Seed Germination and Seedling Growth in Suaeda salsa (Linn.) Pall. (Amaranthaceae) Demonstrate Varying Salinity Tolerance among Different Provenances
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Seed Germination, Seedling Growth, and Germination Recovery
2.3. Statistical Analysis
3. Results
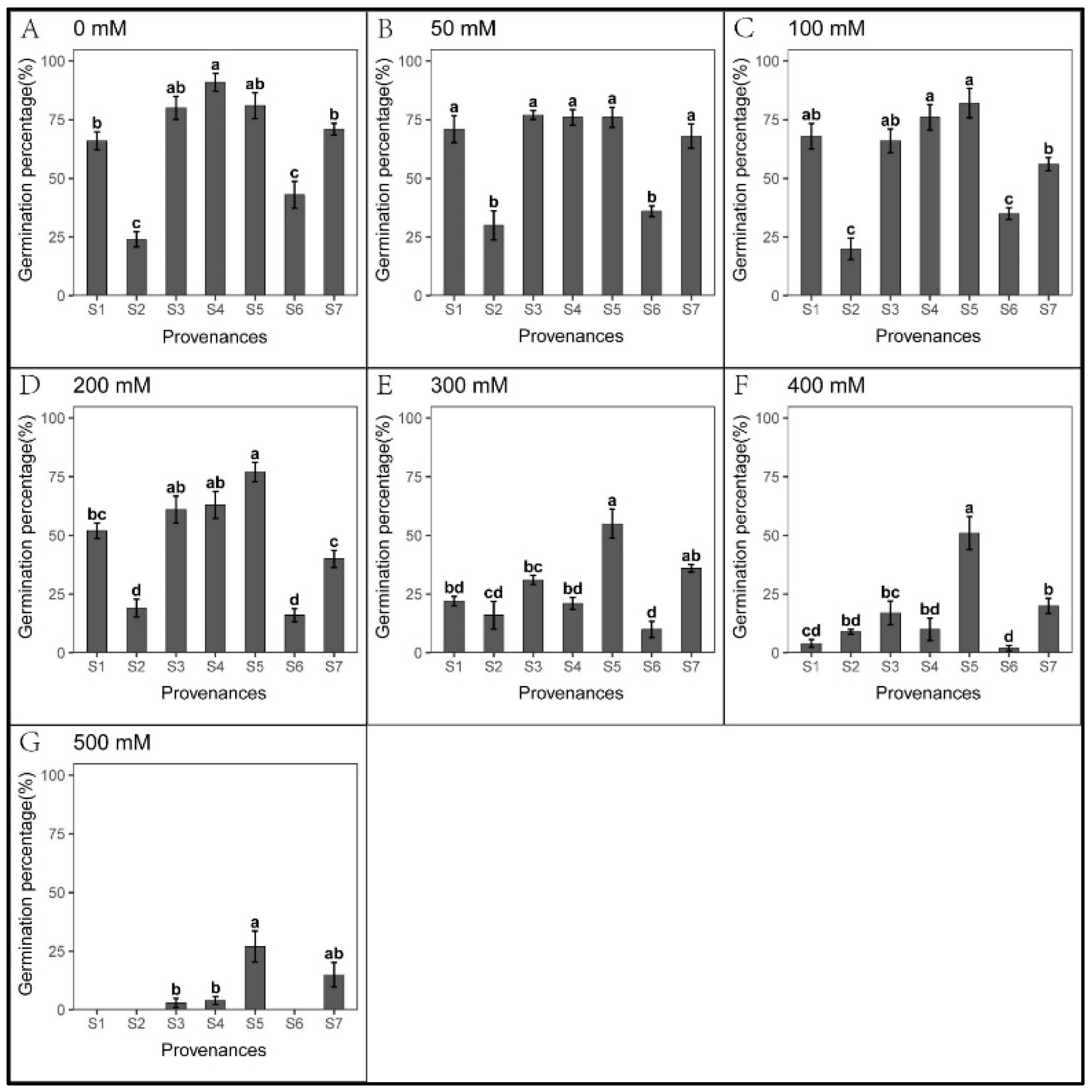
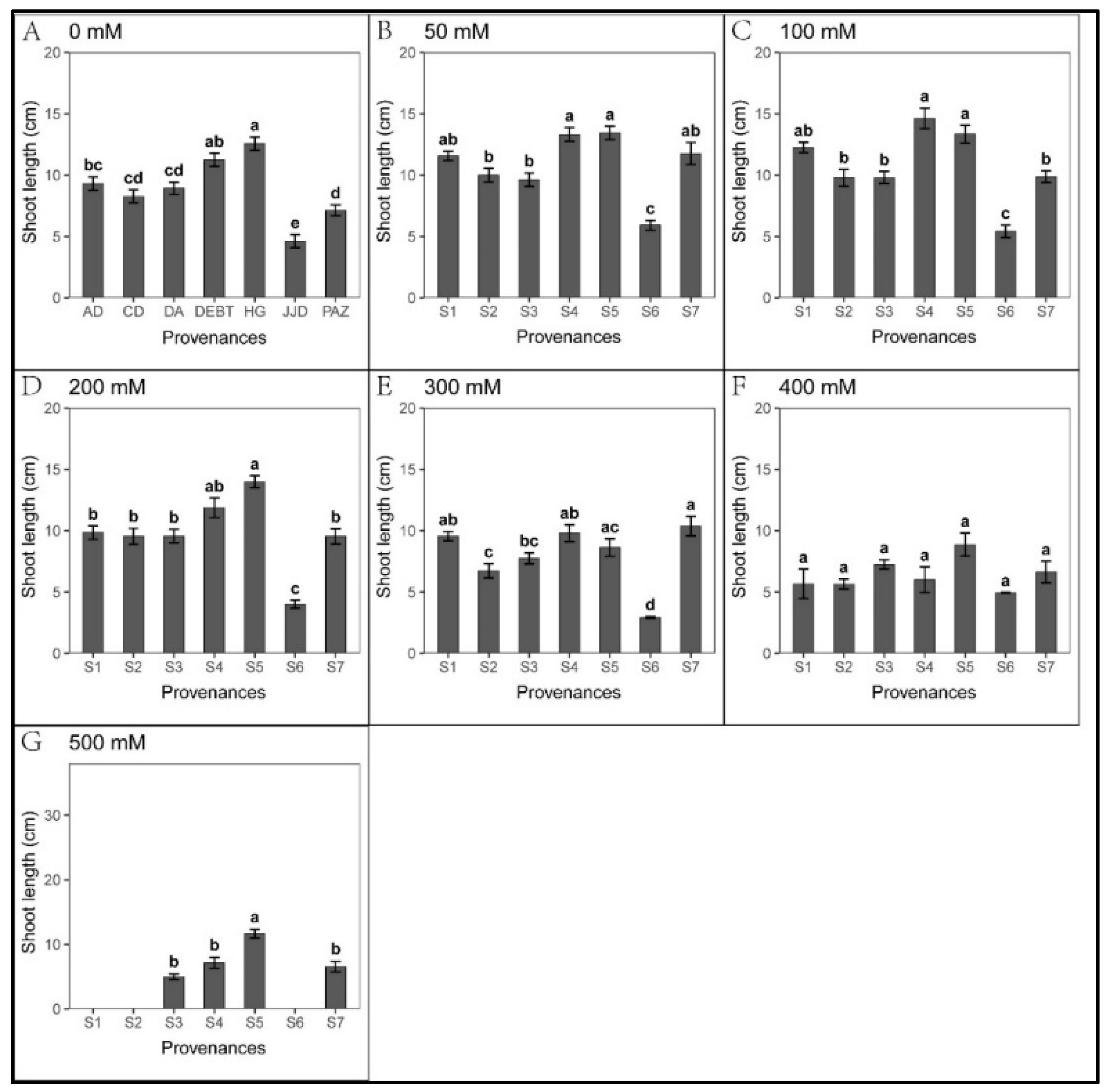
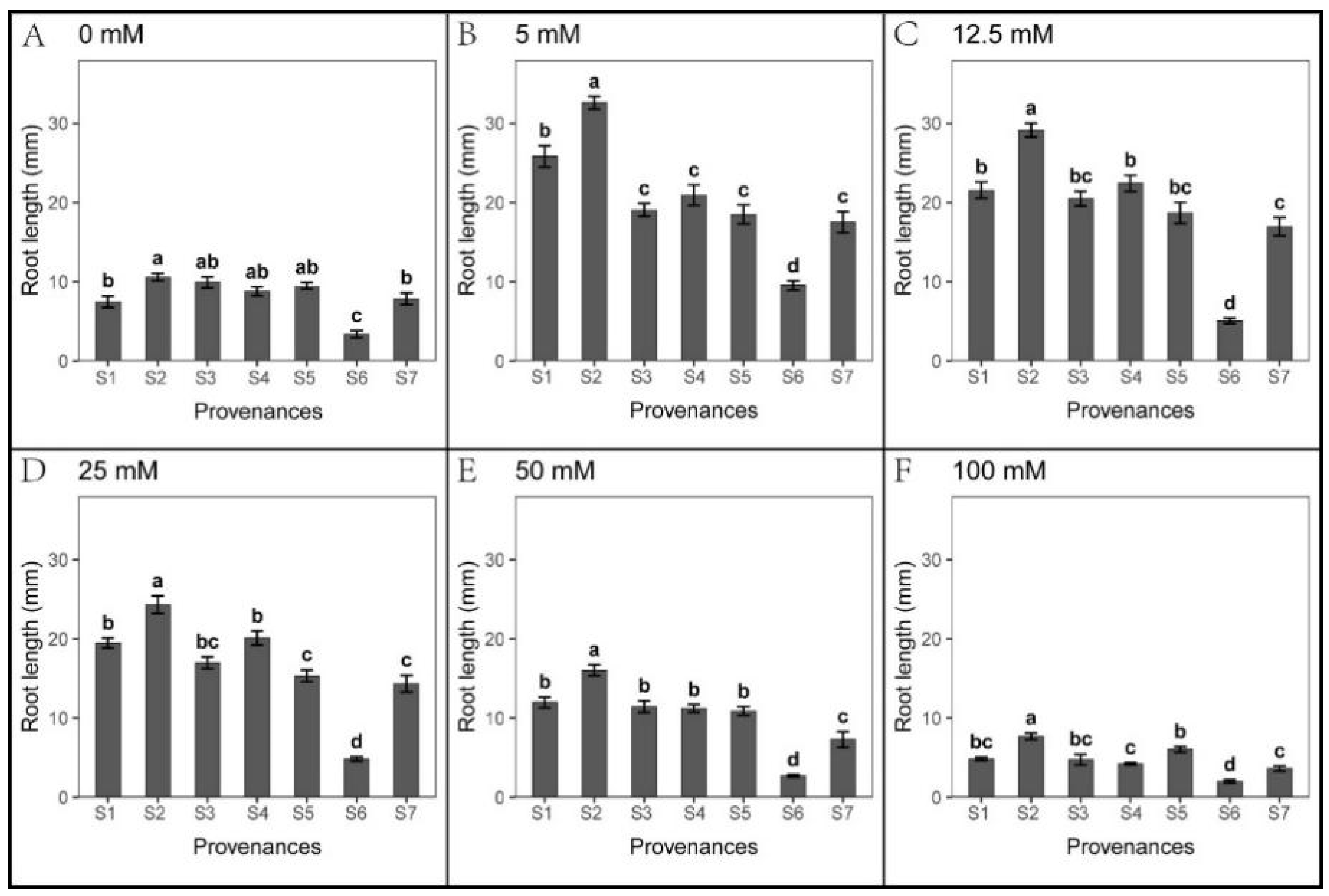
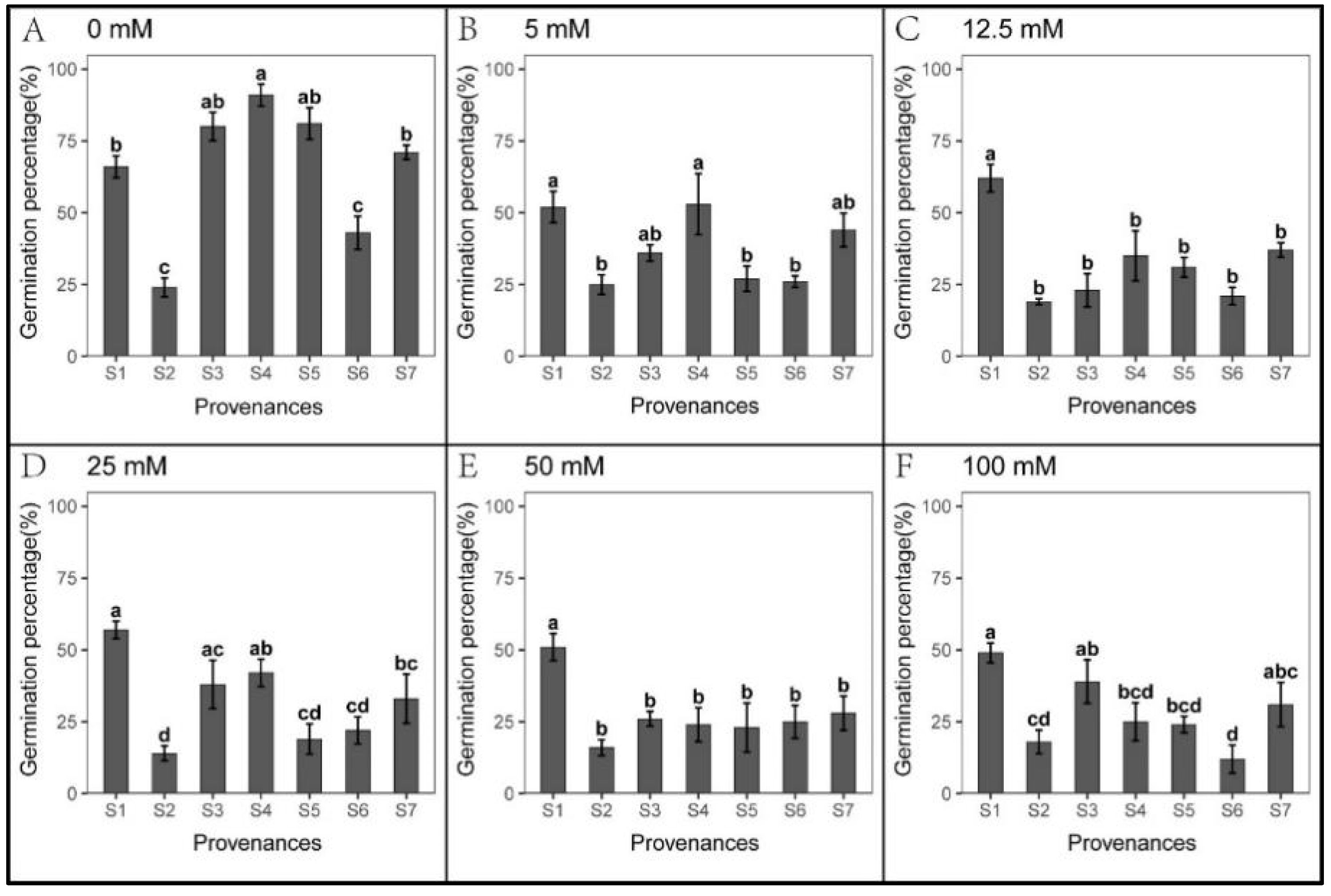
3.1. Effect of Provenance on Seed Germination under NaCl Stress
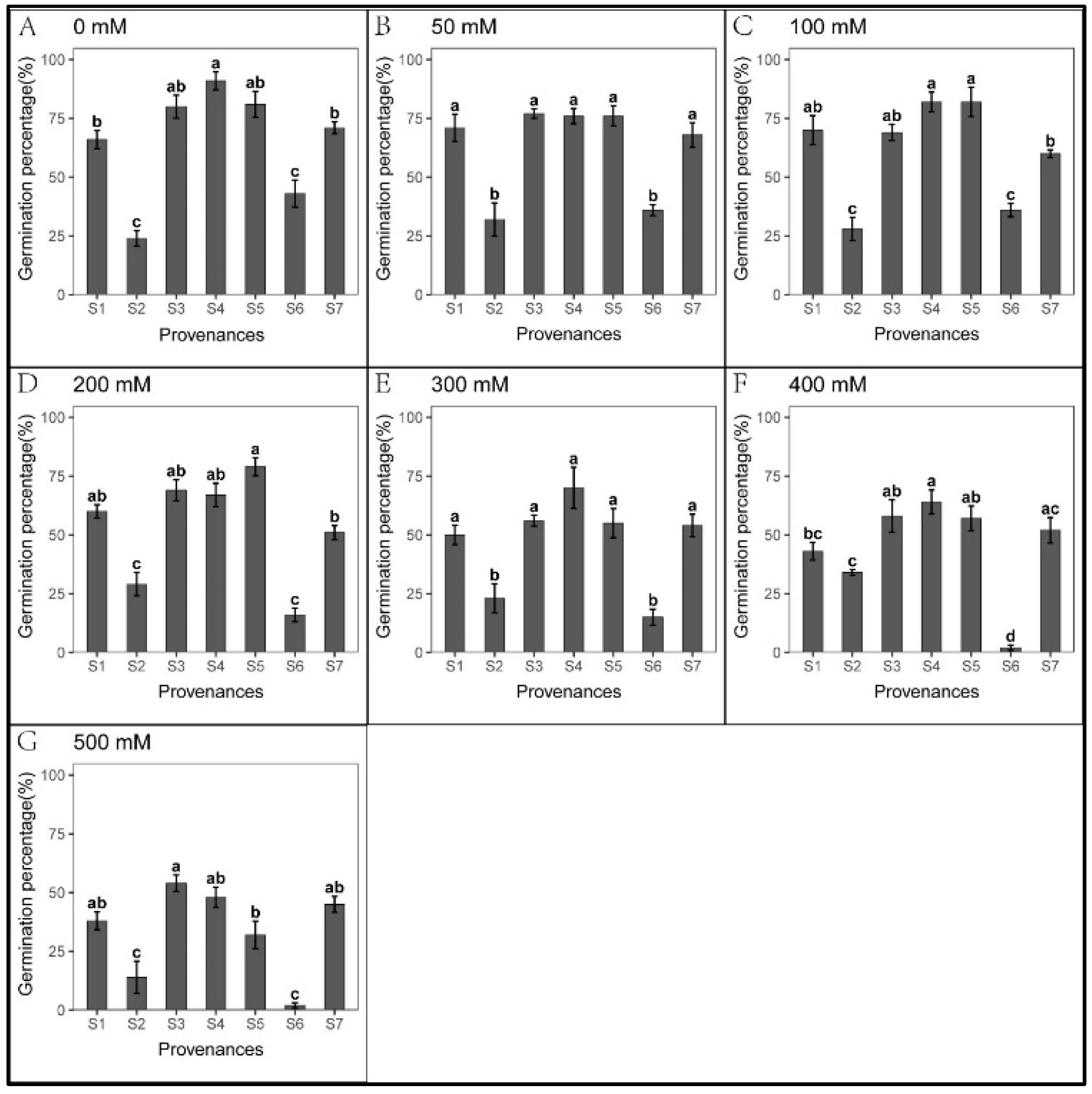
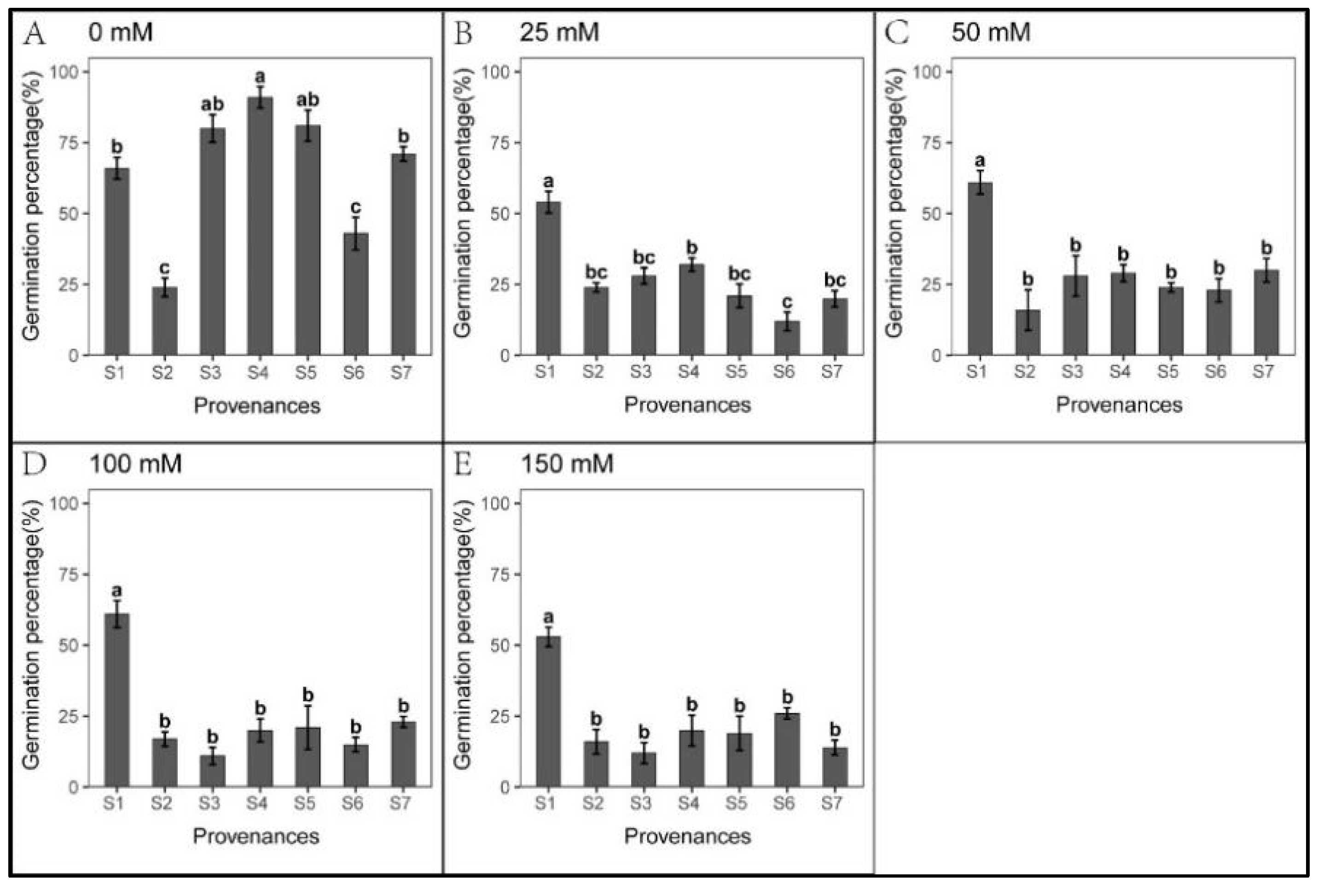
3.2. Effect of Provenances on Seed Germination under Na2CO3 Stress
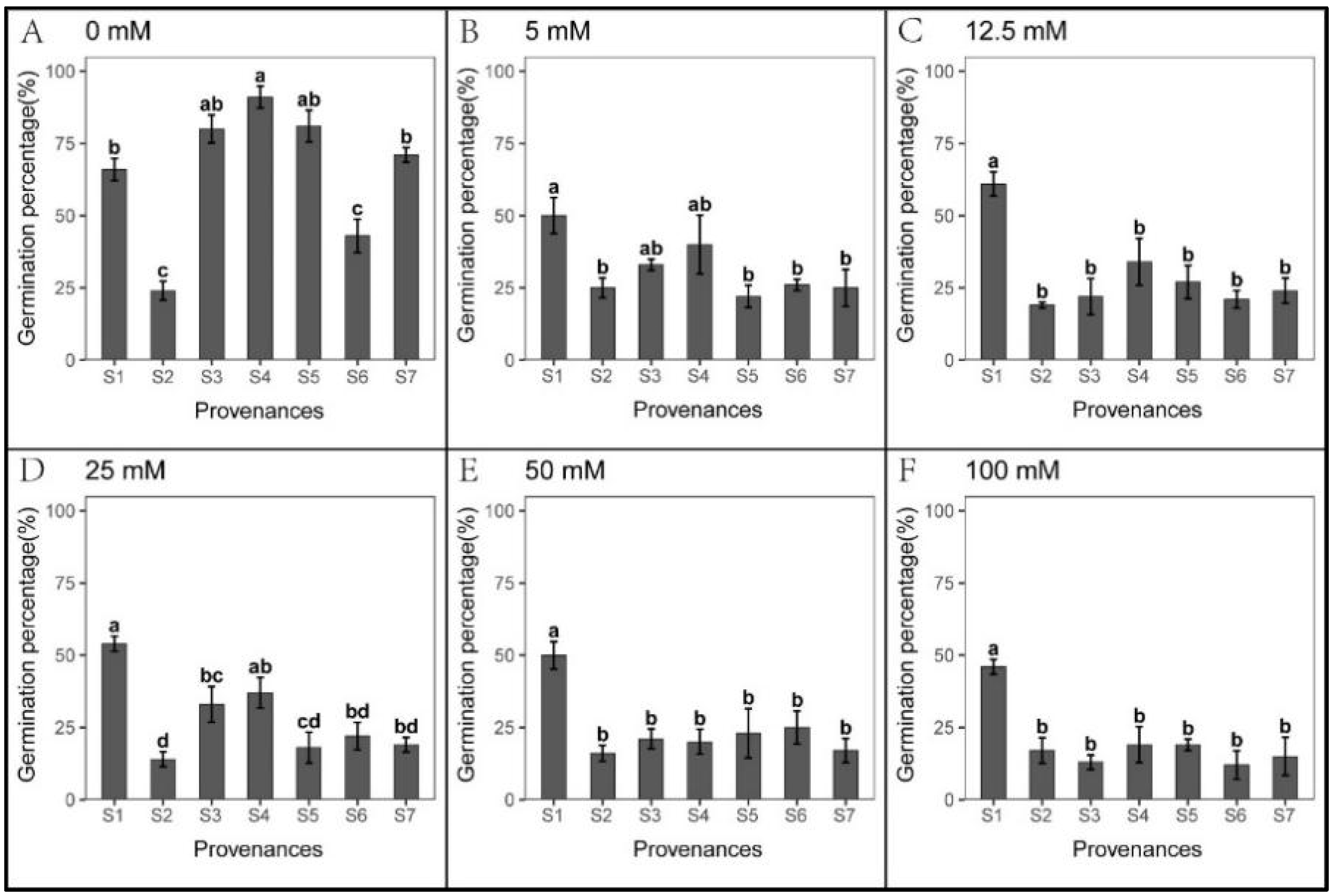
3.3. Effect of Provenance on Seed Germination under NaHCO3 Stress
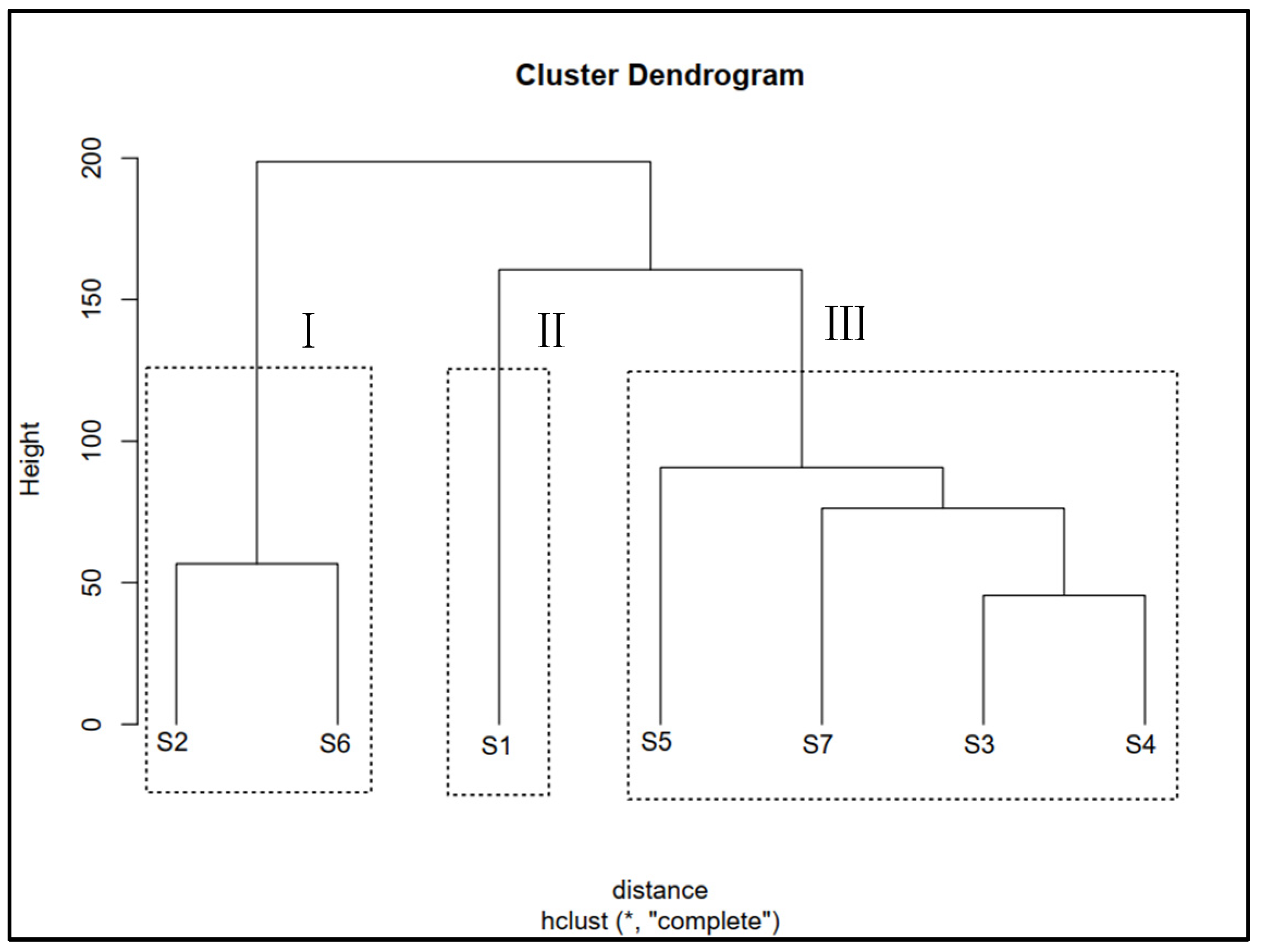
3.4. Cluster Analysis of Seed Germination Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morton, M.J.L.; Awlia, M.; Al-Tamimi, N.; Saade, S.; Pailles, Y.; Negrão, S.; Tester, M. Salt stress under the scalpel—Dissecting the genetics of salt tolerance. Plant J. 2018, 97, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.C.; Liu, C.A.; Zhao, H.L.; Wang, Y. Changes of soil physico-chemical properties and enzyme activities in relation to grassland salinization. Eur. J. Soil Biol. 2013, 55, 13–19. [Google Scholar] [CrossRef]
- Corwin, D.L.; Yemoto, K. Salinity: Electrical conductivity and total dissolved solids. Soil Sci. Soc. Am. J. 2020, 84, 1442–1461. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Lü, B.-S.; Yang, H.-Y.; Yan, C.; Liang, Z.-W. Responses of seed germination of Leymus chinensis to environmental factors in degraded grassland on Songnen Plain in China. Chin. J. Plant Ecol. 2012, 36, 812–818. [Google Scholar] [CrossRef]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as new model plant species for salt tolerance strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C. Effect of natural and artificial afforestation reclamation on soil properties and vegetation in coastal saline silt soils. Catena 2020, 198, 105066. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Liang, F.; Jing, X.; Feng, W. Application of flue gas desulfurization gypsum improves multiple functions of saline-sodic soils across China. Chemosphere 2021, 277, 130345. [Google Scholar] [CrossRef]
- Wang, S.X.; Liu, W.; Li, P.J.; Wu, H.Y. Advances of Researches in Plant-improvement of Saline-alkaline Soil. Chin. Agric. Sci. Bull. 2011, 27, 1–7. [Google Scholar]
- Elnaggar, A.; Mosa, K.A.; El-Keblawy, A.; Tammam, A.; El-Naggar, M. Physiological and biochemical insights for salt stress tolerance in the habitat-indifferent halophyte Salsola drummondii during the vegetative stage. Botany 2020, 98, 673–689. [Google Scholar] [CrossRef]
- Skorupa, M.; Szczepanek, J.; Mazur, J.; Domagalski, K.; Tretyn, A.; Tyburski, J. Salt stress and salt shock differently affect DNA methylation in salt-responsive genes in sugar beet and its wild, halophytic ancestor. PLoS ONE 2021, 16, e0251675. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Aljasmi, M.; Gairola, S.; Mosa, K.A.; Hameed, A. Provenance determines salinity tolerance and germination requirements of the multipurpose tree Prosopis juliflora seeds. Arid Land Res. Manag. 2021, 35, 446–462. [Google Scholar] [CrossRef]
