Effects of Clipping an Invasive Plant Species on the Growth of Planted Plants of Two Co-Occurring Species in a Greenhouse Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Stage I: Culture of S. canadensis Seedlings at Different Densities
2.2. Stage II: Clipping and Sowing Seeds of Co-Occurring Plant Species
2.3. Data Analysis
3. Results
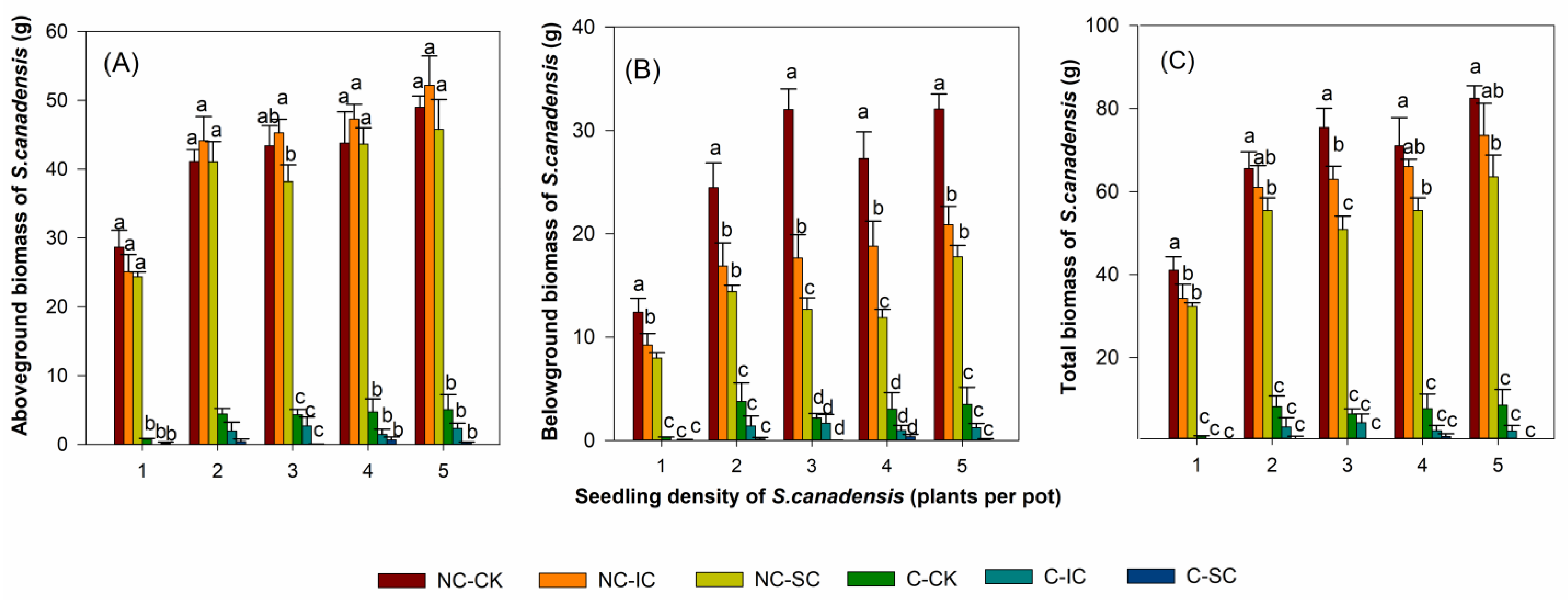
3.1. The Effects of S. canadensis Density, Clipping, and Co-Occurring Species Planting on Biomass Accumulation of S. canadensis
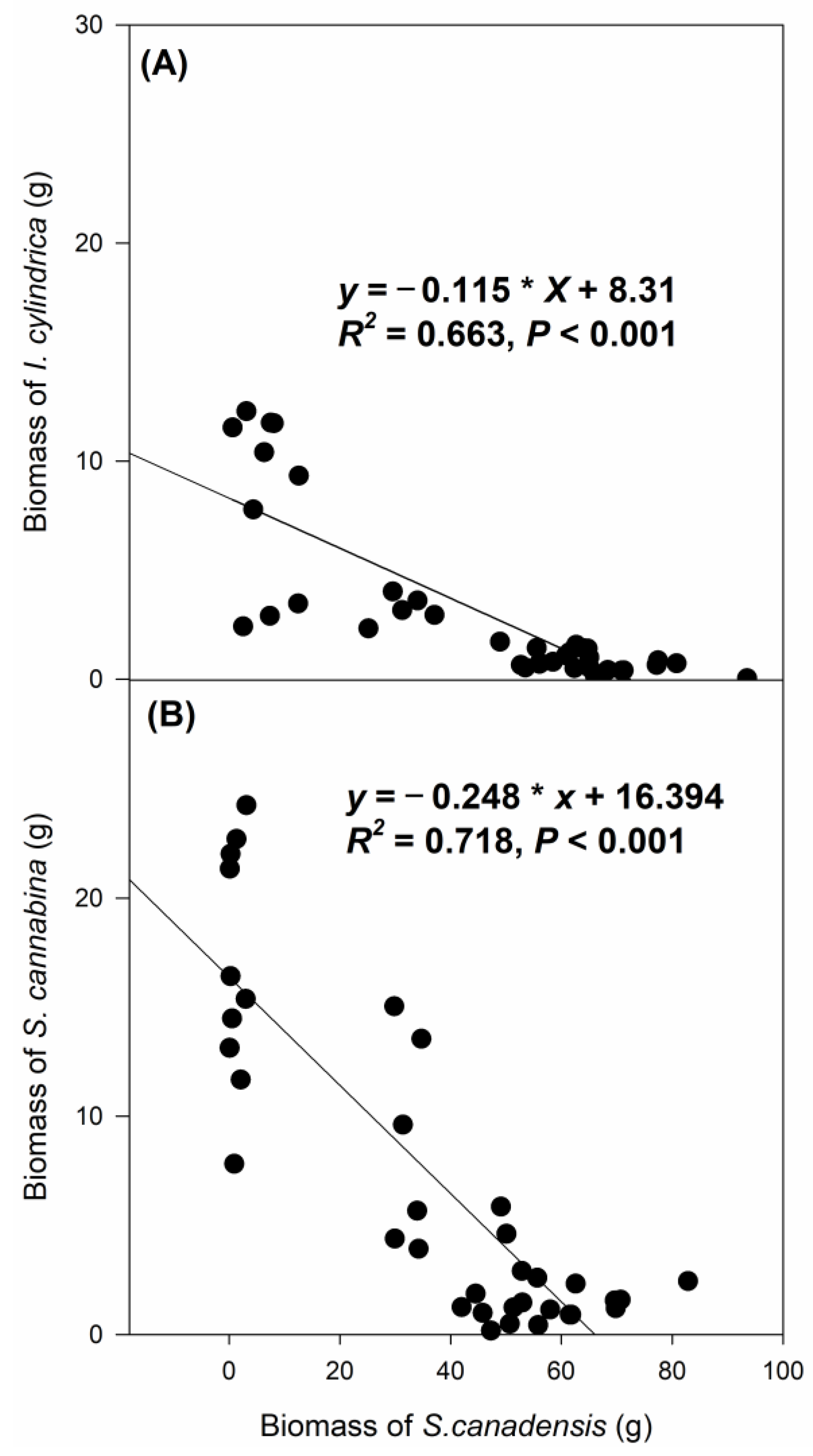
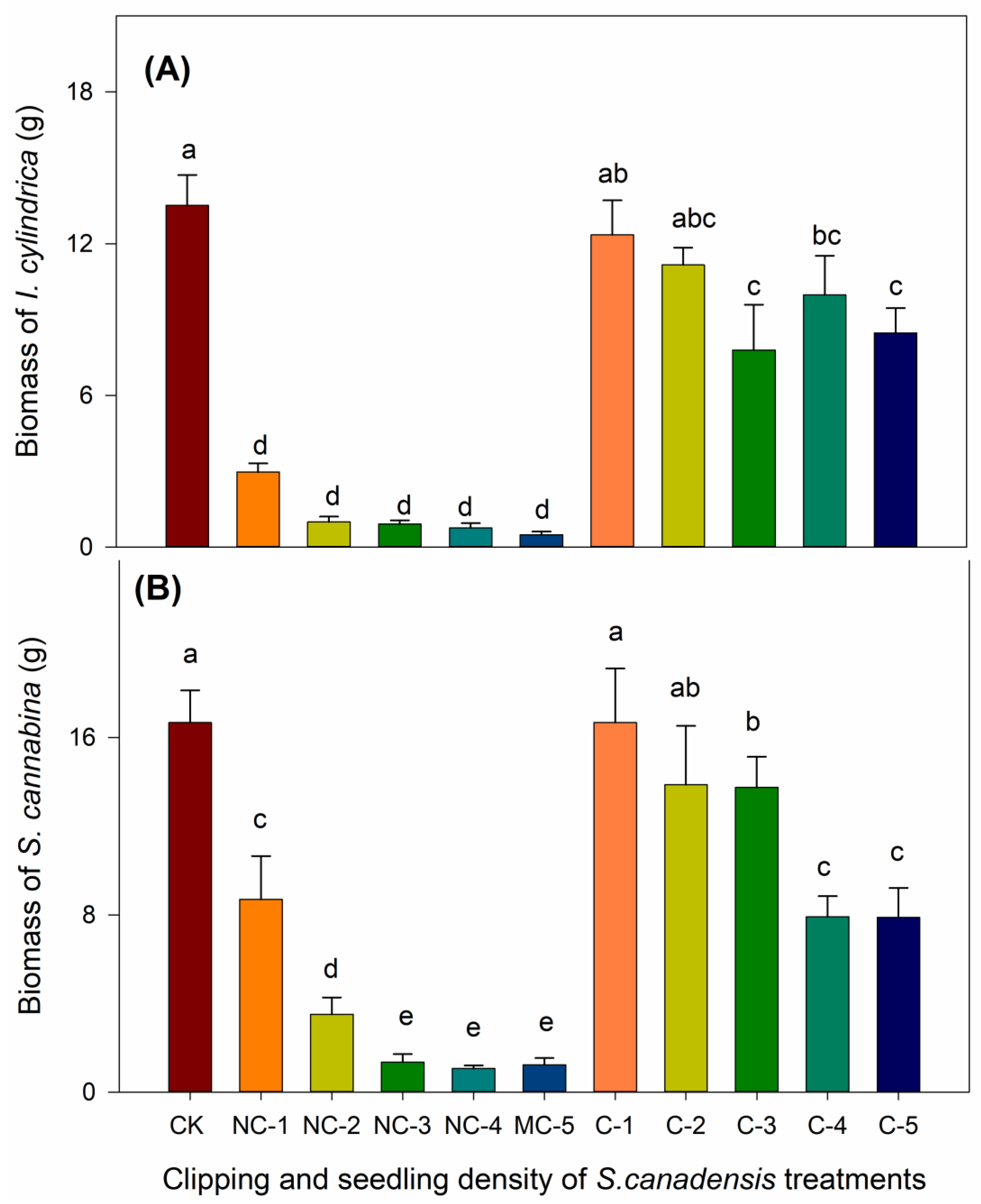
3.2. The Effects of Clipping and Seedling Density on the Biomass of I. cylindrica and S. cannabina
4. Discussion
4.1. The Effects of Clipping and Planting Plants of the Co-Occurring Species on Biomass Accumulation of S. canadensis
4.2. The Effects of S. canadensis on Biomass Accumulation of the Co-Occurring Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Spatial variation in allelopathic inhibition by Ageratina adenophora on growth and yield of two traditional millet crops. Vegetos 2022, 35, 663–673. [Google Scholar] [CrossRef]
- Powell, K.I.; Chase, J.M.; Knight, T.M. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 2013, 339, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Trait plasticity: A key attribute in the invasion success of Ageratina adenophora in different forest types of Kumaun Himalaya, India. Environ. Dev. Sustain. 2023, 1–22. [Google Scholar] [CrossRef]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Phenotypic variation in morphology and associated functional traits in Ageratina adenophora along an altitudinal gradient in Kumaun Himalaya, India. Biologia 2023, 78, 1333–1347. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Eschen, R.; Kadzamira, M.; Stutz, S.; Ogunmodede, A.; Djeddour, D.; Shaw, R.; Pratt, C.; Varia, S.; Constantine, K.; Williams, F. An updated assessment of the direct costs of invasive non-native species to the United Kingdom. Biol. Invasions 2023, 25, 3265–3276. [Google Scholar] [CrossRef]
