3.3. Ovarian and Reproductive Tract Histology of the Koala Interestrous Phase
The koala reproductive tissue in the interestrous phase will be used here to give a general base-line histological description of the ovary and reproductive tract, from which to compare relative changes in histology accompanying the proliferative and luteal phases. The ovaries of the interestrous koala were typically dorso-ventrally flattened ellipsoidal structures and on their surface typically contained one or more Graafian follicles of 3 mm or less in diameter. The koala ovary can be arbitrarily divided into an outer cortical and an inner medullary region, which merged with the vascular connective tissue of the mesovarium at the hilus of the ovary (
Figure 3A). The medulla includes nerves, large blood vessels and lymphatic vessels entering the ovary from the mesovarium and through the hilum. Arteries entered the ovary at the hilum, and in the medulla they formed plexuses, giving rise to small arterial branches to the theca cells of the follicles, corpora lutea, and the stroma cells. The venous return was parallel to the arterial supply. The nerves that supply the ovary appeared to be non-myelinated, followed blood vessels, and terminated in the walls of the vessels and around the follicles, in the corpora lutea (luteal phase), and in the tunica albuginea. The cortex consisted of ovarian follicles at different stages of development embedded within stromal connective tissue. The outer cortex was covered by a superficial mesothelium derived from the visceral peritoneum consisting of single flat or cuboidal (5–8 µm in diameter) epithelial cells. The nuclei of the superficial epithelium contained round to oval hyperchromatic nuclei (2–3 µm in diameter) with dispersed chromatin. Immediately beneath the superficial epithelium lay a thick layer of connective tissue (60–70 µm in width), the tunica albuginea.
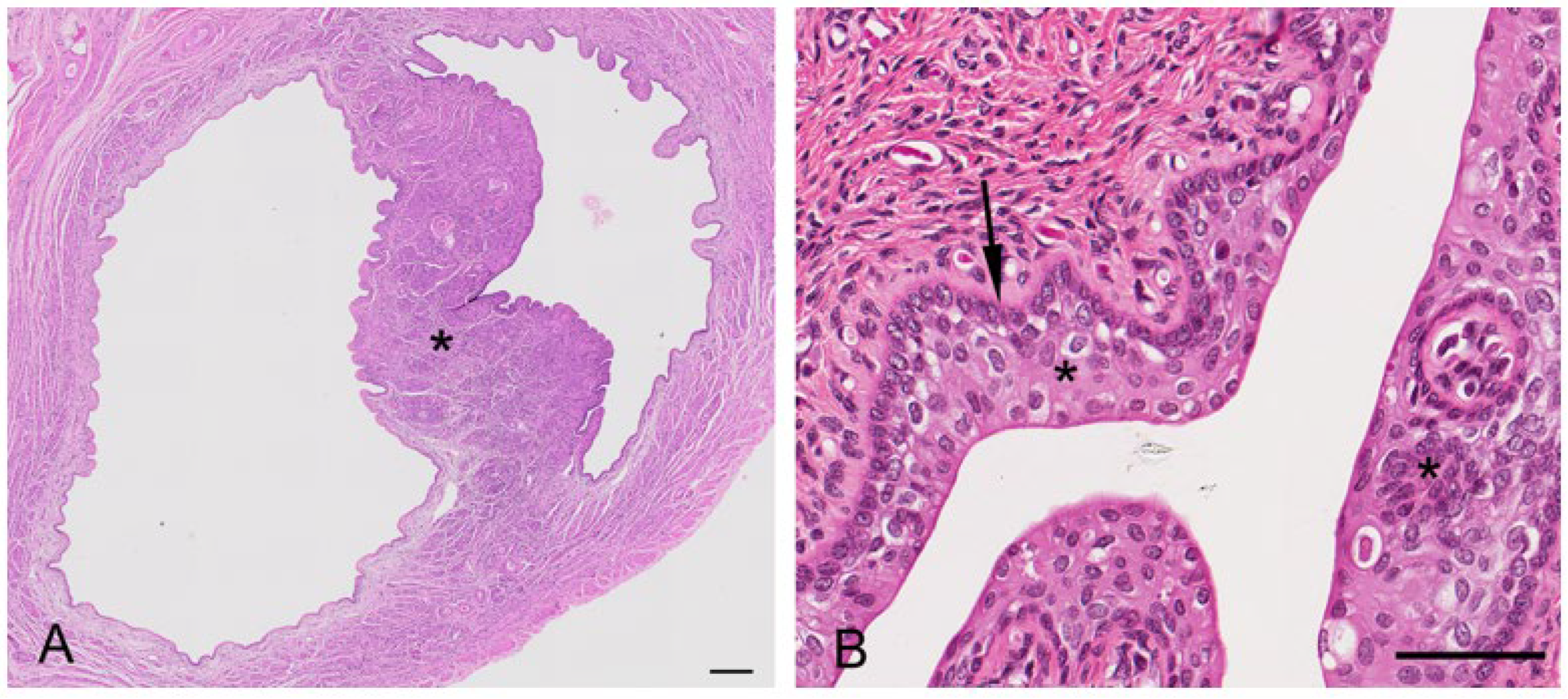
Primordial follicles measured 30–40 µm in diameter and consisted of an oogonium of approximately 7–10 µm diameter, surrounded by a single layer of flattened epithelial-like follicular cells (simple squamous epithelium;
Figure 3B). A thin basal lamina separated the follicle from the ovarian stroma. The primordial follicles were mostly located adjacent to the outer extremity of the cortex and could be found in groups of 5–10 follicles. In the early stages of oocyte growth, follicular cells surrounding the oocyte changed from flattened to cuboidal in appearance to denote the formation of a primary follicle (
Figure 3C). The primary follicles (70–80 µm in diameter) consisted of a primary oocyte encircled by a single layer of now cuboidal follicular cells. By the time the second layer of follicular cells was formed around the oocyte (secondary follicle), the follicular cells were essentially columnar in appearance, and the zona pellucida discernible as a thin eosinophilic layer around the circumference of the oogonium (
Figure 3D). The oocyte of the secondary follicle was encircled by a stratified epithelium of follicular polyhedral granulosa cells. These cells had a round to elongated nucleus, and were occasionally hyperchromatic, but otherwise vesicular with dispersed chromatin and a moderate amount of eosinophilic cytoplasm. The oocyte was characterized by a well-defined round nucleus with a round central to eccentric nucleolus and granular chromatin. Thecal tissue was also clearly delineated into external and internal layers by the time the secondary follicle was 130 µm in diameter. The cells forming the theca interna layer had an elongated nucleus with a scarce amount of eosinophilic cytoplasm.
