Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. Chemical Analyses
2.3. DNA Extraction and the 16S rRNA Gene Library Preparation and Sequencing
2.4. Metagenome Sequencing and MAGs Assembly
2.5. Media Composition for the Enumeration and Isolation of Prokaryotes
2.6. Nucleotide Sequence Accession Number
3. Results
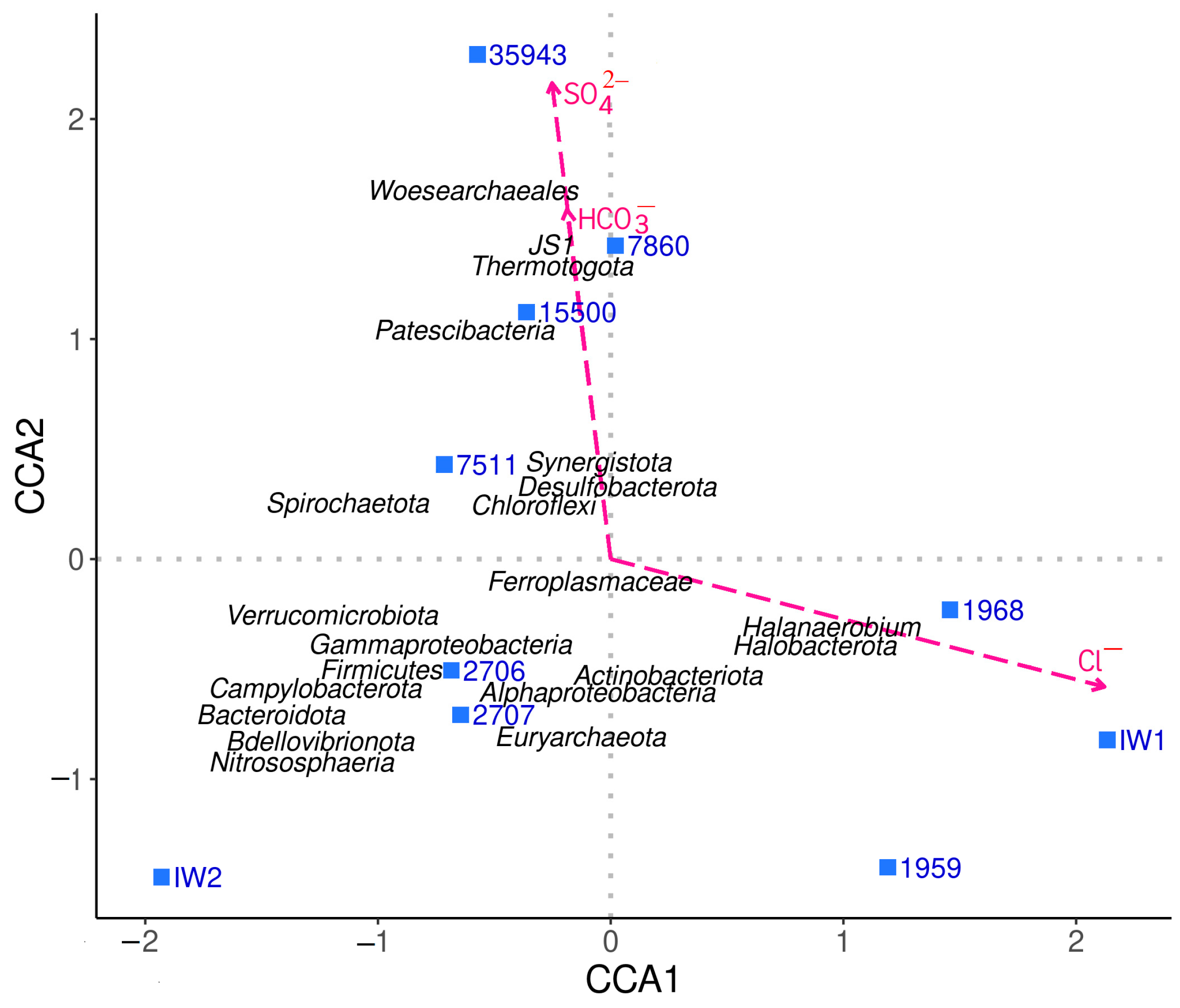
3.1. Formation Water Chemistry and Abundance of Cultivable Microorganisms
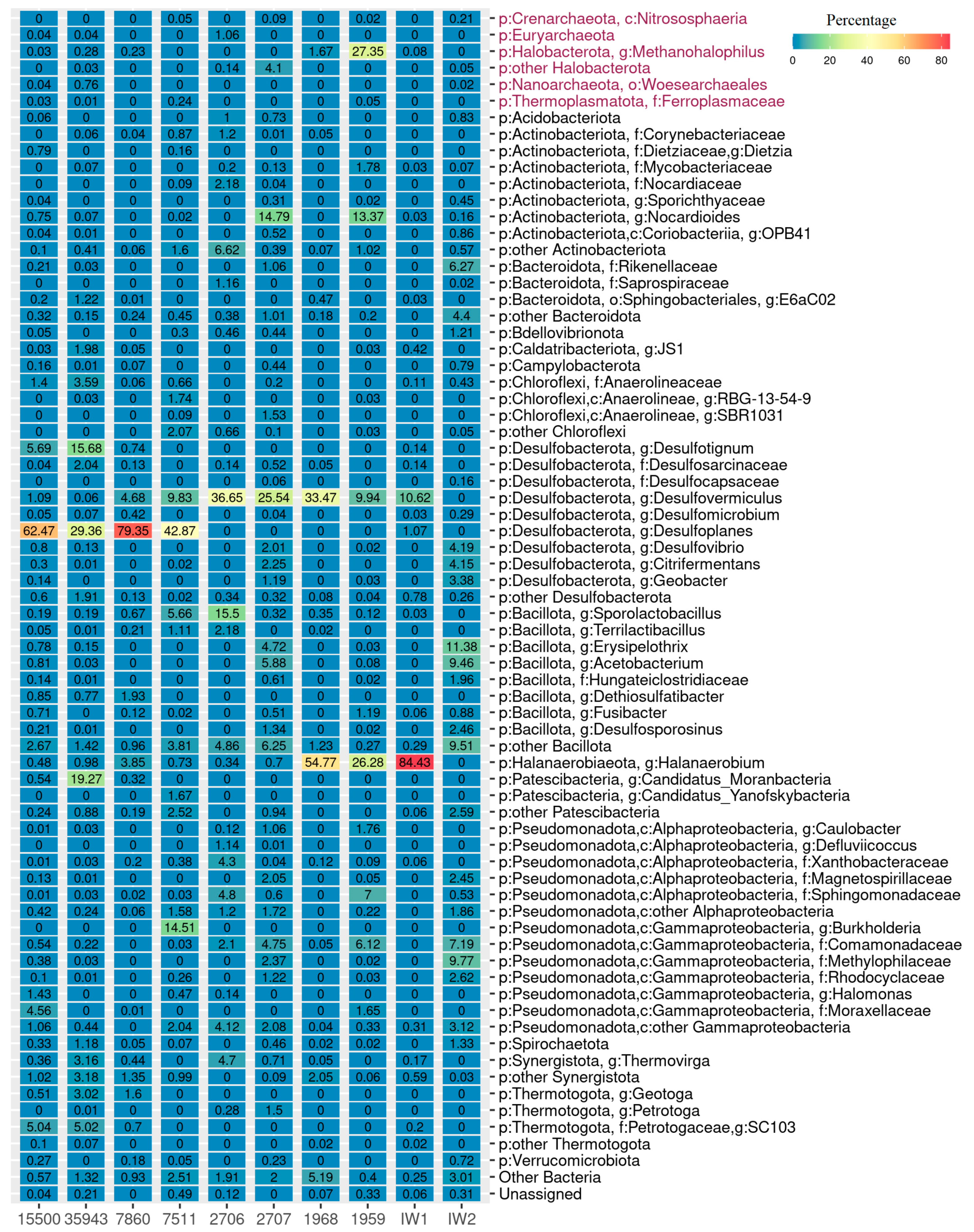
3.2. Microbial Community Composition Based on 16S rRNA Gene Metabarcoding
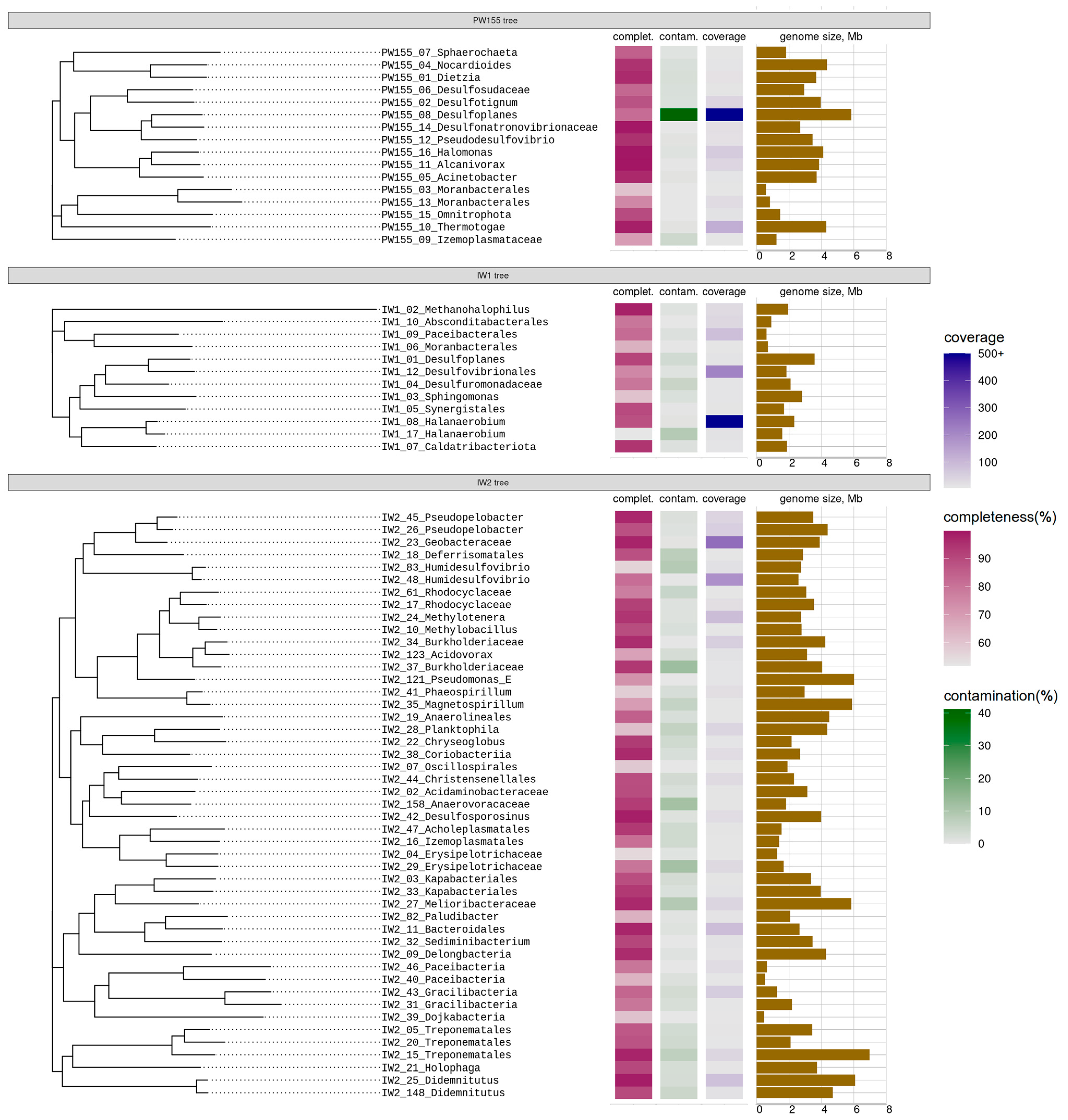
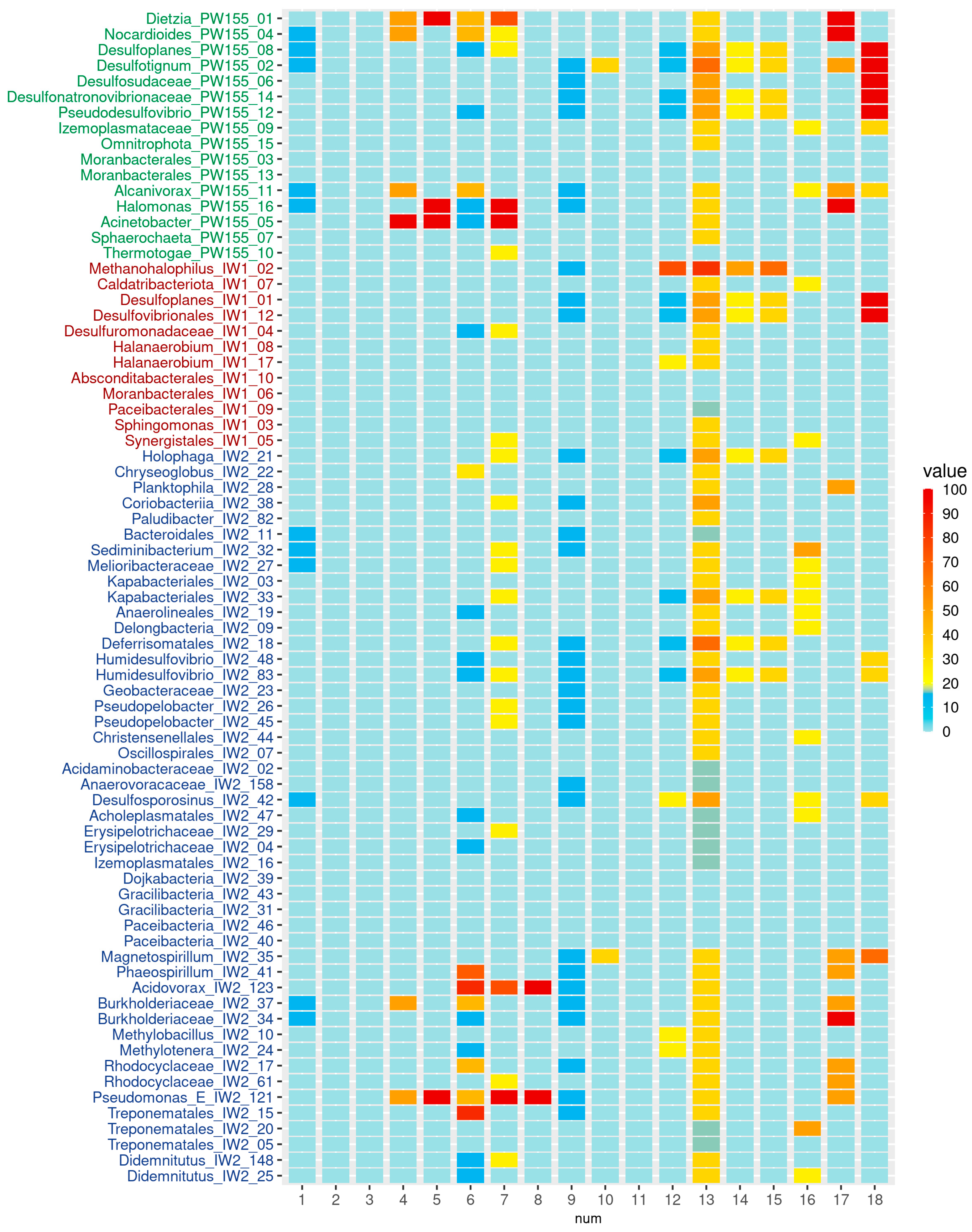
3.3. Genomes Assembled from Microbial Communities’ Metagenomes
3.4. The Genes Responsible for Hydrocarbon Degradation, Osmoprotection, and Sulfur Metabolism Revealed in MAGs
3.5. Diversity of Fermentative Enrichments and Production of Oil-Displacing Compounds
3.6. Influence of Nitrate on Sulfidogenesis by Anaerobic Enrichments
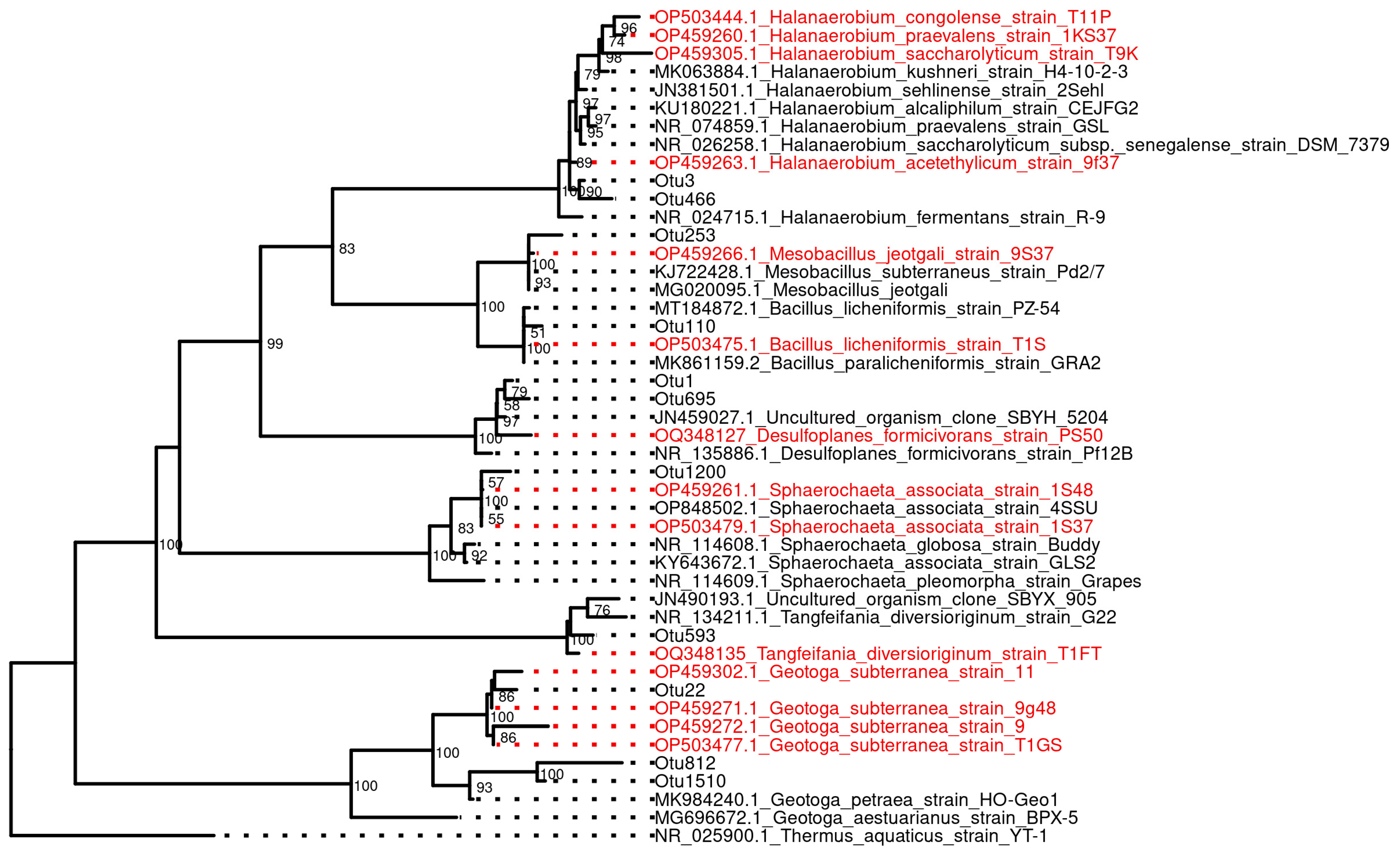
3.7. Isolation and Characterization of Pure Cultures from Oil Fields
4. Discussion
4.1. Microbial Diversity in Oil Fields with Highly Saline Formation Water
4.2. MAG-based Functional Predictions and Their Correspondence to the Features of Isolates
4.3. Biotechnological Potential of Prokaryotes in High-Salinity Petroleum Reservoirs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gieg, L.M.; Jack, T.R.; Foght, J.M. Biological souring and mitigation in oil reservoirs. Appl. Microbiol. Biotechnol. 2011, 92, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Head, I.M.; Gray, N.D.; Larter, S.R. Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Front. Microbiol. 2014, 5, 566. [Google Scholar] [CrossRef] [PubMed]
- Wilhelms, A.; Larter, S.R.; Head, I.; Farrlmond, P.; di-Primio, R.; Zwach, C. Biodegradation of oil in uplifted basins prevented by deep-burial sterilization. Lett. Nat. 2001, 411, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Magot, M.; Ollivier, B.; Patel, B.K.C. Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek 2000, 77, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Grizzle, R.S.; Duncan, K.E.; McInerney, M.J.; Suflita, J.M. Roles of thermophilic thiosulfate-reducing bacteria and methanogenic archaea in the biocorrosion of oil pipelines. Front. Microbiol. 2014, 5, 89. [Google Scholar] [CrossRef]
- Davidova, I.A.; Harmsen, H.J.; Stams, A.J.; Belyaev, S.S.; Zehnder, A.J.B. Taxonomic description of Methanococcoides euhalobius and its transfer to the Methanohalophilus genus. Antonie Van Leeuwenhoek 1997, 71, 313–318. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Hu, B.; Dolfing, J.; Li, Y.; Tang, Y.-Q.; Jiang, Y.; Chi, C.-Q.; Xing, J.; Nie, Y.; Wu, X.-L. Thermodynamically favorable reactions shape the archaeal community affecting bacterial community assembly in oil reservoirs. Sci. Total Environ. 2021, 781, 146506. [Google Scholar] [CrossRef]
- An, D.; Caffrey, S.M.; Soh, J.; Agrawal, A.; Brown, D.; Budwill, K.; Dong, X.; Dunfield, P.F.; Foght, J.; Gieg, L.M. Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environ. Sci. Technol. 2013, 47, 10708–10717. [Google Scholar] [CrossRef]
- Nie, Y.; Zhao, J.-Y.; Tang, Y.-Q.; Guo, P.; Yang, Y.; Wu, X.-L.; Zhao, F. Species divergence vs. functional convergence characterizes crude oil microbial community assembly. Front. Microbiol. 2016, 7, 1254. [Google Scholar] [CrossRef]
