Simple Summary
During insect mating, male insects trigger post-mating responses in females through seminal fluid proteins, inducing a physiological transition in females that is conducive to reproduction. Simultaneously, this process indirectly increases the male’s chances of producing offspring through competition. Females, on the other hand, elicit short- and long-term post-mating responses through proteins and neural networks. Within the intricate regulatory network, seminal fluid proteins and octopamine play pivotal roles. The network spans from the female’s reproductive organs and abdominal ganglion to the brain and operates by activating receptors for seminal fluid proteins and octopamine. This activation leads to the modulation of various signals, influencing the female’s physiological behavior, organ structure, and hormone secretion, and facilitating enhanced nutrient intake, increased expression of reproductive-related genes, ovulation, and sperm storage. Ultimately, these processes aid in the successful oviposition of females.
Abstract
Following insect mating, females often exhibit a series of physiological, behavioral, and gene expression changes. These post-mating responses (PMRs) are induced by seminal fluid components other than sperm, which not only form network proteins to assist sperm localization, supplement female-specific protein requirements, and facilitate the formation of specialized functional structures, but also activate neuronal signaling pathways in insects. This review primarily discusses the roles of seminal fluid proteins (SFPs) and octopamine (OA) in various PMRs in insects. It explores the regulatory mechanisms and mediation conditions by which they trigger PMRs, along with the series of gene expression differences they induce. Insect PMRs involve a transition from protein signaling to neuronal signaling, ultimately manifested through neural regulation and gene expression. The intricate signaling network formed as a result significantly influences female behavior and organ function, contributing to both successful reproduction and the outcomes of sexual conflict.
1. Introduction
Insect mating plays a crucial role in their overall life cycle, as it is essential for their reproduction and evolution. Before mating begins, many insects go through a complex courtship process. The success of courtship is influenced by various factors such as sound [1,2], light [3,4], sex pheromones [5], and sometimes even involves intense competition among individuals of the same species. Male insects dedicate significant efforts to ensure the propagation of their genes, including searching for mates, outcompeting rivals, providing nutritional resources to females, and guarding them post-mating to deter other males, ensuring successful fertilization and oviposition. The ultimate goal of these behaviors is to secure the continuation of their own offspring, evolving in response to ongoing sexual conflict. Female PMRs and the male molecules triggering these responses [6] have evolved under the influence of this conflict, persisting from mating until egg-laying.
Under the activation of SFPs, females undergo a complex transition into a post-mating state. This intricate process involves the recognition and breakdown of SFPs, followed by the activation of neural pathways to regulate changes in the female organ structure and secretions [7]. This continuous process guides females from ovulation to sperm release and even influences behaviors such as feeding and sleeping [8,9,10,11], facilitating successful oviposition. Males consciously adjust the levels of SFPs in response to changing environmental conditions to cope with different forms of competition [12,13]. For instance, males experience reduced reproductive capacity when they age or engage in frequent mating [14]; to compensate for this deficit, they modulate the levels of SFPs [15], thereby influencing female feeding behavior. In some cases, males reduce SFP release and instead utilize SFPs from previous mates to minimize reproductive costs [16,17].
This review will introduce some important SFPs and OA in female insects’ post-mating responses, as well as their effects on female behavior, physiological changes, responses to external stimuli, and alterations in gene expression. It will also discuss the complex interactions between protein networks and neural pathways that exist in post-mating responses. Drosophila melanogaster, a commonly studied model organism, holds a prominent position in understanding insect PMRs. Although other insects have also been investigated, the findings suggest a certain level of commonality in PMRs among different species.
2. Post-Mating Response Induced by SFPs
Insect SFPs are produced by various male reproductive tract (RT) accessory glands [18], the ejaculatory duct [19,20], ejaculatory bulb [21], and testes [22]. During mating, SFPs are transferred along with sperm into the female’s body, where they induce many aspects of the PMR in females. Firstly, SFPs alter the female’s receptivity to mating, forming a mating plug [21] to prevent the adverse effects of repeated mating on reproduction. They also aid in sperm storage and release, acting on the oviducts to enhance reproductive efficiency [23]. SFPs can influence the female’s flight, sleep, and feeding behaviors to acquire the nutrients necessary for sustaining reproduction [8,11]. Some SFPs possess antimicrobial activity [19], assisting females in boosting their immune defenses. Identified SFPs to date encompass a variety of protein categories, such as proteases/protease inhibitors, lectins, prohormone precursors, peptides, and protective proteins (e.g., antioxidants) [7,24]. These protein classes are found in seminal fluid across taxa from arthropods to mammals [25]. Relative molecular weights of different SFPs also vary significantly, ranging from 36 amino acids in sex peptide (SP) to 200–400 amino acids in prohormone-like polypeptides [26] and large glycoproteins [27]. SFPs play a crucial role in sexual conflict competition, and it is precisely for this reason that they face significant selection pressure, requiring rapid evolution to succeed in intense reproductive competition [28,29,30].
SP, one of the most extensively studied and possibly the most important SFP, is involved in almost all PMRs in D. melanogaster (Figure 1) [31]. The N-terminal of SP is linked to sperm, and, during sperm storage, the C-terminal of SP undergoes cleavage at a trypsin cleavage site [32]. A portion of SP enters the female’s hemolymph circulation system, where it inhibits sex peptide sensory neurons expressing the SP receptor (SPR), primarily doublesex (dsx) [33]/fruitless (fru)/pickpocket (ppk) [34,35,36] sensory neurons. This cascade results in long-lasting PMRs [37,38,39]. The SPR receptor is a G-protein-coupled receptor that functions by modulating cAMP levels through Gαi or Gαo signaling [34,35]. SPR is expressed in both the female reproductive organs and the central nervous system [34].

Figure 1.
SP participate in the PMR signaling pathway of D. melanogaster. SP sex peptide, AG accessory gland, FRT female reproductive tract, SR seminal receptacle, SPR sex peptide receptor, SEG subesophageal ganglion, SPSN sex peptide sensory neuron, SAG sex peptide abdominal ganglion, 20E 20-hydroxyecdysone, JH juvenile hormone, CA corpora allata, ETH ecdysis triggering hormone. The symbol “?” represents an unclear pathway, the dashed line represents pathway inhibition.
