |
| 42 | People, men and women, 25.6 ± 4 years | Final angle of the line after adjustment SVV: standard deviation | −12% −12% −12% −12% | 20 60 120 160 | 98 32.8 16.4 12.3 | ~50 μT — — — | 1.5 h — — — | 33 — — — | Eta squared (ηG2) after ANOVAs | Single coil system (1 axis) | Ø 20 cm | Magnetometer, one point (dB/dt = 12.3 T/s) | 0.42 | [135] |
| SVV | +10% +10% +10% +10% | 20 60 120 160 | 98 32.8 16.4 12.3 | — — — — | — — — — | — — — — | ||||||||
| Angle setting time | −70% −70% −70% −70% | 20 60 120 160 | 98 32.8 16.4 12.3 mT | — — — — | — — — — | — — — — | ||||||||
| 43 | Rats 200–250 g body mass, 3 months old, control and after tendon trimming surgery | Muscle mass: control, operated | +10% +25% | 40 — | 1.5 mT — | ~30 μT — | 45 h — | 8 — | ANOVA, Tukey’s post hoc test | Helmholtz coils (1 axis) | Ø 60 cm | Magnetometer, one point | 0.42 | [120] |
| Muscle surface area: control, operated | +2% +12% | — — | — — | — — | — — | — — | ||||||||
| Strength of muscle contraction: control, operated | N/A +50% | — — | — — | — — | — — | — — | ||||||||
| Time of maximum contraction: control, operated | N/A −10% | — — | — — | — — | — — | — — | ||||||||
| Relaxation time at 80% (both) | N/A | — | — | — | — | — | ||||||||
| Contraction force: operated | +60% | 120 | — | — | — | — | ||||||||
| 44 | Human Men and women after SARS-CoV-2 infection, age 50–70 years | Granularity of peripheral blood granulocytes | −10% | 320+780+ 880+ 2600 | 5 μT | ~50 μT | 30 min | 32 | t-test after Shapiro–Wilk test | Ring-shaped portable generator | Ø 50 cm | Magnetometer, one point ELF-MF— GMF | 0.42 | [123] |
| Peripheral blood granulocyte count | −10% | — | — | — | — | — | ||||||||
| 45 | Rats Sprague–Dawley, males, 14–18 days, hippocampal slices | Cell responses to electrical stimulation (normalized amplitude) | −25% −27% −30% −20% −22% −25% −8% −10% −15% | 15 — — 50 — — 100 — — | 0.5 1 2 0.5 1 2 0.5 1 2 mT | ~45 μT — — — — — — — — | 20 min — — — — — — — — | 5 — — — — — — — — | ANOVA on Tukey’s multiple comparisons test | Solenoid (1 axis) | Ø 10 cm | Magnetometer, one point, variation SMF < 5% TVMF < 5% ELF-MF ≫ GMF | 0.93 | [138] |
| 46 | Rats Sprague–Dawley males, 14–18 days, hippocampal slices (CA1 region) | Electrically excited postsynaptic potentials | −30% −25% −20% −35% −25% −25% −35% −25% −25% | 15 — — 50 — — 100 — — | 0.5 1 2 0.5 1 2 0.5 1 2 mT | ~45 μT — — — — — — — — | 10 s — — — — — — — — | 5 — — — — — — — — | Two-way ANOVA, Tukey’s multiple comparisons test | Commercially available systems XcELF (IT’IS Foundation, Zurich, Switzerland) | Not described | Magnetometer, one point, variation SMF < 5% TVMF < 5% ELF-MF ≫ GMF | 0.79 | [139] |
| 47 | Rats Sprague–Dawley, males, 14–18 days, hippocampal slices (CA1 region) | Electrical response to high-frequency electrical stimulation: in MF: control, against the background of receptor blockers NMDAR | −80% −40% | 15 — | 2 mT — | ~45 μT — | 20 min — | 5 — | Two-way ANOVA, Tukey’s multiple comparisons test | Solenoid (1 axis) | Ø 10 cm | Magnetometer, one point, variation SMF < 5% TVMF < 5% ELF-MF≫ GMF | 0.85 | [139] |
| 48 | Rats Sprague–Dawley, males, 14–18 days, hippocampal slices (CA1 region) | Amplitude and slope of the electrical response to electrical stimulation (control): 20 min 40 min 60 min in the presence of AMPA/kainate receptor antagonist (10 μM CNQX) | −5% −20% −25% recovery after washin 100% | 15 — — — | 2 mT — — — | ~45 μT — — — | 20 40 60 min — | 5 — — — | Two-way ANOVA on Tukey’s multiple comparisons test | Solenoid (1 axis) | Ø 10 cm | Magnetometer, one point, variation SMF < 5% AMF < 5% ELF-MF ≫ GMF | 1.04 | [142] |
| 49 | Rats Wistar embryos and newborns, slices of the hippocampus and neocortex | Electrical activity of neurons in response to electro-stimulation: Amplitude between minimum and maximum (bark) embryos, newborns | N/A +10% +15% +30% +45% +45% +50% | 50 — — — — — — | 0.5 1.75 2.0 2.25 2.5 2.75 3.0 mT | 0.5 mT — — — — — — | 7 days — — — — — — | 7 — — — — — — | ANOVA or Student’s t-test | Helmholtz coils (1 axis) | Ø 42 cm | Magnetometer, one point, variation TVFM <25 μT, Variation SMF < 10 μT | 0.64 | [140] |
| Maximum of response: embryos | +80% +100% +100% +100% | — — — — | 2.25 2.5 2.75 3.0 mT | — — — — | — — — — | — — — — | ||||||||
| Maximum of response: newborns | +80% +100% +100% +100% | — — — — | 2.25 2.5 2.75 3.0 mT | — — — — | — — — — | — — — — | ||||||||
| Action potential: embryos | +25% | — | 2 mT | — | — | — | ||||||||
| 50 | Mice BALB/c, males, 12–13 weeks, 20–30 g | Ca2+ concentration in brain tissue: intact: bark cerebellum hippocampus brain stem | +10% +15% +350% +75% | 50 — — — | 1 mT — — — | <1 nT — — — | 10 h — — — | 8 — — — | One-way ANOVA, least significant difference (LSD) test | Helmholtz coils (1 axis) | Ø 40 cm | Magnetometer, one point GMF, magnetic force lines were parallel to the horizontal component of the local GMF | 0.1 | [136] |
| Ca2+ concentration in brain tissue against the background of calcium channel blocker Amlodipine: bark cerebellum hippocampus brain stem | N/A +8% N/A N/A | — — — — | — — — — | — — — — | — — — — | — — — — | ||||||||
| 51 | Rats Wistar, males, 200 g, hippocampal neurons | Electrical response: first peak amplitude, second peak amplitude | +30% +20% | 50 — | 100 μT — | <1 μT — | 180 h — | 5 — | ANOVA Tukey’s test | Solenoid (1 axis) | Ø 20 cm | Magnetometer, one point | 0.85 | [141] |
| 52 | Rats Wistar, males, 21 days, hippocampus | Ca2+ concentration in cells | +200% +300% | 50 — | 50 100 μT | 39 μT — | 90 days — | 3 — | Student’s t-test | Helmholtz coils (3 axes) | 0.5 × 0.5 × 0.5 m | GMF vertical 15.89 ± 0.14 μT horizontal 39.43 ± 0.01 μT | 0.8 | [206] |
| Enzyme activities: Protein kinase C | +15% +50% | — — | 50 100 μT | — — | — — | — — | ||||||||
| Protein kinase A | −55% −75% | — — | 50 100 μT | — — | — — | — — | ||||||||
| Ca2+–calmodulin-dependent protein kinase | +50% +75% | — — | 50 100 μT | — — | — — | — — | ||||||||
| Calcineurin specific activity | N/A N/A | — — | 50 100 μT | — — | — — | — — | ||||||||
| Phosphotases (total) | N/A | — | 50 μT | — — | — — | — — | ||||||||
| Ligand binding NMDAR (3H-L-glutamine) | −25% | — | 100 μT | — — | — — | — — | ||||||||
| 53 | Children living in Mexico City: diagnosed with B-line acute lymphoblastic leukemia and healthy. Age in both groups 16 years | B-lineage acute lymphoblastic leukemia risks (case/control ratio) | +26% +53% +87% +80% +123% | 50- 60 — — — | <200 ≥300 ≥400 ≥500 ≥600 nT | 45 μT | >1 years | 290 407 | Unadjusted ORs, adjusted odds ratios (aORs), and 95% CI were calculated using unconditional logistic regression analysis | ELF-MF in bedrooms | Not applicable | Not applicable | 0.42 | [32] |
| 54 | Honey bees Apis mellifera, from 4 hives | Absolute wing flapping frequency | N/A N/A N/A | 50 — — | 0.1 1 7 mT | 0 μT | 15 min — — — | 120 | One-way and two-way ANOVA, Bonferroni post hoc test | Helmholtz coils (1 axis) | Ø 25 cm | Magnetometer, 3D map ELF-MF ≫ GMF | 0.97 | [131] |
| Proportion of bees successfully trained to forage | −80% | — | 0.1 mT | — | — | — | ||||||||
| 55 | Locust Schistocerca gregaria, 4–9 days, male and females | Absolute wing flapping frequency (slow flying insects) | +20% +5% +10% | 50 — — | 0.1 1 7 mT | <10 μT — — | 10 min — — | 162 — — | Kruskal–Wallis test as the data failed the Brown–Forsythe test, one-way and two-way ANOVA | Helmholtz coils (1 axis) | Ø 25 cm | Magnetometer, 3D map ELF-MF ≫ GMF | 0.42 | [132] |
| Absolute wing flapping frequency (fast flying insects) | −5% −15% −20% | 50 — — | 0.1 1 7 mT | — — — | — — — | — — — | ||||||||
| 56 | Rats Sprague–Dawley, 200–250 g, age 8 weeks | Body mass | N/A N/A N/A | 50 — — | 30 100 500 μT | <10 nT — — | 24 weeks— | 30 — — | One-way ANOVA | Helmholtz coils | 2000× 700× 2000 mm | Magnetometer, 3D map | 0.42 | [222] |
| Water consumption | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Count of the red blood cells | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Protein expression: alanine transaminase, | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| aspartate aminotransferase | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Concentration of micro- and macroelements: Cr | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Ca2+ | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Mg2+ | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Blood urea nitrogen | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Ultrastructure of the kidneys | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Ultrastructure of the liver | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| H2O2 concentration | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| NO concentration | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| Catalase activity | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| SOD activity | N/A N/A N/A | — — — | — — — | — — — | — — — | — — — | ||||||||
| 57 | Sunflower and wheat seedlings | Fresh biomass of sunflowers: Whole plant, Shoots, Roots | +12% +15% +5% | 16.6 — — | 20 μT — — | ~45 μT — — | 12 days — — | 6 — — | Kruskal–Wallis test | Helmholtz coils (1 axis) | Ø 60 cm | Magnetometer, oscilloscope 1 point, temperature variation <0.1% | 0.42 | [150] |
| Fresh biomass of wheat seedlings (whole plant) | −50% | — | — | — | — | — | ||||||||
| 58 | Human Electric train drivers, 40–55 years old, men | Heat rate | −5% | 16.6 | 1.5 μT | 38 μT | 24 h | 7 | Student’s t-test (pilot study) | Workplace | Not applicable | Not applicable | 0.42 | [223] |
| HRV: LF HF | +6% +5% | — — | — — | — — | — — | — — | ||||||||
| 59 | Cardiomyocytes (hiPS line) | Electrical response to Verapamil | N/A | 50 | 400 mT | 0 mT | 60 s | 200 | Student’s t-test | Helmholtz coils (1 axis) iron shield | 50 × 50 cm | Magnetometer, 1 point, variation < 5% | 0.98 | [224] |
| 60 | Human cord blood cells CD34+ pluripotent stem cells | Myeloid differentiation Lymphoid differentiation | N/A N/A | 50 — | 300 mT | 45 μT — | 35 days — | 4 — | Student’s t-test | Helmholtz coils (1 axis) | 50 × 50 cm | Magnetometer, 1 point, variation < 5% | 0.42 | [173] |
| 61 | Mice BALB/c, 22–25 g Peritoneal neutrophils | Membrane peroxidation | +10.2% | 1 +4.4 +16.5 | 600+ 100+ 160 nT | 42 μT | 1 h | 3 | Student’s t-test | Helmholtz coils (2 axes) | Ø 120 cm | Magnetometer, 1 point, variation < 2% GMF~42 μT TVMF 50 Hz 15–50 nT | 0.18 | [126] |
| fMLF-induced ROS generation | +200% | — | — | — | — | — | ||||||||
| 62 | Mice CD-1, males, 22–25 g Peritoneal neutrophils | fMLF-induced ROS generation after cell treatment | +36% | 12.6+ 48.5 | 100 nT | 60 μT | 1 h | 3 | Mann–Whitney test (continuity correction) Benjamini–Hochberg’s correction | Solenoid in a shell made of soft magnetic material | Ø 18 × 36 cm | Magnetometer, 1 point, variation TVMF 50 Hz <5 nT, SMF <10 nT GMF ~44 μT TVMF 50 Hz 15–50 nT | 0.49 | [127] |
| 63 | Mice BALB/c Age 8–10 weeks (25–27 г) Ehrlich ascitic carcinoma | TNF-α secretion: macrophages | −19% | (5.10+ 5.26+ 5.91+ 6.26+ 6.31+ 6.98) | 100 nT | 60 μT | 28 h | 30 | Student’s t-test | Helmholtz coils (2 axes) | Ø 140 cm | Magnetometer, 1 point, variation <2% GMF ~37 μT | 0.4 | [128] |
| fMLF-induced generation of ROS after addition of MF-treated water | +66% | — | — | — | — | — | ||||||||
| TNF-α secretion by macrophages | +270% | — | — | — | — | — | ||||||||
| TNF-α secretion by T-cells | +180% | — | — | — | — | — | ||||||||
| TNF-α secretion by whole blood | +400% | — | — | — | — | — | ||||||||
| IFN-γ secretion by macrophages | +200% | — | — | — | — | — | ||||||||
| IFN-γ secretion by T-cells | +190% | — | — | — | — | — | ||||||||
| IFN-γ secretion by whole blood | +90% | — | — | — | — | — | ||||||||
| Tumor size | −40% | — | — | — | — | — | ||||||||
| Survival rate at 50 days | +900% | — | — | — | — | — | ||||||||
| 64 | Mice Strains Tg and OBE (model of familial and sporadic Alzheimer’s disease) of the C3H and SO lines (appropriate controls) | Spatial memory test (Morris water maze): Tg, C3H, OBE, SO | +25% +25% N/A +25% | 0.38+ 4.88 — — | 80 nT — — — | 42 ± 0.1 μT — — | 40 h — — — | 5 — — — | One-way ANOVA, t-test | Helmholtz coils (1 axis) | Ø 140 × 70 cm | Magnetometer, oscilloscope 1 point, variation < 1% TVMF 50 Hz 20–40 nT GMF~37 μT | 0.4 [144] | |
| Brain Aβ amyloid concentration: Tg, OBE | −25% −50% | — — | — — | — — | — — | — — | ||||||||
| 65 | Spinach Spinacia oleracea 4–5 weeks, insulated membranes | Ca2+ permeability | −6% +4% −9% −4% +9% +15% +4% −5% +5% +5% +1% −4% −1% | 9 16.7 20 25.5 — — — — 30 40 50 60 80 | 25.9 μT — — 20.3 21.0 21.7 22.4 25.9 μT — — — | 37 μT — — 29 30 31 32 37 μT | 1 h — — — — — — — — — — — — | 5 | Student’s t-test | Helmholtz coils (2 axes) | - | Magnetometer, oscilloscope 1 point, variation <2.5% | 0.42 | [202] |
| 66 | Granulocytes differentiated from polypotent CD34+ umbilical cord blood cells | Cell death | +50% | 50 | 1 mT | ~1 nT | 72 h | 3 | Wilcoxon rank-sum test | Helmholtz coils (1 axis) in μ-metallic chamber | 15 × 15 cm | Magnetometer, oscilloscope 1 point, variation < 1%, GMF shielded with μ-metal chamber | 0.97 | [125] |
| Apoptosis | +20% | — | — | — | — | — | ||||||||
| Length of cell cycle phases | N/A | — | — | — | — | — | ||||||||
| Proportion of genes with increased expression | +2% | — | — | — | — | — | ||||||||
| Proportion of genes with reduced expression | +1.5% | — | — | — | — | — | ||||||||
| DNA methylation | −5% | — | — | — | — | — | ||||||||
| 67 | Umbilical Cord Blood Lymphocytes | Cell viability | −15% −16% | 7.8 — | 6.6 12 μT | 4 μT | 72 h | 3 | ANOVA, post hoc Fisher LSD | Coils (2 axes) | 20 × 20 cm | Magnetometer, 3D map, variation < 8%, GMF 33.6–38 μT, GMF shielded with μ-metal chamber | 1.15 | [225] |
| 68 | Cell line U251 | Proliferation rate | +80% | 7–21 | 24 μT | 126 μT | 72 h | 3 | ANOVA | Coils (2 axes) | 20 × 20 cm | Magnetometer, 3D map, variation < 1 μT GMF 33–38 μT | 1.14 | [226] |
| 69 | E. coli strains AB1157 and EMG2 | Anomalous viscosity time dependencies (AVTD) is strains: AB1157 | +26% +23% +21% | 16 30 64 | 21 μT — — | 43 μT — — | 15 min — — | 3 — — | Student’s t-test | Helmholtz coils | Ø 17.6 cm | Magnetometer, one point, variation SMF < 2%, TVMF < 5% | book 0.72 | [227,228] |
| EMG2 | +26% +21% +18% | 16 28 55 | — — — | — — — | — — — | — — — | ||||||||
| 70 | Wheat Triticum aestivum Control and Drought Conditions | Fresh, Control, Drought | N/A +90% | 14.3 — | 18 μT — | 52 μT — | 12 days — | 3 — | Student’s t-test | Helmholtz coils (1 axis) | Ø 20 cm | Magnetometer, one point | 0.79 | [151] |
| Length: Control, Drought | N/A +15% | — — | — — | — — | — — | — — | ||||||||
| Leaf Area: Control, Drought | N/A +80% | — — | — — | — — | — — | — — | ||||||||
| Photosynthesis efficiency: Control, Drought | N/A +60% | — — | — — | — — | — — | — — | ||||||||
| Water content: Control, Drought | N/A +95% | — — | — — | — — | — — | — — | ||||||||
| 71 | Bacillus Iicheniformis α-amylase immobilized on superparamagnetic particle | Enzyme activity | +28% +27% | 5 7 | 12 mT — | 50 μT — | 30 min | 3 | Student’s t-test | System of 4 coils | 10 × 10 cm | Magnetometer, one point | 0.79 | [218] |
| 72 | Fruit fly Drosophila melanogaster wild type, eggs | Mortality: eggs, larvaem, pupae, adult | +350% N/A +140% −33% | 50 — — — | 1 mT — — — | 40 μT 3 — — — | 48 h — — — | 1000 — — — | Two-way ANOVA | Helmholtz coils (1 axis) | Ø 17 cm | Magnetometer, oscilloscope one point ELF-MF— GMF | 0.42 | [156] |
| 73 | Fruit fly Drosophila melanogaster wild type and Cy/Pm mutants (curly wings and plum-colored eyes) hybrids | Percent of frequency of recessive lethal illnesses | N/A N/A | 50 — | 0.5 5 mT | 45 μT — | 500 days (40 generations) | >100 — | ANOVA, Chi-square test of goodness-of-fit, Bartlett’s test | Helmholtz coils (2 axes) | Ø 40 cm | Magnetometer, one point Induction ELF-MF— GMF | 0.43 | [157] |
| Average viability | −15% −20% | — — | 0.5 5 mT | — — | — — | — — | ||||||||
| 74 | Fruit fly Drosophila melanogaster wild type, eggs | Embryo survival | +25% +30% | 50 50 | 5 μT 40 μT | 200 nT 200 nT | 3 h | 30 30 | ANOVA, Student–Newman–Keuls, and Dunnett’s post hoc test | Helmholtz coils (1 axis) | - | Magnetometer, one point | 1.25 | [161] |
| 75 | Fruit fly Drosophila melanogaster wild type, adult | Eggs from Petri dishes: F1, F2, F3 | +100% −30% −60% | 50 — — | 2 mT ↕ — | 48 — — | 3 days | 5 | Student’s t-test | Helmholtz coils (1 axis) | Ø 17 cm | Magnetometer, one point TVMF variation < 0.2 mT GMF (not described) Temperature variation < 1.5 °C | 0.43 | [158] |
| Mature individuals: F1, F2, F3 | +22% −30% −60% | — — — | — — — | — — — | — — — | |||||||||
| Number/% of dead eggs: F1, F2, F3 | +480% +260% +160% | — — — | — — — | — — — | — — — | |||||||||
| 76 | Fruit fly Drosophila melanogaster wild type, adult | Number of F1 pupae per maternal insect Ovarian DNA fragmentation (TUNELpositive eggs): | −2.9% −3.7% −4.3% | 50 — — | 0.1↕ 1.1↕ 1.2 mT↕ | GMF — — | 48 h — — | 12 | ANOVA, Pearson’s correlation analysis | Helmholtz coils (1 axis) | Ø 25 cm | Magnetometer, oscilloscope, spatial distribution, E components 0.13 1.43 2.72 V/m Temperature variation < 1 °C | 0.65 | [159] |
| +5.7% +6.7% +7.5% | — — — | 0.1↕ 1.1↕ 1.2 mT↕ | — — — | — — — | — — — | |||||||||