- Bhatt, A.; Gallacher, D.J.J.; Jarma-Orozco, A.; Fernandes, D.; Pompelli, M.F.F. Seed provenance selection of wild halophyte seeds improves coastal rehabilitation efficiency. Estuar. Coast. Shelf Sci. 2022, 265, 107657. [Google Scholar] [CrossRef]
- Kesh, H.; Devi, S.; Kumar, N.; Kumar, A.; Kumar, A.; Dhansu, P.; Sheoran, P.; Mann, A. Insights into physiological, biochemical and molecular responses in wheat under salt stress. In Wheat; IntechOpen: London, UK, 2022. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes*. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, Y.L.; Mu, Z.J.; Bai, C.L. Hot topics and forntier evolution of plant improvement of saline-alkali land—Visual analysis based on VOSviewer. Acta Agrestia Sin. 2023, 31, 2598–2607. [Google Scholar]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 2020, 154, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.L.; Donovan, L.A. Water potential and ionic effects on germination and seedling growth of two cold desert shrubs. Am. J. Bot. 1999, 86, 1146–1153. [Google Scholar] [CrossRef]
- Li, J.; Guo, K.; Li, X.; Feng, X.; Liu, X. Effects of PEG, NaCl and Na2CO3 stresses on Suaeda glauca and Suaeda salsa seed germination. Chin. J. Eco-Agric. 2018, 26, 1011–1018. [Google Scholar] [CrossRef]
- Ma, H.Y.; Erickson, T.E.; Walck, J.L.; Merritt, D.J. Interpopulation variation in germination response to fire-related cues and after-ripening in seeds of the evergreen perennial Anigozanthos flavidus (Haemodoraceae). Int. J. Wildland Fire 2020, 29, 950–960. [Google Scholar] [CrossRef]
- Elnaggar, A.; El-Keblawy, A.; Mosa, K.A.; Navarro, T. Adaptive drought tolerance during germination of Salsola drummondii seeds from saline and nonsaline habitats of the arid Arabian deserts. Botany 2019, 97, 123–133. [Google Scholar] [CrossRef]
- Mohamed, E.; Kasem, A.; Gobouri, A.A.; Elkelish, A.; Azab, E. Influence of maternal habitat on salt tolerance during germination and growth in Zygophyllum coccineum. Plants 2020, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Mira, S.; Veiga-Barbosa, L.; Gonzalez-Benito, M.E.; Perez-Garcia, F. Inter-population variation in germination characteristics of Plantago lanceolata seeds: Effects of temperature, osmotic stress and salinity. Mediterr. Bot. 2018, 39, 89–96. [Google Scholar] [CrossRef]
- Liu, J.B.; Yang, F.; Wang, Z.C.; Nie, Z.Y.; Zhang, L.; An, F.H.; Guo, L.L. Soil physical-chemical properties and plant biomass in relation tomicrotopography spatial variability in saline-sodic soil area. Soils Crops 2021, 10, 163–176. [Google Scholar]
- Joeckel, R.; Clement, B.A. Soils, surficial geology, and geomicrobiology of saline-sodic wetlands, North Platte River Valley, Nebraska, USA. Catena 2005, 61, 63–101. [Google Scholar] [CrossRef]
- Shang, C.; Wang, L.; Tian, C.; Song, J. Heavy metal tolerance and potential for remediation of heavy metal-contaminated saline soils for the euhalophyte Suaeda salsa. Plant Signal. Behav. 2020, 15, 1805902. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, Z.; Liu, C.; Zuo, Z.; Jiang, L. Research progress on characteristics, utilization and development ofeuhalophyte suaeda salsa resources. Chin. Wild Plant Resour. 2022, 41, 30–37. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Wen, W.; Yang, G. The antioxidant system in Suaeda salsa under salt stress. Plant Signal. Behav. 2020, 15, 1771939. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, M.; Ma, H.; Jiang, L.; Zhao, Z.; Ma, J.; Wang, L. Differential responses of dimorphic seeds and seedlings to abiotic stresses in the halophyte Suaeda salsa. Front Plant Sci. 2021, 12, 630338. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Jiang, L.; Zhang, K.; Tanveer, M.; Tian, C.Y.; Zhao, Z.Y. Reclamation of saline soil by planting annual euhalophyte Suaeda salsa with drip irrigation: A three-year field experiment in arid northwestern China. Ecol. Eng. 2021, 159, 106090. [Google Scholar] [CrossRef]
- Wang, B.; Luttge, U.; Ratajczak, R. Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J. Exp. Bot. 2001, 52, 2355–2365. [Google Scholar] [CrossRef]
- Yang, M.F.; Song, J.; Wang, B.S. Organ-specific responses of vacuolar H-ATPase in the shoots and roots of C halophyte Suaeda salsa to NaCl. J. Integr. Plant Biol. 2010, 52, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2014, 115, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, L.; Tang, X.Y.; Xu, Q.; Yi, H.P.; Tian, H.F. Effects of saline alkali stress and nitrogen supply on germination and seedling growth of Suaeda salsa. Acta Prataculturae Sin. 2015, 24, 216–222. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, M.; Qiu, D.; Xie, T.; Shi, W.; Cui, B. Effect of hydrological characteristics on the recruitmentof Suaeda salsa in coastal salt marshes. J. Nat. Resour. 2019, 34, 2569–2579. [Google Scholar]
- Liu, X.H.; Li, M.; Xu, F. Effects of hydrological interference on Suaeda salsain different saline habitats. Shandong Agric. Sci. 2015, 47, 34–38. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yu, W.Y.; Ji, R.P.; Zhao, Y.J.; Feng, R.; Jia, Q.Y.; Wu, J.W. Dynamic response of phragmites australis and Suaeda salsa to climate change in the Liaohe Delta Wetland. J. Meteorol. Res. 2021, 35, 157–171. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Yang, Y.; Zhang, X.X.; Liu, R.R.; Song, J. Chloride allocation in the euhalophyte Suaeda salsa from different habitats in field and controlled saline conditions. Aquat. Bot. 2020, 167, 103292. [Google Scholar] [CrossRef]
- Gao, R.R.; Wei, X.Y.; He, Z.; Zhao, R.H.; Wang, K.; Yang, X.J.; Walck, J.L. Soil salt and NaCl have different effects on seed germination of the halophyte Suaeda salsa. J. Plant Nutr. Soil Sci. 2018, 181, 488–497. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Z.; Ma, H.; Yang, F.; Tian, C.; An, F. Research and integrated demonstration of ecological amelioration techniques ofsaline-sodic land in northeast China. Acta Ecol. Sin. 2016, 36, 7054–7058. [Google Scholar]
- Wang, Z.M.; Song, K.S.; Zhang, B.; Liu, D.W.; Ren, C.Y.; Luo, L.; Yang, T.; Huang, N.; Hu, L.; Yang, H.J.; et al. Shrinkage and fragmentation of grasslands in the West Songnen Plain, China. Agric. Ecosyst. Environ. 2009, 129, 315–324. [Google Scholar] [CrossRef]
- Yu, R.-P.; Chen, D.-M. Saline soil resources and their development and utilization in China. Chin. J. Soil Sci. 1999, 30, 3. [Google Scholar] [CrossRef]
- Zhang, L. Mechanism of Saline-Sodic Soil Reclamation by Saline Ice Irrigation and Meltwater Leaching in Songnen Plain. Ph.D. Thesis, University of Chinese Academy of Sciences (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences), Beijing, China, 2021. [Google Scholar]
