Biological and Chemical Characterization of Musa paradisiaca Leachate
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Leachate
2.2. Chemical Analysis
2.3. Metabolomic Analysis
2.4. M. paradisiaca Leachate as a Fungicide
2.5. M. paradisiaca Leachate as a Plant Defense Elicitor
2.6. M. paradisiaca Leachate as a Biostimulant
2.7. Image Analysis and Statistical Tests
3. Results and Discussion
3.1. Chemical Composition of Leachate
3.2. Metabolomic Analysis
3.3. M. paradisiaca Leachate as a Fungistatic Agent
3.4. M. paradisiaca Leachate as a Plant Defense Elicitor
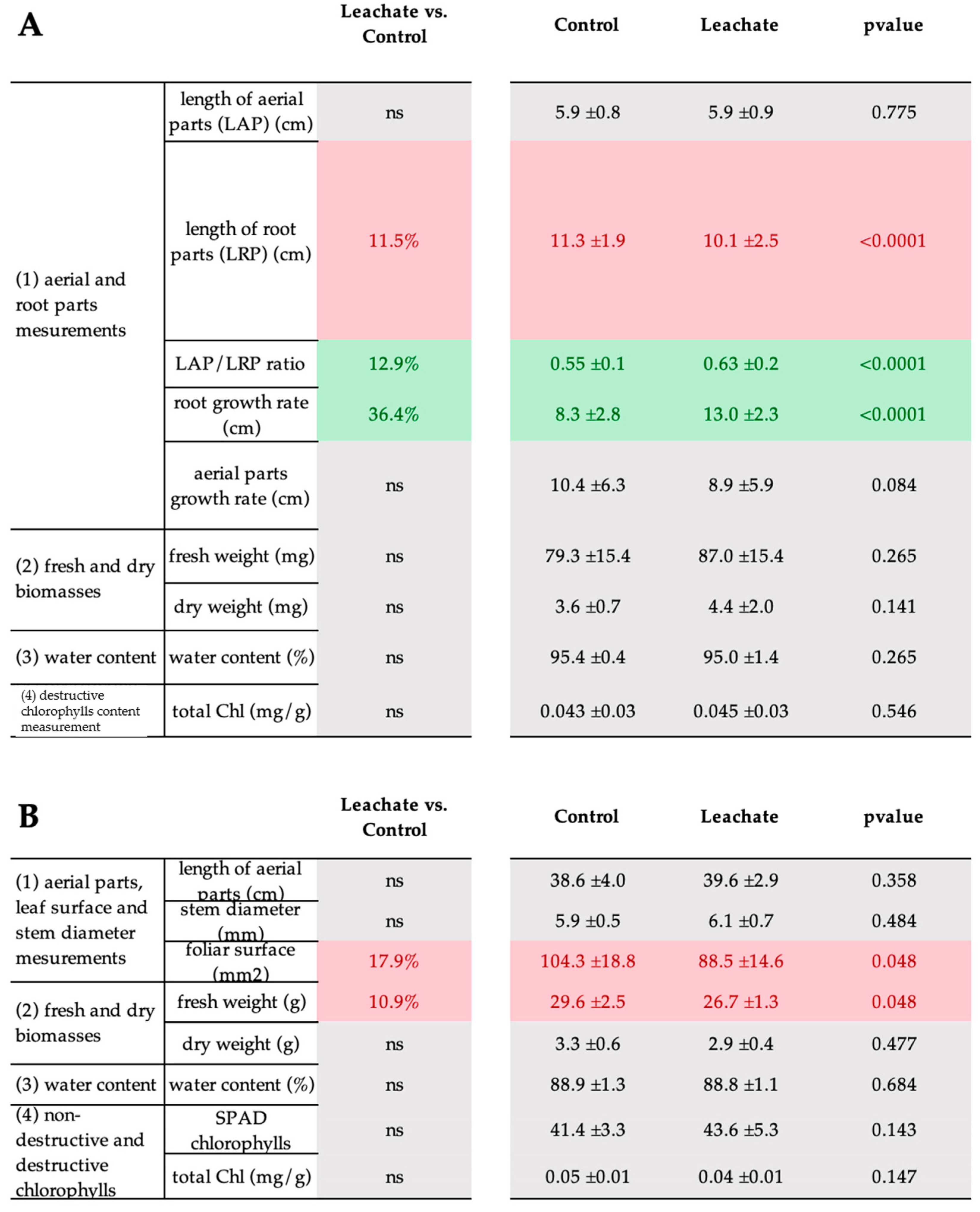
3.5. M. paradisiaca Leachate as a Biostimulant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altieri, M.A. Linking ecologists and traditional farmers in the search for sustainable agriculture. Front. Ecol. Environ. 2004, 2, 35–42. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Tittonell, P.A.; Klerkx, L.W.A.; Baudron, F.; Félix, G.F.; Ruggia, A.P.; van Apeldoorn, D.F.; Dogliotti, S.; Mapfumo, P.; Rossing, W.A.H. Ecological Intensification: Local innovation to address global challenges. Sustain. Agric. Rev. 2016, 19, 1–34. [Google Scholar] [CrossRef]
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Pretty, J.; Sutherland, W.J.; Ashby, J.; Auburn, J.; Baulcombe, D.; Bell, M.; Bentley, J.; Bickersteth, S.; Brown, K.; Burke, J.; et al. The top 100 questions of importance to the future of global agriculture. Int. J. Agric. Sustain. 2010, 8, 219–236. [Google Scholar] [CrossRef]
- Davillerd, Y.; Marchand, P.A. Les substances naturelles à usage biostimulant: Statut réglementaire et état des lieux de ces préparations naturelles peu préoccupantes (PNPP). Cah. Agr. 2022, 31, 28. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs) an introduction. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–30. [Google Scholar] [CrossRef]
- Özer, N.; Köycü, N.D. The ability of plant compost leachates to control black mold (Aspergillus niger) and to induce the accumulation of antifungal compounds in onion following seed treatment. BioControl 2006, 51, 229–243. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Naidu, Y.; Meon, S.; Siddiqui, Y. Foliar application of microbial-enriched compost tea enhances growth, yield and quality of muskmelon (Cucumis melo L.) cultivated under fertigation system. Sci. Hort. 2013, 159, 33–40. [Google Scholar] [CrossRef]
- Chávez-Estudillo, V.; Valencia-Ordoñez, A.; Córdova-Nieto, C.; Flores-Estevéz, N.; Jarillo-Rodríguez, J.; Noa-Carrazana, J. Lixiviados de raquis de plátano: Obtención y usos potenciales. Cuad. Biodivers. 2017, 53, 1–8. [Google Scholar] [CrossRef]
- Pacome, S.S.; Phillipe, G.; Patrice, A.K.; Turquin, L.; Theodore, S.V.; Koutoua, S.; Diallo, H.A. Bio-control of the lesion nematode Pratylenchus coffeae using lixiviate from banana rachis (Musa sp.). Int. J. Phytopathol. 2018, 7, 111–121. [Google Scholar] [CrossRef]
- Seri, S.P.; Kouadio, D.L.; Kabran, A.F.; Gnonhouri, P.; Attioua, K.B.; Turquin, L. Phytochemical screening and nematicidal activity of lixiviate from plantain and banana rachis. Int. J. Phytopathol. 2022, 11, 207–213. [Google Scholar] [CrossRef]
