Synbiotic Agents and Their Active Components for Sustainable Aquaculture: Concepts, Action Mechanisms, and Applications
Abstract
Simple Summary
Abstract
1. Introduction
2. Probiotics
2.1. Definition and Characteristic Features
2.2. Possible Modes of Action of Probiotics in Aquaculture
2.2.1. Probiotics Act as Growth Enhancers in Aquaculture
2.2.2. Biocontrol of Bacterial Diseases in Aquaculture
2.2.3. Biocontrol of Viral Diseases in Aquaculture
2.2.4. Immunostimulant Agents in Aquaculture
2.2.5. Interference of Quorum Sensing in Aquaculture
2.2.6. Stress Improvement in the Aquaculture System
2.2.7. Reducing Heavy Metals in Aquaculture
2.3. Major Probiotic Genera as Biocontrol Agents in Aquaculture
3. Prebiotics
3.1. Action in the Gastrointestinal Tracts of Aquatic Animals
3.2. Regulation in the Immune System of Aquatic Animals
3.2.1. Phagocytosis
3.2.2. Macrophage Activation
3.2.3. Respiratory Burst Activity
3.2.4. Synthesis of Antibodies
3.3. Major Prebiotics with Biocontrol Capabilities in Aquaculture
3.3.1. β-Glucan
3.3.2. Oligosaccharide
3.3.3. Chitosan
3.3.4. Inulin
4. Postbiotics
4.1. Concept, Definition, and Major Components of Postbiotics
4.2. Action Modes and Applications of Postbiotics in Aquaculture
4.2.1. Immunomodulation via Microbial Compounds
4.2.2. Antagonizing Pathogens via Antimicrobial Activities
4.2.3. Inhibition of Oxidation via Antioxidant Enzyme Systems and Metabolites
5. Synbiotics
5.1. Possible Modes of Action of Synbiotics in Aquaculture
5.1.1. Synbiotics Enhance Digestive Enzyme and Growth Performance
5.1.2. Synbiotics Improve Immune Response and Disease Resistance
6. Limitations of the Use of Synbiotic Agents in Aquaculture
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gephart, J.A.; Golden, C.D.; Asche, F.; Belton, B.; Brugere, C.; Froehlich, H.E.; Fry, J.P.; Halpern, B.S.; Hicks, C.C.; Jones, R.C. Scenarios for Global Aquaculture and Its Role in Human Nutrition. Rev. Fish. Sci. Aquac. 2020, 29, 122–138. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S. Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Dias, M. Toxicity, Physiological, Histopathological and Antiparasitic Effects of the Formalin, a Chemotherapeutic of Fish Aquaculture. Aquac. Res. 2021, 52, 1803–1823. [Google Scholar] [CrossRef]
- Mohamed, S.; Nagaraj, G.; Chua, F.H.C.; Wang, Y.G. The Use of Chemicals in Aquaculture in Malaysia and Singapore. In Proceedings of the Use of Chemicals in Aquaculture in Asia, Iloilo, Philippines, 20–22 May 1996; Aquaculture Department, Southeast Asian Fisheries Development Center: Iloilo, Philippines, 2000; pp. 127–140. [Google Scholar]
- Ghelichpour, M.; Rajabiesterabadi, H.; Hoseini, S.M. Gill Histopathological Characteristics of Caspian Roach (Rutilus rutilus caspicus) Fingerlings Treated with Potassium Permanganate and Formalin. Aquac. Res. 2016, 47, 276–282. [Google Scholar] [CrossRef]
- Leal, J.F.; Neves, M.G.P.M.S.; Santos, E.B.H.; Esteves, V.I. Use of Formalin in Intensive Aquaculture: Properties, Application and Effects on Fish and Water Quality. Rev. Aquac. 2018, 10, 281–295. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Han, Q.; Wang, X.; Wang, S.; Yuan, X.; Zhang, B.; Zhao, S. Antibiotics in Mariculture Organisms of Different Growth Stages: Tissue-Specific Bioaccumulation and Influencing Factors. Environ. Pollut. 2021, 288, 117715. [Google Scholar] [CrossRef] [PubMed]
- Alfred, O.; Shaahu, A.; Orban, D.A.; Egwenomhe, M. An Overview on Understanding the Basic Concept of Fish Diseases in Aquaculture. IRE J. 2020, 4, 83–91. [Google Scholar]
- Essawi, T.; Srour, M. Screening of Some Palestinian Medicinal Plants for Antibacterial Activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Zou, H.K.; Miandare, H.K.; Van Doan, H.; Romano, N.; Dadar, M. Enrichment of Common Carp (Cyprinus carpio) Diet with Medlar (Mespilus germanica) Leaf Extract: Effects on Skin Mucosal Immunity and Growth Performance. Fish Shellfish Immunol. 2017, 67, 346–352. [Google Scholar] [CrossRef]
- Musthafa, M.S.; Asgari, S.M.; Kurian, A.; Elumalai, P.; Ali, A.R.J.; Paray, B.A.; Al-Sadoon, M.K. Protective Efficacy of Mucuna pruriens (L.) Seed Meal Enriched Diet on Growth Performance, Innate Immunity, and Disease Resistance in Oreochromis Mossambicus against Aeromonas hydrophila. Fish Shellfish Immunol. 2018, 75, 374–380. [Google Scholar] [CrossRef]
- Safety, S. FDA Needs to Improve Oversight of Imported Seafood and Better Leverage Limited Resources; United States Government Accountability Office: Washington, DC, USA, 2011.
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, Prebiotics, and Synbiotics Used to Control Vibriosis in Fish: A Review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in Fish Gastrointestinal Microbiota Research. Rev. Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Luna, G.M.; Quero, G.M.; Kokou, F.; Kormas, K. Time to Integrate Biotechnological Approaches into Fish Gut Microbiome Research. Curr. Opin. Biotechnol. 2022, 73, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.-P.; Junaid, M.; Wen, P.-P.; Yang, Y.-F.; Li, W.-G.; Yang, X.-G.; Pei, D.-S. Role of Germ-Free Animal Models in Understanding Interactions of Gut Microbiota to Host and Environmental Health: A Special Reference to Zebrafish. Environ. Pollut. 2021, 279, 116925. [Google Scholar] [CrossRef]
- Bates, J.M.; Mittge, E.; Kuhlman, J.; Baden, K.N.; Cheesman, S.E.; Guillemin, K. Distinct Signals from the Microbiota Promote Different Aspects of Zebrafish Gut Differentiation. Dev. Biol. 2006, 297, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Rawls, J.F. Microbial Influences on Gut Development and Gut-Brain Communication. Development 2021, 148, dev194936. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.E.; Yang, S.; Lamers, G.; Stougaard, J.; Spaink, H.P. Intestinal Microbiome Adjusts the Innate Immune Setpoint during Colonization through Negative Regulation of MyD88. Nat. Commun. 2018, 9, 4099. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Yang, W.; Nguyen, X.T.; Zhang, M.; Ma, H.; Zheng, H.; Zhang, Y.; Chan, K.-G.; Li, S. Application of Heat-Killed Probiotics in Aquaculture. Aquaculture 2022, 548, 737700. [Google Scholar] [CrossRef]
- Yang, H.-L.; Sun, Y.-Z.; Hu, X.; Ye, J.; Lu, K.-L.; Hu, L.-H.; Zhang, J.-J. Bacillus Pumilus SE5 Originated PG and LTA Tuned the Intestinal TLRs/MyD88 Signaling and Microbiota in Grouper (Epinephelus coioides). Fish Shellfish Immunol. 2019, 88, 266–271. [Google Scholar] [CrossRef]
- Van Doan, H. Bacillus spp. in Aquaculture-Mechanisms and Applications: An Update View. In Probiotic Bacteria and Postbiotic Metabolites: Role in Animal and Human Health; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–59. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Kim, E.-Y.; Choi, S.; Hossain, M.T.; Oh, R.-K.; Heo, W.-S.; Lee, J.-M.; Cho, Y.-C.; Kong, I.-S. Effect of a Probiotic Strain, Enterococcus Faecium, on the Immune Responses of Olive Flounder (Paralichthys olivaceus). J. Microbiol. Biotechnol. 2012, 22, 526–529. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.; Kimera, F.; Sewilam, H. Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: A Review. Probiotics Antimicrob. Proteins 2021, 14, 130–157. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Shakouri, M.; Yousefi, S.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Mazandarani, M.; Mozanzadeh, M.T.; Tulino, M.G.; Faggio, C. Humoral and Skin Mucosal Immune Parameters, Intestinal Immune Related Genes Expression and Antioxidant Defense in Rainbow Trout (Oncorhynchus mykiss) Fed Olive (Olea europea L.) Waste. Fish Shellfish Immunol. 2020, 100, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Liebl, A.R.; Cáo, M.A.; dos Santos Nascimento, M.; da Castro, P.D.S.; Duncan, W.L.P.; Pantoja-Lima, J.; Aride, P.H.R.; Bussons, M.R.F.M.; Furuya, W.M.; Faggio, C. Dietary Lysine Requirements of Colossoma macropomum (Cuvier, 1818) Based on Growth Performance, Hepatic and Intestinal Morphohistology and Hematology. Vet. Res. Commun. 2022, 46, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Mahboub, H.H.; Faggio, C.; Hendam, B.M.; Algharib, S.A.; Alkafafy, M.; Hashem, M.A.; Mahmoud, Y.K.; Khamis, T.; Abdel-Ghany, H.M.; Masoud, S.R. Immune-Antioxidant Trait, Aeromonas Veronii Resistance, Growth, Intestinal Architecture, and Splenic Cytokines Expression of Cyprinus carpio Fed Prunus armeniaca Kernel-Enriched Diets. Fish Shellfish Immunol. 2022, 124, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The Potential Use of Probiotics to Improve Animal Health, Efficiency, and Meat Quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Adel, M.; Dawood, M.A. Probiotics Application: Implications for Sustainable Aquaculture. In Probiotic Bacteria and Postbiotic Metabolites: Role in Animal and Human Health; Springer: Berlin/Heidelberg, Germany, 2021; pp. 191–219. [Google Scholar]
- FAO/WHO Expert Consultation. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; World Health Organization: Córdoba, Spain, 2001. [Google Scholar]
- Guardiola, F.A.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M.A. Impact of Date Palm Fruits Extracts and Probiotic Enriched Diet on Antioxidant Status, Innate Immune Response and Immune-Related Gene Expression of European Seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 2016, 52, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Ángeles Esteban, M.; Dadar, M.; Dawood, M.A.; Faggio, C. Host-Associated Probiotics: A Key Factor in Sustainable Aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
- Morshedi, V.; Bojarski, B.; Hamedi, S.; Torahi, H.; Hashemi, G.; Faggio, C. Effects of Dietary Bovine Lactoferrin on Growth Performance and Immuno-Physiological Responses of Asian Sea Bass (Lates calcarifer) Fingerlings. Probiotics Antimicrob. Proteins 2021, 13, 1790–1797. [Google Scholar] [CrossRef]
- Mirbakhsh, M.; Ghaednia, B.; Zorriehzahra, M.J.; Esmaeili, F.; Faggio, C. Dietary Mixed and Sprayed Probiotic Improves Growth Performance and Digestive Enzymes of Juvenile Whiteleg Shrimp (Litopenaeus vannamei, Boone, 1931). J. Appl. Aquac. 2022, 35, 823–836. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel Approaches for Co-Encapsulation of Probiotic Bacteria with Bioactive Compounds, Their Health Benefits and Functional Food Product Development: A Review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- El-Kady, A.A.; Magouz, F.I.; Mahmoud, S.A.; Abdel-Rahim, M.M. The Effects of Some Commercial Probiotics as Water Additive on Water Quality, Fish Performance, Blood Biochemical Parameters, Expression of Growth and Immune-Related Genes, and Histology of Nile Tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737249. [Google Scholar] [CrossRef]
- Vijayaram, S.; Kannan, S. Probiotics: The Marvelous Factor and Health Benefits. Biomed. Biotechnol. Res. J. BBRJ 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Cıl, G.I.; Bulut, G.; Budak, D.; Camkerten, G.; Camkerten, I. Probiotics and Functional Feed. In Probiotics, the Natural Microbiota in Living Organisms; CRC Press: Boca Raton, FL, USA, 2021; pp. 315–342. ISBN 1-351-02754-9. [Google Scholar]