- Yang, C.W.; Shi, D.C.; Wang, D.L. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Yang, C.W.; Chong, J.N.; Li, C.Y.; Kim, C.M.; Shi, D.C.; Wang, D.L. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Env. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Hartung, W.; Leport, L.; Ratcliffe, R.G.; Sauter, A.; Duda, R.; Turner, N.C. Abscisic acid concentration, root pH and anatomy do not explain growth differences of chickpea (Cicer arietinum L.) and lupin (Lupinus angustifolius L.) on acid and alkaline soils. Plant Soil 2002, 240, 191–199. [Google Scholar] [CrossRef]
- Bhatt, A.; Batista-Silva, W.; Gallacher, D.J.; Pompelli, M.F. Germination of cenchrus ciliaris, pennisetum divisum, and panicum turgidum is seasonally dependent. Botany 2020, 98, 449–458. [Google Scholar] [CrossRef]
- Huang, Z.X.; Qiu, C.; Zeng, Z.J.; Zhi, S.; Zhang, Y.; Li, F. Research progress of disinfectant application in plant tissue culture. J. Neijtang Norm. Univ. 2013, 28, 5. [Google Scholar]
- Ma, H.Y.; Lv, B.S.; Li, X.W.; Liang, Z.W. Germination response to differing salinity levels for 18 grass species from the saline-alkaline grasslands of the Songnen Plain, China. Pak. J. Bot. 2014, 46, 1147–1152. [Google Scholar]
- Zhao, D.D.; Ma, H.Y.; Wang, L.; Li, S.Y.; Qi, W.W.; Ma, M.Y.; Xia, J.B. Effects of water and nitrogen addition on the seed yield and germination characteristics of the perennial grass Leymus chinensis (Trin.) Tzvel. Front. Environ. Sci. 2021, 9, 404. [Google Scholar] [CrossRef]
- Li, S.; Ma, H.; Ooi, M.K.J. Fire-related cues significantly promote seed germination of some salt-tolerant species from non-fire-prone saline-alkaline grasslands in Northeast China. Plants 2021, 10, 2675. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shi, L.; Yan, C.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.T.; Siddiqui, S.A.; Rathore, M.S. Seed germination, seedling growth and seedling development associated physiochemical changes in Salicornia brachiata (Roxb.) under salinity and osmotic stress. Aquat. Bot. 2020, 166, 103272. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Elnaggar, A.; Tammam, A.; Mosa, K.A. Seed provenance affects salt tolerance and germination response of the habitat-indifferent Salsola drummondii halophyte in the arid Arabian deserts. Flora 2020, 266, 151592. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K. Interactive effects of salt and alkali stresses on seed germination, germination recovery, and seedling growth of a halophyte Spartina alterniflora (Poaceae). S. Afr. J. Bot. 2010, 76, 380–387. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, S.Q.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y.; Mai, W.X. Progress of euhalophyte adaptation to arid areas to remediate salinized soil. Agriculture 2023, 13, 704. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, J.; Wang, Z.; Cheng, K.; Zhang, P.; Tian, G.; Liu, X.; Guo, E.; Du, Y.; Wang, Y. Transcriptome and metabolite analysis reveal the drought tolerance of foxtail millet significantly correlated with phenylpropanoids-related pathways during germination process under PEG stress. BMC Plant Biol. 2020, 20, 274. [Google Scholar] [CrossRef]
- Peng, B.; Xu, W.; Shao, R.; Feng, G.-N.; Shi, W.-Y. Growth of Suaeda salsatin response to salt stress in different habitats. Acta Prataculturae Sin. 2016, 25, 81–90. [Google Scholar] [CrossRef]