2.1. Physiological Structures and Behavioral Changes
After mating, in female D. melanogaster, the SP increases the feeding behavior significantly by either binding to the subesophageal ganglion associated with taste recognition and feeding [38] or through some indirect influence. The feeding amount in mated female D. melanogaster increases up to 2.3 times that of virgin counterparts [8]. The length of the midgut in female D. melanogaster gradually increases after one hour of mating and stabilizes until the sixth day, after which it starts to decrease by the tenth day. The growth of the midgut in females post-mating depends on the nutrient supply; if the nutritional status is poor, it inhibits the effects of SP on post-mating midgut growth [40]. Increased feeding and intestinal length lead to an increase in excretion [41]. Additionally, the sleep deprivation observed in female D. melanogaster after mating lasts for ten days [11], coinciding with the time when SP promotes midgut growth. This suggests that female insects undergo a series of long-term physiological and behavioral responses after mating. Recent studies suggest that post-mating sleep deprivation is regulated by pC1 neurons which inhibit sleep-promoting dorsal fan-shaped body (dFB) neurons to reduce sleep time. However, when the food source is changed to a higher energy sucrose, sleep deprivation disappears [42]. Evidently, females require more nutrients to ensure normal oviposition after mating, and the increase in midgut length facilitates nutrient absorption. However, increasing midgut length in poor nutritional conditions is not the preferred choice for females. The increase in activity and reduction in sleep time in females after mating are essentially aimed at acquiring more nutrients. This complex neural regulation ensures that females have sufficient energy for egg production. The physiological changes in female insects caused by mating are significant to the extent that they can influence the lifespan of females [43]. Females lacking SP in male mating tend to have a longer lifespan [44,45].
2.2. The Protein Network of SFPs Regulates the Storage and Release of Sperm
At the initiation of the event when male sperm enters the female’s body, SFP has no effect on the structure of the uterus or the entry of sperm into the seminal receptacle (SR) or spermathecae [46,47,48]. However, SFP plays a crucial role in the subsequent storage and release of sperm in the SR and spermathecae. On the first day after mating in D. melanogaster, CG33943 is required for stimulating oviposition, but it only affects females 24 h after mating [46]. Thereafter, the long-term persistence of PMRs requires seven SFPs: CG9997, CG1652, CG1656, CG17575, CG10586 (seminase), Antares (Antr), and SP [32,46,49,50]. Knocking out CG9997, CG1652, CG1656, or CG17575 in males results in defects in sperm release at an average interval of 4 and/or 10 days, while the functionality of these sperm remains normal and does not affect the female’s hatching rate [46]. Additionally, the help of these four SFPs is required for the retention of SP in the female reproductive tract (FRT) to locate the sperm storage organ (SSO) and bind to the lumens of sperm and/or SSO [47]. Subsequently, SP separates from the sperm, another critical step for sperm release. The absence of either one of these critical steps will result in defective sperm release, with the appearance of release defects due to the failure of SP detachment occurring later than in females that did not receive SP [10]. The lack of SP also leads to reduced depletion of sperm in the SR and minor damage to the mating female’s SR. Recent studies suggest that there is overlap between SFPs and certain sperm-associated proteins in the FRT fluid. Apart from this overlap, other sperm-associated proteins also contribute to sperm storage [51].
2.3. SPs Influence Egg Production
When SPs are lacking, the storage and release of sperm in females are affected, leading to a slower depletion of sperm and resulting in a reduced egg production [10]. However, SP also reduces female receptivity, causing females lacking SP to have a tendency for multiple matings. This behavior can compensate for some of the negative effects caused by defective sperm storage. The interaction between these factors suggests that the oviposition rate in mated females is not actually influenced by SP [44]. However, it should be noted that the process of multiple matings in females lacking SP implies the possibility of mating with multiple different males. This significantly reduces the probability of producing offspring from a particular male and increases reproductive costs. Therefore, males mix SFPs into their seminal fluid to ensure the continuation of their own offspring, albeit at a slightly increased reproductive cost.
2.4. SP Induced Vitellogenesis
In addition to sperm storage, post-mating vitellogenesis is also induced by SP. In virgin females, sex peptide abdominal ganglion (SAG) neurons stimulate the secretion of AstC by allatostatin C-producing thoracic ganglion (AstC-mTh) neurons, which inhibits the synthesis of juvenile hormone (JH) in corpora allata (CA). By inhibiting SAG, SP renders AstC-mTh neurons inactive [52], thereby inducing the biosynthesis of 20-hydroxyecdysone. The expression and secretion of ecdysis triggering hormone (ETH) and its receptor depend on the activation of Inka cells by 20-hydroxyecdysone. ETH acts as an obligatory allatotropin to promote the production of JH in CA [52,53], ultimately leading to the induction of vitellogenesis. JH is involved in numerous physiological processes during the growth and development of insects [54,55]. Its regulation of female JH synthesis not only affects post-mating vitellogenesis but also reduces female receptivity [56]. JH is so crucial for insects that it may influence even more PMRs. Additionally, research suggests that 20-hydroxyecdysone is produced in male accessory glands [57]. Once 20E enters the female’s body, it localizes in the mating plug and is gradually released [58]; it also impacts the female’s receptivity and oviposition behavior [59].
2.5. SFPs Affect Female Receptivity
SPs ultimately influence female receptivity after mating through the pathways mentioned above, and there are other SFPs that also play a role in this process. In Helicoverpa armigera, when the SPR in females is damaged, long-term receptivity is restored compared to the wild type. However, intriguingly, short-term receptivity remains suppressed [45]. It is PEBII, a protein involved in the formation of mating plug ejaculatory bulb, that affects short-term receptivity [60]. Furthermore, studies have shown variations in the impact of laboratory strains versus wild strains on female receptivity [61]. It is speculated that wild strains have adapted to a more complex and intense competitive environment, resulting in a stronger influence on female post-mating receptivity through SFPs.
2.6. SFPs Constitute the Regulatory Network of Post-Mating Response
When SFPs from the male are transferred into the female through seminal fluid, they initially reach the FRT. From there, most of the SFPs migrate to the spermathecae and SR, while a smaller portion enters the female hemolymph. The SPs that enter the hemolymph can be cleaved at the C-terminus by trypsin, and can be further broken down into smaller fragments by other proteases. However, it is not yet clear whether trypsin is upregulated in the female hemolymph post-mating or if it is derived from male SFPs transferred during mating as a means to supplement endogenous female proteases [62].
The actions of SFPs within the female are not independent, but rather form a complex network, where they interact with each other to elicit subsequent responses. During mating, the transfer of CG1652 and CG1656 from males to females requires the presence of CG9997, while the stability of CG9997 itself depends on CG1652 and CG1656 [47]. Shortly after seminal fluid entry into the female, CG1652, CG1656, CG9997, and Antr accompany SP into the SR and bind with sperm. The transfer of CG1656 and CG1652 relies on Antr and CG17575, after which they become difficult to detect within a day [63]. SP not only interacts with sperm, but also binds to the SR, a process that involves CG1652, CG1656, CG9997, and CG17575 [47]. Another SFP, CG10586, plays a crucial role in regulating protein hydrolysis. It participates in the pro-peptide cleavage of CG11864 and is involved in the proper processing of ovulin and Acp36DE. It then enters the SR and spermathecae to participate in sperm release [49]. This system, where a single protein performs multiple functions, adds to the complexity of the protein network. Geoffrey et al. utilized evolutionary rate covariation to study D. melanogaster SFPs, discovering and predicting several SP-related network proteins, thus demonstrating the effectiveness of evolutionary rate covariation in studying the interactions of insect SFPs [64].