| 77 | Zebrafish Danio rerio embryos | Mortality | N/A N/A N/A | 50 — — | 0.2 0.4 0.8 μT | 13 μT — — | 96 h — — | 100 — — | ANOVA, LSD test | Helmholtz coils (1 axis) | 100× 100× 50 cm | Magnetometer, spatial distribution, variation SMF < 20 nT, TVMF < 1% | 0.73 | [160] |
| Ebryo malformation | N/A N/A N/A | — — — | 0.2 0.4 0.8 | — — — | — — — | — — — | ||||||||
| Heart rate 36 h of development | −5% −15% −12% | — — — | 0.2 0.4 0.8 | — — — | — — — | — — — | ||||||||
| Hatching rate, 48 h of development | −60% −60% −50% | — — — | 0.2 0.4 0.8 | — — — | — — — | — — — | ||||||||
| 54 h of development | −60% −80% −90% | — — — | 0.2 0.4 0.8 | — — — | — — — | — — — | ||||||||
| 60 h of development | −8% −10% | — — | 0.4 0.8 mT | — — | — — | — — | ||||||||
| Gene expression: caspase-3 | +20% +20% +20% | — — — | 0.2 0.4 0.8 mT | — — — | — — — | — — — | ||||||||
| caspase-9 | +35% | — | 0.8 mT | — | — | — | ||||||||
| 78 | Glioblastoma cell line U251 and breast cancer MDA-MB-231 cell line | U251 cell proliferation rate | +12% +14% −60% −55% −40% −30% −40% | 7+14+20 7.8 — — — — | 6 24 6 10 13 17 24 | >17 μT — — — — — — | 7 days — — — — — — | 3 — — — — — — | ANOVA, Dunnet’s post hoc test | Perpendicular coils | ~130× 90 mm | Magnetometer, oscilloscope, 3D map, variation SMF < 2 μT, TVMF <100 nT GMF < 2% GMF 41.7 μT | 1.14 | [226] |
| MDA-MB-231 cell proliferation rate | −10% −15% −20% | — — — | 6 10 13 μT | — — — | — — — | — — — | ||||||||
| 79 | Human SH-SY5Y neuroblastoma cells and mouse primary cortical neurons (PCNs) | PCNs cells: p53 fold change | −10% −20% | 50 — | 1 mT — | 300 nT — | 48 h — | 3 — | Two-way ANOVA, Friedman test | Helmholtz coils (1 axis) | 38 × 12 cm | Magnetometer, 3D map, TVMF and SMF variation < 5%, temperature variation < 0.2% | 1.33 | [208] |
| SH-SY5Y cells: p53 fold change | +30% | — | — | — | 48 h | — | ||||||||
| Proportion of 5-metylcitosine in DNA | +50% | — | — | — | 4 h | — | ||||||||
| Superoxide regeration | +80% | — | — | — | 24 h | — | ||||||||
| H2O2 regeration | +120% | — | — | — | 24 h | — | ||||||||
| Expression of Btg4 (cell cycle regulator): control, DAG-treated cells | 70% N/A | — — | — — | — — | 6 h — | — — | ||||||||
| Mitochondrial potential | −30% −20% | — — | — — | — — | 24 h 48 h | — — | ||||||||
| Alpha-synuclein expression | +25% | — | — | — | 48 h | — | ||||||||
| Alpha-synuclein aggregation | +30% | — | — | — | — | — | ||||||||
| Levels of differentiation regulators miR-34b | −25% −80% −90% | — — — | — — — | — — — | 24 h 48 h 72 h | — — — | ||||||||
| miR-34c | −30% −25% | — — | — — | — — | 48 h 72 h | — — | ||||||||
| 80 | Human SH-SY5Y neuroblastoma cells | DHE-detected ROS generation (superoxide) | +20% +25% +40% | 50 — — | 1 mT — — | 300 nT — — | 24 48 72 h | 3 — — | Two-way ANOVA, Friedman test | Helmholtz coils (1 axis) | 38 × 12 cm | Magnetometer, 3D map, TVMF and SMF variation < 5%, temperature variation < 0.2% | 1.33 | [209] |
| DCF-detected ROS generation (H2O2) | +30% +70% +40% | — — — | — — — | — — — | 24 48 72 h | — — — | ||||||||
| Thiols content (antioxidants) | −20% −25% −15% | — — — | — — — | — — — | 24 48 72 h | — — — | ||||||||
| MPP+ toxin induced: proliferation inhibition | +20% | — | — | — | 72 h | — | ||||||||
| Cell death | +100% | — | — | — | — | — | ||||||||
| Apoptosis | +400% | — | — | — | — | — | ||||||||
| Caspase 3/7 activation | +200% | — | — | — | — | — | ||||||||
| 81 | Calves, adult | Melatonin concentration in saliva: winter, summer | −50% +25% | 50 — | 400 nT | 49 μT — | 80 days — | 80 — | Multivariate general linear mixed model | Custom-built coil, TVMF variation < 10 nT | - | Magnetometer, one point | 0.97 | [198] |
| 82 | Immortalized nontumorigenic human keratinocytes HaCaT | Cell number, | −30% | 60 | 1.5 mT | 0.47 μT | 144 h | 3 | Student’s t-test | Helmholtz coil | Ø 37 cm | Magnetometer, spatial distribution, variation, TVMF < 4.4%, SMF < 30 nT, Temperature variation < 0.3 °C, pH of culture medium variation < 0.02 | 0.89 | [204] |
| Number of colonies | −20% | — | — | — | — | — | ||||||||
| Cell cycle phase duration: G0/G1, S, G2/M | +30% −60% −10% | — — — | — — — | — — — | — — — | — — — | ||||||||
| Proteins levels: phospho-Chk2 (Thr68), | +100% | — | — | — | — | — | ||||||||
| p21 | +100% | — | — | — | — | — | ||||||||
| 83 | Immortalized COS7, CHO, HB2, and MEF, transformed MDA-MB-231 (MDA), HeLa, and PC3, Jurkat and REH cell lines | pERK amount in cells CHO | +50% +200% | 50 — | 7 μT 1 mT | 10 nT — | 71 min — | 3 — | Student’s t-test | sXcELF ELF-MF exposure system | No discribed | Magnetometer, one point | 0.83 | [205] |
| MEF | +500% +450% | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| HB2 | +400% +450% | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| COS7 | +200% +450% | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| HeLa | +80% +80% +90% +200% +350% | — — — — — | 7 μT 15 μT 50 μT 1 mT 10 mT | — — — — — | 71 min 15 min — — — | — — — — — | ||||||||
| Juncat | +100% +200% | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| p-p38 MAPK amount in cells COS7 | N/A N/A | — — | 7 μT 1 mT | — — | 70 min — | — — | ||||||||
| HeLa | N/A N/A | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| pJNK amount in cells COS7 | N/A N/A | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| HeLa | N/A N/A | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| pAKT amount in cells COS7 | N/A N/A | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| HeLa | N/A N/A | — — | 7 μT 1 mT | — — | — — | — — | ||||||||
| 84 | Wistar rats aged 8 weeks old, healthy or with modeled Alzheimer’s disease, hippocampal neurons | Phosphorylation level of NF-κB | +120% +40% +40% N/A | 50 — — — | 400 μT — — — | 35 μT | 6 h 7 14 28 days | 3 | ANOVA, Levene’s test for homogeneity of variances | Helmholtz coils (1 axis) | 140 × 70 cm | Magnetometer, one point, variation, TVMF <20 μT Background TVMF 50 Hz <100 nT, GMF not described | 0.79 | [207] |
| Phosphorylation level of IKK | +40% | — | — | — | 6 h | — | ||||||||
| Expression level of RKIP and TAK1 | −25% −20% −20% | — — — | — — — | — — — | 14 days 6 h 14 days | — — — | ||||||||
| RKIP/TAK1 interaction | −80% −80% −75% N/A | — — — — | — — — — | — — — — | 6 7 14 h 28 days | — — — — | ||||||||
| Behavior Morris water maze test | +30% +25% +25% +25% | — — — — | — — — — | — — — — | 6 7 14 h 28 days | — — — — | ||||||||
| Alzheimer’s disease effect in model rats | −80% −60% −75% −90% | — — — — | — — — — | — — — — | 6 7 14 h 28 days | — — — — | ||||||||
| 85 | Flax Linum bienne upper segments of stems without leaves, 2.5 cm long | Deviation of the apical end of a segment from the horizontal plane (gravitropism) | +15% +20% +32% +40% +44% +36% +29% +4% | 35.8 — — — — — — — | 32.6 41.9 60.5 74.4 83.7 97.7 130.2 158.1 μT | 46.5 — — — — — — — | 2 h | 20 | Student’s t-test | Helmholtz coils | Ø 30 cm | Magnetometer, one point, TVMF 50 Hz 5 nT | 0.18 | [55] |
| 86 | Chromaffin cell cultures from rats | Proportion of cells with neurite-like growth | +220% | 60 | 0.7 mT | 50 μT | 28 h | 6 | Student’s t-test | Helmholtz coil (1 axis) | Ø 18.32 cm | Magnetometer, spatial distribution | 0.99 | [181] |
| Neurite length | +110% | — | — | — | — | — | ||||||||
| Change in potential induced by Ca2+ curren | +110% | — | — | — | — | — | ||||||||
| KCl-evoked catecholamine release | +700% | — | — | — | — | — | ||||||||
| 87 | tT20 D16V neuronal cells | Ca2+ influx | +30% | 50 | 2 mT | 44 μT | 48 h | 500 | Student’s t-test | Solenoid | Ø 10 cm | Magnetometer, one point E = 12 V/m, temperature variation < 0.3 °C, GMG (not described) | 0.42 | [182] |
| Intracellular pH | −0.2 pH units | — | — | — | — | — | ||||||||
| Neurofilament-positive cells count: control, Nifedipine treated (Ca2+ channels antagonist), | +260% −15% | — — | — — | — — | — — | 3 — | ||||||||
| Synaptophysin protein-positive cell count | +3000% | — | — | — | — | — | ||||||||
| NF-200 gene expression | +100% | — | — | — | — | — | ||||||||
| 88 | Neural stem/progenitor cells from the brain cortices of newborn mice | Beta-III-tubulin+ cells: 6 days, 12 days | +90% +90% | 50 — | 1 mT — | 44 μT — | 24 h — | 90 — | Student’s paired and unpaired t-test | Solenoid | Ø 20 cm | Magnetometer and oscilloscope, one point, temperature 37.4 ± 0.1 °C (both control and sham incubators) | 1.29 | [175] |
| MAP2+ cells count: 6 days, 12 days | +15% +20% | — — | — — | — — | — — | — — | ||||||||
| Surface expression of Ca(v)1.2 channel | +100% | — | — | — | — | — | ||||||||
| Surface expression of Ca(v)1.3 channel | +100% | — | — | — | — | — | ||||||||
| Spontaneous Ca2+ transients frequency | +100% | — | — | — | — | — | ||||||||
| Spontaneous Ca2+ transients amplitude | +20% | — | — | — | — | — | ||||||||
| KCl-induced Ca2+ transients frequency | +25% | — | — | — | — | — | ||||||||
| Amplitude of KCl-induced Ca2+ transients | +30% | — | — | — | — | — | ||||||||
| pCREB+ cells count | +400% | — | — | — | — | — | ||||||||
| 89 | CHO-K1 cells transfected Kv1.3 channel | Whole-cell Kv1.3 steady-state conductance | +5% +10% | 20 — | 268 902 μT | 44 μT — | 1 min — | 92 44 | Wilcoxon signed-rank test | Solenoids | Ø 88 mm | Magnetometer, one point | 0.4 | [176] |
| 90 | CA1 pyramidal neurons of young Sprague–Dawley rats | Maximum current density of INa (modulus of pA/pF) | +29% +32% +38% +72% +80% +94% +147% +136% +103% +10% +71% +86% +380% +345% +312% +407% +413% +441% | 15 — — — — — — — — 50 — — — — — — — — | 0.5 — — 1 — — 2 — — 0.5 — — 1 — — 2 — — | 50 μT — — — — — — — — — — — — — — — — — | 10 20 30 10 20 30 10 20 30 10 20 30 10 20 30 10 20 30 | 5 — — — — — — — — — — — — — — — — — | ANOVA on ranks, Tukey’s post hoc test | Coils system (1 axis) | 18 × 69 mm | Magnetometer, spatial distribution, TVMF variation < 8%, ELF-MF— GMF | 0.4 | [177] |
| Maximum current density of Ik (modulus of pA/pF) | −30% −40% −30% −25% −40% −30% −30% −40% −25% −35% −20% −50% −75% −20% −40% −55% | 15 — — — — — — — — — — — — — — — | 0.5 — 1 — — 2 — — 0.5 — 1 — — 2 mT — — | — — — — — — — — — — — — — — — — | 20 30 10 20 30 10 20 30 20 30 10 20 30 10 20 30 | — — — — — — — — — — — — — — — — | ||||||||
| 91 | Neurogenic tumor cell lines (U251, A172, SH-SY5Y) and primary cultured neurogenic cells from rat embryos (astrocytes, microglia, cortical neurons) | γH2AX foci formation (all cells) | N/A | 50 | 2 mT | 50 μT | 24 h | 3 | Student’s t-test | Exposure system (sXc-ELF) on base of Helmholtz coils | - | Magnetometer, oscilloscope, one point, temperature variation <0.1°C | 0.57 | [229] |
| cell cycle phases proportion (all cells) | N/A | — | — | — | — | — | ||||||||
| cell viability (all cells) | N/A | — | — | — | — | — | ||||||||
| total dendritelength | N/A | — | — | — | — | — | ||||||||
| average dendrite branch length | N/A | — | — | — | — | — | ||||||||
| average number of branches | N/A | — | — | — | — | — | ||||||||
| 92 | Children, boys and girls, healthy or with leukemia | Risk of cancer development: leukemia | +70% | 60 | 0.1–10 μT | 50 μT | 10 years | 936 | Chi-squared test | Epidemiological study | Not applicable | Not applicable | 1.81 | [230] |
| lymphoma | +100% | — | — | — | — | — | ||||||||
| nervous system tumors | +80% | — | — | — | — | — | ||||||||
| other tumors | +90% | — | — | — | — | — | ||||||||
| 93 | Humans, adult, men and women, healthy or with leukemia | risk of cancer development | +64% +43% | 60 — | 0.25 0.12 μT | 50 μT — | 7 years — | 56 134 | Chi-square test | Epidemiological study | Not applicable | Not applicable | 1.81 | [231] |
| 94 | Children, boys and girls, <16 years old, healthy or with leukemia | Risk of cancer development: all cancer | +50% +20% +30% | 50 — — | 0.1–0.2 0.2–0.3 >0.3 | 53 μT — — | <15 years — — | 127.383 — — | Spearman rank correlations, confidence intervals, logistic regression model Mantel extension technique | Living <300 m from any of the 220 and 400 kV power lines | Not applicable | Not applicable | 1.81 | [232] |
| leukemia | +110% +50% | — — | 0.1–0.2 0.2–0.3 | — — | — — | — — | ||||||||
| lymphoma | +280% +30% | — — | >0.3 0.2–0.3 μT | — — | — — | — — | ||||||||
| 95 | Humans, adult, men and women, healthy or with cancer | Risk of cancer development: acute myeloid leukemia | +70% | 50 | >0.2 μT | 53 μT | 10–15 years | >300 | Spearman rank correlations, confidence intervals, logistic regression model Mantel extension technique | Living <300 m from any of the 220 and 400 kV power lines | Not applicable | Not applicable | 1.96 | [233] |
| chronic myeloid leukemia | +70% | — | — | — | — | — | ||||||||
| central nervous system tumors | N/A | — | — | — | — | — | ||||||||
| 96 | Humans, adult, men, electric utility workers, healthy or with cancer | Risk of cancer development: all hematopoietic malignancies, | +23% +23% | 60 — | >3.2 3 >7 | 55 μT — | years 2 — | 31.543 — | X2 test | Ontario electric utility power lines | Electric fields were >172 V/m or >345 V/m, respectively | Not applicable | 1.81 | [234] |
| non-Hodgkin’s lymphoma | +27% +29% | — — | >3.2 >7 | — — | — — | — — | ||||||||
| acute nonlymphoid leukemia | +93% +187% | — — | >3.2 >7 | — — | — — | — — | ||||||||
| acute myeloid leukemia | +287% | — | >7 | — | — | — | ||||||||
| chronic lymphoid leukemia | N/A N/A | — — | >3.2 >7 | — — | — — | — — | ||||||||
| malignant brain tumors | N/A N/A | — — | >3.2 >7 | — — | — — | — — | ||||||||
| benign brain tumors | +483% +464% | — — | >3.2 >7 | — — | — — | — — | ||||||||
| malignant melanoma | N/A N/A | — — | >3.2 >7 | — — | — — | — — | ||||||||
| stomach cancer | +123% | — | >3.2 | — | — | — | ||||||||
| lung cancer | +100% +22% | — — | >7 >7 μT | — — | — — | — — | ||||||||
3.3. Effects of Anthropogenic Fields (Epidemiological Studies)
4. Potential Mechanisms of Action of Magnetic Fields
5. Dependence of Quantitative Characteristics of Biological Effects of ELF-MFs on Their Frequency, Induction, and Duration
6. Influence of Environmental Factors
7. Biological Effects of Extremely-Low-Frequency Electrical Fields
8. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELF-MF | extremely-low-frequency magnetic fields |
| EMF | electromagnetic fields |
| EMF | electromagnetic fields |
| f | frequency |
| GMF | geomagnetic field |
| MF | magnetic fields |
| SJR | scientific journal ranking |
| SMF | static magnetic fields |
| TVMF | time-varying magnetic fields |
References
- Finlay, C.C.; Maus, S.; Beggan, C.D.; Bondar, T.N.; Chambodut, A.; Chernova, T.A.; Chulliat, A.; Golovkov, V.P.; Hamilton, B.; Hamoudi, M.; et al. International Geomagnetic Reference Field: The eleventh generation. Geophys. J. Int. 2010, 183, 1216–1230. [Google Scholar] [CrossRef]
- Malakhov, V.V.; Alekseev, V.V.; Golubkov, V.S.; Mayorov, A.G.; Rodenko, S.A.; Yulbarisov, R.F. Magnetic field in the inner near-Earth space. Uspekhi Fiz. Nauk 2022, 193, 1025–1046. [Google Scholar] [CrossRef]
- Strangway, D.W. The magnetic fields of the terrestrial planets. Phys. Earth Planet. Inter. 1977, 15, 121–130. [Google Scholar] [CrossRef]
- Dubrov, A.P. The Geomagnetic Feld and Life: Geomagnetobiology; Plenum Press: New York, NY, USA, 1978; p. 318. [Google Scholar]
- Rycroft, M.J.; Harrison, R.G.; Nicoll, K.A.; Mareev, E.A. An Overview of Earth’s Global Electric Circuit and Atmospheric Conductivity. In Planetary Atmospheric Electricity; Space Sciences Series of ISSI; Springer: Berlin/Heidelberg, Germany, 2008; pp. 83–105. [Google Scholar]
- Kane, R.P. Geomagnetic field variations. Space Sci. Rev. 1976, 18, 413–540. [Google Scholar] [CrossRef]
- Goguitchaichvili, A.; Hernández-Quintero, E.; García, R.; Cejudo, R.; Cifuentes, G.; Cervantes, M. Fluctuation of the Earth’s magnetic field elements in Mexico revealed by archive documents since 1587. Phys. Earth Planet. Inter. 2020, 300, 106433. [Google Scholar] [CrossRef]
- Erdmann, W.; Kmita, H.; Kosicki, J.Z.; Kaczmarek, Ł. How the Geomagnetic Field Influences Life on Earth—An Integrated Approach to Geomagnetobiology. Orig. Life Evol. Biosph. 2021, 51, 231–257. [Google Scholar] [CrossRef] [PubMed]
- Doglioni, C.; Pignatti, J.; Coleman, M. Why did life develop on the surface of the Earth in the Cambrian? Geosci. Front. 2016, 7, 865–873. [Google Scholar] [CrossRef]
- Vidotto, A.A. The evolution of the solar wind. Living Rev. Sol. Phys. 2021, 18, 3. [Google Scholar] [CrossRef]
- Lammer, H.; Bredehöft, J.H.; Coustenis, A.; Khodachenko, M.L.; Kaltenegger, L.; Grasset, O.; Prieur, D.; Raulin, F.; Ehrenfreund, P.; Yamauchi, M.; et al. What makes a planet habitable? Astron. Astrophys. Rev. 2009, 17, 181–249. [Google Scholar] [CrossRef]
- Tarduno, J.A.; Cottrell, R.D.; Watkeys, M.K.; Hofmann, A.; Doubrovine, P.V.; Mamajek, E.E.; Liu, D.; Sibeck, D.G.; Neukirch, L.P.; Usui, Y. Geodynamo, Solar Wind, and Magnetopause 3.4 to 3.45 Billion Years Ago. Science 2010, 327, 1238–1240. [Google Scholar] [CrossRef]
- Michalski, G.; Bhattacharya, S.K.; Girsch, G. NOx cycle and the tropospheric ozone isotope anomaly: An experimental investigation. Atmos. Chem. Phys. 2014, 14, 4935–4953. [Google Scholar] [CrossRef]
- Iwaniuk, A.; Heyers, D.; Manns, M.; Luksch, H.; Güntürkün, O.; Mouritsen, H. A Visual Pathway Links Brain Structures Active during Magnetic Compass Orientation in Migratory Birds. PLoS ONE 2007, 2, e937. [Google Scholar] [CrossRef]