- Gya, R.; Geange, S.R.; Lynn, J.S.; Topper, J.P.; Wallevik, O.; Zernichow, C.; Vandvik, V. A test of local adaptation to drought in germination and seedling traits in populations of two alpine forbs across a 2000 mm/year precipitation gradient. Ecol. Evol. 2023, 13, e9772. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Wang, S.; Yang, L.; Gu, J. Intraspecific variations of anatomical, morphological and chemical traits in leaves and absorptive roots along climate and soil gradients: A case study with Ginkgo biloba and Eucommia Ulmoides. Plant Soil 2021, 469, 73–88. [Google Scholar] [CrossRef]
- Liu, R.; Wang, L.; Tanveer, M.; Song, J. Seed heteromorphism: An important adaptation of halophytes for habitat heterogeneity. Front Plant Sci. 2018, 9, 1515. [Google Scholar] [CrossRef] [PubMed]






| Provenance | Latitude | Longitude | MAT (°C/year) | MAP (mm/year) | pH | EC (mS/cm) | Na+ mmolc/L | Cl− mmolc/L | CO32− mmolc/L | HCO3− mmolc/L |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 46.50 | 125.44 | 5 | 535.32 | 10.39 | 0.830 | 16.63 | 1.05 | 0.60 | 13.20 |
| S2 | 45.58 | 124.76 | 4 | 924.09 | 10.16 | 1.065 | 17.18 | 4.50 | 4.00 | 4.60 |
| S3 | 45.60 | 123.85 | 6 | 559.7 | 9.50 | 2.320 | 21.30 | 23.10 | 3.80 | 15.20 |
| S4 | 46.91 | 124.32 | 10 | 338.06 | 10.45 | 2.800 | 25.31 | 17.25 | 0.20 | 23.20 |
| S5 | 46.32 | 124.95 | 9 | 354.33 | 9.43 | 2.500 | 23.25 | 24.56 | 2.30 | 10.23 |
| S6 | 45.32 | 123.59 | 6 | 559.7 | 8.74 | 0.087 | 2.23 | 0.90 | 0.10 | 4.80 |
| S7 | 45.40 | 123.69 | 6 | 559.7 | 9.52 | 2.260 | 19.30 | 13.22 | 2.50 | 23.60 |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Aab | 100 ± 0 Aa | 100 ± 0 Aab | 100 ± 0 Ab | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aac |
| 50 | 112.3 ± 7.1 Aa | 89.3 ± 1.9 Aa | 93.3 ± 3.6 Ab | 107.8 ± 3.2 Aab | 87.3 ± 1.7 Aab | 42.2 ± 7.4 Bb | 103.5 ± 9.8 Aa |
| 100 | 113.7 ± 8.9 Aa | 83.3 ± 2.5 BCa | 106.7 ± 2.7 ABa | 126.5 ± 7.2 Aa | 102 ± 2.6 ABa | 58.9 ± 11 Cb | 100.7 ± 6.9 ABab |
| 200 | 87.6 ± 1.4 Aab | 58.2 ± 8.6 Bb | 74.7 ± 3.6 ABc | 65.9 ± 4.3 ABc | 70.1 ± 2.8 ABbc | 62.1 ± 4.2 Bb | 76.4 ± 4.8 ABbd |
| 300 | 76.3 ± 4.9 Abc | 43.6 ± 2.6 Cb | 51.1 ± 2.1 BCd | 49.7 ± 2.4 BCc | 79.5 ± 4.7 Ab | 49.2 ± 0.8 BCb | 62.7 ± 6.2 ABde |
| 400 | 50 ± 16.7 Ac | 41.1 ± 4 ABb | 39.1 ± 3.6 ABd | 22.3 ± 4.3 Bd | 43.1 ± 4.4 ABd | 33.4 ± 0.5 ABb | 40.9 ± 1.8 ABe |
| 500 | 0 ± 0 Cd | 0 ± 0 Cc | 36.6 ± 0.5 Bd | 46.7 ± 10.2 Bc | 57.5 ± 8.4 ABcd | 0 ± 0 Cc | 74.7 ± 2.3 Acd |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Ab | 100 ± 0 Aab | 100 ± 0 Aa | 100 ± 0 Abc | 100 ± 0 Aa | 100 ± 0 Aab | 100 ± 0 Ac |
| 50 | 126.7 ± 9 Bab | 119.9 ± 1.4 Ba | 107.6 ± 2.1 Ba | 118.1 ± 3.2 Bab | 107.2 ± 2.8 Ba | 115.7 ± 12.7 Ba | 165.5 ± 6.7 Aa |
| 100 | 135.2 ± 12.4 Aa | 113.4 ± 7.5 Aa | 109.5 ± 2.8 Aa | 129 ± 6.7 Aa | 105.9 ± 3 Aa | 102.6 ± 19.9 Aab | 140.8 ± 7.9 Aab |
| 200 | 107.2 ± 5.5 Bab | 109.2 ± 6.2 Ba | 106.8 ± 2.4 Ba | 105.3 ± 2.2 Bbc | 111.6 ± 1.5 Ba | 73.4 ± 11.2 Cab | 136.8 ± 7.8 Ab |
| 300 | 105 ± 8.1 Bab | 82 ± 4.9 BCbc | 86.4 ± 3.5 BCb | 86.7 ± 1.7 BCcd | 68.6 ± 2.8 CDb | 50 ± 16.7 Dbc | 145.6 ± 4.8 Aab |
| 400 | 48.2 ± 10.9 ABc | 66.6 ± 4.5 ABc | 78.8 ± 1.6 ABb | 50.2 ± 7.3 Ae | 64.3 ± 12.3 ABb | 54.3 ± 0.5 ABbc | 86.9 ± 4 Bc |
