Purification and Characterization of Polyphenol Oxidase in the Fruits of Opuntia ficus-indica
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Chemicals
2.3. Extraction and Purification of the Enzyme
2.4. PPO Activity Test
2.5. Detection of Protein Concentration
2.6. Electrophoresis of PPO
2.7. Effects of pH on the PPO Activity
2.8. Effects of Temperature on the PPO Activity
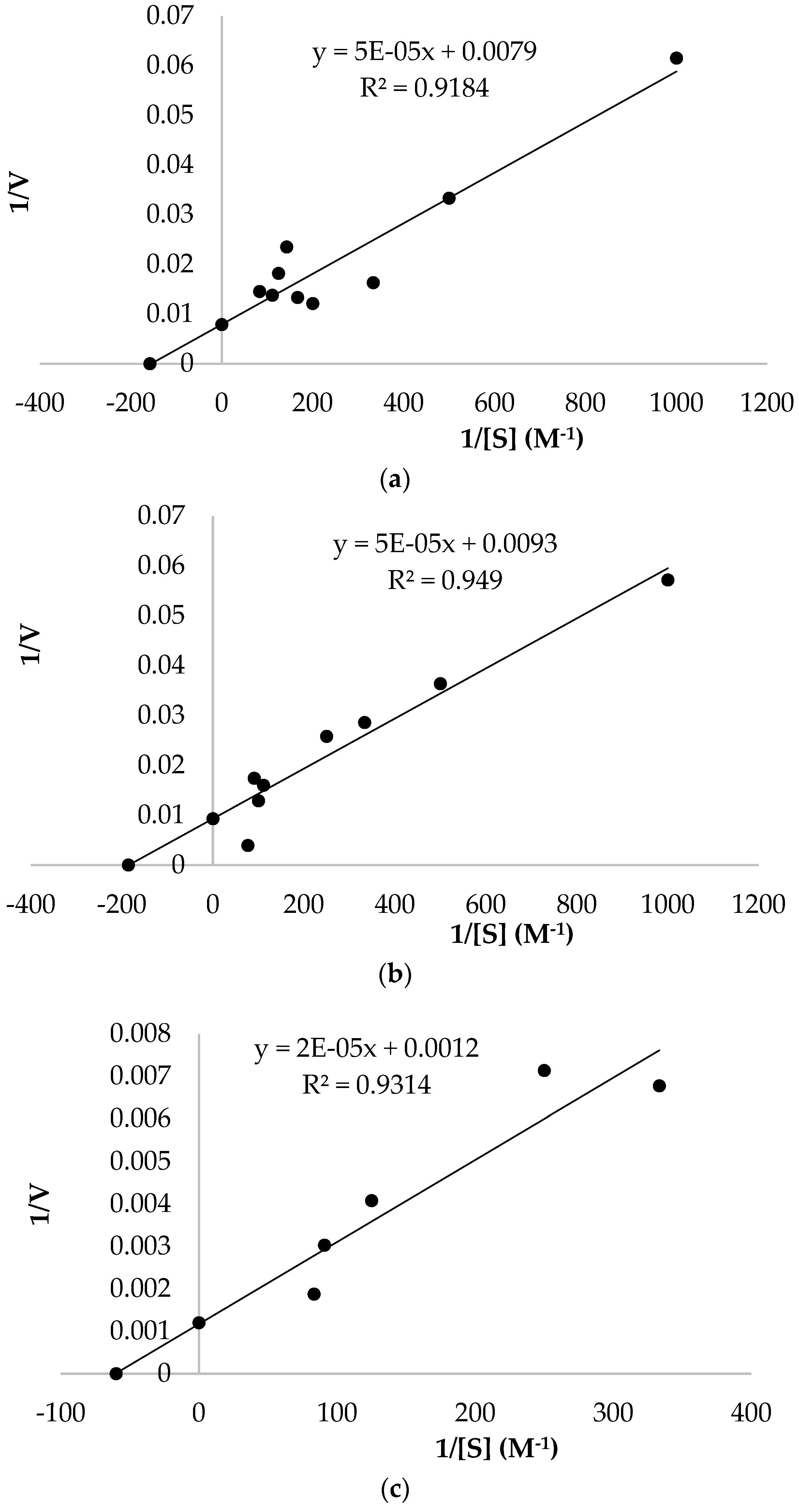
2.9. Enzyme Kinetics Studies
3. Results and Discussion
3.1. Extraction and Purification of the Enzyme
3.2. Effects of Temperature and pH on the PPO Activity
3.3. Kinetic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Missaoui, M.; D’Antuono, I.; D’Imperio, M.; Linsalata, V.; Boukhchina, S.; Logrieco, A.F.; Cardinali, A. Characterization of Micronutrients, Bioaccessibility and Antioxidant Activity of Prickly Pear Cladodes as Functional Ingredient. Molecules 2020, 25, 2176. [Google Scholar] [CrossRef]
- Coria Cayupan, Y.S.; Ochoa, M.J.; Nazareno, M.A. Health-promoting Substances and Antioxidant Properties of Opuntia sp. Fruits. Changes in Bioactive-compound Contents during Ripening Process. Food Chem. 2011, 126, 514–519. [Google Scholar] [CrossRef]
- Isaac, A.A. Overview of Cactus (Opuntia ficus-indica (L): A Myriad of Alternatives. Stud. Ethno-Med. 2016, 10, 195–205. [Google Scholar] [CrossRef]
- Medina, E.M.D.; Rodríguez, E.M.R.; Romero, C.D. Chemical Characterization of Opuntia dillenii and Opuntia ficus-indica Fruits. Food Chem. 2007, 103, 38–45. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Bachir-bey, M. Optimization of Phenolic Compounds Recovery and Antioxidant Activity Evaluation from Opuntia ficus-indica Using Response Surface Methodology. J. Food Meas. Charact. 2022, 16, 1354–1366. [Google Scholar] [CrossRef]
