Do Reactive Oxygen and Nitrogen Species Have a Similar Effect on Digestive Processes in Carnivorous Nepenthes Plants and Humans?
Abstract
Simple Summary
Abstract
1. Carnivorous Plants
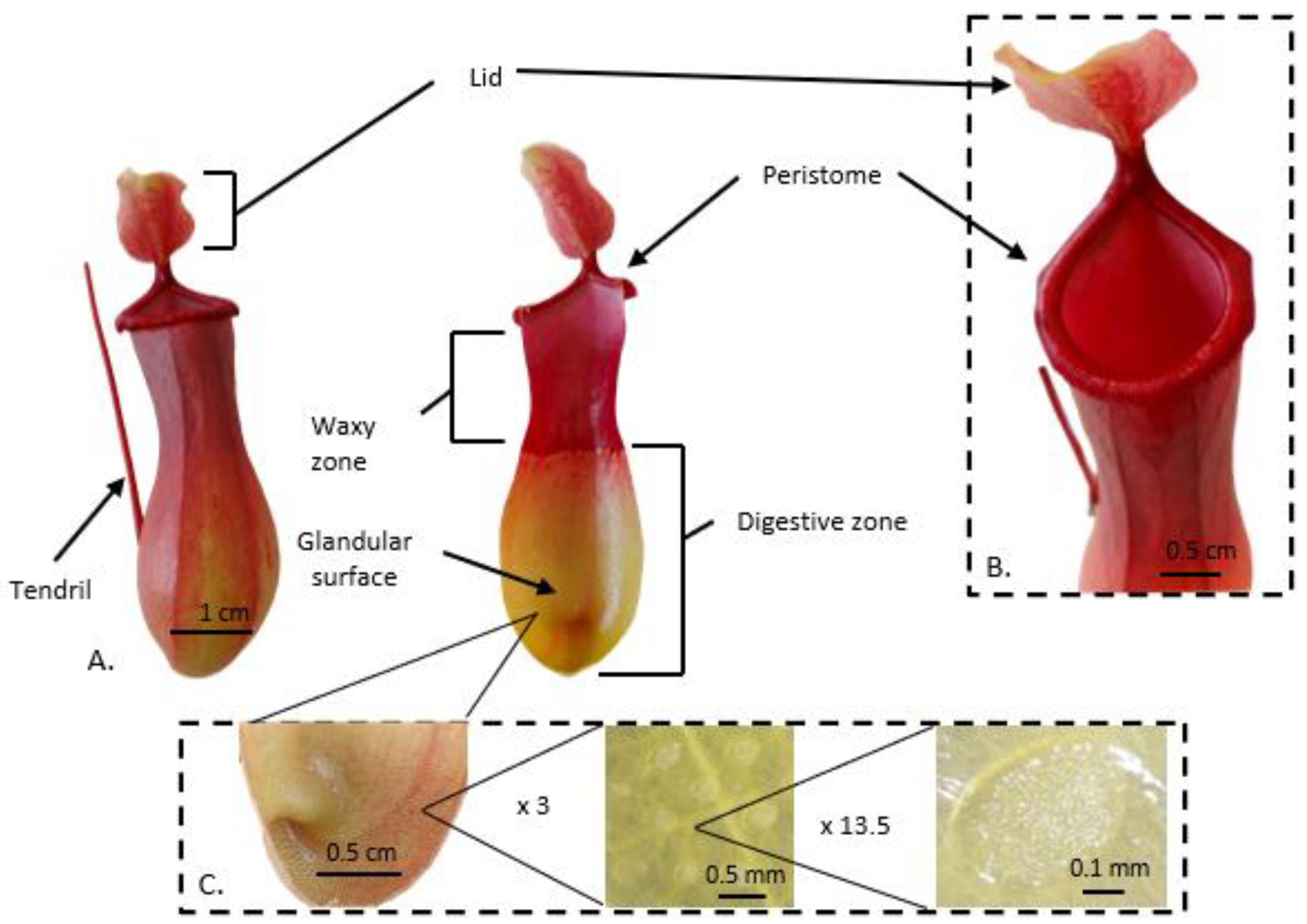
2. Carnivorous Pitcher Plants
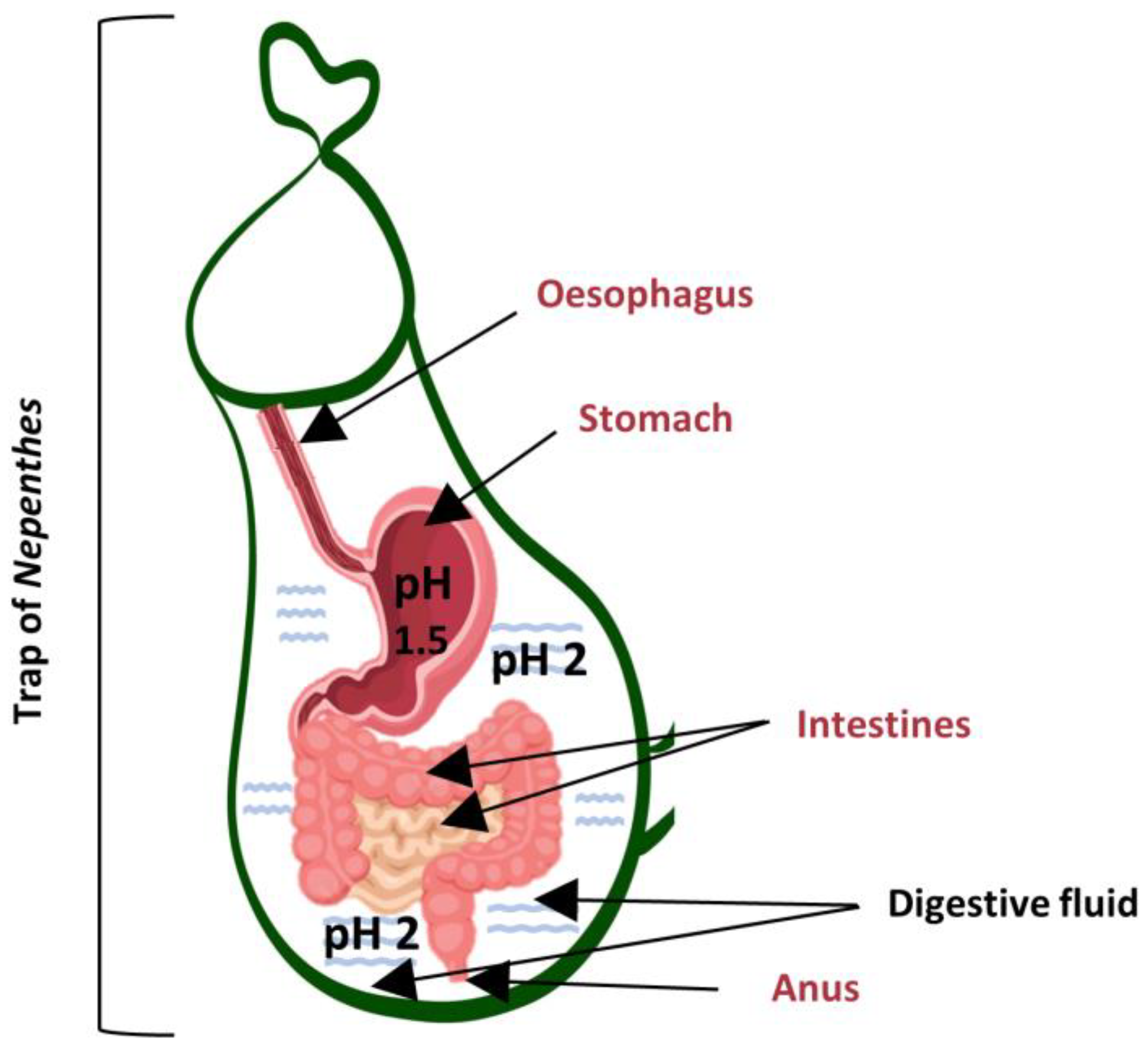
3. Nepenthes Trap as a Human Digestive System
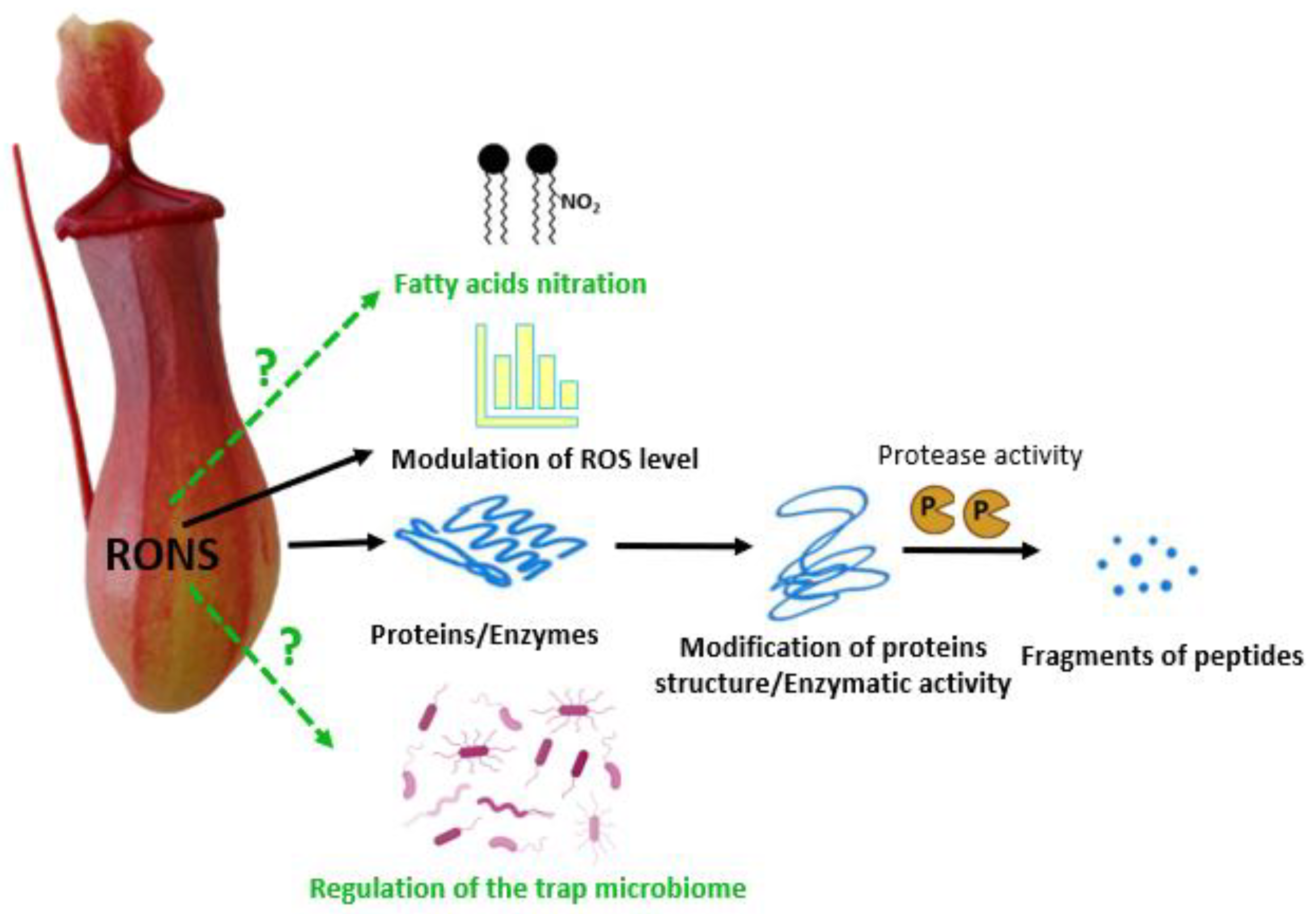
4. The Role of Reactive Oxygen Species in External Digestion by Carnivorous Plants
5. The Significance of Reactive Nitrogen Species in Carnivorous Plant External Digestion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melzig, M.F.; Pertz, H.H.; Krenn, L. Anti-Inflammatory and Spasmolytic Activity of Extracts from Droserae herba. Phytomedicine 2001, 8, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A. Carnivorous Pitcher Plants: Insights in an Old Topic. Phytochemistry 2011, 72, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Morohoshi, T.; Oikawa, M.; Sato, S.; Kikuchi, N.; Kato, N.; Ikeda, T. Isolation and Characterization of Novel Lipases from a Metagenomic Library of the Microbial Community in the Pitcher Fluid of the Carnivorous Plant Nepenthes hybrida. J. Biosci. Bioeng. 2011, 112, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. Insectivorous Plants; D. Appleton and Company: New York, NY, USA, 1875. [Google Scholar]
- Ellison, A.M.; Gotelli, N.J. Energetics and the Evolution of Carnivorous Plants—Darwin’s ‘Most Wonderful Plants in the World’. J. Exp. Bot. 2009, 60, 19–42. [Google Scholar] [CrossRef]
- Armstrong, W. Aeration in Higher Plants. Adv. Bot. Res. 1979, 7, 225–332. [Google Scholar] [CrossRef]
- Jürgens, A.; El-Sayed, A.M.; Suckling, D.M. Do Carnivorous Plants Use Volatiles for Attracting Prey Insects? Funct. Ecol. 2009, 23, 875–887. [Google Scholar] [CrossRef]
- Adlassnig, W.; Steinhauser, G.; Peroutka, M.; Musilek, A.; Sterba, J.H.; Lichtscheidl, I.K.; Bichler, M. Expanding the Menu for Carnivorous Plants: Uptake of Potassium, Iron and Manganese by Carnivorous Pitcher Plants. Appl. Radiat. Isot. 2009, 67, 2117–2122. [Google Scholar] [CrossRef]
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trębacz, K. Quite a Few Reasons for Calling Carnivores ‘the Most Wonderful Plants in the World’. Ann. Bot. 2012, 109, 47–64. [Google Scholar] [CrossRef]
- Płachno, B.J.; Adamec, L.; Huet, H. Mineral Nutrient Uptake from Prey and Glandular Phosphatase Activity as a Dual Test of Carnivory in Semi-Desert Plants with Glandular Leaves Suspected of Carnivory. Ann. Bot. 2009, 104, 649–654. [Google Scholar] [CrossRef]
- Cross, A.T.; Krueger, T.A.; Gonella, P.M.; Robinson, A.S.; Fleischmann, A.S. Conservation of Carnivorous Plants in the Age of Extinction. Glob. Ecol. Conserv. 2020, 24, e01272. [Google Scholar] [CrossRef]
- Millett, J.; Svensson, B.M.; Newton, J.; Rydin, H. Reliance on Prey-Derived Nitrogen by the Carnivorous Plant Drosera rotundifolia Decreases with Increasing Nitrogen Deposition. New Phytol. 2012, 195, 182–188. [Google Scholar] [CrossRef]