- Obleser, P.; Hart, V.; Malkemper, E.P.; Begall, S.; Holá, M.; Painter, M.S.; Červený, J.; Burda, H. Compass-controlled escape behavior in roe deer. Behav. Ecol. Sociobiol. 2016, 70, 1345–1355. [Google Scholar] [CrossRef]
- Hart, V.; Malkemper, E.P.; Kušta, T.; Begall, S.; Nováková, P.; Hanzal, V.; Pleskač, L.; Ježek, M.; Policht, R.; Husinec, V.; et al. Directional compass preference for landing in water birds. Front. Zool. 2013, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Casacci, L.P.; Bianco Dolino, G.; Badolato, G.; Maffei, M.E.; Barbero, F. The Geomagnetic Field (GMF) Is Necessary for Black Garden Ant (Lasius niger L.) Foraging and Modulates Orientation Potentially through Aminergic Regulation and MagR Expression. Int. J. Mol. Sci. 2023, 24, 4387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Zhao, J.; He, J.; Xuanyuan, Z.; Pan, W.; Sword, G.A.; Chen, F.; Wan, G. Probing Transcriptional Crosstalk between Cryptochromes and Iron-sulfur Cluster Assembly 1 (MagR) in the Magnetoresponse of a Migratory Insect. Int. J. Mol. Sci. 2023, 24, 11101. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Urey, H.C. Organic compound synthesis on the primitive Earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef]
- Famiano, M.A.; Boyd, R.N.; Kajino, T.; Onaka, T. Selection of amino acid chirality via neutrino interactions with 14N in crossed electric and magnetic fields. Astrobiology 2018, 18, 190–206. [Google Scholar] [CrossRef]
- Herd, C.D.K.; Blinova, A.; Simkus, D.N.; Huang, Y.; Tarozo, R.; Alexander, C.M.O.D.; Gyngard, F.; Nittler, L.R.; Cody, G.D.; Fogel, M.L.; et al. Origin and Evolution of Prebiotic Organic Matter As Inferred from the Tagish Lake Meteorite. Science 2011, 332, 1304–1307. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for Extraterrestrial Amino-acids and Hydrocarbons in the Murchison Meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef]
- Binhi, V.N. Principles of Electromagnetic Biophysics; Fizmatlit: Moscow, Russia, 2011. [Google Scholar]
- Sarimov, R.; Binhi, V. Low-Frequency Magnetic Fields in Cars and Office Premises and the Geomagnetic Field Variations. Bioelectromagnetics 2020, 41, 360–368. [Google Scholar] [CrossRef]
- Berbri, A.; Younsi, S.; Laghbeche, M. Dust Acoustic Shock Waves in a Warm Magnetized Dusty Plasma with Kappa Distributed Electrons and Ions. Phys. Wave Phenom. 2023, 30, 378–386. [Google Scholar] [CrossRef]
- Lakhina, G.S.; Tsurutani, B.T. Electromagnetic Pulsations and Magnetic Storms. In Encyclopedia of Solid Earth Geophysics; Encyclopedia of Earth Sciences Series; Springer: Berlin/Heidelberg, Germany, 2021; pp. 354–359. [Google Scholar]
- Minamoto, Y.; Fujita, S.; Hara, M. Frequency distributions of magnetic storms and SI+SSC-derived records at Kakioka, Memambetsu, and Kanoya. Earth Planets Space 2015, 67, 191. [Google Scholar] [CrossRef]
- Gurfinkel, Y.I.; Vasin, A.L.; Pishchalnikov, R.Y.; Sarimov, R.M.; Sasonko, M.L.; Matveeva, T.A. Geomagnetic storm under laboratory conditions: Randomized experiment. Int. J. Biometeorol. 2017, 62, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Alabdulgader, A.; McCraty, R.; Atkinson, M.; Dobyns, Y.; Vainoras, A.; Ragulskis, M.; Stolc, V. Long-Term Study of Heart Rate Variability Responses to Changes in the Solar and Geomagnetic Environment. Sci. Rep. 2018, 8, 2663. [Google Scholar] [CrossRef] [PubMed]
- Alipov, Y.D.; Belyaev, I.Y. Difference in frequency spectrum of extremely-low-frequency effects on the genome conformational state of AB 1157 and EMG2 E. coli cells. Bioelectromagnetics 1996, 17, 384–387. [Google Scholar] [CrossRef]
- Zannella, S. Biological Effects of Magnetic Fields. 1998. Available online: https://cds.cern.ch/record/1246526/files/p375.pdf (accessed on 19 October 2023).
- Núñez-Enríquez, J.C.; Correa-Correa, V.; Flores-Lujano, J.; Pérez-Saldivar, M.L.; Jiménez-Hernández, E.; Martín-Trejo, J.A.; Espinoza-Hernández, L.E.; Medina-Sanson, A.; Cárdenas-Cardos, R.; Flores-Villegas, L.V.; et al. Extremely Low-Frequency Magnetic Fields and the Risk of Childhood B-Lineage Acute Lymphoblastic Leukemia in a City With High Incidence of Leukemia and Elevated Exposure to ELF Magnetic Fields. Bioelectromagnetics 2020, 41, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Gajšek, P.; Ravazzani, P.; Grellier, J.; Samaras, T.; Bakos, J.; Thuróczy, G. Review of Studies Concerning Electromagnetic Field (EMF) Exposure Assessment in Europe: Low Frequency Fields (50 Hz–100 kHz). Int. J. Environ. Res. Public Health 2016, 13, 875. [Google Scholar] [CrossRef]
- Bonato, M.; Chiaramello, E.; Parazzini, M.; Gajšek, P.; Ravazzani, P. Extremely Low Frequency Electric and Magnetic Fields Exposure: Survey of Recent Findings. IEEE J. Electromagn. RF Microw. Med. Biol. 2023, 7, 216–228. [Google Scholar] [CrossRef]
- Raz-Steinkrycer, L.S.; Dubnov, J.; Gelberg, S.; Jia, P.; Portnov, B.A. ELF-MF Exposure, Actual and Perceived, and Associated Health Symptoms: A Case Study of an Office Building in Tel Aviv-Yafo, Israel. Sustainability 2022, 14, 11065. [Google Scholar] [CrossRef]
- Choi, S.; Cha, W.; Park, J.; Kim, S.; Kim, W.; Yoon, C.; Park, J.-H.; Ha, K.; Park, D. Extremely Low Frequency-Magnetic Field (ELF-MF) Exposure Characteristics among Semiconductor Workers. Int. J. Environ. Res. Public Health 2018, 15, 642. [Google Scholar] [CrossRef] [PubMed]
- Perov, S.Y.; Bogacheva, E.V.; Bezrukavnikova, L.M.; Lazarashvili, N.A. Experimental Study of Electromagnetic Fields the Meter Band Some Indicators of Oxidative Stress. Chem. Biol. Ecol. 2015, 15, 44–48. [Google Scholar] [CrossRef]
- Perov, S.Y.; Belaya, O.V.; Balzano, Q.; Rubtsova, N.B. The problems of mobile communication electromagnetic field exposure assessment today and tomorrow. Russ. J. Occup. Health Ind. Ecol. 2020, 60, 597–599. [Google Scholar] [CrossRef]
- Fesenko, E.E.; Geletyuk, V.I.; Kazachenko, V.N.; Chemeris, N.K. Preliminary microwave irradiation of water solutions changes their channel-modifying activity. FEBS Lett. 2000, 366, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Perov, S.Y.; Belaya, O.V.; Rubtsova, N.B. The prospects for radiofrequency electromagnetic fields control approaches improvement under 5G wireless communication technologies introduction. Russ. J. Occup. Health Ind. Ecol. 2022, 62, 388–396. [Google Scholar] [CrossRef]
- Sarimov, R.; Malmgren, L.; Markova, E.; Persson, B.; Belyaev, I.Y. Nonthermal GSM Microwaves Affect Chromatin Conformation in Human Lymphocytes Similar to Heat Shock. IEEE Trans. Plasma Sci. 2004, 32, 1600–1608. [Google Scholar] [CrossRef]
- Sarimov, R.; Alipov, E.D.; Belyaev, I.Y. Fifty hertz magnetic fields individually affect chromatin conformation in human lymphocytes: Dependence on amplitude, temperature, and initial chromatin state. Bioelectromagnetics 2011, 32, 570–579. [Google Scholar] [CrossRef]
- Perov, S.Y.; Sazhina, M.V.; Konshina, T.A. The influence of the thermal load of the environment on electrical personnel using shielding personal protective equipment in open areas during the warm season. Russ. J. Occup. Health Ind. Ecol. 2023, 63, 109–115. [Google Scholar] [CrossRef]
- Garvanova, M.; Garvanov, I.; Jotsov, V.; Razaque, A.; Alotaibi, B.; Alotaibi, M.; Borissova, D. A Data-Science Approach for Creation of a Comprehensive Model to Assess the Impact of Mobile Technologies on Humans. Appl. Sci. 2023, 13, 3600. [Google Scholar] [CrossRef]
- Schuermann, D.; Mevissen, M. Manmade Electromagnetic Fields and Oxidative Stress—Biological Effects and Consequences for Health. Int. J. Mol. Sci. 2021, 22, 3772. [Google Scholar] [CrossRef]
- Misek, J.; Jakus, J.; Hamza Sladicekova, K.; Zastko, L.; Veternik, M.; Jakusova, V.; Belyaev, I. Extremely low frequency magnetic fields emitted by cell phones. Front. Phys. 2023, 11, 47. [Google Scholar] [CrossRef]
- Tuor, M.B.; Ebert, S.; Kuster, N. Assessment of ELF Exposure from GSM Handsets and Development of an Optimized RF/ELF Exposure Setup for Studies of Human Volunteers, BAG Reg. No. 2.23.02.-18/02.001778. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=a49b5a5a16bdb63252fb1b30556e8d2d8c42a4ba (accessed on 19 October 2023).
- Krylov, V.V.; Osipova, E.A. Molecular Biological Effects of Weak Low-Frequency Magnetic Fields: Frequency–Amplitude Efficiency Windows and Possible Mechanisms. Int. J. Mol. Sci. 2023, 24, 10989. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.V.; Osipova, E.A.; Talikina, M.G.; Izyumov, Y.G. The influence of magnetic fields on mitotic activity. Cytology 2017, 59, 811–819. [Google Scholar]
- Kukanov, V.Y.; Vasin, A.L.; Demin, A.V.; Schastlivtseva, D.V.; Bubeev, Y.A.; Suvorov, A.V.; Popova, J.A.; Luchitskaya, E.S.; Niiazov, A.R.; Polyakov, A.V.; et al. Effect of Simulated Hypomagnetic Conditions on Some Physiological Paremeters under 8-Hour Exposure. Experiment Arfa-19. Hum. Physiol. 2023, 49, 138–146. [Google Scholar] [CrossRef]
- IARC. Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields. 2002. In IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2002; Volume 80. [Google Scholar]
- Binhi, V.N.; Rubin, A.B. Theoretical Concepts in Magnetobiology after 40 Years of Research. Cells 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. Magnetobiology: Underlying Physical Problems; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Ermakov, A.; Afanasyeva, V.; Ermakova, O.; Blagodatski, A.; Popov, A. Effect of weak alternating magnetic fields on planarian regeneration. Biochem. Biophys. Res. Commun. 2022, 592, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Belova, N.A.; Lednev, V.V. Dependence of gravitotropic reaction in segments of flax stems on frequency and amplitude of variable components of a weak combined magnetic field. Biophysics 2000, 45, 1108–1111. [Google Scholar]
- Blackman, C.F.; Benane, S.G.; Rabinowitz, J.R.; House, D.E.; Joines, W.T. A Role for the magnetic field in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics 1985, 6, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.; Markova, E.; Johansson, F.; Jenssen, D.; Belyaev, I. Exposure to ELF magnetic field tuned to Zn inhibits growth of cancer cells. Bioelectromagnetics 2005, 26, 631–638. [Google Scholar] [CrossRef]
- Gurfinkel, Y.; Baranov, M.; Vasin, A.; Pishchalnikov, R. Evaluation of combined effects of lunar gravity simulation and the altered magnetic field on cardiovascular system of healthy volunteers. Front. Physiol. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Zilli Vieira, C.L.; Alvares, D.; Blomberg, A.; Schwartz, J.; Coull, B.; Huang, S.; Koutrakis, P. Geomagnetic disturbances driven by solar activity enhance total and cardiovascular mortality risk in 263 U.S. cities. Env. Health 2019, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Weydahl, A.; Sothern, R.B.; Cornélissen, G.; Wetterberg, L. Geomagnetic activity influences the melatonin secretion at latitude 70° N. Biomed. Pharmacother. 2000, 55, s57–s62. [Google Scholar] [CrossRef] [PubMed]
- Jaruševičius, G.; Rugelis, T.; McCraty, R.; Landauskas, M.; Berškienė, K.; Vainoras, A. Correlation between Changes in Local Earth’s Magnetic Field and Cases of Acute Myocardial Infarction. Int. J. Env. Res Public Health 2018, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Zenchenko, T.A.; Poskotinova, L.V.; Rekhtina, A.G.; Zaslavskaya, R.M. Relation between microcirculation parameters and Pc3 geomagnetic pulsations. Biophysics 2010, 55, 646–651. [Google Scholar] [CrossRef]
- Astashev, M.E.; Serov, D.A.; Gudkov, S.V. Application of Spectral Methods of Analysis for Description of Ultradian Biorhythms at the Levels of Physiological Systems, Cells and Molecules (Review). Mathematics 2023, 11, 3307. [Google Scholar] [CrossRef]
- Burda, H.; Begall, S.; Červený, J.; Neef, J.; Němec, P. Extremely low-frequency electromagnetic fields disrupt magnetic alignment of ruminants. Proc. Natl. Acad. Sci. USA 2009, 106, 5708–5713. [Google Scholar] [CrossRef] [PubMed]
- Baaken, D.; Dechent, D.; Blettner, M.; Drießen, S.; Merzenich, H. Occupational Exposure to Extremely Low-Frequency Magnetic Fields and Risk of Amyotrophic Lateral Sclerosis: Results of a Feasibility Study for a Pooled Analysis of Original Data. Bioelectromagnetics 2021, 42, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Kuritzky, A.; Zoldan, Y.; Hering, R.; Stoupel, E. Geomagnetic activity and severity of the migraine attacks. Headache 1987, 27, 87–89. [Google Scholar] [CrossRef]
- Stoupel, E. The effect of geomagnetic activity on cardiovascular parameters. Biomed. Pharmacother. 2002, 56 (Suppl. S2), 247s–256s. [Google Scholar] [CrossRef]
- Vencloviene, J.; Babarskiene, R.; Slapikas, R. The association between solar particle events, geomagnetic storms, and hospital admissions for myocardial infarction. Nat. Hazards 2013, 65, 1–12. [Google Scholar] [CrossRef]
- Greenland, S.; Sheppard, A.R.; Kaune, W.T.; Poole, C.; Kelsh, M.A. A Pooled Analysis of Magnetic Fields, Wire Codes, and Childhood Leukemia. Epidemiology 2000, 11, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Ahlbom, A.; Day, N.; Feychting, M.; Roman, E.; Skinner, J.; Dockerty, J.; Linet, M.; McBride, M.; Michaelis, J.; Olsen, J.H.; et al. A pooled analysis of magnetic fields and childhood leukaemia. Br. J. Cancer 2000, 83, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Pishchalnikov, R.Y.; Gurfinkel, Y.I.; Sarimov, R.M.; Vasin, A.L.; Sasonko, M.L.; Matveeva, T.A.; Binhi, V.N.; Baranov, M.V. Cardiovascular response as a marker of environmental stress caused by variations in geomagnetic field and local weather. Biomed. Signal Process. Control 2019, 51, 401–410. [Google Scholar] [CrossRef]
- Tankanag, A.; Chemeris, N. Application of the adaptive wavelet transform for analysis of blood flow oscillations in the human skin. Phys. Med. Biol. 2008, 53, 5967–5976. [Google Scholar] [CrossRef] [PubMed]
- Mizeva, I.; Di Maria, C.; Frick, P.; Podtaev, S.; Allen, J. Quantifying the correlation between photoplethysmography and laser Doppler flowmetry microvascular low-frequency oscillations. J. Biomed. Opt. 2015, 20, 037007. [Google Scholar] [CrossRef] [PubMed]
- Tankanag, A.; Krasnikov, G.; Mizeva, I. A pilot study: Wavelet cross-correlation of cardiovascular oscillations under controlled respiration in humans. Microvasc. Res. 2020, 130, 103993. [Google Scholar] [CrossRef] [PubMed]
- Martín-Montero, A.; Gutiérrez-Tobal, G.C.; Gozal, D.; Barroso-García, V.; Álvarez, D.; del Campo, F.; Kheirandish-Gozal, L.; Hornero, R. Bispectral Analysis of Heart Rate Variability to Characterize and Help Diagnose Pediatric Sleep Apnea. Entropy 2021, 23, 1016. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. Nonspecific magnetic biological effects: A model assuming the spin-orbit coupling. J. Chem. Phys. 2019, 151, 204101. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. Biological effects of the hypomagnetic field: An analytical review of experiments and theories. PLoS ONE 2017, 12, e0179340. [Google Scholar] [CrossRef]
- Mitsutake, G.; Otsuka, K.; Oinuma, S.; Ferguson, I.; Cornélissen, G.; Wanliss, J.; Halberg, F. Does exposure to an artificial ULF magnetic field affect blood pressure, heart rate variability and mood? Biomed. Pharmacother. 2004, 58 (Suppl. S1), S20–S27. [Google Scholar] [CrossRef]
- Gurfinkel, Y.I.; Kuleshova, V.P.; Oraevsky, V.N. Assessment of the influence of geomagnetic storms on the incidence of acute cardiovascular pathology. Biophysics 1998, 43, 654. [Google Scholar]
- Pikin, D.A.; Gurfinkel, Y.I.; Oraevsky, V.N. The influence of geomagnetic disturbances on the blood coagulation system in patients with coronary heart disease and the possibility of drug correction. Biophysics 1998, 43, 617–622. [Google Scholar]
- Stepanova, T.Y.; Nikolaeva, A.V.; Kurmaev, D.P. The influence of geomagnetic disturbances on the aggregation function of platelets in elderly and senile people suffering from coronary artery disease. Clin. Fundam. Asp. Gerontol. 2015, 330–335. [Google Scholar]
- Stoupel, E. Solar-terrestrial prediction: Aspects for preventive medicine. In Solar-Terrestrial Predications Workshop; Boulder, Co., National Ocean Atmospheric Administration: Boulder, CO, USA, 1980; pp. 29–40. [Google Scholar]
- Joshua, H.; Stoupel, E. Geomagnetic activity influences on coagulation system in humans. In Proceedings of the VIII World Congress of Cardiology, Tokyo, Japan, 17–23 September 1978; p. 379. [Google Scholar]
- Stoupel, E.; Joshua, H.; Lahav, J. Human blood coagulation and geomagnetic activity. Europ. J. Int. Med. 1996, 7, 217–220. [Google Scholar]
- Stoupel, E.; Shimshoni, M.; Keret, R.; Silbergeld, A.; Zoldan, Y.; Assa, S.; Gilad, I.; Raps, A.; Hod, M.; Merlob, P.; et al. Some clinical cosmobiological correlations in solar cycle 21. Solar Terrestrial Predictions. In Proceedings of the Workshop at Leura, Australia, 16–20 October 1990; pp. 152–157. [Google Scholar]
- Stoupel, E.; Keret, R.; Assa, S.; Kaufman, H.; Shimshoni, M.; Laron, Z. Secretion of growth hormone, prolactin and corticosteroids during different levels of geomagnetic activity. Neuroendocrinol. Lett. 1983, 5, 365–368. [Google Scholar]
- Stefanovska, A. Physics of the human cardiovascular system. Contemp. Phys. 2010, 40, 31–55. [Google Scholar] [CrossRef]