- Osorio Gutiérrez, L.A.; Castaño Zapata, J.; Gutiérrez Ríos, L.B. In-vitro efficacy of plantain lixiviates on Fusarium oxysporum Schlecht, causal agent of pea (Pisum sativum Linneo) roots rot. Agronomía 2012, 20, 17–25. [Google Scholar]
- Bele, L.; Kouamé, D.K.; Assiri, K.P.; Diallo, H.A. Antifungal activity of banana rachis leachate on some fungi responsible for banana (Musa acuminata Colla) post-harvest diseases. Int. J. Environ. Agric. Res. 2018, 4, 1–6. [Google Scholar]
- Valenzuela-Cobos, J.D.; Rodríguez-Grimón, R.O.; Vargas-Farías, C.; Grijalva-Endara, A.; Mercader-Camejo, O.A. Biodegradation of plantain rachis using phytopathogenic fungi for composting. Rev. Mex. Ing. Quim. 2020, 19, 533–541. [Google Scholar] [CrossRef]
- Duran Eugenio, J.W.; Florencin Sánchez, F.D. Efecto de Dosis de Lixiviado del Raquis de Platano en el Rendimiento del Cultivo de Maíz (Zea mays L.) Híbrido pm 213 en Condiciones de Chanchamayo. Master’s Thesis, Universidad Nacional Daniel Alcides Carrión, La Merced, Perú, 2018. [Google Scholar]
- Petro, D.; Onyeka, T.J.; Etienne, S.; Rubens, S. An intraspecific genetic map of water yam (Dioscorea alata L.) based on AFLP markers and QTL analysis for anthracnose resistance. Euphytica 2011, 179, 405–416. [Google Scholar] [CrossRef]
- Raj, M.; Jeeva, M.L.; Nath, V.S.; Sankar, S.; Vidhyadharan, P.; Archana, P.V.; Hegde, V. A highly sensitive nested-PCR method using a single closed tube for the detection of Colletotrichum gloeosporioides causing greater yam anthracnose. J. Root Crops 2015, 39, 163–167. [Google Scholar] [CrossRef]
- USDA National Agricultural Statistics Service. Vegetables-Tomato Production, Price, and Value by Utilization. Available online: https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Vegetables/index.php (accessed on 25 August 2023).
- Sierra, J.; Desfontaines, L.; Favérial, J.; Loranger-Merciris, G.; Boval, M. Composting and vermicomposting of cattle manure and green wastes under tropical conditions: Carbon and nutrient balances and end-product quality. Soil Res. 2013, 51, 142–151. [Google Scholar] [CrossRef]
- Novozamsky, I.; Houba, V.J.G.; Vaneck, R.; Vanvark, W.A. novel digestion technique for multi-element plant analysis. Commun. Soil Sci. Plant Anal. 1983, 14, 239–248. [Google Scholar] [CrossRef]
- Luna, E.; Flandin, A.; Cassan, C.; Prigent, S.; Chevanne, C.; Kadiri, C.F.; Gibon, Y.; Pétriacq, P. Metabolomics to exploit the primed immune system of tomato fruit. Metabolites 2020, 10, 96. [Google Scholar] [CrossRef]
- Dussarrat, T.; Prigent, S.; Latorre, C.; Bernillon, S.; Flandin, A.; Díaz, F.P.; Cassan, C.; Van Delft, P.; Jacob, D.; Varala, K. Predictive metabolomics of multiple Atacama plant species unveils a core set of generic metabolites for extreme climate resilience. New Phytol. 2022, 234, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Frézal, L.; Desquilbet, L.; Jacqua, G.; Neema, C. Quantification of the aggressiveness of a foliar pathogen, Colletotrichum gloeosporioides, responsible for water yam (Dioscorea alata) anthracnose. Eur. J. Plant Pathol. 2012, 134, 267–279. [Google Scholar] [CrossRef]