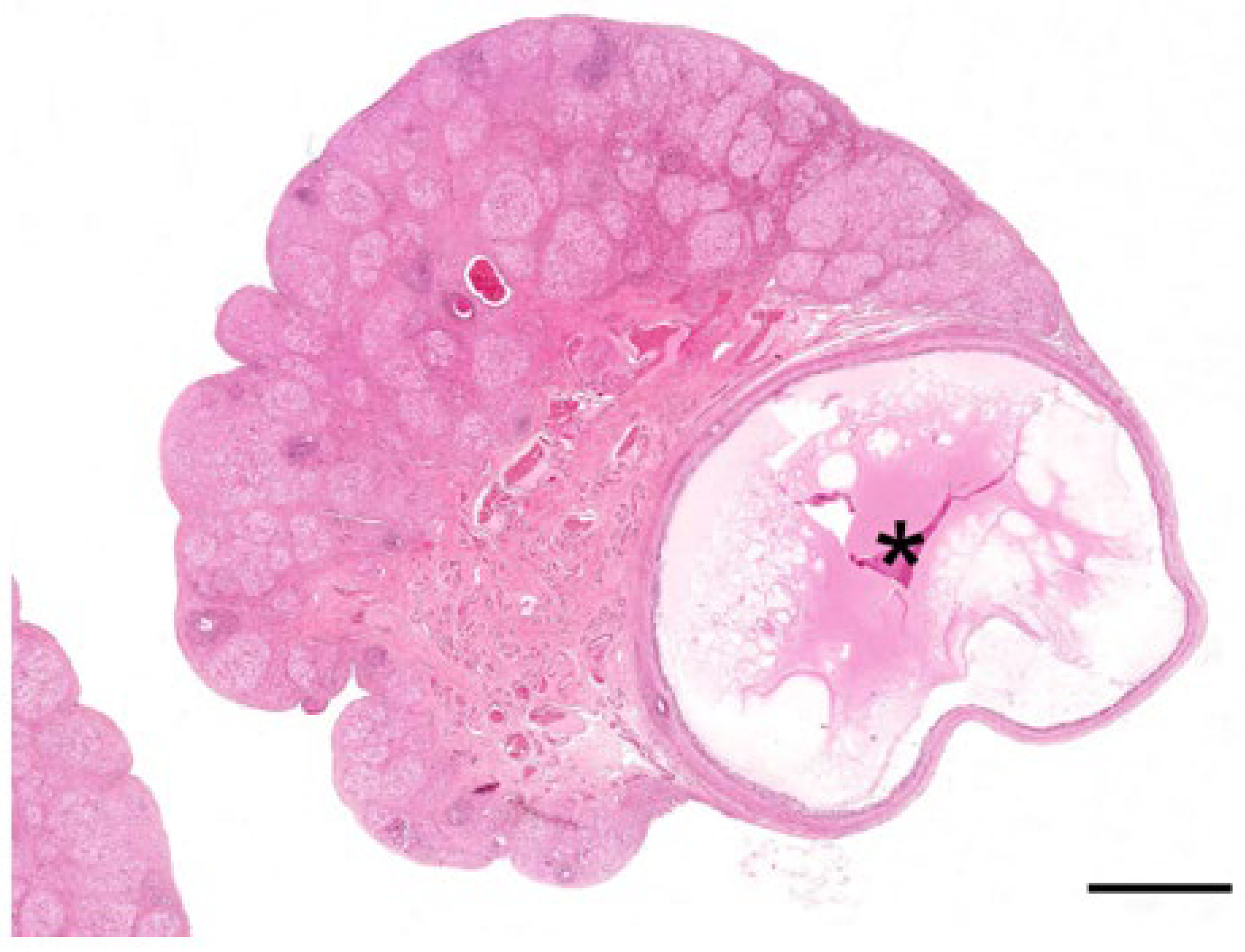
Early stages of antrum formation occurred when the diameters of the follicle and oocyte were approximately 230 µm and 120 µm, respectively. The antrum formation coincided with the appearance of the third and/or fourth layer of follicular cells (
Figure 3E). The antrum formation was characterized by the coalescence of small fluid-filled fissures to form a single cavity containing the follicular fluid (liquor folliculi). The typical koala
Graafian follicle (2 to 3 mm) (
Figure 3F) of the interestrous ovary consisted of an outer theca externa composed of elongated fibrous cells, measuring 42.6 ± 2.7 µm (
n = 51) thick. The outer extremity of the theca externa was often difficult to clearly delineate from the surrounding stromal tissue. The theca interna measured 41.9 ± 2.2 µm (
n = 51) and consisted of slightly enlarged epithelioid stromal cells. Both theca interna and theca externa were supplied with numerous blood vessels. The koala oocyte was usually suspended in the antrum to one side of the fluid-filled follicle by a discus proligerus. A basement membrane separated the cells and blood vessels of the theca from the granulosa cells. The thickness of the zona pellucida, measured from 10 oocytes dissected free from 3 mm follicles, was 14.2 ± 0.6 µm. Incidental histological sections of ovaries from two 6-month-old and two 10-month-old pouch young collected opportunistically also revealed the presence of Graafian follicles. In all koala ovaries examined, at least one Graafian follicle was found per ovary, independent of the koala’s body condition, time of the year the ovary was recovered, or whether or not the koala was lactating.
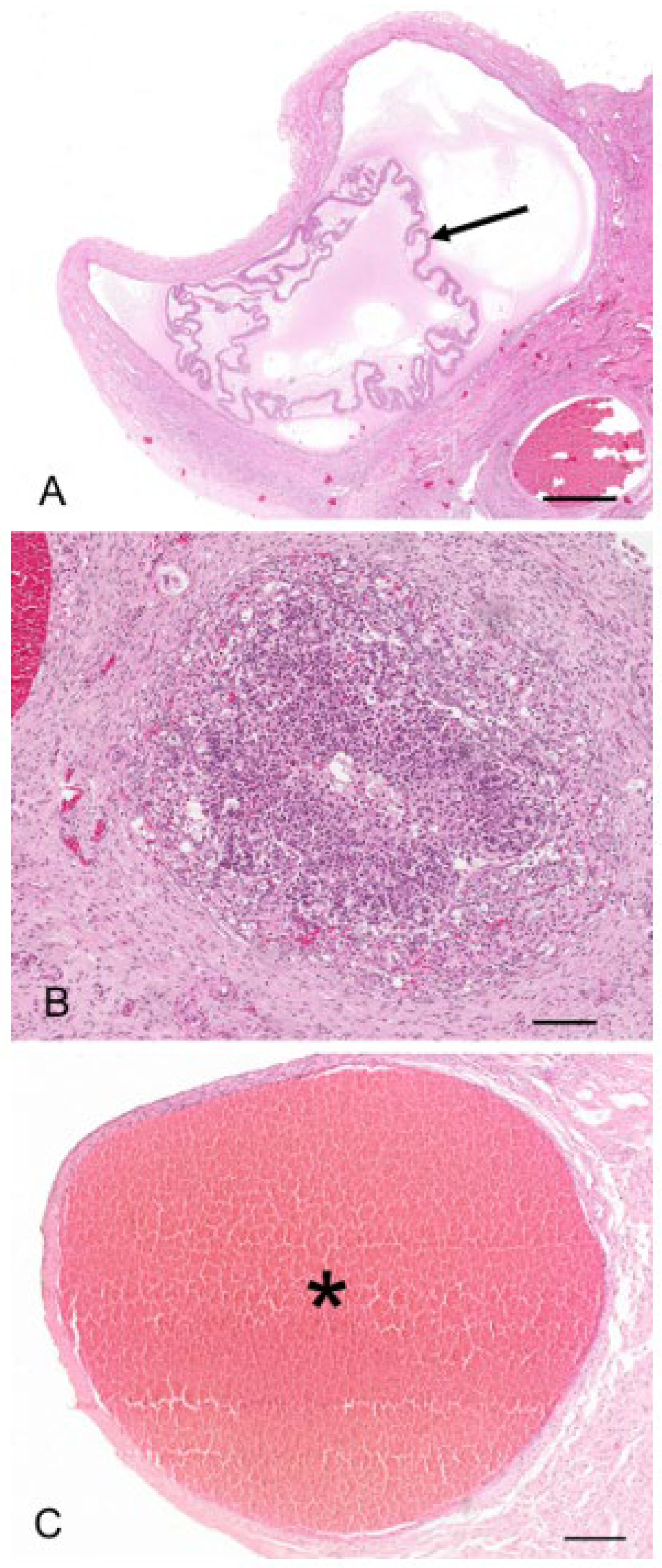
The regulation of the number of follicles in the developing follicular pool includes a normal process known as follicular atresia. The main features of atresia in follicular and granulosa cells are pyknosis and chromatolysis of the nucleus. During atresia, the basal membrane of the stratum granulosum can become thicker, more translucent, and irregular (membrana vitrea) (
Figure 4A). When atretic follicles are reabsorbed, small scarring areas of fibrotic tissue are left. Both types of atresia, obliterative atresia and cystic atresia, were noted in the koala ovary. In obliterative atresia, the granulosa and theca layers were irregular, hypertrophic, and occupied the entire antral space. Hypertrophic cells were spindle-like cells, 5–15 µm in diameter, with clear eosinophilic cytoplasm (
Figure 4B). During cystic atresia, the granulosa layer became atrophic while the theca cells started to develop luteinization features, such as an enlarged polygonal shape, ample eosinophilic cytoplasm, and the presence of scattered lipid droplets.
A typical feature of the koala ovary, but more so in the interestrous phase, was the presence of hemorrhagic follicles (
Figure 4C). The majority of histological sections taken through these structures revealed a thin theca externa encapsulating a blood-filled cavity. Less commonly, blood-filled cavities were also surrounded by a theca interna or membrana granulosa, the cells of which were in various stages of hyperplasia.
