Simple Summary
In Coleoptera, successful female reproduction partially relies on effective vitellogenesis, characterized by vitellogenin (Vg) synthesis in the fat body, secretion into hemolymphs, transport through intercellular channels in follicular epithelium, endocytosis mediated by Vg receptor (VgR), and absorption and storage by mature oocytes. In two representative Coleoptera species, Leptinotarsa decemlineata (Chrysomelidae) and Henosepilachna vigintioctopunctata (Coccinellidae), we performed RNA interference targeting ecdysone receptor (EcR) or ultraspiracle (usp) genes. Depletion of the expression level of EcR or usp inhibited oocyte development and dramatically repressed the transcription of Vg in fat bodies. Our findings indicate that 20E signaling plays an indispensable role in the stimulation of Vg synthesis and uptake in the two beetle species.
Abstract
Coleoptera is the largest taxa of animals by far. The robust reproductive capacity is one of the main reasons for such domination. Successful female reproduction partially relies on effective vitellogenesis. However, the hormone regulation of vitellogenesis remains to be explored. In the present paper, in vitro culture of Leptinotarsa decemlineata 1-day-old adult fat bodies in the 20E-contained median did not activate juvenile hormone production and insulin-like peptide pathways, but significantly stimulated the expression of two LdVg genes, in a cycloheximide-dependent pattern. In vivo RNA interference (RNAi) of either ecdysone receptor (LdEcR) or ultraspiracle (Ldusp) by injection of corresponding dsRNA into 1-day-old female adults inhibited oocyte development, dramatically repressed the transcription of LdVg genes in fat bodies and of LdVgR in ovaries; application of JH into the LdEcR or Ldusp RNAi L. decemlineata females did not restore the oocyte development, partially rescued the decreased LdVg mRNA levels but over-compensated LdVgR expression levels. The same RNAi experiments were performed in another Coleoptera species, Henosepilachna vigintioctopunctata. Little yolk substances were seen in the misshapen oocytes in the HvEcR or Hvusp RNAi ovaries, in contrast to larger amounts of yolk granules in the normal oocytes. Correspondingly, the transcript levels of HvVg in the fat bodies and ovaries decreased significantly in the HvEcR and Hvusp RNAi samples. Our results here show that 20E signaling is indispensable in the activation of vitellogenesis in the developing oocytes of the two beetle species.
1. Introduction
Coleoptera is currently the most species-rich group, including about one fourth of all known insects with more than 360,000 described species on this planet. An enormous reproductive capacity is one of the main reasons for such domination [1]. To some extent, successful female reproduction depends on functioning vitellogenesis, a process that leads to the accumulation of vitellogenin (Vg) in the oocytes [2,3]. Insect Vgs are mainly produced in the fat body [3,4]. In some insect species, Vgs have also been reported to be synthesized in ovarian tissues [5], follicle cells [6], nurse cells [7], and haemocytes [8]. The Vg proteins are transported to the ovary through circulating hemolymph, across the patency among the follicle epithelium cells that enclose oocytes, absorbed through Vg receptor (VgR), and stored by oocytes [3,8].
Cumulative studies have established that ecdysteroids (the most active form, 20-hydroxyecdysone, 20E) and juvenile hormones (JH) regulate the synthesis and absorption of Vgs [3,9,10]. In Coleoptera, however, the reproduction regulation in the female is complex and less explored. JH has even been documented regulating vitellogenesis in some beetles [9,11]. For example, a positive correlation between JH synthesis and ovarian development has been reported in Leptinotarsa decemlineata (Chrysomelidae) [12], Tribolium castaneum (Tenebrionidae) [11], Coccinella septempunctata (Coccinellidae) [13], and Anthonomus grandis (Curculionidae) [14]. In T. castaneum, application of JH III can induce TmVg expression in the previtellogenic females. Conversely, suppressing JH signal impairs Vg gene expression and Vg protein accumulation [11]. Further research displays that the JH signaling pathway promotes insulin-like peptide 2 (ILP2) production through Met. As a result, the expression of the Vg gene is activated. JH also indirectly regulates vitellogenesis by inducing ILP-IGF production, and ILP-IGF subsequently activates an ILP cascade [15]. Moreover, the Bursicon neuropeptide signal also induces Vg expression, possibly by mediating the expression of JH and ILP cascade genes [16]. These data demonstrate that JH is necessary for the production of Vg protein by the beetle fat body.