- Iscan, G.; Kirimer, N.; Kürkcüoglu, M.; Başer, H.C.; Demirci, F. Antimicrobial screening of Mentha piperita essential oils. J. Agric. Food Chem. 2002, 50, 3943–3946. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L. Activity of Bulgarian propolis against 94 Helicobacter pylori strains in vitro by agar-well diffusion, agar dilution and disc diffusion methods. J. Med. Microbiol. 2005, 54, 481–483. [Google Scholar] [CrossRef]
- Zbytniewski, R.; Kosobucki, P.; Kowalkowski, T.; Buszewski, B. The comparison study of compost and natural organic matter samples. Environ. Sci. Pollut. Res. 2002, 9, 68–74. [Google Scholar] [CrossRef]
- Albrecht, R.; Le Petit, J.; Terrom, G.; Périssol, C. Comparison between UV spectroscopy and nirs to assess humification process during sewage sludge and green wastes co-composting. Bioresour. Technol. 2011, 102, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis. Part 3-Chemical Methods; Sparks, D., Page, A., Helmke, P., Loeppert, R., Soltanpour, P., Tabatabai, M., Johnston, C., Sumner, M., Eds.; SSSA Book Series; Soil Science Society of America, Inc.: Madison, WI, USA; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 1011–1069. [Google Scholar] [CrossRef]
- Velez, J.E.; Zapata, J.C. Fulvic acid applications for the management of diseases caused by Mycosphaerella spp. InfoMusa 2005, 14, 15–17. [Google Scholar]
- Ortiz Bastidas, M.F. Evaluación de la Actividad de los Lixiviados de Raquis de Banano (Musa AAA), Plátano (Musa AAB), y Banano Orito (Musa AA) Sobre el Agente Causal de la Sigatoka Negra (Mycosphaerella fijiensis) en Condiciones In vitro. Master’s Thesis, Ingeniero Agropecuaria, Escuela Superior Politécnica del Litoral, Guayaquil, Ecuador, 2009. [Google Scholar]
- Preston, C.M.; Nault, J.R.; Trofymow, J.A. Chemical changes during 6 years of decomposition of 11 litters in some canadian forest sites. Part 2. 13C abundance, solid-state 13C NMR spectroscopy and the meaning of lignin. Ecosystems 2009, 12, 1078–1102. Available online: http://www.jstor.org/stable/25622867 (accessed on 3 September 2023). [CrossRef]
- Lima, M.D.C.; de Sousa, C.P.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A., Eds.; Springer Nature: Singapore, 2020; pp. 517–532. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Khan, M.I., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Contreras-Blancas, E.; Ruíz-Valdiviezo, V.M.; Santoyo-Tepole, F.; Luna-Guido, M.; Meza-Gordillo, R.; Dendooven, L.; Gutiérrez-Miceli, F.A. Evaluation of worm-bed leachate as an antifungal agent against pathogenic fungus, Colletotrichum gloeosporioides. Compost Sci. Util. 2014, 22, 23–32. [Google Scholar] [CrossRef]
- Carlson, M.; Thompson, R.D. Analyte loss due to membrane filter adsorption as determined by high-performance liquid chromatography. J. Chromatogr. Sci. 2000, 38, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Medlen, C.E. Fulvic Acid and Its Use in the Treatment of Various Conditions. Available online: http://www.google.com/patents/US6569900 (accessed on 25 August 2023).
