Aging-Related Behavioral Patterns in Tibetan Macaques
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Site and Subjects
2.2. Data Collection and Behavioral Definition
2.3. Data Analysis
2.4. Ethics Statement
3. Results
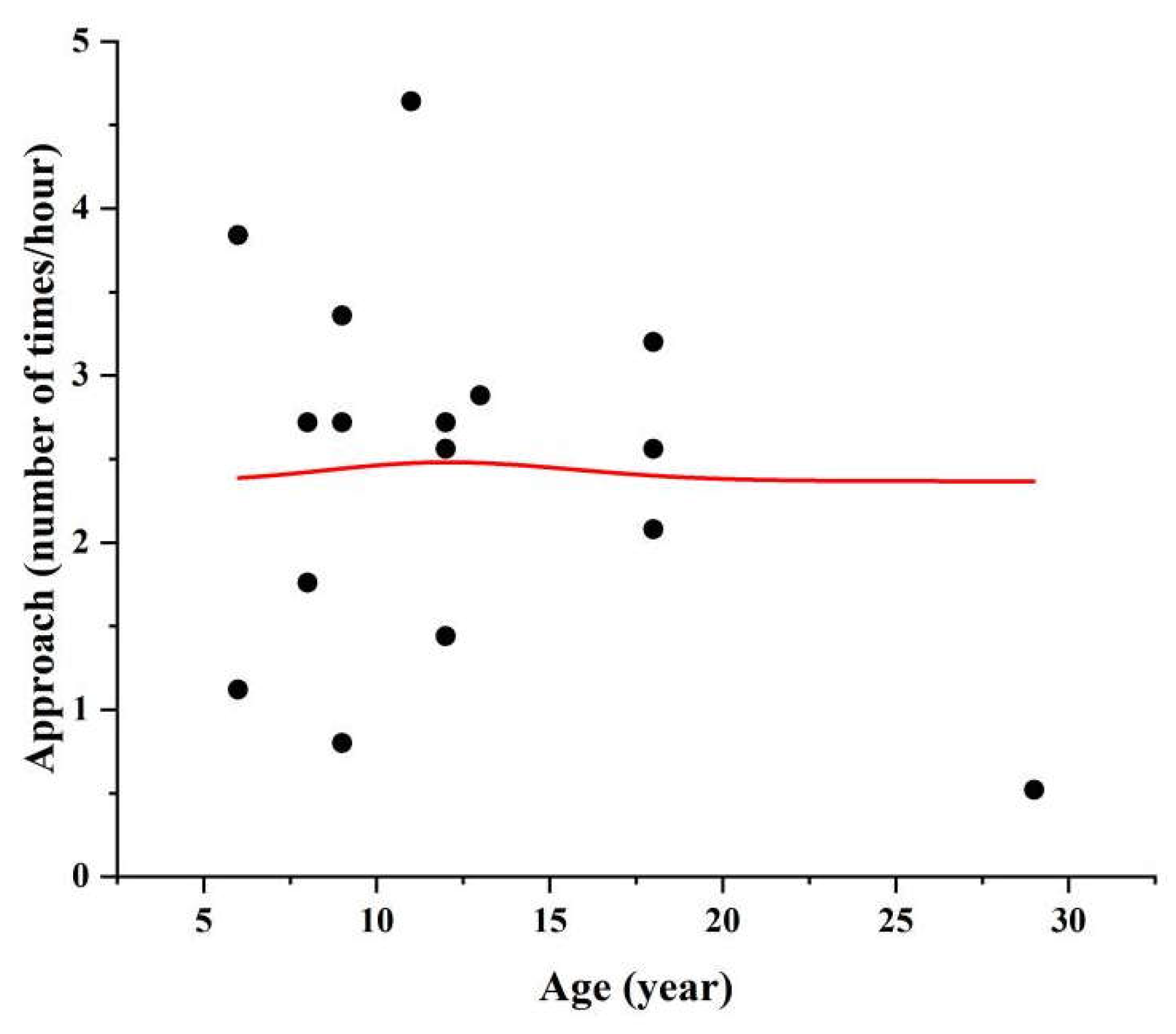
3.1. Influence of Age on Affiliative Behavior
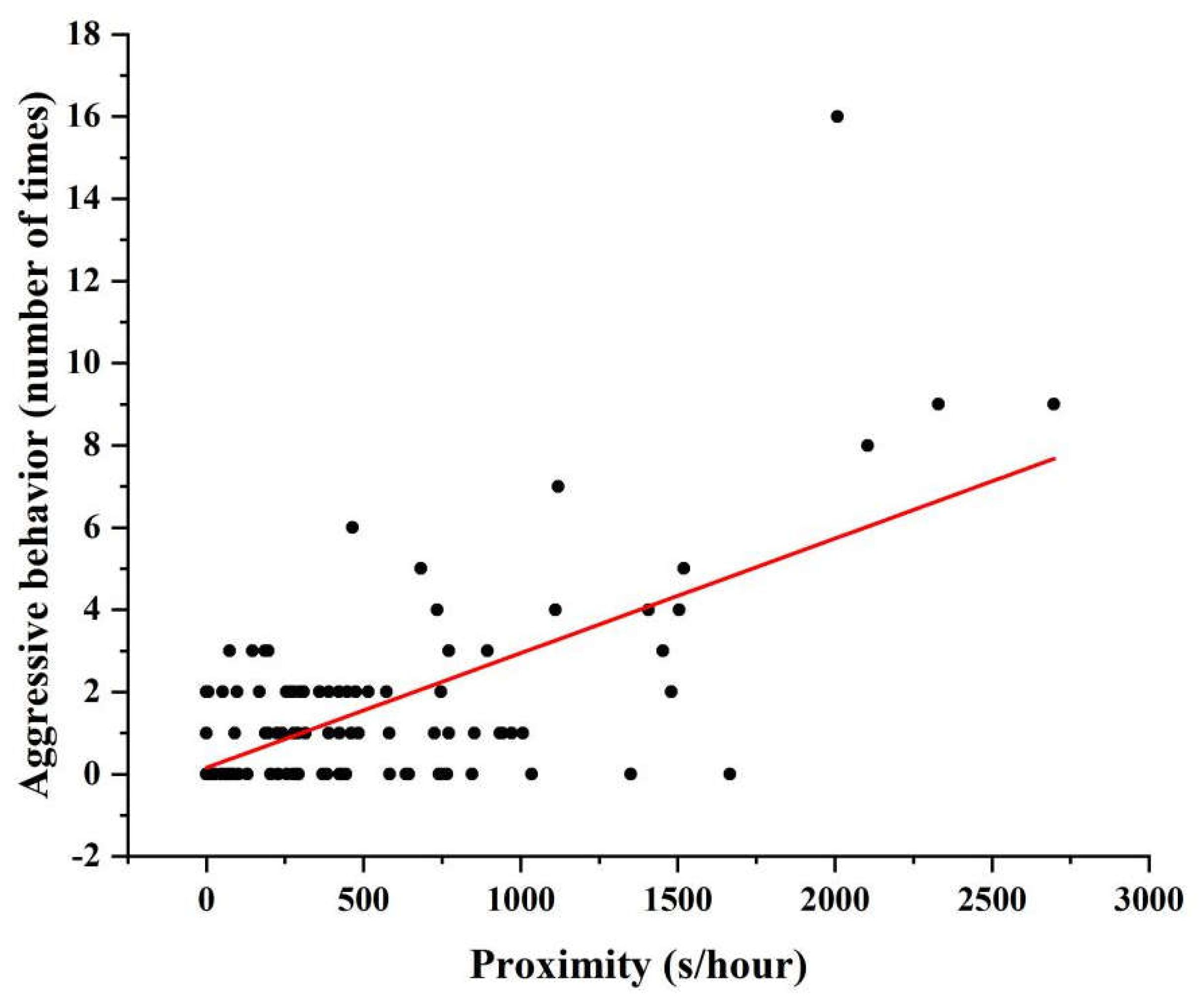
3.2. Influence of Age on Aggressive Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables; Department of Economics and Social Affairs PD, Ed.; United Nations: New York, NY, USA, 2017; p. 46. [Google Scholar]
- Smith, S.G.; Jackson, S.E.; Kobayashi, L.C.; Steptoe, A. Social Isolation, Health Literacy, and Mortality Risk: Findings from the English Longitudinal Study of Ageing. Health Psychol. 2018, 37, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, L.L.; Isaacowitz, D.M.; Charles, S.T. Taking time seriously: A theory of socioemotional selectivity. Am. Psychol. 1999, 54, 165. [Google Scholar] [CrossRef] [PubMed]
- Negrey, J.D.; Frye, B.M.; Craft, S.; Register, T.C.; Baxter, M.G.; Jorgensen, M.J.; Shively, C.A. Executive function mediates age-related variation in social integration in female vervet monkeys (Chlorocebus sabaeus). GeroScience 2023, 1–12. [Google Scholar] [CrossRef]
- Fischer, J. Aging rhesus monkeys stick to friends and family. Proc. Natl. Acad. Sci. USA 2023, 120, e2219062120. [Google Scholar] [CrossRef]
- Rosati, A.G.; Hagberg, L.; Enigk, D.K.; Otali, E.; Emery Thompson, M.; Muller, M.N.; Machanda, Z.P. Social selectivity in aging wild chimpanzees. Science 2020, 370, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.A. Nonhuman primate models in biogerontology. Exp. Gerontol. 2000, 35, 533–541. [Google Scholar] [CrossRef]
- Schino, G.; Pinzaglia, M. Age-related changes in the social behavior of tufted capuchin monkeys. Am. J. Primatol. 2018, 80, e22746. [Google Scholar] [CrossRef]
- Liao, Z.; Sosa, S.; Wu, C.; Zhang, P. The influence of age on wild rhesus macaques’ affiliative social interactions. Am. J. Primatol. 2018, 80, e22733. [Google Scholar] [CrossRef]
- Siracusa, E.R.; Negron-Del Valle, J.E.; Phillips, D.; Platt, M.L.; Higham, J.P.; Snyder-Mackler, N.; Brent, L.J.N. Within-individual changes reveal increasing social selectivity with age in rhesus macaques. Proc. Natl. Acad. Sci. USA 2022, 119, e2209180119. [Google Scholar] [CrossRef]