3. OA and Their Receptors in Activating Post-Mating Responses
Octopamine (OA) possesses a biogenic monoamine structure that acts as a neurotransmitter, neuromodulator, and neurohormone in invertebrates. OA, along with tyramine (TA), is the only known biogenic amine neurotransmitter in invertebrates, and its role in invertebrates is similar to norepinephrine in vertebrates [65]. The production pathway of OA involves tyrosine being decarboxylated into TA by ty-rosine decarboxylase; then, tyramine β-hydroxylase further hydroxylates TA to convert it into OA [66] (Figure 2A). In addition to being an intermediate in OA synthesis, TA also has independent effects and can even act as an antagonist of OA [67,68]. OA is typically found only in the nervous system, while TA exists in both the nervous system and non-nervous tissues [69]. Extensive research has shown that OA and TA play important roles in insect physiology and behavior.
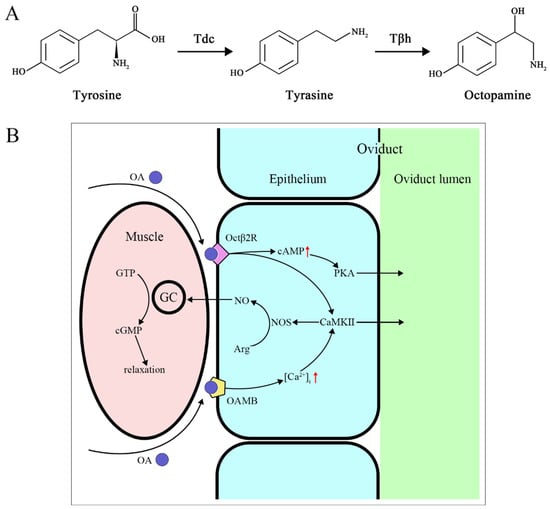
Figure 2.
(A) OA biosynthetic pathway. (B) OA mediates oviduct relaxation through receptors. GC guanylate cyclase, NOS nitric oxide synthases. Red arrow: up-regulation.
OA exerts its effects by binding to receptors on target cells, and the classification system for octopamine receptors is based on structural and signaling similarities to cloned insect octopamine receptors and vertebrate adrenergic receptors [70,71]. As with SPR, OA receptors belong to the G protein-coupled receptor superfamily and can be divided into three subgroups, including α-adrenergic-like octopamine receptors (OA1), β-adrenergic-like octopamine receptors (OA2) [70], and α2-adrenergic-like octopamine receptors (OA3) [72]. In D. melanogaster, five OA-specific G protein-coupled receptors have been identified, including OAMB-K3, OAMB-AS, Octβ1R, Octβ2R, and Octβ3R [73,74,75]. Similarly, five OA receptor genes have been identified in the Nilaparvata lugens, including NlOA2B2, NlOA1, NlOA2B1, NlOA2B3, and NlOA3 [76]. Among them, NlOA2B2 shows a clear sexual dimorphic expression pattern, with higher expression levels in male adults compared to female adults, and other developmental stages. OA and TA significantly increase the cAMP levels in cells expressing NlOA2B2, with OA being eight times more potent than TA and having a lower threshold for receptor activation [77]. In addition to its effects on reproduction, OA receptors also play a positive role in the insect’s stress resistance, providing some level of resilience against high temperatures, starvation, and pesticides [78].
3.1. OA Regulates the Contraction of Oviduct Muscles during Ovulation
The OA signal is necessary for the release of sperm from the storage organ [79], ovulation, and deposition of eggs [69,80,81]. Octopaminergic neurons in the abdominal ganglion regulate the muscle contractions of the oviducts and uterus [82], helping to trigger ovulation [81,83,84]. In Rhodnius prolixus, OA reduces the amplitude of oviduct contractions in a dose-dependent manner. High concentrations of OA can almost completely eliminate bursal contractions, while TA does not affect the amplitude of contractions but can slightly lower the frequency of bursal contractions [85]. Changes in OA receptors can also lead to ovulation defects in females. Following RNA interference of NlO2B2 expression in female N. lugens, retained eggs were observed in the ovaries, indicating ovulation defects [77]. Additionally, the lifespan of females is significantly reduced [78]. The way OA mediates ovulation in D. melanogaster is through the activation of Octβ2R, which increases cAMP production, activating cAMP-dependent protein kinase A (PKA) as a downstream effector. Simultaneously, it interacts with Octβ2R and OAMB to activate Ca2+/calmodulin-sensitive protein kinase II (CaMKII). CaMKII acts on nitric oxide synthase (NOS) to release NO, which relaxes the oviduct muscles. PKA and CaMKII work together to trigger downstream effectors that secrete fluid for egg activation and transport [86,87,88] (Figure 2B).
3.2. OA Causes Receptivity Decline
A decrease in OA levels leads to a decrease in receptivity in D. melanogaster. Compared to wild-type females, virgin females with a mutation in Tyramine β-hydroxylase (TβhnM18) exhibit increased receptivity, while heterozygous mutants show intermediate receptivity, indicating a dose-dependent effect. Additionally, the mutants show some level of oviposition impairment [89]. Similar effects have been observed in Agrotis ipsilon Hufnagel, where injection of OA and 5-hydroxytryptamine (5-HT) increases the sensitivity of antennal lobe neurons in mated males but remains lower than in unmated males. However, newly mated males do not exhibit a significant positive response to sex pheromones after OA or 5-HT injection [90]. Furthermore, changes in receptivity may vary among insect species. Different strains of Callosobruchus chinensis show significant differences in receptivity changes induced by four different monoamines (dopamine, OA, TA, and serotonin) [91].
3.3. OA Mediates Sperm Release
The SSO in D. melanogaster typically consists of a singular SR and paired spermathecae [84,92]. The entry and distribution of sperm within the SSO are regulated by the nervous system [84,92]. The neuromuscular connections at the SSO are governed by the octopaminergic-tyraminergic system, although it remains unclear if the SSO also receives input from other neurotransmitters, such as glutamate [79]. Neurologically induced muscle contractions may potentially facilitate the “pumping” of sperm by the SR or the release of secretions from associated cells [93].
Both OA and TA do not play a role in uterine morphological changes or the accumulation and storage of sperm. Females with lower levels of OA and OA/TA show defects in sperm release, indicating that this is not solely a consequence of egg retention, but rather a result of the involvement of OA and TA in the process of depleting sperm from storage [79]. It is speculated that the release of sperm necessitates sustained activation of neural signals.
3.4. Distribution of Other Neurotransmitters in the Reproductive Organs
In addition to OA, serotonin and dromyosuppressin (DMS) are also neuromodulators present in insects. Hefetz et al. conducted a study on their levels in the reproductive tract nerve endings of female D. melanogaster after mating [94]. These neurotransmitters elicit different responses at various stages after mating in different regions of the reproductive tract in D. melanogaster, depending on the varying levels of signaling molecules. In unmated D. melanogaster, OA, serotonin, and DMS are distributed throughout all regions of the reproductive tract, but their distribution is not uniform. OA is most abundant in the nerve endings of the outermost oviduct, gradually decreasing until reaching the lowest concentration in the nerve endings of the uterus. Serotonin, on the other hand, exhibits more uniform distribution, with relatively higher levels in the SR. DMS is most abundant in the lateral oviducts.