- Galati, E.M.; Tripodo, M.M.; Trovato, A.; Miceli, N.; Monforte, M.T. Biological Effect of Opuntia ficus indica (L.) Mill. (Cactaceae) Waste Matter Note I: Diuretic Activity. J. Ethnopharmacol. 2002, 79, 17–21. [Google Scholar] [CrossRef]
- Loro, J.F.; del Rio, I.; Perez-Santana, L. Preliminary Studies of Analgesic and Anti-inflammatory Properties of Opuntia dillenii Aqueous Extract. J. Ethnopharmacol. 1999, 67, 213–218. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Tripodo, M.M.; d’Aquino, A.; Mondello, M.R. Antiulcer Activity of Opuntia ficus indica (L.) Mill. (Cactaceae): Ultrastructural Study. J. Ethnopharmacol. 2001, 76, 1–9. [Google Scholar] [CrossRef]
- Frati, A.C.; Jimenez, E.; Ariza, R.C. Hypoglycemic Effect of Opuntia ficus indica in Non-insulin-Dependent Diabetes Mellitus Patients. Phytother. Res. 1990, 4, 195–197. [Google Scholar] [CrossRef]
- Roman-Ramos, R.; Flores-Saenz, J.L.; Alarcon-Aguilar, F.J. Anti-hyperglycemic Effect of Some Edible Plants. J. Ethnopharmacol. 1995, 48, 25–32. [Google Scholar] [CrossRef]
- Mansour, R.; Ben Ali, H. Exploring Chitosan as an Ecofriendly Agent to Improve Sustainable Dyeing Properties of Cotton Fabric Dyed with (Opuntia ficus-indica L.) Fruit Peel and Its UV Protection Activity. J. Nat. Fibers 2023, 20, 2134263. [Google Scholar] [CrossRef]
- Felker, P.; Stintzing, F.C.; Müssig, E.; Leitenberger, M.; Carle, R.; Vogt, T.; Bunch, R. Colour Inheritance in Cactus Pear (Opuntia ficus-indica) Fruits. Ann. Appl. Biol. 2008, 152, 307–318. [Google Scholar] [CrossRef]
- Yıldız, S.; Bilen, Ç.; Karakuş, E. Purification of Damson plum Polyphenol Oxidase by Affinity Chromatography and Investigation of Metal Effects on Enzyme Activity. Prep. Biochem. Biotechnol. 2022, 52, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Nixha, A.R.; Arslan, M.; Atalay, Y.; Gençer, N.; Ergün, A.; Arslan, O. Synthesis and Theoretical Calculations of Carbazole Substituted Chalcone Urea Derivatives and Studies Their Polyphenol Oxidase Enzyme Activity. J. Enzym. Inhib. Med. Ch. 2013, 28, 808–815. [Google Scholar] [CrossRef]