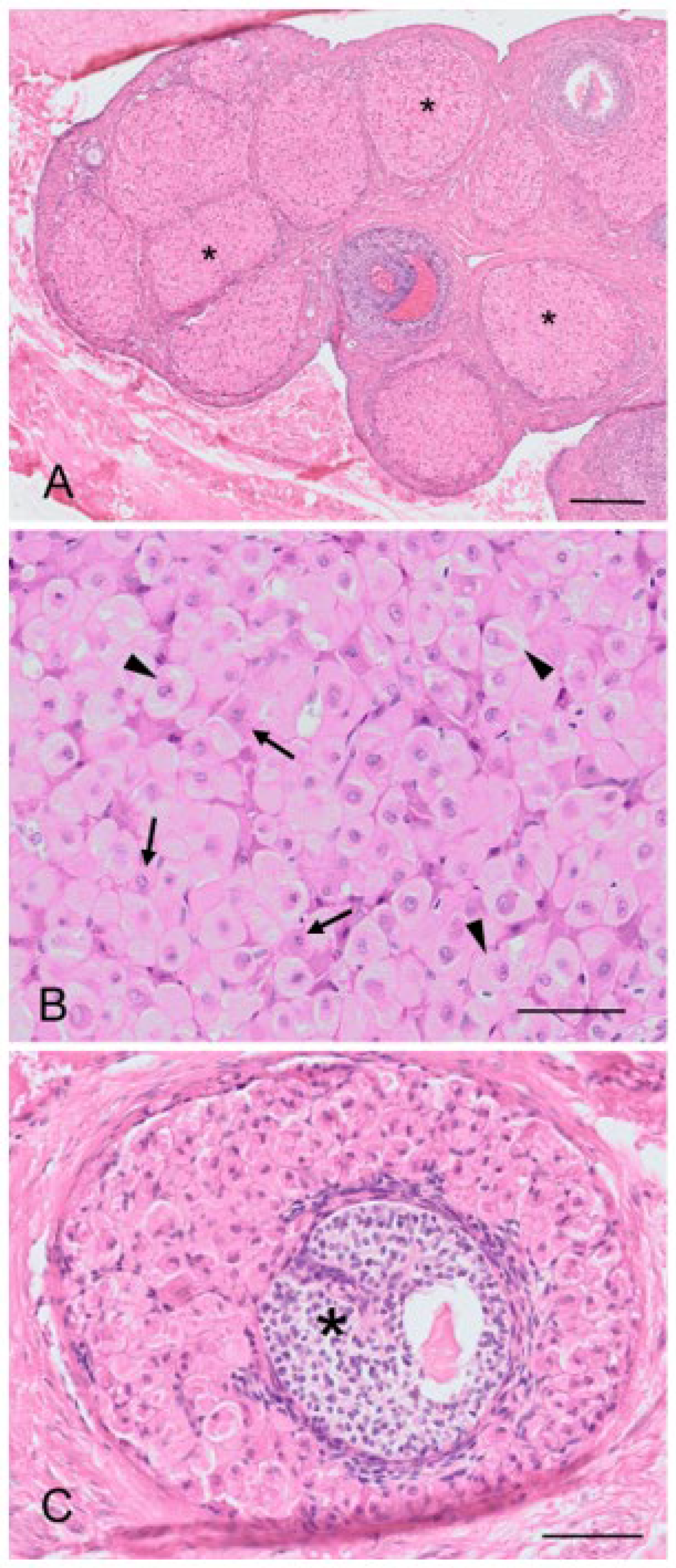
The cortex of most koala interestrous ovaries examined contained discrete foci of “interstitial-like” tissue encapsulated by layers of connective tissue (
Figure 5A). Apart from connective tissue cells and a blood supply, this “interstitial-like” tissue was composed of at least two cell types which were distinguished by their relative size and degree of cytoplasmic eosinophilia (
Figure 5B). Type “I” cells (28–43 µm in diameter) are described as ovoid with a slightly eosinophilic cytoplasm. The nucleus was large (7–8 µm in diameter and occupying 1/3 of the cell cytoplasm), and round with finely dispersed chromatin. These cells were large and often polyhedral in shape, their cytoplasm containing lipid droplets, thus having the histological appearance of steroidogenic cells. Type “II” cells (13–26 µm in diameter) were polygonal, spindle-like, and contained a strongly eosinophilic cytoplasm; the nucleus (3–7 µm in diameter) of these cells was round and had mostly condensed central chromatin. Every aggregate of interstitial cells was surrounded by connective tissue admixed with reticular fibers. From the observation of numerous atretic follicles in various stages of degeneration, it was apparent that the cells of the theca interna, and possibly in some instances, membrana granulosa, may have been the source of this “interstitial-like” tissue (
Figure 5C). Interestingly, no tissue resembling “interstitial-like” tissue was found in the ovaries of a 6-month-old pouch young, but numerous atretic follicles showed evidence of hyperplasia.
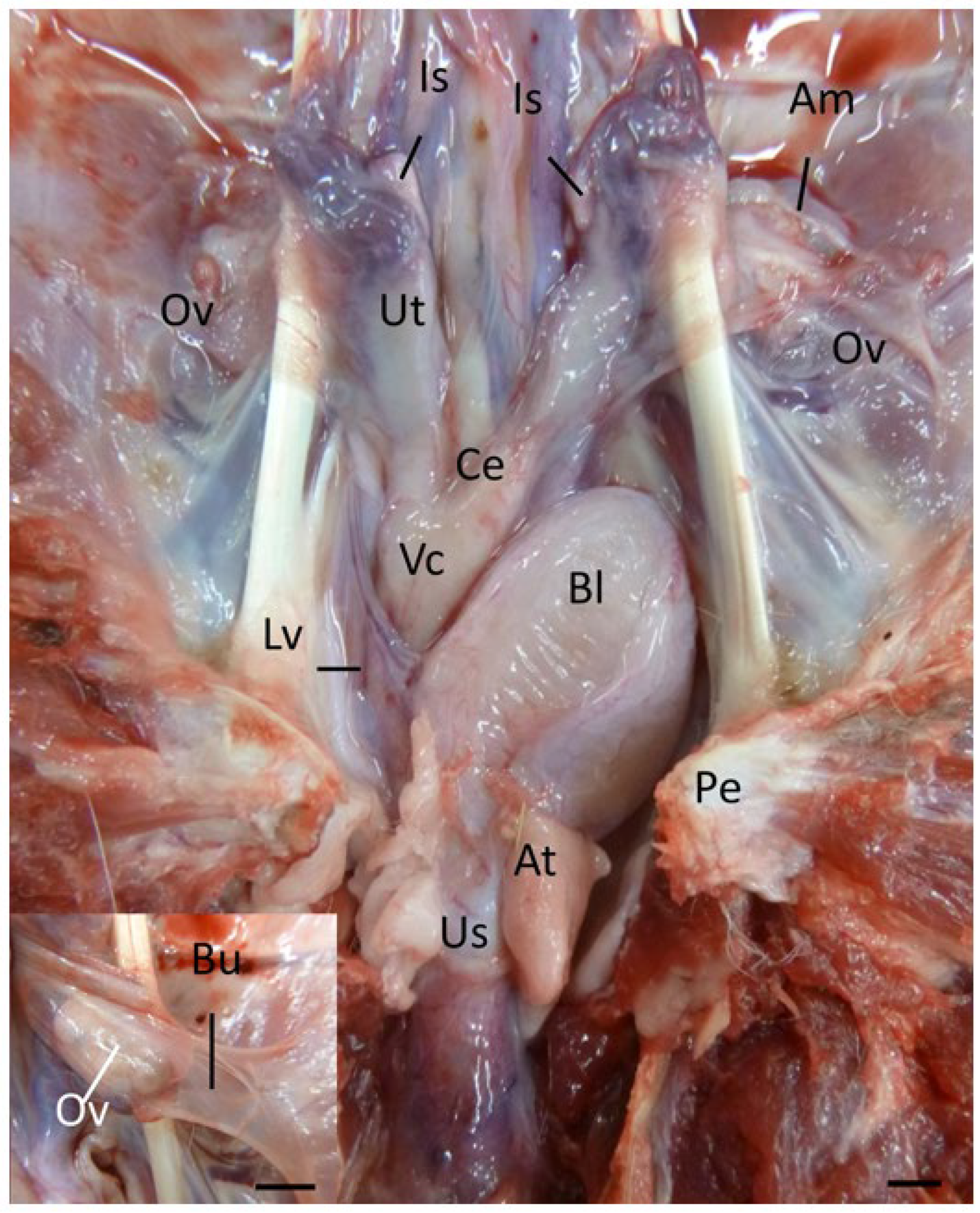
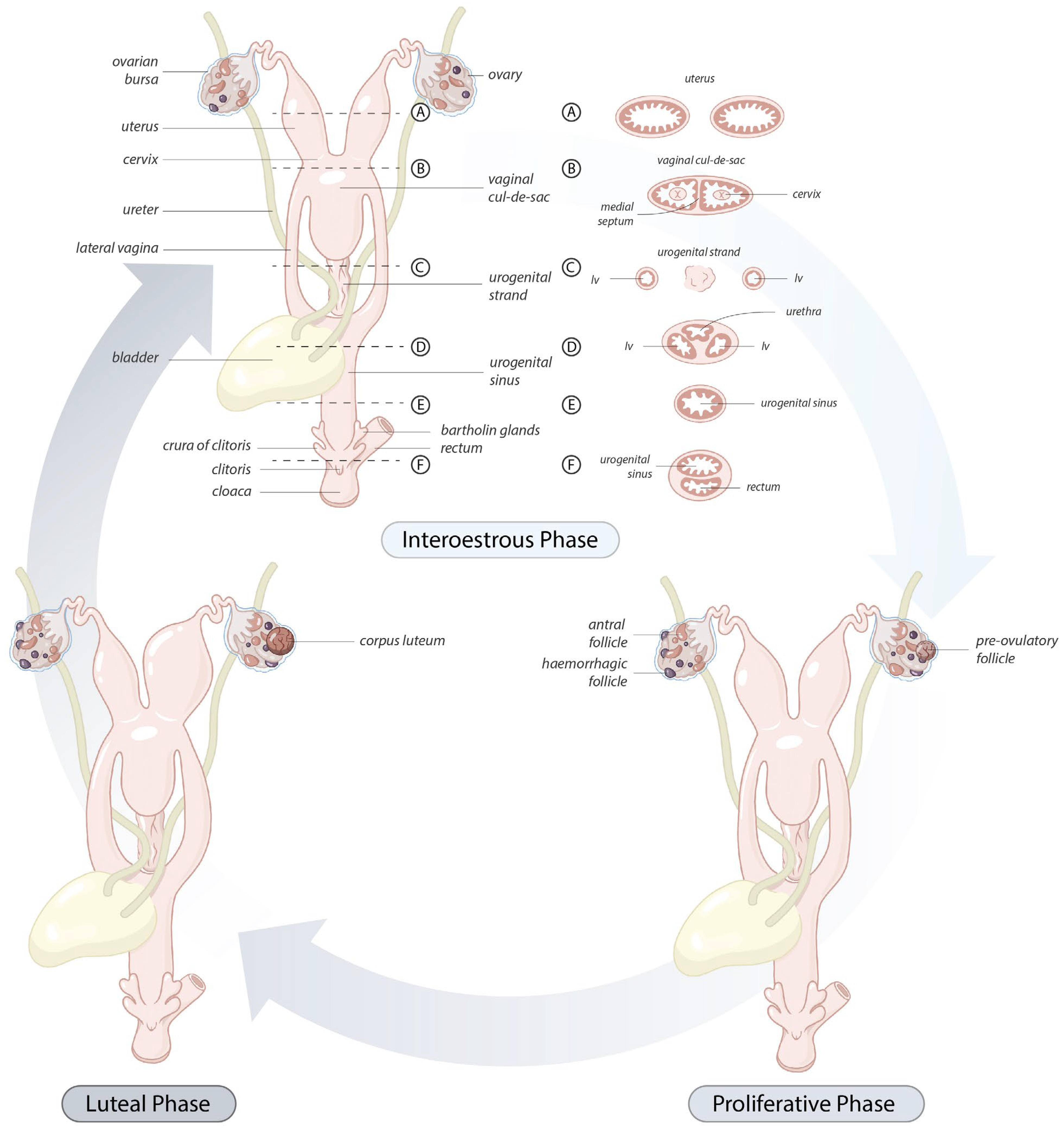
The koala ovary was enclosed within a thin peritoneal
ovarian bursa which was composed of three layers, the bursal epithelium facing the ovary, a connective tissue layer, and the peritoneal mesothelium (
Figure 2 and [
13]). The middle layer contained connective tissue with fibroblasts, bundles of smooth muscle cells, and blood vessels. The bursal epithelium of the koala was a continuous layer of cells resting on a dense mat of collagen fibers. The epithelial cells (12–14 µm in diameter) varied in shape from cuboidal to elongated. The nuclei (1–2 µm in diameter) of cuboidal cells were ovoid, whereas in elongated cells they were more cigar-shaped and smooth. The cell surface was covered with microvilli of a fairly uniform length. The peritoneal surface was covered by a continuous layer of ciliated and non-ciliated mesothelial cells (12–14 µm in diameter), resting on a basal lamina. The nuclei (1–2 µm in diameter) of these cells were also elongated, narrow, and had a smooth outline. On the cranio-medial aspect of the ovarian bursa, there was a peritoneal opening which accommodated the passage of the cranial portion of the oviduct (infundibulum) and ovarian blood supply. As the histology of the bursa did not change with respect to the stage of the estrous cycle, it will not be described in further phases.
The koala oviduct was a tortuous tubular organ extending adjacent to the ovaries within the bursa to the uterine horns. Each oviduct could be divided into three distinctive sections, the infundibulum, an initial large funnel-shaped portion, the ampulla (
