Improving Tannery Wastewater Treatments Using an Additional Microbial Treatment with a Bacterial–Fungal Consortium
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physical and Chemical Properties
2.3. Isolation and Identification of Microorganisms
2.4. Collagenase and Gelatinase Activities
2.5. Tannery Effluent Treatments
2.5.1. Collagen
2.5.2. Sample Volume (mL)
3. Results
3.1. Bacterial and Fungal Species with Collagenase and Gelatinase Activities
3.2. Tannery Effluents
3.3. Tannery Wastewater Treatment Experiments
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, K.; Kumari, M.; Prasad, K.S. Tannery Effluents: Current Practices, Environmental Consequences, Human Health Risks, and Treatment Options. CLEAN Soil Air Water 2023, 51, 2200303. [Google Scholar] [CrossRef]
- Ghorab, R.E.A.; Pugazhendi, A.; Jamal, M.T.; Jeyakumar, R.B.; Godon, J.J.; Mathew, D.K. Tannery wastewater treatment coupled with bioenergy production in upflow microbial fuel cell under saline condition. Environ. Res. 2022, 212, 113304. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Yadav, A.; Raj, A.; Purchase, D.; Ferreira, L.F.R.; Saratale, G.D.; Bharagava, R.N. Phytotoxicity, cytotoxicity and genotoxicity evaluation of organic and inorganic pollutants rich tannery wastewater from a Common Effluent Treatment Plant (CETP) in Unnao district, India using Vigna radiata and Allium cepa. Chemosphere 2019, 224, 324–332. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, W. A review for tannery wastewater treatment: Some thoughts under stricter discharge requirements. Environ. Sci. Pollut. Res. 2019, 26, 26102–26111. [Google Scholar] [CrossRef]
- Mehra, A.; Mehra, R. Analysis of heavy metals and toxicity level in the tannery effluent and the environs. Environ. Monit. Assess. 2023, 195, 554. [Google Scholar] [CrossRef]
- Sallam, A.S.; Usman, A.R.A.; Al-Makrami, H.A.; Al-Wabel, M.I.; Al-Omran, A. Environmental assessment of tannery wastes in relation to dumpsite soil: A case study from Riyadh, Saudi Arabia. Arab. J. Geosci. 2015, 8, 11019–11029. [Google Scholar] [CrossRef]
- Ahsan, M.A.; Satter, F.; Siddique, M.A.B.; Akbor, M.A.; Ahmed, S.; Shajahan, M.; Khan, R. Chemical and physicochemical characterization of effluents from the tanning and textile industries in Bangladesh with multivariate statistical approach. Environ. Monit. Assess. 2019, 191, 575. [Google Scholar] [CrossRef]