- Harrison, C. Primates, like humans, focus their social circle with age. Lab Anim. 2023, 52, 33. [Google Scholar] [CrossRef]
- Ratnayeke, S. The behavior of postreproductive females in a wild population of toque macaques (Macaca sinica) in Sri Lanka. Int. J. Primatol. 1994, 15, 445–469. [Google Scholar] [CrossRef]
- Hauser, M.D.; Tyrrell, G. Old age and its behavioral manifestations: A study on two species of macaque. Folia Primatol. 1984, 43, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Rathke, E.M.; Fischer, J. Social aging in male and female Barbary macaques. Am. J. Primatol. 2021, 83, e23272. [Google Scholar] [CrossRef]
- Almeling, L.; Hammerschmidt, K.; Sennhenn-Reulen, H.; Freund, A.M.; Fischer, J. Motivational shifts in aging monkeys and the origins of social selectivity. Curr. Biol. 2016, 26, 1744–1749. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S. The Influence of Gender, Age, Matriline and Hierarchical Rank on Individual Social Position, Role and Interactional Patterns in Macaca sylvanus at ‘La Forêt des Singes’: A Multilevel Social Network Approach. Front. Psychol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, M. Age-related differences in social grooming among adult female Japanese monkeys (Macaca fuscata). Primates 2003, 44, 239–246. [Google Scholar] [CrossRef]
- Kato, E. Effects of age, dominance and seasonal changes on proximity relationships in female Japanese macaques (Macaca fuscata) in a free-ranging group at Katsuyama. Primates 1999, 40, 291–300. [Google Scholar] [CrossRef]
- Pavelka, M.S.M. Sociability in old female Japanese monkeys: Human versus nonhuman primate aging. Am. Anthropol. 1991, 93, 588–598. [Google Scholar] [CrossRef]
- Pavelka, M.S.M. Do old female monkeys have a specific social role. Primates 1990, 31, 363–373. [Google Scholar] [CrossRef]
- Baker, K.C. Advanced age influences chimpanzee behavior in small social groups. Zoo Biol. 2000, 19, 111–119. [Google Scholar] [CrossRef]
- Corr, J. Social behavior in aged rhesus macaques. Coll. Antropol. 2003, 27, 87–94. [Google Scholar] [PubMed]