It has also been known that 20E signaling is associated with vitellogenesis in two Coleoptera, T. castaneum (Tenebrionidae) and Colaphellus bowringi (Chrysomelidae) [9,11,17]. In T. castaneum, RNA interference (RNAi) studies show that vitellogenesis and oocyte maturation requires 20E response genes, such as Ecdysone-induced protein 75 (E75), Ecdysone-induced protein 93F (E93), hormone receptor 3 (HR3), hormone receptor 4 (HR4), Ecdysone receptor (EcR), ultraspiracle (USP), and βFTZ transcription factor 1 (βFTZ-F1) [18,19]. Moreover, Cap ‘n’collar isoform C, a transcription factor that activates the genes encoding for ecdysteroid biosynthesis enzymes, regulates the tradeoff between detoxification and reproduction [20]. In C. bowringi, exogenous application of either 20E or ecdysis-triggering hormone peptide (ETH) induces vitellogenesis. Any 20E deficiency not only decreases ETH level, but also reduces JH production, while knockdown of the ETH gene decreases the expression of Vg1, Vg2, and a few JH biosynthetic genes [17]. The mitogen-activated protein kinase signaling pathway possibly regulates ecdysone biosynthesis; disruption of this pathway delays ovarian development and causes low fecundity [21]. Similarly, knockdown of the coat protein II complex gene represses the ecdysone signaling pathway. As a result, yolk deposition and ovarian growth are considerably inhibited [22].
In T. castaneum and C. bowringi, 20E signaling triggers JH and/or ILP signaling to activate vitellogenesis. Whether the same hormonal regulative mode is conserved in other Coleoptera species remains to be determined. In order to compare the similarities within a family and the differences between the families in terms of oogenesis, in the present paper we selected two Coleoptera potato pests, L. decemlineata (Chrysomelidae) and Henosepilachna vigintioctopunctata (Coccinellidae). We intended to determine the role of 20E signaling in the regulation of vitellogenesis and tried to address two long-standing issues. (1). Is the 20E cascade necessary for the activation of vitellogenesis? (2). Is the gonadotrophic role of 20E signaling independent of the JH cascade? Our results here suggest the importance of 20E signaling in the vitellogenesis in the two Coleoptera pests.
2. Materials and Methods
2.1. Insect Rearing
H. vigintioctopunctata beetles were collected on Solanum melongena L. during the summer in Nanjing (24°32′00.00″ N; 117°22′00.00″ E), Jiangsu Province in China. The beetles were cultured in a laboratory at 28 ± 1 °C, 16 h:8 h light–dark photoperiod and under 50–60% relative humidity conditions, using fresh potato leaves. Under such feeding conditions, the duration from egg-laying to adult eclosion took about 20 days.
L. decemlineata adults were collected in spring, on the potato fields of Urumqi (43°82′55″ N, 87°61′68″ E), Xinjiang Uygur autonomous region in China. The insects were routinely fed on fresh potato leaves in an insectary at 28 ± 1 °C, 14 h:10 h light–dark photoperiod and 50–60% relative humidity. Under such feeding conditions, the duration from egg to adults was around 28 days.
2.2. Molecular Cloning
The Henosepilachna vigintioctopunctata and Leptinotarsa decemlineata EcR isoforms were downloaded from the National Center for Biotechnology Information (NCBI) (accession numbers: HvEcRA, BAP15927.1; HvEcRB1, BAP15926.1; LdEcRA, AB211191; LdEcRB1, AB211192). The usp isoforms were obtained from NCBI (accession numbers: Hvusp1, AB506671.1; Hvusp2, AB506672.1; Ldusp1, MH492017; Ldusp2, MH492018). Polymerase chain reaction (PCR) was performed using the primers in Table S1 to verify the sequence correctness of these target genes.