- Liu, Y.F.; Galzerani, D.D.; Mbadinga, S.M.; Zaramela, L.S.; Gu, J.D.; Mu, B.Z.; Zengler, K. Metabolic capability and in situ activity of microorganisms in an oil reservoir. Microbiome 2018, 6, 5. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Yu, L.; Huang, L.; Xiu, J.; Lin, W.; Zhang, Y. Characterizing the microbiome in petroleum reservoir flooded by different water sources. Sci. Total Environ. 2019, 653, 872–885. [Google Scholar] [CrossRef]
- Song, Z.; Chen, S.; Zhao, F.; Zhu, W. Whole metagenome of injected and produced fluids reveal the heterogenetic characteristics of the microbial community in a water-flooded oil reservoir. J. Pet. Sci. Eng. 2019, 176, 1198–1207. [Google Scholar] [CrossRef]
- Jiao, Y.; An, L.; Wang, W.; Ma, J.; Wu, C.; Wu, X. Microbial communities and their roles in the Cenozoic sulfurous oil reservoirs in the Southwestern Qaidam Basin, Western China. Sci. Rep. 2023, 13, 7988. [Google Scholar] [CrossRef]
- Hu, P.; Tom, L.; Singh, A.; Thomas, B.C.; Baker, B.J.; Piceno, Y.M.; Andersen, G.L.; Banfield, J.F. Genome-resolved metagenomic analysis reveals roles for candidate phyla and other microbial community members in biogeochemical transformations in oil reservoirs. mBio 2016, 7, e01669-15. [Google Scholar] [CrossRef]
- Vigneron, A.; Alsop, E.B.; Lomans, B.P.; Kyrpides, N.C.; Head, I.M.; Tsesmetzis, N. Succession in the petroleum reservoir microbiome through an oil field production lifecycle. ISME J. 2017, 11, 2141–2154. [Google Scholar] [CrossRef]
- Sierra-Garcia, I.N.; Dellagnezze, B.M.; Santos, V.P.; Chaves B, M.R.; Capilla, R.; Santos Neto, E.V.; Gray, N.; Oliveira, V.M. Microbial diversity in degraded and non-degraded petroleum samples and comparison across oil reservoirs at local and global scales. Extremophiles 2017, 21, 211–229. [Google Scholar] [CrossRef]
- Hidalgo, K.J.; Sierra-Garcia, I.N.; Zafra, G.; de Oliveira, V.M. Genome-resolved meta-analysis of the microbiome in oil reservoirs worldwide. Microorganisms 2021, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, B.L.; Mbadinga, S.M.; Liu, J.F.; Gu, J.D.; Mu, B.Z. Functional genes (dsr) approach reveals similar sulphidogenic prokaryotes diversity but different structure in saline waters from corroding high temperature petroleum reservoirs. Appl. Microbiol. Biotechnol. 2014, 98, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Tian, H.; Wang, Y.; Li, Y.; Li, Y.; Xie, J.; Zeng, B.; Zhou, J.; Li, G.; Ma, T. Spatial isolation and environmental factors drive distinct bacterial and archaeal communities in different types of petroleum reservoirs in China. Sci. Rep. 2016, 6, 20174. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Sun, S.S.; Zhang, Z.Z.; Wang, J.M.; Qiu, L.W.; Sun, H.Y.; Song, Z.Z.; Zhang, B.Y.; Gao, D.L.; Zhang, G.Q. Analysis of bacterial diversity in two oil blocks from two low-permeability reservoirs with high salinities. Sci. Rep. 2016, 6, 19600. [Google Scholar] [CrossRef]
- Pereira, G.F.; Pilz-Junior, H.L.; Corção, G. The impact of bacterial diversity on resistance to biocides in oilfields. Sci. Rep. 2021, 11, 23027. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, G.; Hubert, C.R.J.; Enning, D.R.; Lahme, S.; Mand, J.; de Rezende, J.R. Metagenomic investigation of a low diversity, high salinity offshore oil reservoir. Microorganisms 2021, 9, 2266. [Google Scholar] [CrossRef] [PubMed]
- Piceno, Y.M.; Reid, F.C.; Tom, L.M.; Conrad, M.E.; Bill, M.; Hubbard, C.G.; Fouke, B.W.; Graff, C.J.; Han, J.; Stringfellow, W.T.; et al. Temperature and injection water source influence microbial community structure in four Alaskan North Slope hydrocarbon reservoirs. Front. Microbiol. 2014, 5, 409. [Google Scholar] [CrossRef]