- Hulme, P.E.; Bernard-Verdier, M. Comparing traits of native and alien plants: Can we do better? Funct. Ecol. 2018, 32, 117–125. [Google Scholar] [CrossRef]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Trait variability in co-occurring invasive and native plant species in road side population of Kumaun Himalaya. Braz. J. Bot. 2022, 45, 1099–1110. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. Crit. Rev. Plant Sci. 2004, 23, 431–452. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Borer, E.T.; Buckley, Y.; Cleland, E.E.; Davies, K.; Firn, J.; Harpole, W.S.; Hautier, Y.; Lind, E.; MacDougall, A.; et al. Predicting invasion in grassland ecosystems: Is exotic dominance the real embarrassment of richness? Glob. Chang. Biol. 2013, 19, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Iannone, B.V., III; Heneghan, L.; Rijal, D.; Wise, D.H. Below-ground causes and consequences of woodland shrub invasions: A novel paired-point framework reveals new insights. J. Appl. Ecol. 2015, 52, 78–88. [Google Scholar] [CrossRef]
- Hess, M.C.M.; Mesléard, F.; Buisson, E. Priority effects: Emerging principles for invasive plant species management. Ecol. Eng. 2019, 127, 48–57. [Google Scholar] [CrossRef]
- Vilà, M.; Weiner, J. Are invasive plant species better competitors than native plant species?–Evidence from pair-wise experiments. Oikos 2004, 105, 229–238. [Google Scholar] [CrossRef]
- Golivets, M.; Wallin, K.F. Neighbour tolerance, not suppression, provides competitive advantage to non-native plants. Ecol. Lett. 2018, 21, 745–759. [Google Scholar] [CrossRef]
- Byun, C.; Kettenring, K.M.; Tarsa, E.E.; de Blois, S. Applying ecological principles to maximize resistance to invasion in restored plant communities. Ecol. Eng. 2023, 190, 106926. [Google Scholar] [CrossRef]
- Byun, C.; de Blois, S.; Brisson, J. Management of invasive plants through ecological resistance. Biol. Invasions 2018, 20, 13–27. [Google Scholar] [CrossRef]
- Weidlich, E.W.A.; Flórido, F.G.; Sorrini, T.B.; Brancalion, P.H.S. Controlling invasive plant species in ecological restoration: A global review. J. Appl. Ecol. 2020, 57, 1806–1817. [Google Scholar] [CrossRef]
- Li, Y.M.; Munson, S.M.; Lin, Y.C.; Grissom, P. Effectiveness of a decade oftreatments to reduce invasive buffelgrass (Pennisetum ciliare). Invasive Plant Sci. Manag. 2023, 16, 27–37. [Google Scholar] [CrossRef]
- Wang, S.; Martin, P.A.; Hao, Y.; Sutherland, W.J.; Shackelford, G.E.; Wu, J.H.; Ju, R.T.; Zhou, W.N.; Li, B. A global synthesis of the effectiveness and ecological impacts of management interventions for Spartina species. Front. Environ. Sci. Eng. 2023, 17, 141. [Google Scholar] [CrossRef]
