In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice
Abstract
Simple Summary
Abstract
1. Introduction
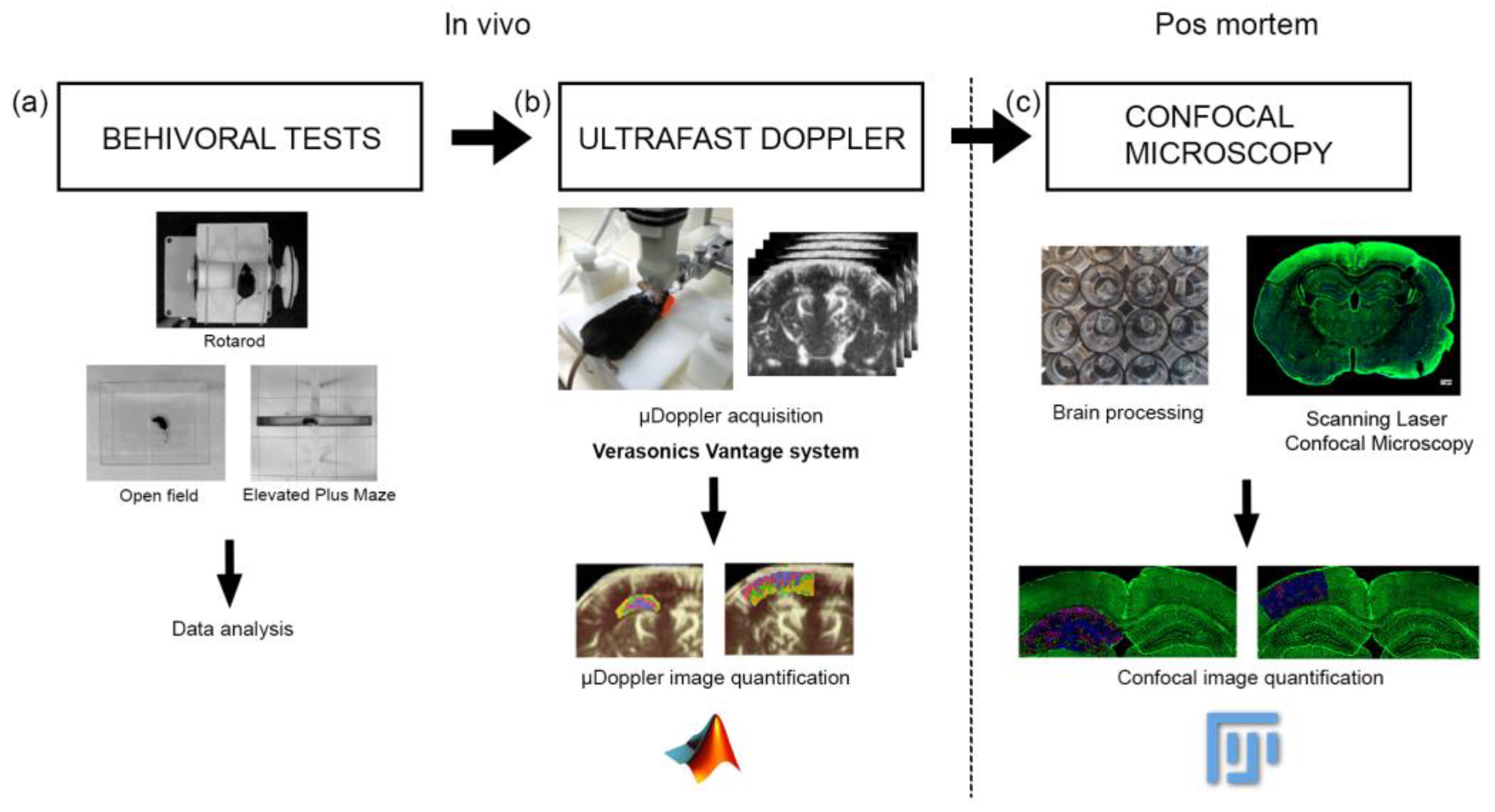
2. Materials and Methods
2.1. Animals
2.2. µDoppler Images Acquisition
2.3. µDoppler Images Analysis
2.4. Brain Processing for Vibratome Sectioning
2.5. Scanning Laser Confocal Microscopy
2.6. Confocal Image Analysis
2.7. Behavioral Tests
2.7.1. Open Field Test
2.7.2. Elevated Plus Maze Test
2.7.3. Rotarod Test
2.8. Statistical Analysis
3. Results
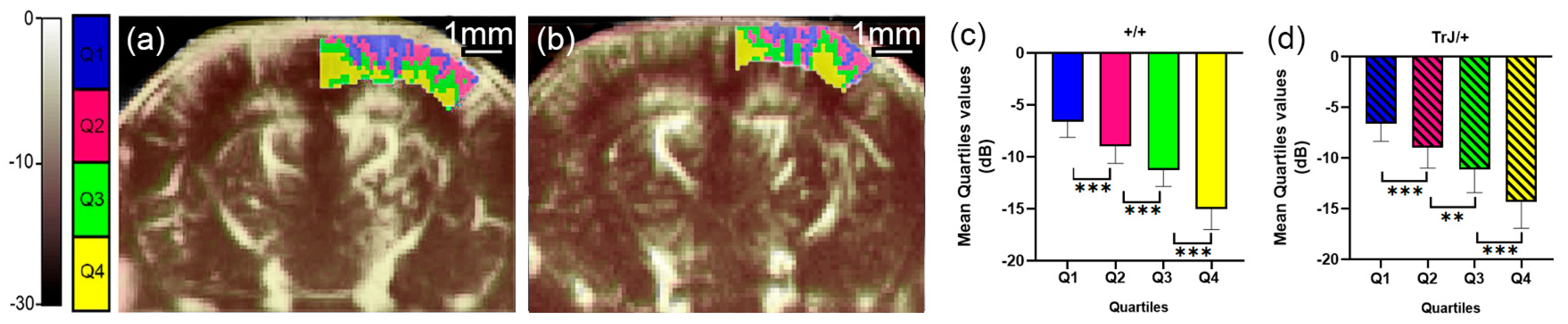
3.1. µDoppler Images Quantification
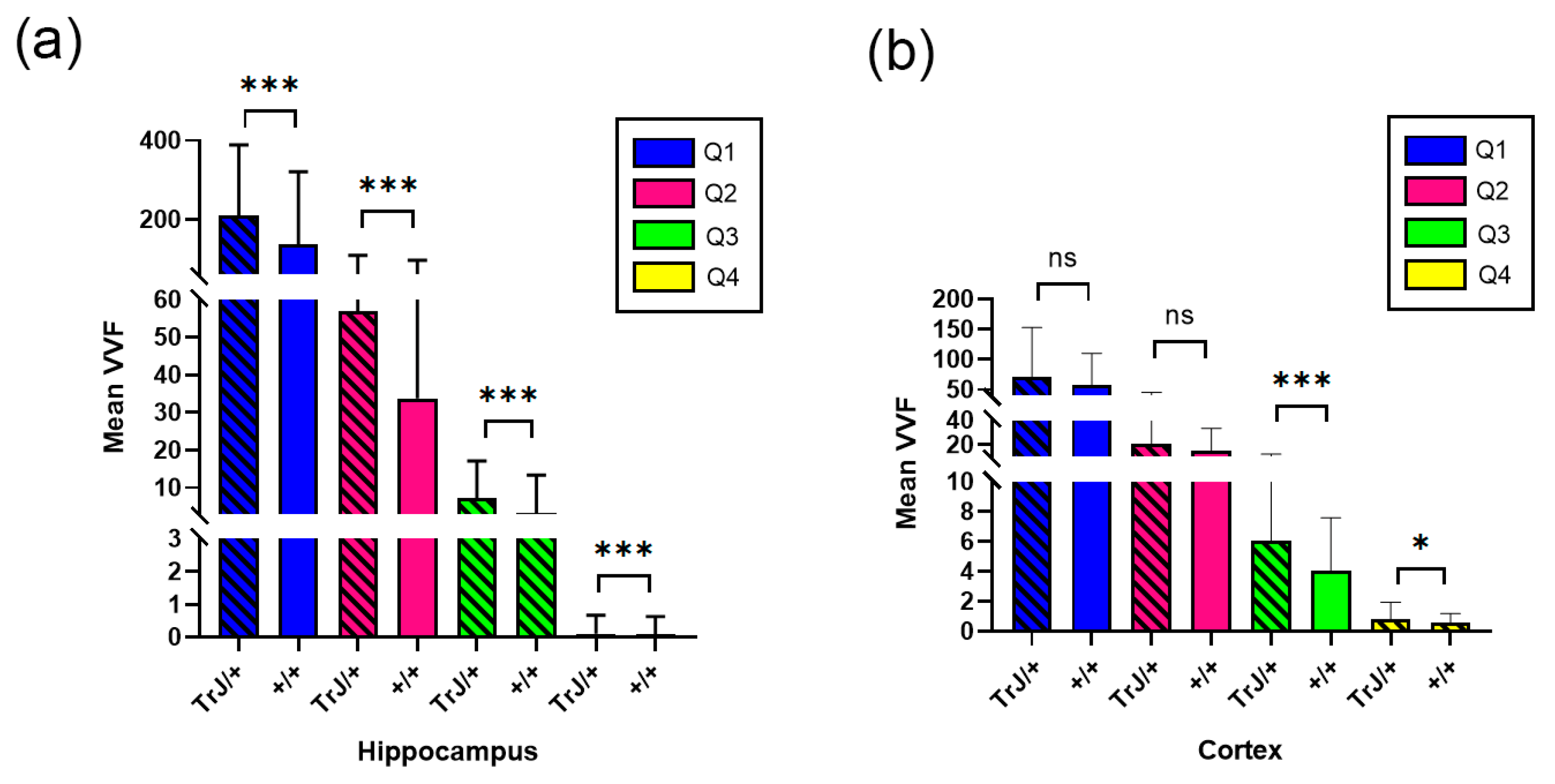
3.2. Confocal Microscopy Vascular Visualization
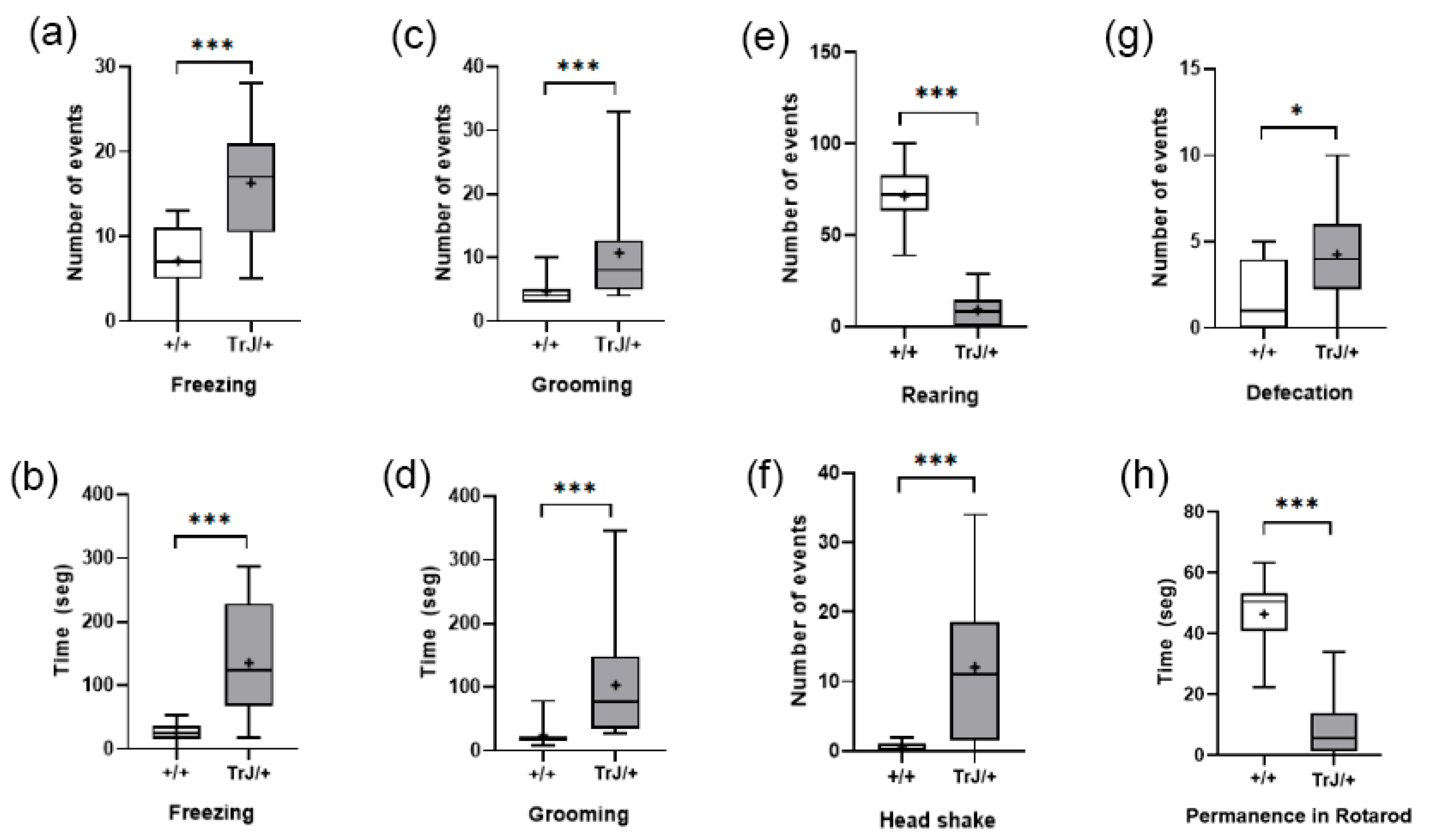
3.3. Behavioral Tests
3.3.1. Elevated Plus Maze
3.3.2. Open Field Test and Rotarod
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stavrou, M.; Kagiava, A.; Sargiannidou, I.; Georgiou, E.; Kleopa, K.A. Charcot-Marie-Tooth Neuropathies: Current Gene Therapy Advances and the Route toward Translation. J. Peripher. Nerv. Syst. 2023, 28, 150–168. [Google Scholar] [CrossRef]
- Fridman, V.; Saporta, M.A. Mechanisms and Treatments in Demyelinating CMT. Neurotherapeutics 2021, 18, 2236–2268. [Google Scholar] [CrossRef]
- Bird, T.D. Charcot-Marie-Tooth (CMT) Hereditary Neuropathy Overview; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Timmerman, V.; Strickland, A.V.; Züchner, S. Genetics of Charcot-Marie-Tooth (CMT) Disease within the Frame of the Human Genome Project Success. Genes 2014, 5, 13. [Google Scholar] [CrossRef]
- Li, J.; Parker, B.; Martyn, C.; Natarajan, C.; Guo, J. The PMP22 Gene and Its Related Diseases. Mol. Neurobiol. 2013, 47, 673–698. [Google Scholar] [CrossRef]
- Higuchi, Y.; Takashima, H. Clinical Genetics of Charcot–Marie–Tooth Disease. J. Hum. Genet. 2022, 68, 199–214. [Google Scholar] [CrossRef]
- Morena, J.; Gupta, A.; Hoyle, J.C. Charcot-Marie-Tooth: From Molecules to Therapy. Int. J. Mol. Sci. 2019, 20, 3419. [Google Scholar] [CrossRef]
- Reilly, M.M.; Murphy, S.M.; Laurá, M. Charcot-Marie-Tooth Disease. J. Peripher. Nerv. Syst. 2011, 16, 1–14. [Google Scholar] [CrossRef]
- Schneider, C.; King, R.M.; Philipson, L. Genes Specifically Expressed at Growth Arrest of Mammalian Cells. Cell 1988, 54, 787–793. [Google Scholar] [CrossRef]