2.3. Synthesis of dsRNAs
Using the online siRNA design website (http://sidirect2.rnai.jp/) URL (accessed on 22 April 2022), highly efficient and specific siRNA fragments originating from the common HvEcR or Hvusp fragments or HvEcRB1 isoform-specific sequence were selected. Then, primer premier 5.0 software was used to design a pair of primers (Table S1) to amplify cDNA containing siRNA fragments. Further BLASTN searches of target cDNA sequences with H. vigintioctopunctata transcriptome data [23] were performed to determine any off-target sequences that might have an identical match of 20 bp or more. For L. decemlineata, primer pairs for LdEcRA, LdEcRB1, and Ldusp (dsLdusp-1, dsLdusp-2) are listed in Table S1. In addition, a cDNA fragment of enhanced green fluorescent protein (egfp) gene was obtained from Aequorea victoria. PCR amplification was performed with specific primers conjugated with the T7 RNA polymerase promoter sequence (Table S1). According to the instructions provided by the kit, dsRNA was synthesized in vitro using a MEGAscript T7 high-yield transcription kit (Ambion, Austin, TX, USA). The quality of synthetized dsRNA was detected by agarose gel electrophoresis, and the concentration (5–8 μg/μL) of the synthetized dsRNA was measured by a NanoDrop1000 spectrophotometer. The resultant dsRNA products were stored at −80 °C before experiments.
2.4. Injection of dsRNAs and Bioassay
Injecting dsRNA was performed according to a documented method [24,25]. In short, 0.1 μL of solution containing 500 ng dsRNA was injected into the newly emerged female adults. Negative control adults were injected with the same volume of dsegfp solution.
Juvenile hormone III (JH) (purity > 65%) and ecdysteroid 20-hydroxyecdysone (20E) (purity > 93%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). JH was further purified by reverse-phase high-performance liquid chromatography (RP-HPLC); its final purity reached 90%. 20E and JH were dissolved in distilled water containing surfactant (Tween 20, 1 g/L) to obtain, respectively, 10−3 M and 100 ng/mL stock solutions.
For L. decemlineata, a total of 900 newly emerged female adults were used to carry out two independent biological experiments. A replicate contains 10 individuals, and a total of 90 replicates were set. The first was intended to determine the function of Ldusp; 450 newly emerged female adults were randomly separated into 45 replicates (groups); 9 groups were injected with dsegfp, 18 replicates with dsLdusp-1 and another 18 groups with dsLdusp-2. Nine dsegfp-, 9 dsLdusp-1- and 9 dsLdusp-2-treated groups were fed on normal potato leaves. Nine dsLdusp-1- and 9 dsLdusp-2-treated groups were fed on potato leaves immersed with 30 mL JH (100 ng/mL). The second experiment was intended to evaluate the function of LdEcR; a total of 450 newly emerged female adults were randomly separated into 45 replicates; 9 groups were injected with dsegfp, 18 groups with dsLdEcRA and 18 groups with dsLdEcRB1. Nine dsegfp-, 9 dsLdEcRA- and 9 dsLdEcRB1-treated female beetles were fed on normal potato leaves. Nine dsLdEcRA- and 9 dsLdEcRB1-treated groups were fed on potato leaves immersed with 30 mL JH (100 ng/mL). One day later, these female adults were fed with fresh potato foliage. Three days after injection, three repeated samples were taken for qRT-PCR to detect the efficacy of RNA interference. Ten days after injection, three repeats were used to measure the expression levels of vitellogenesis genes (LdVg1, LdVg2 and LdVgR). Six days after injection, another three replicates were used for dissection for observation under a microscope.