| 500 | 0 ± 0 Cd | 0 ± 0 Cd | 49.5 ± 0.5 Bc | 66.2 ± 6.9 Bde | 92.7 ± 2.6 Aa | 0 ± 0 Cc | 99.6 ± 6.4 Ac |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 0 ± 0 Abc | 0 ± 0 Aab | 0 ± 0 Aab | 0 ± 0 Abc | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aab |
| 50 | 0 ± 0 Bbc | 3.3 ± 3.3 Ab | 0 ± 0 Bab | 0 ± 0 Bbc | 0 ± 0 Ba | 0 ± 0 Ba | 0 ± 0 Bab |
| 100 | 7.8 ± 4.5 Ab | 10.3 ± 2.4 Aab | 7.5 ± 4.4 Aa | 25 ± 10.4 Aab | 0 ± 0 Ba | 1.5 ± 1.5 Ba | 8.5 ± 3.5 Aa |
| 200 | 16.5 ± 3 Aab | 12.5 ± 4.3 Aab | 20 ± 4.3 Aa | 9.5 ± 5.5 Ab | 7.3 ± 7.3 Aa | 0 ± 0 Aa | 18.3 ± 1.3 Aab |
| 300 | 35.8 ± 5.8 Bbc | 8.5 ± 3.9 Cb | 35.8 ± 4.3 Bab | 61.3 ± 12.3 Ac | 0 ± 0 Ca | 5.5 ± 2.2 Ca | 28.8 ± 5.9 Bab |
| 400 | 40.5 ± 3.9 ACc | 27.3 ± 0.9 BCa | 50 ± 6.4 ABb | 59.8 ± 5.6 Ac | 10.5 ± 4.5 Ca | 0 ± 0 Da | 39.8 ± 7.1 ACb |
| 500 | 38 ± 3.8 Bac | 14 ± 6.8 Bab | 52.5 ± 4 Ab | 45.8 ± 4.3 Aac | 7 ± 2.7 Ba | 2 ± 1.2 Ba | 35.3 ± 1.7 Ab |
| Provenance | NaCl | Provenance × NaCl | ||
|---|---|---|---|---|
| Germination | d.f | 6 | 6 | 36 |
| χ2 | 1496.38 | 311.11 | 158.97 | |
| p | <0.001 | <0.001 | <0.001 | |
| Total germination | d.f. | 6 | 6 | 36 |
| χ2 | 280.52 | 1020.26 | 167.26 | |
| p | <0.001 | <0.001 | <0.001 | |
| Root length | d.f. | 6 | 6 | 29 |
| F | 33.38 | 69.72 | 2.25 | |
| p | <0.001 | <0.001 | <0.001 | |
| Shoot length | d.f. | 6 | 6 | 29 |
| F | 58.39 | 36.25 | 3.25 | |
| p | <0.001 | <0.001 | <0.001 | |
| Recovery germination percentage | d.f. | 6 | 6 | 36 |
| χ2 | 473.92 | 180.19 | 117.76 | |
| p | <0.001 | <0.001 | 0.004 |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Aab | 100 ± 0 Aab | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa |
| 50 | 107.3 ± 4.3 ABa | 128.1 ± 19.2 Aa | 97 ± 4 ABab | 83.6 ± 1.8 Bbc | 94.1 ± 2.1 ABa | 86.1 ± 7 Ba | 95.4 ± 4.3 ABa |
| 100 | 105.5 ± 4.5 ABab | 116.7 ± 11.3 Aab | 86.5 ± 1.6 Bb | 90.1 ± 2 Bab | 101.1 ± 1.1 ABa | 86.1 ± 8.3 Ba | 84.6 ± 1 Bab |
| 200 | 91.3 ± 3.7 Bb | 118.8 ± 7.1 Aa | 86.3 ± 2.2 BCb | 73.4 ± 3.3 Cc | 98 ± 3 Ba | 36.7 ± 1.9 Db | 71.7 ± 2.5 Cbc |
| 300 | 75.5 ± 2.7 c | 91.7 ± 13.2 Aab | 70.4 ± 3.1 Ac | 76.1 ± 6.8 Abc | 67.4 ± 3.6 Ab | 33.9 ± 4.2 Bb | 75.7 ± 4.7 Abc |
| 400 | 64.8 ± 2.6 Bcd | 149 ± 18.7 Aa | 71.8 ± 4.8 Bc | 70.1 ± 3.3 Bc | 70.2 ± 3 Bb | 4.2 ± 2.5 Cc | 72.7 ± 5.5 Bbc |
| 500 | 57.3 ± 3.5 Ad | 50 ± 21.5 Ab | 67.5 ± 1.5 Ac | 52.6 ± 3.3 Ad | 38.6 ± 5.2 ABc | 4.2 ± 2.5 Bc | 63.1 ± 2.8 Ac |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa |
| 5 | 78.7 ± 5.8 ABbc | 104.2 ± 4.2 Aa | 45 ± 1.8 CDb | 57.3 ± 9.9 BDb | 32.7 ± 3.4 Db | 62.5 ± 7 BCb | 61.3 ± 6.4 BCb |
| 12.5 | 93.7 ± 2.6 Aab | 82.3 ± 7.7 Aab | 28 ± 5.3 Cb | 37.4 ± 8.5 BCbc | 38 ± 1.9 BCb | 48.9 ± 3.7 BCbc | 52 ± 2 Bb |
| 25 | 86.6 ± 2.6 Aac | 57.3 ± 4.3 Bb | 46.1 ± 8.2 BCb | 45.7 ± 3.6 BCbc | 22.5 ± 5.6 Cb | 49.7 ± 3.7 BCbc | 45.4 ± 10.7 BCb |
| 50 | 77.1 ± 4.4 Ac | 65.6 ± 6.9 ABb | 32.3 ± 1.5 CDb | 25.9 ± 5.7 Dc | 26.6 ± 8.8 Db | 56.7 ± 7.9 ACb | 38.8 ± 7 BDb |
| 100 | 74.1 ± 1.4 Ac | 71.9 ± 8.6 Ab | 47.6 ± 6.9 ABb | 26.7 ± 6.5 Bc | 29.3 ± 2 Bb | 26.4 ± 10.5 Bc | 42.9 ± 9.7 ABb |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Ad | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ab | 100 ± 0 Ad | 100 ± 0 Ab | 100 ± 0 Ab |
| 5 | 346 ± 15 Aa | 312.7 ± 13.4 ABa | 193.2 ± 9.2 Cab | 238.4 ± 7.3 BCa | 194.4 ± 4 Ca | 260.4 ± 41 BCa | 220.6 ± 10.3 Ca |
| 12.5 | 291 ± 16.4 Ab | 278.4 ± 8.4 ABa | 208.3 ± 8.3 BDa | 260.3 ± 21.7 ACa | 197.1 ± 3.3 CDa | 136.4 ± 23.3 Db | 223.7 ± 19.3 aC |