- Roy, S.; Nagarchi, L.; Das, I.; Mangalam Achuthananthan, J.; Krishnamurthy, S. Cytotoxicity, Genotoxicity and Phytotoxicity of tannery effluent discharged into Palar River Basin, Tamil Nadu, India. J. Toxicol. 2015, 2015, 504360. [Google Scholar] [CrossRef]
- Mwinyihija, M. Main Pollutants and Environmental Impacts of the Tanning Industry. In Ecotoxicological Diagnosis in the Tanning Industry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–35. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Q.; Tang, Y.; Zhou, J.; Guo, H. Tannery wastewater treatment: Conventional and promising processes, an updated 20-year review. J. Leather Sci. Eng. 2022, 4, 10. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, S.; Singh, D. Tannery effluent treatment and its environmental impact: A review of current practices and emerging technologies. Water Qual. Res. J. 2023, 58, 128–152. [Google Scholar] [CrossRef]
- Guo, L.; Xie, Y.; Sun, W.; Xu, Y.; Sun, Y. Research Progress of High-Salinity Wastewater Treatment Technology. Water 2023, 15, 684. [Google Scholar] [CrossRef]
- Giacobbo, A.; Feron, G.L.; Rodrigues, M.A.S.; Ferreira, J.Z.; Meneguzzi, A.; Bernardes, A.M. Integration of membrane bioreactor and advanced oxidation processes for water recovery in leather industry. Desalin. Water Treat. 2015, 56, 1712–1721. [Google Scholar] [CrossRef]
- Alemu, A.; Gabbiye, N.; Lemma, B. Evaluation of Tannery Wastewater Treatment by Integrating Vesicular Basalt with Local Plant Species in a Constructed Wetland System. Front. Environ. Sci. 2021, 9, 721014. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Abu Sayid Mia, M.; Ahmad, F.; Mafizur Rahman, M. Adsorption of chromium (Cr) from tannery wastewater using low-cost spent tea leaves adsorbent. Appl. Water Sci. 2018, 8, 129. [Google Scholar] [CrossRef]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E.R. Microbial technologies for heavy metal remediation: Effect of process conditions and current practices. Clean Technol. Environ. Policy 2023, 25, 1485–1507. [Google Scholar] [CrossRef]
- Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment—New Approaches and Recent Advances; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Sundar, V.J.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K.; Mandal, A.B. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Barathi, J.; Hemapriya, I.; Gunasekaran, R.; Vadakkan, K.; Shyamala, A.; Ravi, A.; Vijayanand, S. Bioremediation of Textile and Tannery Effluents—An Overview. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 3782–3790. [Google Scholar] [CrossRef]
- Liu, X.; Mei, S.; Salles, J.F. Inoculated microbial consortia perform better than single strains in living soil: A meta-analysis. Appl. Soil Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- Ma, X.-K.; Li, T.-T.; Fam, H.; Peterson, E.C.; Zhao, W.-W.; Guo, W.; Zhou, B. The influence of heavy metals on the bioremediation of polycyclic aromatic hydrocarbons in aquatic system by a bacterial–fungal consortium. Environ. Technol. 2018, 39, 2128–2137. [Google Scholar] [CrossRef]
- Kim, I.-S.; Ekpeghere, K.; Ha, S.-Y.; Kim, S.-H.; Kim, B.-S.; Song, B.; Chun, J.; Chang, J.-S.; Kim, H.-G.; Koh, S.-C. An eco-friendly treatment of tannery wastewater using bioaugmentation with a novel microbial consortium. J. Environ. Sci. Health Part A 2013, 48, 1732–1739. [Google Scholar] [CrossRef]
- Reyes-Romero, B.; Gutierrez-López, A.N.; Hernandez-Altamirano, R.; Mena-Cervantes, V.Y.; Ruiz-Baca, E.; Neri-Torres, E.E.; Chairez, I.; Garcia-Solares, S.M.; Vazquez-Arenas, J. Removal of concentrated Cr(III) from real tannery wastewater using abiotic and anaerobic processes with native microbial consortia. J. Environ. Chem. Eng. 2021, 9, 104626. [Google Scholar] [CrossRef]
- Cheng, Y.; Chon, K.; Ren, X.; Kou, Y.; Hwang, M.; Chae, K. Bioaugmentation treatment of a novel microbial consortium for degradation of organic pollutants in tannery wastewater under a full-scale oxic process. Biochem. Eng. J. 2021, 175, 108131. [Google Scholar] [CrossRef]
- Mawad, A.M.; Hesham, A.E.-L.; Yousef, N.M.H.; Shoreit, A.A.M.; Gathergood, N.; Gupta, V.K. Role of Bacterial-Fungal Consortium for Enhancement in the Degradation of Industrial Dyes. Curr. Genom. 2020, 21, 283–294. [Google Scholar] [CrossRef]
