How the Adequate Choice of Plant Species Favors the Restoration Process in Areas Susceptible to Extreme Frost Events
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
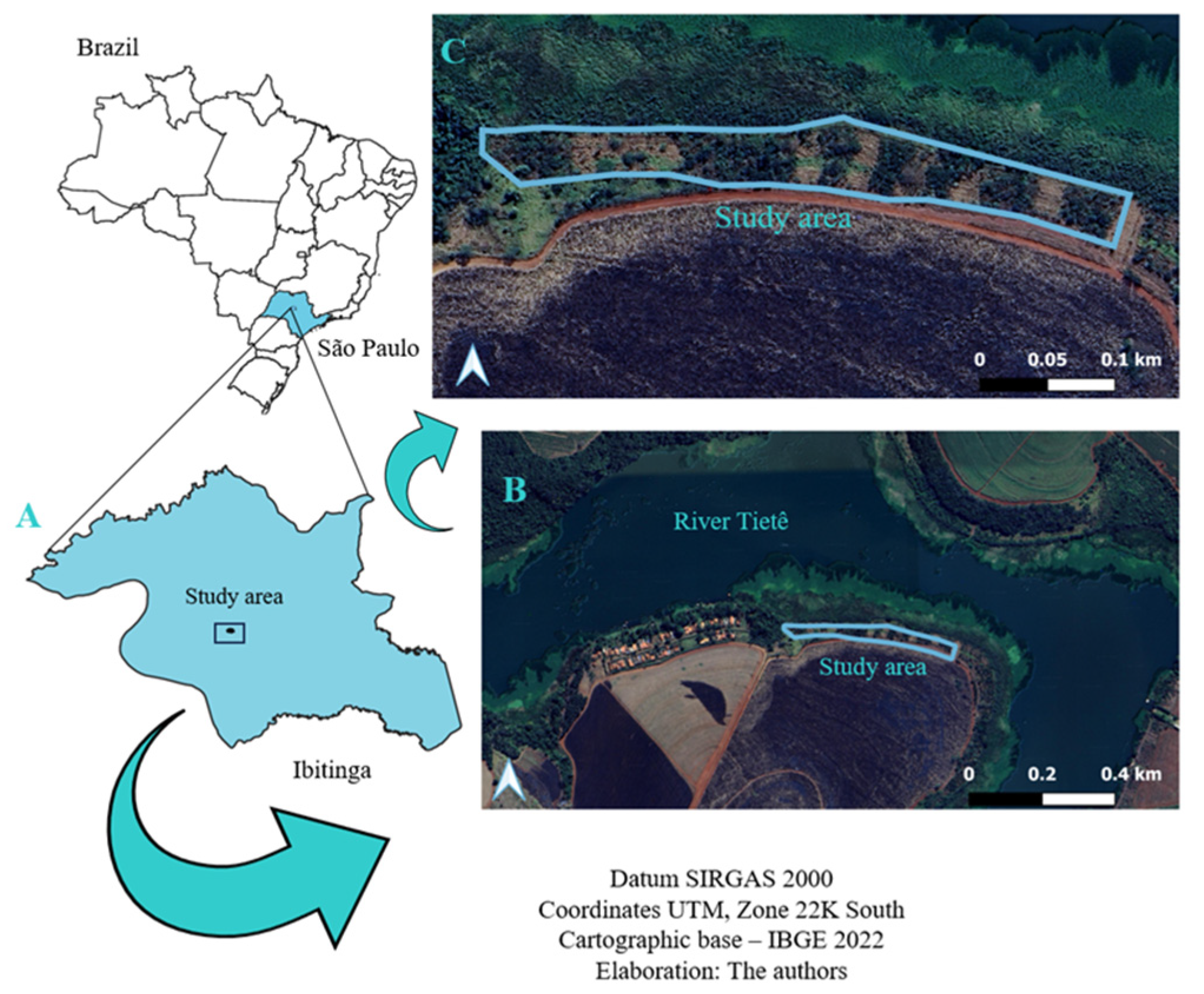
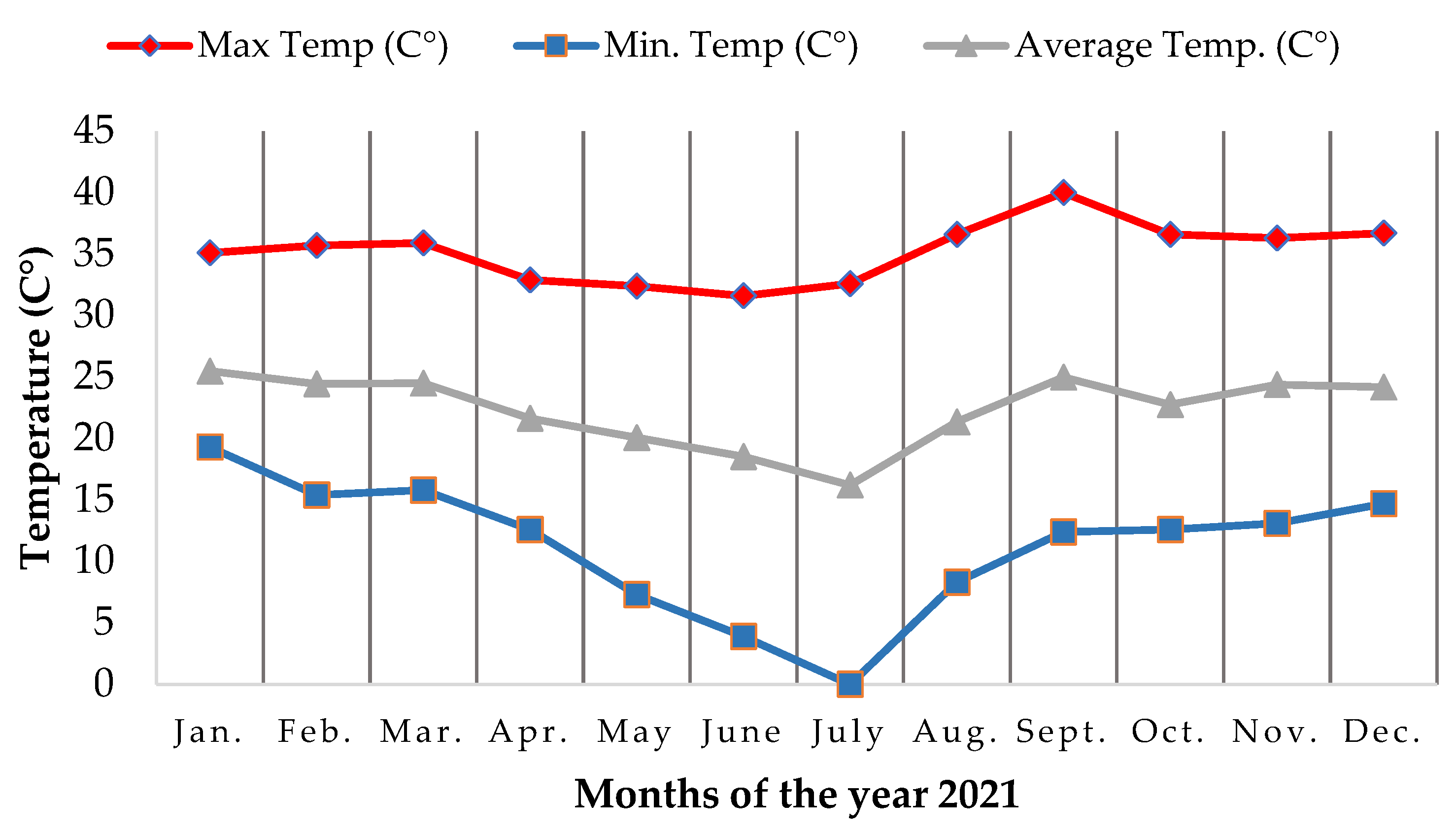
2.1. Geographical Location
2.2. Sampling
2.3. Data Analysis
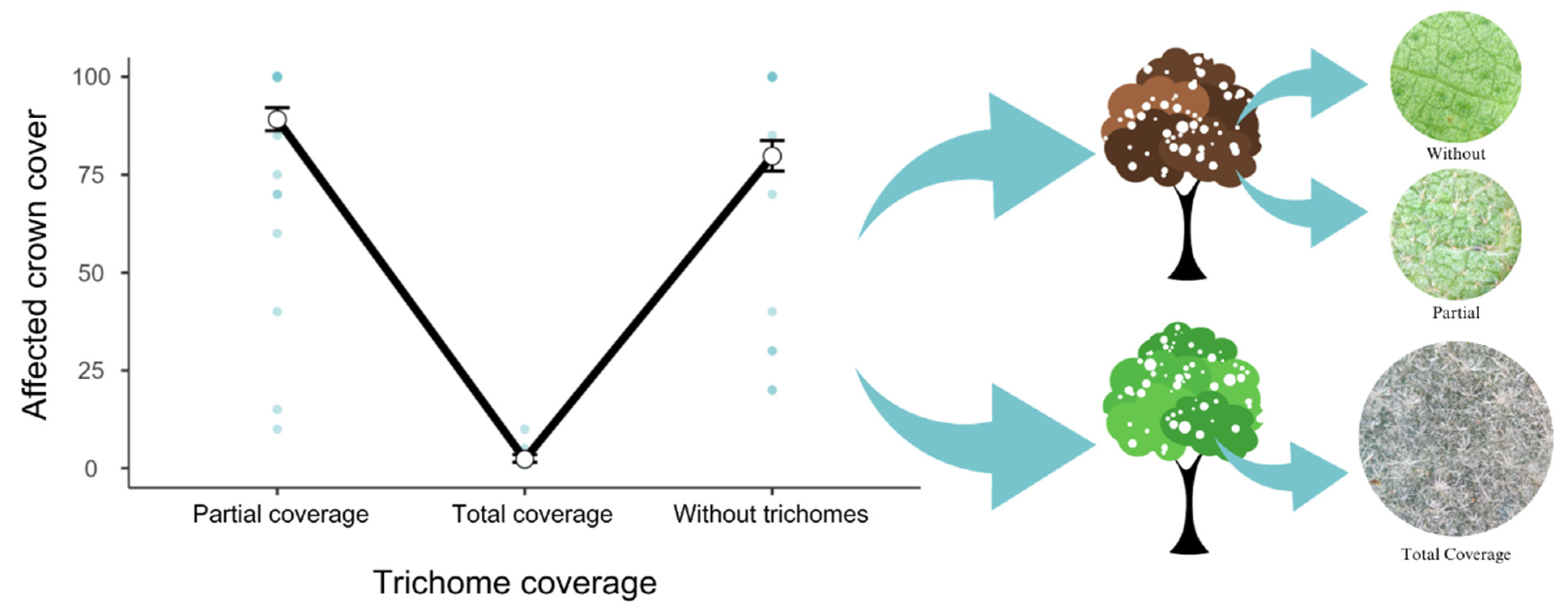
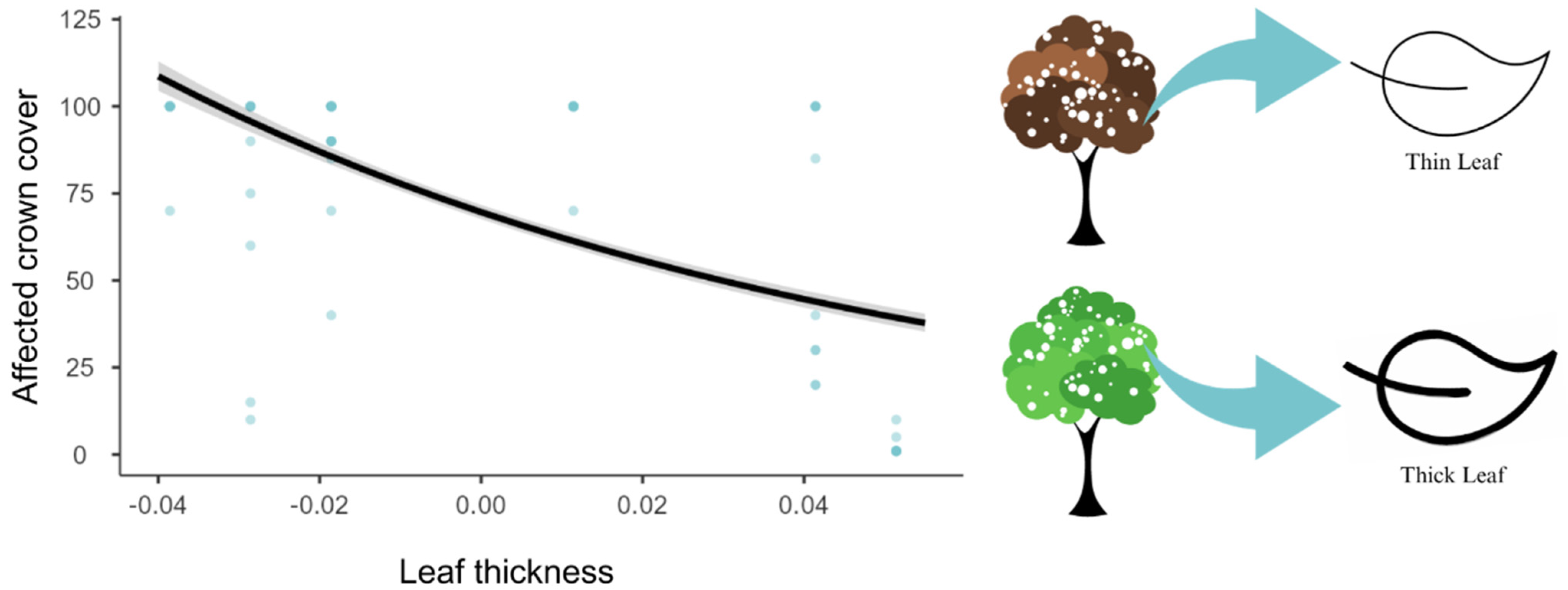
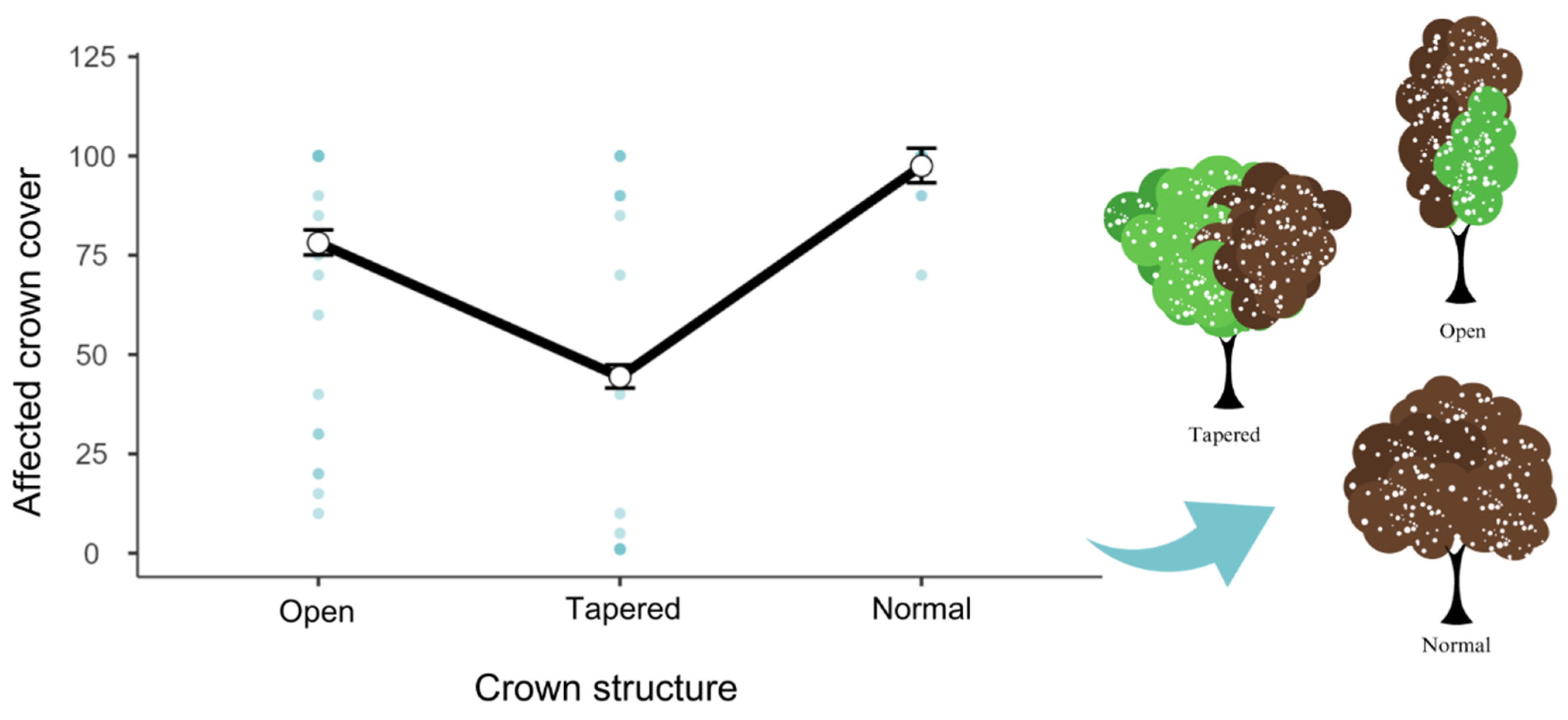
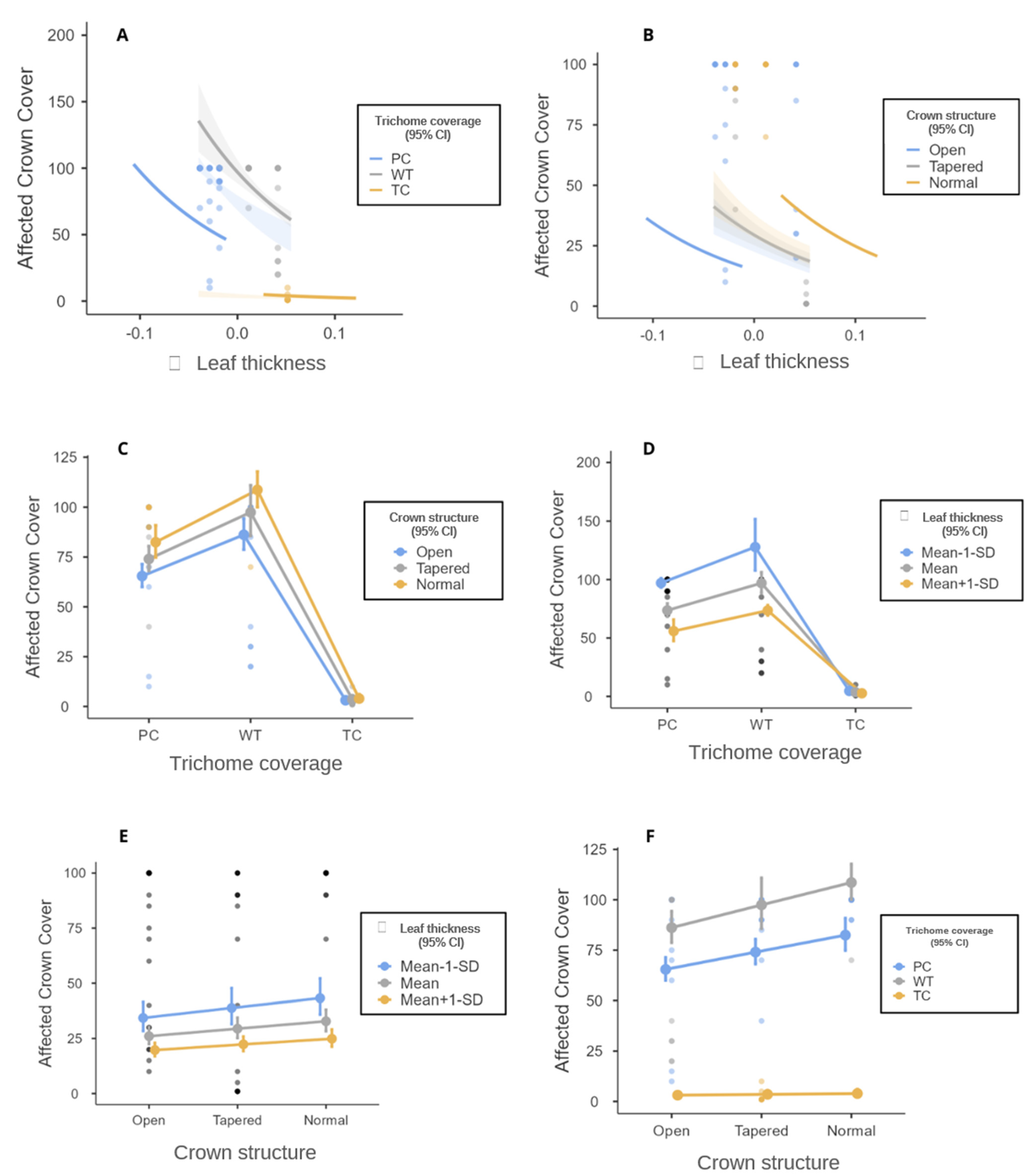
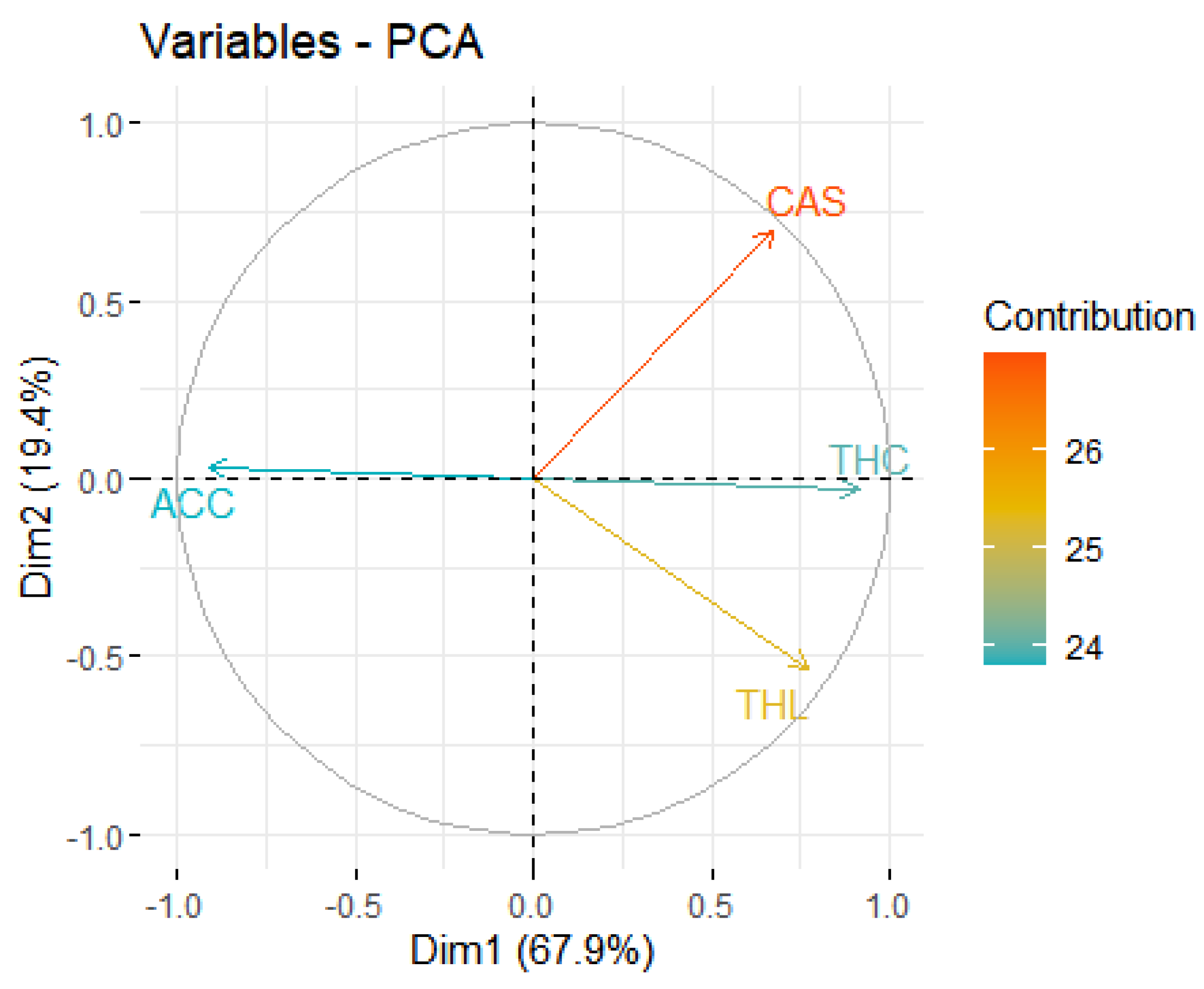
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.C.; Sandve, S.R. Adaptation to seasonality and the winter freeze. Front. Plant Sci. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Adhikari, L.; Baral, R.; Paudel, D.; Min, M.; Makaju, S.O.; Poudel, H.P.; Acharya, J.P.; Missaoui, A.M. Cold