- Wilson, M.V.; Clark, D.L. Controlling invasive Arrhenatherum elatius and promoting native prairie grasses through mowing. Appl. Veg. Sci. 2001, 4, 129–138. [Google Scholar] [CrossRef]
- Tang, L.; Yang, G.; Wang, J.Q.; Wang, C.H.; Li, B.; Chen, J.K.; Zhao, B. Designing an effective clipping regime for controlling the invasive plant Spartina alterniflora in an estuarine salt marsh. Ecol. Eng. 2009, 35, 874–881. [Google Scholar] [CrossRef]
- Renz, M.; Heflin, R. Impact of clipping timing on Japanese hedgeparsley (Torilis japonica) seed production and viability. Invasive Plant Sci. Manag. 2014, 7, 511–516. [Google Scholar] [CrossRef]
- Li, W.H.; Luo, J.N.; Tian, X.S.; Chow, W.S.; Sun, Z.Y.; Zhang, T.J.; Peng, S.L.; Peng, C.L. A new strategy for controlling invasive weeds: Selecting valuable native plants to defeat them. Sci. Rep. 2015, 5, 11004. [Google Scholar] [CrossRef] [PubMed]
- Szymura, M.; Świerszcz, S.; Szymura, T.H. Restoration of ecologically valuable grassland on sites degraded by invasive Solidago: Lessons from a 6-year experiment. Land Degrad. Dev. 2022, 33, 1985–1998. [Google Scholar] [CrossRef]
- Lishawa, S.C.; Lawrence, B.A.; Albert, D.A.; Tuchman, N.C. Biomass harvest of invasive Typha promotes plant diversity in a Great Lakes coastal wetland. Restor. Ecol. 2015, 23, 228–237. [Google Scholar] [CrossRef]
- Körner, C.; Stöcklin, J.; Reuther-Thiébaud, L.; Pelaez-Riedl, S. Small differences in arrival time influence composition and productivity of plant communities. New Phytol. 2008, 177, 698–705. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, W.L.; Li, B.; Zhao, B.; Li, P.; Li, Z.B.; Tang, L. The substantial influences of non-resource conditions on recovery of plants: A case study of clipped Spartina alterniflora asphyxiated by submergence. Ecol. Eng. 2014, 73, 345–352. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Fan, Z.W.; Li, X.X.; Wang, Y.; Liu, Y.; Shen, Y.D. Effects of nutrient addition and clipping on biomass production of invasive and native annual Asteraceae plants. Weed Res. 2018, 58, 318–326. [Google Scholar] [CrossRef]
- van Kleunen, M.V.; Ramponi, G.; Schmid, B. Effects of herbivory simulated by clipping and jasmonic acid on Solidago canadensis. Basic Appl. Ecol. 2004, 5, 173–181. [Google Scholar] [CrossRef]