- Manfioletti, G.; Ruaro, M.E.; Del Sal, G.; Philipson, L.; Schneider, C. A Growth Arrest-Specific (Gas) Gene Codes for a Membrane Protein. Mol. Cell. Biol. 1990, 10, 2924–2930. [Google Scholar] [PubMed]
- Spreyer, P.; Kuhn, G.; Hanemann, C.O.; Gillen, C.; Schaal, H.; Kuhn, R.; Lemke Muller, G.H.W. Axon-Regulated Expression of a Schwann Cell Transcript That Is Homologous to a “growth Arrest-Specific” Gene. EMBO J. 1991, 10, 3661. [Google Scholar] [CrossRef]
- Welcher, A.A.; Suter, U.; De Leon, M.; Snipes, G.J.; Shooter, E.M. A Myelin Protein Is Encoded by the Homologue of a Growth Arrest-Specific Gene. Proc. Natl. Acad. Sci. USA 1991, 88, 7195–7199. [Google Scholar] [CrossRef]
- Welcher, A.A.; De Leon, M.; Suter, U.; Snipes, G.J.; Meakin, S.O.; Shooter, E.M. Isolation of Transcriptionally Regulated Sequences Associated with Neuronal and Non-Neuronal Cell Interactions. Prog. Brain Res. 1992, 94, 163–176. [Google Scholar] [CrossRef]
- Snipes, G.J.; Suter, U.; Welcher, A.A.; Shooter, E.M. Characterization of a Novel Peripheral Nervous System Myelin Protein (PMP22/SR13). J. Cell Biol. 1992, 117, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Pareek, S.; Notterpek, L.; Snipes, G.J.; Naef, R.; Sossin, W.; Laliberté, J.; Iacampo, S.; Suter, U.; Shooter, E.M.; Murphy, R.A. Neurons Promote the Translocation of Peripheral Myelin Protein 22 into Myelin. J. Neurosci. 1997, 17, 7754–7762. [Google Scholar] [CrossRef] [PubMed]
- Suter, U.; Patel, P.I. Genetic Basis of Inherited Peripheral Neuropathies. Hum. Mutat. 1994, 3, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Van De Wetering, R.A.C.; Gabreëls-Festen, A.A.W.M.; Kremer, H.; Kalscheuer, V.M.; Gabreëls, F.J.M.; Mariman, E.C. Regulation and Expression of the Murine PMP22 Gene. Mamm. Genome 1999, 10, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Suter, U.; Moskow, J.J.; Welcher, A.A.; Snipes, G.J.; Kosaras, B.; Sidman, R.L.; Buchberg, A.M.; Shooter, E.M. A Leucine-to-Proline Mutation in the Putative First Transmembrane Domain of the 22-KDa Peripheral Myelin Protein in the Trembler-J Mouse. Proc. Natl. Acad. Sci. USA 1992, 89, 4382–4386. [Google Scholar] [CrossRef]
- Bronstein, J.M. Function of Tetraspan Proteins in the Myelin Sheath. Curr. Opin. Neurobiol. 2000, 10, 552–557. [Google Scholar] [CrossRef]
- Hammer, M. An Identified Neuron Mediates the Unconditioned Stimulus in Associative Olfactory Learning in Honeybees. Nature 1993, 366, 59–63. [Google Scholar] [CrossRef]