stress in plants: Strategies to improve cold tolerance in forage species. Plant Stress 2022, 4, 100081. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E. Introduction: Plant Cold Acclimation and Freezing Tolerance. In Cold Acclimation Methods in Molecular Biology; Hincha, D., Zuther, E., Eds.; Humana Press: New York, NY, USA, 2014; Volume 1166, pp. 1–6. [Google Scholar] [CrossRef]
- di Francescantonio, D.; Villagra, M.; Goldstein, G.; Campanello, P.I. Drought and frost resistance vary between evergreen and deciduous Atlantic Forest canopy trees. Funct. Plant Biol. 2020, 47, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Inouye, D.W. The ecological and evolutionary significance of frost in the context of climate change. Ecol. Lett. 2000, 3, 457–463. [Google Scholar] [CrossRef]
- Whitecross, M.A.; Archibald, S.; Witkowski, E.T.F. Do freeze events create a demographic bottleneck for Colophospermum mopane? S. Afr. J. Bot. 2012, 83, 9–18. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Conner, J.K.; Stinchcombe, J.R. Evolution of plant resistance and tolerance to frost damage. Ecol. Lett. 2004, 7, 1199–1208. [Google Scholar] [CrossRef]
- Nautiyal, P.C.; Rajgopal, K.; Zala, P.V.; Pujari, D.S.; Basu, M.; Dhadhal, B.A.; Nandre, B.M. Evaluation of wild Arachis species for abiotic stress tolerance: I. Thermal stress and leaf water relations. Euphytica 2007, 159, 43–57. [Google Scholar] [CrossRef]
- Pereira, A.R.; Angelocci, L.R.; Sentelhas, P.C. (Eds.) Geada. In Agrometeorologia: Fundamentos e Aplicações Práticas; Agropecuária; ESALQ: Guaíba, Brazil, 2002; pp. 385–411. [Google Scholar]