- Stefanovska, A.; Bracic, M.; Kvernmo, H.D. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans. Biomed. Eng. 1999, 46, 1230–1239. [Google Scholar] [CrossRef]
- Stewart, J.M.; Taneja, I.; Goligorsky, M.S.; Medow, M.S. Noninvasive Measure of Microvascular Nitric Oxide Function in Humans Using Very Low-Frequency Cutaneous Laser Doppler Flow Spectra. Microcirculation 2007, 14, 169–180. [Google Scholar] [CrossRef]
- Kolosova, N.G.; Geppe, N.A.; Lozko, N.I.; Denisova, V.D.; Gerasimov, A.N.; Sidorov, V.V. Laser Doppler Flowmetry in Microcirculation Assessment in Children with Bronchial Asthma. Doctor. Ru 2018, 149, 37–41. [Google Scholar] [CrossRef]
- Tikhonova, I.V.; Tankanag, A.V.; Chemeris, N.K. Time–amplitude analysis of skin blood flow oscillations during the post-occlusive reactive hyperemia in human. Microvasc. Res. 2010, 80, 58–64. [Google Scholar] [CrossRef]
- Iskhakova, I.S.; Ruyatkina, L.A.; Nikolaev, K.Y.; Ruyatkin, D.S. Myogenic vasomotions in postmenopausal women with normoglycemia, prediabetes and diabetes mellitus type 2. Reg. Blood Circ. Microcirc. 2016, 15, 36–43. [Google Scholar] [CrossRef]
- Tikhonova, I.V.; Kosyakova, N.I.; Tankanag, A.V.; Chemeris, N.K. Oscillations of Skin Microvascular Blood Flow in Patients with Asthma. Microcirculation 2016, 23, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Velasco, M.; Ruiz-Hurtado, G.; Gómez, A.M.; Rueda, A. Ca2+ handling alterations and vascular dysfunction in diabetes. Cell Calcium 2014, 56, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric Oxide Synthesis Is Reduced in Subjects With Type 2 Diabetes and Nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, I.V.; Tankanag, A.V.; Guseva, I.E.; Grinevich, A.A. Analysis of interactions between cardiovascular oscillations for discrimination of early vascular disorders in arterial hypertension and type 2 diabetes. Biomed. Signal Process. Control 2023, 79, 104222. [Google Scholar] [CrossRef]
- Brazhe, A.R.; Brazhe, N.A.; Sosnovtseva, O.V.; Pavlov, A.N.; Mosekilde, E.; Maksimov, G.V. Wavelet-based analysis of cell dynamics measured by interference microscopy. Comput. Res. Model. 2009, 1, 77–83. [Google Scholar] [CrossRef]
- Ghione, S.; Mezzasalma, L.; Del Seppia, C.; Papi, F. Do geomagnetic disturbances of solar origin affect arterial blood pressure? J. Hum. Hypertens. 1998, 12, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, S.; Angelov, I.; Petrova, E. Solar and geomagnetic activity effects on heart rate variability. Nat. Hazards 2013, 69, 25–37. [Google Scholar] [CrossRef]
- Kember, G.C.; Fenton, G.A.; Collier, K.; Armour, J.A. Aperiodic stochastic resonance in a hysteretic population of cardiac neurons. Phys. Rev. E 2000, 61, 1816–1824. [Google Scholar] [CrossRef]
- Kember, G.C.; Fenton, G.A.; Armour, J.A.; Kalyaniwalla, N. Competition model for aperiodic stochastic resonance in a Fitzhugh-Nagumo model of cardiac sensory neurons. Phys. Rev. E 2001, 63, 041911. [Google Scholar] [CrossRef]
- Bigger, J.T.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Freedland, K.E.; Stein, P.K.; Miller, G.E.; Steinmeyer, B.; Rich, M.W.; Duntley, S.P. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J. Psychosom. Res. 2007, 62, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Lampert, R.; Bremner, J.D.; Su, S.; Miller, A.; Lee, F.; Cheema, F.; Goldberg, J.; Vaccarino, V. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am. Heart J. 2008, 156, 759.e1–759.e7. [Google Scholar] [CrossRef] [PubMed]
- Stoupel, E.; Wittenberg, C.; Zabludowski, J.; Boner, G. Ambulatory blood pressure monitoring in patients with hypertension on days of high and low geomagnetic activity. J. Hum. Hypertens. 1995, 9, 293–294. [Google Scholar] [PubMed]
- Mikhaylis, A.A.; Mikulyak, N.I.; Vershinina, O.D. Influence of solar flare activity and geomagnetic storms on the manifestation cyclicity of cerebral and coronary vascular catastrophes. Univ. Proc. Volga Reg. Med. Sci. 2019, 2, 152–163. [Google Scholar] [CrossRef]
- Vencloviene, J.; Antanaitiene, J.; Babarskiene, R. The association between space weather conditions and emergency hospital admissions for myocardial infarction during different stages of solar activity. J. Atmos. Sol.-Terr. Phys. 2016, 149, 52–58. [Google Scholar] [CrossRef]
- Vencloviene, J.; Radisauskas, R.; Vaiciulis, V.; Kiznys, D.; Bernotiene, G.; Kranciukaite-Butylkiniene, D.; Tamosiunas, A. Associations between Quasi-biennial Oscillation phase, solar wind, geomagnetic activity, and the incidence of acute myocardial infarction. Int. J. Biometeorol. 2020, 64, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Stoupel, E.; Hod, M.; Shimshoni, M.; Friedman, S.; Ovadia, J.; Keith, L. Monthly cosmic activity and pregnancy induced hypertension. Clin Exp Obs. Gynecol 1990, 17, 7–12. [Google Scholar]
- Stoupel, E. Cardiac arrhythmia and geomagnetic activity. Indian Pacing Electrophysiol. J. 2006, 6, 49–53. [Google Scholar]
- Gordon, C.; Berk, M. The effect of geomagnetic storms on suicide. S. Afr. Psychiatry Rev. 2003, 6, 24–27. [Google Scholar]
- Spivak, A.; Rybnov, Y.S.; Riabova, S.; Kharlamov, V. Wave Disturbances in the Near-Surface Atmosphere during Magnetic Storms. In Doklady Earth Sciences; Pleiades Publishing: New York, NY, USA, 2021; pp. 487–490. [Google Scholar]
- Kleimenova, N.; Kozyreva, O.; Michnowski, S.; Kubicki, M. Effect of magnetic storms in variations in the atmospheric electric field at midlatitudes. Geomagn. Aeron. 2008, 48, 622–630. [Google Scholar] [CrossRef]
- Alabdulgade, A.; Maccraty, R.; Atkinson, M.; Vainoras, A.; Berškienė, K.; Mauricienė, V.; Daunoravičienė, A.; Navickas, Z.; Šmidtaitė, R.; Landauskas, M. Human heart rhythm sensitivity to earth local magnetic field fluctuations. J. Vibroengineer. 2015, 17, 3271–3278. [Google Scholar]
- Sastre, A.; Graham, C.; Cook, M.R. Brain frequency magnetic fields alter cardiac autonomic control mechanisms. Clin. Neurophysiol. 2000, 111, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Stoupel, E.; Martfel, J.; Rotenberg, Z. Paroxysmal atrial fibrillation and stroke in males and females above and below age 65 on days of different geomagnetic activity levels. J. Basic Clin. Physiol. Pharmacol. 1994, 5, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, G.; Otsuka, K.; Hayakawa, M.; Sekiguchi, M.; Cornélissen, G.; Halberg, F. Does Schumann resonance affect our blood pressure? Biomed. Pharmacother. 2005, 59, S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhu, H.; Gao, C.; Shi, M.; Yang, D.; Jin, M.; Wang, F.; Sui, X. System-level biological effects of extremely low-frequency electromagnetic fields: An in vivo experimental review. Front. Neurosci. 2023, 17, 1247021. [Google Scholar] [CrossRef] [PubMed]
- Klimek, A.; Rogalska, J. Extremely Low-Frequency Magnetic Field as a Stress Factor—Really Detrimental?—Insight into Literature from the Last Decade. Brain Sci. 2021, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Cicek, F.; Tastekin, B.; Baldan, I.; Tokus, M.; Pelit, A.; Ocal, I.; Gunay, I.; Ogur, H.U.; Cicek, H. Effect of 40 Hz Magnetic Field Application in Posttraumatic Muscular Atrophy Development on Muscle Mass and Contractions in Rats. Bioelectromagnetics 2022, 43, 453–461. [Google Scholar] [CrossRef]
- Kanat, E.; Alp, A.; Yurtkuran, M. Magnetotherapy in hand osteoarthritis: A pilot trial. Complement. Ther. Med. 2013, 21, 603–608. [Google Scholar] [CrossRef]
- Binboğa, E.; Tok, S.; Munzuroğlu, M. The Short-Term Effect of Occupational Levels of 50 Hz Electromagnetic Field on Human Heart Rate Variability. Bioelectromagnetics 2021, 42, 60–75. [Google Scholar] [CrossRef]
- Cuppen, J.J.M.; Gradinaru, C.; Raap-van Sleuwen, B.E.; de Wit, A.C.E.; van der Vegt, T.A.A.J.; Savelkoul, H.F.J. LF-EMF Compound block type signal activates human neutrophilic granulocytes in vivo. Bioelectromagnetics 2022, 43, 309–316. [Google Scholar] [CrossRef]
- Zastko, L.; Makinistian, L.; Moravčíková, A.; Jakuš, J.; Belyaev, I. Effect of Intermittent ELF MF on Umbilical Cord Blood Lymphocytes. Bioelectromagnetics 2020, 41, 649–655. [Google Scholar] [CrossRef]
- Manser, M.; Sater, M.R.A.; Schmid, C.D.; Noreen, F.; Murbach, M.; Kuster, N.; Schuermann, D.; Schär, P. ELF-MF exposure affects the robustness of epigenetic programming during granulopoiesis. Sci. Rep. 2017, 7, 43345. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.V.; Yablokova, E.V.; Novikov, G.V.; Fesenko, E.E. The role of lipid peroxidation and myeloperoxidase in priminga respiratory burst in neutrophils under the actionof combined constant and alternating magnetic fields. Biophysics 2017, 62, 926–931. [Google Scholar] [CrossRef]
- Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. The Role of Water in the Effect of Weak Combined Magnetic Fields on Production of Reactive Oxygen Species (ROS) by Neutrophils. Appl. Sci. 2020, 10, 3326. [Google Scholar] [CrossRef]
- Novoselova, E.G.; Novikov, V.V.; Lunin, S.M.; Glushkova, O.V.; Novoselova, T.V.; Parfenyuk, S.B.; Novoselov, S.V.; Khrenov, M.O.; Fesenko, E.E. Effects of low-level combined static and weak low-frequency alternating magnetic fields on cytokine production and tumor development in mice. Electromagn. Biol. Med. 2018, 38, 74–83. [Google Scholar] [CrossRef]
- Thomas, J.R.; Schrot, J.; Liboff, A.R. Low-intensity magnetic fields alter operant behavior in rats. Bioelectromagnetics 1986, 7, 349–357. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Padmanabha, S.; Boyce, C.K.; Oglesby, J. Measurement of the Threshold Sensitivity of Honeybees to Weak, Extremely Low-Frequency Magnetic Fields. J. Exp. Biol. 1997, 200, 1363–1368. [Google Scholar] [CrossRef]
- Shepherd, S.; Lima, M.A.P.; Oliveira, E.E.; Sharkh, S.M.; Jackson, C.W.; Newland, P.L. Extremely Low Frequency Electromagnetic Fields impair the Cognitive and Motor Abilities of Honey Bees. Sci. Rep. 2018, 8, 7923. [Google Scholar] [CrossRef]
- Shepherd, S.; Jackson, C.W.; Sharkh, S.M.; Aonuma, H.; Oliveira, E.E.; Newland, P.L. Extremely Low-Frequency Electromagnetic Fields Entrain Locust Wingbeats. Bioelectromagnetics 2021, 42, 296–308. [Google Scholar] [CrossRef]
- Acosta-Avalos, D.; Pinho, A.T.; de Souza Barbosa, J.; Belova, N. Alternating magnetic fields of 60 Hz affect magnetic orientation and magnetosensitivity of fire ants. J. Insect Behav. 2015, 28, 664–673. [Google Scholar] [CrossRef]
- Lai, H.; Levitt, B.B. Cellular and molecular effects of non-ionizing electromagnetic fields. Rev. Environ. Health 2023, 1–11. [Google Scholar] [CrossRef]
- Bouisset, N.; Villard, S.; Legros, A. Vestibular Extremely Low-Frequency Magnetic and Electric Stimulation Effects on Human Subjective Visual Vertical Perception. Bioelectromagnetics 2022, 43, 355–367. [Google Scholar] [CrossRef]
- Freije, A.; Saknini, L.; Abdul Ghafoor, R. Effects of short-term exposure to (1 mT, 50 Hz) electromagnetic fields on calcium concentration in different brain regions of mice: The role of calcium channel blocker. Bahrain Med. Bull. 2015, 37, 92–96. [Google Scholar] [CrossRef]
- Dong, L.; Xia, P.; Tian, L.; Tian, C.; Zhao, W.; Zhao, L.; Duan, J.; Zhao, Y.; Zheng, Y. A Review of Aspects of Synaptic Plasticity in Hippocampus via mT Extremely Low-Frequency Magnetic Fields. Bioelectromagnetics 2023, 44, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, C.; Dong, L.; Ma, X.; Gao, Y.; Xiong, C.; Zhang, K.; Li, C. Extreme Low Frequency Electromagnetic Field Stimulation Induces Metaplastic-Like Effects on LTP/ LTD. IEEE Access 2019, 7, 152919–152927. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, J.; Dong, L.; Ma, X.; Kong, Q. Effects of exposure to extremely low frequency electromagnetic fields on hippocampal long-term potentiation in hippocampal CA1 region. Biochem. Biophys. Res. Commun. 2019, 517, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Balassa, T.; Varró, P.; Elek, S.; Drozdovszky, O.; Szemerszky, R.; Világi, I.; Bárdos, G. Changes in synaptic efficacy in rat brain slices following extremely low-frequency magnetic field exposure at embryonic and early postnatal age. Int. J. Dev. Neurosci. 2013, 31, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Komaki, A.; Khalili, A.; Salehi, I.; Shahidi, S.; Sarihi, A. Effects of exposure to an extremely low frequency electromagnetic field on hippocampal long-term potentiation in rat. Brain Res. 2014, 1564, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, G.; Gao, Y.; Lin, L.; Zheng, Y.; Cao, X.b. Exploring the form- And time-dependent effect of low-frequency electromagnetic fields on maintenance of hippocampal long-term potentiation. Eur. J. Neurosci. 2020, 52, 3166–3180. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Liu, Y.; Xie, J.-C.; Liu, N.-N.; Tian, X. Effects of repetitive transcranial magnetic stimulation on synaptic plasticity and apoptosis in vascular dementia rats. Behav. Brain Res. 2015, 281, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Bobkova, N.V.; Novikov, V.V.; Medvinskaya, N.I.; Aleksandrova, I.Y.; Nesterova, I.V.; Fesenko, E.E. Effect of weak combined static and extremely low-frequency alternating magnetic fields on spatial memory and brain amyloid-β in two animal models of Alzheimer’s disease. Electromagn. Biol. Med. 2018, 37, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (MF) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Miñano, H.L.A.; Silva, A.C.d.S.; Souto, S.; Costa, E.J.X. Magnetic Fields in Food Processing Perspectives, Applications and Action Models. Processes 2020, 8, 814. [Google Scholar] [CrossRef]
- Vashisth, A.; Meena, N.; Krishnan, P. Magnetic Field Affects Growth and Yield of Sunflower Under Different Moisture Stress Conditions. Bioelectromagnetics 2021, 42, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Belova, N.A.; Ermakov, A.M.; Znobishcheva, A.V.; Srebnitskaya, L.K.; Lednev, V.V. The influence of extremely weak alternating magnetic fields on the regeneration of planarians and the gravitropic response of plants. Biophysics 2010, 55, 704–709. [Google Scholar] [CrossRef]
- Smith, S.D.; McLeod, B.R.; Liboff, A.R.; Cooksey, K. Calcium cyclotron resonance and diatom mobility. Bioelectromagnetics 1987, 8, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Tausz, M.; Kock, M.; Grill, D. Effects of weak 16 3/2 Hz magnetic fields on growth parameters of young sunflower and wheat seedlings. Bioelectromagnetics 2004, 25, 638–641. [Google Scholar] [CrossRef]
- Mshenskaya, N.S.; Grinberg, M.A.; Kalyasova, E.A.; Vodeneev, V.A.; Ilin, N.V.; Slyunyaev, N.N.; Mareev, E.A.; Sinitsyna, Y.V. The Effect of an Extremely Low-Frequency Electromagnetic Field on the Drought Sensitivity of Wheat Plants. Plants 2023, 12, 826. [Google Scholar] [CrossRef]
- Grinberg, M.A.; Vodeneev, V.A.; Il’in, N.V.; Mareev, E.A. Laboratory Simulation of Photosynthesis in a Wide Range of Electromagnetic and Radiation Environment Parameters. Astron. Rep. 2023, 67, 71–77. [Google Scholar] [CrossRef]
- Safronova, V.G.; Vakarsina, G.S.; Chemeris, N.K. Damaging effects of magnetic fields in the early stages of embryonic development of sea urchins Strongylocentrotus intermedius. Biochem. (Mosc.) Suppl. Ser. A Membrane. Cell Biol. 1992, 9, 1169–1171. [Google Scholar]
- Li, Y.; Sun, C.; Zhou, H.; Huang, H.; Chen, Y.; Duan, X.; Huang, S.; Li, J. Extremely Low-Frequency Electromagnetic Field Impairs the Development of Honeybee (Apis cerana). Animals 2022, 12, 2420. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.L.; Mullins, J.M.; Litovitz, T.A. Thresholds for electromagnetic field-induced hypoxia protection: Evidence for a primary electric field effect. Bioelectrochemistry 2000, 52, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, E.; Monteagudo, J.L.; Garcia-Gracia, M.; Delgado, J.M.R. Oviposition and development of Drosophila modified by magnetic fields. Bioelectromagnetics 2005, 4, 315–326. [Google Scholar] [CrossRef]
- Kikuchi, T.; Ogawa, M.; Otaka, Y.; Furuta, M. Multigeneration exposure test of Drosophila melanogaster to ELF magnetic fields. Bioelectromagnetics 1998, 19, 335–340. [Google Scholar] [CrossRef]
- Gonet, B.; Kosik-Bogacka, D.I.; Kuźna-Grygiel, W. Effects of extremely low-frequency magnetic fields on the oviposition of Drosophila melanogasterover three generations. Bioelectromagnetics 2009, 30, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Karabarbounis, A.; Lioliousis, C. ELF Alternating Magnetic Field Decreases Reproduction by DNA Damage Induction. Cell Biochem. Biophys. 2013, 67, 703–716. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Liu, K.; Miao, W.; Zhou, C.; Li, Y.; Wu, H. Extremely Low-Frequency Magnetic Fields Induce Developmental Toxicity and Apoptosis in Zebrafish (Danio rerio) Embryos. Biol. Trace Elem. Res. 2014, 162, 324–332. [Google Scholar] [CrossRef]
- Ma, T.-H.; Chu, K.-C. Effect of the extremely low frequency (ELF) electromagnetic field (EMF) on developing embryos of the fruit fly (Drosophila melanogaster L.). Mutat. Res. Lett. 1993, 303, 35–39. [Google Scholar] [CrossRef]
- Verheyen, G.R.; Pauwels, G.; Verschaeve, L.; Schoeters, G. Effect of coexposure to 50 Hz magnetic fields and an aneugen on human lymphocytes, determined by the cytokinesis block micronucleus assay. Bioelectromagnetics 2003, 24, 160–164. [Google Scholar] [CrossRef]