- Li, X.X.; Mbadinga, S.M.; Liu, J.F.; Zhou, L.; Yang, S.Z.; Gu, J.D.; Mu, B.Z. Microbiota and their affiliation with physiochemical characteristics of different subsurface petroleum reservoirs. Int. Biodeterior. Biodegrad. 2017, 120, 170–185. [Google Scholar] [CrossRef]
- Sokolova, D.S.; Semenova, E.M.; Grouzdev, D.S.; Bidzhieva, S.K.; Babich, T.L.; Loiko, N.G.; Ershov, A.P.; Kadnikov, V.V.; Beletsky, A.V.; Mardanov, A.V.; et al. Sulfidogenic microbial communities of the Uzen high-temperature oil field in Kazakhstan. Microorganisms 2021, 9, 1818. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, J.; Liu, Q.; Zhang, Q.; Wu, J.; Zhou, M.; Nie, Y.; Wu, X.-L. pH and nitrate drive bacterial diversity in oil reservoirs at a localized geographic scale. Microorganisms 2023, 11, 151. [Google Scholar] [CrossRef]
- Orphan, V.J.; Taylor, L.T.; Hafenbradl, D.; Delong, E.F. Culture-dependent and culture-independent characterization of microbial assemblages associated with high-temperature petroleum reservoirs. Appl. Environ. Microbiol. 2000, 66, 700–711. [Google Scholar] [CrossRef]
- Grabowski, A.; Nercessian, O.; Fayolle, F.; Blanchet, D.; Jeanthon, C. Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol. Ecol. 2005, 54, 427–443. [Google Scholar] [CrossRef]
- Gieg, L.M.; Davidova, I.A.; Duncan, K.E.; Suflita, J.M. Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ. Microbiol. 2010, 12, 3074–3086. [Google Scholar] [CrossRef]
- Nazina, T.N.; Shestakova, N.M.; Semenova, E.M.; Korshunova, A.V.; Kostrukova, N.K.; Tourova, T.P.; Min, L.; Feng, Q.; Poltaraus, A.B. Diversity of metabolically active Bacteria in water-flooded high-temperature heavy oil reservoir. Front. Microbiol. 2017, 8, 707. [Google Scholar] [CrossRef]
- Belyaev, S.S.; Laurinavichus, K.S.; Obraztsova, A.Y.; Gorlatov, S.N.; Ivanov, M.V. Microbiological processes in the near-bottom zone of injection wells of oil fields. Microbiology 1982, 51, 997–1001. [Google Scholar]
- Ivanov, M.V.; Belyaev, S.S. Biotechnology of Enhancement of Oil Recovery Based on the Geochemical Activity of Microorganisms (Field Experiments), Microbial Enhancement of Oil Recovery—Recent Advances; Donaldson, E.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 421–432. [Google Scholar]
- Lenchi, N.; İnceoğlu, Ö.; Kebbouche-Gana, S.; Gana, M.L.; Llirós, M.; Servais, P.; García-Armisen, T. Diversity of microbial communities in production and injection waters of Algerian oilfields revealed by 16S rRNA gene amplicon 454 pyrosequencing. PLoS ONE 2013, 8, e66588. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, S.S.; Borzenkov, I.A. Microbial transformation of low-molecular-weight carbon compounds in the deep subsurface. In Biogeochemistry of Global Change; Chapman & Hall: New York, NY, USA; London, UK, 1993; pp. 825–838. [Google Scholar]
- Nazina, T.N.; Shestakova, N.M.; Ivoilov, V.S.; Kostrukova, N.K.; Belyaev, S.S.; Ivanov, M.V. Radiotracer assay of microbial processes in petroleum reservoirs. Adv. Biotech. Microbiol. 2017, 2, 555591. [Google Scholar] [CrossRef]
- Belyaev, S.S.; Borzenkov, I.A.; Nazina, T.N.; Rozanova, E.P.; Glumov, I.F.; Ibatullin, R.R.; Ivanov, M.V. Use of microorganisms in the biotechnology for the enhancement of oil recovery. Microbiology 2004, 73, 590–598. [Google Scholar] [CrossRef]