- Machanda, Z.P.; Rosati, A.G. Shifting sociality during primate ageing. Philos Trans. R. Soc. B 2020, 375, 20190620. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, L.A.; McGuire, M. Age, reproductive value, and dominance-related behaviour in vervet monkey females: Cross-generational influences on social relationships and reproduction. Anim. Behav. 1986, 34, 1710–1721. [Google Scholar] [CrossRef]
- Veenema, H.C.; Spruijt, B.M.; Gispen, W.H.; Van Hooff, J. Aging, dominance history, and social behavior in Java-monkeys (Macaca fascicularis). Neurobiol. Aging 1997, 18, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Sun, L.X.; Kappeler, P.M. The Behavioral Ecology of the Tibetan Macaque; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Wang, T.; Wang, X.; Garber, P.A.; Sun, B.-H.; Sun, L.; Xia, D.-P.; Li, J.-H. Sex-specific variation of social play in wild immature Tibetan macaques, Macaca thibetana. Animals 2021, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Berman, C.M.; Ionica, C.S.; Li, J. Dominance style among Macaca thibetana on Mt. Huangshan, China. Int. J. Primatol. 2004, 25, 1283–1312. [Google Scholar] [CrossRef]
- Gammell, M.P.; Vries, H.D.; Jennings, D.J.; Carlin, C.M.; Hayden, T.J. David’s score: A more appropriate dominance ranking method than Clutton-Brock et al.’s index. Anim. Behav. 2003, 66, 601–605. [Google Scholar] [CrossRef]
- Bernstein, I.S.; Ehardt, C. The influence of kinship and socialization on aggressive behaviour in rhesus monkeys (Macaca mulatta). Anim. Behav. 1986, 34, 739–747. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Arseneau-Robar, T.J.M.; Joyce, M.M.; Stead, S.M.; Teichroeb, J.A. Proximity and grooming patterns reveal opposite-sex bonding in Rwenzori Angolan colobus monkeys (Colobus angolensis ruwenzorii). Primates 2018, 59, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.T.; Piazza, J.R.; Luong, G.; Almeida, D.M. Now you see it, now you don’t: Age differences in affective reactivity to social tensions. Psychol. Aging 2009, 24, 645. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.J. Early-life physical performance predicts the aging and death of elite athletes. Sci. Adv. 2023, 9, eadf1294. [Google Scholar] [CrossRef] [PubMed]