- Marcos, A.; Fisher, G.; Ree, G.; Hill, S.J. Preliminary study using trace element concentrations and a chemometrics approach to determine the geological origin of tea. J. Anal. At. Spectr. 1998, 113, 521–525. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A. Oily bilge water treatment using indigenous soil bacteria: Implications for recycling the treated sludge in vegetable farming. Chemosphere 2023, 334, 139040. [Google Scholar] [CrossRef]
- Ameen, F.; AlNAdhari, S.; Yassin, M.A.; Al-Sabri, A.; Almansob, A.; Alqahtani, N.; Stephenson, S.L. Desert soil fungi isolated from Saudi Arabia: Cultivable fungal community and biochemical production. Saudi J. Biol. Sci. 2022, 29, 2409–2420. [Google Scholar] [CrossRef]
- Costa, O.Y.; Pijl, A.; Houbraken, J.; van Lith, W.; Kuramae, E.E. Soil substrate source drives the microbes involved in the degradation of gelatin used as a biostimulant. Appl. Soil Ecol. 2023, 189, 104906. [Google Scholar] [CrossRef]
- Savita, K.; Pethe, A. Production of collagenase by Bacillus KM369985 isolated from leather sample. Int. J. Biosci. IJB 2015, 4, 81–87. [Google Scholar]
- Saxena, G.; Chandra, R.; Bharagava, R.N. Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2016; 240p. [Google Scholar] [CrossRef]
- Muthukrishnan, L. Nanotechnology for cleaner leather production: A review. Environ. Chem. Lett. 2021, 19, 2527–2549. [Google Scholar] [CrossRef]
- Saravanabhavan, S.; Thanikaivelan, P.; Rao, J.R.; Nair, B.U.; Ramasami, T. Reversing the Conventional Leather Processing Sequence for Cleaner Leather Production. Environ. Sci. Technol. 2006, 40, 1069–1075. [Google Scholar] [CrossRef]
- Sivaram, N.; Barik, D. Toxic Waste from Leather Industries. In Energy from Toxic Organic Waste for Heat and Power Generatio; Woodhead Publishing: Cambridge, UK, 2019; pp. 55–67. [Google Scholar] [CrossRef]
- Chaudhary, P.; Chhokar, V.; Kumar, A.; Beniwal, V. Bioremediation of tannery waste water. In Advances in Enviornmental Biotechnology; Kumar, R., Sharma, A., Ahluwalia, S., Eds.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Chen, L.; Qiang, T.; Chen, X.; Ren, W.; Zhang, H.J. Gelatin from leather waste to tough biodegradable packaging film: One valuable recycling solution for waste gelatin from leather industry. Waste Manag. 2022, 145, 10–19. [Google Scholar] [CrossRef]