- Adlassnig, W.; Peroutka, M.; Lambers, H.; Lichtscheidl, I.K. The Roots of Carnivorous Plants. Plant Soil. 2005, 274, 127–140. [Google Scholar] [CrossRef]
- Lloyd, F.E. The Carnivorous Plants; Chronica Botanica Company: Waltham, MA, USA, 1942. [Google Scholar]
- Albert, V.A.; Williams, S.E.; Chase, M.W. Carnivorous Plants: Phylogeny and Structural Evolution. Science (1979) 1992, 257, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.; Moran, J.A.; Lee, C.C. Nepenthes baramensis (Nepenthaceae)—A New Species from North-Western Borneo. Blumea Biodivers. Evol. Biogeogr. Plants 2011, 56, 229–233. [Google Scholar] [CrossRef]
- Meimberg, H.; Wistuba, A.; Dittrich, P.; Heubl, G. Molecular Phylogeny of Nepenthaceae Based on Cladistic Analysis of Plastid TrnK Intron Sequence Data. Plant Biol. 2001, 3, 164–175. [Google Scholar] [CrossRef]
- Meimberg, H.; Heubl, G. Introduction of a Nuclear Marker for Phylogenetic Analysis of Nepenthaceae. Plant Biol. 2006, 8, 831–840. [Google Scholar] [CrossRef]
- Bauer, U.; Grafe, T.U.; Federle, W. Evidence for Alternative Trapping Strategies in Two Forms of the Pitcher Plant. Nepenthes rafflesiana. J. Exp. Bot. 2011, 62, 3683–3692. [Google Scholar] [CrossRef]
- Clarke, C. Nepenthes of Borneo; Natural History Publications (Borneo): Kota Kinabalu, Malaysia, 1997; ISBN 9789838120159. [Google Scholar]
- Dančák, M.; Majeský, Ľ.; Čermák, V.; Golos, M.R.; Płachno, B.J.; Tjiasmanto, W. First Record of Functional Underground Traps in a Pitcher Plant: Nepenthes pudica (Nepenthaceae), a New Species from North Kalimantan, Borneo. PhytoKeys 2022, 201, 77–97. [Google Scholar] [CrossRef]
- Adlassnig, W.; Peroutka, M.; Lendl, T. Traps of Carnivorous Pitcher Plants as a Habitat: Composition of the Fluid, Biodiversity and Mutualistic Activities. Ann. Bot. 2011, 107, 181–194. [Google Scholar] [CrossRef]
- Gaume, L.; Di Giusto, B. Adaptive Significance and Ontogenetic Variability of the Waxy Zone in Nepenthes rafflesiana. Ann. Bot. 2009, 104, 1281–1291. [Google Scholar] [CrossRef]
- Gaume, L.; Gorb, S.; Rowe, N. Function of Epidermal Surfaces in the Trapping Efficiency of Nepenthes alata Pitchers. New Phytol. 2002, 156, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Gaume, L.; Perret, P.; Gorb, E.; Gorb, S.; Labat, J.J.; Rowe, N. How Do Plant Waxes Cause Flies to Slide? Experimental Tests of Wax-Based Trapping Mechanisms in Three Pitfall Carnivorous Plants. Arthropod Struct. Dev. 2004, 33, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tao, D.; Dong, S.; Li, S.; Tian, Y. Contributions of Lunate Cells and Wax Crystals to the Surface Anisotropy of Nepenthes Slippery Zone. R. Soc. Open Sci. 2018, 5, 180766. [Google Scholar] [CrossRef] [PubMed]
- Gorb, E.; Haas, K.; Henrich, A.; Enders, S.; Barbakadze, N.; Gorb, S. Composite Structure of the Crystalline Epicuticular Wax Layer of the Slippery Zone in the Pitchers of the Carnivorous Plant Nepenthes alata and Its Effect on Insect Attachment. J. Exp. Biol. 2005, 208, 4651–4662. [Google Scholar] [CrossRef]