- Swisshelm, K.; Machl, A.; Planitzer, S.; Robertson, R.; Kubbies, M.; Hosier, S. SEMP1, a Senescence-Associated CDNA Isolated from Human Mammary Epithelial Cells, Is a Member of an Epithelial Membrane Protein Superfamily. Gene 1999, 226, 285–295. [Google Scholar] [CrossRef]
- Zoidl, G.; Blass-Kampmann, S.; D’Urso, D.; Schmalenbach, C.; Müller, H.W. Retroviral-Mediated Gene Transfer of the Peripheral Myelin Protein PMP22 in Schwann Cells: Modulation of Cell Growth. EMBO J. 1995, 14, 1122–1128. [Google Scholar] [CrossRef]
- Bosse, F.; Zoidl, G.; Wilms, S.; Gillen, C.P.; Kuhn, H.G.; Müller, H.W. Differential Expression of Two MRNA Species Indicates a Dual Function of Peripheral Myelin Protein PMP22 in Cell Growth and Myelination. J. Neurosci. Res. 1994, 37, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Parmantier, E.; Cabon, F.; Braun, C.; D’Urso, D.; Müller, H.W.; Zalc, B. Peripheral Myelin Protein-22 Is Expressed in Rat and Mouse Brain and Spinal Cord Motoneurons. Eur. J. Neurosci. 1995, 7, 1080–1088. [Google Scholar] [CrossRef]
- Ohsawa, Y.; Murakami, T.; Miyazaki, Y.; Shirabe, T.; Sunada, Y. Peripheral Myelin Protein 22 Is Expressed in Human Central Nervous System. J. Neurol. Sci. 2006, 247, 11–15. [Google Scholar] [CrossRef]
- Sacco, S.; Totaro, R.; Bastianello, S.; Marini, C.; Carolei, A. Brain White Matter Lesions in an Italian Family with Charcot-Marie-Tooth Disease. Eur. Neurol. 2004, 51, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Koros, C.; Evangelopoulos, M.-E.; Kilidireas, C.; Andreadou, E. Central Nervous System Demyelination in a Charcot-Marie-Tooth Type 1A Patient. Case Rep. Neurol. Med. 2013, 2013, 243652. [Google Scholar] [CrossRef]
- Pontillo, G.; Tozza, S.; Perillo, T.; Cocozza, S.; Dubbioso, R.; Severi, D.; Iodice, R.; Tedeschi, E.; Elefante, A.; Brunetti, A.; et al. Diffuse Brain Connectivity Changes in Charcot–Marie–Tooth Type 1a Patients: A Resting-State Functional Magnetic Resonance Imaging Study. Eur. J. Neurol. 2021, 28, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Tobler, A.; Liu, N.; Mueller, L.; Shooter, E.M. Differential Aggregation of the Trembler and Trembler J Mutants of Peripheral Myelin Protein 22. J. Peripher. Nerv. Syst. 2002, 7, 206–207. [Google Scholar] [CrossRef][Green Version]