Figure 6A), a thinner section extending caudally from the infundibulum, and the isthmus, a narrow muscular segment connecting to each uterus. The epithelium of all sections was flattened to simple cuboidal (12–14 µm in diameter), with the majority of cells, especially those of the infundibulum, possessing mobile cilia (and some microvilli). The nucleus (5–8 µm in diameter) of these epithelial cells was mostly eccentric, and round with condensed chromatin. A few cup-like cells, goblet cells, were present amongst the epithelial cells of the infundibulum; these cells were simple columnar epithelial cells (10 µm in diameter), having a height of four times their width. The cytoplasm was displaced toward the basal end of the cell body by large mucin granules accumulating at the apical surface of the cell. The mostly basophilic basal part of the cell contained a large round to oval nucleus (4–5 µm in diameter). The oviduct submucosa was composed of loose connective tissue rich in plasma cells, eosinophils, and a few mast cells. The mucosal-submucosal layer of the koala ampulla consisted of several longitudinal folds, gradually reducing in size and number closer to the isthmus-uterine junction (
Figure 6A insert). While the ampullary epithelium contained numerous goblet cells (secretory epithelium), ciliated cells were not common in the oviduct of the interestrous koala. The external diameter of the isthmus increased proximally as it approached the utero-tubal junction. The lumen of this region was thrown into five to six longitudinal folds of a simple cuboidal epithelium. The outer diameter of the isthmus increased as it looped towards the uterus, which appeared to be due to a corresponding increase in thickness of the muscularis and outer connective tissue.
The wall of the uterus was composed of three layers, the mucosa-submucosa or endometrium, the myometrium, and the serosal layer or perimetrium (
Figure 6B). The endometrium consisted of two sections, being distinctive by their structure and function: a superficial layer or “functional zone, and a thin deeper layer or “basal zone”. The lining epithelium of the functional zone was simple columnar in the koala (15–20 µm in diameter), with an oval to round nucleus (4–5 µm in diameter) and condensed chromatin. The columnar epithelium included secreting cells and ciliated non-secreting cells. The myometrium consisted of a circular inner thicker layer (800 µm in diameter) and a longitudinal layer of smooth muscular cells (250 µm in diameter); in-between these layers, a vascular area composed of large arteries, veins, and lymphatic vessels was present. The perimetrium, or
tunica serosa, of the uterus consisted of loose connective tissue covered by the peritoneal mesothelium; it included smooth muscular cells, vessels, and nerves. Interestrous females had small uteri with a narrow uterine endometrium with few folds, and short and relatively narrow glands with small lumens that occupied a small percentage area of the endometrium. The endometrial epithelium of interestrous koalas was composed of tall columnar cells and scattered polymorphonuclear cells infiltrated the lamina propria. The uterine glands in this phase were sparse and relatively small (diameter 70–80 µm). The endometrial epithelium of the sections showed scattered areas of intracytoplasmic vacuolar degeneration, and low numbers of mitotic figures were generally observed in the luminal uterine epithelium. The epithelial cells in the endometrial lumen had scattered ciliated cells. While the uteri of females in lactational anestrus were similar in size to those in other stages of interestrus, they also possessed non-ciliated uterine and glandular epithelial cells with irregular nuclei, with large amounts of degenerated cells (leukocytes) accumulated in the uterine gland lumen; mitotic activity was also noted more frequently in the uterine epithelial cells of these animals.