- Nursyam, H.; Prihanto, A.A.; Warasari, N.I.; Saadah, M.; Masrifa, R.E.; Nabila, N.A.; Istiqfarin, N.; Siddiq, I.J. The isolation and identification of endophytic bacteria from mangrove (Sonneratia alba) that produces gelatinase. IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 012056. [Google Scholar] [CrossRef]
- Balan, S.S.; Nethaji, R.; Sankar, S.; Jayalakshmi, S. Production of gelatinase enzyme from Bacillus spp. isolated from the sediment sample of Porto Novo Coastal sites. Asian Pac. J. Trop. Biomed. 2012, 2, S1811–S1816. [Google Scholar] [CrossRef]
- Joseph, J.; Sasidharan, H.; Chithira, O.S. Isolation, Production and Characterisation of Novel Gelatinase Enzyme from Bacillus Spp. Biol. Sci. 2018, 5, 111–120. [Google Scholar] [CrossRef]
- Batroukha, Y.A.; Nour El Dein, M.M.; Abou-Dobara, M.I.; El-Sayed, A.K. Production and Optimization of Gelatinase Producing Lentzea sp. Strain Isolated from the Soil Rhizosphere. Sci. J. Damietta Fac. Sci. 2022, 12, 124–131. [Google Scholar] [CrossRef]
- Hoppe, I.J.; Brandstetter, H.; Schönauer, E. Biochemical characterisation of a collagenase from Bacillus cereus strain Q1. Sci. Rep. 2021, 11, 4187. [Google Scholar] [CrossRef]
- Suphatharaprateep, W.; Cheirsilp, B.; Jongjareonrak, A. Production and properties of two collagenases from bacteria and their application for collagen extraction. N. Biotechnol. 2011, 28, 649–655. [Google Scholar] [CrossRef]
- Tohar, R.; Ansbacher, T.; Sher, I.; Weinberg, E.; Gal, M. Screening Collagenase Activity in Bacterial Lysate for Directed Enzyme Applications. Int. J. Mol. Sci. 2021, 22, 8552. [Google Scholar] [CrossRef]
- Yakovleva, M.B.; Khoang, T.L.; Nikitina, Z.K. Collagenolytic activity in several species of deuteromycetes under various storage conditions. Appl. Biochem. Microbiol. 2006, 42, 431–434. [Google Scholar] [CrossRef]
- Sharma, S.; Malaviya, P. Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecol. Eng. 2016, 91, 419–425. [Google Scholar] [CrossRef]
- Abioye, O.P.; Oyewole, O.A.; Oyeleke, S.B.; Adeyemi, M.O.; Orukotan, A.A. Biosorption of lead, chromium and cadmium in tannery effluent using indigenous microorganisms. Braz. J. Biol. Sci. 2018, 5, 25–32. [Google Scholar] [CrossRef]