- Carvalho, A.L.; Santos, D.V.; Marengo, J.A.; Coutinho, S.M.V.; Maia, S.M.F. Impacts of extreme climate events on Brazilian agricultural production. Sustain. Debate 2020, 11, 211–224. Available online: https://periodicos.unb.br/index.php/sust/article/view/33814/28556 (accessed on 10 August 2023). [CrossRef]
- Brando, P.M.; Durigan, G. Changes in cerrado vegetation after disturbance by frost (São Paulo State, Brazil). Plant Ecol. 2005, 175, 205–215. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Flake, S.W.; Abreu, R.C.R.; Pilon, N.A.; Rossatto, D.R.; Durigan, G.D. Rare frost events reinforce tropical savanna–forest boundaries. J. Ecol. 2019, 107, 468–477. [Google Scholar] [CrossRef]
- De Antonio, A.C.; Scalon, M.C.; Rossatto, D.R. The role of bud protection and bark density in frost resistance of savanna trees. Plant Biol. 2020, 22, 55–61. [Google Scholar] [CrossRef]
- Rorato, D.G.; Araujo, M.M.; Tabaldi, L.A.; Turchetto, F.; Griebeler, A.M.; Berghetti, A.L.P.; Barbosa, F.M. Tolerance and resilience of forest species to frost in restoration planting in southern Brazil. Restor. Ecol. 2018, 26, 537–542. [Google Scholar] [CrossRef]
- Koppen, W. Climatologia: Com um Estudio de los Climas de la Tierra; Fundo de Cultura Economica: Mexico City, Mexico, 1948; 478p. [Google Scholar]
- Climate-data.org. Clima: Borborema. Available online: https://pt.climate-data.org/america-do-sul/brasil/sao-paulo/borborema-34940/ (accessed on 20 March 2023).