For H. vigintioctopunctata, three independent biological experiments were carried out using the newly emerged female adults, with two treatments: (1) dsegfp and (2) dsHvEcR, dsHvEcRB1 or dsHvusp. A group of 10 injected newly emerged female adults was set as a replicate. For dsHvEcR RNAi experiment, each treatment had 21 replicates. A total of nine replicates were harvested 3, 10 and 20 days after treatment. Three days after injection, three repeated samples were taken for qRT-PCR to detect the efficacy of RNAi. Ten and twenty days after treatment, the other six replicates were used to measure the expression levels of vitellogenesis genes (HvVg and HvVgR). Another 12 repeated treatments were collected 10, 15 or 30 days after injection and used to dissect and observe the ovaries under a microscope or used for sectioning and HE staining. For dsHvRcRB1 RNAi experiment, each treatment had nine replicates. Three and ten days after treatment, a total of six replicates were harvested to extract total RNA. The other three repeats were used to observe the 10-day-old ovaries after dissection. For dsHvusp RNAi experiment, each treatment had 21 replicates. A total of nine replicates were harvested 3, 10 and 20 days after treatment. Three days after injection, three repeated samples were taken for qRT-PCR to detect the efficacy of RNA interference. Ten and twenty days after treatment, the other six replicates were used to measure the expression levels of vitellogenesis genes (HvVg and HvVgR). Another 12 repeated samples were collected and dissected 10, 20 and 30 days after injection, observed under microscope or stained with HE.
2.5. In Vitro Fat Body Culture
One-day-old L. decemlineata adult fat bodies were dissected in saline. The isolated fat bodies were washed with culture medium four times and then cultured independently in 25 °C EX-CELL® 405 Serum-Free Medium for Insect Cells (Sigma-Aldrich, USA). The fat bodies from 12 individuals were cultured in culture medium (control), 10−4 M cycloheximide (Chx) (Sigma-Aldrich, USA), 10−6 M 20E, or 10−4 M Chx + 10−6 M 20E. The treatment was repeated three times. Samples were randomly selected at 0, 3, 6, 12 and 24 h after incubation for mRNA expression analysis.
2.6. Quantitative Real-Time PCR
For analysis of the effects of treatments, total RNA was extracted from treated adults. Each sample contained 10 individuals and were repeated three times. The collected RNA samples were extracted using SV Total RNA Isolation system Kit (Promega, Madison, WI, USA), and DNase I was used to remove any residual DNA from the purified RNA. For H. vigintioctopunctata, using two internal control genes (HvRPS18 and HvRPL13, primers listed in Table S1) according to the published results [26]. For L. decemlineata, using four internal control genes (LdRP4, LdRP18, LdARF1 and LdARF4, the primers listed in Table S1) according to our published results [27]. Quantitative measurements of mRNA by qRT-PCR in a triplicate technique. The 2−ΔΔCT method was used for calculating the relative value, using the geometric mean of internal control genes for normalization.
2.7. Hematoxylin-Eosin (HE) Staining
The phenotypic defects of ovaries were observed by HE staining. Simply, 10-, 20- and 30-day-old ovaries were dissected after the adults were treated with dsegfp-, dsEcR and dsusp, and were then fixed with 4% paraformaldehyde and embedded in paraffin. Then, the paraffin-embedded ovarian tissue was cut into 6-μm sections. Ovarian tissue sections were hydrated and stained with Mayer’s H&E (Yeasen, Shanghai, China), and then observed under an Olympus BH2 light microscope (Olympus, Tokyo, Japan).
2.8. Data Analysis
SPSS for Windows (Chicago, IL, USA) were used for statistical analysis. After ensuring the normal distribution of the data, multiple comparisons were made by one-way analysis of variance (ANOVA) and the Tukey–Kramer post-test. Some data were compared by unpaired Student’s t-test. Values (mean ± SE) of p < 0.05 were regarded as significant.
3. Results
3.1. In Vitro Induction of Hormonal and Vitellogenesis Genes by 20E in L. decemlineata
The fat bodies of newly emerged female adults were dissected and cultured with 20E/cycloheximide (Chx) (Figure 1). In order to determine whether the incubation in the presence of 10−6 M 20E activates 20E signaling, the expression of late ecdysone response gene LdFTZ-F1 was measured. As expected, an addition of 20E greatly induced LdFTZ-F1 transcription 3, 6, 12 and 24 h after treatment. However, an addition of 10−4 M Chx (the protein synthesis inhibitor) in the 20E-containing medium almost completely abrogated the induction. It appears that the stimulation of 20E signaling needs protein synthesis (Figure 1A).