- Youssef, N.; Elshahed, M.S.; McInerney, M.J. Microbial processes in oil fields: Culprits, problems and opportunities. Adv. Appl. Microbiol. 2009, 66, 141–251. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ji, K.; Zhang, Y.; Liu, X.; Dai, X.; Zhi, B.; Cao, Y.; Liu, D.; Wu, M.; Li, G.; et al. Microbial enhanced oil recovery through deep profile control using a conditional bacterial cellulose-producing strain derived from Enterobacter sp. FY-07. Microb. Cell Factories 2020, 19, 59. [Google Scholar] [CrossRef]
- Wu, B.; Xiu, J.; Yu, L.; Huang, L.; Yi, L.; Ma, Y. Research advances of microbial enhanced oil recovery. Heliyon 2022, 8, e11424. [Google Scholar] [CrossRef]
- Ivanov, M.V.; Belyaev, S.S.; Borzenkov, I.A.; Glumov, I.F.; Ibatullin, R.R. Additional oil production during field trials in Russia. Dev. Pet. Sci. 1993, 39, 373–381. [Google Scholar]
- Nazina, T.N.; Ivanova, A.E.; Ivoilov, V.S.; Miller, Y.M.; Kandaurova, G.F.; Ibatullin, R.R.; Belyaev, S.S.; Ivanov, M.V. Results of the trial of the microbiological method for the enhancement of oil recovery at the carbonate collector of the Romashkinskoe oil field: Biogeochemical and production characteristics. Microbiology 1999, 68, 222–226. [Google Scholar]
- Nazina, T.; Sokolova, D.; Grouzdev, D.; Semenova, E.; Babich, T.; Bidzhieva, S.; Serdukov, D.; Volkov, D.; Bugaev, K.; Ershov, A.; et al. The potential application of microorganisms for sustainable petroleum recovery from heavy oil reservoirs. Sustainability 2020, 12, 15. [Google Scholar] [CrossRef]
- Nazina, T.N.; Shestakova, N.M.; Pavlova, N.K.; Tatarkin, Y.V.; Ivoilov, V.S.; Khisametdinov, M.R.; Sokolova, D.S.; Babich, T.L.; Tourova, T.P.; Poltaraus, A.B.; et al. Functional and phylogenetic microbial diversity in formation waters of a low-temperature carbonate petroleum reservoir. Int. Biodeterior. Biodegradation 2013, 81, 71–81. [Google Scholar] [CrossRef]
- Kadnikov, V.V.; Frank, Y.A.; Mardanov, A.V.; Beletskii, A.V.; Ivasenko, D.A.; Pimenov, N.V.; Karnachuk, O.V.; Ravin, N.V. Uncultured bacteria and methanogenic archaea predominate in the microbial community of Western Siberian deep subsurface aquifer. Microbiology 2017, 86, 412–415. [Google Scholar] [CrossRef]
- Semenova, E.M.; Ershov, A.P.; Sokolova, D.S.; Tourova, T.P.; Nazina, T.N. Diversity and biotechnological potential of nitrate-reducing bacteria from heavy-oil reservoirs (Russia). Microbiology 2020, 89, 685–696. [Google Scholar] [CrossRef]
- Trüper, H.G.; Schlegel, H.G. Sulfur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Van Leeuwenhoek 1964, 30, 321–323. [Google Scholar] [CrossRef]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 7, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kang, D.D.; Froula, J.; Egan, R.; Wang, Z. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 2015, 3, e1165. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Simmons, B.A.; Singer, S.W. MaxBin 2.0: An automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 2016, 32, 605–607. [Google Scholar] [CrossRef]
- Alneberg, J.; Bjarnason, B.S.; de Bruijn, I.; Schirmer, M.; Quick, J.; Ijaz, U.Z.; Lahti, L.; Loman, N.J.; Andersson, A.F.; Quince, C. Binning metagenomics contigs by coverage and composition. Nat. Methods 2014, 11, 1144–1146. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2020, 36, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.A.; Hugenholtz, P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018, 36, 996–1004. [Google Scholar] [CrossRef]
- Palù, M.; Basile, A.; Zampieri, G.; Treu, L.; Rossi, A.; Morlino, M.S.; Campanaro, S. KEMET–A python tool for KEGG Module evaluation and microbial genome annotation expansion. Comput. Struct. Biotechnol. J. 2022, 20, 1481–1486. [Google Scholar] [CrossRef]