- Jouaud, M.; Mathis, S.; Richard, L.; Lia, A.S.; Magy, L.; Vallat, J.M. Rodent Models with Expression of PMP22: Relevance to Dysmyelinating CMT and HNPP. J. Neurol. Sci. 2019, 398, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Quarles, R.H.; Macklin, W.B.; Morell, P. Myelin Structure and Biochemistry. In Basic Neurochemistry; Siegel, G.J., Albers, R.W., Brady, S.T., Price, D.L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2006; pp. 51–71. ISBN 9780123749475. [Google Scholar]
- Opalach, K.; Rangaraju, S.; Madorsky, I.; Leeuwenburgh, C.; Notterpek, L. Lifelong Calorie Restriction Alleviates Age-Related Oxidative Damage in Peripheral Nerves. Rejuvenation Res. 2010, 13, 65–74. [Google Scholar] [CrossRef]
- Rangaraju, S.; Hankins, D.; Madorsky, I.; Madorsky, E.; Lee, W.H.; Carter, C.S.; Leeuwenburgh, C.; Notterpek, L. Molecular Architecture of Myelinated Peripheral Nerves Is Supported by Calorie Restriction with Aging. Aging Cell 2009, 8, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Laurá, M.; Polke, J.M.; Davis, M.B.; Blake, J.; Brandner, S.; Hughes, R.A.C.; Houlden, H.; Bennett, D.L.H.; Lunn, M.P.T.; et al. Variable Phenotypes Are Associated with PMP22 Missense Mutations. Neuromuscul. Disord. 2011, 21, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Notterpek, L.; Tolwani, R.J. Experimental Models of Peripheral Neuropathies. Lab AnimSci 1999, 49, 588–599. [Google Scholar]
- Chin, L.; Olzmann, J.; Li, L. Aggresome Formation and Neurodegenerative Diseases: Therapeutic Implications. Curr. Med. Chem. 2008, 15, 47–60. [Google Scholar] [CrossRef]
- Sinclair, D.A. Toward a Unified Theory of Caloric Restriction and Longevity Regulation. Mech. Ageing Dev. 2005, 126, 987–1002. [Google Scholar] [CrossRef]
- Henry, E.W.; Sidman, R.L. The Murine Mutation Trembler-j: Proof of Semidominant Expression by Use of the Linked Vestigial Tail Marker. J. Neurogenet. 1983, 1, 39–52. [Google Scholar] [CrossRef]
- Robertson, A.M.; Huxley, C.; King, R.H.M.; Thomas, P.K. Development of Early Postnatal Peripheral Nerve Abnormalities in Trembler-J and PMP22 Transgenic Mice. J. Anat. 1999, 195, 331. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, L.J.; Baas, F.; Wolterman, R.A.; Hoogendijk, J.E.; van den Bosch, N.H.A.; Zorn, I.; Gabreëls-Festen, A.A.W.M.; de Visser, M.; Bolhuis, P.A. Identical Point Mutations of PMP–22 in Trembler–J Mouse and Charcot–Marie–Tooth Disease Type 1A. Nat. Genet. 1992, 2, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Rosso, G.; Negreira, C.; Sotelo, J.R.; Kun, A. Myelinating and Demyelinating Phenotype of Trembler-J Mouse (a Model of Charcot-Marie-Tooth Human Disease) Analyzed by Atomic Force Microscopy and Confocal Microscopy. J. Mol. Recognit. 2012, 25, 247–255. [Google Scholar] [CrossRef]