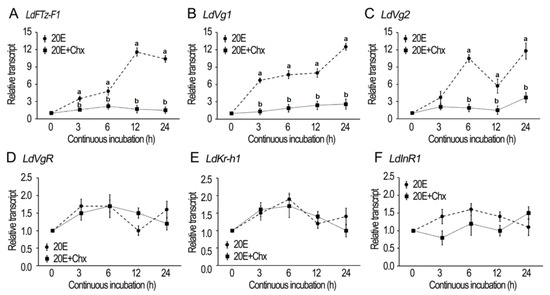
Figure 1.
20E-induced expression of hormone response and vitellogenesis genes in Leptinotarsa decemlineata. The fat bodies from newly emerged female adults were incubated with a mixture of culture medium, 10−4 M cycloheximide (Chx), 10−6 M 20E, and 10−4 M Chx + 10−6 M 20E for 3, 6, 12, and 24 h. The expression levels of LdFTZ-F1, LdVg1, LdVg2, LdVgR, LdKr-h1 and LdInR1 genes were detected respectively (A–F). The expression level of the culture medium–incubation group was similar to that of the 10−4 M Chx-incubated group at each time point, and the average value was defined as 1. The dot represents the 2−ΔΔCT method value (±SD), normalized to the geometrical mean of housekeeping gene expression. Different letters indicate significant difference at p value < 0.05.
Similarly, the incubation in the presence of 10−6 M 20E activated the expression of LdVg1 and 2 in the fat bodies. The activation of transcription of LdVg1 and 2 was also inhibited by Chx (Figure 1B,C).
In contrast, the incubation in the presence of 20E or 20E + Chx could not up-regulate the expression of LdVgR, Krüppel homolog 1 (LdKr-h1) and Insulin receptor (LdInR1) (Figure 1D–F).
3.2. RNAi of usp Represses Vitellogenesis in L. decemlineata
Introduction of two dsRNAs (dsLdusp-1 and dsLdusp-2) targeting different regions of Ldusp into the 1-day-old female adults reduced the expression levels to similar extents (Figure 2A).
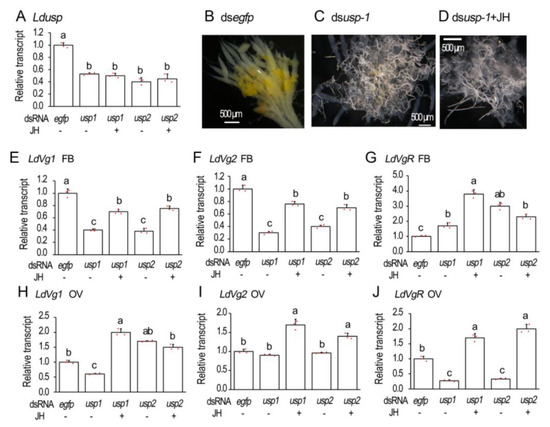
Figure 2.
Knockdown of Ldusp represses vitellogenesis in Leptinotarsa decemlineata. The newly emerged female adults were treated with dsegfp, dsLdusp-1, dsLdusp-2, dsusp-1 + 100 ng/mL JH and dsLdusp-2 + 100 ng/mL JH. The treated beetles were fed on fresh potato foliage. Three and ten days after treatment, transcript levels of Ldusp (A, 3d), LdVg1 (E,H, 10d), LdVg2 (F,I, 10d) and LdVgR (G,J, 10d) were determined. The relative transcripts refers to the ratios of relative copy numbers of the treated individuals to dsegfp-treated controls, which are set to 1. Different letters indicate significant difference at p < 0.05 using ANOVA with the Tukey–Kramer test. The ovaries of 6-day-old females were dissected and imaged (B–D).