- Foysal, M.J.; Alam, M.; Kawser, A.R.; Hasan, F.; Rahman, M.M.; Tay, C.-Y.; Prodhan, M.S.H.; Gupta, S.K. Meta-Omics Technologies Reveals Beneficiary Effects of Lactobacillus plantarum as Dietary Supplements on Gut Microbiota, Immune Response and Disease Resistance of Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 520, 734974. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Dawood, M.A.; Dossou, S.; Yukun, Z.; Mzengereza, K. Singular Effects of Bacillus Subtilis C-3102 or Saccharomyces Cerevisiae Type 1 on the Growth, Gut Morphology, Immunity, and Stress Resistance of Red Sea Bream (Pagrus major); Mzuzu University: Mzuzu, Malawi, 2021. [Google Scholar] [CrossRef]
- Kong, Y.; Gao, C.; Du, X.; Zhao, J.; Li, M.; Shan, X.; Wang, G. Effects of Single or Conjoint Administration of Lactic Acid Bacteria as Potential Probiotics on Growth, Immune Response and Disease Resistance of Snakehead Fish (Channa argus). Fish Shellfish Immunol. 2020, 102, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Kord, M.I.; Maulu, S.; Srour, T.M.; Omar, E.A.; Farag, A.A.; Nour, A.A.M.; Hasimuna, O.J.; Abdel-Tawwab, M.; Khalil, H.S. Impacts of Water Additives on Water Quality, Production Efficiency, Intestinal Morphology, Gut Microbiota, and Immunological Responses of Nile tilapia Fingerlings under a Zero-Water-Exchange System. Aquaculture 2022, 547, 737503. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; da Silva, R.C.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative Stress Tolerance and Antioxidant Capacity of Lactic Acid Bacteria as Probiotic: A Systematic Review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Dicks, L.M.; Popov, I.V.; Karaseva, A.; Ermakov, A.M.; Suvorov, A.; Tagg, J.R.; Weeks, R.; Chikindas, M.L. Probiotics at War against Viruses: What Is Missing from the Picture? Front. Microbiol. 2020, 11, 1877. [Google Scholar] [CrossRef]
- Govindaraj, K.; Samayanpaulraj, V.; Narayanadoss, V.; Uthandakalaipandian, R. Isolation of Lactic Acid Bacteria from Intestine of Freshwater Fishes and Elucidation of Probiotic Potential for Aquaculture Application. Probiotics Antimicrob. Proteins 2021, 13, 1598–1610. [Google Scholar] [CrossRef]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a Probiotic in Animal Feed: A Review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Vallesi, A.; Pucciarelli, S.; Buonanno, F.; Fontana, A.; Mangiagalli, M. Bioactive Molecules from Protists: Perspectives in Biotechnology. Eur. J. Protistol. 2020, 75, 125720. [Google Scholar] [CrossRef]
- Butt, U.D.; Lin, N.; Akhter, N.; Siddiqui, T.; Li, S.; Wu, B. Overview of the Latest Developments in the Role of Probiotics, Prebiotics and Synbiotics in Shrimp Aquaculture. Fish Shellfish Immunol. 2021, 114, 263–281. [Google Scholar] [CrossRef]
- Labba, I.-C.M.; Andlid, T.; Lindgren, Å.; Sandberg, A.-S.; Sjöberg, F. Isolation, Identification, and Selection of Strains as Candidate Probiotics and Starters for Fermentation of Swedish Legumes. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Rajyalakshmi, K.; Babu, M.K.; Shabana, S.; Satya, A.K. Identification and Screening of Probiotics as a Biocontrol Agent against Pathogenic Vibriosis in Shrimp Aquaculture. Ann. Rom. Soc. Cell Biol. 2021, 25, 12292–12305. [Google Scholar]
- Chang, X.; Kang, M.; Shen, Y.; Yun, L.; Yang, G.; Zhu, L.; Meng, X.; Zhang, J.; Su, X. Bacillus Coagulans SCC-19 Maintains Intestinal Health in Cadmium-Exposed Common Carp (Cyprinus carpio L.) by Strengthening the Gut Barriers, Relieving Oxidative Stress and Modulating the Intestinal Microflora. Ecotoxicol. Environ. Saf. 2021, 228, 112977. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, G.; Li, Z.; Chen, J.; Ren, Y. Effects of Probiotics (Bacillus coagulans) Supplementation after Antibiotic Administration on Growth, Immunity, and Intestinal Microflora in Turbot Scophthalmus maximus. Aquac. Int. 2023. [Google Scholar] [CrossRef]
- Galagarza, O.A.; Smith, S.A.; Drahos, D.J.; Eifert, J.D.; Williams, R.C.; Kuhn, D.D. Modulation of Innate Immunity in Nile tilapia (Oreochromis niloticus) by Dietary Supplementation of Bacillus subtilis Endospores. Fish Shellfish Immunol. 2018, 83, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.U.; Xiang, J.; Xiong, F.; Wang, G.; Zou, H.; Li, W.; Li, M.; Wu, S. Effects of Bacillus Licheniformis on the Growth, Antioxidant Capacity, Intestinal Barrier and Disease Resistance of Grass Carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2020, 97, 344–350. [Google Scholar] [CrossRef]
- Das, S.; Mondal, K.; Sengupta, C. Evaluation of the Probiotic Potential of Streptomyces antibioticus and Bacillus cereus on Growth Performance of Freshwater Catfish Heteropneustes Fossilis. Aquac. Rep. 2021, 20, 100752. [Google Scholar] [CrossRef]
- Loghmani, H.; Khalili Hadad, B.; Kazempoor, R.; Sh, A.S. Investigation of the Effects of Bifidobacterium Bifidum as a Probiotic on Liver Function Enzymes Due to Exposure to E. Coli. O157H7 in Koi Fish (Cyprinus rubrofuscus). J. Surv. Fish. Sci. 2022, 5, 27-3. [Google Scholar] [CrossRef]
- Puvanendran, V.; Rud, I.; Breiland, M.S.W.; Arnesen, J.-A.; Axelsson, L. Probiotic Carnobacterium Divergens Increase Growth Parameters and Disease Resistance in Farmed Atlantic Cod (Gadus morhua) Larvae without Influencing the Microbiota. Aquaculture 2021, 532, 736072. [Google Scholar] [CrossRef]
- Tachibana, L.; Telli, G.S.; de Carla Dias, D.; Goncalves, G.S.; Ishikawa, C.M.; Cavalcante, R.B.; Natori, M.M.; Hamed, S.B.; Ranzani-Paiva, M.J.T. Effect of Feeding Strategy of Probiotic Enterococcus Faecium on Growth Performance, Hematologic, Biochemical Parameters and Non-Specific Immune Response of Nile Tilapia. Aquac. Rep. 2020, 16, 100277. [Google Scholar] [CrossRef]
- Tian, J.-X.; Kang, Y.-H.; Chu, G.-S.; Liu, H.-J.; Kong, Y.-D.; Zhao, L.-H.; Kong, Y.-X.; Shan, X.-F.; Wang, G.-Q. Oral Administration of Lactobacillus Casei Expressing Flagellin A Protein Confers Effective Protection against Aeromonas Veronii in Common Carp, Cyprinus carpio. Int. J. Mol. Sci. 2019, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Sagada, G.; Gray, N.; Wang, L.; Xu, B.; Zheng, L.; Zhong, Z.; Ullah, S.; Tegomo, A.F.; Shao, Q. Effect of Dietary Inactivated Lactobacillus plantarum on Growth Performance, Antioxidative Capacity, and Intestinal Integrity of Black Sea Bream (Acanthopagrus schlegelii) Fingerlings. Aquaculture 2021, 535, 736370. [Google Scholar] [CrossRef]
- Noshair, I.; Kanwal, Z.; Jabeen, G.; Arshad, M.; Yunus, F.-U.-N.; Hafeez, R.; Mairaj, R.; Haider, I.; Ahmad, N.; Alomar, S.Y. Assessment of Dietary Supplementation of Lactobacillus rhamnosus Probiotic on Growth Performance and Disease Resistance in Oreochromis niloticus. Microorganisms 2023, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Z.; Li, D.; Chen, W.-J.; Ban, S.-N.; Liu, T.; Wen, H.; Jiang, M. Effects of Dietary Host-Associated Lactococcus lactis on Growth Performance, Disease Resistance, Intestinal Morphology and Intestinal Microbiota of Mandarin Fish (Siniperca chuatsi). Aquaculture 2021, 540, 736702. [Google Scholar] [CrossRef]
- Al-Hisnawi, A.; Rodiles, A.; Rawling, M.D.; Castex, M.; Waines, P.; Gioacchini, G.; Carnevali, O.; Merrifield, D.L. Dietary Probiotic Pediococcus acidilactici MA18/5M Modulates the Intestinal Microbiota and Stimulates Intestinal Immunity in Rainbow Trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2019, 50, 1133–1151. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, G.; Guo, Z.; Wang, Y.; Xu, Z.; Ren, Y.; Zhang, Q.; Cui, M.; Zhao, X.; Xu, D. Application of Potential Probiotic Strain Streptomyces sp. SH5 on Anti-Aeromonas Infection in Zebrafish Larvae. Fish Shellfish Immunol. 2022, 127, 375–385. [Google Scholar] [CrossRef]
- Boonanuntanasarn, S.; Ditthab, K.; Jangprai, A.; Nakharuthai, C. Effects of Microencapsulated Saccharomyces cerevisiae on Growth, Hematological Indices, Blood Chemical, and Immune Parameters and Intestinal Morphology in Striped Catfish, Pangasianodon hypophthalmus. Probiotics Antimicrob. Proteins 2019, 11, 427–437. [Google Scholar] [CrossRef]
- Zamini, A.; Tehranifard, A. Effects of Lactococcus lactis and Weissella Cibaria as Probiotic on Growth Performance, Intestinal Bacterial Flora, Digestive Enzymes and Intestinal Histology in Common Carp (Cyprinus Carpio). J. Aquac. Dev. 2021, 15, 13–29. [Google Scholar]
- Mingmongkolchai, S.; Panbangred, W. Bacillus Probiotics: An Alternative to Antibiotics for Livestock Production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Ringø, E. Probiotics in Shellfish Aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, E.; Lu, M.; Xie, J. Effects of Probiotic Supplementation on Gut Microbiota as Well as Metabolite Profiles within Nile tilapia, Oreochromis niloticus. Aquaculture 2020, 527, 735428. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut Microbiota Metagenomics in Aquaculture: Factors Influencing Gut Microbiome and Its Physiological Role in Fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Wu, G. Nutrition and Metabolism: Foundations for Animal Growth, Development, Reproduction, and Health. In Recent Advances in Animal Nutrition and Metabolism; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–24. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Hardy, R.W.; Kaushik, S.J.; Mai, K.; Bai, S.C. Fish Nutrition—History and Perspectives. In Fish Nutrition; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–16. [Google Scholar]
- Uma, A.; Subash, P.; Abraham, T.J. Importance of Gut Microbiota in Fish–A Review. Indian J. Anim. Health 2020, 59, 181–194. [Google Scholar] [CrossRef]
- Singh, S.K.; Bhandari, M.P.; Shrestha, S.; Koirala, U.; Gurung, G.B. Supplementation of Commercial Probiotics in Feed for Growth and Survival of Rainbow Trout (Oncorhynchus mykiss). In Proceedings of the National Workshop on Livestock and Fisheries Research in Nepal, Kathmandu, Nepal, 3–4 March 2021; Volume 3, p. 275. [Google Scholar]
- Chen, J.; Sun, D.; Cui, H.; Rao, C.; Li, L.; Guo, S.; Yang, S.; Zhang, Y.; Cao, X. Toxic Effects of Carbon Quantum Dots on the Gut–Liver Axis and Gut Microbiota in the Common Carp Cyprinus carpio. Environ. Sci. Nano 2022, 9, 173–188. [Google Scholar] [CrossRef]
- Phianphak, W.; Rengpipat, S.; Piyatiratitivorakul, S.; Menasveta, P. Probiotic Use of Lactobacillus spp. for Black Tiger Shrimp, Penaeus monodon. J. Sci. Res. Chula Univ. 1999, 24, 41–58. [Google Scholar]
- Tuan, T.N.; Duc, P.M.; Hatai, K. Overview of the Use of Probiotics in Aquaculture. Int. J. Res. Fish. Aquac. 2013, 3, 89–97. [Google Scholar]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.; Dhama, K.; Abdel-Latif, H.M. The Functionality of Probiotics in Aquaculture: An Overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Yassir, R.Y.; Adel, M.E.; Azze, A. Use of Probiotic Bacteria as Growth Promoters, Antibacterial and the Effect on Physiological Parameters of Orechromis niloticus. J. Fish Dis. 2002, 22, 633–642. [Google Scholar]
- Swain, S.; Hauzoukim, S.K.G.; Das, S.K.; Roy, A. Application of Probiotics in Aquaculture. Pharma innov. 2021, 10, 146–149. Available online: https://www.thepharmajournal.com/archives/2021/vol10issue7S/PartC/S-10-6-136-245.pdf (accessed on 1 November 2023).