At the end of mating in D. melanogaster, SFPs stimulate the release of octopamine at the ovarian nerve endings and trigger its accumulation at the uterine nerve endings. Serotonin levels significantly decrease in the ovary and SR. DMS decreases in the lateral oviducts and the upper and lower common oviducts, while it increases in the ovary and uterus. During the peak period of sperm storage and initiation of ovulation in D. melanogaster, sperm influences the level of octopamine in the nerve endings of the lateral oviducts, leading to a decrease in the lateral oviducts, upper common oviducts, SR, and uterus. Serotonin decreases in the ovary and lateral oviducts. DMS decreases in the ovary and common oviducts, while it increases in the uterus.
4. Regulation of Gene Expression in Insect Post-Mating Responses
Following mating, both females and males exhibit sex-specific differences in gene expression. In males, there is no differential expression of PMR genes in the head-thorax, with only a few overlapping differentially expressed genes in the abdomen. Notably, among these genes, the expression of JH esterase stands out, as it plays a crucial role in regulating JH levels and contributes to the production of SFPs. Male D. melanogaster demonstrates a significantly higher number of differentially expressed genes in the abdomen compared to the head-thorax, tallying up to 2068 genes with differential expression. Conversely, females exhibit the opposite pattern, with a greater number of differentially expressed genes in the head-thorax after mating compared to the abdomen [95,96].
The differentially expressed genes in males predominantly cluster around protein synthesis, which is understandable, considering the need for replenishing depleted sperm and Acps post-mating. Females, on the other hand, rely on neuroregulation to modulate their behavior after mating. Therefore, despite accepting SFPs from males via the abdomen, the communication of signals through neural pathways triggers changes in the brain. Similar findings have been observed in previous studies examining the expression profiles of honeybee queens’ brains and ovaries after mating. Ovaries respond more rapidly to mating, demonstrating gene expression primarily associated with physiological changes, while the brain’s gene expression is more behavior-related [97].
Moreover, it has been discovered that the production of SFPs is regulated by specific genes. For instance, the Hox gene Abd-B in the male accessory gland is instrumental in maintaining the morphology and functionality of secondary cells. These cells secrete SFPs, which are indispensable for the sustained response following female mating [50,98]. Transcriptomic analysis comparing the influence of Acps in Mexican fruit flies on females [99], along with the identification of heat-shock factor (HSF) binding sites and Hsfisoforms regulating Anopheles gambiae’s Acps expression [100], further support these findings.
Research indicates that the number of differentially expressed genes in the FRT of Aedes aegypti gradually increases after mating [101]. Similar observations have been made in female Cephalcia chuxiongica, where reproductive-related genes are upregulated from 1 to 24 h after mating, while immune genes remain downregulated. After 24 h post-mating, longevity-related genes start to decline [102]. It is worth mentioning that the expression of female immune genes after mating shows species-specific, and even interspecies-specific, differences [103]. In the PMR of ant queens, elevated expression of reproductive-related vitellogenin genes has been found, while defensin genes also show an increase followed by a return to normal levels within five days [104]. Similarly, in the PMR of Spodoptera frugiperda, aside from the downregulation of longevity-related genes, both female and male immune genes exhibit a similar pattern of upregulation followed by downregulation over time [105].
Some studies suggest that immune response genes in female D. melanogaster are upregulated after mating [95,106,107], while others indicate lower expression of antimicrobial peptides genes in mated female D. melanogaster [108]. Fedorka et al. proposed a different explanation for the changes in post-mating immune responses in D. melanogaster. They suggested that the observed increase in immune gene expression in mated females might be due to a discrepancy between their actual immune capabilities and their potential immune capabilities. The increased expression of immune genes in mated female D. melanogaster could be attributed to self-inhibition for accepting sperm or depletion of immune reserves [107]. On the contrary, Sarah et al. found that post-mating immune defense ability in female D. melanogaster is independent of their genetic makeup, and, interestingly, mated females display immunosuppression in a pathogen-dependent manner [109]. These differing results suggest that the expression of immune-related genes after female mating is regulated by a complex system, not solely due to the induction and regulation by SFPs. This complexity may arise from sexual conflict, where males induce females into a post-mating state while females maintain their own survival. Alternatively, the conclusions drawn by researchers could be influenced by factors such as pathogens, experimental conditions, female nutritional levels, or physiological states, resulting in different responses when faced with pathogen-induced stress.
Regarding research on D. melanogaster PMR, it has been found that the number of mating events can also influence gene expression. Metabolic regulatory genes involved in the metabolism of glucose, amino acids (including dopamine precursors), carboxylic acids, and the metabolic pathway of JH tend to be downregulated after the second mating event. The expression of long-term response-related genes is insensitive to the number of matings. Genes related to cell proliferation processes and reproductive cell development show an increased expression level after the second mating, and some genes exhibit significant responses specifically to the second mating event. However, this might be a delayed response to the initial mating or an overlapping effect with the aforementioned responses [110].
5. Conclusions
The process of post-mating reactions in female insects essentially involves adjusting their bodies to a more suitable reproductive state, but it also comes with a trade-off of compromising their chances of survival in the natural environment. The changes induced by reproductive behavior are disadvantageous for female individuals in terms of survival [111,112], whereas males, in order to ensure the production of their own offspring, may offer “gifts” or compensate for the losses incurred by females in other forms [113,114]. Both male and female insects consider the costs of mating, seeking a balance between reproduction and survival. Female insects exhibit post-mating immune suppression, which may be related to the energy expenditure involved in egg-laying [115]. Proteins secreted by the accessory gland and ejaculatory duct in male D. melanogaster have been found to possess antimicrobial effects [19], thus mitigating the introduction of additional pathogens through seminal fluid. The immune response exhibited by females after mating clearly represents a complex system, and the role played by SFPs in this process remains to be further discovered.
The transformation of females from never having mated to post-mating is a complex triggering process that is not exclusively mediated by SFPs or neurotransmitters alone. Instead, it involves the collaborative action of both, with the two often leading to similar outcomes. For example, SFPs can inhibit sleep after mating, while octopamine (OA), by acting on insulin-secreting neurons, can achieve the same effect [88]. Additionally, the activation of OA neurons is not necessarily directly caused by SFPs, but may occur indirectly. Acp62F, a serine protease inhibitor, participates in protein hydrolysis cascades by regulating the processing rate of ovulin [116]. After mating, ovulin in female D. melanogaster induces an increase in OA neuron activity, indirectly leading to an increase in the number of axonal boutons and relaxing the muscles of the oviduct to increase ovulation rates [117]. However, ovulin only produces a short-term response in oviposition, lasting 24 h [26]. The PMRs in females can be divided into neural regulation and physiological reactions, with the latter exhibiting a significantly faster response rate compared to neural regulation. Neural regulation tends to affect long-term behaviors such as feeding and sleep in females. SFPs begin processing within the male body [49] and can immediately trigger PMRs in females once transferred. In contrast, neural regulation requires more preparatory steps before activation. The involvement of a complex system of short-term PMRs may be a necessary step, as the precise and rapid initiation of PMR in females greatly increases the success rate of reproduction; after transitioning from an unmated to a mated state, maintaining the ‘switch’ in the open position is sufficient to sustain long-term responses.