| 25 | 263.1 ± 15.4 Ab | 233.1 ± 8.6 ABb | 173.1 ± 8.4 CDb | 230.9 ± 9.8 ACa | 161.6 ± 3.5 Db | 133 ± 23.5 Db | 185.5 ± 11.6 BDa |
| 50 | 160 ± 3.3 Ac | 151.7 ± 5.6 ABc | 115.9 ± 2.3 BCc | 129.4 ± 8 ACb | 115.5 ± 3.3 BDc | 77.7 ± 14.8 Db | 99.4 ± 11.9 CDb |
| 100 | 65.3 ± 2.3 ABd | 73.3 ± 1.8 Ad | 45.1 ± 2.4 Cd | 48.9 ± 2.6 BCc | 64 ± 1.3 ACe | 54.9 ± 9.2 ACb | 47.1 ± 5.1 BCc |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Ab | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Ab | 100 ± 0 Ab | 100 ± 0 Aa | 100 ± 0 Ab |
| 5 | 104.9 ± 7.4 Cab | 147.5 ± 4.1 Ab | 114.3 ± 2.7 ACab | 106.3 ± 2.6 BCb | 107.7 ± 4 BCb | 138.6 ± 16 ABa | 141.2 ± 2.8 Aa |
| 12.5 | 115.5 ± 8.2 Bab | 173.7 ± 2.9 Aa | 120.6 ± 3 Ba | 120.4 ± 3 Ba | 115.4 ± 6.6 Bb | 136.6 ± 19 ABa | 146.8 ± 10.3 ABa |
| 25 | 114.9 ± 5.7 BCab | 175.8 ± 3.7 Aa | 105.3 ± 3.1 Cbc | 121.2 ± 3.8 BCa | 116.3 ± 3 BCb | 109.2 ± 10.5 Ca | 141.5 ± 7.5 Ba |
| 50 | 132.7 ± 6.8 BCa | 179.4 ± 0.9 Aa | 116 ± 0.7 BCa | 125.4 ± 2.9 BCa | 136.9 ± 3.4 BCa | 106.2 ± 14.8 Ca | 146.1 ± 10.9 ABa |
| 100 | 130.8 ± 9.3 Bab | 171.2 ± 4.6 Aa | 116.9 ± 2.7 BCa | 125.7 ± 1.5 Ba | 137.7 ± 3.8 Ba | 89.4 ± 12.8 Ca | 143.3 ± 7.3 ABa |
| Provenance | Na2CO3 | Provenance × Na2CO3 | ||
|---|---|---|---|---|
| Germination | d.f | 6 | 5 | 30 |
| χ2 | 736.86 | 319.25 | 176.57 | |
| p | <0.001 | <0.001 | <0.001 | |
| Total germination | d.f. | 6 | 5 | 30 |
| χ2 | 628.11 | 3416.47 | 187.63 | |
| p | <0.001 | <0.001 | <0.001 | |
| Root length | d.f. | 6 | 5 | 30 |
| F | 192.18 | 447.42 | 11.31 | |
| p | <0.001 | <0.001 | <0.001 | |
| Shoot length | d.f. | 6 | 5 | 30 |
| F | 181.04 | 32.36 | 3.66 | |
| p | <0.001 | <0.001 | <0.001 | |
| Recovery germination percentage | d.f. | 6 | 5 | 30 |
| χ2 | 193.25 | 142.3 | 93.36 | |
| p | <0.001 | <0.001 | 0.016 |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aab | 0 ± 0 Ab | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Ab |
| 5 | 3.5 ± 2 Ba | 0 ± 0 Ba | 4.8 ± 3.1 Bb | 24.3 ± 6 Aa | 6.8 ± 1.4 Ba | 0 ± 0 Ba | 25.3 ± 3.9 Aa |
| 12.5 | 3.3 ± 3.3 Aa | 0 ± 0 Aa | 1.3 ± 1.3 Ab | 2 ± 2 Ab | 5 ± 3.3 Aa | 0 ± 0 Aa | 17 ± 2.5 Aa |
| 25 | 6.8 ± 2.3 Aa | 0 ± 0 Aa | 8.5 ± 4.4 Ab | 7.3 ± 5.2 Ab | 1.3 ± 1.3 Aa | 0 ± 0 Aa | 18 ± 8.2 Aa |
| 50 | 2 ± 2 Aa | 0 ± 0 Aa | 6.3 ± 0.9 Ab | 5.5 ± 2.5 Ab | 0 ± 0 Aa | 0 ± 0 Aa | 13.8 ± 3 Aa |
| 100 | 5.8 ± 1.9 Ba | 1.3 ± 1.3 Ba | 30.3 ± 6.8 Aa | 7.5 ± 3 Bb | 6.3 ± 2.4 Ba | 0 ± 0 ABa | 19.5 ± 4.4 ABa |
| Provenance | NaHCO3 | Provenance × NaHCO3 | ||
|---|---|---|---|---|
| Germination | d.f | 6 | 4 | 24 |
| χ2 | 676.09 | 273.27 | 112.49 | |
| p | <0.001 | <0.001 | <0.001 | |
| Total germination | d.f. | 6 | 4 | 24 |
| χ2 | 534.47 | 253.52 | 111.55 | |
| p | <0.001 | <0.001 | <0.001 | |
| Root length | d.f. | 6 | 4 | 24 |
| F | 204.4 | 262.89 | 7.76 | |
| p | <0.001 | <0.001 | <0.001 | |
| Shoot length | d.f. | 6 | 4 | 24 |
| F | 129.55 | 46.22 | 5.1 | |
| p | <0.001 | <0.001 | <0.001 | |
| Recovery germination percentage | d.f. | 6 | 4 | 24 |
| χ2 | 141.99 | 90.54 | 61.19 | |
| p | <0.001 | <0.001 | 0.207 |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Ac | 100 ± 0 Ad | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Ac | 100 ± 0 Aa | 100 ± 0 Ac |
| 25 | 296.6 ± 19.8 Aa | 285 ± 2.5 Aa | 226.9 ± 8.1 ABa | 244.2 ± 20.4 ABa | 195.3 ± 3.5 BCa | 124.6 ± 24 Ca | 240.3 ± 16 ABa |