- Endo, I.; Galbiatti, H.F.; Delgado, C.E.R.; de Oliveira, M.M.F.; Zapparoli, A.D.C.; Moura, L.G.B.; de Oliveira, A.H.; Zavaglia, G.; Danderfer, F.A.; Gomes, C.J.S.; et al. Mapa Geológico do Quadrilátero Ferrífero, Minas Gerais, Brasil. Escala 1: 150.000: Uma Celebração do Cinquentenário da Obra de Dorr (1969). In O Quadrilátero Ferrífero: Avanços do conhecimento nos últimos 50 anos, 1st ed.; Castro, P.T.A., Endo, I., Gandini, A.L., Eds.; Quadrilatero Ferrifero: Belo Horizonte City, Brazil, 2020; Volume 1, pp. 236–261. [Google Scholar]
- Soares, P.C.; Landim, P.M.B.; Fúlfaro, V.J.; Sobreiro Neto, A.F. Ensaio de caracterizaç ao estratigráfica do Cretáceo no Estado de Sao Paulo: Grupo Bauru. Rev. Bras. Geociênc. 1980, 10, 177–185. [Google Scholar] [CrossRef]
- Almeida, F.D.; Hasui, Y.; Ponçano, W.L.; Dantas, A.S.L.; Carneiro, C.D.R.; Melo, M.D.; Bistrichi, C.A. Mapa geológico do estado de São Paulo. IPT-Sér. Monogr. 1981, 6, 126. [Google Scholar]
- De Antonio, A.D.; Scalon, M.C.; Rossato, D.R. Leaf size and thickness are related to frost damage in ground layer species of Neotropical savannas. Flora 2023, 299, 152208. [Google Scholar] [CrossRef]
- Felfili, J.M.; Eisenlohr, P.V.; Melo, M.M.R.F.; Andrade, L.A.; Meira Neto, J.A.A. Fitossociologia no Brasil: Métodos e estudos de casos. Viçosa UFV 2011, 1, 556. [Google Scholar]
- INMET, Instituto Nacional de Meteorologia. Normais Climatológicas do Brasil 1961–1990. Available online: http://www.inmet.gov.br/portal/index.php?r=clima/normaisclimatologicas (accessed on 21 September 2023).
- Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 27 August 2023).
- Li, P.; Zhang, Y.; Ye, Z.; Zuo, H.; Li, P.; Zhao, X.; Chen, Z.; Chen, C.; Zhao, J. Roles of trichomes in tea plant resistance against multiple abiotic and biotic stresses. Beverage Plant Res. 2022, 2, 14. [Google Scholar] [CrossRef]
- Lima, M.T. Funções Ecohidrológicas do Escoamento pelo Tronco em Espécies Florestais em Ambientes Urbanos. Ph.D. Thesis, Universidade Federal de São Carlos, Sorocaba, São Paulo, 2021. [Google Scholar]
- Kassambara, A.; Mundt, F.; Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.6. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 26 August 2023).
- The Jamovi Project. Jamovi (Version 2.3) [Computer Software]. 2022. Available online: https://www.jamovi.org (accessed on 26 August 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 26 August 2023).