Currently, most research on PMR focuses on D. melanogaster, while investigations on other species are limited. It remains unclear whether the regulatory mechanisms and signaling pathways of PMR are universally applicable to various insect species. The numerous SFPs pose a challenge to constructing protein signaling networks. The functions exerted by SFPs from sources other than the male accessory glands in the signaling network, as well as the interactions among SFPs downstream, have not been fully elucidated. The functions of genes such as dsx/fru/ppk and their contributions to neural signal transmission among expressing neurons remain unclear. The functionalities mediated by the activation of downstream neural systems require further refinement. Additionally, the functions of numerous differentially expressed genes after mating need to be validated. Current research has shifted from investigating the transformation of female insects after mating to understanding the changes in gene expression and their functional significance. This includes studying the co-evolution of proteins secreted by males and receptors in the female reproductive system, as well as the interconnectedness between sexual maturity and post-mating in insects.
Author Contributions
Conceptualization, G.-X.G. and D.-T.L.; writing—original draft preparation, G.-X.G.; writing—review and editing, G.-X.G., X.-P.Y. and D.-T.L.; supervision, D.-T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32001895 and 32372526), the National Natural Science Foundation of China Regional Innovation and Development Joint Fund Key Support Project (U21A20223) and the Natural Science Foundation of Zhejiang Province (LY23C140007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baker, C.A.; Clemens, J.; Murthy, M. Acoustic Pattern Recognition and Courtship Songs: Insights from Insects. Annu. Rev. Neurosci. 2019, 42, 129–147. [Google Scholar] [CrossRef]
- Derlink, M.; Abt, I.; Mabon, R.; Julian, C.; Virant-Doberlet, M.; Jacquot, E. Mating behavior of Psammotettix alienus (Hemiptera: Cicadellidae). Insect Sci. 2018, 25, 148–160. [Google Scholar] [CrossRef]
- Woods, W.A., Jr.; Hendrickson, H.; Mason, J.; Lewis, S.M. Energy and predation costs of firefly courtship signals. Am. Nat. 2007, 170, 702–708. [Google Scholar] [CrossRef]
- Godfrey, J.A.; Murray, T.M.; Rypstra, A.L. The effects of environmental light on the role of male chemotactile cues in wolf spider mating interactions. Behav. Ecol. Sociobiol. 2022, 76, 39. [Google Scholar] [CrossRef]
- Guo, H.; Mo, B.T.; Li, G.C.; Li, Z.L.; Huang, L.Q.; Sun, Y.L.; Dong, J.F.; Smith, D.P.; Wang, C.Z. Sex pheromone communication in an insect parasitoid, Campoletis chlorideae Uchida. Proc. Natl. Acad. Sci. USA 2022, 119, e2215442119. [Google Scholar] [CrossRef]
- Hollis, B.; Koppik, M.; Wensing, K.U.; Ruhmann, H.; Genzoni, E.; Erkosar, B.; Kawecki, T.J.; Fricke, C.; Keller, L. Sexual conflict drives male manipulation of female postmating responses in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2019, 116, 8437–8444. [Google Scholar] [CrossRef]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.A.; Rubinstein, C.D.; Wolfner, M.F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef]
- Carvalho, G.B.; Kapahi, P.; Anderson, D.J.; Benzer, S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Curr. Biol. 2006, 16, 692–696. [Google Scholar] [CrossRef]
- Bloch Qazi, M.C.; Heifetz, Y.; Wolfner, M.F. The developments between gametogenesis and fertilization: Ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 2003, 256, 195–211. [Google Scholar] [CrossRef]
- Avila, F.W.; Ravi Ram, K.; Bloch Qazi, M.C.; Wolfner, M.F. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 2010, 186, 595–600. [Google Scholar] [CrossRef]
- Isaac, R.E.; Li, C.; Leedale, A.E.; Shirras, A.D. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. Biol. Sci. 2010, 277, 65–70. [Google Scholar] [CrossRef]
- Moschilla, J.A.; Tomkins, J.L.; Simmons, L.W. Males adjust their manipulation of female remating in response to sperm competition risk. Proc. Biol. Sci. 2020, 287, 20201238. [Google Scholar] [CrossRef]
- Tuni, C.; Weber, S.; Bilde, T.; Uhl, G. Male spiders reduce pre- and postmating sexual investment in response to sperm competition risk. Behav. Ecol. 2017, 28, 1030–1036. [Google Scholar] [CrossRef]
- Bath, E.; Buzzoni, D.; Ralph, T.; Wigby, S.; Sepil, I. Male condition influences female post mating aggression and feeding in Drosophila. Funct. Ecol. 2021, 35, 1288–1298. [Google Scholar] [CrossRef]
- Koppik, M.; Fricke, C. Gene expression changes in male accessory glands during ageing are accompanied by reproductive decline in Drosophila melanogaster. Mol. Ecol. 2017, 26, 6704–6716. [Google Scholar] [CrossRef]
- Misra, S.; Wolfner, M.F. Drosophila seminal sex peptide associates with rival as well as own sperm, providing SP function in polyandrous females. Elife 2020, 9, e58322. [Google Scholar] [CrossRef]
- Sirot, L.K.; Wolfner, M.F.; Wigby, S. Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2011, 108, 9922–9926. [Google Scholar] [CrossRef]
- Wolfner, M.F. The gifts that keep on giving: Physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity 2002, 88, 85–93. [Google Scholar] [CrossRef]
- Lung, O.; Kuo, L.; Wolfner, M.F. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J. Insect Physiol. 2001, 47, 617–622. [Google Scholar] [CrossRef]
- Saudan, P.; Hauck, K.; Soller, M.; Choffat, Y.; Ottiger, M.; Sporri, M.; Ding, Z.; Hess, D.; Gehrig, P.M.; Klauser, S.; et al. Ductus ejaculatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur. J. Biochem. 2002, 269, 989–997. [Google Scholar] [CrossRef]
- Lung, O.; Wolfner, M.F. Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem. Mol. Biol. 2001, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Takami, Y.; Sasabe, M.; Nagata, N.; Sota, T. Dual function of seminal substances for mate guarding in a ground beetle. Behav. Ecol. 2008, 19, 1173–1178. [Google Scholar] [CrossRef]
- Ravi Ram, K.; Ji, S.; Wolfner, M.F. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem. Mol. Biol. 2005, 35, 1059–1071. [Google Scholar] [CrossRef]
- Ravi Ram, K.; Wolfner, M.F. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 2007, 47, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Poiani, A. Complexity of seminal fluid: A review. Behav. Ecol. Sociobiol. 2006, 60, 289–310. [Google Scholar] [CrossRef]