- Koch, A.L. Most probable numbers. In Methods for General and Molecular Bacteriology; Gerhardt, P., Murray, R.G.E., Wood, W.A., Krieg, N.R., Eds.; American Society for Microbiology: Washington, DC, USA, 1994; pp. 257–260. [Google Scholar]
- Pfennig, N.; Lippert, K.D. Über das vitamin B12—Bedürfnis phototropher Schweferelbakterien. Arch. Microbiol. 1966, 55, 245–256. [Google Scholar]
- Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Formation of methane by bacterial extracts. J. Biol. Chem. 1963, 238, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Bidzhieva, S.K.; Sokolova, D.S.; Grouzdev, D.S.; Kostrikina, N.A.; Poltaraus, A.B.; Tourova, T.P.; Shcherbakova, V.A.; Troshina, O.Y.; Nazina, T.N. Sphaerochaeta halotolerans sp. nov., a novel spherical halotolerant spirochete from a Russian heavy oil reservoir, emended description of the genus Sphaerochaeta, reclassification of Sphaerochaeta coccoides to a new genus Parasphaerochaeta gen. nov. as Parasphaerochaeta coccoides comb. nov. and proposal of Sphaerochaetaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 4748–4759. [Google Scholar] [CrossRef] [PubMed]
- Jezbera, J.; Sharma, A.K.; Brandt, U.; Doolittle, W.F.; Hahn, M.W. ‘Candidatus Planktophila limnetica’, an actinobacterium representing one of the most numerically important taxa in freshwater bacterioplankton. Int. J. Syst. Evol. Microbiol. 2009, 59, 2864–2869. [Google Scholar] [CrossRef][Green Version]
- Lopera, J.; Miller, I.J.; McPhail, K.L.; Kwan, J.C. Increased biosynthetic gene dosage in a genome-reduced defensive bacterial symbiont. mSystems 2017, 2, e00096-17. [Google Scholar] [CrossRef]
- Eisenberg, T.; Muhldorfer, K.; Erhard, M.; Fawzy, A.; Kehm, S.; Ewers, C.; Semmler, T.; Blom, J.; Lipski, A.; Rau, J.; et al. Erysipelothrix anatis sp. nov., Erysipelothrix aquatica sp. nov. and Erysipelothrix urinaevulpis sp. nov., three novel species of the genus, and emended description of Erysipelothrix. Int. J. Syst. Evol. Microbiol. 2022, 72, 005454. [Google Scholar] [CrossRef]
- Gregson, B.H.; Metodieva, G.; Metodiev, M.V.; McKew, B.A. Differential protein expression during growth on linear versus branched alkanes in the obligate marine hydrocarbon-degrading bacterium Alcanivorax borkumensis SK2T. Environ. Microbiol. 2019, 21, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.T.; Li, M.S.M.; McDowell, T.; MacDonald, J.; Yuan, Z.C. Characterization and genomic analysis of a diesel-degrading bacterium, Acinetobacter calcoaceticus CA16, isolated from Canadian soil. BMC Biotechnol. 2020, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Malathi, M.; Devi, P.R. Characterization of Dietzia maris AURCCBT01 from oil-contaminated soil for biodegradation of crude oil. 3 Biotech. 2021, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Mitzscherling, J.; MacLean, J.; Lipus, D.; Bartholomäus, A.; Mangelsdorf, K.; Lipski, A.; Roddatis, V.; Liebner, S.; Wagner, D. Nocardioides alcanivorans sp. nov., a novel hexadecane-degrading species isolated from plastic waste. Int. J. Syst. Evol. Bacteriol. 2022, 72, 005319. [Google Scholar] [CrossRef]
- Bødtker, G.; Lysnes, K.; Torsvik, T.; Bjørnestad, E.Ø.; Sunde, E. Microbial analysis of backflowed injection water from a nitrate-treated North Sea oil reservoir. J. Ind. Microbiol. Biotechnol. 2009, 36, 439–450. [Google Scholar] [CrossRef]
- Gittel, A.; Sorensen, K.B.; Skovhus, T.L.; Ingvorsen, K.; Schramm, A. Prokaryotic community structure and activity of sulfate reducers in production water from high-temperature oil reservoirs with and without nitrate treatment. Appl. Environ. Microbiol. 2009, 75, 7086–7096. [Google Scholar] [CrossRef]