The caudal extremities of both muscular cervices protruded into their respective compartments of the vaginal cul-de-sac complex (
Figure 6C). The longitudinal and transverse sections of the cervices revealed the lumen to be narrow and convoluted. The columnar epithelium lining the lumen of the cervices was arranged in shallow longitudinal folds, lined by a single layer of cuboidal epithelium (15–20 µm in diameter), with several muciparous cells, including goblet-shaped cells scattered amongst them; the secreting cells in the cervix were similar to those described in the oviductal infundibulum. The epithelial cells were characterized by an oval nucleus (5 µm in diameter) with condensed chromatin. The propria-submucosa consisted of thick irregular connective tissue, subject to changes in its thickness during the different phases of the cycle. The tunica muscularis consisted of an inner circular layer, rich in elastic fibers, and an external layer of smooth muscle cells. The tunica serosa of the cervix consisted of loose connective tissue and lining mesothelial cells.
The vaginal cul-de-sac of the interestrous koala was lined by a simple cuboidal epithelium (10–15 µm in diameter), with a round to oval regular nucleus (5–6 µm in diameter) and condensed chromatin, as was the medial septum partitioning the vaginal cul-de-sac (
Figure 7A). The basal lamina of the cul-de-sac containing loose connective tissue separated the epithelium from the submucosa, consisting of connective tissue, small blood vessels, and a large amount of lymphoid tissue. Scattered polymorphonuclear cells were also observed in the submucosa during the interestrous stage. Longitudinally arranged smooth muscle cells were present in the highly vascular muscular layer (
tunica muscularis). In addition to the connective tissue and vascular structure, the submucosa of the medial septum was also composed of several layers of longitudinally arranged muscle fibers. No patent birth canal or opening was observed between the caudal extremities of the cul-de-sac and the urogenital sinus in any of the interestrous reproductive tracts examined.
The lateral vaginae of the koala were two tubular muscular organs, extending from the cervices to the common urogenital sinus, lined by flat longitudinal folds of the mucosa and submucosa layers. The lumina of koala lateral vaginae featured 6 to 11 longitudinal folds and were lined for almost their entire length by a stratified squamous epithelium (5–8 µm length). The deeper cellular layer, the stratum basale, consisted of a single sheet of cuboidal cells (6–8 µm in diameter), with an apical nucleus containing uniform condensed chromatin (
Figure 7B); and a stratum spinosum, made of a variable number of highly interconnected polyhedral cell (5–8 µm in diameter). As these cells reach the outer layers, they become flatter. This specific layer is then called stratum granulosum, which is not present when the epithelium is non-cornified. The propria-submucosa consisted of loose or irregular connective tissue, containing lymphatic nodules in the caudal section of the vagina. The tunica muscularis was made of two or three layers of smooth muscular tissue; the innermost layer was thickened, separated by connective tissue and covered by a thin external longitudinal layer. The tunica adventitia (or tunica serosa more cranially) consisted of loose connective tissue and major vessels, nerve bundles, and ganglia.
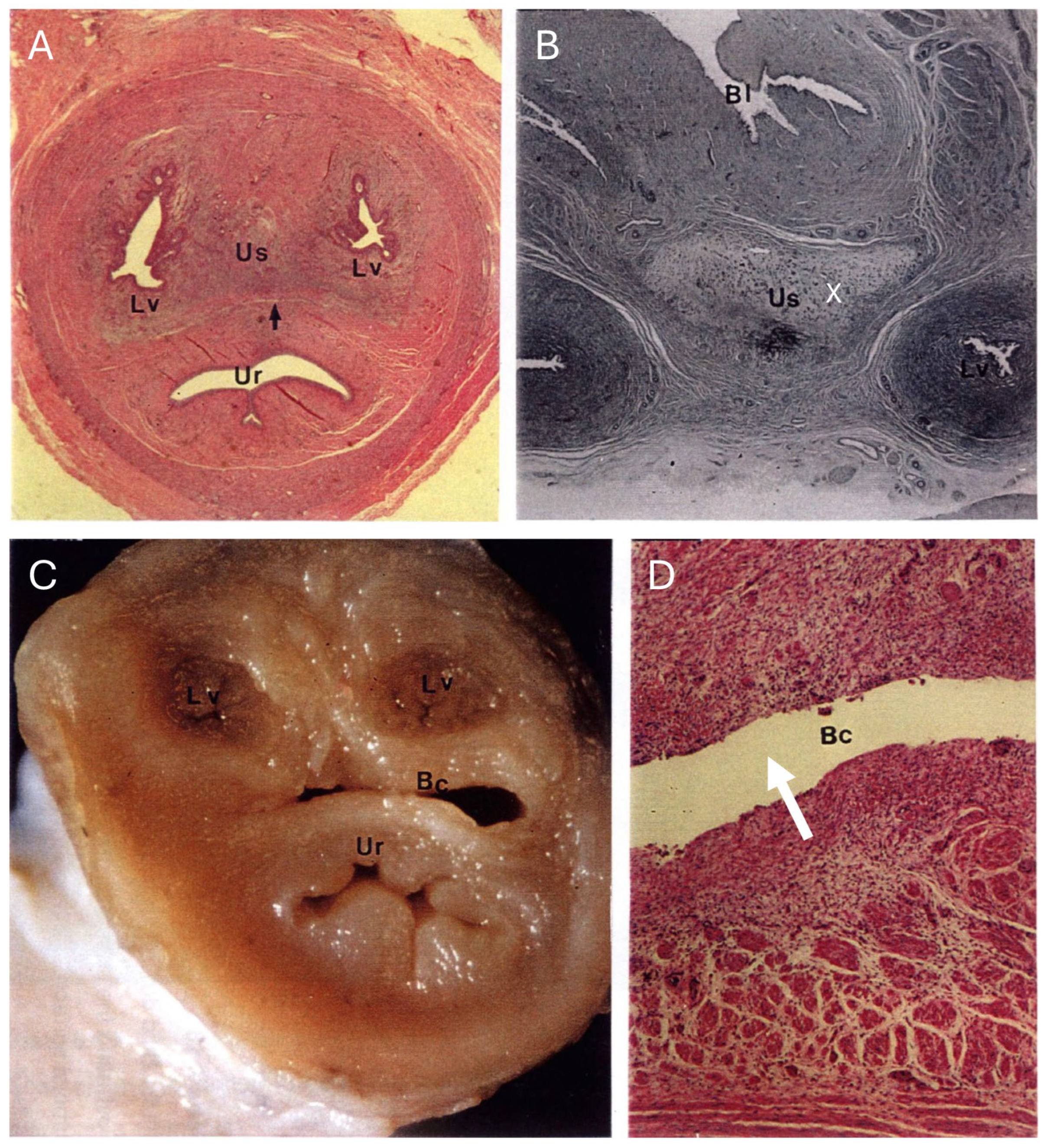
The urogenital sinus of the interestrous koala was widest at its cranial and caudal extremities, in association with distinct swellings at the junction of the canal with the lateral vaginae and urethra (‘urogenital swelling’) and the opening of the canal into the common vestibule, respectively. The most characteristic feature of the urogenital sinus was the presence of 9 to 10 prominent longitudinal mucosal folds which ran over the length of the canal (
Figure 8A). These folds of tissue appeared to define a pathway cranially, to the point where they ultimately coalesced to join the distinctive lumina of the lateral vaginae and urethra. The mucosal folds observed throughout the entire luminal surface of the urogenital sinus were lined with four/five layers of stratified non-cornified/cornified squamous epithelium (each cell 6–9 µm in diameter), with only occasional degenerated cells, showing hydropic changes and pyknotic nuclei.