- Kun, A.; Rosso, G.; Canclini, L.; Bresque, M.; Romeo, C.; Cal, K.; Calliari, A.; Hanuz, A.; Roberto, J.; Roberto, J. The Schwann Cell-Axon Link in Normal Condition or Neuro-Degenerative Diseases: An Immunocytochemical Approach. In Applications of Immunocytochemistry; Dehghani, H., Ed.; InTech: London, UK, 2012; pp. 249–266. ISBN 978-953-51-5235-4. [Google Scholar]
- Kun, A.; Canclini, L.; Rosso, G.; Bresque, M.; Romeo, C.; Hanusz, A.; Cal, K.; Calliari, A.; Sotelo Silveira, J.; Sotelo, J.R. F-Actin Distribution at Nodes of Ranvier and Schmidt-Lanterman Incisures in Mammalian Sciatic Nerves. Cytoskeleton 2012, 69, 486–495. [Google Scholar] [CrossRef]
- Lafon Hughes, L.I.; Romeo, C.J.; Cal, K.B.; Vilchez, S.C.; Sotelo Sosa, J.R.; Folle, G.A.; Fernández, S.H.; Kun, A. Poly(ADP-Ribosylation) Is Present in Murine Sciatic Nerve Fibers and Is Altered in a Charcot-Marie-Tooth-1E Neurodegenerative Model. PeerJ 2017, 5, e3318. [Google Scholar] [CrossRef]
- Vázquez Alberdi, L.; Rosso, G.; Velóz, L.; Romeo, C.; Farias, J.; Di Tomaso, M.V.; Calero, M.; Kun, A. Curcumin and Ethanol Effects in Trembler-J Schwann Cell Culture. Biomolecules 2022, 12, 515. [Google Scholar] [CrossRef]
- Di Tomaso, M.V.; Vázquez Alberdi, L.; Olsson, D.; Cancela, S.; Fernández, A.; Rosillo, J.C.; Reyes Ábalos, A.L.; Álvarez Zabaleta, M.; Calero, M.; Kun, A. Colocalization Analysis of Peripheral Myelin Protein-22 and Lamin-B1 in the Schwann Cell Nuclei of Wt and TrJ Mice. Biomolecules 2022, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Damián, J.P.; Vázquez Alberdi, L.; Canclini, L.; Rosso, G.; Bravo, S.O.; Martínez, M.; Uriarte, N.; Ruiz, P.; Calero, M.; Di Tomaso, M.V.; et al. Central Alteration in Peripheral Neuropathy of Trembler-J Mice: Hippocampal Pmp22 Expression and Behavioral Profile in Anxiety Tests. Biomolecules 2021, 11, 601. [Google Scholar] [CrossRef]