- Fida, T.T.; Gassara, F.; Voordouw, G. Biodegradation of isopropanol and acetone under denitrifying conditions by Thauera sp. TK001 for nitrate-mediated microbially enhanced oil recovery. J. Hazard. Mater. 2017, 334, 68. [Google Scholar] [CrossRef]
- An, B.A.; Shen, Y.; Voordouw, G. Control of sulfide production in high salinity Bakken Shale oil reservoirs by halophilic bacteria reducing nitrate to nitrite. Front. Microbiol. 2017, 8, 1164. [Google Scholar] [CrossRef]
- Watanabe, M.; Kojima, H.; Fukui, M. Desulfoplanes formicivorans gen. nov., sp. nov., a novel sulfate-reducing bacterium isolated from a blackish meromictic lake, and emended description of the family Desulfomicrobiaceae. Int. J. Syst. Evol. Microbiol. 2015, 65, 1902–1907, Erratum in Int. J. Syst. Evol. Microbiol. 2015, 65, 2775.. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.M.; Goulart, F.R.V.; Marques, J.M.; Bizzo, H.R.; Blank, A.F.; Groposo, C.; Sousa, M.P.; Vólaro, V.; Alviano, C.S.; Moreno, D.S.A.; et al. Growth inhibition of sulfate-reducing bacteria in produced water from the petroleum industry using essential oils. Molecules 2017, 22, 648. [Google Scholar] [CrossRef]
- Kuever, J.; Konneke, M.; Galushko, A.; Drzyzga, O. Reclassification of Desulfobacterium phenolicum as Desulfobacula phenolica comb. nov. and description of strain SaxT as Desulfotignum balticum gen. nov., sp. nov. Int. J. Syst. Evol. Bacteriol. 2001, 51, 171–177. [Google Scholar] [CrossRef][Green Version]
- Schink, B.; Thiemann, V.; Laue, H.; Friedrich, M.W. Desulfotignum phosphitoxidans sp. nov., a new marine sulfate reducer that oxidizes phosphite to phosphate. Arch. Microbiol. 2002, 177, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Ommedal, H.; Torsvik, T. Desulfotignum toluenicum sp. nov., a novel toluene-degrading, sulphate-reducing bacterium isolated from an oil-reservoir model column. Int. J. Syst. Evol. Microbiol. 2007, 57, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Liu, J.-F.; Yao, F.; Wu, W.-L.; Yang, S.-Z.; Mbadinga, S.M.; Gu, J.-D.; Mu, B.Z. Dominance of Desulfotignum in sulfate-reducing community in high sulfate production-water of high temperature and corrosive petroleum reservoirs. Int. Biodeterior. Biodegrad. 2016, 114, 45–56. [Google Scholar] [CrossRef]
- Belyakova, E.V.; Rozanova, E.P.; Borzenkov, I.A.; Tourova, T.P.; Pusheva, M.A.; Lysenko, A.M.; Kolganova, T.V. The new facultatively chemolithoautotrophic, moderately halophilic, sulfate-reducing bacterium Desulfovermiculus halophilus gen. nov., sp. nov., isolated from an oil field. Microbiology 2006, 75, 161–171. [Google Scholar] [CrossRef]
- Davey, M.E.; Wood, W.A.; Key, R.; Nakamura, K.; Stahl, D.A. Isolation of three species of Geotoga and Petrotoga: Two new genera, representing a new lineage in the bacterial line of descent distantly related to the “Thermotogales”. Syst. Appl. Microbiol. 1993, 16, 191–200. [Google Scholar] [CrossRef]
- Liang, R.; Davidova, I.A.; Marks, C.R.; Stamps, B.W.; Harriman, B.H.; Stevenson, B.S.; Duncan, K.E.; Suflita, J.M. Metabolic capability of a predominant Halanaerobium sp. in hydraulically fractured gas wells and its implication in pipeline corrosion. Front. Microbiol. 2016, 7, 988. [Google Scholar] [CrossRef]
- Booker, A.E.; Borton, M.A.; Daly, R.A.; Welch, S.A.; Nicora, C.D.; Hoyt, D.W.; Wilson, T.; Purvine, S.O.; Wolfe, R.A.; Sharma, S.; et al. Sulfide generation by dominant Halanaerobium microorganisms in hydraulically fractured shales. mSphere 2017, 2, e00257–e00317. [Google Scholar] [CrossRef]