- Schuster, M.J.; Wragg, P.D.; Reich, P.B. Using revegetation to suppress invasive plants in grasslands and forests. J. Appl. Ecol. 2018, 55, 2362–2373. [Google Scholar] [CrossRef]
- Perera, P.C.D.; Szymura, T.H.; Zając, A.; Chmolowska, D.; Szymura, M. Drivers of Solidago species invasion in Central Europe—Case study in the landscape of the Carpathian Mountains and their foreground. Ecol. Evol. 2021, 11, 12429–12444. [Google Scholar] [CrossRef]
- Mantoani, M.C.; Osborne, B.A. Post-invasion recovery of plant communities colonised by Gunnera tinctoria after mechanical removal or herbicide application and its interaction with an extreme weather event. Plants 2022, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Blank, R.R.; Morgan, T. Suppression of Bromus tectorum L. by established perennial grasses: Potential mechanisms—Part one. Appl. Environ. Soil Sci. 2012, 2012, 632172. [Google Scholar] [CrossRef]
- Blank, R.R.; Morgan, T.; Allen, F. Suppression of annual Bromus tectorum by perennial Agropyron cristatum: Roles of soil nitrogen availability and biological soil space. AoB Plants 2015, 7, plv006. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.G.; Cronin, S.A.; Paschke, M.W. Native cover crops suppress exotic annuals and favor native perennials in a greenhouse competition experiment. Plant Ecol. 2009, 204, 247–259. [Google Scholar] [CrossRef]
- Möhrle, K.; Reyes-Aldana, H.E.; Kollmann, J.; Teixeira, L.H. Suppression of an invasive native plant species by designed grassland communities. Plants 2021, 10, 775. [Google Scholar] [CrossRef]
- Puliafico, K.; Schwarzländer, M.; Price, W.; Harmon, B.; Hinz, H. Native and exotic grass competition with invasive hoary cress (Cardaria draba). Invasive Plant Sci. Manag. 2011, 4, 38–49. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Barber, K.; Martin, L.M. Exotic grassland species have stronger priority effects than natives regardless of whether they are cultivated or wild genotypes. New Phytol. 2015, 205, 928–937. [Google Scholar] [CrossRef]
- Young, T.P.; Stuble, K.L.; Balachowski, J.A.; Werner, C.M. Using priority effects to manipulate competitive relationships in restoration. Restor. Ecol. 2017, 25, S114–S123. [Google Scholar] [CrossRef]