- Durigan, G.; Siqueira, M.F.; Daher, G.A.; Franco, C.; Contieri, W.A. A flora arbustivo-arbórea do Médio Paranapanema: Base para a restauração dos ecossistemas naturais. In Pesquisas em Conservação e Recuperação Ambiental no Oeste Paulista: Resultados da Cooperação Braso; Vilas Bôas, O., Durigan, G., Eds.; Páginas & Letras: São Paulo City, Brazil, 2004; pp. 199–239. [Google Scholar]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef]
- Amada, G.; Onoda, Y.; Ichie, T.; Kitayama, K. Influence of leaf trichomes on boundary layer conductance and gas-exchange characteristics in Metrosideros polymorpha (Myrtaceae). Biotropica 2017, 49, 482–492. [Google Scholar] [CrossRef]
- Li, C.; Mo, Y.; Wang, N.; Xing, L.Y.; Qu, Y.; Chen, Y.L.; Yuan, Z.; Ali, A.; Qi, J.; Fernandez, V.; et al. The overlooked functions of trichomes: Water absorption and metal detoxication. Plant Cell Environ. 2023, 46, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Amme, S.; Rutten, T.; Melzer, M.; Sonsmann, G.; Vissers, V.P.C.; Schlesier, B.; Mock, H.P. A proteome approach defines protective functions of tobacco leaf trichomes. Proteomics 2005, 5, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tossens, T.; Harley, P.C.; Jiang, Y.; Kanagerdran, A.; Grosberg, M.; Jaamets, K.; Niinemets, U. Glandular trichomes as a barrier against atmospheric oxidative stress: Relationships with ozone uptake, leaf damage, and emission of LOX products across a diverse set of species. Plant Cell Environ. 2018, 41, 1263–1277. [Google Scholar] [CrossRef]
- Giordano, C.; Maleci, L.; Agati, G.; Petruccilli, R. Ficus carica L. leaf anatomy: Trichomes and solid inclusions. Ann. Appl. Biol. 2020, 176, 47–54. [Google Scholar] [CrossRef]
- De Campos, B.H.; Guimarães, E.; Canaveze, Y.; Machado, S.R. Epicormic bud protection traits vary along a latitudinal gradient in a neotropical savanna. Sci. Nat. 2021, 108, 11. [Google Scholar] [CrossRef]
- Bickford, C.P. Ecophysiology of leaf trichomes. Funct. Plant Biol. 2016, 43, 807–814. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Muravnik, L.E. The structural peculiarities of the leaf glandular trichomes: A review. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K., Ekiert, H., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2021; pp. 1–35. [Google Scholar]
- Morales, F.; Abadia, A.; Abadia, J.; Montserrat, G.; Gil-Pelegrin, E. Trichomes and photosynthetic pigment composition changes: Responses of Quercus ilex subsp. ballota (Desf.) Samp. and Quercus coccifera L. to Mediterranean stress conditions. Trees 2002, 16, 504–510. [Google Scholar] [CrossRef][Green Version]