The connective tissue between the caudal extremity of the vaginal cul-de-sac and the cranial limit of the urogenital sinus is known as the urogenital strand. The urogenital strand begins in the region where the lateral vaginae and urethra coalesce with the longitudinal folds of the urogenital sinus and terminates at the caudal extremity of the vaginal cul-de-sac. The strand consisted of a section of tissue positioned between the surrounding musculature of the lateral vaginae, urethra, and outer muscularis of the urogenital sinus, and was composed of densely packed connective tissue, smooth muscle fibers, and blood vessels (
Figure 8B).
Due to the high variability in the gross appearance and location of the vestibular glands (
Figure 2), only two pairs of vestibular glands during the interestrous phase were examined. The tubulo-acinar glandular component was lined by a layer of basal cells beneath the columnar epithelium. The basal cells were flat to cuboidal, 7–10 µm in diameter, with scarce to moderate cytoplasm, a central vesicular round nucleus, and granular chromatin. The columnar epithelial cells were approximately 7 µm wide and 20 µm long, with a central to paracentral nucleus and abundant eosinophilic cytoplasm. Scattered degenerated epithelial cells were described in the glands (pyknotic nuclei, hydropic degeneration). The glandular lumen did not contain any secretory material, only scattered leukocytes and cell debris in one specimen, and highly basophilic secretory material with a few leukocytes in the other. The superficial fibrous capsule was continuous with an extensive stroma internally supporting lobules of secretory parenchyma. Each vestibular gland was drained by an excretory duct lined by a single layer of cuboidal epithelial cells, which emptied into the urogenital lumen via a small papilla-like opening in the caudal extremity of the urogenital canal.
Protruding from the ventral floor of the koala urogenital sinus into the common vestibule was the clitoris. The koala clitoris was a dorso-ventrally flattened bifid structure contained within the walls of the vestibule. It consisted of broad longitudinal folds of the mucous membrane, lined by a stratified non-cornified squamous epithelium. The supporting stroma was made of loose connective tissue. The clitoris consisted of several lymphatic nodules and nerve ganglia.
3.4. Ovarian and Reproductive Tract Histology of the Koala Proliferative Phase
The main feature of the koala ovary in the proliferative phase was the presence of a large tertiary follicle in the ovarian cortex (
Figure 9). Protruding from the surface of the ovary, the presumptive pre-ovulatory follicle in the koala was remarkably large, and in one koala measured 6.8 mm in diameter. This large fluid-filled structure consisted of a theca externa, a theca interna composed of one to two cell layers, and a granulosa cell layer of 5 to 8 cells thickness. The primary oocyte in the tertiary follicle of the koala was 110–130 µm in diameter, spheroidal, and contained a central nucleus with condensed chromatin and a thick zona pellucida (6–8 µm in diameter). As the antrum enlarged, the oocyte became eccentric, and two or three layers of granulosa cells (30–40 µm in diameter) called the cumulus oophorus surrounded the oocyte. Polyhedral granulosa cells formed the internal layer of the follicle which is known as the stratum granulosum (granulosa layer). Diffuse papillary hyperplasia was also observed on the superficial epithelium of the ovary during the proliferative phase of the cycle in several koala specimens. Oocytes recovered directly from two 6 mm follicles measured 166 and 167 μm in diameter, while the zonae pellucidae of these oocytes were 14.4 and 15.6 μm thick, respectively; no polar bodies or metaphase plates were observed in either oocyte.
The oviducts of the koalas in the proliferative phase of the reproductive cycle showed marked changes in the histological appearance of the epithelium. The mucosa of the isthmus was highly convoluted, and the epithelium consisted of a single layer of hyperplastic columnar cells. Similarly, the ampullary region showed hyperplastic changes resembling those of the isthmus. The epithelial lining of the ampulla contained numerous goblet and ciliated cells.
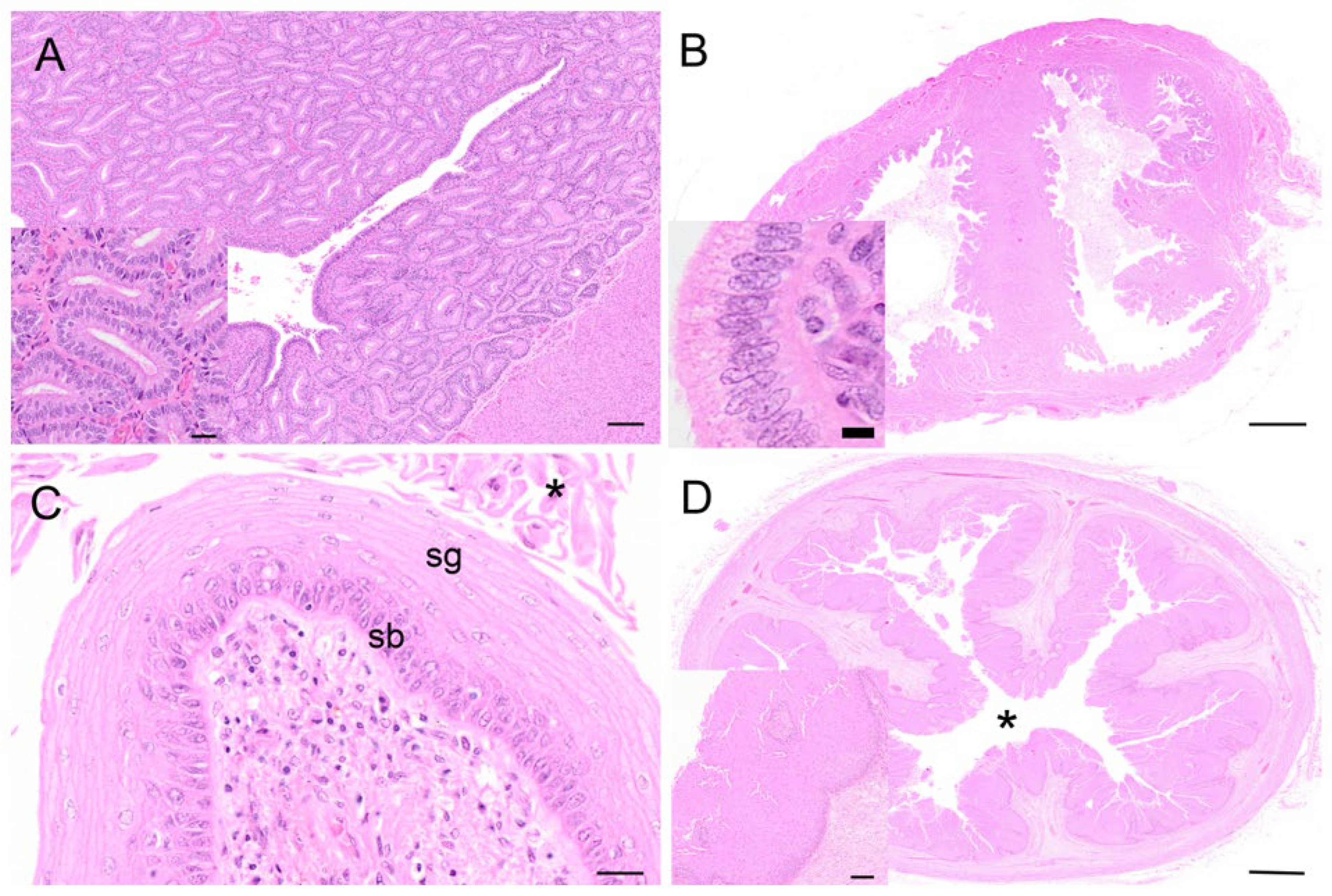
The endometrium of the koalas in the proliferative phase of the cycle consisted of a highly vascular submucosa, tightly packed with glandular tissue (
Figure 10A). The lumen of the uterus was lined by a simple epithelium of cuboidal cells with occasional goblet cells. The glandular lumina were open and lined with simple hyperplastic ciliated columnar epithelium cells. Diffuse stromal oedema, ranging from mild to marked, was also observed. The epithelial cells were about 32 µm in diameter, with eosinophilic cytoplasm, and contained basally located nuclei with condensed chromatin. The koala proliferative uteri were characterized by mitoses apparent in both the uterine and glandular epithelium. Invaginations in the endometrial lining were also observed in this phase. The myometrium was unremarkable, about 1 mm thick, and composed of elongated muscle fibers.