- Demir, D.; Eken, C.; Çelik, E.; Alkan, N. Effect of Rhizoctonia spp. On Polyphenol Oxidase Enzyme Activity in Alfalfa Seedling. Res. J. Biotechnol. 2021, 16, 47–50. [Google Scholar] [CrossRef]
- Nixha, A.R.; Ergun, A.; Gencer, N.; Arslan, O.; Arslan, M. Development of Carbazole-bearing Pyridopyrimidine-substituted Urea/Thiourea as Polyphenol Oxidase Inhibitors: Synthesis, Biochemistry, and Theoretical Studies. Arch. Physiol. Biochem. 2019, 125, 263–269. [Google Scholar] [CrossRef]
- Arslan, O.; Erzengin, M.; Sinan, S.; Ozensoy, O. Purification of Mulberry (Morus alba L.) Polyphenol Oxidase by Affinity Chromatography and Investigation of Its Kinetic and Electrophoretic Properties. Food Chem. 2004, 88, 479–484. [Google Scholar] [CrossRef]
- Ozlem, F. Purification and Characterization of a Polyphenol Oxidase from Cimin Grape (Vitis vinifera spp., Cimin). Res. J. Biotechnol. 2016, 11, 87–94. [Google Scholar]
- Farouk, B.; Aref, N.; Rachid, C.; Mourad, L.; Emna, K.; Fethi, B.; Rania, B.; Wafa, N.; Kenza, B.; Boumediene, M.; et al. Characterization of Three Polyphenol Oxidase Isoforms in Royal dates and Inhibition of Its Enzymatic Browning Reaction by Indole-3-acetic acid. Int. J. Biol. Macromol. 2020, 145, 894–903. [Google Scholar] [CrossRef]
- Sajjad, N.; Naqvi, S.M.S.; Asad, M.J.; Raja, N.I.; Pusztai-Carey, M.; Ahmad, M.S. Biochemical, Purification, Sequencing and Alignment Studies of The Novel Polyphenol Oxidase Isoforms from Musa Acuminata Fruit Pulp. J. Anim. Plant Sci. 2021, 31, 542–555. [Google Scholar] [CrossRef]
- Çınar, F.; Aksay, S. Purification and Characterization of Polyphenol Oxidase from Myrtle berries (Myrtus communis L.). J. Food Meas. Charact. 2022, 16, 2282–2291. [Google Scholar] [CrossRef]