- Simkó, M. Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem. 2007, 14, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Benane, S.G.; Kinney, L.S.; Joines, W.T.; House, D.E. Effects of ELF fields on calcium-ion efflux from brain tissue in vitro. Radiat. Res. 1982, 92, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Kinney, L.S.; House, D.E.; Joines, W.T. Multiple power-density windows and their possible origin. Bioelectromagnetics 1989, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Benane, S.G.; House, D.E.; Elliott, D.J. Importance of alignment between local DC magnetic field and an oscillating magnetic field in responses of brain tissue in vitro and in vivo. Bioelectromagnetics 1990, 11, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Benane, S.G.; House, D.E.; Joines, W.T. Effects of ELF (1–120 Hz) and modulated (50 Hz) RF fields on the efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics 1985, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. Empirical test of an ion parametric resonance model for magnetic field interactions with PC-12 cells. Bioelectromagnetics 1994, 15, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E. Effect of ac and dc magnetic field orientation on nerve cells. Biochem. Biophys. Res. Commun. 1996, 220, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Blackman, C.F.; Blanchard, J.P.; Benane, S.G.; House, D.E.; Elder, J.A. Double blind test of magnetic field effects on neurite outgrowth. Bioelectromagnetics 1998, 19, 204–209. [Google Scholar] [CrossRef]
- Fitzsimmons, R.J.; Ryaby, J.T.; Magee, F.P.; Baylink, D.J. Combined magnetic fields increased net calcium flux in bone cells. Calcif. Tissue Int. 1994, 55, 376–380. [Google Scholar] [CrossRef]
- Takahashi, M.; Furuya, N. Evaluation of the Effects of Exposure to Power-Frequency Magnetic Fields on the Differentiation of Hematopoietic Stem/Progenitor Cells Using Human-Induced Pluripotent Stem Cells. Bioelectromagnetics 2022, 43, 174–181. [Google Scholar] [CrossRef]
- Takahashi, M.; Furuya, N. Evaluation of the effects of power-frequency magnetic field exposure on b-cell differentiation from human hematopoietic stem/progenitor cells. Bioelectromagnetics 2023, 44, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.Q.; Sun, W.J.; Lu, D.Q.; Fu, Y.T.; Chiang, H. 50-Hz magnetic field induces EGF-receptor clustering and activates RAS. Int. J. Radiat. Biol. 2008, 84, 413–420. [Google Scholar] [CrossRef]
- Piacentini, R.; Ripoli, C.; Mezzogori, D.; Azzena, G.B.; Grassi, C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J. Cell Physiol. 2008, 215, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, C.; Maschietto, M.; Boccaccio, P.; Vassanelli, S. Electromagnetic field affects the voltage-dependent potassium channel Kv1.3. Electromagn. Biol. Med. 2020, 39, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xia, P.; Dong, L.; Tian, L.; Xiong, C. Effects of modulation on sodium and potassium channel currents by extremely low frequency electromagnetic fields stimulation on hippocampal CA1 pyramidal cells. Electromagn. Biol. Med. 2021, 40, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Lewis, R.; Silva, S.R.P.; McFadden, J.; Jeevaratnam, K. Effects of electromagnetic fields on neuronal ion channels: A systematic review. Ann. N. Y. Acad. Sci. 2021, 1499, 82–103. [Google Scholar] [CrossRef]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef]
- Barnes, S.; Buckner, C.A.; Buckner, A.L.; Koren, S.A.; Persinger, M.A.; Lafrenie, R.M. Inhibition of Cancer Cell Growth by Exposure to a Specific Time-Varying Electromagnetic Field Involves T-Type Calcium Channels. PLoS ONE 2015, 10, e0124136. [Google Scholar] [CrossRef]
- Morgado-Valle, C.; Verdugo-Diaz, L.; Garcia, D.E.; Morales-Orozco, C.; Drucker-Colin, R. The role of voltage-gated Ca2+ channels in neurite growth of cultured chromaffin cells induced by extremely low frequency (ELF) magnetic field stimulation. Cell Tissue Res 1998, 291, 217–230. [Google Scholar] [CrossRef]
- Lisi, A.; Ledda, M.; Rosola, E.; Pozzi, D.; D’Emilia, E.; Giuliani, L.; Foletti, A.; Modesti, A.; Morris, S.J.; Grimaldi, S. Extremely low frequency electromagnetic field exposure promotes differentiation of pituitary corticotrope-derived AtT20 D16V cells. Bioelectromagnetics 2006, 27, 641–651. [Google Scholar] [CrossRef]
- Blank, M.; Goodman, R. Electromagnetic fields stress living cells. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2009, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Liburdy, R.P.; Sloma, T.R.; Sokolic, R.; Yaswen, P. ELF magnetic fields, breast cancer, and melatonin: 60 Hz fields block melatonin’s oncostatic action on ER+breast cancer cell proliferation. J. Pineal Res. 1993, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Persson, B.R.R. Response of cells to electromagnetic fields of extremely low frequency and microwaves. In Proceedings of the International Symposium Endogenous Physical Fields in Biology, Prague, Czech Republic, 1–3 July 2002. [Google Scholar]
- Strašák, L.k.; Bártová, E.; Krejčı, J.; Fojt, L.; Vetterl, V. Effects of ELF-EMF on Brain Proteins in Mice. Electromagn. Biol. Med. 2009, 28, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Alipov, E.D. Frequency-dependent effects of ELF magnetic field on chromatin conformation in Escherichia coli cells and human lymphocytes. Biochim. Et Biophys. Acta 2001, 1526, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.; Torudd, J.; Harms-Ringdahl, M. Effects of Weak ELF on Human Lymphocytes; Munich, Germany; The BEMS: Frederick, MD, USA, 2000; pp. 169–170. [Google Scholar]
- Mahmoudinasab, H.; Sanie-Jahromi, F.; Saadat, M. Effects of extremely low-frequency electromagnetic field on expression levels of some antioxidant genes in human MCF-7 cells. Mol. Biol. Res. Commun. 2016, 5, 77–85. [Google Scholar] [PubMed]
- Blank, M.; Soo, L. The threshold for Na,K-ATPase stimulation by electromagnetic fields. Bioelectrochem. Bioenerg. 1996, 40, 63–65. [Google Scholar] [CrossRef]
- Blank, M.; Soo, L. Enhancement of cytochrome oxidase activity in 60 Hz magnetic fields. Bioelectrochem. Bioenerg. 1998, 45, 253–259. [Google Scholar] [CrossRef]
- Mullins, J.M.; Penafiel, L.M.; Juutilainen, J.; Litovitz, T.A. Dose–response of electromagnetic field-enhanced ornithine decarboxylase activity. Bioelectrochem. Bioenerg. 1999, 48, 193–199. [Google Scholar] [CrossRef]
- Lin, H.; Head, M.; Blank, M.; Han, L.; Jin, M.; Goodman, R. Myc-mediated transactivation of HSP70 expression following exposure to magnetic fields. J. Cell. Biochem. 1998, 69, 181–188. [Google Scholar] [CrossRef]
- Blank, M.; Soo, L.; Lin, H.; Henderson, A.S.; Goodman, R. Changes in transcription in HL-60 cells following exposure to alternating currents from electric fields. Bioelectrochem. Bioenerg. 1992, 28, 301–309. [Google Scholar] [CrossRef]
- Bakos, J.; Nagy, N.; Thuróczy, G.; Szabó, L.D. Urinary 6-sulphatoxymelatonin excretion is increased in rats after 24 hours of exposure to vertical 50 Hz, 100 μT magnetic field. Bioelectromagnetics 1997, 18, 190–192. [Google Scholar] [CrossRef]
- Kato, M.; Honma, K.-I.; Shigemitsu, T.; Shiga, Y. Effects of exposure to a circularly polarized 50-Hz magnetic field on plasma and pineal melatonin levels in rats. Bioelectromagnetics 1993, 14, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, S.; Abdolmaleki, P.; Movahedi, M.M. Comparing performances of logistic regression and neural networks for predicting melatonin excretion patterns in the rat exposed to ELF magnetic fields. Bioelectromagnetics 2010, 31, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kolbabová, T.; Pascal Malkemper, E.; Bartoš, L.; Vanderstraeten, J.; Turčáni, M.; Burda, H. Effect of exposure to extremely low frequency magnetic fields on melatonin levels in calves is seasonally dependent. Sci. Rep. 2015, 5, 14206. [Google Scholar] [CrossRef] [PubMed]
- Chacón, L. 50-Hz sinusoidal magnetic field effect onin vitropinealn-acetyltransferase activity. Electro-Magnetobiology 2000, 19, 339–343. [Google Scholar] [CrossRef]
- Aparicio-Bautista, D.I.; Chávez-Valenzuela, D.; Ambriz-Álvarez, G.; Córdova-Fraga, T.; Reyes-Grajeda, J.P.; Medina-Contreras, Ó.; Rodríguez-Cruz, F.; García-Sierra, F.; Zúñiga-Sánchez, P.; Gutiérrez-Gutiérrez, A.M.; et al. An Extremely Low-Frequency Vortex Magnetic Field Modifies Protein Expression, Rearranges the Cytoskeleton, and Induces Apoptosis of a Human Neuroblastoma Cell Line. Bioelectromagnetics 2022, 43, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetyan, G.; Grigoryan, A.; Dadasyan, E.; Ayrapetyan, S. The comparative study of the effects of 4 Hz Electromagnetic Fields-, Infrasound-treated and hydrogen peroxide containing physiological solutions on Na pump-induced inhibition of heart muscle contractility. Environ. 2007, 27, 483–488. [Google Scholar] [CrossRef]
- Bauréus Koch, C.L.; Sommarin, M.; Persson, B.R.; Salford, L.G.; Eberhardt, J.L. Interaction between weak low frequency magnetic fields and cell membranes. Bioelectromagnetics 2003, 24, 395–402. [Google Scholar] [CrossRef]
- Tekutskaya, E.E.; Baryshev, M.G.; Ilchenko, G.P.; Gusaruk, L.R. Oxidative damage to DNA under the action of an alternating magnetic field. Biophysics 2020, 65, 664–669. [Google Scholar] [CrossRef]
- Migliaccio, A.; Huang, C.-Y.; Chang, C.-W.; Chen, C.-R.; Chuang, C.-Y.; Chiang, C.-S.; Shu, W.-Y.; Fan, T.-C.; Hsu, I.C. Extremely Low-Frequency Electromagnetic Fields Cause G1 Phase Arrest through the Activation of the ATM-Chk2-p21 Pathway. PLoS ONE 2014, 9, e104732. [Google Scholar] [CrossRef]
- Kapri-Pardes, E.; Hanoch, T.; Maik-Rachline, G.; Murbach, M.; Bounds, P.L.; Kuster, N.; Seger, R. Activation of Signaling Cascades by Weak Extremely Low Frequency Electromagnetic Fields. Cell. Physiol. Biochem. 2017, 43, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Manikonda, P.K.; Rajendra, P.; Devendranath, D.; Gunasekaran, B.; Channakeshava; Aradhya, R.S.S.; Sashidhar, R.B.; Subramanyam, C. Influence of extremely low frequency magnetic fields on Ca2+ signaling and NMDA receptor functions in rat hippocampus. Neurosci. Lett. 2007, 413, 145–149. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, X.; Wang, D.; Li, Y.; Xu, X.; Peng, R.; Song, T. RKIP-Mediated NF-κB Signaling is involved in ELF-MF-mediated improvement in AD rat. Int. J. Med. Sci. 2018, 15, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Consales, C.; Cirotti, C.; Filomeni, G.; Panatta, M.; Butera, A.; Merla, C.; Lopresto, V.; Pinto, R.; Marino, C.; Benassi, B. Fifty-Hertz Magnetic Field Affects the Epigenetic Modulation of the miR-34b/c in Neuronal Cells. Mol. Neurobiol. 2017, 55, 5698–5714. [Google Scholar] [CrossRef] [PubMed]
- Benassi, B.; Filomeni, G.; Montagna, C.; Merla, C.; Lopresto, V.; Pinto, R.; Marino, C.; Consales, C. Extremely Low Frequency Magnetic Field (ELF-MF) Exposure Sensitizes SH-SY5Y Cells to the Pro-Parkinson’s Disease Toxin MPP+. Mol. Neurobiol. 2015, 53, 4247–4260. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Ghadiri Moghaddam, F.; Valipour, M. Insights in the biology of extremely low-frequency magnetic fields exposure on human health. Mol. Biol. Rep. 2020, 47, 5621–5633. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Chen, A.W.; Varner, J.D. A review of the mammalian unfolded protein response. Biotechnol. Bioeng. 2011, 108, 2777–2793. [Google Scholar] [CrossRef]
- Blank, M.; Soo, L. Electromagnetic acceleration of electron transfer reactions. J. Cell Biochem. 2001, 81, 278–283. [Google Scholar] [CrossRef]
- Morris, J.I.; Morrison, R.C.; Smith, D.W.; Garst, J.F. Chemically induced dynamic nuclear polarization. General solution of CKO [Closs-Kaptein-Oosterhoff] model. Applicability to reactions run in low magnetic fields. J. Am. Chem. Soc. 2002, 94, 2406–2414. [Google Scholar] [CrossRef]
- Penkov, N.V. Influence of the Combined Magnetic Field and High Dilution Technology on the Intrinsic Emission of Aqueous Solutions. Water 2023, 15, 599. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Bolotskova, P.N.; Bondarchuk, E.V.; Gryaznov, V.G.; Gudkov, S.V.; Kozlov, V.A.; Okuneva, M.A.; Ovchinnikov, O.V.; Smoliy, O.P.; Turkanov, I.F. Long-term effect of low-frequency electromagnetic irradiation in water and isotonic aqueous solutions as studied by photoluminescence from polymer membrane. Polymers 2021, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Astashev, M.E.; Serov, D.A.; Sarimov, R.M.; Gudkov, S.V. Influence of the Vibration Impact Mode on the Spontaneous Chemiluminescence of Aqueous Protein Solutions. Phys. Wave Phenom. 2023, 31, 189–199. [Google Scholar] [CrossRef]
- Blank, M. Protein and DNA reactions stimulated by electromagnetic fields. Electromagn. Biol. Med. 2008, 27, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Mizuki, T.; Watanabe, N.; Nagaoka, Y.; Fukushima, T.; Morimoto, H.; Usami, R.; Maekawa, T. Activity of an enzyme immobilized on superparamagnetic particles in a rotational magnetic field. Biochem. Biophys. Res. Commun. 2010, 393, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Mannerling, A.-C.; Simkó, M.; Mild, K.H.; Mattsson, M.-O. Effects of 50-Hz magnetic field exposure on superoxide radical anion formation and HSP70 induction in human K562 cells. Radiat. Environ. Biophys. 2010, 49, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez de la Fuente, A.O.; Alcocer-González, J.M.; Antonio Heredia-Rojas, J.; Balderas-Candanosa, I.; Rodríguez-Flores, L.E.; Rodríguez-Padilla, C.; Taméz-Guerra, R.S. Effect of 60 Hz electromagnetic fields on the activity of hsp70 promoter: An in vitro study. Cell Biol. Int. 2009, 33, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, R.R.; Bonelli, M.A.; Pedrazzi, G.; Desenzani, S.; Ghillani, M.; Fumarola, C.; Ghibelli, L.; Borghetti, A.F.; Petronini, P.G. Increased Levels of Inducible HSP70 in Cells Exposed to Electromagnetic Fields. Radiat. Res. 2006, 165, 95–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Liu, X.; Ding, L.; Wu, X.; He, M.; Hou, H.; Ruan, G.; Lai, J.; Chen, C. An Investigation Into the Effects of Long-Term 50-Hz Power-Frequency Electromagnetic Field Exposure on Hematogram, Blood Chemistry, Fibrosis, and Oxidant Stress Status in the Liver and the Kidney From Sprague–Dawley Rats. Bioelectromagnetics 2020, 41, 511–525. [Google Scholar] [CrossRef]
- Hansson Mild, K.; Bergling, R.; Hörnsten, R. Heart Rate Variability and Magnetic Field Exposure Among Train Engine Drivers—A Pilot Study. Bioelectromagnetics 2021, 42, 259–264. [Google Scholar] [CrossRef]
- Takahashi, M.; Saito, A.; Jimbo, Y.; Nakasono, S. Evaluation of the effects of power-frequency magnetic fields on the electrical activity of cardiomyocytes differentiated from human induced pluripotent stem cells. J. Toxicol. Sci. 2017, 42, 223–231. [Google Scholar] [CrossRef]
- Zastko, L.; Makinistian, L.; Tvarožná, A.; Belyaev, I. Intermittent ELF-MF Induce an Amplitude-Window Effect on Umbilical Cord Blood Lymphocytes. Int. J. Mol. Sci. 2022, 23, 14391. [Google Scholar] [CrossRef] [PubMed]
- Makinistian, L.; Marková, E.; Belyaev, I. A high throughput screening system of coils for ELF magnetic fields experiments: Proof of concept on the proliferation of cancer cell lines. BMC Cancer 2019, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Alipov, Y.D.; Harms-Ringdahl, M. Effects of weak ELF on E. coli cells and human lymphocytes: Role of genetic, physiological, and physical parameters. In Electricity and Magnetism in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 1999; pp. 481–484. [Google Scholar]
- Alipov, Y.D.; Belyaev, I.Y.; Aizenberg, O.A. Systemic reaction of Escherichia coli cells to weak electromagnetic fields of extremely low frequency. Bioelectrochem. Bioenerg. 1994, 34, 5–12. [Google Scholar] [CrossRef]
- Su, L.; Yimaer, A.; Wei, X.; Xu, Z.; Chen, G. The effects of 50 Hz magnetic field exposure on DNA damage and cellular functions in various neurogenic cells. J. Radiat. Res. 2017, 58, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, N.; Leeper, E.D. Electrical Wiring Configurations and Childhood Cancer. Am. J. Epidemiol. 1979, 109, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, N.; Leeper, E.D. Adult Cancer Related to Electrical Wires Near the Home. Int. J. Epidemiol. 1982, 11, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Feychting, M.; Alhbom, M. Magnetic Fields and Cancer in Children Residing Near Swedish High-voltage Power Lines. Am. J. Epidemiol. 1993, 138, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Feychting, M.; Ahlbom, A. Magnetic fields, leukemia, and central nervous system tumors in Swedish adults residing near high-voltage power lines. Epidemiology 1994, 5, 501–509. [Google Scholar]
- Miller, A.B.; To, T.; Agnew, D.A.; Wall, C.; Green, L.M. Leukemia following occupational exposure to 60-Hz electric and magnetic fields among Ontario electric utility workers. Am. J. Epidemiol. 1996, 144, 150–160. [Google Scholar] [CrossRef]
- Park, D.-U. Review on the Association between Exposure to Extremely Low Frequency-Magnetic Fields (ELF-MF) and Childhood Leukemia. J. Environ. Health Sci. 2023, 49, 57–65. [Google Scholar] [CrossRef]