- Franca, F.M.; Danielle de Carla, D.; Teixeira, P.C.; Marcantonio, A.S.; de Stefani, M.V.; Antonucci, A.; Da Rocha, G.; Ranzani-PAIVA, M.J.T.; Ferreira, C.M. Efeito Do Probiótico Bacillus Subtilis No Crescimento, Sobrevivência e Fisiologia de Rãs-Touro (Rana catesbeiana). Bol. Inst. Pesca 2008, 34, 403–412. [Google Scholar]
- Wang, Z.; Yang, M.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Bacillus Subtilis LCBS1 Supplementation and Replacement of Fish Meal with Fermented Soybean Meal in Bullfrog (Lithobates catesbeianus) Diets: Effects on Growth Performance, Feed Digestibility and Gut Health. Aquaculture 2021, 545, 737217. [Google Scholar] [CrossRef]
- Assan, D.; Kuebutornye, F.K.A.; Hlordzi, V.; Chen, H.; Mraz, J.; Mustapha, U.F.; Abarike, E.D. Effects of Probiotics on Digestive Enzymes of Fish (Finfish and Shellfish); Status and Prospects: A Mini Review. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2022, 257, 110653. [Google Scholar] [CrossRef] [PubMed]
- Lara-Flores, M.; Olivera-Castillo, L.; Olvera-Novoa, M.A. Effect of the Inclusion of a Bacterial Mix (Streptococcus faecium and Lactobacillus acidophilus), and the Yeast (Saccharomyces cerevisiae) on Growth, Feed Utilization and Intestinal Enzymatic Activity of Nile Tilapia (Oreochromis niloticus). Int. J. Fish. Aquac. 2010, 2, 93–101. [Google Scholar]
- Carnevali, O.; Sun, Y.-Z.; Merrifield, D.L.; Zhou, Z.; Picchietti, S. Probiotic Applications in Temperate and Warm Water Fish Species. In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; Wiley: New York, NY, USA, 2014; pp. 253–289. [Google Scholar] [CrossRef]
- Loh, J.Y.; Chan, H.K.; Yam, H.C.; In, L.L.A.; Lim, C.S.Y. An Overview of the Immunomodulatory Effects Exerted by Probiotics and Prebiotics in Grouper Fish. Aquac. Int. 2020, 28, 729–750. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Liu, Y.; Miao, L.-L.; Li, E.-W.; Hou, T.-T.; Liu, Z.-P. Mechanism of Anti-Vibrio Activity of Marine Probiotic Strain Bacillus Pumilus H2, and Characterization of the Active Substance. AMB Express 2017, 7, 23. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y.; Hlordzi, V.; Sakyi, M.E.; Afriyie, G.; Wang, Z.; Li, Y.; Xie, C.X. Mechanisms and the Role of Probiotic Bacillus in Mitigating Fish Pathogens in Aquaculture. Fish Physiol. Biochem. 2020, 46, 819–841. [Google Scholar] [CrossRef]
- Emam, A.M.; Dunlap, C.A. Genomic and Phenotypic Characterization of Bacillus velezensis AMB-Y1; a Potential Probiotic to Control Pathogens in Aquaculture. Antonie Leeuwenhoek 2020, 113, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-M.; Rong, Y.-J.; Zhao, M.-X.; Song, B.; Chi, Z.-M. Antibacterial Activity of the Lipopetides Produced by Bacillus Amyloliquefaciens M1 against Multidrug-Resistant Vibrio spp. Isolated from Diseased Marine Animals. Appl. Microbiol. Biotechnol. 2014, 98, 127–136. [Google Scholar] [CrossRef]
- Chau, K.M.; Van, T.T.H.; Quyen, D.V.; Le, H.D.; Phan, T.H.T.; Ngo, N.D.T.; Vo, T.D.T.; Dinh, T.T.; Le, H.T.; Khanh, H.H.N. Molecular Identification and Characterization of Probiotic Bacillus Species with the Ability to Control Vibrio spp. in Wild Fish Intestines and Sponges from the Vietnam Sea. Microorganisms 2021, 9, 1927. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, Q.; Liu, Y.; Gao, S.; He, Y.; Yao, C.; Huang, W.; Gong, Y.; Mai, K.; Ai, Q. Early Life Intervention Using Probiotic Clostridium butyricum Improves Intestinal Development, Immune Response, and Gut Microbiota in Large Yellow Croaker (Larimichthys crocea) Larvae. Front. Immunol. 2021, 12, 640767. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, B.; Liu, C.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of Dietary Raw or Enterococcus faecium Fermented Soybean Meal on Growth, Antioxidant Status, Intestinal Microbiota, Morphology, and Inflammatory Responses in Turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2020, 100, 261–271. [Google Scholar] [CrossRef]
- Kamei, Y.; Yoshimizu, M.; Ezura, Y.; Kimura, T. Screening of Bacteria with Antiviral Activity from Fresh Water Salmonid Hatcheries. Microbiol. Immunol. 1988, 32, 67–73. [Google Scholar] [CrossRef]
- Hasan, K.N.; Banerjee, G. Recent Studies on Probiotics as Beneficial Mediator in Aquaculture: A Review. J. Basic Appl. Zool. 2020, 81, 53. [Google Scholar] [CrossRef]
- Maeda, M.; Nogami, K.; Kanematsu, M.; Hirayama, K. The Concept of Biological Control Methods in Aquaculture. Hydrobiologia 1997, 358, 285–290. [Google Scholar] [CrossRef]
- Mondal, H.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Viral Infections in Cultured Fish and Shrimps: Current Status and Treatment Methods. Aquac. Int. 2022, 30, 227–262. [Google Scholar] [CrossRef]
- De Andrade Belo, M.A.; Charlie-Silva, I. Teleost Fish as an Experimental Model for Vaccine Development. In Vaccine Design: Methods and Protocols, Volume 2. Vaccines for Veterinary Diseases; Springer: Berlin/Heidelberg, Germany, 2022; pp. 175–194. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S. Recent Advances in the Role of Probiotics and Prebiotics in Carp Aquaculture: A Review. Aquaculture 2016, 454, 243–251. [Google Scholar] [CrossRef]
- Akbari, H.; Shekrabi, S.P.H.; Soltani, M.; Mehrgan, M.S. Effects of Potential Probiotic Enterococcus Casseliflavus (EC-001) on Growth Performance, Immunity, and Resistance to Aeromonas Hydrophila Infection in Common Carp (Cyprinus carpio). Probiotics Antimicrob. Proteins 2021, 13, 1316–1325. [Google Scholar] [CrossRef]
- Rachmawati, R.A.; Mulyani, Y.; Rochima, E.; Grandiosa, R. The Effect of Induction of Bacteria Bacillus Subtilis in Feed on the Immune System of Carp (Cyprinus carpio Linnaeus, 1758). World Sci. News 2021, 160, 203–216. [Google Scholar]
- Shah, S.; Chesti, A.; Rather, M.; Manzoor, S.; Malik, R.; Khan, J. Effect of Probiotic (Bacillus Subtilis) on the Immune System of Fingerlings of Grass Carp, Ctenopharyngodon idella. Pharma Innov. J. 2021, 10, 769–772. [Google Scholar]
- Yan, Y.-Y.; Xia, H.-Q.; Yang, H.-L.; Hoseinifar, S.H.; Sun, Y.-Z. Effects of Dietary Live or Heat-inactivated Autochthonous Bacillus pumilus SE 5 on Growth Performance, Immune Responses and Immune Gene Expression in Grouper Epinephelus coioides. Aquac. Nutr. 2016, 22, 698–707. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Yang, H.-L.; Hu, L.-H.; Yang, W.; Ai, C.-X.; Sun, Y.-Z. Dose-Dependent Effects of Histamine on Growth, Immunity and Intestinal Health in Juvenile Grouper (Epinephelus coioides). Front. Mar. Sci. 2021, 8, 685720. [Google Scholar] [CrossRef]
- Yang, H.-L.; Xia, H.-Q.; Ye, Y.-D.; Zou, W.-C.; Sun, Y.-Z. Probiotic Bacillus Pumilus SE5 Shapes the Intestinal Microbiota and Mucosal Immunity in Grouper Epinephelus coioides. Dis. Aquat. Organ. 2014, 111, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Hu, X.; Ye, J.-D.; Seerengaraj, V.; Yang, W.; Ai, C.-X.; Sun, Y.-Z. Cell Wall Components of Bacillus Pumilus SE5 Improved the Growth, Digestive and Immunity of Grouper (Epinephelus coioides). Curr. Chin. Sci. 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Boo, A.; Amaro, R.L.; Stan, G.-B. Quorum Sensing in Synthetic Biology: A Review. Curr. Opin. Syst. Biol. 2021, 28, 100378. [Google Scholar] [CrossRef]
- Saeki, E.K.; Kobayashi, R.K.T.; Nakazato, G. Quorum Sensing System: Target to Control the Spread of Bacterial Infections. Microb. Pathog. 2020, 142, 104068. [Google Scholar] [CrossRef]
- Defoirdt, T.; Boon, N.; Bossier, P.; Verstraete, W. Disruption of Bacterial Quorum Sensing: An Unexplored Strategy to Fight Infections in Aquaculture. Aquaculture 2004, 240, 69–88. [Google Scholar] [CrossRef]
- Alexpandi, R.; Abirami, G.; Satish, L.; Swasthikka, R.P.; Krishnaveni, N.; Jayakumar, R.; Pandian, S.K.; Ravi, A.V. Tocopherol and Phytol Possess Anti-Quorum Sensing Mediated Anti-Infective Behavior against Vibrio campbellii in Aquaculture: An in Vitro and in Vivo Study. Microb. Pathog. 2021, 161, 105221. [Google Scholar] [CrossRef]
- Samrot, A.V.; Abubakar Mohamed, A.; Faradjeva, E.; Si Jie, L.; Hooi Sze, C.; Arif, A.; Chuan Sean, T.; Norbert Michael, E.; Yeok Mun, C.; Xiao Qi, N. Mechanisms and Impact of Biofilms and Targeting of Biofilms Using Bioactive Compounds—A Review. Medicina 2021, 57, 839. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.-Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of Probiotics in Aquaculture of China—A Review of the Past Decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, S.; Sarkodie, E.K.; Chu, W. The Effects of Bacillus cereus QSI-1 on Intestinal Barrier Function and Mucosal Gene Transcription in Crucian Carp (Carassius auratus gibelio). Aquac. Rep. 2020, 17, 100356. [Google Scholar] [CrossRef]
- Chu, W.; Zhou, S.; Zhu, W.; Zhuang, X. Quorum Quenching Bacteria Bacillus Sp. QSI-1 Protect Zebrafish (Danio rerio) from Aeromonas hydrophila Infection. Sci. Rep. 2014, 4, 5446. [Google Scholar] [CrossRef] [PubMed]
- Shaheer, P.; Sreejith, V.N.; Joseph, T.C.; Murugadas, V.; Lalitha, K.V. Quorum Quenching Bacillus spp.: An Alternative Biocontrol Agent for Vibrio harveyi Infection in Aquaculture. Dis. Aquat. Organ. 2021, 146, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xi, B.; Qin, T.; Chen, K.; Xie, J. Isolation and Characterization of AHL-Degrading Bacteria from Fish and Pond Sediment. J. Oceanol. Limnol. 2019, 37, 1460–1467. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Nasren, S.; Abhiman, P.B.; Rathore, S.S.; Sowndarya, N.S.; Ramesh, K.S.; Shankar, K.M. Investigation of Production, Formation and Characterization of Biofilm Cells of Aeromonas hydrophila for Oral Vaccination of Fish. J. Exp. Zool. India 2019, 22, 1115–1123. [Google Scholar]
- Assan, D.; Huang, Y.; Mustapha, U.F.; Addah, M.N.; Li, G.; Chen, H. Fish Feed Intake, Feeding Behavior, and the Physiological Response of Apelin to Fasting and Refeeding. Front. Endocrinol. 2021, 12, 798903. [Google Scholar] [CrossRef]
- Mohapatra, S.; Chakraborty, T.; Kumar, V.; DeBoeck, G.; Mohanta, K.N. Aquaculture and Stress Management: A Review of Probiotic Intervention. J. Anim. Physiol. Anim. Nutr. 2013, 97, 405–430. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Vianello, S.; Brazzoduro, L.; Dalla Valle, L.; Belvedere, P.; Colombo, L. Myostatin Expression during Development and Chronic Stress in Zebrafish (Danio rerio). J. Endocrinol. 2003, 176, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, E.; Basili, D.; Falcinelli, S.; Morillas, L.; Carnevali, O.; Capilla, E.; Navarro, I. The Probiotic Lactobacillus Rhamnosus Mimics the Dark-Driven Regulation of Appetite Markers and Melatonin Receptors’ Expression in Zebrafish (Danio Rerio) Larvae: Understanding the Role of the Gut Microbiome. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 256, 110634. [Google Scholar] [CrossRef]
- Carnevali, O.; de Vivo, L.; Sulpizio, R.; Gioacchini, G.; Olivotto, I.; Silvi, S.; Cresci, A. Growth Improvement by Probiotic in European Sea Bass Juveniles (Dicentrarchus labrax, L.), with Particular Attention to IGF-1, Myostatin and Cortisol Gene Expression. Aquaculture 2006, 258, 430–438. [Google Scholar] [CrossRef]