- Sarsenova, A.; Demir, D.; Çağlayan, K.; Abiyev, S.; Darbayeva, T.; Eken, C. Purification and Properties of Polyphenol Oxidase of Dried Volvariella bombycina. Biology 2023, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Doğan, S.; Salman, Ü. Partial characterization of Lettuce (Lactuca sativa L.) Polyphenol Oxidase. Eur. Food Res. Technol. 2007, 226, 93–103. [Google Scholar] [CrossRef]
- Bradford, M.A. A Rapid and Sensitive Method for the Quantitaion of Microgram Quantites of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavege of Structural Proteins During in Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constant. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Kaya, E.D.; Bağci, O. Purification and Biochemical Characterization of Polyphenol Oxidase Extracted from Kirmizi Kismis grape (Vitis vinifera L.). J. Food Biochem. 2021, 45, e13627. [Google Scholar] [CrossRef]
- Oktay, M.; Kufrevioglu, I.; Kocacaliskan, I.; Sakirolu, H. Polyphenol Oxidase from Amasya apple. J. Food Sci. 1995, 60, 495–499. [Google Scholar] [CrossRef]
- Atrooz, O.M.; AlKhamaisa, N.K.; AlRawashdeh, I.M. Determination of the Activity and Kinetic Parameters of Polyphenol Oxidase Enzyme in Crude Extracts of Some Jordanian Plants. J. Appl. Biol. Biotechnol. 2020, 8, 69–74. [Google Scholar] [CrossRef]
- Rayan, A.; Morsy, N. Thermal Inactivation Kinetics of Peroxidase and Polyphenol Oxidase from Pomegranate arils (Punica granatum L. cv. Wonderful). J. Food Biochem. 2020, 44, e13428. [Google Scholar] [CrossRef]
- Mazzafera, P.; Robinson, S.P. Characterization of Polyphenol Oxidase in Coffee. Phytochemistry 2000, 55, 285–296. [Google Scholar] [CrossRef] [PubMed]
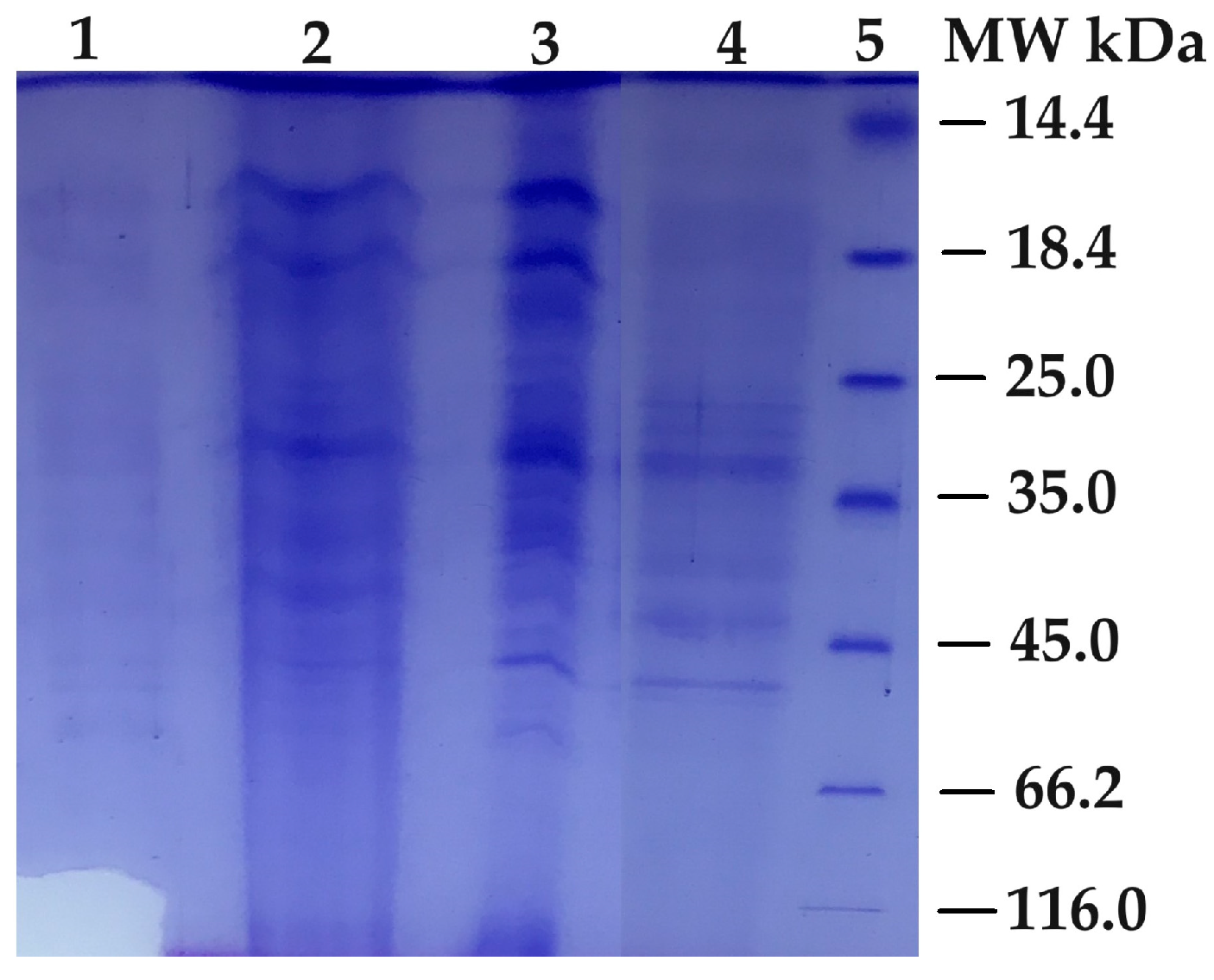
| Purification Steps | Volume (mL) | Activity (U/mLmin) | Total Activity | Total Protein (mg) | Specific Activity (U/mg Protein) | Purification Fold |
|---|---|---|---|---|---|---|
| Crude Extract | 130 | 45.00 | 5850.00 | 3.7126 | 1575.72 | |