| Male ID | Social Rank (DS) | Age Group | Mother | Immigrate/Birth Time | Female ID | Social Rank (DS) | Age | Mother |
|---|---|---|---|---|---|---|---|---|
| YXK | 1 (27.00) | Middle | YH | 14 February 2013 | YXX | 1 (81.00) | 11 | YH |
| TRG | 2 (19.88) | Middle | # | 24 April 2010 | YH | 2 (67.67) | 18 | YM |
| ZB | 3 (13.00) | Old | # | 25 August 2008 | YCY | 3 (56.67) | 12 | YM |
| DS | 4 (4.71) | Old | # | 10 August 2013 | YXY | 4 (40.75) | 6 | YH |
| TQ | 5 (−3.33) | Middle | # | 27 November 2018 | YM | 5 (35.37) | 29 | # |
| WM | 6 (−3.67) | Middle | # | 20 November 2018 | YCH | 6 (30.00) | 9 | # |
| TQS | 7 (−10.83) | Young | TXH | 4 May 2015 | TXH | 7 (9.90) | 12 | TH |
| JM | 8 (−19.88) | Young | # | 3 September 2020 | YCL | 8 (8.17) | 9 | YM |
| SJT | 9 (−26.88) | Old | # | 18 September 2020 | TH | 9 (−10.00) | 18 | TG * |
| TXX | 10 (−23.77) | 13 | TH | |||||
| TQL | 11 (−36.00) | 8 | TXX | |||||
| HH | 12 (−44.53) | 18 | H * | |||||
| THY | 13 (−45.27) | 12 | TR * | |||||
| HXY | 14 (−45.46) | 6 | HH | |||||
| THX | 15 (−63.58) | 9 | TH | |||||
| HXW | 16 (−80.57) | 8 | HH |
| Behavior | Definition |
|---|---|
| Affiliative | |
| Grooming | An individual uses his/her fingers and palms to groom the fur of another individual. The groomer may pick out small objects from the recipient’s fur and eat them. |
| Proximity | Two or more individuals keep a sitting or lying posture within a certain distance; the distance in this study was 2 m. |
| Approach | Focal subject came from beyond to ≤1 m of another individual, or vice versa. |
| Aggressive | |
| Threat | An individual directs an open mouth threat gesture or any of its components, e.g., stare, raised eyebrows, lowered jaw, ground slap, to another individual. |
| Short lunge | An individual directs a lunge <2 body lengths to another individual. |
| Long lunge | An individual directs a lunge >2 body lengths to another individual but does not go into a full chase. |
| Chase | An individual runs rapidly after another individual. |
| Bite | An individual grabs and bites hard, either releasing the victim quickly or hanging on for several seconds. |