- Semenova, E.M.; Grouzdev, D.S.; Tourova, T.P.; Nazina, T.N. Physiology and genomic characteristics of Geotoga petraea, a bacterium isolated from a low-temperature oil reservoir (Russia). Microbiology 2019, 88, 662–670. [Google Scholar] [CrossRef]
- Oren, A. “Halanaerobium” in Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–8. [Google Scholar] [CrossRef]
- Davis, J.P.; Struchtemeyer, C.G.; Elshahed, M.S. Bacterial communities associated with production facilities of two newly drilled thermogenic natural gas wells in the Barnett Shale (Texas, USA). Microb. Ecol. 2012, 64, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Murali Mohan, A.; Hartsock, A.; Bibby, K.J.; Hammack, R.W.; Vidic, R.D.; Gregory, K.B. Microbial community changes in hydraulic fracturing fluids and produced water from shale gas extraction. Environ. Sci. Technol. 2013, 47, 13141–13150. [Google Scholar] [CrossRef] [PubMed]
- Cluff, M.A.; Hartsock, A.; Macrae, J.D.; Carter, K.; Mouser, P.J. Temporal changes in microbial ecology and geochemistry in produced water from hydraulically fractured marcellus shale gas wells. Environ. Sci. Technol. 2014, 48, 6508–6517. [Google Scholar] [CrossRef]
- Bhupathiraju, V.K.; Oren, A.; Sharma, P.K.; Tanner, R.S.; Woese, C.R.; McInerney, M.J. Haloanaerobium salsugo sp. nov., a moderately halophilic, anaerobic bacterium from a subterranean brine. Int. J. Syst. Bacteriol. 1994, 44, 565–572. [Google Scholar] [CrossRef]
- Bhupathiraju, V.K.; McInerney, M.J.; Woese, C.R.; Tanner, R.S. Haloanaerobium kushneri sp. nov., an obligately halophilic, anaerobic bacterium from an oil brine. Int. J. Syst. Bacteriol. 1999, 49, 953–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravot, G.; Magot, M.; Ollivier, B.; Patel, B.K.; Ageron, E.; Grimont, P.A.; Thomas, P.; Garcia, J.L. Haloanaerobium congolense sp. nov., an anaerobic, moderately halophilic, thiosulfate- and sulfur-reducing bacterium from an African oil field. FEMS Microbiol. Lett. 1997, 147, 81–88. [Google Scholar] [CrossRef]
- Tourova, T.P.; Sokolova, D.S.; Semenova, E.M.; Ershov, A.P.; Grouzdev, D.S.; Nazina, T.N. Genomic and physiological characterization of halophilic bacteria of the genera Halomonas and Marinobacter from petroleum reservoirs. Microbiology 2022, 91, 235–248. [Google Scholar] [CrossRef]
- Yang, N.; Ding, R.; Liu, J. Synthesizing glycine betaine via choline oxidation pathway as an osmoprotectant strategy in Haloferacales. Gene 2022, 847, 146886. [Google Scholar] [CrossRef] [PubMed]
- Obrazstova, A.Y.; Shipin, O.V.; Bezrukova, L.V.; Belyaev, S.S. Properties of the coccoid methylotrophic methanogen. Microbiology 1988, 56, 523–527. [Google Scholar]
- Christman, G.D.; León-Zayas, R.I.; Summers, Z.M.; Biddle, J.F. Methanogens within a high salinity oil reservoir from the Gulf of Mexico. Front. Microbiol. 2020, 11, 570714. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Yoshioka, H.; Mochimaru, H.; Meng, X.-Y.; Muramoto, Y.; Usami, J.; Ikeda, H.; Kamagata, Y.; Sakata, S. Methanohalophilus levihalophilus sp. nov., a slightly halophilic, methylotrophic methanogen isolated from natural gas-bearing deep aquifers, and emended description of the genus Methanohalophilus. Int. J. Syst. Evol. Microbiol. 2014, 64, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Wilharm, T.; Zhilina, T.N.; Hummel, P. DNA-DNA hybridization of methylotrophic halophilic methanogenic bacteria and transfer of Methanococcus halophilus VP to the genus Methanohalophilus as Methanohalophilus halophilus comb. nov. Int. J. Syst. Bacteriol. 1991, 41, 558–562. [Google Scholar] [CrossRef][Green Version]