- Wang, Y.; Sun, Y.; Wang, J.; Zhou, M.; Wang, M.; Feng, J. Antifungal activity and action mechanism of the natural product cinnamic acid against Sclerotinia sclerotiorum. Plant Dis. 2019, 103, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.C.; Ferreira, A.R.; Silva, D.F.; Lima, E.O.; de Sousa, D.P. Antifungal activity of cinnamic acid and benzoic acid esters against Candida albicans strains. Nat. Prod. Res. 2018, 32, 572–575. [Google Scholar] [CrossRef]
- Behiry, S.I.; Okla, M.K.; Alamri, S.A.; EL-Hefny, M.; Salem, M.Z.M.; Alaraidh, I.A.; Ali, H.M.; Al-Ghtani, S.M.; Monroy, J.C.; Salem, A.Z.M. Antifungal and antibacterial activities of Musa paradisiaca L. peel extract: HPLC analysis of phenolic and flavonoid contents. Processes 2019, 7, 215. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, Y.; Yi, G.; Li, M.; Liao, L.; Yang, C.; Cho, C.; Zhang, B.; Zhu, J.; Zou, K.; et al. Quinic acid: A potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa. Chin. Med. 2021, 16, 72. [Google Scholar] [CrossRef]
- Mackrill, J.J.; Kehoe, R.A.; Zheng, L.; McKee, M.M.; O’Sullivan, E.C.; Doyle, B.M.; McCarthy, F.O. Inhibitory properties of aldehydes and related compounds against Phytophthora infestans—Identification of a new lead. Pathogens 2020, 9, 542. [Google Scholar] [CrossRef]
- McKee, M.L.; Zheng, L.; O’Sullivan, E.C.; Kehoe, R.A.; Doyle Prestwich, B.M.; Mackrill, J.J.; McCarthy, F.O. Synthesis and evaluation of novel ellipticines and derivatives as inhibitors of Phytophthora infestans. Pathogens 2020, 9, 558. [Google Scholar] [CrossRef]
- Lemar, K.; Turner, M.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Chavez-Navarrete, T.; Sanchez-Timm, L.; Pacheco-Coello, R.; Baisakh, N.; Santos-Ordóñez, E. Identification of differential-expressed genes in banana-biostimulant interaction using suppression subtractive hybridization. Agronomy 2023, 13, 415. [Google Scholar] [CrossRef]
- Xu, D.; Deng, Y.; Xi, P.; Yu, G.; Wang, Q.; Zeng, Q.; Jiang, Z.; Gao, L. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chem. 2019, 286, 226–233. [Google Scholar] [CrossRef]
- Lamar, R.T. Possible role for electron shuttling capacity in elicitation of PB activity of humic substances on plant growth enhancement. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 97–121. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Ezquer, I.; Salameh, I.; Colombo, L.; Kalaitzis, P. Plant cell walls tackling climate change: Biotechnological strategies to improve crop adaptations and photosynthesis in response to global warming. Plants 2020, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Carreras, A.; Bernard, S.; Durambur, G.; Gügi, B.; Loutelier, C.; Pawlak, B.; Boulogne, I.; Vicré, M.; Goffner, D.; Follet-Gueye, M.L. In vitro characterization of root extracellular trap and exudates of three Sahelian woody plant species. Planta 2020, 251, 19. [Google Scholar] [CrossRef] [PubMed]