- Moran, J.A.; Clarke, C.M.; Hawkins, B.J. From Carnivore to Detritivore? Isotopic Evidence for Leaf Litter Utilization by the Tropical Pitcher Plant Nepenthes ampullaria. Int. J. Plant Sci. 2003, 164, 635–639. [Google Scholar] [CrossRef]
- Bohn, H.F.; Federle, W. Insect Aquaplaning: Nepenthes Pitcher Plants Capture Prey with the Peristome, a Fully Wettable Water-Lubricated Anisotropic Surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef]
- Moran, J.A.; Clarke, C.M. The Carnivorous Syndrome in Nepenthes Pitcher Plants. Plant Signal Behav. 2010, 5, 644–648. [Google Scholar] [CrossRef]
- Kang, V.; Isermann, H.; Sharma, S.; Wilson, D.I.; Federle, W. How a Sticky Fluid Facilitates Prey Retention in a Carnivorous Pitcher Plant (Nepenthes rafflesiana). Acta Biomater. 2021, 128, 357–369. [Google Scholar] [CrossRef]
- Hatano, N.; Hamada, T. Proteomic Analysis of Secreted Protein Induced by a Component of Prey in Pitcher Fluid of the Carnivorous Plant Nepenthes alata. J. Proteom. 2012, 75, 4844–4852. [Google Scholar] [CrossRef]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced Production of Antifungal Naphthoquinones in the Pitchers of the Carnivorous Plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef]
- Raj, G.; Kurup, R.; Hussain, A.A.; Baby, S. Distribution of Naphthoquinones, Plumbagin, Droserone, and 5-O-Methyl Droserone in Chitin-Induced and Uninduced Nepenthes khasiana: Molecular Events in Prey Capture. J. Exp. Bot. 2011, 62, 5429–5436. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.H.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Ahmed, A.A.; Pare, P.W.; Mabry, T.J. Phenolic Constituents from the Leaves of the Carnivorous Plant Nepenthes gracilis. Fitoterapia 2002, 73, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.M.; Bauer, U.; Lee, C.C.; Tuen, A.A.; Rembold, K.; Moran, J.A. Tree Shrew Lavatories: A Novel Nitrogen Sequestration Strategy in a Tropical Pitcher Plant. Biol. Lett. 2009, 5, 632–635. [Google Scholar] [CrossRef]
- Grafe, T.U.; Schöner, C.R.; Kerth, G.; Junaidi, A.; Schöner, M.G. A Novel Resource-Service Mutualism between Bats and Pitcher Plants. Biol. Lett. 2011, 7, 436–439. [Google Scholar] [CrossRef]
- Freund, M.; Graus, D.; Fleischmann, A.; Gilbert, K.J.; Lin, Q.; Renner, T.; Stigloher, C.; Albert, V.A.; Hedrich, R.; Fukushima, K. The Digestive Systems of Carnivorous Plants. Plant Physiol. 2022, 190, 44–59. [Google Scholar] [CrossRef]
- Schleiffer, R.; Raul, F. Nitric Oxide and the Digestive System in Mammals and Non-Mammalian Vertebrates. Comp. Biochem. Physiol. 1997, 118, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Pecere, S.; Giorgio, V.; Gasbarrini, A.; Cammarota, G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr. Drug Metab. 2016, 17, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M. Neural and Hormonal Stimulation of Gastric Secretion of Acid. In Handbook of Physiology. Section 6: Alimentary Canal; American Physiological Society: Washington, DC, USA, 1987; pp. 845–863. [Google Scholar]