The histology of the cervix from females in the proliferative phase was similar to that of the interestrous or luteal phases. The propria-submucosa, made of thick irregular connective tissue, was the only section subjected to changes in its thickness (increased) during the proliferative phase of the cycle.
During the proliferative phase of the koala reproductive cycle, there was an increase in the degree of folding of the epithelium over the surface of the medial septum and of the upper regions of the vaginal cul-de-sac adjacent to the cervical ostia (
Figure 10B). The vaginal cul-de-sac epithelium was characterized by increased hyperplasia, with the cells transitioning from a cuboidal to a columnar appearance. The cul-de-sac was filled with an eosinophilic mucoid substance mixed with cellular debris and a few leukocytes. Several mitotic figures were detected within the epithelial layer, and little if any degeneration or desquamation was observed. Very few polymorphonuclear cells were described. Marked folding and hyperplasia of the luminal epithelium was observed during this phase.
The vaginal epithelium during the proliferative phase marked the formation of the stratum granulosum over the stratum germinativum (stratum basale) of the epithelium (
Figure 10C). This consisted of flattened epithelial cells (15–20 µm in diameter), containing many keratohyalin granules within their cytoplasm and no nucleus. The formation of a stratum corneum of dense, cornified cells also characterized this phase. Some specimens, likely at the end of the proliferative stage, showed a fully cornified epithelium exhibiting some desquamation of superficial cells. Occasional scattered stromal leukocytic infiltrates were observed. In the cornified layers, if present, the superficial cells (stratum corneum) either had a pyknotic nucleus or lacked one (and possessed a high amount of keratin), while in the non-cornified epithelium those cells possessed a nucleus. The propria-submucosa consisted of a thinner layer of loose connective tissue, muscle fibers, and blood vessels compared to the lateral vaginae. The tunica muscularis consisted of two or three layers of smooth muscle fibers. The outer tunica adventitia consisted of loose connective tissue and large vessels.
Hypertrophy and hyperplasia of the epithelial layer of the urogenital sinus, with formation of the stratum corneum (8–10 µm in diameter), was observed during the proliferative phase (
Figure 10D); in some sections of the urogenital sinus, up to 80 cell layers were observed. Slight luminal dilatation was also described. The stratum lucidum consisted of a layer of flattened translucent cornified cells (12–15 µm in diameter, not possessing a nucleus).
Only one pair of vestibular glands were collected during the proliferative phase of the koala estrous cycle. The epithelium of the vestibular glands in the proliferative phase of the ovarian cycle of the koala was composed of hyperplastic columnar cells interspersed with the occasional goblet cell. The lumina of these glands were filled with degenerating epithelial cells, secretory material, cellular debris, and leukocytes. Basophilic secretory vesicles were also observed in the apical cytoplasm of some epithelial cells.
3.5. Ovarian and Reproductive Tract Histology of the Koala Luteal Phase
Two reproductive tracts were recovered from pregnant koalas estimated to be at the pharyngeal arch stage and considered to be in the last third of gestation. The corpora lutea associated of these pregnant tracts measured 7.3 mm and 6.5 mm in diameter, respectively. Histologically, each CL consisted of a theca externa which measured approximately 45 µm thick and which encapsulated a solid mass of luteal cells (
Figure 11A). There were also prominent blood vessels, grossly observable on the surface of the CL, which, after histological examination, were found embedded in the tissue of the theca interna. The lutein elements were presumably granulosa in origin and consisted of large polygonal cells with granulated cytoplasm, indicative of cells with secretory activity (
Figure 11A, inset). Based on the developmental stage of the fetus and endocrinological data from pregnant koala progesterone profiles [
15], it is likely that this CL was producing peak levels of progesterone. The respective measurements of the longest and widest dimensions of the luteal cells were 61.5 ± 3.9 mm and 37.0 ± 2.9 mm. The luteal cell nuclei were conspicuous and had a diameter of 12.0 ± 0.5 mm. Interspersed amongst the lutein cells were strands of connective tissue cells and small blood vessels. The CLs of pregnancy were both filled completely with luteal tissue.
Corpora lutea (up to 8 mm in diameter) were also observed from non-pregnant koalas as large spherical structures, grossly protruding from the surface of the ovary and surrounded by connective tissue. The “large luteal cells” of the corpus luteum of non-pregnancy were polyhedral, with an approximate diameter of 30–50 µm, and possessed a large spheroidal nucleus (7–9 µm in diameter) and multiple lipid granular inclusions in the foamy eosinophilic cytoplasm. The stroma of the corpus luteum was formed by a few collagen fibers and many reticular fibers located around the structure together with small blood vessels. The “small luteal cells” had an approximate average diameter of 15 µm and possessed an irregular-shaped nucleus (5–8 µm in diameter) and multiple lipid droplets in the cytoplasm.
Although the glandular ipsilateral gravid uterus of the two pregnant koalas observed in this study was grossly larger than the contralateral non-gravid uterus, the endometrial (4.3 mm) and myometrial (0.8 mm) layers of the gravid uterus were smaller than those of the non-gravid uterus (6.3 mm and 1.2 mm), respectively. However, the most notable aspect of the endometrial tissue in both uteri was its bi-layered appearance (
Figure 11B). The basal layer of the endometrium was composed of non-ciliated, hyperplastic, glandular epithelial cells; these cells contained basally located nuclei and eosinophilic cytoplasm. The lumina of the glands of the basal endometrium contained secretory products, referred to by Shorey and Hughes [
17] as uterine milk. The submucosa, in the region of the basal endometrium, was filled with a homogeneous eosinophilic material and blood vessels. A superficial layer of the endometrium was clearly differentiated from the basal layer by an increase in density of the glandular tissue and a marked increase in cytoplasmic eosinophilia. The nuclei of these cells were centrally located, and the lumina of glands in this region appeared narrower. Although both gravid and non-gravid uteri of the pregnant koala exhibited a bi-layered appearance, the basal layer of the gravid uterus was not as pronounced (1.0 mm) as that of the non-gravid uterus (2.6 mm). The most distinctive histological difference between the two uteri was the increase in the degree of oedema associated with the superficial layer of the non-gravid uterus (
Figure 11B).
The observation of non-pregnant koalas revealed that both glandular uteri of the non-pregnant luteal phase were similar in size and histology. The myometrium consisted of an outer longitudinal layer and inner circular layer which measured 0.1 ± 0.03 mm and 0.3 ± 0.01 mm thick, respectively. The endometrium was 3.8 ± 0.6 mm thick and was composed of basal and superficial layers of 1.9 ± 0.2 mm and 1.9 ± 0.1 mm, respectively. The histology of the endometrium was similar to that described for the pregnant uteri, although there was not the same degree of oedema or presence of eosinophilic secretory material in either the superficial or basal layers. The glandular epithelial cells of the basal layer measured 57.0 ± 2.5 µm high, while those of the superficial layer were 44.3 ± 1.5 µm high. The uterine endometrial epithelium also showed frequent vacuolar degeneration and high mitotic activity, with associated detachment of the most external layer and presence of cellular debris and leukocytes (neutrophils) in the uterine lumen.
The histological features of the vaginal cul-de-sac of non-pregnant koalas during the luteal phase were similar to the ones of the interestrous stage, lined with a single layer of epithelial cuboidal or columnar cells. The muscularis of the lateral vaginae was thickened during the luteal phase to such an extent that the lumen of the canal was constricted. The epithelium lining the lateral vaginae was composed of five to nine layers of non-cornified cells and showed some evidence of deterioration. There was an accompanying variable polymorphonuclear cells infiltration. The epithelium lining the urogenital sinus in the non-pregnant luteal phase showed hyperplasia of the layers. Cornified epithelium was still observed in some specimens that also showed diffuse edema of the submucosal layers. The submucosa and muscularis of the urogenital sinus were heavily vascularized. The epithelial lining of the urogenital sinus of the koalas in this phase was composed of three to five layers of non-keratinized cuboidal cells.