- Busont, O.; Durambur, G.; Bernard, S.; Plasson, C.; Joudiou, C.; Baude, L.; Chefdor, F.; Depierreux, C.; Héricourt, F.; Larcher, M.; et al. Black poplar (Populus nigra L.) root extracellular trap, structural and molecular remodeling in response to osmotic stress. Cells 2023, 12, 858. [Google Scholar] [CrossRef]
- Gao, M.; Showalter, A.M. Immunolocalization of LeAGP-1, a modular arabinogalactan-protein, reveals its developmentally regulated expression in tomato. Planta 2000, 210, 865–874. [Google Scholar] [CrossRef]
- Chen, Y.; Aviad, T. Effects of humic substances on plant growth. In Humic Substances in Soil and Crop Sciences: Selected Readings; MacCarthy, P., Clapp, C.E., Malcolm, R.L.P., Bloom, R., Eds.; ASA, CSSA, and SSSA Books: Madison, WI, USA, 1990; pp. 161–186. [Google Scholar] [CrossRef]
- Thirumalaikumar, V.P.; Devkar, V.; Mehterov, N.; Ali, S.; Ozgur, R.; Turkan, I.; Mueller-Roeber, B.; Balazadeh, S. NAC transcription factor JUNGBRUNNEN 1 enhances drought tolerance in tomato. Plant Biotechnol. J. 2018, 16, 354–366. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Piro, G.; Leucci, M.R.; Waldron, K.; Dalessandro, G. Exposure to water stress causes changes in the biosynthesis of cell wall polysaccharides in roots of wheat cultivars varying in drought tolerance. Plant Sci. 2003, 165, 559–569. [Google Scholar] [CrossRef]
- Lamport, D.T.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 156, 11–19. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Zouari, M.; Hassena, A.B.; Trabelsi, L.; Rouina, B.B.; Decou, R.; Labrousse, P. Exogenous proline-mediated abiotic stress tolerance in plants: Possible mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 99–121. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.H.; Li, C.Q.; Liu, L.; Han, G.Y.; Zhang, Y.L.; Wang, C.M. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.A.; Ahmed, M.E.; El-Nagar, A. Benzoic acid and its hydroxylated derivatives suppress early blight of tomato (Alternaria solani) via the induction of salicylic acid biosynthesis and enzymatic and nonenzymatic antioxidant defense machinery. J. Fungi 2021, 7, 663. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef]
- Jones, L.; Seymour, G.B.; Knox, J.P. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1 [->] 4)-β-D-Galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef]
- Willats, W.G.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1→5)-α-l-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- Willats, W.G.; McCartney, L.; Steele-King, C.G.; Marcus, S.E.; Mort, A.; Huisman, M.; van Alebeek, G.J.; Shols, H.A.; Voragen, A.G.J.; Le Goff, A.; et al. A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 2004, 218, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.L.; Fangel, J.U.; McCleary, B.; Ruzanski, C.; Rydahl, M.G.; Ralet, M.C.; Farkas, V.; von Schantz, L.; Marcus, S.E.; Andersen, M.C.; et al. Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 2012, 287, 39429–39438. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef]