- Feychting, M.; Ahlbom, A. Childhood leukemia and residential exposure to weak extremely low frequency magnetic fields. Environ. Health Perspect. 1995, 103, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Magnetic Fields and Cancer: Epidemiology, Cellular Biology, and Theranostics. Int. J. Mol. Sci. 2022, 23, 1339. [Google Scholar] [CrossRef] [PubMed]
- Struchen, B.; Liorni, I.; Parazzini, M.; Gängler, S.; Ravazzani, P.; Röösli, M. Analysis of personal and bedroom exposure to ELF-MFs in children in Italy and Switzerland. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Perov, S.Y.; Kon’shina, T.y.A.; Sazhina, M.V.; Levchenkov, D.I. Heart rate variability assessment during work in personal protective equipment under environmental thermal load. Russ. J. Occup. Health Ind. Ecol. 2023, 63, 308–314. [Google Scholar] [CrossRef]
- Pophof, B.; Henschenmacher, B.; Kattnig, D.R.; Kuhne, J.; Vian, A.; Ziegelberger, G. Biological Effects of Electric, Magnetic, and Electromagnetic Fields from 0 to 100 MHz on Fauna and Flora: Workshop Report. Health Phys. 2023, 124, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Prato, F.S. A physical mechanism of magnetoreception: Extension and analysis. Bioelectromagnetics 2017, 38, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. Nuclear spins in the primary mechanisms of biological action of magnetic fields. Biophysics 1995, 40, 677–691. [Google Scholar]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Buchachenko, A.L. Magnetic field-dependent molecular and chemical processes in biochemistry, genetics and medicine. Russ. Chem. Rev. 2014, 83, 1–12. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002, 298, 95–102. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Messini, N.; Karabarbounis, A.; Philippetis, A.L.; Margaritis, L.H. A Mechanism for Action of Oscillating Electric Fields on Cells. Biochem. Biophys. Res. Commun. 2000, 272, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Cortassa, S.; Aon, M.A.; Marbán, E.; Winslow, R.L.; O’Rourke, B. An Integrated Model of Cardiac Mitochondrial Energy Metabolism and Calcium Dynamics. Biophys. J. 2003, 84, 2734–2755. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.A.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Helekar, S.A. Rotating Magnetic Fields Inhibit Mitochondrial Respiration, Promote Oxidative Stress and Produce Loss of Mitochondrial Integrity in Cancer Cells. Front. Oncol. 2021, 11, 768758. [Google Scholar] [CrossRef] [PubMed]
- Sarimov, R.M.; Simakin, A.V.; Matveeva, T.A.; Gudkov, S.V.; Lyakhov, G.A.; Pustovoy, V.I.; Troitskii, A.V.; Shcherbakov, I.A. Influence of Magnetic Fields with Induction of 7 T on Physical and Chemical Properties of Aqueous NaCl Solutions. Appl. Sci. 2021, 11, 11466. [Google Scholar] [CrossRef]
- Gudkova, O.Y.; Gudkov, S.V.; Gapeyev, A.B.; Bruskov, V.I.; Rubanik, A.V.; Chemeris, N.K. The study of the mechanisms of formation of reactive oxygen species in aqueous solutions on exposure to high peak-power pulsed electromagnetic radiation of extremely high frequencies. Biophisics 2005, 50, 773–779. [Google Scholar]
- Skumiel, A.; Kopcansky, P.; Timko, M.; Molcan, M.; Paulovicova, K.; Wojciechowski, R. The influence of a rotating magnetic field on the thermal effect in magnetic fluid. Int. J. Therm. Sci. 2022, 171, 107258. [Google Scholar] [CrossRef]
- Racuciu, M.; Miclaus, S.; Creanga, D. On the thermal effect induced in tissue samples exposed to extremely low-frequency electromagnetic field. J. Environ. Health Sci. Eng. 2015, 13, 85. [Google Scholar] [CrossRef]
- Shcherbakov, I.A.; Baimler, I.V.; Gudkov, S.V.; Lyakhov, G.A.; Mikhailova, G.N.; Pustovoy, V.I.; Sarimov, R.M.; Simakin, A.V.; Troitsky, A.V. Influence of a Constant Magnetic Field on Some Properties of Water Solutions. Dokl. Phys. 2020, 65, 273–275. [Google Scholar] [CrossRef]
- Henderson, B.R.; Pfister, G.; Boeck, G.; Kind, M.; Wick, G. Expression levels of heat shock protein 60 in human endothelial cells in vitro are unaffected by exposure to 50 Hz magnetic fields. Cell Stress Chaperones 2003, 8, 172. [Google Scholar] [CrossRef]
- Afanasyev, G.N. Old and new problems in the theory of the Aharonov-Bohm effect (In Russ). Phys. Elem. Part. At. Nucl. 1990, 21, 172–250. (In Russian) [Google Scholar]
- Yang, X.; Li, Z.; Polyakova, T.; Dejneka, A.; Zablotskii, V.; Zhang, X. Effect of static magnetic field on DNA synthesis: The interplay between DNA chirality and magnetic field left-right asymmetry. FASEB BioAdvances 2020, 2, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Prato, F.S.; Carson, J.J.; Ossenkopp, K.P.; Kavaliers, M. Possible mechanisms by which extremely low frequency magnetic fields affect opioid function. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Adey, W.R. Cell and molecular biology associated with radiation fields of mobile telephones. In Review of Radio Science; Stone, W.R., Ueno, S., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 845–872. [Google Scholar]
- Liboff, A.R. Geomagnetic cyclotron resonance in living cells. J. Biol. Phys. 1985, 13, 99–102. [Google Scholar] [CrossRef]
- Liboff, A.R. Ion cyclotron resonance interactions in living systems. Soc. Ital. Biofisica Elettrodinamica 2013, 1, 1–14. [Google Scholar]
- Liboff, A.R. ION cyclotron resonance: Geomagnetic strategy for living systems? Electromagn. Biol. Med. 2019, 38, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Jenrow, K.A.; Smith, C.H.; Liboff, A.R. Weak extremely-low-frequency magnetic fields and regeneration in the planarian Dugesia tigrina. Bioelectromagnetics 1995, 16, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, O.; Babusiak, M.; Vyoral, D.; Petrák, J. Role of zinc in eukaryotic cells, zinc transporters and zinc-containing proteins. Review article. Sb. Lek. 2003, 104, 157–170. [Google Scholar]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Liboff, A.R.; Williams, T., Jr.; Strong, D.M.; Wistar, R., Jr. Time-varying magnetic fields: Effect on DNA synthesis. Science 1984, 223, 818–820. [Google Scholar] [CrossRef]
- McLeod, B.R.; Liboff, A.R. Cyclotron resonance in cell membranes: The theory of the mechanism. In Mechanistic Approaches to Interactions of Electric and Electromagnetic Fields with Living Systems; Springer: Berlin/Heidelberg, Germany, 1987; pp. 97–108. [Google Scholar]
- Ross, S.M. Combined DC and ELF magnetic fields can alter cell proliferation. Bioelectromagnetics 1990, 11, 27–36. [Google Scholar] [CrossRef]
- Halle, B. On the cyclotron resonance mechanism for magnetic field effects on transmembrane ion conductivity. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1988, 9, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.V.; Zhadin, M.N. Combined action of weak constant and variable low-frequency magnetic fields on ionic currents in aqueous solutions of amino acids. Biophysics 1994, 39, 41–45. [Google Scholar]
- Zhadin, M.N. Combined action of static and alternating magnetic fields on ion motion in a macromolecule: Theoretical aspects. Bioelectromagnetics 1998, 19, 279–292. [Google Scholar] [CrossRef]
- Blanchard, J.P.; Blackman, C.F. Clarification and application of an ion parametric resonance model for magnetic field interactions with biological systems. Bioelectromagnetics 1994, 15, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Lednev, V.V. Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 1991, 12, 71–75. [Google Scholar] [CrossRef]
- Adair, R.K. Criticism of Lednev’s mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 1992, 13, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Batcioglu, K.; Uyumlu, A.B.; Satilmis, B.; Yildirim, B.; Yucel, N.; Demirtas, H.; Onkal, R.; Guzel, R.M.; Djamgoz, M.B. Oxidative stress in the in vivo DMBA rat model of breast cancer: Suppression by a voltage-gated sodium channel inhibitor (RS100642). Basic Clin Pharmacol Toxicol 2012, 111, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Vazquez-Sanchez, A.Y.; Carrion-Robalino, N.; Camacho, J. Ion Channels and Oxidative Stress as a Potential Link for the Diagnosis or Treatment of Liver Diseases. Oxid Med. Cell Longev 2016, 2016, 3928714. [Google Scholar] [CrossRef]
- Akbarali, H.I. Oxidative Stress and Ion Channels. In Systems Biology of Free Radicals and Antioxidants; Springer: Berlin, Germany, 2014; pp. 355–373. [Google Scholar] [CrossRef]
- Panagopoulos, D.; Karabarbounis, A.; Yakymenko, I.; Chrousos, G. Human-made electromagnetic fields: Ion forced-oscillation and voltage-gated ion channel dysfunction, oxidative stress and DNA damage (Review). Int. J. Oncol. 2021, 59, 92. [Google Scholar] [CrossRef]
- Lednev, V.V. Bioeffects of weak combined, constant and alternating magnetic fields (in Russ). Biophysics 1996, 41, 224–232. (In Russian) [Google Scholar]
- Del Giudice, E.; Fleischmann, M.; Preparata, G.; Talpo, G. On the “unreasonable” effects of ELF magnetic fields upon a system of ions. Bioelectromagnetics 2002, 23, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wikfeldt, K.T.; Tokushima, T.; Nordlund, D.; Harada, Y.; Bergmann, U.; Niebuhr, M.; Weiss, T.M.; Horikawa, Y.; Leetmaa, M.; et al. The inhomogeneous structure of water at ambient conditions. Proc. Natl. Acad. Sci. USA 2009, 106, 15214–15218. [Google Scholar] [CrossRef] [PubMed]
- Taschin, A.; Bartolini, P.; Eramo, R.; Righini, R.; Torre, R. Evidence of two distinct local structures of water from ambient to supercooled conditions. Nat. Commun. 2013, 4, 2401. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.V.; Papchenkova, G.A.; Golovanova, I.L. Influence of Calcium Resonance-Tuned Low-Frequency Magnetic Fields on Daphnia magna. Int. J. Mol. Sci. 2022, 23, 15727. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, K.; Füllekrug, M. Weltweite Ortung von Blitzen: 50 Jahre Schumann-Resonanzen. Phys. Unserer Zeit 2002, 33, 256–261. [Google Scholar] [CrossRef]
- Barr, R.; Jones, D.L.; Rodger, C.J. ELF and VLF radio waves. J. Atmos. Sol.-Terr. Phys. 2000, 62, 1689–1718. [Google Scholar] [CrossRef]
- Persinger, M.A. Schumann Resonance Frequencies Found within Quantitative Electroencephalographic Activity: Implications for Earth-Brain Interactions. Int. Lett. Chem. Phys. Astron. 2014, 11, 24. [Google Scholar] [CrossRef]
- Nuñez, A.; Buño, W. The Theta Rhythm of the Hippocampus: From Neuronal and Circuit Mechanisms to Behavior. Front. Cell. Neurosci. 2021, 15, 649262. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Gondeck, A.R.; Smith, J.R. Dynamics of human sleep sigma spindles. Electroencephalogr. Clin. Neurophysiol. 1974, 37, 293–297. [Google Scholar] [CrossRef]
- Nayak, C.S.; Anilkumar, A.C. EEG Normal Waveforms. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Barone, J.; Rossiter, H.E. Understanding the Role of Sensorimotor Beta Oscillations. Front. Syst. Neurosci. 2021, 15, 655886. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. Neural Synchrony in Brain Disorders: Relevance for Cognitive Dysfunctions and Pathophysiology. Neuron 2006, 52, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M.; Saroka, K.S.; Vares, D.E.; Persinger, M.A. Similar Spectral Power Densities within the Schumann Resonance and a Large Population of Quantitative Electroencephalographic Profiles: Supportive Evidence for Koenig and Pobachenko. PLoS ONE 2016, 11, e0146595. [Google Scholar] [CrossRef]
- Kirschvink, J.L.; Walker, M.M.; Diebel, C.E. Magnetite-based magnetoreception. Curr. Opin. Neurobiol. 2001, 11, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Chernavsi, D.S. The stochastic resonance of magnetosomes fixed in the cytoskeleton. Biofizika 2005, 50, 684–688. (In Russian) [Google Scholar]
- Kirschvink, J.L.; Winklhofer, M.; Walker, M.M. Biophysics of magnetic orientation: Strengthening the interface between theory and experimental design. J. R. Soc. Interface 2010, 7 (Suppl. S2), S179–S191. [Google Scholar] [CrossRef] [PubMed]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef]
- Doktorov, A.B.; Lukzen, N.N. Magnetic Field Effect in Bimolecular Rate Constant of Radical Recombination. Int. J. Mol. Sci. 2023, 24, 7555. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Radical pairs can explain magnetic field and lithium effects on the circadian clock. Sci. Rep. 2022, 12, 269. [Google Scholar] [CrossRef]
- Gegear, R.J.; Foley, L.E.; Casselman, A.; Reppert, S.M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 2010, 463, 804–807. [Google Scholar] [CrossRef]
- Bernard, G.D.; Wehner, R. Functional similarities between polarization vision and color vision. Vis. Res. 1977, 17, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Marshak, D.W.; Mills, S.L. Short-wavelength cone-opponent retinal ganglion cells in mammals. Vis. Neurosci. 2014, 31, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.L.; Kattnig, D.R. Magnetoreception in cryptochrome enabled by one-dimensional radical motion. AVS Quantum Sci. 2023, 5, 022601. [Google Scholar] [CrossRef]
- Schulten, K.; Swenberg, C.E.; Weller, A. A Biomagnetic Sensory Mechanism Based on Magnetic Field Modulated Coherent Electron Spin Motion. Z. Für Phys. Chem. 1978, 111, 1–5. [Google Scholar] [CrossRef]
- Wiltschko, W.; Wiltschko, R. Magnetic Compass of European Robins. Science 1972, 176, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Prato, F.S. Rotations of macromolecules affect nonspecific biological responses to magnetic fields. Sci. Rep. 2018, 8, 13495. [Google Scholar] [CrossRef] [PubMed]
- Richards, O.W.; Davies, R.G. Imms’ General Textbook of Entomology: Structure, Physiology and Development, 10th ed.; Chapman and Hall: London, UK, 1977. [Google Scholar]
- Binhi, V.N. A limit in the dynamic increase in the accuracy of group migration. Biosystems 2018, 166, 19–25. [Google Scholar] [CrossRef]
- Steiner, U.E.; Ulrich, T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 2002, 89, 51–147. [Google Scholar] [CrossRef]
- Shcherbakov, I.A. Current Trends in the Studies of Aqueous Solutions. Phys. Wave Phenom. 2022, 30, 129–134. [Google Scholar] [CrossRef]
- Lyakhov, G.A.; Man’ko, V.I.; Suyazov, N.V.; Shcherbakov, I.A.; Shermeneva, M.A. Physical mechanisms of activation of radical reactions in aqueous solutions under mechanical and magnetic effect: Problem of singlet oxygen. Phys. Wave Phenom. 2022, 30, 174–181. [Google Scholar] [CrossRef]
- Afanasyeva, M.S.; Taraban, M.B.; Purtov, P.A.; Leshina, T.V.; Grissom, C.B. Magnetic Spin Effects in Enzymatic Reactions: Radical Oxidation of NADH by Horseradish Peroxidase. J. Am. Chem. Soc. 2006, 128, 8651–8658. [Google Scholar] [CrossRef] [PubMed]
- Brocklehurst, B.; McLauchlan, K.A. Free radical mechanism for the effects of environmental electromagnetic fields on biological systems. Int. J. Radiat. Biol. 1996, 69, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Afanasyeva, M.S.; Purtov, P.A.; Taraban, M.B.; Leshina, T.V. Spin chemistry of enzymatic processes. Russ. Chem. Rev. 2007, 76, 599. [Google Scholar] [CrossRef]
- Antill, L.M.; Ramsay, J.; Kattnig, D.R. Radical triads, not pairs, may explain effects of hypomagnetic fields on neurogenesis. PLoS Comput. Biol. 2022, 18, e1010519. [Google Scholar] [CrossRef]
- Shaev, I.A.; Novikov, V.V.; Yablokova, E.V.; Fesenko, E.E. A Brief Review of the Current State of Research on the Biological Effects of Weak Magnetic Fields. Biophysics 2022, 67, 245–251. [Google Scholar] [CrossRef]
- Binhi, V.N. Interference of ion quantum states within a protein explains weak magnetic field’s effect on biosystems. Electromagn. Biol. Med. 1997, 16, 203–214. [Google Scholar]
- Binhi, V.N.; Savin, A.V. Effects of weak magnetic fields on biological systems: Physical aspects. Phys. Uspekhi 2003, 46, 259–291. [Google Scholar] [CrossRef]
- Dhiman, S.K.; Galland, P. Effects of weak static magnetic fields on the gene expression of seedlings of Arabidopsis thaliana. J. Plant Physiol. 2018, 231, 9–18. [Google Scholar] [CrossRef]
- Svidlov, A.; Drobotenko, M.; Basov, A.; Gerasimenko, E.; Malyshko, V.; Elkina, A.; Baryshev, M.; Dzhimak, S. DNA Dynamics under Periodic Force Effects. Int. J. Mol. Sci. 2021, 22, 7873. [Google Scholar] [CrossRef]
- Binhi, V.N. Stochastic dynamics of magnetosomes and a mechanism of biological orientation in the geomagnetic field. Bioelectromagnetics 2006, 27, 58–63. [Google Scholar] [CrossRef]
- Hore, P.J. Upper bound on the biological effects of 50/60 Hz magnetic fields mediated by radical pairs. eLife 2019, 8, e44179. [Google Scholar] [CrossRef]
- Lewis, A.M.; Fay, T.P.; Manolopoulos, D.E.; Kerpal, C.; Richert, S.; Timmel, C.R. On the low magnetic field effect in radical pair reactions. J. Chem. Phys. 2018, 149, 034103. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, D.; Morariu, V.V. Life in zero magnetic field. Iii. Activity of aspartate aminotransferase and alanine aminotransferase during in vitro aging of human blood. Electro-Magnetobiology 2009, 20, 313–321. [Google Scholar] [CrossRef]
- Katiukhin, L.N. Rheological properties of the erythrocytes in weakened static magnetic field of the earth in vitro study. J. Sci. Res. Rep. 2019, 22, 1–12. [Google Scholar] [CrossRef]
- Binhi, V.N. Magnetobiology: Experiments and Models; MILTA: Moscow, Russia, 2002; p. 592. [Google Scholar]
- Okabe, M.; Ito, K. How to Make Figures and Presentations That Are Friendly to Color Blind People. 2002. Available online: https://jfly.uni-koeln.de/html/color_blind/ (accessed on 16 October 2023).