- Knudson, J. Canada thistle (Cirsium arvense) response to clipping and seeding of competitive grasses. Am. J. Plant Sci. 2012, 3, 1252–1259. [Google Scholar] [CrossRef][Green Version]
- Abraham, J.K.; Corbin, J.D.; D’Antonio, C.M. California native and exotic perennial grasses differ in their response to soil nitrogen, exotic annual grass density, and order of emergence. Plant Ecol. 2009, 201, 445–456. [Google Scholar] [CrossRef]
- Yannelli, F.A.; Karrer, G.; Hall, R.; Kollmann, J.; Heger, T. Seed density is more effective than multi-trait limiting similarity in controlling grassland resistance against plant invasions in mesocosms. Appl. Veg. Sci. 2018, 21, 411–418. [Google Scholar] [CrossRef]
- Goldberg, D.E. Neighborhood competition in an old-field plant community. Ecology 1987, 68, 1211–1223. [Google Scholar] [CrossRef]
- He, L.; Bakker, E.S.; Nunez, M.M.A.; Hilt, S. Combined effects of shading and clipping on the invasive alien macrophyte Elodea nuttallii. Aquat. Bot. 2019, 154, 24–27. [Google Scholar] [CrossRef]
- Huang, H.; Guo, S.; Chen, G. Reproductive biology in an invasive plant Solidago canadensis. Front. Biol. China 2007, 2, 196–204. [Google Scholar] [CrossRef]
- Chen, G.Q.; Zhang, C.B.; Ma, L.; Qiang, S.; Silander, J.A.; Qi, L.L. Biotic homogenization caused by the invasion of Solidago canadensis in China. J. Integr. Agric. 2013, 12, 835–845. [Google Scholar] [CrossRef]
- Szymura, M.; Szymura, T. Interactions between alien goldenrods (Solidago and Euthamia species) and comparison with native species in Central Europe. Flora 2015, 218, 51–61. [Google Scholar] [CrossRef]
- Zubek, S.; Majewska, M.L.; Kapusta, P.; Stefanowicz, A.M.; Błaszkowski, J.; Rożek, K.; Stanek, M.; Karpowicz, F.; Zalewska-Gałosz, J. Solidago canadensis invasion in abandoned arable fields induces minor changes in soil properties and does not affect the performance of subsequent crops. Land Degrad. Dev. 2020, 31, 334–345. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Scheiner, S.M. ANOVA and ANCOVA: Field competition experiments. In Design and Analysis of Ecological Experiments, 2nd ed.; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 77–98. ISBN 9780195131888. [Google Scholar]
- Poorter, L.; Kitajima, K.; Mercado, P.; Chubiña, J.; Melgar, I.; Prins, H.H.T. Resprouting as a persistence strategy of tropical forest trees: Relations with carbohydrate storage and shade tolerance. Ecology 2010, 91, 2613–2627. [Google Scholar] [CrossRef]