- Liu, Y.-B.; Long, M.-X.; Yin, Y.-J.; Si, M.-R.; Zhang, L.; Lu, Z.-Q.; Wang, Y.; Shen, X.-H. Physiological roles of mycothiol in detoxification and tolerance to multiple poisonous chemicals in Corynebacterium glutamicum. Arch. Microbiol. 2013, 195, 419–429. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Won, S.W.; Mao, J.; Yun, Y.-S. Chemical modification of Corynebacterium glutamicum to improve methylene blue biosorption. Chem. Eng. J. 2008, 145, 1–6. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Utilization of fermentation waste (Corynebacterium glutamicum) for biosorption of Reactive Black 5 from aqueous solution. J. Hazard. Mater. 2007, 141, 45–52. [Google Scholar] [CrossRef]
- Fanous, A.; Hecker, M.; Görg, A.; Parlar, H.; Jacob, F. Corynebacterium glutamicum as an indicator for environmental cobalt and silver stress–A proteome analysis. J. Environ. Sci. Health Part B 2010, 45, 666–675. [Google Scholar] [CrossRef]
- Podder, M.S.; Majumder, C.B. Corynebacterium glutamicum MTCC 2745 immobilized on granular activated carbon/MnFe2O4 composite: A novel biosorbent for removal of As(III) and As(V) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 159–179. [Google Scholar] [CrossRef]
- Choi, S.; Yun, Y.S. Lead biosorption by waste biomass of Corynebacterium glutamicum generated from lysine fermentation process. Biotechnol. Lett. 2004, 26, 331–336. [Google Scholar] [CrossRef]
- Ray, D.; Anand, U.; Jha, N.K.; Korzeniewska, E.; Bontempi, E.; Proćków, J.; Dey, A. The soil bacterium, Corynebacterium glutamicum, from biosynthesis of value-added products to bioremediation: A master of many trades. Environ. Res. 2022, 213, 113622. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Removal of Cr(VI) from aqueous solution by fungal biomass. Eng. Life Sci. 2010, 10, 480–485. [Google Scholar] [CrossRef]
- Mohammadian, E.; Ahari, A.B.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]
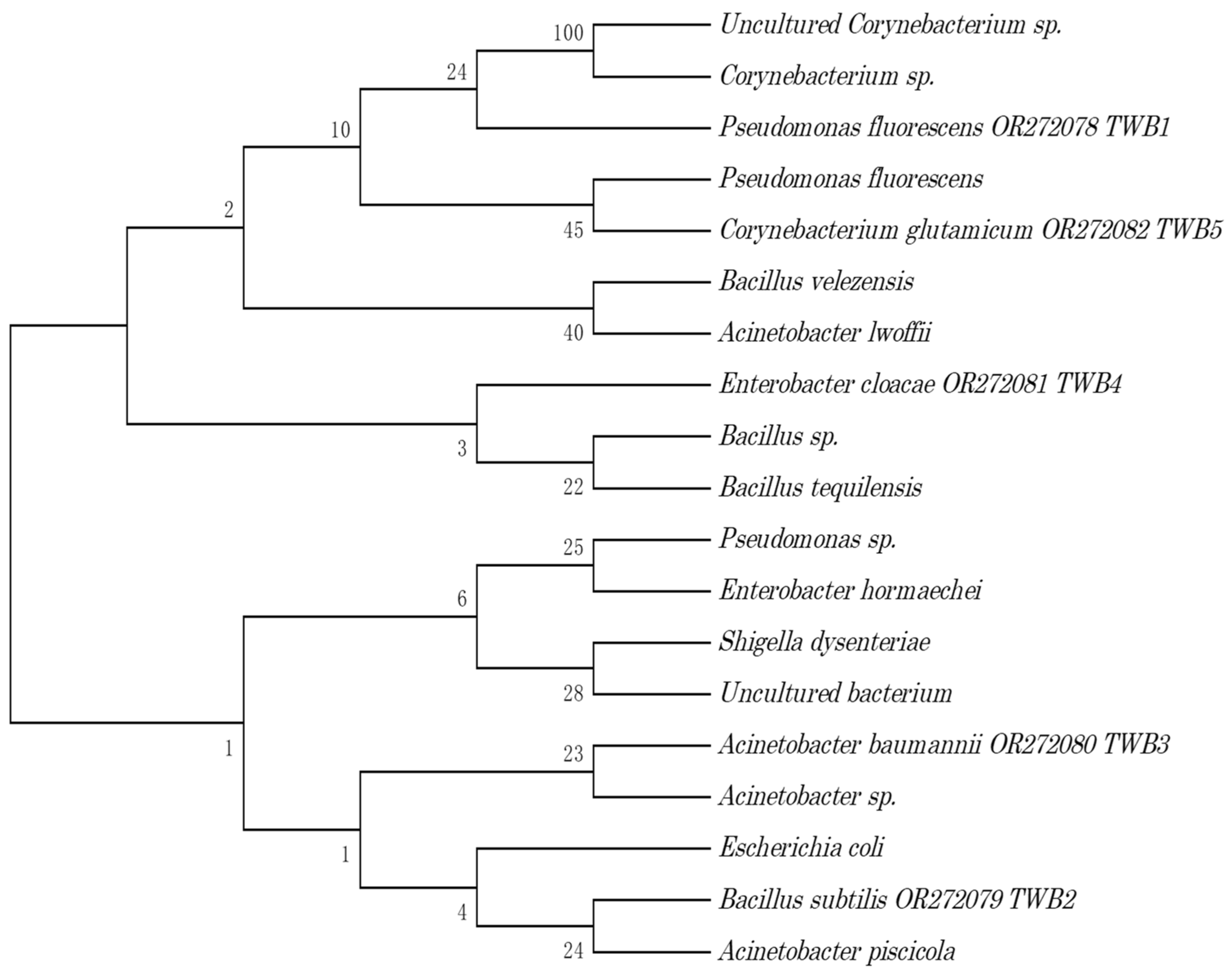
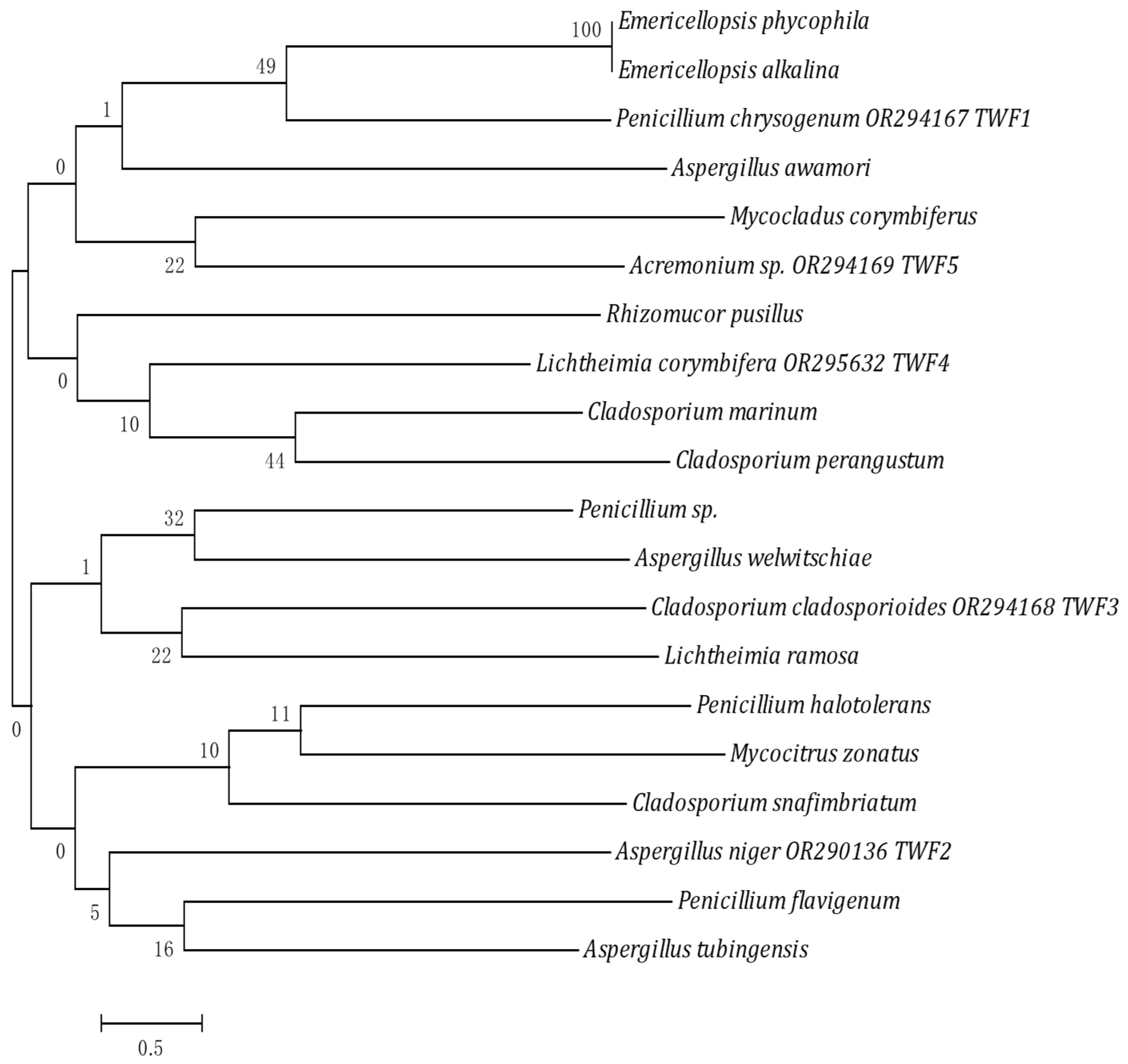
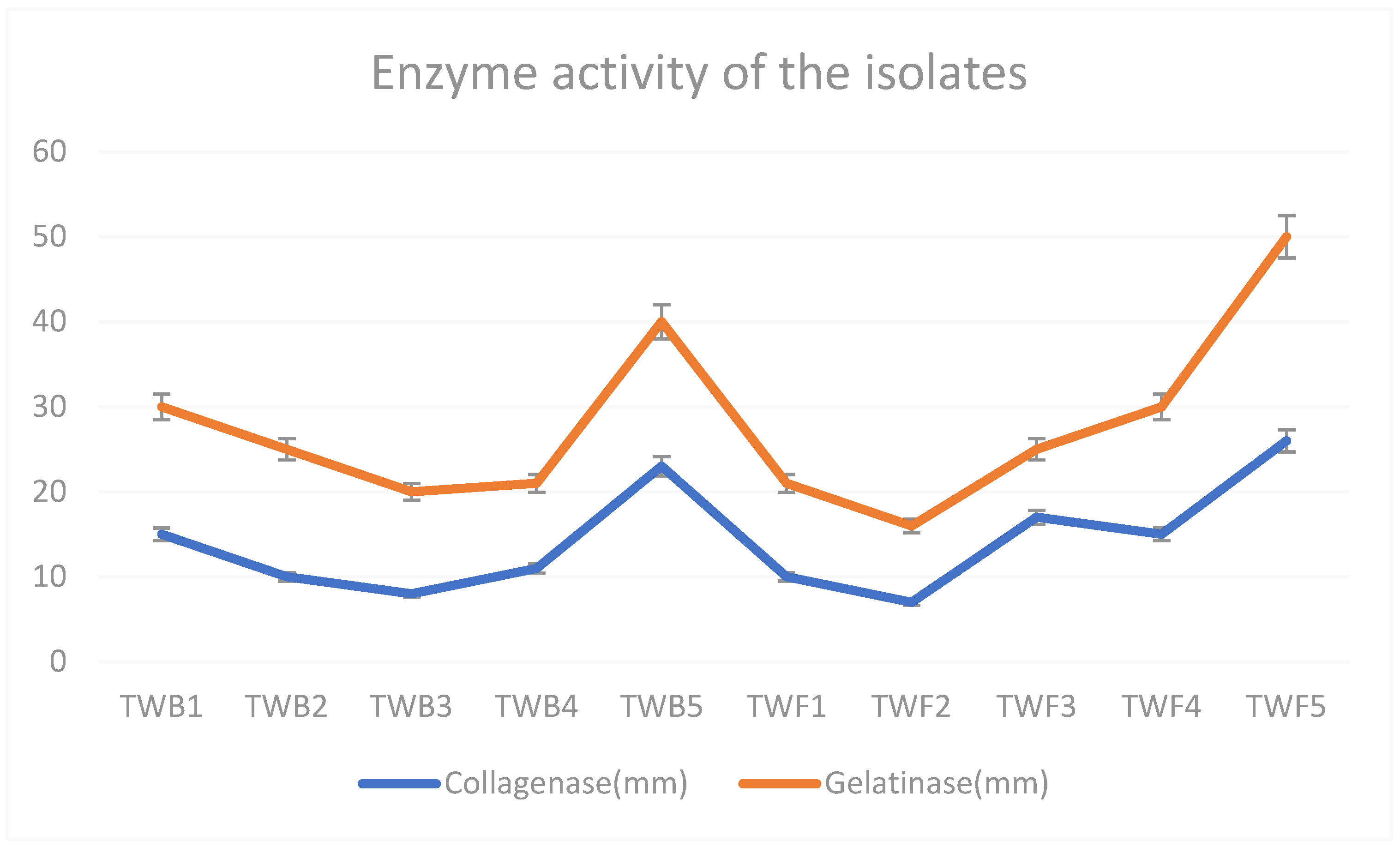
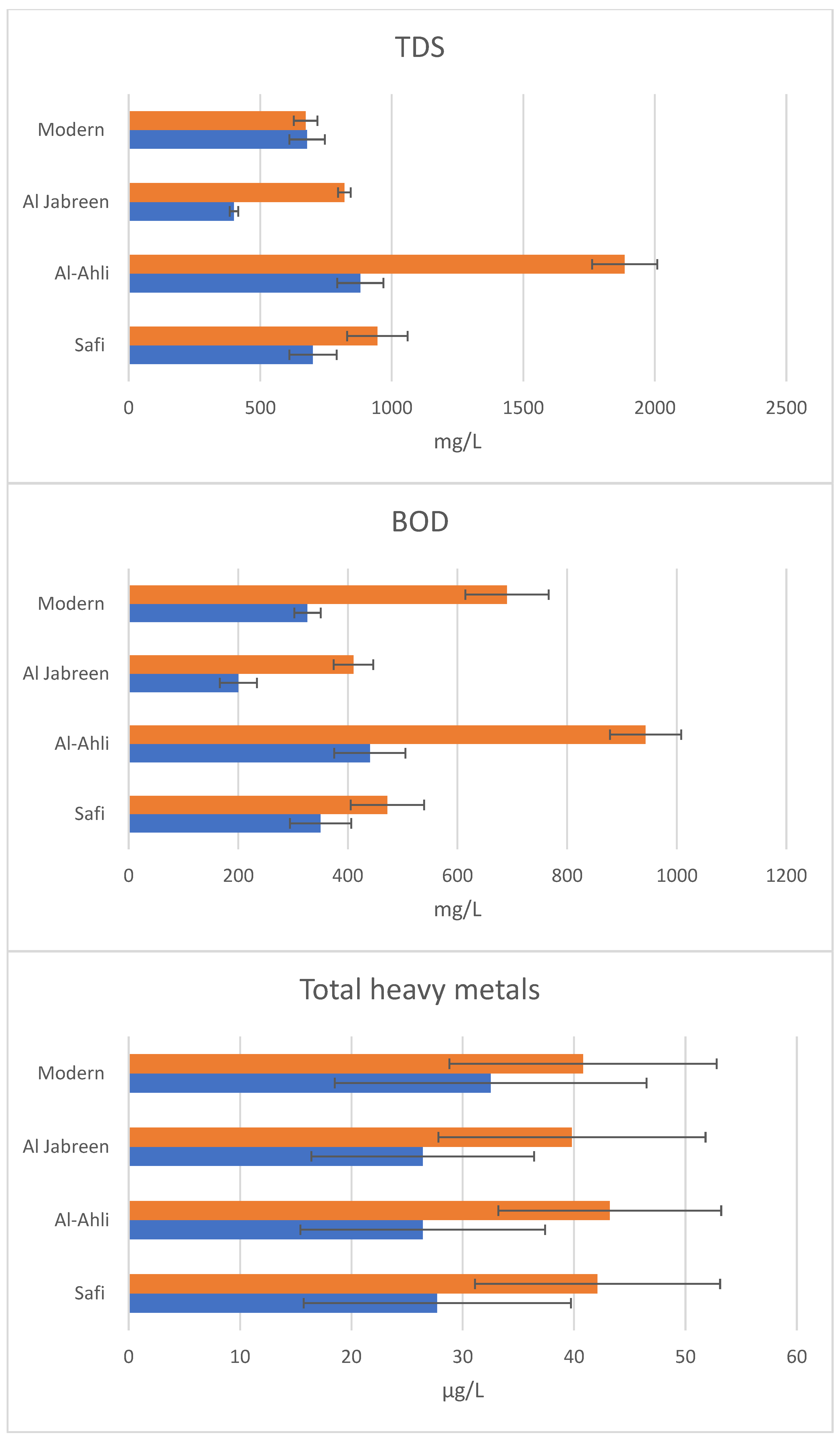
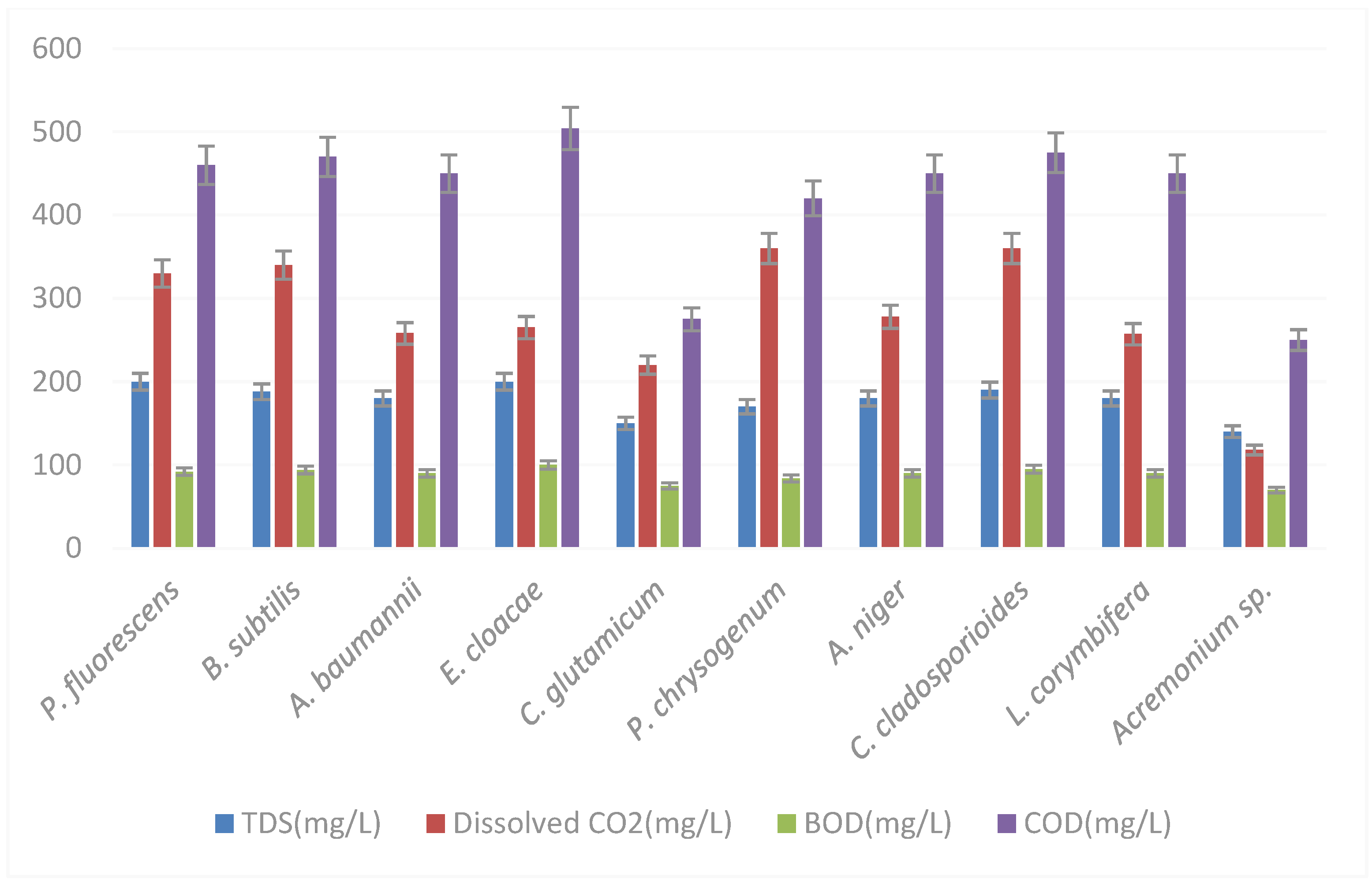
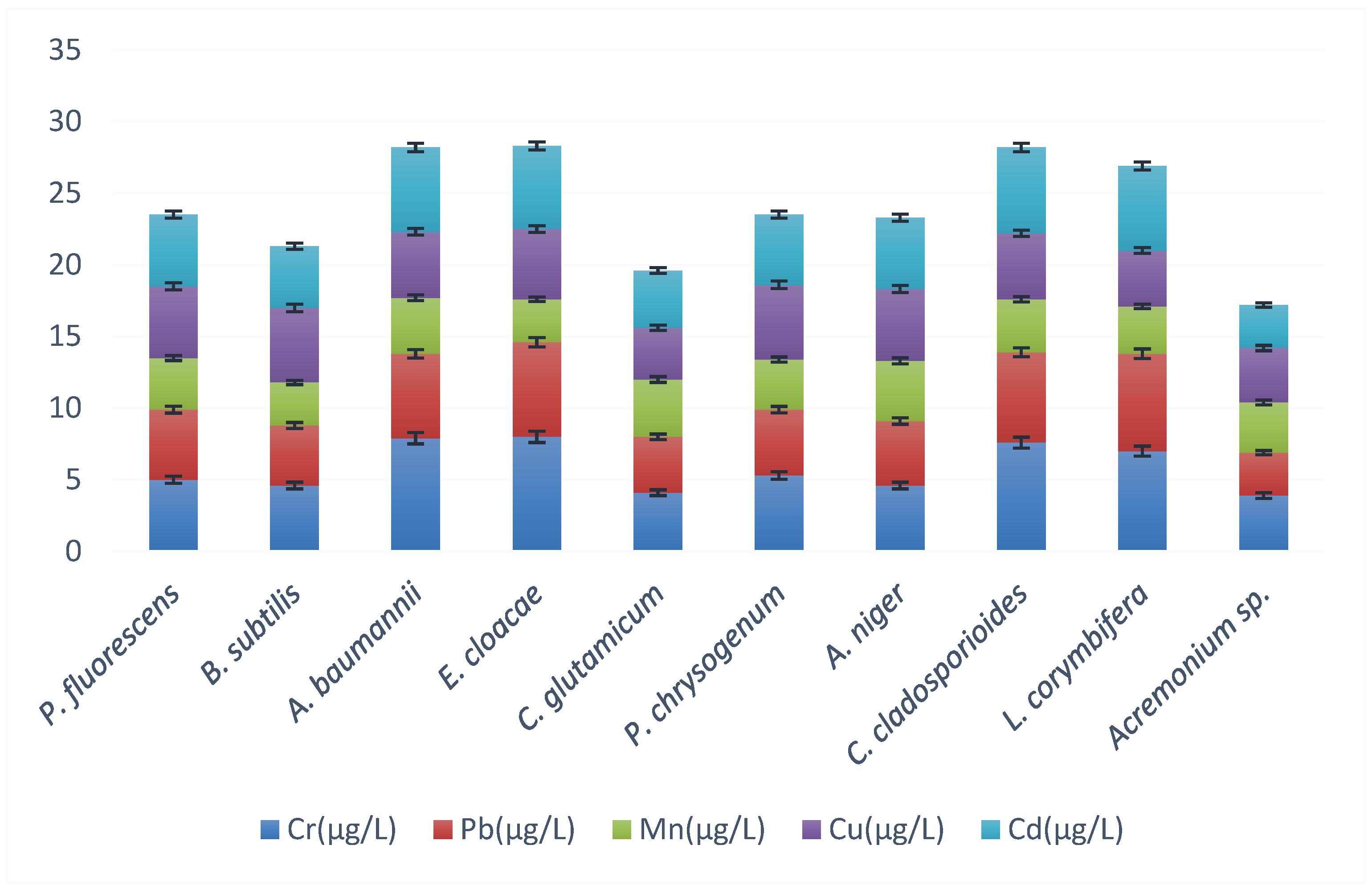
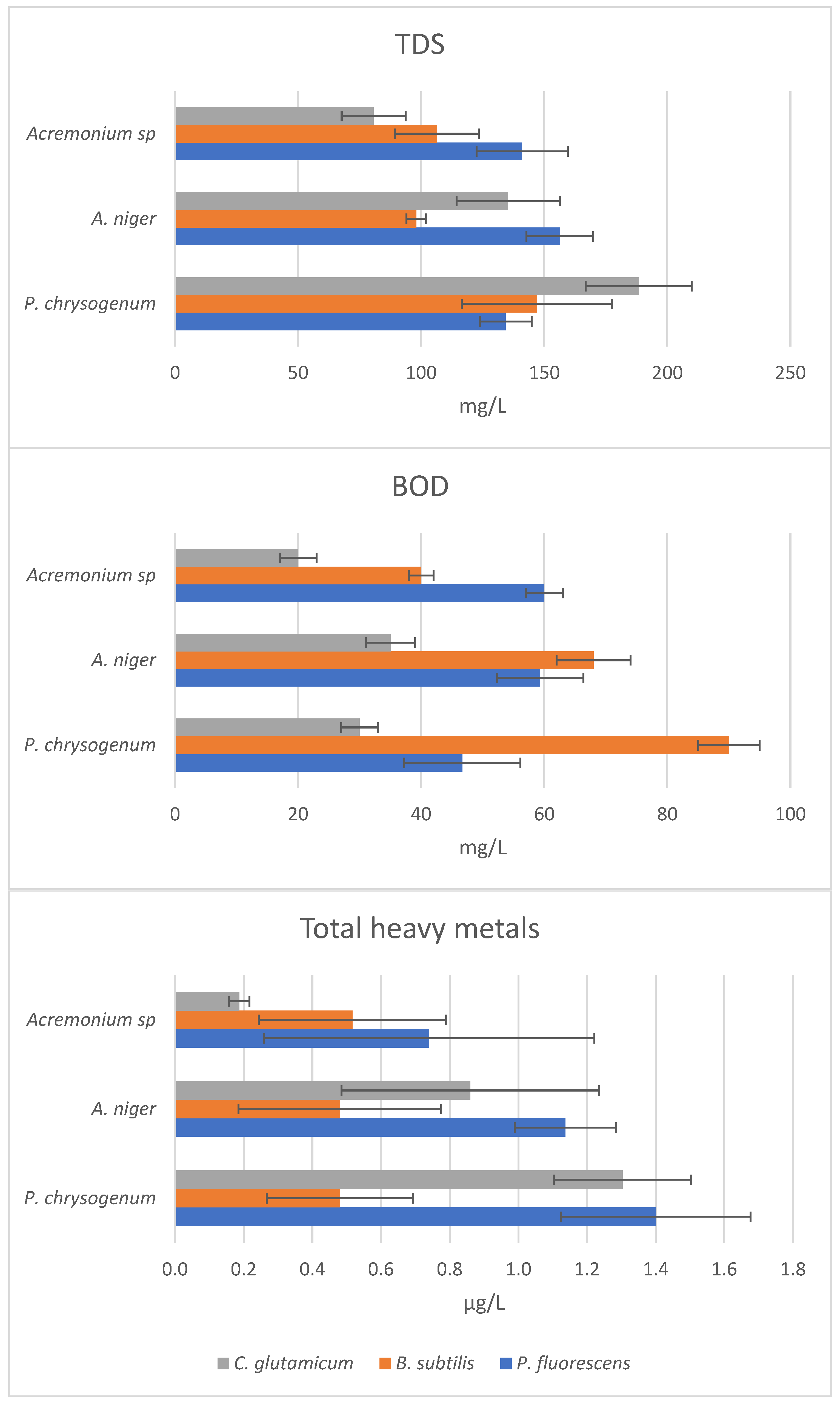
| Isolate | Organism | Name | Accession Number |
|---|---|---|---|
| TWB1 | Bacterium | Pseudomonas fluorescens | OR272078 |