The 6-day-old ovaries were dissected and their structures were observed. In the control ovarioles, the absorption of the yolk was initiated but not completed. The yellow-colored yolk molecules were accumulated in the developing oocytes (Figure 2B). The accumulation of yellow-colored yolk substances was hindered in the Ldusp RNAi beetles. The hindrance could not be relieved by the introduction of JH (Figure 2C,D).
The transcript levels of genes involved in the vitellogenesis were measured 10 days after dsRNA injection. The expression levels of both LdVg1 and LdVg2 were reduced in the Ldusp RNAi fat bodies. The reduction could be partially restored by JH application (Figure 2E,F).
For vitellogenin receptor gene LdVgR, its transcript levels declined in the Ldusp knockdown ovaries, which could be over-rescued by JH introduction (Figure 2J).
3.3. RNAi of EcR Isoforms Mirrors the Inhibition of Vitellogenesis in L. decemlineata
Isoform-specific dsRNA (dsLdEcRA, dsLdEcRB1) [24] were individually introduced into the 1-day-old adult females. In the females having suffered from a dsLdEcRA injection, LdEcRA + LdEcRB1 (hereafter LdEcR) and LdEcRA mRNA levels were significantly decreased. In beetles injected with dsLdEcRB1, transcription levels of LdEcR and LdEcRB1 were significantly reduced, while expression levels of LdEcRA were significantly increased (Figure 3A–C). Treatment with JH did not significantly change the expression levels of LdEcRA, LdEcRB1, or LdEcR in the treated females (Figure 3A–C).
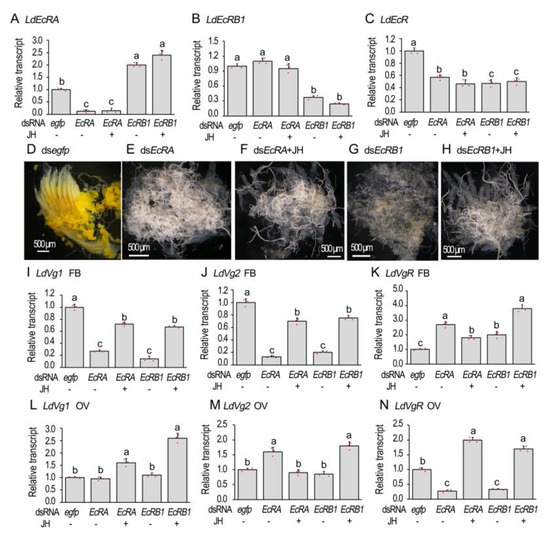
Figure 3.
RANi of LdEcR inhibits vitellogenesis in Leptinotarsa decemlineata. The newly emerged female adults were treated with dsegfp, dsLdEcRA, dsLdEcRB1, dsLdEcRA + 100 ng/mL JH and dsLdEcRB1 + 100 ng/mL JH. The treated beetles were fed on fresh potato foliage. Three and ten days after treatment, transcript levels of LdEcRA, LdEcRB1 and both isoforms (A–C, 3d), LdVg1 (I,L, 10d), LdVg2 (J,M, 10d) and LdVgR (K,N, 10d) were determined. The relative transcripts refers to the ratios of relative copy numbers of the treated individuals to dsegfp-treated controls, which are set to 1. Different letters indicate significant differences at p < 0.05 using ANOVA with the Tukey–Kramer test. The ovaries of 6-day-old females were dissected and imaged (D–H).
Knockdown of either LdEcRA or LdEcRB1 delayed the accumulation of yolk; the ovarioles appeared to be almost transparent (Figure 3E,G). Introducing JH into the LdEcRA or LdEcRB1RNAi females did not rescue the delay of the yolk accumulation, estimating by the almost transparent ovarioles (Figure 3F,H).
In the fat bodies, knockdown of either LdEcRA or LdEcRB1 significantly reduced the mRNA levels of both vitellogenin genes LdVg1 and LdVg2. Introduction of JH into the LdEcRA or LdEcRB1 RNAi females partially alleviated the reduction of the mRNA contents (Figure 3I,J). In the ovaries, RNAi of LdEcRA or LdEcRB1 did not reduce the expression levels of LdVg1 or LdVg2, whereas application of JH stimulated the transcription of LdVg1 (Figure 3L,M).