- Shibata, R.; Shibata, M.; Tanaka, H.; Iida, S.; Masaki, T.; Hatta, F.; Kurokawa, H.; Nakashizuka, T. Interspecific variation in the size-dependent resprouting ability of temperate woody species and its adaptive significance. J. Ecol. 2014, 102, 209–220. [Google Scholar] [CrossRef]
- Konlechner, T.M.; Orlovich, D.A.; Hilton, M.J. Restrictions in the sprouting ability of an invasive coastal plant, Ammophila arenaria, from fragmented rhizomes. Plant Ecol. 2016, 217, 521–532. [Google Scholar] [CrossRef]
- Smith, M.G.; Arndt, S.K.; Miller, R.E.; Kasel, S.; Bennett, L.T. Trees use more non-structural carbohydrate reserves during epicormic than basal resprouting. Tree Physiol. 2018, 38, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Liu, Y.; Chu, C.J.; Xu, H.; Fang, S.Q. Fast seedling root growth leads to competitive superiority of invasive plants. Biol. Invasions 2018, 20, 1821–1832. [Google Scholar] [CrossRef]
- Ren, G.Q.; Li, Q.; Li, Y.; Li, J.; Adomako, M.; Dai, Z.C.; Li, G.L.; Wan, L.Y.; Zhang, B.; Zou, C.; et al. The enhancement of root biomass increases the competitiveness of an invasive plant against a co-occurring native plant under elevated nitrogen deposition. Flora 2019, 261, 151486. [Google Scholar] [CrossRef]
- Reader, R.J.; Wilson, S.D.; Belcher, J.W.; Wisheu, I.; Keddy, P.A.; Tilman, D.; Morris, E.C.; Grace, J.B.; McGraw, J.B.; Olff, H.; et al. Plant competition in relation to neighbor biomass: An intercontinental study with Poa pratensis. Ecology 1994, 75, 1753–1760. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Grewell, B.J.; D’Antonio, C.M.; Funk, J.L.; James, J.J.; Molinari, N.; Parker, I.M.; Richards, C.L. A functional trait perspective on plant invasion, Ann. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef]
- Hao, Q.; Ma, J.S. Invasive alien plants in China: An update. Plant Divers. 2023, 45, 117–121. [Google Scholar] [CrossRef]
- Becker, M.; George, T. Nitrogen fixation response of stem and root-nodulating Sesbania species to flooding and mineral nitrogen. Plant Soil 1995, 175, 189–196. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.Y.; Liu, Y.J.; Wang, E.T.; Ren, C.G.; Liu, W.; Xu, H.L.; Wu, H.L.; Jiang, N.; Li, Y.Z.; et al. Genetic diversity and community structure of rhizobia nodulating Sesbania cannabina in saline-alkaline soils. Syst. Appl. Microbiol. 2016, 39, 195–202. [Google Scholar] [CrossRef]