- Marcus, S.E.; Blake, A.W.; Benians, T.A.; Lee, K.J.; Poyser, C.; Donaldson, L.; Leroux, O.; Rogowski, A.; Petersen, H.L.; Boraston, A.; et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010, 64, 191–203. [Google Scholar] [CrossRef]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef]
- Yates, E.A.; Valdor, J.F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, J.P. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef]
- Smallwood, M.; Martin, H.; Knox, J.P. An epitope of rice threonine-and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta 1995, 196, 510–522. [Google Scholar] [CrossRef]


| Dried Weight | N | C | P | K | Ca | Mg | Na | N-NH4 | N-NO3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Liquid leachate | 1.09 | 0.008 | 0.30 | 0.51 | 33.61 | 0.39 | 0.009 | 0.031 | 0.041 | 0.00 |
| Freeze-drying leachate | - | 0.731 | 16.04 | 0.06 | 29.57 | 0.07 | 0.00 | 0.14 | 0.12 | 0.00 |
| OD 664 | OD 472 | OD 280 | Q2/4 | Q2/6 | Q4/6 | |
|---|---|---|---|---|---|---|
| Liquid leachate | 3.56 | 12.50 | 82.50 | 6.60 | 23 | 3.50 |
| Freeze-drying leachate | 0.12 | 0.76 | 6.06 | 6.60 | 43.42 | 6.40 |
| No Sterilization | With Streptomycin (200 µg·mL−1) | Sterilization with 0.2 μm Filter | Autoclaved | UV Sterilization | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 7 days | 24 h | 7 days | 24 h | 7 days | 24 h | 7 days | 24 h | 7 days | |
| Liquid leachate | + | − | + | − | − | − | − | − | − | − |
| Fungistop FL | + | + | + | + | + | + | − | − | + | + |
| Distilled water | − | − | − | − | − | − | − | − | − | − |
| LOX-D | PAL5 | PPO-D | Pti-5 | Worky28 | Worky70-80 | |
|---|---|---|---|---|---|---|
| Leachate vs. Control (water) | 2.80 * | 1.37 | 6.34 * | 1.45 | −1.30 | 1.54 * |
| P5CS | ProDH | AREB1 | |
|---|---|---|---|
| Leachate vs. Control (water) (in vitro) | 1.4 | 1.35 | −1.15 |
| Leachate vs. Control (water) (phytotron) | −1.25 | 4.45 * | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulogne, I.; Petit, P.; Desfontaines, L.; Durambur, G.; Deborde, C.; Mirande-Ney, C.; Arnaudin, Q.; Plasson, C.; Grivotte, J.; Chamot, C.; et al. Biological and Chemical Characterization of Musa paradisiaca Leachate. Biology 2023, 12, 1326. https://doi.org/10.3390/biology12101326
Boulogne I, Petit P, Desfontaines L, Durambur G, Deborde C, Mirande-Ney C, Arnaudin Q, Plasson C, Grivotte J, Chamot C, et al. Biological and Chemical Characterization of Musa paradisiaca Leachate. Biology. 2023; 12(10):1326. https://doi.org/10.3390/biology12101326
Chicago/Turabian StyleBoulogne, Isabelle, Philippe Petit, Lucienne Desfontaines, Gaëlle Durambur, Catherine Deborde, Cathleen Mirande-Ney, Quentin Arnaudin, Carole Plasson, Julie Grivotte, Christophe Chamot, and et al. 2023. "Biological and Chemical Characterization of Musa paradisiaca Leachate" Biology 12, no. 10: 1326. https://doi.org/10.3390/biology12101326
APA StyleBoulogne, I., Petit, P., Desfontaines, L., Durambur, G., Deborde, C., Mirande-Ney, C., Arnaudin, Q., Plasson, C., Grivotte, J., Chamot, C., Bernard, S., & Loranger-Merciris, G. (2023). Biological and Chemical Characterization of Musa paradisiaca Leachate. Biology, 12(10), 1326. https://doi.org/10.3390/biology12101326