There were two koalas in this study which were processed for histology that showed evidence of patent birth canals that were presumed to have recently given birth, one with a pouch young and one without. The CLs from both reproductive tracts were hollow, and the cavity was lined by a layer of eosinophilic homogeneous substance (
Figure 12A), beneath which was a connective tissue layer, presumably laid down by the original theca interna, which measured 250 µm to 325 µm thick. The outer edge of the mature CL was lined by what appeared to be the original theca externa and measured 125 µm to 210 µm thick. The majority of the CL was composed of eosinophilic polygonal luteal cells of slightly shrunken appearance, surrounded by a blood capillary network. The longest and widest dimensions of cells from the CL of each koala were 36.3 ± 1.8/20.8 ± 1.5 mm and 56.3 ± 3.0/31.3 ± 3.0 mm, respectively. The cell nuclei of both CLs were small and pyknotic and had mean diameters of 8.0 ± 0.5 and 10.8 ± 0.5 mm, respectively. Based on their size and the lack of other structures which resembled CLs, there was little doubt that these CLs were those of a recent pregnancy.
The contralateral ovaries of both recently post-partum koalas also showed evidence of follicular atresia. Each ovary had numerous Graafian follicles (2 to 3 mm in diameter) in the process of degeneration (
Figure 12B,C). It is likely that these Graafian follicles grew during pregnancy but became atretic during the latter stages of pregnancy or very early post partum. The granulosa cells of the more mature follicles in these ovaries were in the process of collapsing away from their basement membrane. Blood vessels were prominent in the stromal tissue immediately outside the theca and within and between the cells of the theca externa and theca interna. Other follicles in the same ovaries showed evidence of slightly more advanced atresia, as indicated by the rupture of thecal blood vessels and the influx of red blood corpuscles into the antrum of the follicle. One female koala died when her pouch young had a head length of approximately 12.0 mm. We estimated a head length of 12.0 mm corresponded to a pouch young age of approximately 1 to 2 weeks. The ovary supporting the old CL of pregnancy in this animal contained numerous Graafian and blood-filled follicles. Although a solid structure, the CL consisted of luteal cells in the process of regression which were surrounded by invading connective tissue and blood vessels.
At the time of death, a captive koala had a pouch young estimated to be approximately 4 to 6 weeks old. While the ovary of this animal had numerous Graafian and hemorrhagic follicles, the luteal cells of the old CL were almost completely regressed. Connective tissue had thoroughly invaded the CL, forming a lattice network around what were originally luteal elements, but which had now become hollow cavities. The CL of a second captive female which died 57 d after giving birth had regressed to 2.3 mm in diameter. Numerous Graafian follicles had formed on both ovaries, some of which had become atretic and formed blood-filled follicles. It is likely that this female had passed through at least one interestrous period since the pregnancy and prior to her death.
The uteri of both recently post-partum koalas showed evidence of a marked regression of both superficial and basal endometrial layers (
Figure 12D). The nuclei of cells in both layers were pyknotic, and the cytoplasm vacuolated and degenerated. The uterine lumen was devoid of epithelium. In the uterus of one koala, retained fetal membranes were still present. Approximately 12 d post-partum, both glandular uteri in one koala had regressed such that they were not histologically different from interestrous uteri.
The vaginal cul-de-sacs of both recently post-partum koalas were similar to those in the interestrous phase. The lateral vaginae, however, appeared thickened, similar to those observed during the luteal phase. The urogenital sinuses of the same koalas were lined with five to nine layers of non-cornified epithelium. There was also evidence in both animals of recent trauma to the mucosa of the urogenital sinus, the submucosa of which was heavily vascularized.
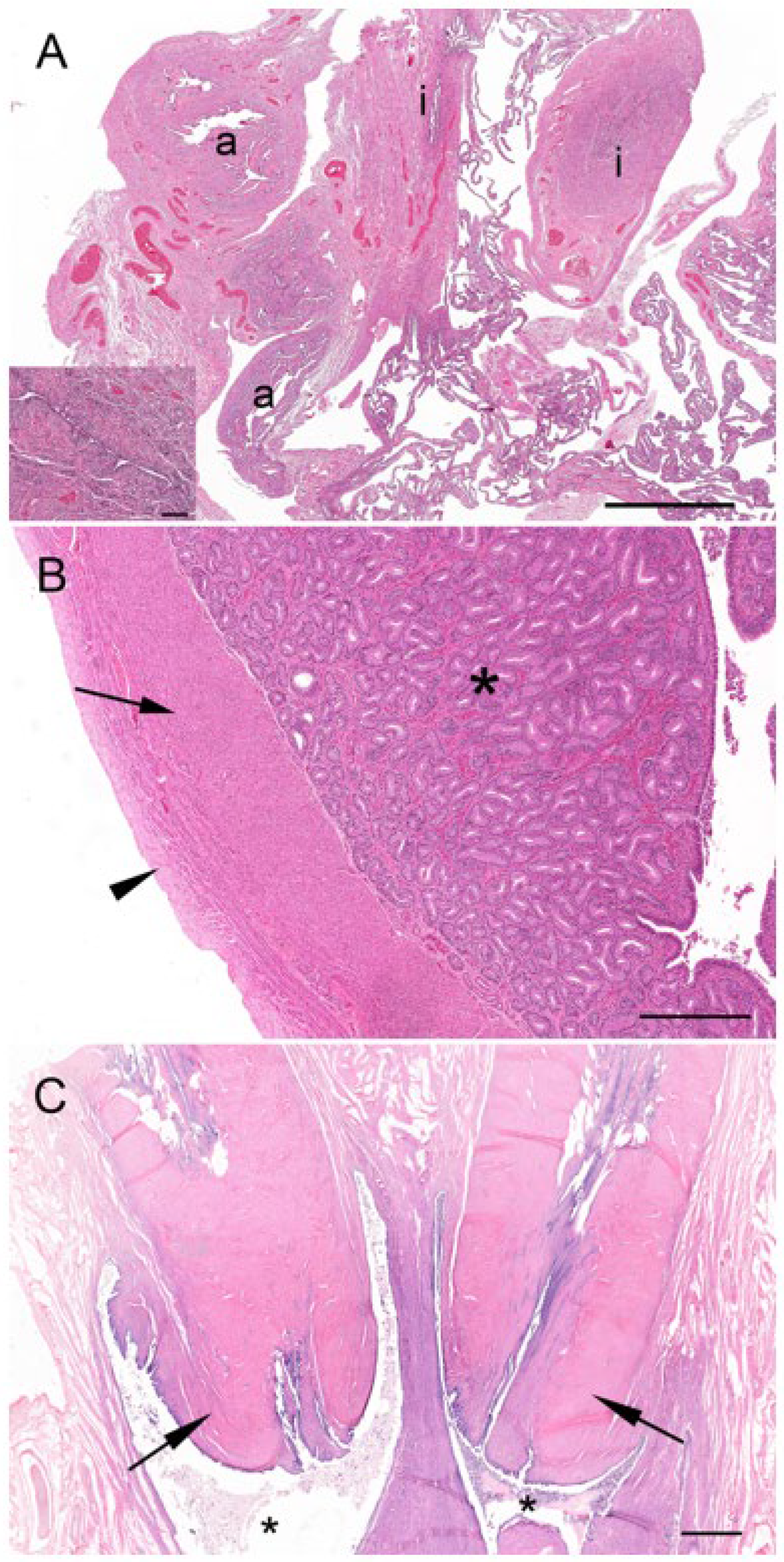
In both recently post-partum koalas, a presumptive patent birth canal was still present. The birth canal was formed in the connective tissue as a slit-like lumen between the lateral vaginae and urethra (
Figure 13A–D). In a transverse section of the urogenital sinus in one koala, the birth canal measured 4.8 to 4.9 mm wide and 0.7 to 0.85 mm deep. There was no epithelium lining the lumen of the canal. In the female which had presumably given birth most recently, the lumen of the canal was large and defined by the distribution of adjacent muscle, lateral vaginae, and the urethra. A second female showed evidence of partial tissue repair in the region of the birth canal, with the lumen of the canal appearing to be in the process of closure. The tissue filling the birth canal was composed of loose connective tissue, muscular bundles, blood vessels, leukocytes, and extravasated red blood cells. There was no evidence of retained fetal membrane remnants. A birth canal was not present in the urogenital strand of a female koala 1 to 2 weeks post partum, suggestive of the rapid closure of the canal post partum. The urogenital strand tissue in this animal was in a state of re-organization and was composed of loosely arranged connective tissue, blood vessels, and small bundles of muscle fibers.