- An, X.; Sun, M.; Ren, K.; Xu, M.; Wang, Z.; Li, Y.; Liu, H.; Lian, B. Effect and mechanism of the improvement of coastal silt soil by application of organic fertilizer and gravel combined with Sesbania cannabina cultivation. Front. Plant Sci. 2022, 13, 1092089. [Google Scholar] [CrossRef]
- Li, R.; Tang, N.; Jia, X.; Xu, Y.J.; Cheng, Y.Q. Antidiabetic activity of galactomannan from Chinese Sesbania cannabina and its correlation of regulating intestinal microbiota. J. Funct. Foods 2021, 83, 104530. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, J.; Huang, C.; Lai, C.; Ling, Z.; Yong, Q. Rheological properties of Sesbania cannabina galactomannan as a new source of thickening agent. J. Food Sci. 2022, 87, 1527–1539. [Google Scholar] [CrossRef]
- Ploughe, L.W.; Carlyle, C.N.; Fraser, L.H. Priority effects: How the order of arrival of an invasive grass, Bromus tectorum, alters productivity and plant community structure when grown with native grass species. Ecol. Evol. 2020, 10, 13173–13181. [Google Scholar] [CrossRef]
- Ye, X.Q.; Ma, R.X.; Lei, S.T.; Wu, M.; Yu, F.H. Maintained nutrient accumulation in invasive Solidago canadensis in response to competition. Flora 2022, 295, 152136. [Google Scholar] [CrossRef]
- Meira-Neto, J.A.A.; da Silva, M.C.N.A.; Tolentino, G.S.; Gastauer, M.; Buttschardt, T.; Ulm, F.; Máguas, C. Early Acacia invasion in a sandy ecosystem enables shading mediated by soil, leaf nitrogen and facilitation. Biol. Invasions 2018, 20, 1567–1575. [Google Scholar] [CrossRef]
- Mckinney, A.M.; Goodell, K. Shading by invasive shrub reduces seed production and pollinator services in a native shrub. Biol. Invasions 2010, 12, 2751–2763. [Google Scholar] [CrossRef]
- Schenk, H.J. Root competition: Beyond resource depletion. J. Ecol. 2006, 94, 725–739. [Google Scholar] [CrossRef]
- Phillips, M.L.; Mcnellis, B.E.; Allen, M.F.; Allen, E.B. Differences in root phenology and water depletion by an invasive grass explains persistence in a Mediterranean ecosystem. Am. J. Bot. 2019, 106, 1210–1218. [Google Scholar] [CrossRef]
- Grman, E.; Suding, K.N. Within-year soil legacies contribute to strong priority effects of exotics on native California grassland communities. Restor. Ecol. 2010, 18, 664–670. [Google Scholar] [CrossRef]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef]