| TWB2 | Bacterium | Bacillus subtilis | OR272079 |
| TWB3 | Bacterium | Acinetobacter baumannii | OR272080 |
| TWB4 | Bacterium | Enterobacter cloacae | OR272081 |
| TWB5 | Bacterium | Corynebacterium glutamicum | OR272082 |
| TWF1 | Fungus | Penicillium chrysogenum | OR294167 |
| TWF2 | Fungus | Aspergillus niger | OR290136 |
| TWF3 | Fungus | Cladosporium cladosporioides | OR294168 |
| TWF4 | Fungus | Lichtheimia corymbifera | OR295632 |
| TWF5 | Fungus | Acremonium sp. | OR294169 |
| Parameters | Sample Sites | |||||||
|---|---|---|---|---|---|---|---|---|
| Modern Leather Factory | Al Jabreen Leather Factory | Al-Ahli Leather Factory | Safi Universal for Leather Tanning | |||||
| Before | After | Before | After | Before | After | Before | After | |
| EC (mmhos/cm) | 4.5 ± 2 | 3.6 ± 3 | 3.5 ± 1 | 2.8 ± 3 | 4.7 ± 1 | 3.9 ± 2 | 3.9 ± 2 | 2.9 ± 1 |
| pH | 6.2 ± 2 | 7.0 ± 3 | 6.5 ± 1 | 6.7 ± 2 | 5.9 ± 2 | 6.4 ± 3 | 6.5 ± 3 | 6.9 ± 2 |
| CO2 (mg/L) | 745 ± 88 | 465 ± 49 | 812 ± 68 | 486 ± 46 | 888 ± 98 | 599 ± 47 | 672 ± 25 | 478 ± 3 |
| COD (mg/L) | 3430 ± 99 | 1630 ± 98 | 2050 ± 78 | 1000 ± 90 | 4712 ± 130 | 2200 ± 122 | 2362 ± 123 | 1750 ± 78 |
| Cr (μg/L) | 10 ± 3 | 7.4 ± 2 | 9.4 ± 3 | 5 ± 2 | 12 ± 3 | 6 ± 2 | 10 ± 3 | 9 ± 2 |
| Pb (μg/L) | 8 ± 2 | 6.9 ± 3 | 5.2 ± 3 | 3 ± 3 | 8.2 ± 2 | 5.9 ± 3 | 8 ± 2 | 4 ± 3 |
| Mn (μg/L) | 7.8 ± 2 | 7.4 ± 2 | 11.2 ± 2 | 9.7 ± 3 | 6 ± 1 | 3.6 ± 1 | 7.2 ± 3 | 3.9 ± 4 |
| Cu (μg/L) | 8.2 ± 3 | 6 ± 3 | 8 ± 2 | 4.8 ± 1 | 8 ± 2 | 5.9 ± 2 | 7.7 ± 1 | 4 ± 1 |
| Cd (μg/L) | 6.8 ± 2 | 4.8 ± 4 | 6 ± 2 | 3.9 ± 1 | 9 ± 2 | 5 ± 3 | 9.2 ± 2 | 6.6 ± 2 |
| Bacterium | Fungus | ||
|---|---|---|---|
| P. chrysogenum | A. niger | Acremonium sp. | |
| Parameter | Original | Factory-treated | |
| EC mmhos/cm | 4.7 ± 1 | 3.9 ± 2 | |
| P. fluorescens | 1.6 ± 0.2 | 1.9 ± 0.1 | 1.6 ± 0.3 |
| B. subtilis | 1.2 ± 0.2 | 1.5 ± 0.3 | 1.3 ± 0.2 |
| C. glutamicum | 1.6 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.1 |
| pH | 5.9 ± 2 | 6.4 ± 3 | |
| P. fluorescens | 7.2 ± 0.2 | 7.2 ± 0.1 | 7.1 ± 0.3 |
| B. subtilis | 7.2 ± 0.1 | 7.2 ± 0.1 | 7.3 ± 0.1 |
| C. glutamicum | 7.2 ± 0.1 | 7.1 ± 0.2 | 7.2 ± 0.2 |
| Dissolved CO2 (mg/L) | 888 ± 98 | 599 ± 47 | |
| P. fluorescens | 548 ± 32 | 520 ± 24 | 440 ± 13 |