- Sandvik, A.K.; Brenna, E.; Waldum, H.L. The Pharmacological Inhibition of Gastric Acid Secretion-Tolerance and Rebound. Aliment. Pharmacol. Ther. 1997, 11, 1013–1018. [Google Scholar] [CrossRef]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Wal, A.; Staszek, P.; Pakula, B.; Paradowska, M.; Krasuska, U. ROS and RNS Alterations in the Digestive Fluid of Nepenthes × Ventrata Trap at Different Developmental Stages. Plants 2022, 11, 3304. [Google Scholar] [CrossRef]
- Bauer, U.; Willmes, C.; Federle, W. Effect of Pitcher Age on Trapping Efficiency and Natural Prey Capture in Carnivorous Nepenthes rafflesiana Plants. Ann. Bot. 2009, 103, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Bazile, V.; Le Moguédec, G.; Marshall, D.J.; Gaume, L. Fluid Physico-Chemical Properties Influence Capture and Diet in Nepenthes Pitcher Plants. Ann. Bot. 2015, 115, 705–716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonhomme, V.; Gounand, I.; Alaux, C.; Jousselin, E.; Barthélémy, D.; Gaume, L. The Plant-Ant Camponotus Schmitzi Helps Its Carnivorous Host-Plant Nepenthes bicalcarata to Catch Its Prey. J. Trop. Ecol. 2011, 27, 15–24. [Google Scholar] [CrossRef]
- An, C.I.; Fukusaki, E.I.; Kobayashi, A. Plasma-Membrane H+-ATPases Are Expressed in Pitchers of the Carnivorous Plant Nepenthes alata Blanco. Planta 2001, 212, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Buch, F.; Kaman, W.E.; Bikker, F.J.; Yilamujiang, A.; Mithöfer, A. Nepenthesin Protease Activity Indicates Digestive Fluid Dynamics in Carnivorous Nepenthes Plants. PLoS ONE 2015, 10, e0118853. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, P.; Hogan, J. Cloning and Characterization of a Ribonuclease, a Cysteine Proteinase, and an Aspartic Proteinase from Pitchers of the Carnivorous Plant Nepenthes Ventricosa Blanco. Int. J. Plant Sci. 2006, 167, 239–248. [Google Scholar] [CrossRef]
- Rottloff, S.; Miguel, S.; Biteau, F.; Nisse, E.; Hammann, P.; Kuhn, L.; Chicher, J.; Bazile, V.; Gaume, L.; Mignard, B.; et al. Proteome Analysis of Digestive Fluids in Nepenthes Pitchers. Ann. Bot. 2016, 117, 479–495. [Google Scholar] [CrossRef]
- An, C.I.; Fukusaki, E.I.; Kobayashi, A. Aspartic Proteinases Are Expressed in Pitchers of the Carnivorous Plant Nepenthes alata Blanco. Planta 2002, 214, 661–667. [Google Scholar] [CrossRef]
- Athauda, S.B.P.; Matsumoto, K.; Rajapakshe, S.; Kuribayashi, M.; Kojima, M.; Kubomura-Yoshida, N.; Iwamatsu, A.; Shibata, C.; Inoue, H.; Takahashi, K. Enzymic and Structural Characterization of Nepenthesin, a Unique Member of a Novel Subfamily of Aspartic Proteinases. Biochem. J. 2004, 381, 295–306. [Google Scholar] [CrossRef]
- Kadek, A.; Tretyachenko, V.; Mrazek, H.; Ivanova, L.; Halada, P.; Rey, M.; Schriemer, D.C.; Man, P. Expression and Characterization of Plant Aspartic Protease Nepenthesin-1 from Nepenthes gracilis. Protein Expr. Purif. 2014, 95, 121–128. [Google Scholar] [CrossRef]