| 50 | 287.5 ± 4.4 Aa | 237.7 ± 3.6 ABb | 202.3 ± 10.9 BCa | 218.3 ± 10.4 BCa | 181.1 ± 6.1 Ca | 115.2 ± 19.8 Da | 197.8 ± 11.9 BCab |
| 100 | 188.1 ± 7.2 Ab | 161 ± 5.7 ABc | 141.5 ± 6.5 Bb | 154.8 ± 9.2 ABb | 135.7 ± 2.1 Bb | 71.3 ± 9.3 Ca | 146.6 ± 12.6 Bbc |
| 150 | 188.1 ± 7.2 Ab | 161 ± 5.7 ABc | 141.5 ± 6.5 Bb | 154.8 ± 9.2 ABb | 135.7 ± 2.1 Bb | 71.3 ± 9.3 Ca | 146.6 ± 12.6 Bbc |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Ab | 100 ± 0 Ab | 100 ± 0 Ab | 100 ± 0 Ab | 100 ± 0 Ab | 100 ± 0 Aa | 100 ± 0 Ab |
| 25 | 117.6 ± 8.8 BCab | 179.3 ± 5.4 Aa | 121.1 ± 3.3 BCa | 104.1 ± 1.3 Cab | 106.1 ± 8.2 Cb | 116.8 ± 16.9 BCa | 153.5 ± 6.4 ABa |
| 50 | 127.6 ± 5.9 BCa | 177.3 ± 4.4 Aa | 122.4 ± 3.1 BCa | 102.7 ± 3.6 Cab | 120.1 ± 5.8 Cb | 139.1 ± 22.3 ACa | 166.3 ± 6.9 ABa |
| 100 | 134.6 ± 6.1 BCa | 182.6 ± 7.4 Aa | 123.7 ± 5.7 BCa | 116.6 ± 4.5 Ca | 153.8 ± 4.9 ABa | 126.7 ± 15.2 BCa | 148.4 ± 7.1 ACa |
| 150 | 134.6 ± 6.1 BCa | 182.6 ± 7.4 Aa | 123.7 ± 5.7 BCa | 116.6 ± 4.5 Aa | 153.8 ± 4.9 ABa | 126.7 ± 15.2 BCa | 148.4 ± 7.1 ACa |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aa | 0 ± 0 Aab |
| 25 | 5 ± 2.9 Aa | 0 ± 0 Aa | 7 ± 2.6 Aa | 2.8 ± 2.8 Aa | 5.3 ± 2.1 Aa | 0 ± 0 Aa | 14.3 ± 4 Aa |
| 50 | 7.5 ± 4.8 Ba | 0 ± 0 ABa | 5.3 ± 2.1 Ba | 8.3 ± 2.6 Ba | 13.5 ± 1.8 Ba | 0 ± 0 ABa | 40.5 ± 4.3 Ab |
| 100 | 2.5 ± 2.5 Ba | 0 ± 0 Ba | 7.8 ± 3.7 Ba | 5.3 ± 2.1 Ba | 2.3 ± 1.3 Ba | 0 ± 0 Ba | 39.3 ± 3.6 Ab |
| 150 | 7.8 ± 2.9 Ba | 2.3 ± 1.3 Ba | 12.5 ± 1.7 Ba | 14.5 ± 3.7 ABa | 1.3 ± 1.3 Ba | 0 ± 0 Ba | 34.3 ± 6.9 Ab |
| Concentration (mM) | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 0 Aa | 100 ± 0 Aab | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa | 100 ± 0 Aa |
| 25 | 84.6 ± 4 Ac | 103.1 ± 7.9 Aa | 41.2 ± 0.8 Bb | 37.3 ± 0.9 Bb | 30 ± 4.5 Bb | 26.9 ± 5.3 Bd | 43.1 ± 6.5 Bc |
| 50 | 96.8 ± 1.9 Aab | 59.4 ± 19.9 ACb | 39.1 ± 4.9 Cb | 38.3 ± 1.4 Cb | 41.9 ± 0.6 Cb | 53.3 ± 7.2 BCbc | 81.3 ± 4.5 ABab |
| 100 | 93.6 ± 3 Aac | 70.8 ± 2.4 Bab | 21.8 ± 4.9 Cc | 26.1 ± 4 Cc | 27.1 ± 6.5 Cb | 35 ± 4.8 Ccd | 74.5 ± 3.7 ABb |
| 150 | 86.8 ± 2.3 Abc | 74 ± 4.9 ABab | 28.5 ± 1.7 Cbc | 34.9 ± 2.8 Cbc | 23.4 ± 6.5 Cb | 62.5 ± 7 ABb | 59.9 ± 8.2 Bbc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, W.; Ma, H.; Li, S.; Wu, H.; Zhao, D. Seed Germination and Seedling Growth in Suaeda salsa (Linn.) Pall. (Amaranthaceae) Demonstrate Varying Salinity Tolerance among Different Provenances. Biology 2023, 12, 1343. https://doi.org/10.3390/biology12101343
Qi W, Ma H, Li S, Wu H, Zhao D. Seed Germination and Seedling Growth in Suaeda salsa (Linn.) Pall. (Amaranthaceae) Demonstrate Varying Salinity Tolerance among Different Provenances. Biology. 2023; 12(10):1343. https://doi.org/10.3390/biology12101343
Chicago/Turabian StyleQi, Wenwen, Hongyuan Ma, Shaoyang Li, Haitao Wu, and Dandan Zhao. 2023. "Seed Germination and Seedling Growth in Suaeda salsa (Linn.) Pall. (Amaranthaceae) Demonstrate Varying Salinity Tolerance among Different Provenances" Biology 12, no. 10: 1343. https://doi.org/10.3390/biology12101343
APA StyleQi, W., Ma, H., Li, S., Wu, H., & Zhao, D. (2023). Seed Germination and Seedling Growth in Suaeda salsa (Linn.) Pall. (Amaranthaceae) Demonstrate Varying Salinity Tolerance among Different Provenances. Biology, 12(10), 1343. https://doi.org/10.3390/biology12101343