- Castex, M.; Lemaire, P.; Wabete, N.; Chim, L. Effect of Dietary Probiotic Pediococcus Acidilactici on Antioxidant Defences and Oxidative Stress Status of Shrimp Litopenaeus Stylirostris. Aquaculture 2009, 294, 306–313. [Google Scholar] [CrossRef]
- Chang, X.; Chen, Y.; Feng, J.; Huang, M.; Zhang, J. Amelioration of Cd-Induced Bioaccumulation, Oxidative Stress and Immune Damage by Probiotic Bacillus coagulans in Common Carp (Cyprinus carpio L.). Aquac. Rep. 2021, 20, 100678. [Google Scholar] [CrossRef]
- Merola, C.; Bisegna, A.; Angelozzi, G.; Conte, A.; Abete, M.C.; Stella, C.; Pederiva, S.; Faggio, C.; Riganelli, N.; Perugini, M. Study of Heavy Metals Pollution and Vitellogenin Levels in Brown Trout (Salmo trutta trutta) Wild Fish Populations. Appl. Sci. 2021, 11, 4965. [Google Scholar] [CrossRef]
- Jyoti, D.; Sinha, R.; Faggio, C. Advances in Biological Methods for the Sequestration of Heavy Metals from Water Bodies: A Review. Environ. Toxicol. Pharmacol. 2022, 94, 103927. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of Heavy Metals on Fish Physiology–A Review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud Univ.-Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Stefanescu, I.A. Bioaccumulation of Heavy Metals by Bacillus megaterium from Phosphogypsum Waste. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2015, 16, 93. [Google Scholar]
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water Contamination by Heavy Metals and Their Toxic Effect on Aquaculture and Human Health through Food Chain. Lett. Appl. NanoBioSci. 2020, 10, 2148–2166. [Google Scholar] [CrossRef]
- Fatima, S.; Muzammal, M.; Rehman, A.; Rustam, S.A.; Shehzadi, Z.; Mehmood, A.; Waqar, M. Water Pollution on Heavy Metals and Its Effects on Fishes. Int. J. Fish Aquat. Stud. 2020, 8, 6–14. [Google Scholar]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and Bioaccumulation of Heavy Metals (Hg, Cd and Pb) in Fish: Influence of the Aquatic Environment and Climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. Bioremediation of Toxic Heavy Metals (THMs) Contaminated Sites: Concepts, Applications and Challenges. Environ. Sci. Pollut. Res. 2020, 27, 27563–27581. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.; Śliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzyński, J. Bioremediation of Heavy Metals by the Genus Bacillus. Int. J. Environ. Res. Public. Health 2023, 20, 4964. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Al-Turki, I.A.; Ramadan, M.F. Application of Lactic Acid Bacteria in Removing Heavy Metals and Aflatoxin B1 from Contaminated Water. Water Sci. Technol. 2016, 74, 625–638. [Google Scholar] [CrossRef]
- Wang, Y.; Han, J.; Ren, Q.; Liu, Z.; Zhang, X.; Wu, Z. The Involvement of Lactic Acid Bacteria and Their Exopolysaccharides in the Biosorption and Detoxication of Heavy Metals in the Gut. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef]
- Murthy, S.; Bali, G.; Sarangi, S.K. Effect of Lead on Metallothionein Concentration in Leadresistant Bacteria Bacillus cereus Isolated from Industrial Effluent. Afr. J. Biotechnol. 2011, 10, 15966–15972. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Biosequestration of Heavy Metals by Microbially Induced Calcite Precipitation of Ureolytic Bacteria. Rom. Biotechnol. Lett. 2018, 24, 147–153. [Google Scholar] [CrossRef]
- Fakhar, A.; Gul, B.; Gurmani, A.R.; Khan, S.M.; Ali, S.; Sultan, T.; Chaudhary, H.J.; Rafique, M.; Rizwan, M. Heavy Metal Remediation and Resistance Mechanism of Aeromonas, Bacillus, and Pseudomonas: A Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1868–1914. [Google Scholar] [CrossRef]
- Rekadwad, B. Microbial Systematics; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Ringø, E.; Van Doan, H.; Lee, S.H.; Soltani, M.; Hoseinifar, S.H.; Harikrishnan, R.; Song, S.K. Probiotics, Lactic Acid Bacteria and Bacilli: Interesting Supplementation for Aquaculture. J. Appl. Microbiol. 2020, 129, 116–136. [Google Scholar] [CrossRef]
- Valipour, A.; Nedaei, S.; Noori, A.; Khanipour, A.A.; Hoseinifar, S.H. Dietary Lactobacillus Plantarum Affected on Some Immune Parameters, Air-Exposure Stress Response, Intestinal Microbiota, Digestive Enzyme Activity and Performance of Narrow Clawed Crayfish (Astacus leptodactylus, Eschscholtz). Aquaculture 2019, 504, 121–130. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, Z.; Zhao, F.; Liu, H.; Yu, L.; Zha, J.; Wang, G. Probiotic Potential of Bacillus velezensis JW: Antimicrobial Activity against Fish Pathogenic Bacteria and Immune Enhancement Effects on Carassius auratus. Fish Shellfish Immunol. 2018, 78, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-B.; Wu, Y.-C.; Chi, S.-C. Dietary Supplementation of Pediococcus Pentosaceus Enhances Innate Immunity, Physiological Health and Resistance to Vibrio anguillarum in Orange-Spotted Grouper (Epinephelus coioides). Fish Shellfish Immunol. 2014, 39, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Dejene, F.; Regasa Dadi, B.; Tadesse, D. In Vitro Antagonistic Effect of Lactic Acid Bacteria Isolated from Fermented Beverage and Finfish on Pathogenic and Foodborne Pathogenic Microorganism in Ethiopia. Int. J. Microbiol. 2021, 2021, 5370556. [Google Scholar] [CrossRef]
- Huy, N.D.; Ngoc, L.M.T.; Loc, N.H.; Lan, T.T.; Quang, H.T.; Dung, T. Isolation of Weissella Cibaria from Pacific White Shrimp (Litopenaeus vannamei) Gastrointestinal Tract and Evaluation of Its Pathogenic Bacterial Inhibition. Indian J. Sci. Technol. 2020, 13, 1200–1212. [Google Scholar] [CrossRef]
- Puvanasundram, P.; Chong, C.M.; Sabri, S.; Yusoff, M.S.; Karim, M. Multi-Strain Probiotics: Functions, Effectiveness and Formulations for Aquaculture Applications. Aquac. Rep. 2021, 21, 100905. [Google Scholar] [CrossRef]
- Midhun, S.J.; Neethu, S.; Arun, D.; Vysakh, A.; Divya, L.; Radhakrishnan, E.K.; Jyothis, M. Dietary Supplementation of Bacillus Licheniformis HGA8B Improves Growth Parameters, Enzymatic Profile and Gene Expression of Oreochromis niloticus. Aquaculture 2019, 505, 289–296. [Google Scholar] [CrossRef]
- Yang, G.; Tian, X.; Dong, S.; Peng, M.; Wang, D. Effects of Dietary Bacillus Cereus G19, B. Cereus BC-01, and Paracoccus marcusii DB11 Supplementation on the Growth, Immune Response, and Expression of Immune-Related Genes in Coelomocytes and Intestine of the Sea Cucumber (Apostichopus japonicus selenka). Fish Shellfish Immunol. 2015, 45, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, L.; Wan, J.; Sun, Z.; Wang, Y.; Sun, H. Effects of Potential Probiotic Bacillus Cereus EN25 on Growth, Immunity and Disease Resistance of Juvenile Sea Cucumber Apostichopus Japonicus. Fish Shellfish Immunol. 2016, 49, 237–242. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Yang, H.-L.; Yan, Y.-Y.; Zhang, C.-X.; Ye, J.; Sun, Y.-Z. Effects of Fish Origin Probiotics on Growth Performance, Immune Response and Intestinal Health of Shrimp (Litopenaeus vannamei) Fed Diets with Fish Meal Partially Replaced by Soybean Meal. Aquac. Nutr. 2020, 26, 1255–1265. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Adesiyun, A.A.; Mutani, A.; Ramsubhag, A.; Brunt, J.; Austin, B. Bacillus Subtilis AB1 Controls Aeromonas Infection in Rainbow Trout (Oncorhynchus mykiss, Walbaum). J. Appl. Microbiol. 2007, 103, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Sahandi, J.; Jafaryan, H.; Soltani, M.; Ebrahimi, P. The Use of Two Bifidobacterium Strains Enhanced Growth Performance and Nutrient Utilization of Rainbow Trout (Oncorhynchus mykiss) Fry. Probiotics Antimicrob. Proteins 2019, 11, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Seppola, M.; Berg, A.; Olsen, R.E.; Schillinger, U.; Holzapfel, W. Characterization of Carnobacterium divergens Strain 6251 Isolated from Intestine of Arctic Charr (Salvelinus alpinus L.). Syst. Appl. Microbiol. 2002, 25, 120–129. [Google Scholar] [CrossRef]
- Ringø, E. The Ability of Carnobacteria Isolated from Fish Intestine to Inhibit Growth of Fish Pathogenic Bacteria: A Screening Study. Aquac. Res. 2008, 39, 171–180. [Google Scholar] [CrossRef]
- Vendrell, D.; Balcazar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Gironés, O.; Muzquiz, J.L. Protection of Rainbow Trout (Oncorhynchus mykiss) from Lactococcosis by Probiotic Bacteria. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 337–345. [Google Scholar] [CrossRef]
- Al-Dohail, M.A.; Hashim, R.; Aliyu-Paiko, M. Evaluating the Use of Lactobacillus acidophilus as a Biocontrol Agent against Common Pathogenic Bacteria and the Effects on the Haematology Parameters and Histopathology in African Catfish Clarias gariepinus Juveniles: L. Acidophilus as a Probiotic in African Catfish. Aquac. Res. 2011, 42, 196–209. [Google Scholar] [CrossRef]
- Zheng, C.N.; Wang, W. Effects of Lactobacillus Pentosus on the Growth Performance, Digestive Enzyme and Disease Resistance of White Shrimp, Litopenaeus vannamei (Boone, 1931). Aquac. Res. 2017, 48, 2767–2777. [Google Scholar] [CrossRef]
- Lee, S.H.; Beck, B.R.; Hwang, S.-H.; Song, S.K. Feeding Olive Flounder (Paralichthys olivaceus) with Lactococcus lactis BFE920 Expressing the Fusion Antigen of Vibrio OmpK and FlaB Provides Protection against Multiple Vibrio Pathogens: A Universal Vaccine Effect. Fish Shellfish Immunol. 2021, 114, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Balcázar, J.L.; Vendrell, D.; De Blas, I.; Ruiz-Zarzuela, I.; Múzquiz, J.L. Effect of Lactococcus lactis CLFP 100 and Leuconostoc mesenteroides CLFP 196 on Aeromonas salmonicida Infection in Brown Trout (Salmo trutta). Microb. Physiol. 2009, 17, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Thao, T.T.P.; Lan, T.T.P.; Phuong, T.V.; Truong, H.T.H.; Khoo, K.S.; Manickam, S.; Hoa, T.T.; Tram, N.D.Q.; Show, P.L.; Huy, N.D. Characterization Halotolerant Lactic Acid Bacteria Pediococcus pentosaceus HN10 and in Vivo Evaluation for Bacterial Pathogens Inhibition. Chem. Eng. Process.-Process Intensif. 2021, 168, 108576. [Google Scholar] [CrossRef]
- Tarkhani, R.; Imani, A.; Hoseinifar, S.H.; Moghanlou, K.S.; Manaffar, R. The Effects of Host-Associated Enterococcus faecium CGMCC1. 2136 on Serum Immune Parameters, Digestive Enzymes Activity and Growth Performance of the Caspian Roach (Rutilus rutilus caspicus) Fingerlings. Aquaculture 2020, 519, 734741. [Google Scholar] [CrossRef]
- Safari, R.; Adel, M.; Lazado, C.C.; Caipang, C.M.A.; Dadar, M. Host-Derived Probiotics Enterococcus casseliflavus Improves Resistance against Streptococcus Iniae Infection in Rainbow Trout (Oncorhynchus mykiss) via Immunomodulation. Fish Shellfish Immunol. 2016, 52, 198–205. [Google Scholar] [CrossRef]
- Ali, M.; Soltanian, S.; Mirghaed, A.T.; Akhlaghi, M.; Hoseinifar, S.H.; Esmailnejad, A. The Effect of Oral Administration of Lactic Acid Bacteria Isolated from Kefir on Intestinal Microbiota, Growth Performance and Survival in Juvenile Rainbow Trout, Oncorhynchus mykiss. Int. J. Aquat. Biol. 2020, 8, 35–49. [Google Scholar] [CrossRef]