- Hatano, N.; Hamada, T. Proteome Analysis of Pitcher Fluid of the Carnivorous Plant Nepenthes alata. J. Proteome Res. 2008, 7, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Dkhar, J.; Bhaskar, Y.K.; Lynn, A.; Pareek, A. Pitchers of Nepenthes khasiana Express Several Digestive-Enzyme Encoding Genes, Harbor Mostly Fungi and Probably Evolved through Changes in the Expression of Leaf Polarity Genes. BMC Plant Biol. 2020, 20, 524. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.; Kawahara, M.; Kodaira, R.; Kume, M.; Arai, N.; Nishikawa, J.; Ohyama, T. S-like Ribonuclease Gene Expression in Carnivorous Plants. Planta 2013, 238, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Ciacka, K.; Krasuska, U.; Staszek, P.; Wal, A.; Zak, J.; Gniazdowska, A. Effect of Nitrogen Reactive Compounds on Aging in Seed. Front. Plant Sci. 2020, 11, 1011. [Google Scholar] [CrossRef]
- Horn, A.; Jaiswal, J.K. Cellular Mechanisms and Signals That Coordinate Plasma Membrane Repair. Cell. Mol. Life Sci. 2018, 75, 3751–3770. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production Sites of Reactive Oxygen Species (ROS) in Organelles from Plant Cells. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Corpas, F.J., Gupta, D.K., Palma, J.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–22. [Google Scholar]
- Thiel, J.; Rolletschek, H.; Friedel, S.; Lunn, J.E.; Nguyen, T.H.; Feil, R.; Tschiersch, H.; Müller, M.; Borisjuk, L. Seed-Specific Elevation of Non-Symbiotic Hemoglobin AtHb1: Beneficial Effects and Underlying Molecular Networks in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 48. [Google Scholar] [CrossRef]
- Møller, I.M.; Rogowska-Wrzesinska, A.; Rao, R.S.P. Protein Carbonylation and Metal-Catalyzed Protein Oxidation in a Cellular Perspective. J. Proteomics 2011, 74, 2228–2242. [Google Scholar] [CrossRef]
- Kumar, J.S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed Birth to Death: Dual Functions of Reactive Oxygen Species in Seed Physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef]
- Tamura, M.; Mutoh, M.; Fujii, G.; Matsui, H. Involvement of Mitochondrial Reactive Oxygen Species in Gastric Carcinogenesis. J. Gastrointest. Dig. Syst. 2013, 3, 150. [Google Scholar] [CrossRef]
- Toyokuni, S.; Mori, T.; Hiai, H.; Dizdaroglu, M. Treatment of Wistar Rats with a Renal Carcinogen, Ferric Nitrilotriacetate, Causes DNA-protein Cross-linking between Thymine and Tyrosine in Their Renal Chromatin. Int. J. Cancer 1995, 62, 309–313. [Google Scholar] [CrossRef]
- Kasai, H.; Nishimura, S. Hydroxylation of Deoxyguanosine at the C-8 Position by Ascorbic Acid and Other Reducing Agents. Nucleic Acids Res. 1984, 12, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Ciacka, K.; Tymiński, M.; Gniazdowska, A.; Krasuska, U. Carbonylation of Proteins—An Element of Plant Ageing. Planta 2020, 252, 12. [Google Scholar] [CrossRef] [PubMed]
- Morscher, R.J.; Aminzadeh-Gohari, S.; Feichtinger, R.G.; Mayr, J.A.; Lang, R.; Neureiter, D.; Sperl, W.; Kofler, B. Inhibition of Neuroblastoma Tumor Growth by Ketogenic Diet and/or Calorie Restriction in a CD1-Nu Mouse Model. PLoS ONE 2015, 10, e0129802. [Google Scholar] [CrossRef]