- Anzibar Fialho, M.; Vázquez Alberdi, L.; Martínez, M.; Calero, M.; Baranger, J.; Tanter, M.; Damián, J.P.; Negreira, C.; Rubido, N.; Kun, A.; et al. Intensity Distribution Segmentation in Ultrafast Doppler and Correlative Scanning Laser Confocal Microscopy for Assessing Vascular Changes Associated with Ageing in Murine Hippocampi. Sci. Rep. 2022, 12, 6784. [Google Scholar] [CrossRef]
- Hertzog, N.; Jacob, C. Mechanisms and Treatment Strategies of Demyelinating and Dysmyelinating Charcot-Marie-Tooth Disease. Neural Regen. Res. 2023, 18, 1931. [Google Scholar] [CrossRef]
- Bunik, V. The Therapeutic Potential of Vitamins B1, B3 and B6 in Charcot-Marie-Tooth Disease with the Compromised Status of Vitamin-Dependent Processes. Biology 2023, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Bolino, A.; D’Antonio, M. Recent Advances in the Treatment of Charcot-Marie-Tooth Neuropathies. J. Peripher. Nerv. Syst. 2023, 28, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, C.; Pareyson, D. Gene Therapy and Other Novel Treatment Approaches for Charcot-Marie-Tooth Disease. Neuromuscul. Disord. 2023, 33, 627–635. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C.; Weiner, M.W.; Aisen, P.; Petersen, R.; Jack, C.R.; Jagust, W.; et al. Early Role of Vascular Dysregulation on Late-Onset Alzheimer’s Disease Based on Multifactorial Data-Driven Analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef]
- Iadecola, C.; Smith, E.E.; Anrather, J.; Gu, C.; Mishra, A.; Misra, S.; Perez-Pinzon, M.A.; Shih, A.Y.; Sorond, F.A.; van Veluw, S.J.; et al. The Neurovasculome: Key Roles in Brain Health and Cognitive Impairment: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke 2023, 54, e251–e271. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; Oâ Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary Pericytes Regulate Cerebral Blood Flow in Health and Disease. Nature 2014, 508, 55. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral Blood Flow Regulation and Neurovascular Dysfunction in Alzheimer Disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Wiesmann, M.; Timmer, N.M.; Zinnhardt, B.; Reinhard, D.; Eligehausen, S.; Königs, A.; Ben Jeddi, H.; Dederen, P.J.; Jacobs, A.H.; Kiliaan, A.J. Effect of a Multinutrient Intervention after Ischemic Stroke in Female C57Bl/6 Mice. J. Neurochem. 2018, 144, 549–564. [Google Scholar] [CrossRef]
- Tarawneh, R. Microvascular Contributions to Alzheimer Disease Pathogenesis: Is Alzheimer Disease Primarily an Endotheliopathy? Biomolecules 2023, 13, 830. [Google Scholar] [CrossRef]