- Herndon, L.A.; Wolfner, M.F. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl. Acad. Sci. USA 1995, 92, 10114–10118. [Google Scholar] [CrossRef]
- Neubaum, D.M.; Wolfner, M.F. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 1999, 153, 845–857. [Google Scholar] [CrossRef]
- Chapman, T. The soup in my fly: Evolution, form and function of seminal fluid proteins. PLoS Biol. 2008, 6, e179. [Google Scholar] [CrossRef]
- Ramm, S.A.; McDonald, L.; Hurst, J.L.; Beynon, R.J.; Stockley, P. Comparative proteomics reveals evidence for evolutionary diversification of rodent seminal fluid and its functional significance in sperm competition. Mol. Biol. Evol. 2009, 26, 189–198. [Google Scholar] [CrossRef]
- Sirot, L.K.; Wong, A.; Chapman, T.; Wolfner, M.F. Sexual conflict and seminal fluid proteins: A dynamic landscape of sexual interactions. Cold Spring Harb. Perspect. Biol. 2014, 7, a017533. [Google Scholar] [CrossRef]
- Kubli, E. Sex-peptides: Seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 2003, 60, 1689–1704. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, S.; Busser, S.; Liu, H.; Honegger, T.; Kubli, E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 2005, 15, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Rezaval, C.; Pavlou, H.J.; Dornan, A.J.; Chan, Y.B.; Kravitz, E.A.; Goodwin, S.F. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 2012, 22, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Yapici, N.; Kim, Y.J.; Ribeiro, C.; Dickson, B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 2008, 451, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Rumpf, S.; Xiang, Y.; Gordon, M.D.; Song, W.; Jan, L.Y.; Jan, Y.N. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 2009, 61, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hasemeyer, M.; Yapici, N.; Heberlein, U.; Dickson, B.J. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 2009, 61, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Soller, M.; Haussmann, I.U.; Hollmann, M.; Choffat, Y.; White, K.; Kubli, E.; Schafer, M.A. Sex-peptide-regulated female sexual behavior requires a subset of ascending ventral nerve cord neurons. Curr. Biol. 2006, 16, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Ottiger, M.; Soller, M.; Stocker, R.F.; Kubli, E. Binding sites of Drosophila melanogaster sex peptide pheromones. J. Neurobiol. 2000, 44, 57–71. [Google Scholar] [CrossRef]
- Avila, F.W.; Mattei, A.L.; Wolfner, M.F. Sex peptide receptor is required for the release of stored sperm by mated Drosophila melanogaster females. J. Insect Physiol. 2015, 76, 1–6. [Google Scholar] [CrossRef]
- White, M.A.; Bonfini, A.; Wolfner, M.F.; Buchon, N. Drosophila melanogaster sex peptide regulates mated female midgut morphology and physiology. Proc. Natl. Acad. Sci. USA 2021, 118, e2018112118. [Google Scholar] [CrossRef]
- Apger-McGlaughon, J.; Wolfner, M.F. Post-mating change in excretion by mated Drosophila melanogaster females is a long-term response that depends on sex peptide and sperm. J. Insect Physiol. 2013, 59, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Duhart, J.M.; Buchler, J.R.; Inami, S.; Kennedy, K.J.; Jenny, B.P.; Afonso, D.J.S.; Koh, K. Modulation and Neural Correlates of Postmating Sleep Plasticity in Drosophila Females. Curr. Biol. 2023, 33, 2702–2716. [Google Scholar] [CrossRef] [PubMed]
- Civetta, A.; Clark, A.G. Correlated effects of sperm competition and postmating female mortality. Proc. Natl. Acad. Sci. USA 2000, 97, 13162–13165. [Google Scholar] [CrossRef] [PubMed]
- Wigby, S.; Chapman, T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005, 15, 316–321. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Liu, W.; Liu, Y.; Ren, B.; Wang, G. Sex peptide receptor mediates the post-mating switch in Helicoverpa. armigera (Lepidoptera: Noctuidae) female reproductive behavior. Pest Manag. Sci. 2021, 77, 3427–3435. [Google Scholar] [CrossRef]
- Ravi Ram, K.; Wolfner, M.F. Sustained Post-Mating Response in Drosophila melanogaster Requires Multiple Seminal Fluid Proteins. PLoS Genet. 2005; preprint. [Google Scholar] [CrossRef][Green Version]
- Ram, K.R.; Wolfner, M.F. A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 15384–15389. [Google Scholar] [CrossRef]
- Wolfner, M.F. Battle and ballet: Molecular interactions between the sexes in Drosophila. J. Hered. 2009, 100, 399–410. [Google Scholar] [CrossRef]
- LaFlamme, B.A.; Ram, K.R.; Wolfner, M.F. The Drosophila melanogaster seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genet. 2012, 8, e1002435. [Google Scholar] [CrossRef]
- Gligorov, D.; Sitnik, J.L.; Maeda, R.K.; Wolfner, M.F.; Karch, F. A novel function for the Hox gene Abd-B in the male accessory gland regulates the long-term female post-mating response in Drosophila. PLoS Genet. 2013, 9, e1003395. [Google Scholar] [CrossRef]
- McCullough, E.L.; Whittington, E.; Singh, A.; Pitnick, S.; Wolfner, M.F.; Dorus, S. The life history of Drosophila sperm involves molecular continuity between male and female reproductive tracts. Proc. Natl. Acad. Sci. USA 2022, 119, e2119899119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kim, A.J.; Rivera-Perez, C.; Noriega, F.G.; Kim, Y.J. The insect somatostatin pathway gates vitellogenesis progression during reproductive maturation and the post-mating response. Nat. Commun. 2022, 13, 969. [Google Scholar] [CrossRef]
- Meiselman, M.; Lee, S.S.; Tran, R.T.; Dai, H.; Ding, Y.; Rivera-Perez, C.; Wijesekera, T.P.; Dauwalder, B.; Noriega, F.G.; Adams, M.E. Endocrine network essential for reproductive success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E3849–E3858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, S.; Liu, S. Juvenile Hormone Studies in Drosophila melanogaster. Front. Physiol. 2021, 12, 785320. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shi, J.; Jiang, X.; Song, Y.; Du, J.; Zhao, Z. Neuropeptide F regulates feeding via the juvenile hormone pathway in Ostrinia furnacalis larvae. Pest Manag. Sci. 2023, 79, 1193–1203. [Google Scholar] [CrossRef]
- Meiselman, M.R.; Ganguly, A.; Dahanukar, A.; Adams, M.E. Post-mating Refractoriness in Drosophila melanogaster Depends Upon Ecdysis Triggering Hormone Signaling. bioRxiv 2021. [Google Scholar] [CrossRef]
- Pondeville, E.; Maria, A.; Jacques, J.C.; Bourgouin, C.; Dauphin-Villemant, C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc. Natl. Acad. Sci. USA 2008, 105, 19631–19636. [Google Scholar] [CrossRef]