- Blackman, C.F.; Benane, S.G.; House, D.E. Evidence for direct effect of magnetic fields on neurite outgrowth. FASEB J. 1993, 7, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Alipov, Y.D.; Belyaev, I.Y. Effect of static magnetic field on E. coli cells and individual rotations of ion-protein complexes. Bioelectromagnetics 2001, 22, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, S.; Tallgren, P.; Andersson, S.; Sainio, K.; Voipio, J.; Kaila, K. DC-EEG discloses prominent, very slow activity patterns during sleep in preterm infants. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2002, 113, 1822–1825. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, S.; Palva, J.M.; Holmes, M.D.; Miller, J.W.; Voipio, J.; Kaila, K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc. Natl. Acad. Sci. USA 2004, 101, 5053–5057. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Shcheglov, V.S.; Alipov, E.D.; Ushakov, V.D. Nonthermal effects of extremely high-frequency microwaves on chromatin conformation in cells in vivo-dependence on physical, physiological, and genetic factors. IEEE Trans. Microw. Theory Tech. 2000, 48, 2172–2179. [Google Scholar]
- Belyaev, I.Y.; Shcheglov, V.S.; Alipov, Y.D.; Polunin, V.A. Resonance effect of millimeter waves in the power range from 10(−19) to 3 × 10(−3) W/cm2 on Escherichia coli cells at different concentrations. Bioelectromagnetics 1996, 17, 312–321. [Google Scholar] [CrossRef]
- Juutilainen, J.; Höytö, A.; Kumlin, T.; Naarala, J. Review of possible modulation-dependent biological effects of radiofrequency fields. Bioelectromagnetics 2011, 32, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Hinrikus, H.; Bachmann, M.; Lass, J.; Karai, D.; Tuulik, V. Effect of low frequency modulated microwave exposure on human EEG: Individual sensitivity. Bioelectromagnetics 2008, 29, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Alipov, Y.D.; Polunin, V.A.; Shcheglov, V.S. Evidence for dependence of resonant frequency of millimeter wave interaction with Escherichia coli K12 cells on haploid genome length. Electro-Magnetobiology 1993, 12, 39–49. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.; Yun, S.; Park, G.S.; Lee, H.J.; Song, K. Time-varying magnetic fields of 60 Hz at 7 mT induce DNA double-strand breaks and activate DNA damage checkpoints without apoptosis. Bioelectromagnetics 2012, 33, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Ivancsits, S.; Diem, E.; Pilger, A.; Rüdiger, H.W.; Jahn, O. Induction of DNA strand breaks by intermittent exposure to extremely-low-frequency electromagnetic fields in human diploid fibroblasts. Mutat. Res. 2002, 519, 1–13. [Google Scholar] [CrossRef]
- Giorgi, G.; Lecciso, M.; Capri, M.; Lukas Yani, S.; Virelli, A.; Bersani, F.; Del Re, B. An evaluation of genotoxicity in human neuronal-type cells subjected to oxidative stress under an extremely low frequency pulsed magnetic field. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 775–776, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, I.Y.; Alipov, Y.D.; Matronchik, A.Y.; Radko, S.P. Cooperativity in E. coli cell response to resonance effect of weak extremely low frequency electromagnetic field. Bioelectrochem. Bioenerg. 1995, 37, 85–90. [Google Scholar] [CrossRef]
- Balanis, C.A. Antenna Theory: Analysis and Design; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Poynting, J.H. XV. On the transfer of energy in the electromagnetic field. Philos. Trans. R. Soc. Lond. 1884, 36, 343–361. [Google Scholar]
- Tenforde, T. Biological interactions of extremely-low-frequency electric and magnetic fields. J. Electroanal. Chem. Interfacial Electrochem. 1991, 320, 1–17. [Google Scholar] [CrossRef]
- Gholipour Hamedani, B.; Goliaei, B.; Shariatpanahi, S.P.; Nezamtaheri, M. An overview of the biological effects of extremely low frequency electromagnetic fields combined with ionizing radiation. Prog. Biophys. Mol. Biol. 2022, 172, 50–59. [Google Scholar] [CrossRef]
- Wilson, J.G.; Cummins, K.L. Thunderstorm and fair-weather quasi-static electric fields over land and ocean. Atmos. Res. 2021, 257, 105618. [Google Scholar] [CrossRef]
- Mareev, E.A. Formation of Charge Layers in the Planetary Atmospheres. Space Sci. Rev. 2008, 137, 373–397. [Google Scholar] [CrossRef]
- European Commission. Non-Ionizing Radiation-Sources, Exposure and Health Effects; Office for Official Publications of the European Communities: Luxembourg, 1996. [Google Scholar]
- Maruvada, P.S. Electric field and ion current environment of HVdc transmission lines: Comparison of calculations and measurements. IEEE Trans. Power Deliv. 2011, 27, 401–410. [Google Scholar] [CrossRef]