| Ammonium sulfate precipitation | 5 | 793.75 | 3968.75 | 1.1007 | 3605.68 | 2.29 |
| Dialysis | 7 | 217.50 | 1522.50 | 0.1748 | 8710.65 | 5.53 |
| Affinity chromatography | 2 | 105.00 | 210.00 | 0.0065 | 32,449.13 | 20.59 |
| Temperature (°C) | Catechol | 4-Methyl Catechol | Pyrogallol | |||
|---|---|---|---|---|---|---|
| Optimum pH | Activity (U/mLmin) | Optimum pH | Activity (U/mLmin) | Optimum pH | Activity (U/mLmin) | |
| 15 | 7.5 | 108.75 | 6.0 | 150.00 | 4.5 | 293.75 |
| 20 | 7.5 | 173.33 | 5.5 | 140.75 | 9.0 | 2466.25 |
| 25 | 6.5 | 132.50 | 7.0 | 50.00 | 7.0 | 1640.00 |
| 30 | 5.0 | 83.33 | 7.5 | 330.00 | 6.5 | 131.25 |
| 35 | 9.0 | 35.00 | 6.5 | 41.68 | 8.5 | 442.50 |
| 40 | 8.5 | 125.00 | 7.5 | 97.50 | 6.0 | 172.50 |
| 45 | 5.0 | 162.50 | 6.5 | 85.00 | 6.5 | 201.25 |
| Substrates | Km (mM) | Vmax (U/mLmin) | Vmax/Km (U/mLminmM) |
|---|---|---|---|
| Catechol | 6.33 | 126.58 | 20.00 |
| 4-methyl catechol | 5.38 | 107.53 | 20.00 |
| Pyrogallol | 16.67 | 833.33 | 50.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demir, D.; Kabak, S.; Çağlayan, K. Purification and Characterization of Polyphenol Oxidase in the Fruits of Opuntia ficus-indica. Biology 2023, 12, 1339. https://doi.org/10.3390/biology12101339
Demir D, Kabak S, Çağlayan K. Purification and Characterization of Polyphenol Oxidase in the Fruits of Opuntia ficus-indica. Biology. 2023; 12(10):1339. https://doi.org/10.3390/biology12101339
Chicago/Turabian StyleDemir, Dudu, Selda Kabak, and Kardelen Çağlayan. 2023. "Purification and Characterization of Polyphenol Oxidase in the Fruits of Opuntia ficus-indica" Biology 12, no. 10: 1339. https://doi.org/10.3390/biology12101339
APA StyleDemir, D., Kabak, S., & Çağlayan, K. (2023). Purification and Characterization of Polyphenol Oxidase in the Fruits of Opuntia ficus-indica. Biology, 12(10), 1339. https://doi.org/10.3390/biology12101339