- Baldini, F.; Gabrieli, P.; South, A.; Valim, C.; Mancini, F.; Catteruccia, F. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 2013, 11, e1001695. [Google Scholar] [CrossRef]
- Gabrieli, P.; Kakani, E.G.; Mitchell, S.N.; Mameli, E.; Want, E.J.; Mariezcurrena Anton, A.; Serrao, A.; Baldini, F.; Catteruccia, F. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2014, 111, 16353–16358. [Google Scholar] [CrossRef]
- Bretman, A.; Lawniczak, M.K.; Boone, J.; Chapman, T. A mating plug protein reduces early female remating in Drosophila melanogaster. J. Insect Physiol. 2010, 56, 107–113. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Mendiola-Díaz, F.J.; Echave-Sanabria, A.C. Do male seminal donations shape female post-mating receptivity in a usually monandrous moth? Behav. Ecol. Sociobiol. 2019, 73, 163. [Google Scholar] [CrossRef]
- Pilpel, N.; Nezer, I.; Applebaum, S.W.; Heifetz, Y. Mating-increases trypsin in female Drosophila hemolymph. Insect Biochem. Mol. Biol. 2008, 38, 320–330. [Google Scholar] [CrossRef]
- Singh, A.; Buehner, N.A.; Lin, H.; Baranowski, K.J.; Findlay, G.D.; Wolfner, M.F. Long-term interaction between Drosophila sperm and sex peptide is mediated by other seminal proteins that bind only transiently to sperm. Insect Biochem. Mol. Biol. 2018, 102, 43–51. [Google Scholar] [CrossRef]
- Findlay, G.D.; Sitnik, J.L.; Wang, W.; Aquadro, C.F.; Clark, N.L.; Wolfner, M.F. Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster female post-mating responses. PLoS Genet. 2014, 10, e1004108. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 59, 533–561. [Google Scholar] [CrossRef]
- White, M.A.; Chen, D.S.; Wolfner, M.F. She’s got nerve: Roles of octopamine in insect female reproduction. J. Neurogenet. 2021, 35, 132–153. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.B. Tyramine: From octopamine precursor to neuroactive chemical in insects. Gen. Comp. Endocrinol. 2009, 162, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Fox, L.E.; Soll, D.R.; Wu, C.F. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J. Neurobiol. 2004, 58, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.H.; Carney, G.E.; McClung, C.A.; Willard, S.S.; Taylor, B.J.; Hirsh, J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: Distinct roles for neural tyramine and octopamine in female fertility. J. Biol. Chem. 2005, 280, 14948–14955. [Google Scholar] [CrossRef]
- Evans, P.D.; Maqueira, B. Insect octopamine receptors: A new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert. Neurosci. 2005, 5, 111–118. [Google Scholar] [CrossRef]
- Farooqui, T. Review of octopamine in insect nervous systems. Open Access Insect Physiol. 2012, 1–17. [Google Scholar] [CrossRef]
- Wu, S.F.; Xu, G.; Qi, Y.X.; Xia, R.Y.; Huang, J.; Ye, G.Y. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J. Neurochem. 2014, 129, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Han, K.A.; Millar, N.S.; Davis, R.L. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J. Neurosci. 1998, 18, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Maqueira, B.; Chatwin, H.; Evans, P.D. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 2005, 94, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Balfanz, S.; Strunker, T.; Frings, S.; Baumann, A. A family of octopamine [corrected] receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 93, 440–451. [Google Scholar] [CrossRef]
- Wu, S.F.; Jv, X.M.; Huang, J.M.; Gao, C.F. Molecular features and expression profiles of octopamine receptors in the brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2019, 75, 2663–2671. [Google Scholar] [CrossRef]
- Wu, S.F.; Jv, X.M.; Li, J.; Xu, G.J.; Cai, X.Y.; Gao, C.F. Pharmacological characterisation and functional roles for egg-laying of a beta-adrenergic-like octopamine receptor in the brown planthopper Nilaparvata lugens. Insect Biochem. Mol. Biol. 2017, 87, 55–64. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Jiang, L.; Ahamd, S.; Chen, Y.; Zhang, J.Y.; Stanley, D.; Miao, H.; Ge, L.Q. The octopamine receptor, OA2B2, modulates stress resistance and reproduction in Nilaparvata lugens Stål (Hemiptera: Delphacidae). Insect Mol. Biol. 2022, 31, 33–48. [Google Scholar] [CrossRef]
- Avila, F.W.; Bloch Qazi, M.C.; Rubinstein, C.D.; Wolfner, M.F. A requirement for the neuromodulators octopamine and tyramine in Drosophila melanogaster female sperm storage. Proc. Natl. Acad. Sci. USA 2012, 109, 4562–4567. [Google Scholar] [CrossRef]
- Monastirioti, M.; Linn, C.E., Jr.; White, K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J. Neurosci. 1996, 16, 3900–3911. [Google Scholar] [CrossRef]
- Monastirioti, M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev. Biol. 2003, 264, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.L.; Riccio, M.L.; Avila, F.W.; Wolfner, M.F. Correction for Mattei et al., Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro-computed tomography scanning. Proc. Natl. Acad. Sci. USA 2017, 114, E5485. [Google Scholar] [CrossRef]
- Middleton, C.A.; Nongthomba, U.; Parry, K.; Sweeney, S.T.; Sparrow, J.C.; Elliott, C.J. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006, 4, 17. [Google Scholar] [CrossRef]
- Rodriguez-Valentin, R.; Lopez-Gonzalez, I.; Jorquera, R.; Labarca, P.; Zurita, M.; Reynaud, E. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J. Cell. Physiol. 2006, 209, 183–198. [Google Scholar] [CrossRef]
- Hana, S.; Lange, A.B. Octopamine and tyramine regulate the activity of reproductive visceral muscles in the adult female blood-feeding bug, Rhodnius prolixus. J. Exp. Biol. 2017, 220, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Sabandal, P.R.; Fernandez, A.; Sabandal, J.M.; Lee, H.G.; Evans, P.; Han, K.A. The octopamine receptor Octbeta2R regulates ovulation in Drosophila melanogaster. PLoS ONE 2014, 9, e104441. [Google Scholar] [CrossRef]
- Louis, M.; Lee, H.-G.; Rohila, S.; Han, K.-A. The Octopamine Receptor OAMB Mediates Ovulation via Ca2+/Calmodulin-Dependent Protein Kinase II in the Drosophila Oviduct Epithelium. PLoS ONE 2009, 4, e4716. [Google Scholar] [CrossRef]
- Crocker, A.; Shahidullah, M.; Levitan, I.B.; Sehgal, A. Identification of a Neural Circuit that Underlies the Effects of Octopamine on Sleep:Wake Behavior. Neuron 2010, 65, 670–681. [Google Scholar] [CrossRef]
- Rezaval, C.; Nojima, T.; Neville, M.C.; Lin, A.C.; Goodwin, S.F. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 2014, 24, 725–730. [Google Scholar] [CrossRef]