- Sakai, M.; Yoshida, T.; Atsuta, S.; Kobayashi, M. Enhancement of Resistance to Vibriosis in Rainbow Trout, Oncorhynchus mykiss (Walbaum), by Oral Administration of Clostridium butyricum Bacterin. J. Fish Dis. 1995, 18, 187–190. [Google Scholar] [CrossRef]
- Meng, X.; Wu, S.; Hu, W.; Zhu, Z.; Yang, G.; Zhang, Y.; Qin, C.; Yang, L.; Nie, G. Clostridium butyricum Improves Immune Responses and Remodels the Intestinal Microbiota of Common Carp (Cyprinus carpio L.). Aquaculture 2021, 530, 735753. [Google Scholar] [CrossRef]
- Kahyani, F.; Pirali-Kheirabadi, E.; Shafiei, S.; Shenavar Masouleh, A. Effect of Dietary Supplementation of Potential Probiotic Weissella Confusa on Innate Immunity, Immune-related Genes Expression, Intestinal Microbiota and Growth Performance of Rainbow Trout (Oncorhynchus mykiss). Aquac. Nutr. 2021, 27, 1411–1420. [Google Scholar] [CrossRef]
- Rengpipat, S.; Rueangruklikhit, T.; Piyatiratitivorakul, S. Evaluations of Lactic Acid Bacteria as Probiotics for Juvenile Seabass Lates calcarifer. Aquac. Res. 2008, 39, 134–143. [Google Scholar] [CrossRef]
- Jinendiran, S.; Archana, R.; Sathishkumar, R.; Kannan, R.; Selvakumar, G.; Sivakumar, N. Dietary Administration of Probiotic Aeromonas veronii V03 on the Modulation of Innate Immunity, Expression of Immune-Related Genes and Disease Resistance against Aeromonas Hydrophila Infection in Common Carp (Cyprinus carpio). Probiotics Antimicrob. Proteins 2021, 13, 1709–1722. [Google Scholar] [CrossRef]
- Abd El-Rhman, A.M.; Khattab, Y.A.E.; Shalaby, A.M.E. Micrococcus Luteus and Pseudomonas Species as Probiotics for Promoting the Growth Performance and Health of Nile Tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2009, 27, 175–180. [Google Scholar] [CrossRef]
- ABdel-Tawwab, M.; Mousa, M.A.A.; Mohammed, M.A. Use of Live Baker’s Yeast, Saccharomyces Cerevisiae, in Practical Diet to Enhance the Growth Performance of Galilee Tilapia, Sarotherodon galilaeus (L.), and Its Resistance to Environmental Copper Toxicity. J. World Aquac. Soc. 2010, 41, 214–223. [Google Scholar] [CrossRef]
- Banu, M.R.; Akter, S.; Islam, M.R.; Mondol, M.N.; Hossain, M.A. Probiotic Yeast Enhanced Growth Performance and Disease Resistance in Freshwater Catfish Gulsa Tengra, Mystus Cavasius. Aquac. Rep. 2020, 16, 100237. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Alamillo, E.; Angulo, C. Probiotic and Immunomodulatory Activity of Marine Yeast Yarrowia lipolytica Strains and Response against Vibrio parahaemolyticus in Fish. Probiotics Antimicrob. Proteins 2021, 13, 1292–1305. [Google Scholar] [CrossRef]
- Raida, M.K.; Larsen, J.L.; Nielsen, M.E.; Buchmann, K. Enhanced Resistance of Rainbow Trout, Oncorhynchus mykiss (Walbaum), against Yersinia Ruckeri Challenge Following Oral Administration of Bacillus subtilis and B. licheniformis (BioPlus2B). J. Fish Dis. 2003, 26, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Sayed Hassani, M.H.; Jourdehi, A.Y.; Zelti, A.H.; Masouleh, A.S.; Lakani, F.B. Effects of Commercial Superzist Probiotic on Growth Performance and Hematological and Immune Indices in Fingerlings Acipenser baerii. Aquac. Int. 2020, 28, 377–387. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, L.; Fan, D.; He, J.; Su, C.; Gao, S.; Zhang, M. Study of Fermented Feed by Mixed Strains and Their Effects on the Survival, Growth, Digestive Enzyme Activity and Intestinal Flora of Penaeus vannamei. Aquaculture 2021, 530, 735703. [Google Scholar] [CrossRef]
- Joseph, T.C.; Kumar, A.; Basha, K.A. Prophylactic Health Products in Aquaculture; ICAR-Central Institute of Fisheries Technology: Kerala, India, 2017. [Google Scholar]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for Enriching Next-Generation Health-Promoting Gut Bacteria through Prebiotics and Other Dietary Components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health Promoting Microbial Metabolites Produced by Gut Microbiota after Prebiotics Metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Lu, Y.; Hao, H.; Liu, J.; Huang, R. Structural Features, Interaction with the Gut Microbiota and Anti-Tumor Activity of Oligosaccharides. RSC Adv. 2020, 10, 16339–16348. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Kurian, A.; Lakshmi, S.; Musthafa, M.S.; Ringo, E.; Faggio, C. Effect of Leucas Aspera against Aeromonas Hydrophila in Nile Tilapia (Oreochromis niloticus): Immunity and Gene Expression Evaluation. Turk. J. Fish. Aquat. Sci. 2021, 22, TRJFAS19802. [Google Scholar] [CrossRef]
- Kumar, J.; Priyadharshini, M.; Madhavi, M.; Begum, S.S.; Ali, A.J.; Musthafa, M.S.; Faggio, C. Impact of Hygrophila Auriculata Supplementary Diets on the Growth, Survival, Biochemical and Haematological Parameters in Fingerlings of Freshwater Fish Cirrhinus mrigala (Hamilton, 1822). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2022, 263, 111097. [Google Scholar] [CrossRef] [PubMed]
- Aliab, S.S.R.; Ambasankar, K.; Praveena, P.E. Effect of Dietary Mannanoligosaccharide on Intestinal Microbiota and Immune Parameters of Asian Seabass (Lates calcarifer) Juveniles. J. Fish Res. 2021, 5, 16–21. [Google Scholar]
- Asaduzzaman, M.D.; Iehata, S.; Akter, S.; Kader, M.A.; Ghosh, S.K.; Khan, M.N.A.; Abol-Munafi, A.B. Effects of Host Gut-Derived Probiotic Bacteria on Gut Morphology, Microbiota Composition and Volatile Short Chain Fatty Acids Production of Malaysian Mahseer Tor Tambroides. Aquac. Rep. 2018, 9, 53–61. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Wu, S. The Growth Performance and Non-Specific Immunity of Juvenile Grass Carp (Ctenopharyngodon idella) Affected by Dietary Alginate Oligosaccharide. 3 Biotech 2021, 11, 46. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, Y.; Xu, N.; Ding, T.; Cui, K.; Chen, Q.; Zhang, J.; Fang, W.; Mai, K.; Ai, Q. Effects of Dietary Astragalus Polysaccharides (APS) on Survival, Growth Performance, Activities of Digestive Enzyme, Antioxidant Responses and Intestinal Development of Large Yellow Croaker (Larimichthys crocea) Larvae. Aquaculture 2020, 517, 734752. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Won, S.; Lee, S.; Lee, S.; Farris, N.W.; Bai, S.C. Nutrition and Feeding of Olive Flounder Paralichthys olivaceus: A Review. Rev. Fish. Sci. Aquac. 2020, 28, 340–357. [Google Scholar] [CrossRef]
- Kazuń, B.; Małaczewska, J.; Kazuń, K.; Kamiński, R.; Adamek-Urbańska, D.; Żylińska-Urban, J. Dietary Administration of β-1, 3/1, 6-Glucan and Lactobacillus Plantarum Improves Innate Immune Response and Increases the Number of Intestine Immune Cells in Roach (Rutilus rutilus). BMC Vet. Res. 2020, 16, 216. [Google Scholar] [CrossRef]
- Deon, M.P.P.; Bicudo, Á.J.A.; Sado, R.Y. Performance, Hematology, and Immunology of Pacu in Response to Dietary Supplementation with Fructooligosaccharides. Pesqui. Agropecuária Bras. 2021, 56, e02460. [Google Scholar] [CrossRef]
- Yousefi, S.; Shokri, M.M.; Noveirian, H.A.; Hoseinifar, S.H. Effects of Dietary Yeast Cell Wall on Biochemical Indices, Serum and Skin Mucus Immune Responses, Oxidative Status and Resistance against Aeromonas hydrophila in Juvenile Persian Sturgeon (Acipenser persicus). Fish Shellfish Immunol. 2020, 106, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Serradell, A.; Torrecillas, S.; Makol, A.; Valdenegro, V.; Fernández-Montero, A.; Acosta, F.; Izquierdo, M.S.; Montero, D. Prebiotics and Phytogenics Functional Additives in Low Fish Meal and Fish Oil Based Diets for European Sea Bass (Dicentrarchus labrax): Effects on Stress and Immune Responses. Fish Shellfish Immunol. 2020, 100, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Priya, P.S.; Ashwitha, A.; Thamizharasan, K.; Harishkumar, M.; Dinesh, S.; Nithya, T.G.; Kamaraj, M. Synergistic Effect of Durian Fruit Rind Polysaccharide Gel Encapsulated Prebiotic and Probiotic Dietary Supplements on Growth Performance, Immune-Related Gene Expression, and Disease Resistance in Zebrafish (Danio rerio). Heliyon 2021, 7, e06669. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, T.; Ghanei-Motlagh, R.; Molayemraftar, T.; Mesbah, M.; Zarea, M.; Mohtashamipour, H.; Nejad, A.J. Modulation of Growth Performance, Gut Microflora, Non-Specific Immunity and Gene Expression of Proinflammatory Cytokines in Shabout (Tor grypus) upon Dietary Prebiotic Supplementation. Fish Shellfish Immunol. 2021, 112, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Hoseinifar, S.H.; Faggio, C.; Chitmanat, C.; Mai, N.T.; Jaturasitha, S.; Ringø, E. Effects of Corncob Derived Xylooligosaccharide on Innate Immune Response, Disease Resistance, and Growth Performance in Nile Tilapia (Oreochromis niloticus) Fingerlings. Aquaculture 2018, 495, 786–793. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K. Efficiency of Resistant Starch and Dextrins as Prebiotics: A Review of the Existing Evidence and Clinical Trials. Nutrients 2021, 13, 3808. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; González, M.; Murado, M.A. Effects of Lactic Acid Bacteria Cultures on Pathogenic Microbiota from Fish. Aquaculture 2005, 245, 149–161. [Google Scholar] [CrossRef]
- Poolsawat, L.; Li, X.; Xu, X.; Rahman, M.M.; Boonpeng, N.; Leng, X. Dietary Xylooligosaccharide Improved Growth, Nutrient Utilization, Gut Microbiota and Disease Resistance of Tilapia (Oreochromis niloticus x O. Aureus). Anim. Feed Sci. Technol. 2021, 275, 114872. [Google Scholar] [CrossRef]
- Maas, R.M.; Deng, Y.; Dersjant-Li, Y.; Petit, J.; Verdegem, M.C.; Schrama, J.W.; Kokou, F. Exogenous Enzymes and Probiotics Alter Digestion Kinetics, Volatile Fatty Acid Content and Microbial Interactions in the Gut of Nile Tilapia. Sci. Rep. 2021, 11, 8221. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, J.; Selma-Royo, M.; Simal-Gandara, J.; Collado, M.C.; Barba, F.J. Potential Benefits of High-Added-Value Compounds from Aquaculture and Fish Side Streams on Human Gut Microbiota. Trends Food Sci. Technol. 2021, 112, 484–494. [Google Scholar] [CrossRef]
- Maas, R.M.; Verdegem, M.C.; Wiegertjes, G.F.; Schrama, J.W. Carbohydrate Utilisation by Tilapia: A Meta-analytical Approach. Rev. Aquac. 2020, 12, 1851–1866. [Google Scholar] [CrossRef]
- Wee, W.; Hamid, N.K.A.; Mat, K.; Khalif, R.I.A.R.; Rusli, N.D.; Rahman, M.M.; Kabir, M.A.; Wei, L.S. The Effects of Mixed Prebiotics in Aquaculture: A Review. Aquac. Fish. 2022, 9, 28–34. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.-L.; Yan, Y.-Y.; Zhang, C.-X.; Ye, J.; Lu, K.-L.; Hu, L.-H.; Zhang, J.-J.; Ruan, L.; Sun, Y.-Z. Effects of Fructooligosaccharide on Growth, Immunity and Intestinal Microbiota of Shrimp (Litopenaeus vannamei) Fed Diets with Fish Meal Partially Replaced by Soybean Meal. Aquac. Nutr. 2019, 25, 194–204. [Google Scholar] [CrossRef]
- Mustafa, A.; Buentello, A.; Gatlin, D., III; Lightner, D.; Hume, M.; Lawrence, A. Effects of Fructooligosaccharides (FOS) on Growth, Survival, Gut Microflora, Stress, and Immune Response in Pacific White Shrimp, Litopenaeus vannamei, Cultured in a Recirculating System. J. Immunoass. Immunochem. 2020, 41, 45–59. [Google Scholar] [CrossRef]