| Grooming Given | Grooming Received | Approach Given | Approach Received | Aggression Given | Aggression Given | Grooming Given Concentration Index | Grooming Received Concentration Index | |
|---|---|---|---|---|---|---|---|---|
| X2 | 1.238 | 2.250 | 4.950 | 2.250 | 2.285 | 0.375 | 0.225 | 1.238 |
| df | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| p | 0.539 | 0.325 | 0.084 | 0.425 | 0.325 | 0.829 | 0.894 | 0.539 |
| Model | Predictors | Estimate | SE | Z | p |
|---|---|---|---|---|---|
| GLMM1: Frequency of approach | |||||
| Intercept | 3.919 | 0.375 | 10.446 | <0.001 | |
| Age | −0.071 | −0.029 | 2.360 | 0.018 | |
| Rank | −0.078 | 0.043 | −1.812 | 0.070 | |
| GLMM2: Frequency of being the object of approach | |||||
| Intercept | 2.717 | 0.371 | 7.319 | <0.001 | |
| Age | −0.005 | 0.026 | −0.187 | 0.852 | |
| Rank | −0.012 | 0.041 | −0.283 | 0.777 | |
| GLMM3: The average number of monkeys | |||||
| Intercept | 1.982 | 0.768 | 2.582 | 0.009 | |
| Age | −0.048 | 0.061 | −0.791 | 0.429 | |
| Rank | −0.046 | 0.089 | −0.518 | 0.605 | |
| GLMM4: The number of aggressive acts received | |||||
| Intercept | 4.985 | 1.367 | 3.647 | <0.001 | |
| Age | −0.346 | 0.128 | −2.696 | 0.007 | |
| Rank | −0.277 | 0.129 | −2.135 | 0.033 | |
| GLMM5: The proportion of mild aggression | |||||
| Intercept | 0.481 | 0.117 | 4.098 | 0.002 | |
| Age | 0.011 | 0.001 | 0.597 | 0.619 | |
| Rank | 0.104 | 1.001 | 0.104 | 0.917 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Liu, S.-Q.; Xia, Y.-N.; Li, B.-W.; Wang, X.; Li, J.-H. Aging-Related Behavioral Patterns in Tibetan Macaques. Biology 2023, 12, 1325. https://doi.org/10.3390/biology12101325
Zhang T, Liu S-Q, Xia Y-N, Li B-W, Wang X, Li J-H. Aging-Related Behavioral Patterns in Tibetan Macaques. Biology. 2023; 12(10):1325. https://doi.org/10.3390/biology12101325
Chicago/Turabian StyleZhang, Tong, Shen-Qi Liu, Ying-Na Xia, Bo-Wen Li, Xi Wang, and Jin-Hua Li. 2023. "Aging-Related Behavioral Patterns in Tibetan Macaques" Biology 12, no. 10: 1325. https://doi.org/10.3390/biology12101325
APA StyleZhang, T., Liu, S.-Q., Xia, Y.-N., Li, B.-W., Wang, X., & Li, J.-H. (2023). Aging-Related Behavioral Patterns in Tibetan Macaques. Biology, 12(10), 1325. https://doi.org/10.3390/biology12101325