- Barrozo, R.B.; Jarriault, D.; Simeone, X.; Gaertner, C.; Gadenne, C.; Anton, S. Mating-induced transient inhibition of responses to sex pheromone in a male moth is not mediated by octopamine or serotonin. J. Exp. Biol. 2010, 213, 1100–1106. [Google Scholar] [CrossRef]
- Yamane, T. Genetic variation in the effect of monoamines on female mating receptivity and oviposition in the adzuki bean beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). BMC Evol. Biol. 2014, 14, 172. [Google Scholar] [CrossRef]
- Arthur, B.; Hauschteck-Jungen, E.; Nöthiger, R.; Ward, P.I. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: A masculinized nervous system is as good as none. Proc. R. Soc. Lond Ser. B Biol. Sci. 1998, 265, 1749–1753. [Google Scholar] [CrossRef]
- Allen, A.K.; Spradling, A.C. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development 2008, 135, 311–321. [Google Scholar] [CrossRef]
- Heifetz, Y.; Lindner, M.; Garini, Y.; Wolfner, M.F. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr. Biol. 2014, 24, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.K.; Bradley, T.; Moxon, S.; Chapman, T. Divergence in Transcriptional and Regulatory Responses to Mating in Male and Female Fruitflies. Sci. Rep. 2019, 9, 16100. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.E.; Kacheria, T.S.; Knott, S.R.; Lebo, M.S.; Nishitani, A.; Sanders, L.E.; Stirling, E.J.; Winbush, A.; Arbeitman, M.N. Dynamic, mating-induced gene expression changes in female head and brain tissues of Drosophila melanogaster. BMC Genom. 2010, 11, 541. [Google Scholar] [CrossRef]
- Kocher, S.D.; Richard, F.J.; Tarpy, D.R.; Grozinger, C.M. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera). BMC Genom. 2008, 9, 232. [Google Scholar] [CrossRef]
- Sitnik, J.L.; Gligorov, D.; Maeda, R.K.; Karch, F.; Wolfner, M.F. The Female Post-Mating Response Requires Genes Expressed in the Secondary Cells of the Male Accessory Gland in Drosophila melanogaster. Genetics 2016, 202, 1029–1041. [Google Scholar] [CrossRef]
- Sirot, L.; Bansal, R.; Esquivel, C.J.; Arteaga-Vazquez, M.; Herrera-Cruz, M.; Pavinato, V.A.C.; Abraham, S.; Medina-Jimenez, K.; Reyes-Hernandez, M.; Dorantes-Acosta, A.; et al. Post-mating gene expression of Mexican fruit fly females: Disentangling the effects of the male accessory glands. Insect Mol. Biol. 2021, 30, 480–496. [Google Scholar] [CrossRef]
- Dottorini, T.; Persampieri, T.; Palladino, P.; Baker, D.A.; Spaccapelo, R.; Senin, N.; Crisanti, A. Regulation of Anopheles gambiae male accessory gland genes influences postmating response in female. FASEB J. 2013, 27, 86–97. [Google Scholar] [CrossRef]
- Alfonso-Parra, C.; Ahmed-Braimah, Y.H.; Degner, E.C.; Avila, F.W.; Villarreal, S.M.; Pleiss, J.A.; Wolfner, M.F.; Harrington, L.C. Mating-Induced Transcriptome Changes in the Reproductive Tract of Female Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004451. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, M.R.; Xu, J.; Chen, P.; Liu, J.H. Mating-Induced Trade-Offs upon Egg Production versus Fertilization and Offspring’s Survival in a Sawfly with Facultative Parthenogenesis. Insects 2021, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Newell, N.R.; Ray, S.; Dalton, J.E.; Fortier, J.C.; Kao, J.Y.; Chang, P.L.; Nuzhdin, S.V.; Arbeitman, M.N. The Drosophila Post-mating Response: Gene Expression and Behavioral Changes Reveal Perdurance and Variation in Cross-Tissue Interactions. G3 Genes Genomes Genet. 2020, 10, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Cherasse, S.; Dacquin, P.; Aron, S. Mating triggers an up-regulation of vitellogenin and defensin in ant queens. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2019, 205, 745–753. [Google Scholar] [CrossRef]
- Wu, T.; Cao, D.H.; Liu, Y.; Yu, H.; Fu, D.Y.; Ye, H.; Xu, J. Mating-Induced Common and Sex-Specific Behavioral, Transcriptional Changes in the Moth Fall Armyworm (Spodoptera frugiperda, Noctuidae, Lepidoptera) in Laboratory. Insects 2023, 14, 209. [Google Scholar] [CrossRef]
- McGraw, L.A.; Clark, A.G.; Wolfner, M.F. Post-mating Gene Expression Profiles of Female Drosophila melanogaster in Response to Time and to Four Male Accessory Gland Proteins. Genetics 2008, 179, 1395–1408. [Google Scholar] [CrossRef]
- Fedorka, K.M.; Linder, J.E.; Winterhalter, W.; Promislow, D. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc. Biol. Sci. 2007, 274, 1211–1217. [Google Scholar] [CrossRef]
- Short, S.M.; Wolfner, M.F.; Lazzaro, B.P. Female Drosophila melanogaster suffer reduced defense against infection due to seminal fluid components. J. Insect Physiol. 2012, 58, 1192–1201. [Google Scholar] [CrossRef]
- Short, S.M.; Lazzaro, B.P. Female and male genetic contributions to post-mating immune defence in female Drosophila melanogaster. Proc. Biol. Sci. 2010, 277, 3649–3657. [Google Scholar] [CrossRef]
- Innocenti, P.; Morrow, E.H. Immunogenic males: A genome-wide analysis of reproduction and the cost of mating in Drosophila melanogaster females. J. Evol. Biol. 2009, 22, 964–973. [Google Scholar] [CrossRef]
- Wing, S.R. Cost of Mating for Female Insects: Risk of Predation in Photinus collustrans (Coleoptera: Lampyridae). Am. Nat. 1988, 131, 139–142. [Google Scholar] [CrossRef]
- Arnqvist, G.; Ronn, J.; Watson, C.; Goenaga, J.; Immonen, E. Concerted evolution of metabolic rate, economics of mating, ecology, and pace of life across seed beetles. Proc. Natl. Acad. Sci. USA 2022, 119, e2205564119. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, F.; Simmons, L.W. Male-induced costs of mating for females compensated by offspring viability benefits in an insect. J. Evol. Biol. 2010, 23, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Martínez Villar, M.; Germil, M.; Pavón-Peláez, C.; Tomasco, I.H.; Bilde, T.; Toft, S.; Albo, M.J. Lack of Female Preference for Nuptial Gifts May Have Led to Loss of the Male Sexual Trait. Evol. Biol. 2023, 50, 318–331. [Google Scholar] [CrossRef]
- Short, S.M.; Lazzaro, B.P. Reproductive status alters transcriptomic response to infection in female Drosophila melanogaster. G3 Genes Genomes Genet. 2013, 3, 827–840. [Google Scholar] [CrossRef][Green Version]
- Mueller, J.L.; Linklater, J.R.; Ravi Ram, K.; Chapman, T.; Wolfner, M.F. Targeted Gene Deletion and Phenotypic Analysis of the Drosophila melanogaster Seminal Fluid Protease Inhibitor Acp62F. Genetics 2008, 178, 1605–1614. [Google Scholar] [CrossRef]
- Rubinstein, C.D.; Wolfner, M.F. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 17420–17425. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).