- Kranner, I.; Birtić, S.; Anderson, K.M.; Pritchard, H.W. Glutathione Half-Cell Reduction Potential: A Universal Stress Marker and Modulator of Programmed Cell Death? Free Radic. Biol. Med. 2006, 40, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Maddalena, F.; Laudiero, G.; Esposito, F. Adaptation to Oxidative Stress, Chemoresistance, and Cell Survival. Antioxid. Redox Signal. 2009, 11, 2701–2716. [Google Scholar] [CrossRef]
- Babior, B.M. Oxidants from Phagocytes: Agents of Defense and Destruction. Blood 1984, 64, 959–966. [Google Scholar] [CrossRef]
- Takaki, A.; Kawano, S.; Uchida, D.; Takahara, M.; Hiraoka, S.; Okada, H. Paradoxical Roles of Oxidative Stress Response in the Digestive System before and after Carcinogenesis. Cancers 2019, 11, 213. [Google Scholar] [CrossRef]
- Wetscher, G.J.; Hinder, P.R.; Bagchi, D.; Perdikis, G.; Redmond, E.J.; Glaser, K.; Adrian, T.E.; Hinder, R.A. Free Radical Scavengers Prevent Reflux Esophagitis in Rats. Dig. Dis. Sci. 1995, 40, 1292–1296. [Google Scholar] [CrossRef]
- Meining, A.; Classen, M. The Role of Diet and Lifestyle Measures in the Pathogenesis and Treatment of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2000, 95, 2692–2697. [Google Scholar] [CrossRef]
- Van Hecke, T.; Van Camp, J.; De Smet, S. Oxidation during Digestion of Meat: Interactions with the Diet and Helicobacter Pylori Gastritis, and Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2017, 16, 214–233. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J. Protein Damage and Degradation by Oxygen Radicals. I. General. Aspects. J. Biol. Chem. 1987, 262, 9895–9901. [Google Scholar] [PubMed]
- Davies, K.J.A.; Lin, S.W. Degradation of Oxidatively Denatured Proteins in Escherichia Coli. Free Radic. Biol. Med. 1988, 5, 215–223. [Google Scholar] [CrossRef]
- Basset, G.; Raymond, P.; Malek, L.; Brouquisse, R. Changes in the Expression and the Enzymic Properties of the 20S Proteasome in Sugar-Starved Maize Roots. Evidence for an in Vivo Oxidation of the Proteasome. Plant Physiol. 2002, 128, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Krasuska, U.; Ciacka, K.; Dębska, K.; Bogatek, R.; Gniazdowska, A. Dormancy Alleviation by NO or HCN Leading to Decline of Protein Carbonylation Levels in Apple (Malus domestica Borkh.). Embryos. J. Plant Physiol. 2014, 171, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.F.; Aung, H.H.; Osipov, A.N.; Goh, N.K.; Chia, L.S. Carnivorous Pitcher Plant Uses Free Radicals in the Digestion of Prey. Redox Report 2004, 9, 255–261. [Google Scholar] [CrossRef]
- Durzan, D.J.; Pedroso, M.C. Nitric Oxide and Reactive Nitrogen Oxide Species in Plants. Biotechnol. Genet. Eng. Rev. 2002, 19, 293–338. [Google Scholar] [CrossRef][Green Version]
- Del Castello, F.; Nejamkin, A.; Cassia, R.; Correa-Aragunde, N.; Fernández, B.; Foresi, N.; Lombardo, C.; Ramirez, L.; Lamattina, L. The Era of Nitric Oxide in Plant Biology: Twenty Years Tying up Loose Ends. Nitric Oxide 2019, 85, 17–27. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef]