- Zhu, W.; Su, J. Immune Functions of Phagocytic Blood Cells in Teleost. Rev. Aquac. 2022, 14, 630–646. [Google Scholar] [CrossRef]
- Tran, N.T.; Li, S. Potential Role of Prebiotics and Probiotics in Conferring Health Benefits in Economically Important Crabs. Fish Shellfish Immunol. Rep. 2022, 3, 100041. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Zhao, Y.; Mai, K.; Xu, W.; Zhang, W.; Ai, Q.; Zhang, Y.; Wang, X.; Liufu, Z. Influence of Dietary Probiotic Bacillus TC22 and Prebiotic Fructooligosaccharide on Growth, Immune Responses and Disease Resistance against Vibrio Splendidus Infection in Sea Cucumber Apostichopus japonicus. J. Ocean Univ. China 2011, 10, 293–300. [Google Scholar] [CrossRef]
- Park, Y.; Zhang, Q.; Wiegertjes, G.F.; Fernandes, J.M.; Kiron, V. Adherent Intestinal Cells from Atlantic Salmon Show Phagocytic Ability and Express Macrophage-Specific Genes. Front. Cell Dev. Biol. 2020, 8, 580848. [Google Scholar] [CrossRef]
- Picchietti, S.; Miccoli, A.; Fausto, A.M. Gut Immunity in European Sea Bass (Dicentrarchus labrax): A Review. Fish Shellfish Immunol. 2021, 108, 94–108. [Google Scholar] [CrossRef]
- Maldonado, E.; Rojas, D.A.; Morales, S.; Miralles, V.; Solari, A. Dual and Opposite Roles of Reactive Oxygen Species (ROS) in Chagas Disease: Beneficial on the Pathogen and Harmful on the Host. Oxid. Med. Cell. Longev. 2020, 2020, 8867701. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, D.L.; Ringo, E. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 0-470-67271-4. [Google Scholar]
- Ghafarifarsani, H.; Rashidian, G.; Bagheri, T.; Hoseinifar, S.H.; Van Doan, H. Study on Growth Enhancement and the Protective Effects of Dietary Prebiotic Inulin on Immunity Responses of Rainbow Trout (Oncorhynchus mykiss) Fry Infected with Aeromonas Hydrophila. Ann. Anim. Sci. 2021, 21, 543–559. [Google Scholar] [CrossRef]
- Dong, C.; Wang, J. Immunostimulatory Effects of Dietary Fructooligosaccharides on Red Swamp Crayfish, Procambarus clarkii (Girard). Aquac. Res. 2013, 44, 1416–1424. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Ghaedi, G. β-Glucan as a Promising Food Additive and Immunostimulant in Aquaculture Industry. Ann. Anim. Sci. 2022, 22, 817–827. [Google Scholar] [CrossRef]
- Raa, J. The Use of Immune-Stimulants in Fish and Shellfish Feeds. In Avances en Nutrición Acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola. 19–22 Noviembre, 2000; Cruz -Suárez, L.E., Ricque-Marie, D., Tapia-Salazar, M., Olvera-Novoa, M.A., y Civera-Cerecedo, R., Eds.; Mérida: Yucatán, Mexico, 2000. [Google Scholar]
- Song, H.; Zhang, S.; Yang, B.; Liu, Y.; Kang, Y.; Li, Y.; Qian, A.; Yuan, Z.; Cong, B.; Shan, X. Effects of Four Different Adjuvants Separately Combined with Aeromonas veronii Inactivated Vaccine on Haematoimmunological State, Enzymatic Activity, Inflammatory Response and Disease Resistance in Crucian Carp. Fish Shellfish Immunol. 2022, 120, 658–673. [Google Scholar] [CrossRef]
- Geraylou, Z.; Souffreau, C.; Rurangwa, E.; De Meester, L.; Courtin, C.M.; Delcour, J.A.; Buyse, J.; Ollevier, F. Effects of Dietary Arabinoxylan-Oligosaccharides (AXOS) and Endogenous Probiotics on the Growth Performance, Non-Specific Immunity and Gut Microbiota of Juvenile Siberian Sturgeon (Acipenser bábaerii). Fish Shellfish Immunol. 2013, 35, 766–775. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Ghaedi, G.; Sharifinia, M. Effects of Diets Containing Β-glucan on Survival, Growth Performance, Haematological, Immunity and Biochemical Parameters of Rainbow Trout (Oncorhynchus mykiss) Fingerlings. Aquac. Res. 2022, 53, 1842–1850. [Google Scholar] [CrossRef]
- Marcos-López, M.; Rodger, H.D. Amoebic Gill Disease and Host Response in Atlantic Salmon (Salmo salar L.): A Review. Parasite Immunol. 2020, 42, e12766. [Google Scholar] [CrossRef]
- Reis, B.; Gonçalves, A.T.; Santos, P.; Sardinha, M.; Conceição, L.E.; Serradeiro, R.; Pérez-Sánchez, J.; Calduch-Giner, J.; Schmid-Staiger, U.; Frick, K. Immune Status and Hepatic Antioxidant Capacity of Gilthead Seabream Sparus aurata Juveniles Fed Yeast and Microalga Derived β-Glucans. Mar. Drugs 2021, 19, 653. [Google Scholar] [CrossRef]
- Pogue, R.; Murphy, E.J.; Fehrenbach, G.W.; Rezoagli, E.; Rowan, N.J. Exploiting Immunomodulatory Properties of β-Glucans Derived from Natural Products for Improving Health and Sustainability in Aquaculture-Farmed Organisms: Concise Review of Existing Knowledge, Innovation and Future Opportunities. Curr. Opin. Environ. Sci. Health 2021, 21, 100248. [Google Scholar] [CrossRef]
- Mameloco, E.J.; Traifalgar, R.F. Supplementation of Combined Mannan Oligosaccharide and β-Glucan Immunostimulants Improves Immunological Responses and Enhances Resistance of Pacific Whiteleg Shrimp, Penaeus vannamei, against Vibrio parahaemolyticus Infection. Int. Aquat. Res. 2020, 12, 291. [Google Scholar] [CrossRef]
- Cornet, V.; Khuyen, T.D.; Mandiki, S.N.; Betoulle, S.; Bossier, P.; Reyes-López, F.E.; Tort, L.; Kestemont, P. GAS1: A New β-Glucan Immunostimulant Candidate to Increase Rainbow Trout (Oncorhynchus mykiss) Resistance to Bacterial Infections with Aeromonas salmonicida achromogenes. Front. Immunol. 2021, 12, 693613. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.Ø.; Lagos, L.; Lei, P.; Reveco-Urzua, F.E.; Morales-Lange, B.; Hansen, L.D.; Schiavone, M.; Mydland, L.T.; Arntzen, M.Ø.; Mercado, L. Down-Stream Processing of Baker’s Yeast (Saccharomyces cerevisiae)–Effect on Nutrient Digestibility and Immune Response in Atlantic Salmon (Salmo salar). Aquaculture 2021, 530, 735707. [Google Scholar] [CrossRef]
- El-Nobi, G.; Hassanin, M.; Khalil, A.A.; Mohammed, A.Y.; Amer, S.A.; Montaser, M.M.; El-Sharnouby, M.E. Synbiotic Effects of Saccharomyces cerevisiae, Mannan oligosaccharides, and β-Glucan on Innate Immunity, Antioxidant Status, and Disease Resistance of Nile Tilapia, Oreochromis niloticus. Antibiotics 2021, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, G.; Ouraji, H.; Khalesi, M.K.; Sudagar, M.; Barari, A.; Zarei Dangesaraki, M.; Jani Khalili, K.H. Effects of a Prebiotic, Immunogen®, on Feed Utilization, Body Composition, Immunity and Resistance to Aeromonas Hydrophila Infection in the Common Carp Cyprinus carpio (Linnaeus) Fingerlings. J. Anim. Physiol. Anim. Nutr. 2012, 96, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Mirvaghefi, A.; Mojazi Amiri, B.; Rostami, H.K.; Merrifield, D.L. The Effects of Oligofructose on Growth Performance, Survival and Autochthonous Intestinal Microbiota of Beluga (Huso huso) Juveniles. Aquac. Nutr. 2011, 17, 498–504. [Google Scholar] [CrossRef]
- Ziółkowska, E.; Bogucka, J.; Dankowiakowska, A.; Rawski, M.; Mazurkiewicz, J.; Stanek, M. Effects of a Trans-Galactooligosaccharide on Biochemical Blood Parameters and Intestine Morphometric Parameters of Common Carp (Cyprinus carpio L.). Animals 2020, 10, 723. [Google Scholar] [CrossRef]
- Akter, M.N.; Zahan, K.; Zafar, M.A.; Khatun, N.; Rana, M.S.; Mursalin, M.I. Effects of Dietary Mannan Oligosaccharide on Growth Performance, Feed Utilization, Body Composition and Haematological Parameters in Asian Catfish (Clarias batrachus) Juveniles. Turk. J. Fish. Aquat. Sci. 2021, 21, 559–567. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of Dietary Supplementation of Bacillus subtilis and Fructooligosaccharide on Growth Performance, Survival, Non-Specific Immune Response and Disease Resistance of Juvenile Large Yellow Croaker, Larimichthys Crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Hu, Y.; Winter, V.; Gänzle, M. In Vitro Digestibility of Commercial and Experimental Isomalto-Oligosaccharides. Food Res. Int. 2020, 134, 109250. [Google Scholar] [CrossRef]
- Oushani, A.K.; Soltani, M.; Sheikhzadeh, N.; Mehrgan, M.S.; Islami, H.R. Effects of Dietary Chitosan and Nano-Chitosan Loaded Clinoptilolite on Growth and Immune Responses of Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2020, 98, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-Based Drug Delivery System: Applications in Fish Biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef] [PubMed]
- Andresen, A.M.S.; Gjøen, T. Chitosan Nanoparticle Formulation Attenuates Poly (I: C) Induced Innate Immune Responses against Inactivated Virus Vaccine in Atlantic Salmon (Salmo Salar). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 40, 100915. [Google Scholar] [CrossRef]
- Kamilya, D.; Khan, M.I.R. Chitin and Chitosan as Promising Immunostimulant for Aquaculture. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 761–771. [Google Scholar]
- De Campos, C.M.; Zanuzzo, F.S.; Gimbo, R.Y.; Favero, G.C.; Soares, M.P.; Pilarski, F.; Urbinati, E.C. Dietary Inulin Modulated the Cortisol Response and Increased the Protection against Pathogens in Juvenile Pacu (Piaractus mesopotamicus). Aquac. Res. 2022, 53, 860–869. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Yu, X.-B.; Qiao, F.; Chen, L.-Q.; Du, Z.-Y.; Zhang, M.-L. Inulin Alleviates Adverse Metabolic Syndrome and Regulates Intestinal Microbiota Composition in Nile Tilapia (Oreochromis niloticus) Fed with High-Carbohydrate Diet. Br. J. Nutr. 2021, 126, 161–171. [Google Scholar] [CrossRef]
- Xue, S.; Xia, B.; Zhang, B.; Li, L.; Zou, Y.; Shen, Z.; Xiang, Y.; Han, Y.; Chen, W. Mannan Oligosaccharide (MOS) on Growth Performance, Immunity, Inflammatory and Antioxidant Responses of the Common Carp (Cyprinus carpio) under Ammonia Stress. Front. Mar. Sci. 2022, 9, 1062597. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Qin, J.G.; Wang, X.; Chen, L.; Xu, C.; Li, E. Dietary Prebiotic Inulin Benefits on Growth Performance, Antioxidant Capacity, Immune Response and Intestinal Microbiota in Pacific White Shrimp (Litopenaeus vannamei) at Low Salinity. Aquaculture 2020, 518, 734847. [Google Scholar] [CrossRef]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sabharwal, V.; Kaushik, P.; Joshi, A.; Aayushi, A.; Suri, M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022, 6, 887642. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics against Pathogens Commonly Involved in Pediatric Infectious Diseases. Microorganisms 2020, 8, 1510. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, R.; Graczyk, D.; Siedlecki, P. Molecular and Cellular Mechanisms Influenced by Postbiotics. Int. J. Mol. Sci. 2021, 22, 13475. [Google Scholar] [CrossRef]
- Vallejo-Cordoba, B.; Castro-López, C.; García, H.S.; González-Córdova, A.F.; Hernández-Mendoza, A. Postbiotics and Paraprobiotics: A Review of Current Evidence and Emerging Trends. Adv. Food Nutr. Res. 2020, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, S.A.; Roher, N.; Boltaña, S.; Goetz, F.W. Peptidoglycan, Not Endotoxin, Is the Key Mediator of Cytokine Gene Expression Induced in Rainbow Trout Macrophages by Crude LPS. Mol. Immunol. 2010, 47, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, K.; Xu, D.; Wu, M.; Mai, K.; Ai, Q. Molecular Identification of Peptidoglycan Recognition Protein 5 and Its Functional Characterization in Innate Immunity of Large Yellow Croaker, Larimichthys crocea. Dev. Comp. Immunol. 2021, 124, 104130. [Google Scholar] [CrossRef] [PubMed]