- Hart, F.X.; Marino, A.A. Penetration of electric fields into a concentric-sphere model of biological tissue. Med. Biol. Eng. Comput. 1986, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Canaday, D.J.; Doong, H. A Review of the Biophysical Basis for the Clinical Application of Electric Fields in Soft-Tissue Repair. J. Burn Care Rehabil. 1993, 14, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Coghill, R.W.; Steward, J.; Philips, A. Extra low frequency electric and magnetic fields in the bedplace of children diagnosed with leukaemia. Eur. J. Cancer Prev. 1996, 5, 153–158. [Google Scholar] [CrossRef]
- Stoot, L.J.; Gibson, D.P.; Cooke, S.J.; Power, M. Assessing the potential for using low-frequency electric deterrent barriers to reduce lake sturgeon (Acipenser fulvescens) entrainment. Hydrobiologia 2018, 813, 223–235. [Google Scholar] [CrossRef]
- Harakawa, S.; Nedachi, T.; Suzuki, H. Extremely low-frequency electric field suppresses not only induced stress response but also stress-related tissue damage in mice. Sci. Rep. 2020, 10, 20930. [Google Scholar] [CrossRef]
- Harakawa, S.; Takahashi, I.; Doge, F.; Martin, D.E. Effect of a 50 Hz electric field on plasma ACTH, glucose, lactate, and pyruvate levels in stressed rats. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2004, 25, 346–351. [Google Scholar] [CrossRef]
- Mathewson, N.S.; Oosta, G.M.; Levin, S.G.; Ekstrom, M.E.; Diamond, S.S. Extremely low frequency (ELF) vertical electric field exposure of rats: A search for growth, food consumption and blood metabolite alterations. Compil. Navy Spons. ELF Biomed. Ecol. Res. Rep. 1977, 3, AD A035954. [Google Scholar]
- Solov’ev, N. Experimental study of the biological action of a low-frequency electric field. Nov. Meditsinskogo Priborostr. 1969, 3, 101–107. [Google Scholar]
- Altmann, G.; Warnke, U. Der Stoffwechsel von Bienen (Apis mellifica L.) im 50-Hz-Hochspannungsfeld. Z. Für Angew. Entomol. 1976, 80, 267–271. [Google Scholar] [CrossRef]
- Shinba, T.; Nedachi, T.; Harakawa, S. Alterations in Heart Rate Variability and Electroencephalogram during 20-Minute Extremely Low Frequency Electric Field Treatment in Healthy Men during the Eyes-Open Condition. IEEJ Trans. Electr. Electron. Eng. 2023, 18, 38–44. [Google Scholar] [CrossRef]
- Shinba, T.; Nedachi, T.; Harakawa, S. Extremely low-frequency electric field exposure increases theta power of EEG in both eyes-open and eyes-closed resting conditions in healthy male subjects. IEEJ Trans. Electr. Electron. Eng. 2021, 16, 592–599. [Google Scholar] [CrossRef]
- Barron, C.; Dreher, J. Effects of electric fields and negative ion concentrations on test pilots. Aerosp. Med. 1964, 35, 20–23. [Google Scholar] [PubMed]
- Haupt, R.C.; Nolfi, J.R. The effects of high voltage transmission lines on the health of adjacent resident populations. Am. J. Public Health 1984, 74, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Bartha, C.; Jipa, M.; Caramitu, A.-R.; Voina, A.; Tókos, A.; Circiumaru, G.; Micu, D.-D.; Lingvay, I. Behavior of Microorganisms from Wastewater Treatments in Extremely Low-Frequency Electric Field. Biointerface Res. Appl. Chem. 2021, 12, 5071–5080. [Google Scholar]
- Xu, L.; Chen, H.; Liang, Z.; Chen, S.; Xia, Y.; Zhu, S.; Yu, M. Effect of low-frequency electric field on microbial community structure of Pacific white shrimp (Penaeus vannamei) during ice-temperature storage. Food Bioprocess Technol. 2023, preprint. [Google Scholar]
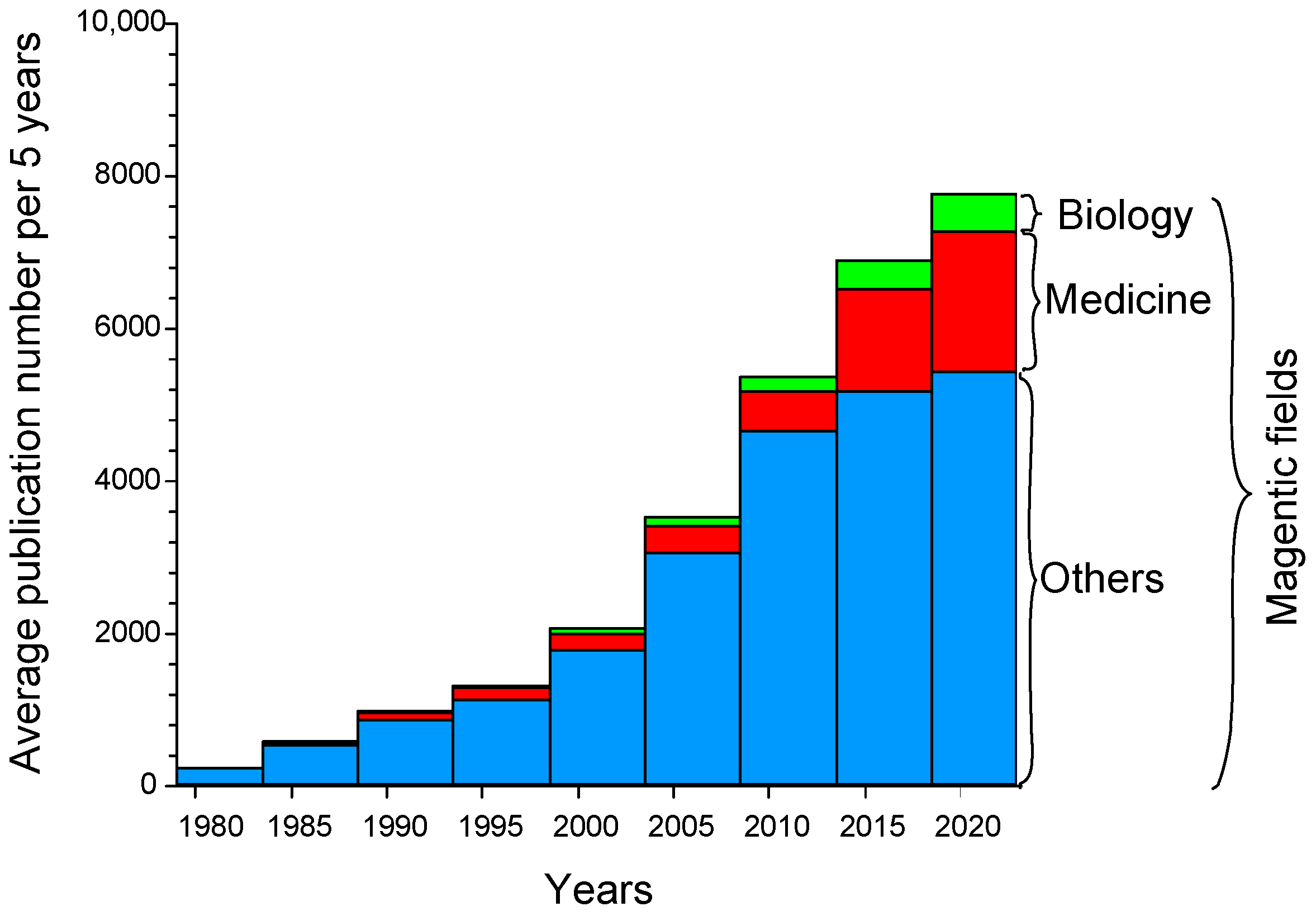
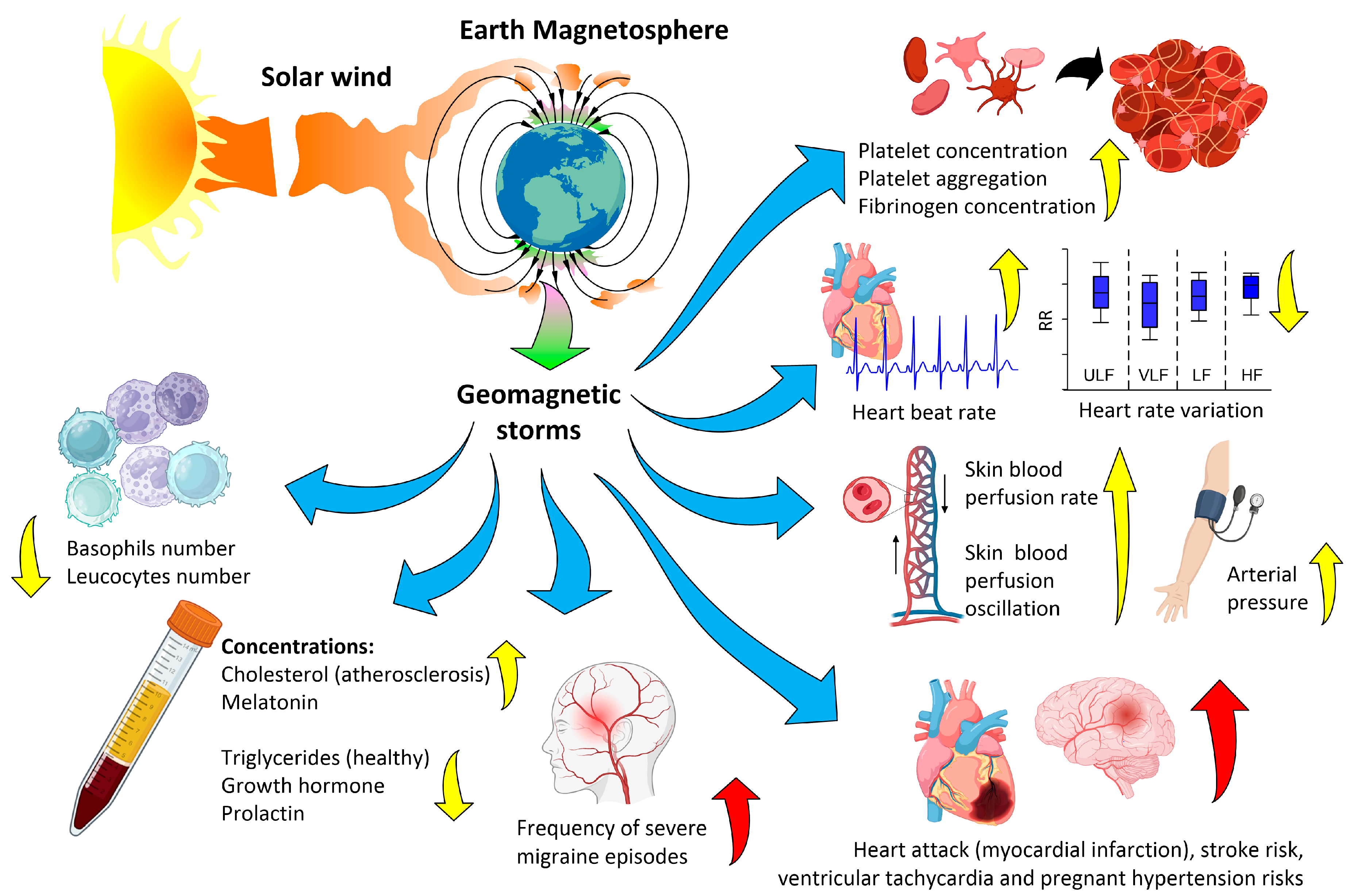
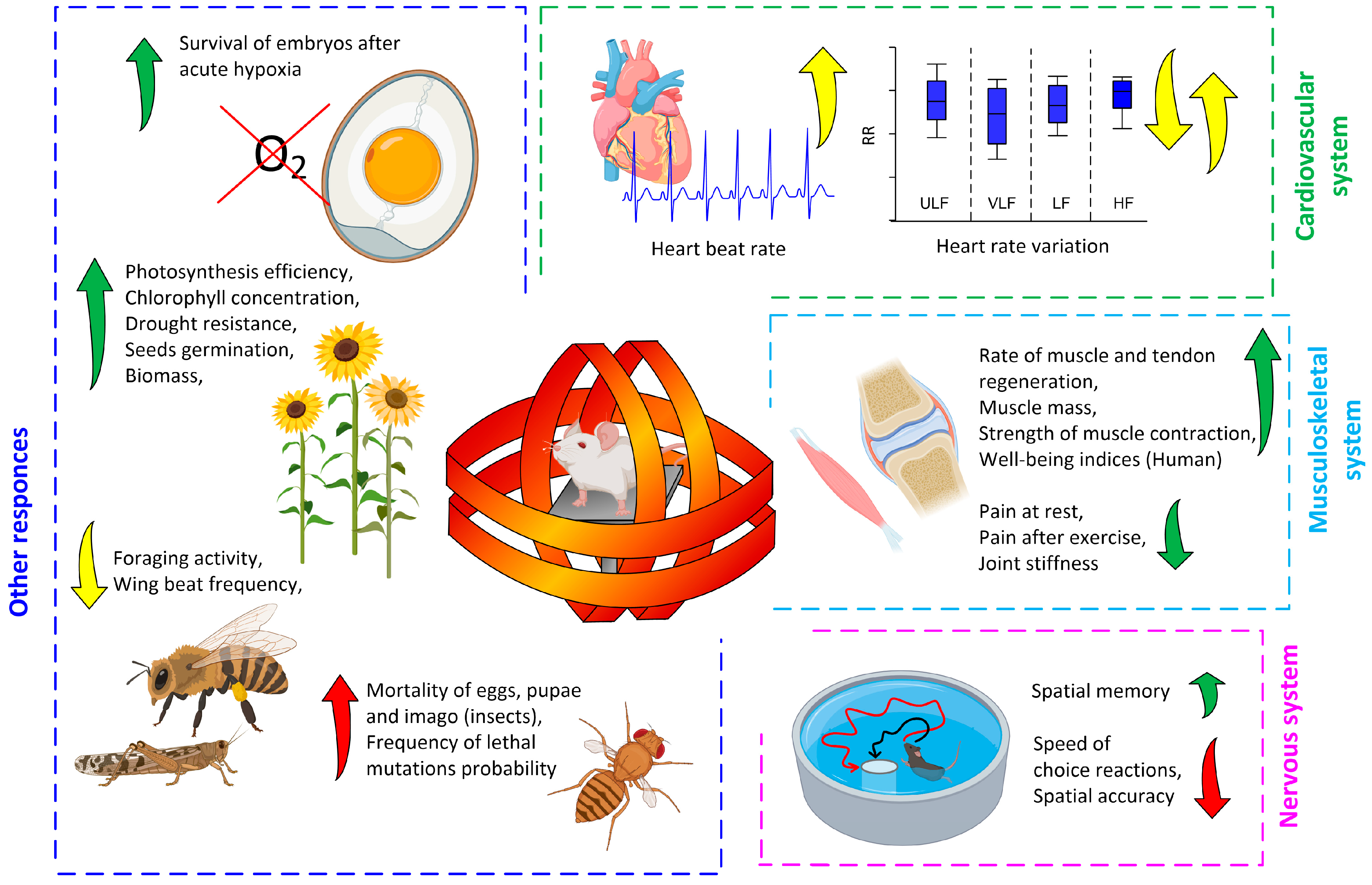
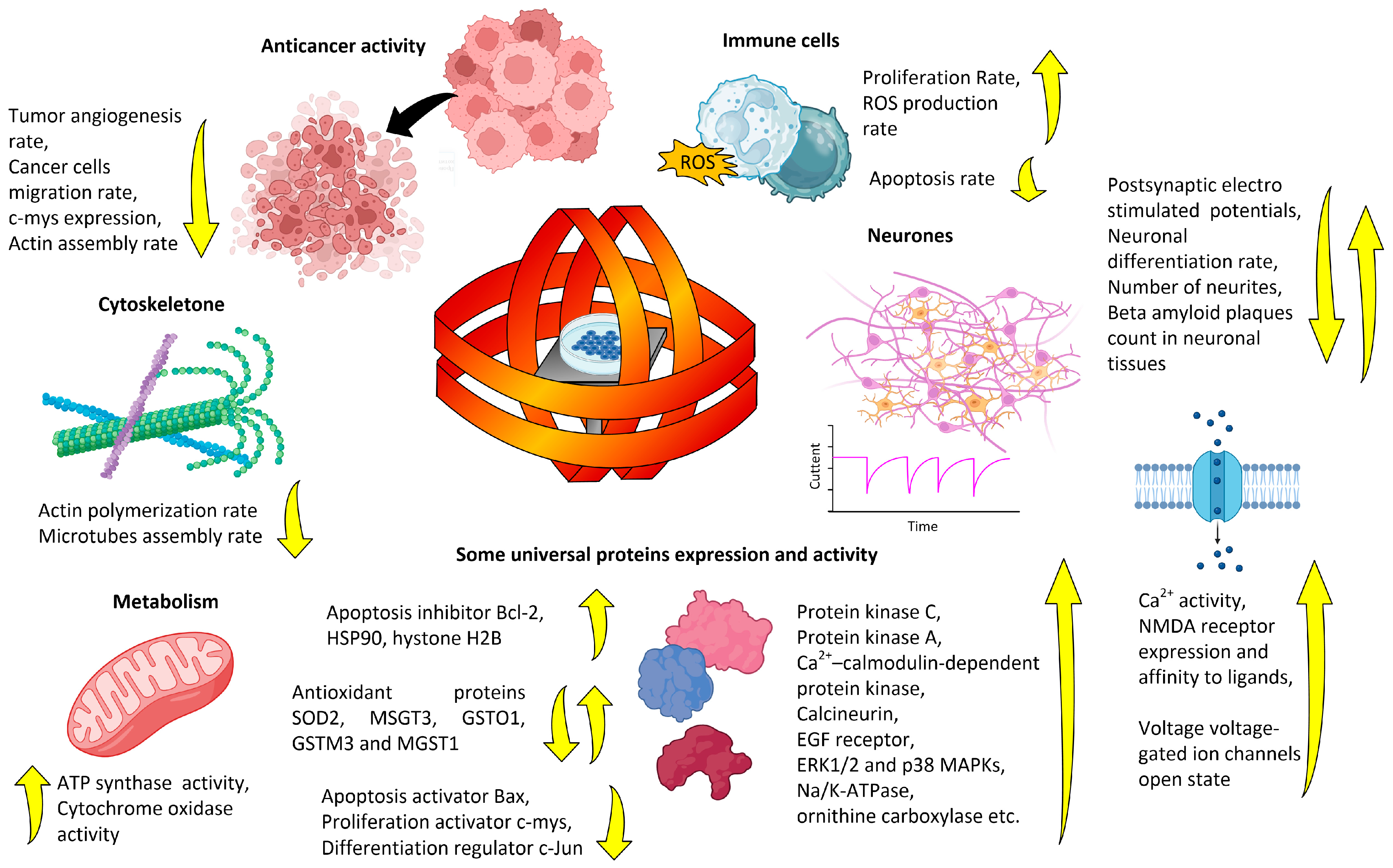
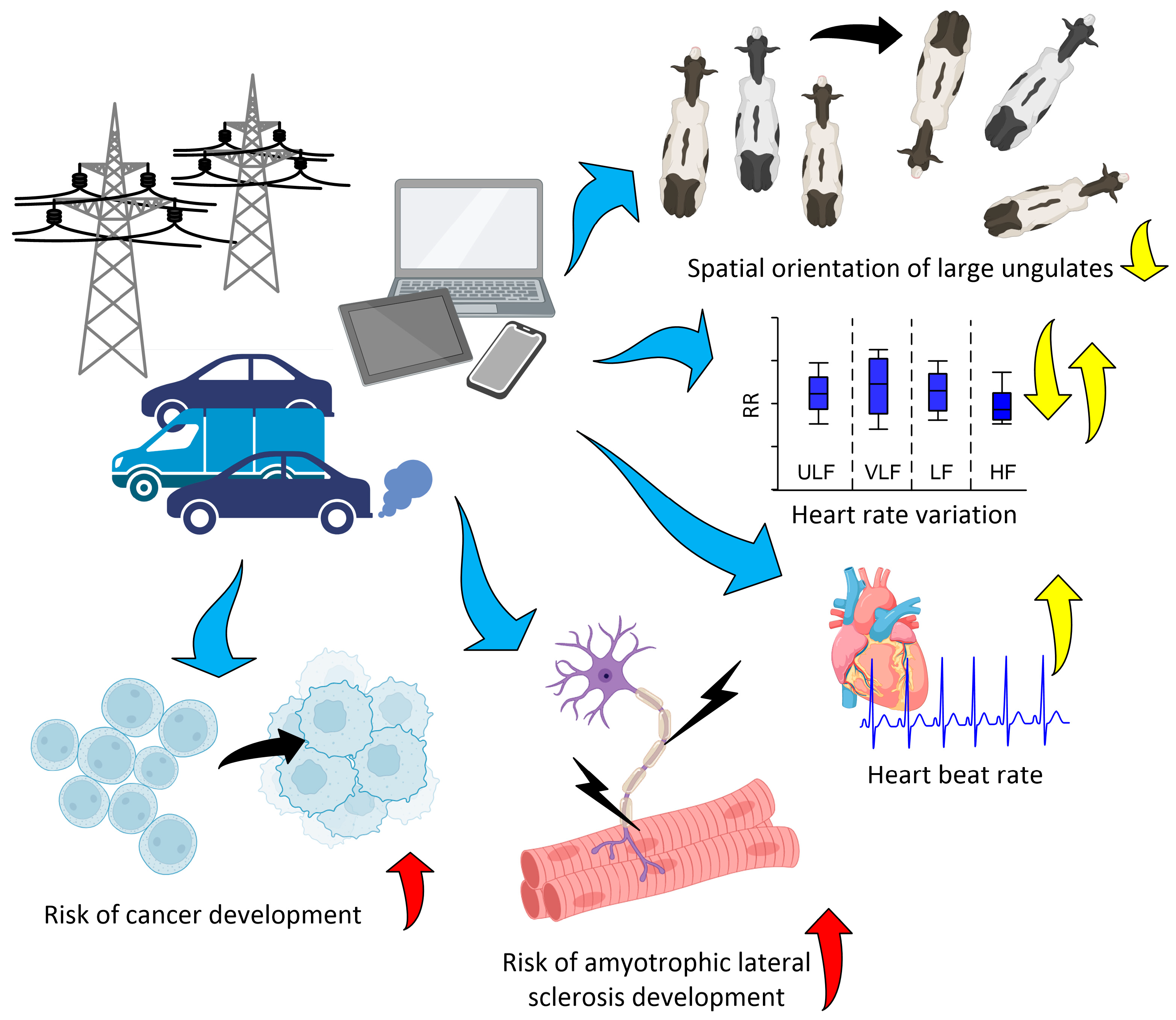
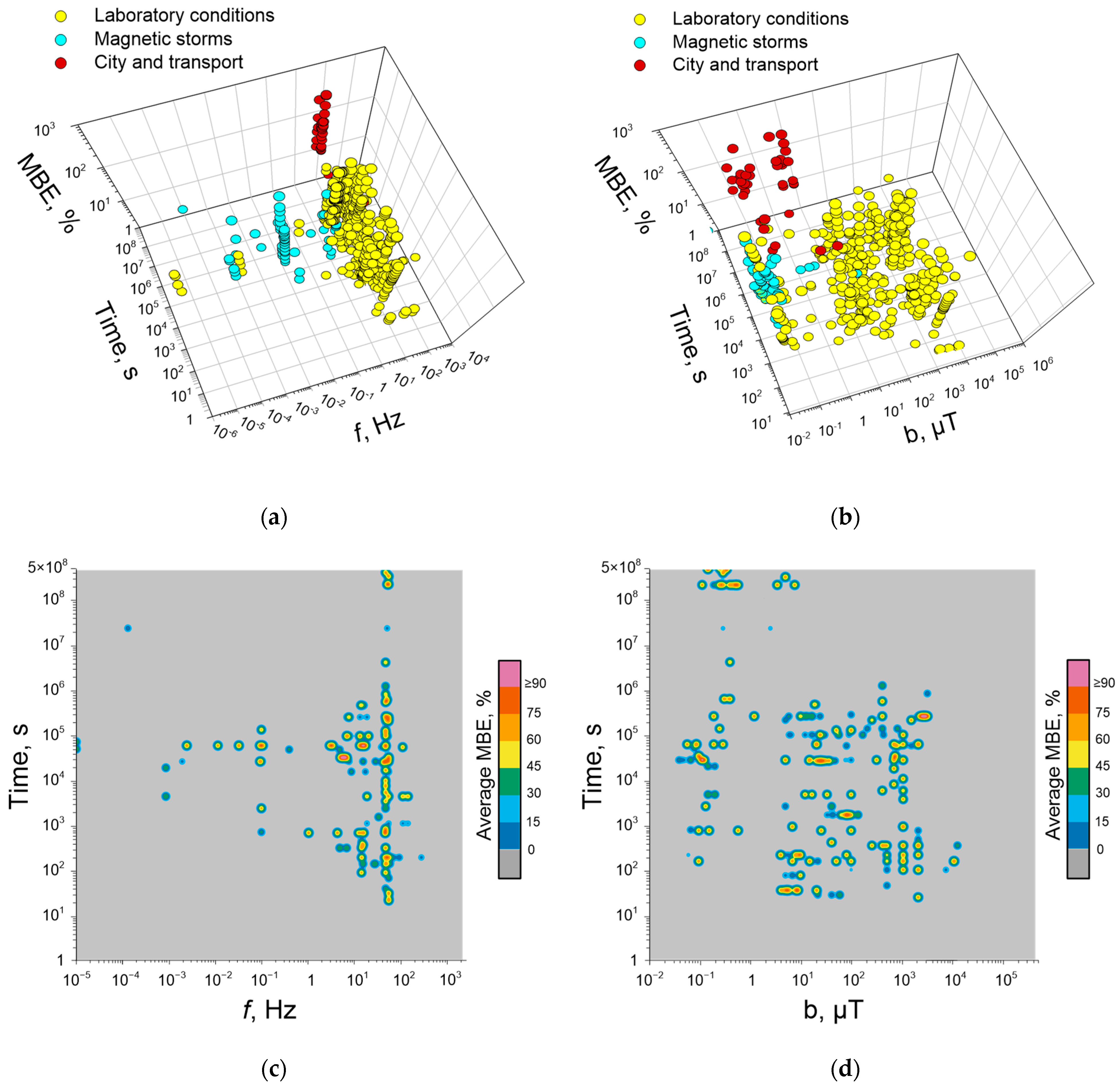
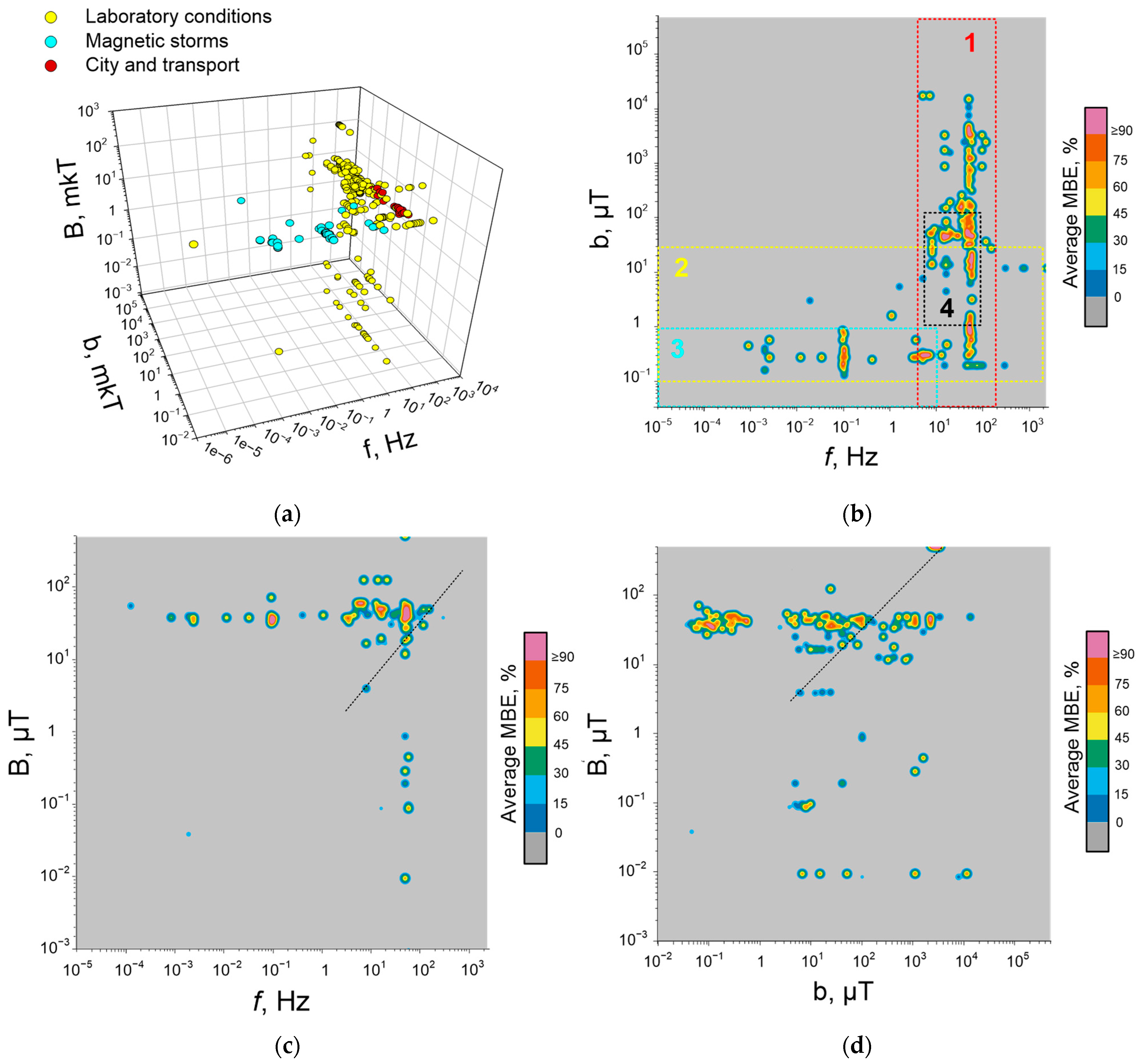
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarimov, R.M.; Serov, D.A.; Gudkov, S.V. Biological Effects of Magnetic Storms and ELF Magnetic Fields. Biology 2023, 12, 1506. https://doi.org/10.3390/biology12121506
Sarimov RM, Serov DA, Gudkov SV. Biological Effects of Magnetic Storms and ELF Magnetic Fields. Biology. 2023; 12(12):1506. https://doi.org/10.3390/biology12121506
Chicago/Turabian StyleSarimov, Ruslan M., Dmitry A. Serov, and Sergey V. Gudkov. 2023. "Biological Effects of Magnetic Storms and ELF Magnetic Fields" Biology 12, no. 12: 1506. https://doi.org/10.3390/biology12121506
APA StyleSarimov, R. M., Serov, D. A., & Gudkov, S. V. (2023). Biological Effects of Magnetic Storms and ELF Magnetic Fields. Biology, 12(12), 1506. https://doi.org/10.3390/biology12121506