- Simin, T.; Devie-Martin, C.L.; Petersen, J.; Hoye, T.T.; Rinnan, R. Impacts of elevation on plant traits and volatile organic compound emissions in deciduous tundra shrubs. Sci. Total Environ. 2022, 837, 155783. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.; Niittynen, P.; Soininen, J.; Virkkala, A.M.; Luoto, M. Plant trait-environment relationships in tundra are consistent across spatial scales. Ecography 2023, 2023, e06397. [Google Scholar] [CrossRef]
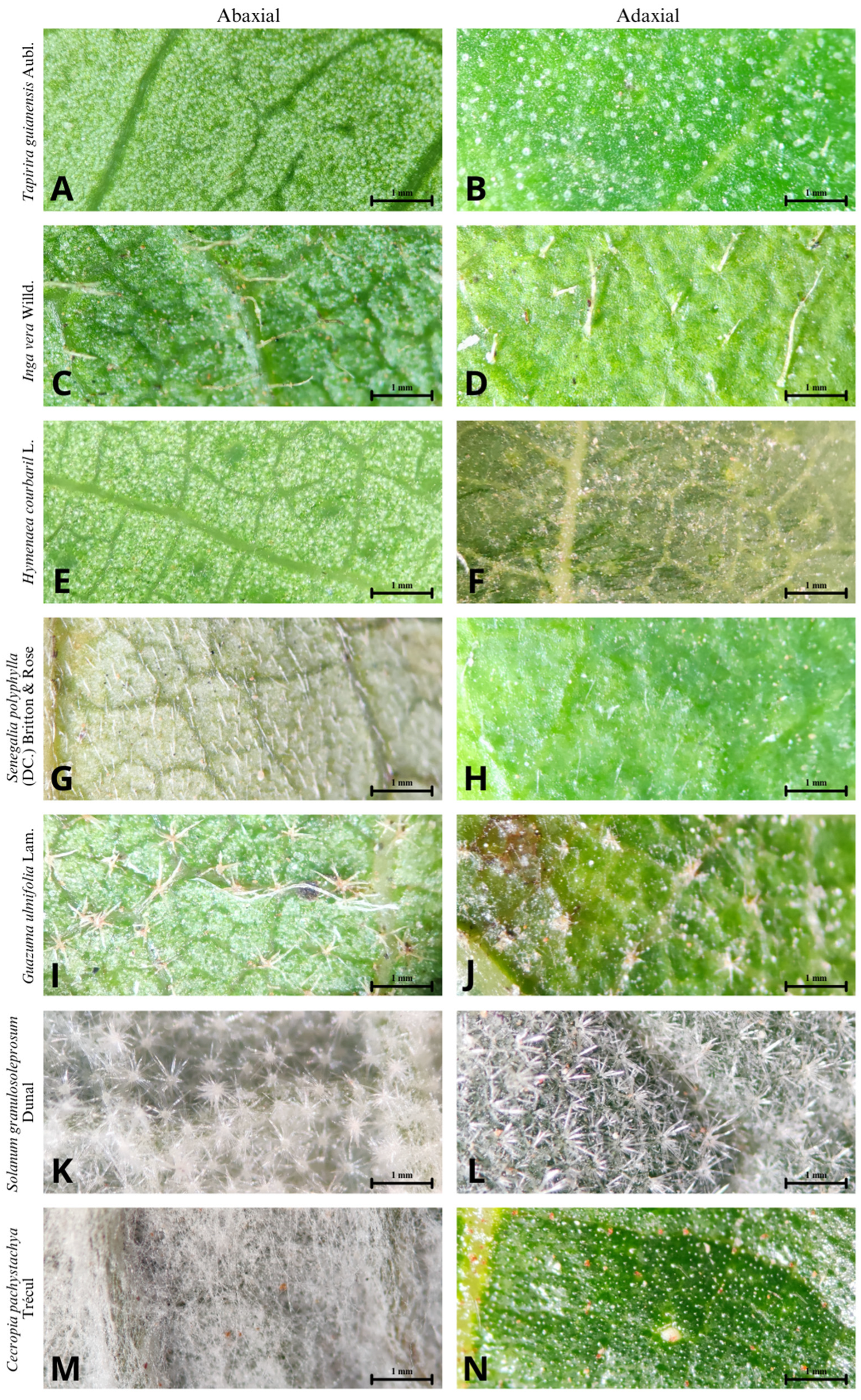
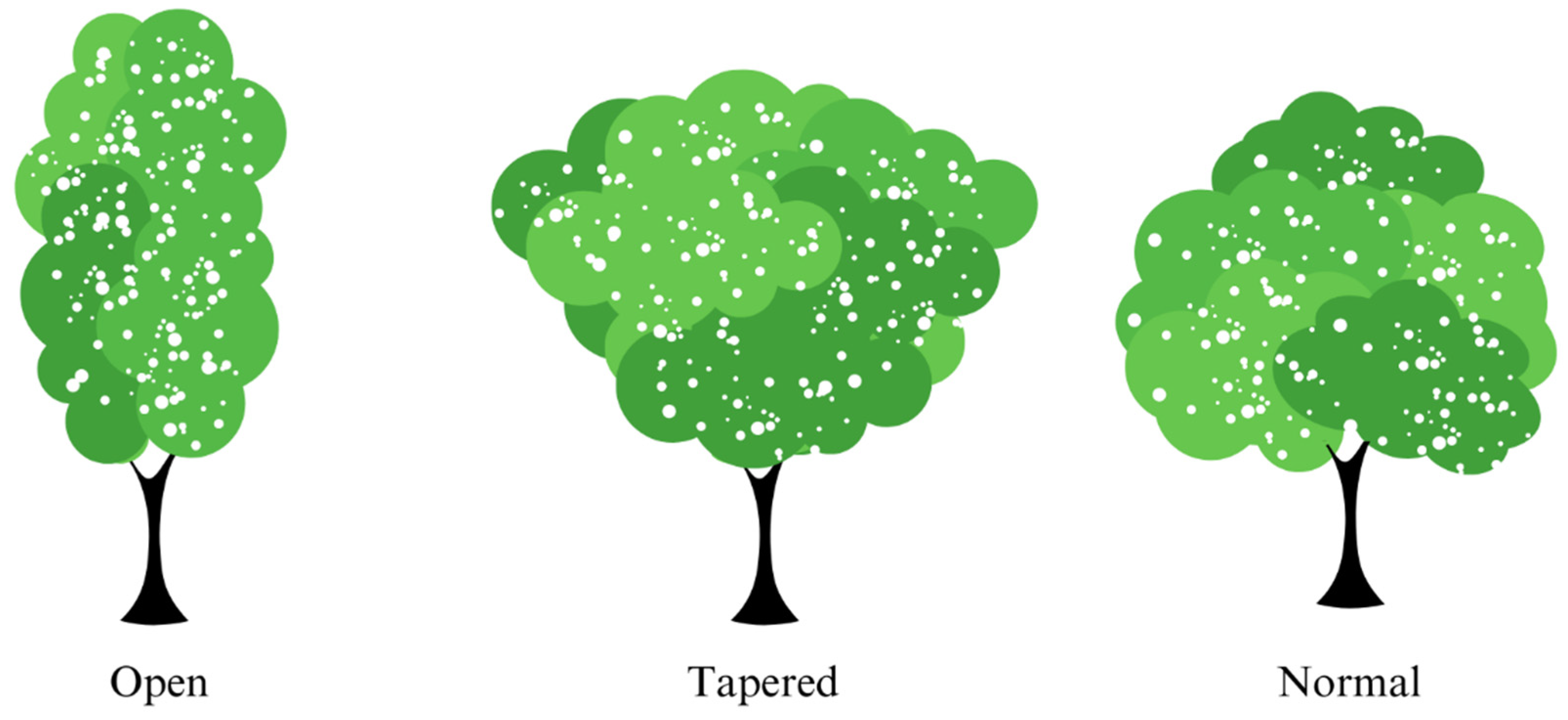
| Family | Species | Crown Structure | Class of TC | Damage Category |
|---|---|---|---|---|
| Anacardiaceae | Tapirira guianensis Aubl. | O | Nt | I |
| Fabaceae | Inga vera Willd. | Ta | Pa | Va |
| Fabaceae | Hymenaea courbaril L. | N | Nt | Va |
| Fabaceae | Senegalia polyphylla (DC.) Britton & Rose | O | Pa | Va |
| Malvaceae | Guazuma ulmifolia Lam. | N | Pa | Va |
| Solanaceae | Solanum granulosoleprosum Dunal | Ta | T | La |
| Urticaceae | Cecropia pachystachya Trécul | O | Pa | Va |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viveiros, E.; Francisco, B.S.; Dutra, F.B.; de Souza, L.A.; Inocente, M.C.; Bastos, A.C.V.; Costa, G.F.L.d.; Barbosa, M.C.; Martins, R.P.; Passaretti, R.A.; et al. How the Adequate Choice of Plant Species Favors the Restoration Process in Areas Susceptible to Extreme Frost Events. Biology 2023, 12, 1369. https://doi.org/10.3390/biology12111369
Viveiros E, Francisco BS, Dutra FB, de Souza LA, Inocente MC, Bastos ACV, Costa GFLd, Barbosa MC, Martins RP, Passaretti RA, et al. How the Adequate Choice of Plant Species Favors the Restoration Process in Areas Susceptible to Extreme Frost Events. Biology. 2023; 12(11):1369. https://doi.org/10.3390/biology12111369
Chicago/Turabian StyleViveiros, Emerson, Bruno Santos Francisco, Felipe Bueno Dutra, Lindomar Alves de Souza, Mariane Cristina Inocente, Aline Cipriano Valentim Bastos, Glória Fabiani Leão da Costa, Maycon Cristiano Barbosa, Rafael Paranhos Martins, Raquel Aparecida Passaretti, and et al. 2023. "How the Adequate Choice of Plant Species Favors the Restoration Process in Areas Susceptible to Extreme Frost Events" Biology 12, no. 11: 1369. https://doi.org/10.3390/biology12111369
APA StyleViveiros, E., Francisco, B. S., Dutra, F. B., de Souza, L. A., Inocente, M. C., Bastos, A. C. V., Costa, G. F. L. d., Barbosa, M. C., Martins, R. P., Passaretti, R. A., Fernandes, M. J. P., Oliveira, J. S. T. d., Shiguehara, A. P. P., Manzoli, E. C., Teração, B. S., Piotrowski, I., Piña-Rodrigues, F. C. M., & Silva, J. M. S. d. (2023). How the Adequate Choice of Plant Species Favors the Restoration Process in Areas Susceptible to Extreme Frost Events. Biology, 12(11), 1369. https://doi.org/10.3390/biology12111369