- Casadei, E.; Bird, S.; Wadsworth, S.; Vecino, J.L.G.; Secombes, C.J. The Longevity of the Antimicrobial Response in Rainbow Trout (Oncorhynchus mykiss) Fed a Peptidoglycan (PG) Supplemented Diet. Fish Shellfish Immunol. 2015, 44, 316–320. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Wei, S.; Huang, J. Effects of Different Enzymatic Hydrolysis Methods on the Bioactivity of Peptidoglycan in Litopenaeus vannamei. Chin. J. Oceanol. Limnol. 2013, 31, 374–383. [Google Scholar] [CrossRef]
- Itami, T.; Asano, M.; Tokushige, K.; Kubono, K.; Nakagawa, A.; Takeno, N.; Nishimura, H.; Maeda, M.; Kondo, M.; Takahashi, Y. Enhancement of Disease Resistance of Kuruma Shrimp, Penaeus Japonicus, after Oral Administration of Peptidoglycan Derived from Bifidobacterium thermophilum. Aquaculture 1998, 164, 277–288. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic lactobacilli, Their Positive Effects on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Du, Y.; Wang, B.; Jiang, K.; Wang, M.; Zhou, S.; Liu, M.; Wang, L. Exploring the Influence of the Surface Proteins on Probiotic Effects Performed by Lactobacillus Pentosus HC-2 Using Transcriptome Analysis in Litopenaeus vannamei Midgut. Fish Shellfish Immunol. 2019, 87, 853–870. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, G.; Guru, A.; Haridevamuthu, B.; Murugan, R.; Arshad, A.; Arockiaraj, J. Molecular Properties of Postbiotics and Their Role in Controlling Aquaculture Diseases. Aquac. Res. 2022, 53, 3257–3273. [Google Scholar] [CrossRef]
- Kim, D.-H.; Subramanian, D.; Park, S.-H.; Jang, Y.-H.; Heo, M.-S. Assessment and Potential Application of the Probiotic Strain, Bacillus Amyloliquefaciens JFP2, Isolated from Fermented Seafood-Jeotgal in Flounder Paralichthys olivaceus Juveniles. Isr. J. Aquac.-Bamidgeh 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Mora-Sánchez, B.; Balcázar, J.L.; Pérez-Sánchez, T. Effect of a Novel Postbiotic Containing Lactic Acid Bacteria on the Intestinal Microbiota and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss). Biotechnol. Lett. 2020, 42, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Güngören, A.; Koluman, A.; İlhak, O.İ.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of Lactic Acid Bacteria Postbiotics, Evaluation in-Vitro Antibacterial Effect, Microbial and Chemical Quality on Chicken Drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef]
- Chang, H.M.; Foo, H.L.; Loh, T.C.; Lim, E.T.C.; Abdul Mutalib, N.E. Comparative Studies of Inhibitory and Antioxidant Activities, and Organic Acids Compositions of Postbiotics Produced by Probiotic Lactiplantibacillus Plantarum Strains Isolated from Malaysian Foods. Front. Vet. Sci. 2021, 7, 602280. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Tian, X.; Wang, B.; Wei, C.; Wang, L.; Zhang, S.; Liu, Y.; Li, T.; Dong, S. Evaluation of Paraprobiotic Applicability of Clostridium butyricum CBG01 in Improving the Growth Performance, Immune Responses and Disease Resistance in Pacific White Shrimp, Penaeus vannamei. Aquaculture 2021, 544, 737041. [Google Scholar] [CrossRef]
- Wu, X.; Teame, T.; Hao, Q.; Ding, Q.; Liu, H.; Ran, C.; Yang, Y.; Zhang, Y.; Zhou, Z.; Duan, M. Use of a Paraprobiotic and Postbiotic Feed Supplement (HWFTM) Improves the Growth Performance, Composition and Function of Gut Microbiota in Hybrid Sturgeon (Acipenser baerii x Acipenser Schrenckii). Fish Shellfish Immunol. 2020, 104, 36–45. [Google Scholar] [CrossRef]
- Del Valle, J.C.; Bonadero, M.C.; Gimenez, A.V.F. Saccharomyces cerevisiae as Probiotic, Prebiotic, Synbiotic, Postbiotics and Parabiotics in Aquaculture: An Overview. Aquaculture 2023, 569, 739342. [Google Scholar] [CrossRef]
- Sousa, J.M.G.; Louvado, A.; Coelho, F.; Oliveira, V.; Oliveira, H.; Cleary, D.F.R.; Gomes, N.C.M. In Vitro Study of the Modulatory Effects of Heat-Killed Bacterial Biomass on Aquaculture Bacterioplankton Communities. Sci. Rep. 2022, 12, 19699. [Google Scholar] [CrossRef]
- Feng, J.; Cai, Z.; Chen, Y.; Zhu, H.; Chang, X.; Wang, X.; Liu, Z.; Zhang, J.; Nie, G. Effects of an Exopolysaccharide from Lactococcus lactis Z-2 on Innate Immune Response, Antioxidant Activity, and Disease Resistance against Aeromonas hydrophila in Cyprinus Carpio L. Fish Shellfish Immunol. 2020, 98, 324–333. [Google Scholar] [CrossRef]
- Gao, Q.; Gao, Q.; Min, M.; Zhang, C.; Peng, S.; Shi, Z. Ability of Lactobacillus plantarum Lipoteichoic Acid to Inhibit Vibrio anguillarum-Induced Inflammation and Apoptosis in Silvery Pomfret (Pampus argenteus) Intestinal Epithelial Cells. Fish Shellfish Immunol. 2016, 54, 573–579. [Google Scholar] [CrossRef]
- Meng, D.; Hao, Q.; Zhang, Q.; Yu, Z.; Liu, S.; Yang, Y.; Ran, C.; Zhang, Z.; Zhou, Z. A Compound of Paraprobiotic and Postbiotic Derived from Autochthonous Microorganisms Improved Growth Performance, Epidermal Mucus, Liver and Gut Health and Gut Microbiota of Common Carp (Cyprinus carpio). Aquaculture 2023, 570, 739378. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gini, E.; Koch, J.F.A.; Iannini, F.; Brambilla, F.; Terova, G. Effects of Hydrolyzed Fish Protein and Autolyzed Yeast as Substitutes of Fishmeal in the Gilthead Sea Bream (Sparus aurata) Diet, on Fish Intestinal Microbiome. BMC Vet. Res. 2020, 16, 118. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Onoda, S.; Van Khanh, T.; Hai, P.D.; Trung, N.T.; Hoang, L.; Koshio, S. Evaluation of Dietary Heat-Killed Lactobacillus plantarum Strain L-137 Supplementation on Growth Performance, Immunity and Stress Resistance of Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 498, 371–379. [Google Scholar] [CrossRef]
- Dawood, M.A.; Abo-Al-Ela, H.G.; Hasan, M.T. Modulation of Transcriptomic Profile in Aquatic Animals: Probiotics, Prebiotics and Synbiotics Scenarios. Fish Shellfish Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef]
- Kuo, H.-W.; Chang, C.-C.; Cheng, W. Synbiotic Combination of Prebiotic, Cacao Pod Husk Pectin and Probiotic, Lactobacillus plantarum, Improve the Immunocompetence and Growth of Litopenaeus vannamei. Fish Shellfish Immunol. 2021, 118, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Talebi, M.; Yousefi, M.; Van Doan, H.; Rufchaei, R.; Paolucci, M. Combined and Singular Effects of Ethanolic Extract of Persian Shallot (Allium Hirtifolium Boiss) and Synbiotic Biomin® IMBO on Growth Performance, Serum-and Mucus-Immune Parameters and Antioxidant Defense in Zebrafish (Danio rerio). Animals 2021, 11, 2995. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Hafezieh, M.; Karimi, A.A.; Azra, M.N.; Van Doan, H.; Tapingkae, W.; Abdelrahman, H.A.; Dawood, M.A. The Synergistic Effects of Plant Polysaccharide and Pediococcus Acidilactici as a Synbiotic Additive on Growth, Antioxidant Status, Immune Response, and Resistance of Nile Tilapia (Oreochromis niloticus) against Aeromonas Hydrophila. Fish Shellfish Immunol. 2022, 120, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Dehaghani, P.G.; Baboli, M.J.; Moghadam, A.T.; Ziaei-Nejad, S.; Pourfarhadi, M. Effect of Synbiotic Dietary Supplementation on Survival, Growth Performance, and Digestive Enzyme Activities of Common Carp (Cyprinus carpio) Fingerlings. Czech J. Anim. Sci. 2015, 60, 224–232. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, P.; Sharma, M.M.M.; Kumari, A.; Kumar, R. Microbes in Pharmaceutical Industry. In Microbial Diversity, Interventions and Scope; Springer: Berlin/Heidelberg, Germany, 2020; pp. 259–299. [Google Scholar] [CrossRef]
- Elabd, H.; Faggio, C.; Mahboub, H.H.; Emam, M.A.; Kamel, S.; El Kammar, R.; Abdelnaeim, N.S.; Shaheen, A.; Tresnakova, N.; Matter, A. Mucuna Pruriens Seeds Extract Boosts Growth, Immunity, Testicular Histology, and Expression of Immune-Related Genes of Mono-Sex Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2022, 127, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lumsangkul, C.; Linh, N.V.; Chaiwan, F.; Abdel-Tawwab, M.; Dawood, M.A.; Faggio, C.; Jaturasitha, S.; Van Doan, H. Dietary Treatment of Nile Tilapia (Oreochromis niloticus) with Aquatic Fern (Azolla caroliniana) Improves Growth Performance, Immunological Response, and Disease Resistance against Streptococcus agalactiae Cultured in Bio-Floc System. Aquac. Rep. 2022, 24, 101114. [Google Scholar] [CrossRef]
- Jamal, M.T.; Sumon, A.A.; Pugazhendi, A.; Al Harbi, M.; Hussain, A.; Haque, F. Use of Probiotics in Commercially Important Finfish Aquaculture. Int. J. Probiotics Prebiotics 2020, 15, 7–21. [Google Scholar] [CrossRef]
- Prabawati, E.; Hu, S.-Y.; Chiu, S.-T.; Balantyne, R.; Risjani, Y.; Liu, C.-H. A Synbiotic Containing Prebiotic Prepared from a By-Product of King Oyster Mushroom, Pleurotus eryngii and Probiotic, Lactobacillus plantarum Incorporated in Diet to Improve the Growth Performance and Health Status of White Shrimp, Litopenaeus Vannamei. Fish Shellfish Immunol. 2022, 120, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.; Das, R.R.; Sivakumar, M.R.; Saravanan, A.; Saranya, C.; Sudheer, N.S.; Vasagam, K.K.; Mahalakshmi, P.; Kannappan, S.; Gopikrishna, G. Bio-Augmentation of Heterotrophic Bacteria in Biofloc System Improves Growth, Survival, and Immunity of Indian White Shrimp Penaeus Indicus. Fish Shellfish Immunol. 2020, 98, 477–487. [Google Scholar] [CrossRef]
- Rajeev, R.; Adithya, K.K.; Kiran, G.S.; Selvin, J. Healthy Microbiome: A Key to Successful and Sustainable Shrimp Aquaculture. Rev. Aquac. 2021, 13, 238–258. [Google Scholar] [CrossRef]
- Safari, O.; Sarkheil, M.; Shahsavani, D.; Paolucci, M. Effects of Single or Combined Administration of Dietary Synbiotic and Sodium Propionate on Humoral Immunity and Oxidative Defense, Digestive Enzymes and Growth Performances of African Cichlid (Labidochromis lividus) Challenged with Aeromonas hydrophila. Fishes 2021, 6, 63. [Google Scholar] [CrossRef]
- Ye, J.-D.; Wang, K.; Li, F.-D.; Sun, Y.-Z. Single or Combined Effects of Fructo- and Mannan Oligosaccharide Supplements and Bacillus clausii on the Growth, Feed Utilization, Body Composition, Digestive Enzyme Activity, Innate Immune Response and Lipid Metabolism of the Japanese Flounder Paralichth: Dietary Benefits of FOS, MOS and B. clausii in the Japanese Flounder. Aquac. Nutr. 2011, 17, e902–e911. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Hoseini, S.M.; Bagheri, D. Effects of Galactooligosaccharide and Pediococcus Acidilactici on Antioxidant Defence and Disease Resistance of Rainbow Trout, Oncorhynchus Mykiss. Ann. Anim. Sci. 2017, 17, 217–227. [Google Scholar] [CrossRef][Green Version]
- Wan, J.-J.; Pan, J.; Shen, M.-F.; Xue, H.; Sun, M.; Zhang, M.-Q.; Zhu, X.-H.; Ma, X. Changes in the Growth Performance, Antioxidant Enzymes and Stress Resistance Caused by Dietary Administration of Synbiotic (Fructooligosaccharide and Probiotics) in Juvenile Chinese Mitten Crab, Eriocheir Sinensis. Aquac. Int. 2022, 30, 467–481. [Google Scholar] [CrossRef]