- Tegeder, I. Nitric Oxide Mediated Redox Regulation of Protein Homeostasis. Cell. Signal. 2019, 53, 348–356. [Google Scholar] [CrossRef]
- Marzetti, E.; Csiszar, A.; Dutta, D.; Balagopal, G.; Calvani, R.; Leeuwenburgh, C. Role of Mitochondrial Dysfunction and Altered Autophagy in Cardiovascular Aging and Disease: From Mechanisms to Therapeutics. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, 459–476. [Google Scholar] [CrossRef]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)Ever after: Aging in the Context of Oxidative Stress, Proteostasis Loss and Cellular Senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef]
- Eisenmenger, L.B.; Peret, A.; Famakin, B.M.; Spahic, A.; Roberts, G.S.; Bockholt, J.H.; Johnson, K.M.; Paulsen, J.S. Vascular Contributions to Alzheimer’s Disease. Transl. Res. 2023, 254, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Charcot, J.M.; Marie, P. Sur Une Form Particulière D′atrophie Musculaire Progressive, Souvant Familiale, Debutant Par Les Pieds et Les Jambes, et Atteignant plus Tard Les Mains. Rev. Méd. 1886, 6, 97–138. [Google Scholar]
- Brooks, A.P. Abnormal Vascular Reflexes in Charcot-Marie-Tooth Disease. J. Neurol. Neurosurg. Psychiatry 1980, 43, 348–350. [Google Scholar] [CrossRef]
- Rosso, G.; Cal, K.; Canclini, L.; Damián, J.P.; Ruiz, P.; Rodríguez, H.; Sotelo, J.R.; Vazquez, C.; Kun, A. Early Phenotypical Diagnoses in Trembler-J Mice Model. J. Neurosci. Methods 2010, 190, 14–19. [Google Scholar] [CrossRef]
- Damián, J.P.; Acosta, V.; Da Cuña, M.; Ramírez, I.; Oddone, N.; Zambrana, A.; Bervejillo, V.; Benech, J.C. Effect of Resveratrol on Behavioral Performance of Streptozotocin-Induced Diabetic Mice in Anxiety Tests. Exp. Anim. 2014, 63, 277–287. [Google Scholar] [CrossRef][Green Version]
- Bouchard, J.; Bedard, P.; Bouchard, R. Study of a Family with Progressive Ataxia, Tremor and Severe Distal Amyotrophy. Can. J. Neurol. Sci. 1980, 7, 345–349. [Google Scholar] [CrossRef]
- Hill, C.M.; Hogan, A.M.; Onugha, N.; Harrison, D.; Cooper, S.; McGrigor, V.J.; Datta, A.; Kirkham, F.J. Increased Cerebral Blood Flow Velocity in Children with Mild Sleep-Disordered Breathing: A Possible Association with Abnormal Neuropsychological Function. Pediatrics 2006, 118, e1100–e1108. [Google Scholar] [CrossRef] [PubMed]