- Stamler, J.; Singel, D.; Loscalzo, J. Biochemistry of Nitric Oxide and Its Redox-Activated Forms. Science (1979) 1992, 258, 1898–1902. [Google Scholar] [CrossRef]
- Mohn, M.; Thaqi, B.; Fischer-Schrader, K. Isoform-Specific NO Synthesis by Arabidopsis thaliana Nitrate Reductase. Plants 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.; Schwarz, G. Nitrite-Dependent Nitric Oxide Synthesis by Molybdenum Enzymes. FEBS Lett. 2018, 592, 2126–2139. [Google Scholar] [CrossRef]
- Foresi, N.; Correa-Aragunde, N.; Parisi, G.; Calo, G.; Salerno, G.; Lamattina, L. Characterization of a Nitric Oxide Synthase from the Plant Kingdom: NO Generation from the Green Alga Ostreococcus tauri Is Light Irradiance and Growth Phase Dependent. Plant Cell 2010, 22, 3816–3830. [Google Scholar] [CrossRef] [PubMed]
- Jeandroz, S.; Wipf, D.; Stuehr, D.J.; Lamattina, L.; Melkonian, M.; Tian, Z.; Zhu, Y.; Carpenter, E.J.; Wong, G.K.-S.; Wendehenne, D. Occurrence, Structure, and Evolution of Nitric Oxide Synthase–like Proteins in the Plant Kingdom. Sci. Signal 2016, 9, re2. [Google Scholar] [CrossRef]
- Hancock, J.T.; Neill, S.J. Nitric Oxide: Its Generation and Interactions with Other Reactive Signaling Compounds. Plants 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H. Nitrite-Dependent Nitric Oxide Production Pathway: Implications for Involvement of Active Nitrogen Species in Photoinhibition in Vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Pereira, C.; Barbosa, R.M.; Bartesaghi, S.; Lundberg, J.O.; Radi, R.; Laranjinha, J. Dietary Nitrite in Nitric Oxide Biology: A Redox Interplay with Implications for Pathophysiology and Therapeutics. Curr. Drug Targets 2011, 12, 1351–1363. [Google Scholar] [CrossRef]
- Gupta, K.J.; Hancock, J.T.; Petrivalsky, M.; Kolbert, Z.; Lindermayr, C.; Durner, J.; Barroso, J.B.; Palma, J.M.; Brouquisse, R.; Wendehenne, D.; et al. Recommendations on Terminology and Experimental Best Practice Associated with Plant Nitric Oxide Research. New Phytol. 2020, 225, 1828–1834. [Google Scholar] [CrossRef]
- Corpas, F.J.; Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Barroso, J.B. Nitration and S-Nitrosylation: Two Post-Translational Modifications (PTMs) Mediated by Reactive Nitrogen Species (RNS) and Their Role in Signalling Processes of Plant Cells. In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants, Signaling and Communication in Plants; Gupta, K.J., Igamberdiev, A.U., Eds.; Springer: Cham, Switzerland, 2015; pp. 267–281. [Google Scholar]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Padilla, M.N.; Begara-Morales, J.C.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitro-Fatty Acids in Plant Signaling: New Key Mediators of Nitric Oxide Metabolism. Redox Biol. 2017, 11, 554–561. [Google Scholar] [CrossRef]
- Izbiańska, K.; Floryszak-Wieczorek, J.; Gajewska, J.; Meller, B.; Kuźnicki, D.; Arasimowicz-Jelonek, M. RNA and mRNA Nitration as a Novel Metabolic Link in Potato Immune Response to Phytophthora Infestans. Front. Plant Sci. 2018, 9, 672. [Google Scholar] [CrossRef]