- Maniat, M.; Salati, A.P.; Zanguee, N.; Mousavi, S.M.; Hoseinifar, S.H. Effects of Dietary Pediococcus acidilactici and Isomaltooligosaccharide on Growth Performance, Immunity, and Antioxidant Defense in Juvenile Common Carp. Aquac. Nutr. 2023, 2023, e1808640. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic Application for Sustainable Aquaculture. Rev. Aquac. 2019, 11, 907–924. [Google Scholar] [CrossRef]
- Goh, J.X.H.; Tan, L.T.-H.; Law, J.W.-F.; Ser, H.-L.; Khaw, K.-Y.; Letchumanan, V.; Lee, L.-H.; Goh, B.-H. Harnessing the Potentialities of Probiotics, Prebiotics, Synbiotics, Paraprobiotics, and Postbiotics for Shrimp Farming. Rev. Aquac. 2022, 14, 1478–1557. [Google Scholar] [CrossRef]
- Carbone, D.; Faggio, C. Importance of Prebiotics in Aquaculture as Immunostimulants. Effects on Immune System of Sparus Aurata and Dicentrarchus Labrax. Fish Shellfish Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, F.M.; Hussein, S.H.; Awad, S.M.; El-Makhzangy, A.A. Growth Promoter, Immune Response, and Histopathological Change of Prebiotic, Probiotic and Synbiotic Bacteria on Nile Tilapia. Saudi J. Biol. Sci. 2023, 30, 103539. [Google Scholar] [CrossRef]
- Jahangiri, L.; Esteban, M.Á. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- Subedi, B.; Shrestha, A. A Review: Application of Probiotics in Aquaculture. Int. J. For. Anim. Fish. Res. 2020, 4, 5. [Google Scholar]
- Peñalosa-Martinell, D.; Araneda-Padilla, M.; Dumas, S.; Martinez-Díaz, S.; Vela-Magaña, M. The Use of Probiotics in Larval Whiteleg Shrimp ( Litopenaeus vannamei ) Production: A Marginal Analysis of Bioeconomic Feasibility. Aquac. Res. 2021, 52, 943–951. [Google Scholar] [CrossRef]
- De Azevedo, R.V.; Fosse Filho, J.C.; Cardoso, L.D.; da Mattos, D.C.; Vidal Júnior, M.V.; de Andrade, D.R. Economic Evaluation of Prebiotics, Probiotics and Symbiotics in Juvenile Nile Tilapia. Rev. Ciênc. Agronômica 2015, 46, 72–79. [Google Scholar] [CrossRef]
- Dias, D.d.C.; Furlaneto, F.d.P.B.; Sussel, F.R.; Tachibana, L.; Gonçalves, G.S.; Ishikawa, C.M.; Natori, M.M.; Ranzani-Paiva, M.J.T. Economic Feasibility of Probiotic Use in the Diet of Nile Tilapia, Oreochromis niloticus, during the Reproductive Period. Acta Sci. Anim. Sci. 2020, 42, e47960. [Google Scholar] [CrossRef]
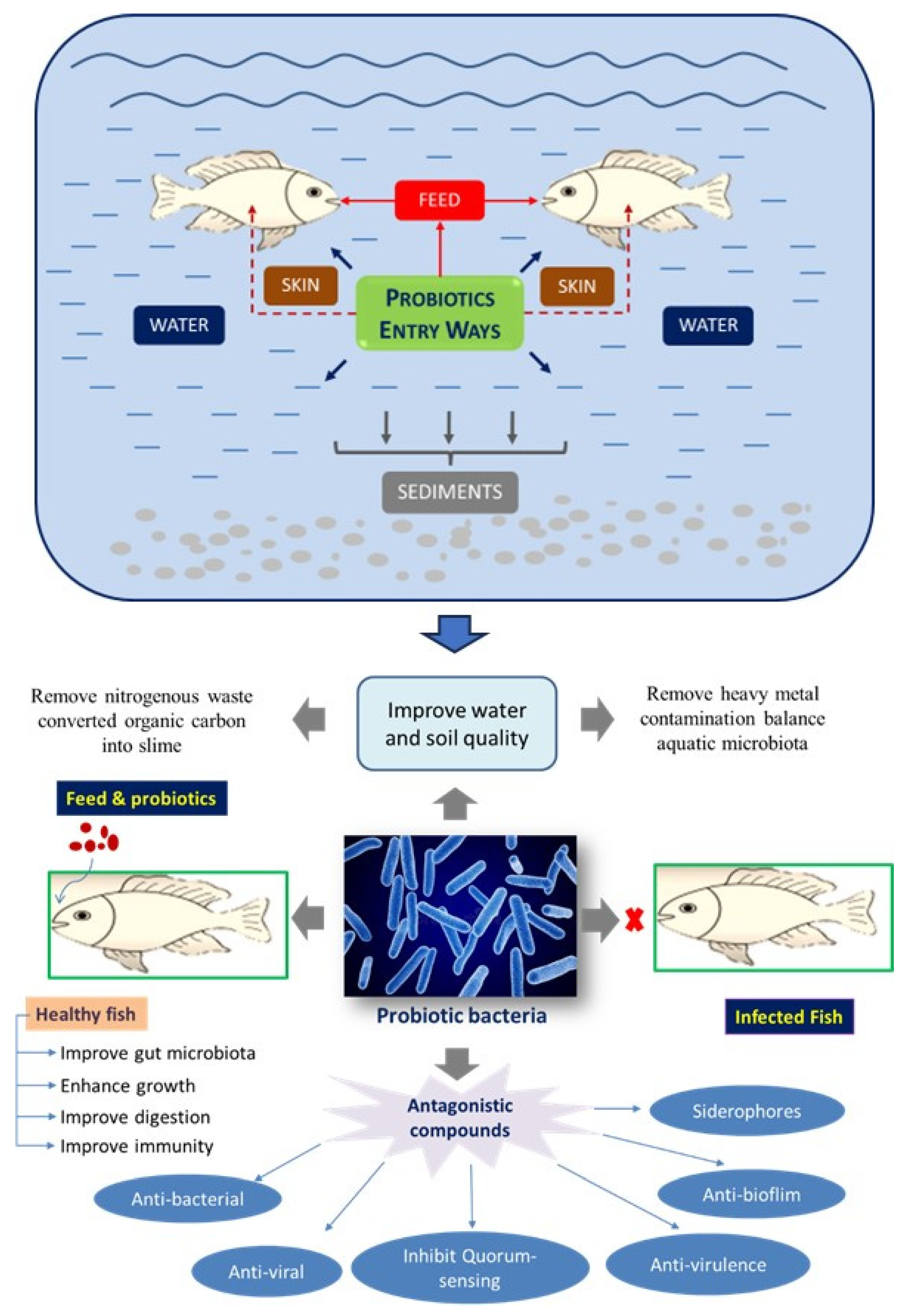
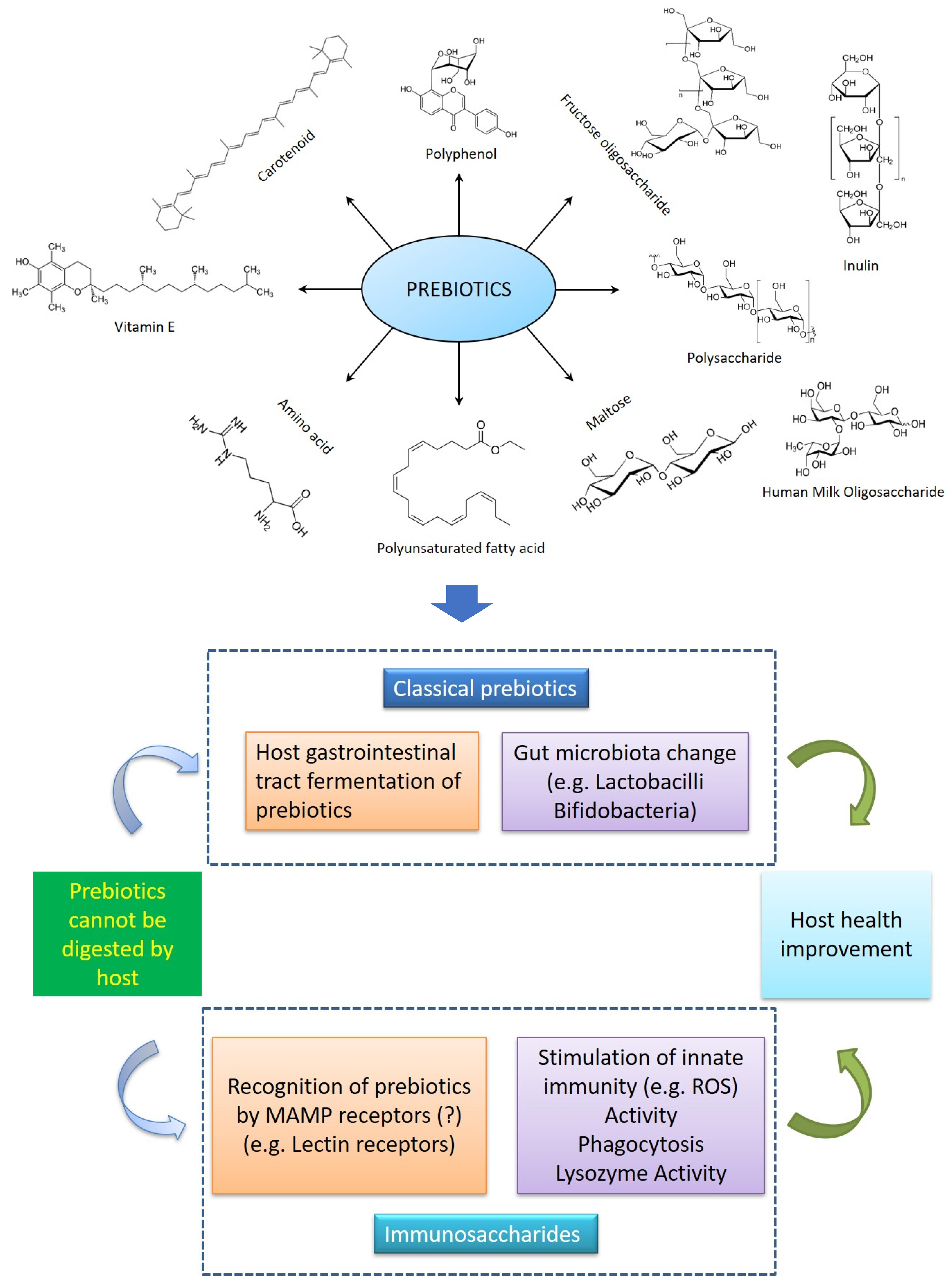
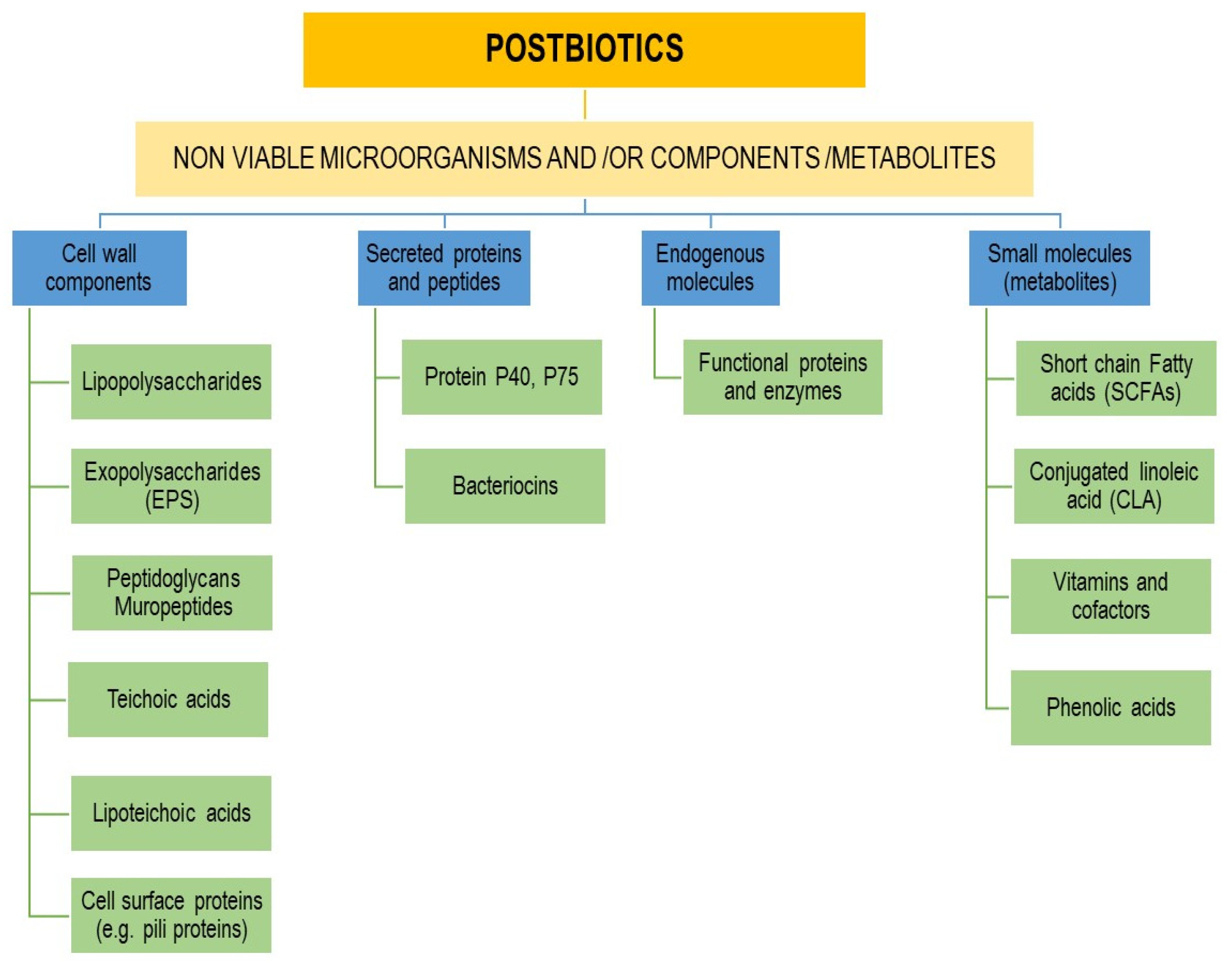
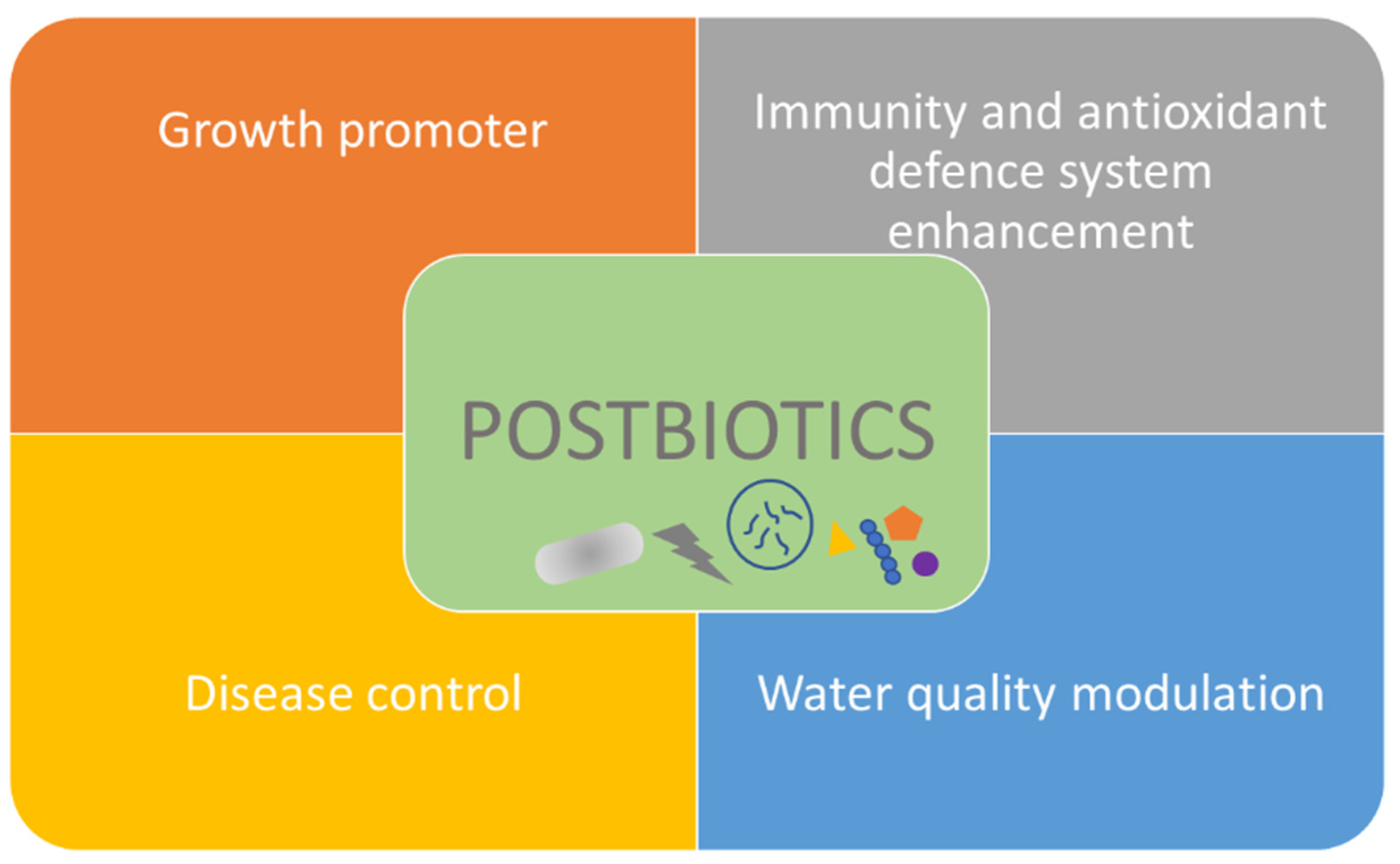

| Genus | Probiotics | Example of Target Fish Species | References |
|---|---|---|---|
| Bacillus | Bacillus coagulans | Common carp (Cyprinus carpio), turbot (Scophthalmus Maximus) | [52,53] |
| Bacillus subtilis | Nile tilapia (O. niloticus) | [54] | |
| Bacillus licheniformis | Grass carp (Ctenopharyngodon idella) | [55] | |
| Bacillus cereus | Catfish (Heteropneustes fossilis) | [56] | |
| Bifidobacterium | Bifidobacterim bifidus | Koi fish (Cyprinus rubrofuscus) | [57] |
| Carnobacterium | Carnobacterium divergens | Atlantic cod (Gadus morhua) | [58] |
| Enterococcus | Enterococcus faecium | Nile tilapia (O. niloticus) | [59] |
| Lactobacillus | Lactobacillus casei | Common carp (Cyprinus carpio) | [60] |
| L. plantarum | Black sea bream (Acanthopagrus schlegelii) | [61] | |
| L. rhamnosus | Nile tilapia (O. niloticus) | [62] | |
| Lactococcus | L. lactis | Mandarin fish (Siniperca chuatsi) | [63] |
| Pediococcus | Pediococcus acidilactici | Rainbow trout (Oncorhynchus mykiss) | [64] |
| Streptomyces | Streptomyces sp. | Zebrafish (Danio rerio) | [65] |
| Saccharomyces | Saccharomyces cerevisiae | Striped catfish (Pangasianodon hypophthalmus) | [66] |
| Weissella | Weissella cibaria | Common carp (Cyprinus carpio) | [67] |
| Probiotics Organisms | Functions | Aquatic Organisms | References |
|---|---|---|---|
| Bacillus | |||
| B. licheniformis HGA8B | ↑ growth performance and ↓ feed conversion ratio Up-regulation of immune genes | O. niloticus | [153] |
| B. cereus G19 B. cereus BC-01 | ↑ growth and immunity | Apostichopus japonicus | [154] |
| B. cereus EN25 | Immunity and resistance against Vibrio splendidus | A. japonicus | [155] |
| B. pumilus SE5 | ↑ growth and immunity | L. vannamei | [156] |
| B. subtilis AB1 | Bactericidal activity against Aeromonas infection | O. mykiss | [157] |
| Bifidobacterium | |||
| Bifidobacterium animalis PTCC-1631 | ↑ growth performance, digestion, and nutrient utilization | O. mykiss | [158] |
| B. lactis PTCC-1736 | ↑ growth, nutrient digestibility, and carcass composition | O. mykiss | [158] |
| Carnobacterium | |||
| C. divergens C. maltaromaticum | Antagonistic effects against V. anguillarum, V. viscosus, and A. salmonicida | - | [159,160] |
| Lactobacillus | |||
| L. plantarum CLFP | ↓ mortality against harmful strain L. garvieae | O. mykiss | [161] |
| L. acidophilus | Survival against Staphylococcus xylosus, Aeromonas hydrophila gr.2, and Streptococcus agalactiae infection | Clarias gariepinus | [162] |
| L. pentosus | ↑ growth performance and feed conversion ratio ↑ survival against Vibrio species | L. vannamei | [163] |
| Lactococcus | |||
| Lactococcus lactis BFE920 | Activation of nonspecific immune system Bactericidal activity against S. iniae | Paralichthys olivaceus | [164] |
| Leuconostoc | |||
| Lc. Mesenteroides CLFP 196 | ↑ survival against A. salmonicida infection | Salmo trutta | [165] |
| Pediococcus | |||
| P. pentosaceus HN10 | ↑ feed utilization, digestive enzyme activity, and anti-Vibrio activity | L. vannamei | [166] |
| Enterococcus | |||
| E. casseliflavus CGMCC1.2136 | ↑ growth performance, immunity, and digestive enzyme activity | Rutilus rutilus caspicus | [167] |
| E. casseliflavus | ↑ growth performance and disease resistance against S. iniae | O. mykiss | [168] |
| E. durans | ↑ growth performance and survival rate | O. mykiss | [169] |
| Clostridium | |||
| C. butyricum | ↑ antibacterial activity against Vibriosis infection | O. mykiss | [170] |
| C. butyricum | ↑ immunity; regulation of gut microbiota; antagonistic effects against Aeromonas sp., Vibrio sp., and Pseudomonas sp. | C. carpio | [171] |
| Weissella | |||
| W. confusa | ↑ growth performance | O. mykiss | [172] |
| W. confusa | ↑ growth performance and antibacterial activity against A. hydrophila | Lates calcarifer | [173] |
| Other strains | |||
| A. veronii BA-1 | ↑ immune system and antibacterial activity | C. carpio | [174] |
| Micrococcus luteus | ↑ growth performance and feed conversion ratio | O. niloticus | [175] |
| Pseudoalteromonas undina VKM-124 | ↑ survival and antiviral activity | Carangoides bartholomaei | [99] |
| Yeast | |||
| S.cerevisiae | ↑ growth performance and resistance against waterborne Cu toxicity | Sarotherodon galilaeus | [176] |
| S. cerevisiae | ↑ immunity and ↓ mortality against P. fluorescens | Mystus cavasius | [177] |
| Yarrowia lipolytica | ↑ immune response, antioxidant status, and disease resistance against V. parahaemolyticus infection | Lutjanus peru | [178] |
| Multi-strain | |||
| B. subtilis and Bacillus licheniformis (BioPlus2B) | ↑ resistance against Y. ruckeri | O. mykiss | [179] |
| Lactobacillus delbrueckii Lactobacillus rhamnosus L. plantarum B. bifidum | ↑ growth performance and immunity | Acipenser baerii | [180] |
| Lactobacillus plantarum (STBL1), Saccharomyces cerevisiae (STBS1), and Bacillus safensis (SQVG18) | ↑ growth, antioxidant capacity, digestion, and gut microflora | P. vannamei | [181] |
| Prebiotics | Functions | Aquatic Species | References |
|---|---|---|---|
| FOS | ↑ growth, survival, and gut microbiota section | L. vannamei | [208] |
| β-glucan | ↑ growth, survival, and immune system | Sparus aurata | [225] |
| MOS | ↑ growth, immune system, antioxidant capacity, and intestinal health | Cyprinus carpio | [243] |
| Chitosan | ↑ growth, feed utilization, lipid metabolism, gut microbiota composition, and immune system | Cyprinus carpio koi | [240] |
| Inulin | ↑ growth, antioxidant capacity, immunity, and gut microbiota at low salinity | L. vannamei | [244] |
| Postbiotics | Microorganism Producer | Aquatic Species | Applications | References |
|---|---|---|---|---|
| Exopolysaccharides | Lactococcus lactis Z-2 | Common carp (C. carpio) | Immunity enhancement Resistance against A. hydrophila | [268] |
| Cell surface proteins | L. pentosus | Shrimp (Litopenaeus vannamei) | Immune response improvement | [258] |
| Cell wall components (PGs and LTA) | B. pumilus SE5 | Grouper (E. coioides) | Growth performance improvement Innate and adaptive immunity amelioration | [109] |
| Lipoteichoic acids | L. plantarum LTA | Silvery pomfret (Pampus argenteus) | Resistance against V. anguillarum-caused vibriosis | [269] |
| Non-living microorganisms | S. cerevisiae, B. velezensis and Cetobacterium somerae | Common carp (C. carpio) | Gut microbiota improvement Enhancement of nonspecific immunity Antioxidant status improvement | [270] |
| Dried autolyzed yeast | Gilthead sea bream (Sparus aurata) | Intestinal microbiota improvement | [271] | |
| Rhodotorula minuta and Cetobacterium somerae | Hybrid sturgeon (Acipenser baerii × Acipenser schrencki) | Growth performance improvement Nonspecific immunity improvement | [265] | |
| Heat-killed L. plantarum L-137 | Nile tilapia (O. niloticus) | Growth performance stress resistance and immunity enhancement | [272] |
| Synbiotics | Functions | Aquatic Organisms | References |
|---|---|---|---|
| P.acidilactici + GOS | ↑ growth, survival, and digestive enzyme function | Labidochromis lividus | [285] |
| B. clausii + FOS, MOS | ↑ growth, survival, and digestive enzyme function | Paralichthys olivaceus | [286] |
| P.acidilactici + GOS | ↑ immunity and antagonistic activity against S. iniae infections | Oncorhynchus mykiss | [287] |
| B. subtilis + L. acidophilus + S. cerevisiae + FOS | ↑ growth and feed efficiency ratio | Eriocheir sinensis | [288] |
| P.acidilactici + IMO | ↑ growth, immune response, and antioxidant capacity | C. carpio | [289] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srirengaraj, V.; Razafindralambo, H.L.; Rabetafika, H.N.; Nguyen, H.-T.; Sun, Y.-Z. Synbiotic Agents and Their Active Components for Sustainable Aquaculture: Concepts, Action Mechanisms, and Applications. Biology 2023, 12, 1498. https://doi.org/10.3390/biology12121498
Srirengaraj V, Razafindralambo HL, Rabetafika HN, Nguyen H-T, Sun Y-Z. Synbiotic Agents and Their Active Components for Sustainable Aquaculture: Concepts, Action Mechanisms, and Applications. Biology. 2023; 12(12):1498. https://doi.org/10.3390/biology12121498
Chicago/Turabian StyleSrirengaraj, Vijayaram, Hary L. Razafindralambo, Holy N. Rabetafika, Huu-Thanh Nguyen, and Yun-Zhang Sun. 2023. "Synbiotic Agents and Their Active Components for Sustainable Aquaculture: Concepts, Action Mechanisms, and Applications" Biology 12, no. 12: 1498. https://doi.org/10.3390/biology12121498
APA StyleSrirengaraj, V., Razafindralambo, H. L., Rabetafika, H. N., Nguyen, H.-T., & Sun, Y.-Z. (2023). Synbiotic Agents and Their Active Components for Sustainable Aquaculture: Concepts, Action Mechanisms, and Applications. Biology, 12(12), 1498. https://doi.org/10.3390/biology12121498