- Levine, J.M.; Pachepsky, E.; Kendall, B.E.; Yelenik, S.G.; Lambers, J.H.R. Plant-soil feedbacks and invasive spread. Ecol. Lett. 2006, 9, 1005–1014. [Google Scholar] [CrossRef]
- Zhang, G.L.; Bai, J.H.; Wang, W.; Jia, J.; Huang, L.B.; Kong, F.L.; Xi, M. Plant invasion reshapes the latitudinal pattern of soil microbial necromass and its contribution to soil organic carbon in coastal wetlands. Catena 2023, 222, 106859. [Google Scholar] [CrossRef]
- Ye, X.Q.; Yan, Y.N.; Wu, M.; Yu, F.H. High capacity of nutrient accumulation by invasive Solidago canadensis in a coastal grassland. Front. Plant Sci. 2019, 10, 575. [Google Scholar] [CrossRef]
| Total Biomass | Aboveground Biomass | Belowground Biomass | |||||
|---|---|---|---|---|---|---|---|
| D.f. | F | p | F | p | F | p | |
| C | 1 | 2094.975 | 0.000 | 2180.523 | 0.000 | 1134.113 | 0.000 |
| S | 2 | 25.144 | 0.000 | 5.678 | 0.004 | 72.507 | 0.000 |
| D | 4 | 31.018 | 0.000 | 26.545 | 0.000 | 25.248 | 0.000 |
| C × S | 2 | 5.224 | 0.007 | 1.873 | 0.158 | 34.010 | 0.000 |
| C × D | 4 | 20.753 | 0.000 | 17.601 | 0.000 | 16.271 | 0.000 |
| S × D | 8 | 0.840 | 0.569 | 0.419 | 0.908 | 2.661 | 0.010 |
| C × S × D | 8 | 0.478 | 0.870 | 0.413 | 0.912 | 1.991 | 0.052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Meng, J.; Ma, R.; Wu, M. Effects of Clipping an Invasive Plant Species on the Growth of Planted Plants of Two Co-Occurring Species in a Greenhouse Study. Biology 2023, 12, 1282. https://doi.org/10.3390/biology12101282
Ye X, Meng J, Ma R, Wu M. Effects of Clipping an Invasive Plant Species on the Growth of Planted Plants of Two Co-Occurring Species in a Greenhouse Study. Biology. 2023; 12(10):1282. https://doi.org/10.3390/biology12101282
Chicago/Turabian StyleYe, Xiaoqi, Jinliu Meng, Ruixiang Ma, and Ming Wu. 2023. "Effects of Clipping an Invasive Plant Species on the Growth of Planted Plants of Two Co-Occurring Species in a Greenhouse Study" Biology 12, no. 10: 1282. https://doi.org/10.3390/biology12101282
APA StyleYe, X., Meng, J., Ma, R., & Wu, M. (2023). Effects of Clipping an Invasive Plant Species on the Growth of Planted Plants of Two Co-Occurring Species in a Greenhouse Study. Biology, 12(10), 1282. https://doi.org/10.3390/biology12101282