- Rocha, B.S.; Correia, M.G.; Fernandes, R.C.; Gonçalves, J.S.; Laranjinha, J. Dietary Nitrite Induces Occludin Nitration in the Stomach. Free Radic. Res. 2016, 50, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Lundberg, J.O.; Mann, G.E.; Radi, R.; Laranjinha, J. Pepsin Is Nitrated in the Rat Stomach, Acquiring Antiulcerogenic Activity: A Novel Interaction between Dietary Nitrate and Gut Proteins. Free Radic. Biol. Med. 2013, 58, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Barbosa, R.M.; Laranjinha, J. Dietary Nitrite Induces Nitrosation of the Gastric Mucosa: The Protective Action of the Mucus and the Modulatory Effect of Red Wine. J. Nutr. Biochem. 2015, 26, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Gangolli, S.D.; van den Brandt, P.A.; Feron, V.J.; Janzowsky, C.; Koeman, J.H.; Speijers, G.J.A.; Spiegelhalder, B.; Walker, R.; Wishnok, J.S. Nitrate, Nitrite and N-Nitroso Compounds. Eur. J. Pharmacol Environ. Toxicol. Pharmacol. 1994, 292, 1–38. [Google Scholar] [CrossRef]
- Honikel, K.-O. The Use and Control of Nitrate and Nitrite for the Processing of Meat Products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pérez, C.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Padilla, M.N.; Valderrama, R.; Corpas, F.J.; Barroso, J.B. Nitric Oxide Release from Nitro-Fatty Acids in Arabidopsis Roots. Plant Signal Behav. 2016, 11, e1154255. [Google Scholar] [CrossRef]
- Fazzari, M.; Trostchansky, A.; Schopfer, F.J.; Salvatore, S.R.; Sánchez-Calvo, B.; Vitturi, D.; Valderrama, R.; Barroso, J.B.; Radi, R.; Freeman, B.A.; et al. Olives and Olive Oil Are Sources of Electrophilic Fatty Acid Nitroalkenes. PLoS ONE 2014, 9, e84884. [Google Scholar] [CrossRef]
- Buch, F.; Rott, M.; Rottloff, S.; Paetz, C.; Hilke, I.; Raessler, M.; Mithöfer, A. Secreted Pitfall-Trap Fluid of Carnivorous Nepenthes Plants Is Unsuitable for Microbial Growth. Ann. Bot. 2013, 111, 375–383. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasuska, U.; Wal, A.; Staszek, P.; Ciacka, K.; Gniazdowska, A. Do Reactive Oxygen and Nitrogen Species Have a Similar Effect on Digestive Processes in Carnivorous Nepenthes Plants and Humans? Biology 2023, 12, 1356. https://doi.org/10.3390/biology12101356
Krasuska U, Wal A, Staszek P, Ciacka K, Gniazdowska A. Do Reactive Oxygen and Nitrogen Species Have a Similar Effect on Digestive Processes in Carnivorous Nepenthes Plants and Humans? Biology. 2023; 12(10):1356. https://doi.org/10.3390/biology12101356
Chicago/Turabian StyleKrasuska, Urszula, Agnieszka Wal, Paweł Staszek, Katarzyna Ciacka, and Agnieszka Gniazdowska. 2023. "Do Reactive Oxygen and Nitrogen Species Have a Similar Effect on Digestive Processes in Carnivorous Nepenthes Plants and Humans?" Biology 12, no. 10: 1356. https://doi.org/10.3390/biology12101356
APA StyleKrasuska, U., Wal, A., Staszek, P., Ciacka, K., & Gniazdowska, A. (2023). Do Reactive Oxygen and Nitrogen Species Have a Similar Effect on Digestive Processes in Carnivorous Nepenthes Plants and Humans? Biology, 12(10), 1356. https://doi.org/10.3390/biology12101356