| B. subtilis | 654 ± 56 | 580 ± 34 | 340 ± 25 |
| C. glutamicum | 488 ± 76 | 600 ± 35 | 270 ± 32 |
| COD (mg/L) | 4712 ± 130 | 2200 ± 122 | |
| P. fluorescens | 200 ± 12 | 220 ± 14 | 250 ± 8 |
| B. subtilis | 270 ± 19 | 290 ± 13 | 170 ± 17 |
| C. glutamicum | 199 ± 12 | 240 ± 78 | 98 ± 15 |
| Cr (μg/L) | 12 ± 3 | 6 ± 2 | |
| P. fluorescens | 0.9 ± 0.4 | 0.6 ± 0.4 | 0.9 ± 0.3 |
| B. subtilis | 0.3 ± 0.2 | 0.4 ± 0.1 | 0.2 ± 0.4 |
| C. glutamicum | 0.6 ± 0.2 | 0.8 ± 0.1 | 0.05 ± 0.1 |
| Pb (μg/L) | 8.2 ± 2 | 5.9 ± 3 | |
| P. fluorescens | 0.09 ± 0.01 | 0.07 ± 0.02 | 0.09 ± 0.01 |
| B. subtilis | 0.06 ± 0.04 | 0.03 ± 0.02 | 0.06 ± 0.02 |
| C. glutamicum | 0.05 ± 0. 1 | 0.03 ± 0. 2 | 0.02 ± 0.2 |
| Mn (μg/L) | 6 ± 1 | 3.6 ± 1 | |
| P. fluorescens | 0.6 ± 0.4 | 0.4 ± 0.04 | 0.09 ± 0.02 |
| B. subtilis | 0.04 ± 0.03 | 0.05 ± 0.02 | 0.04 ± 0.02 |
| C. glutamicum | 0.09 ± 0.03 | 0.08 ± 0.02 | 0.04 ± 0.01 |
| Cu (μg/L) | 8 ± 2 | 5.9 ± 2 | |
| P. fluorescens | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.06 ± 0.02 |
| B. subtilis | 0.05 ± 0.04 | 0.06 ± 0.04 | 0.09 ± 0.03 |
| C. glutamicum | 0.5 ± 0.2 | 0.03 ± 0.01 | 0.02 ± 0.02 |
| Cd (μg/L) | 9 ± 2 | 5 ± 3 | |
| P. fluorescens | 0.03 ± 0.02 | 0.05 ± 0.03 | 0.03 ± 0.01 |
| B. subtilis | 0.07 ± 0.02 | 0.03 ± 0.03 | 0.08 ± 0.04 |
| C. glutamicum | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.03 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, F. Improving Tannery Wastewater Treatments Using an Additional Microbial Treatment with a Bacterial–Fungal Consortium. Biology 2023, 12, 1507. https://doi.org/10.3390/biology12121507
Ameen F. Improving Tannery Wastewater Treatments Using an Additional Microbial Treatment with a Bacterial–Fungal Consortium. Biology. 2023; 12(12):1507. https://doi.org/10.3390/biology12121507
Chicago/Turabian StyleAmeen, Fuad. 2023. "Improving Tannery Wastewater Treatments Using an Additional Microbial Treatment with a Bacterial–Fungal Consortium" Biology 12, no. 12: 1507. https://doi.org/10.3390/biology12121507
APA StyleAmeen, F. (2023). Improving Tannery Wastewater Treatments Using an Additional Microbial Treatment with a Bacterial–Fungal Consortium. Biology, 12(12), 1507. https://doi.org/10.3390/biology12121507