- Deussing, J.M.; Kühne, C.; Pütz, B.; Panhuysen, M.; Breu, J.; Stenzel-Poore, M.P.; Holsboer, F.; Wurst, W. Expression Profiling Identifies the CRH/CRH-R1 System as a Modulator of Neurovascular Gene Activity. J. Cereb. Blood Flow Metab. 2007, 27, 1476–1495. [Google Scholar] [CrossRef]
- Carvalho, C.; Machado, N.; Mota, P.C.; Correia, S.C.; Cardoso, S.; Santos, R.X.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I. Type 2 Diabetic and Alzheimer’s Disease Mice Present Similar Behavioral, Cognitive, and Vascular Anomalies. J. Alzheimer’s Dis. 2013, 35, 623–635. [Google Scholar] [CrossRef]
- Nemeth, C.L.; Miller, A.H.; Tansey, M.G.; Neigh, G.N. Inflammatory Mechanisms Contribute to Microembolism-Induced Anxiety-like and Depressive-like Behaviors. Behav. Brain Res. 2016, 303, 160–167. [Google Scholar] [CrossRef]




| Genotype | Behavioral Tests | µDoppler | Confocal Microscopy |
|---|---|---|---|
| +/+ | 12 | 12 | 4 |
| TrJ/+ | 12 | 12 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Barreiro, M.; Vázquez Alberdi, L.; De León, L.; Avellanal, G.; Duarte, A.; Anzibar Fialho, M.; Baranger, J.; Calero, M.; Rubido, N.; Tanter, M.; et al. In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice. Biology 2023, 12, 1324. https://doi.org/10.3390/biology12101324
Martínez Barreiro M, Vázquez Alberdi L, De León L, Avellanal G, Duarte A, Anzibar Fialho M, Baranger J, Calero M, Rubido N, Tanter M, et al. In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice. Biology. 2023; 12(10):1324. https://doi.org/10.3390/biology12101324
Chicago/Turabian StyleMartínez Barreiro, Mariana, Lucia Vázquez Alberdi, Lucila De León, Guadalupe Avellanal, Andrea Duarte, Maximiliano Anzibar Fialho, Jérôme Baranger, Miguel Calero, Nicolás Rubido, Mickael Tanter, and et al. 2023. "In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice" Biology 12, no. 10: 1324. https://doi.org/10.3390/biology12101324
APA StyleMartínez Barreiro, M., Vázquez Alberdi, L., De León, L., Avellanal, G., Duarte, A., Anzibar Fialho, M., Baranger, J., Calero, M., Rubido, N., Tanter, M., Negreira, C., Brum, J., Damián, J. P., & Kun, A. (2023). In Vivo Ultrafast Doppler Imaging Combined with Confocal Microscopy and Behavioral Approaches to Gain Insight into the Central Expression of Peripheral Neuropathy in Trembler-J Mice. Biology, 12(10), 1324. https://doi.org/10.3390/biology12101324