- L’Haridon, S.; Haroun, H.; Corre, E.; Roussel, E.; Chalopin, M.; Pignet, P.; Balière, C.; la Cono, V.; Jebbar, M.; Yakimov, M.; et al. Methanohalophilus profundi sp. nov., a methylotrophic halophilic piezophilic methanogen isolated from a deep hypersaline anoxic basin. Syst. Appl. Microbiol. 2020, 43, 126107. [Google Scholar] [CrossRef]
- Guan, Y.; Ngugi, D.K.; Vinu, M.; Blom, J.; Alam, I.; Guillot, S.; Ferry, J.G.; Stingl, U. Comparative genomics of the genus Methanohalophilus, including a newly isolated strain from Kebrit Deep in the Red Sea. Front. Microbiol. 2019, 10, 839. [Google Scholar] [CrossRef] [PubMed]
| Oil Field, Well | Total Salinity, g L−1 | pH | Content, g L−1 | Acetate, mg L−1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Na+ + K+ | Ca2+ | Mg2+ | Cl− | HCO3− | SO42− | ||||
| Romashkinskoe | |||||||||
| PW15500 * | 75.8533 | 7.55 | 24.0550 | 3.0060 | 1.7020 | 42.5440 | 0.5120 | 4.0343 | 3.2 |
| PW35943 * | 62.5517 | 7.52 | 22.0020 | 1.4030 | 0.608 | 31.9080 | 0.2680 | 6.3627 | 0 |
| Arkhangelskoe | |||||||||
| PW7860 * | 116.3550 | 6.52 | 36.7690 | 6.4130 | 1.8240 | 70.1970 | 0.3840 | 0.7680 | 1.8 |
| IW2 | 192.6470 | 6.2 | 59.5190 | 10.0200 | 4.3780 | 117.7040 | 0.2200 | 0.8060 | 11.0 |
| Novo-Elhovskoe | |||||||||
| PW7511 | 107.2180 | 6.11 | 30.2490 | 7.3590 | 2.9770 | 65.9430 | 0.3900 | 0.3000 | 4.4 |
| PW2706 | 97.6970 | 6.47 | 26.4370 | 8.7900 | 1.9850 | 60.2700 | 0.1950 | 0.0200 | 0 |
| PW2707 | 138.6900 | 6.55 | 38.4240 | 8.8180 | 4.8640 | 86.5050 | 0.0730 | 0.0060 | 7.0 |
| Sabanchinskoe | |||||||||
| IW1 | 165.9610 | 6.55 | 55.0580 | 7.7580 | 2.8230 | 99.8770 | 0.2250 | 0.2250 | 5.5 |
| PW1959 | 156.8480 | 6.73 | 48.3090 | 9.2180 | 2.9180 | 95.7230 | 0.2320 | 0.4480 | 5.9 |
| PW1968 | 157.8584 | 6.52 | 46.1510 | 8.4170 | 5.1070 | 97.4960 | 0.2320 | 0.4554 | 6.1 |
| Standard error, mg L−1 | ±0.1 | ±0.02 | ±0.09 | ±0.09 | ±0.004 | ±0.001 | ±0.02 | ±0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadnikov, V.V.; Ravin, N.V.; Sokolova, D.S.; Semenova, E.M.; Bidzhieva, S.K.; Beletsky, A.V.; Ershov, A.P.; Babich, T.L.; Khisametdinov, M.R.; Mardanov, A.V.; et al. Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential. Biology 2023, 12, 1300. https://doi.org/10.3390/biology12101300
Kadnikov VV, Ravin NV, Sokolova DS, Semenova EM, Bidzhieva SK, Beletsky AV, Ershov AP, Babich TL, Khisametdinov MR, Mardanov AV, et al. Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential. Biology. 2023; 12(10):1300. https://doi.org/10.3390/biology12101300
Chicago/Turabian StyleKadnikov, Vitaly V., Nikolai V. Ravin, Diyana S. Sokolova, Ekaterina M. Semenova, Salimat K. Bidzhieva, Alexey V. Beletsky, Alexey P. Ershov, Tamara L. Babich, Marat R. Khisametdinov, Andrey V. Mardanov, and et al. 2023. "Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential" Biology 12, no. 10: 1300. https://doi.org/10.3390/biology12101300
APA StyleKadnikov, V. V., Ravin, N. V., Sokolova, D. S., Semenova, E. M., Bidzhieva, S. K., Beletsky, A. V., Ershov, A. P., Babich, T. L., Khisametdinov, M. R., Mardanov, A. V., & Nazina, T. N. (2023). Metagenomic and Culture-Based Analyses of Microbial Communities from Petroleum Reservoirs with High-Salinity Formation Water, and Their Biotechnological Potential. Biology, 12(10), 1300. https://doi.org/10.3390/biology12101300