Consistently, LdVgR levels declined in the LdEcRA and LdEcRB1 RNAi ovaries. JH application over-compensated LdVgR expression levels (Figure 2N). In contrast, the levels of LdVgR were higher in the dsLdEcRA, dsLdEcRB1, dsLdEcRA + JH, and dsLdEcRB1 + JH-treated fat bodies than that in the control sample (Figure 3K).
3.4. Depletion of HvEcR Impairs Vitellogenesis in H. vigintioctopunctata
After knockdown of both HvEcR isoforms (Figure 4A), the phenotypic defects of the resultant females were examined.
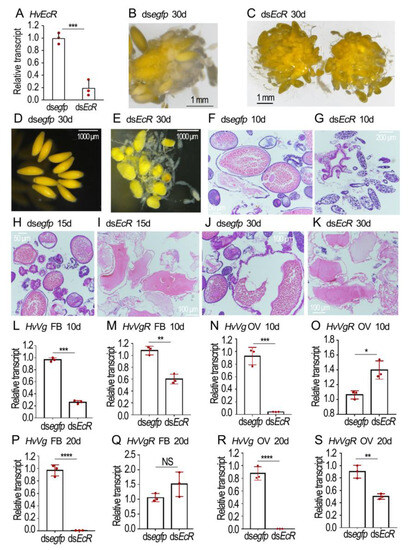
Figure 4.
Depletion of HvECR impairs vitellogenesis in Henosepilachna vigintioctopunctata. The newly emerged female adults were treated with 0.1 μL dsegfp and dsHvEcR (400 ng) by injection. The treated beetles were fed on fresh potato foliage. Three, ten and twenty days (3d, 10d and 20d) after treatment, transcript levels of HvEcR (A), HvVg (L,M,P,Q) and HvVgR (N,O,R,S) were determined. The relative transcripts refers to the ratios of relative copy numbers of the treated individuals to dsegfp-treated controls, which are set to 1. Different instars indicate significant difference at p < 0.05 (*), 0.01 (**), 0.001 (***), or 0.0001 (****) using t-test. These columns use vertical lines to represent averages and indicating SD. NS, no significance. The ovaries of 30-day-old females were dissected and imaged (B–E). The ovaries from 10-, 15- and 30-day-old adults were sectioned, stained with hematoxylin-eosin staining (HE), and imaged (F–K).
In the normal eggs, the yellow-colored yolk substances were evenly distributed (Figure 4B,D). Conversely, the HvEcR RNAi eggs were misshapen and light-colored; the yellow matters were irregularly dispersed, with some colorless patches here and there (Figure 4C,E).
HE staining revealed that the normal eggs contained large amounts of yolk granules when examined 10, 15 and 30 days after eclosion (Figure 4F,H,J). Conversely, little yolk was accumulated in the 10-day-old HvEcR RNAi eggs (Figure 4G). Although some yolk substances were seen in the 15- and 30-day-old HvEcR RNAi eggs, they did not form granules (Figure 4I,K).
In order to determine the effects of HvEcR depletion on vitellogenin accumulation, the expression levels of vitellogenin and vitellogenin receptor genes HvVg and HvVgR in the fat body and ovary were compared at the age of 10 (Figure 4L–O) and 20 (Figure 4P–S) days. Knockdown of HvEcR significantly decreased the expression levels of HvVg in both the fat body and ovary (Figure 4L,N,P,R). The expression of HvVgR was complex: its levels were diminished in 10-day-old fat bodies and 20-day-old ovaries (Figure 4M,S), but were increased in 20-day-old fat bodies and 10-day-old ovaries (Figure 4O,Q) in the HvEcR knockdown samples, compared with the dsegfp-treated group.
3.5. RNAi of HvEcRB1 Delays Vitellogenesis in H. vigintioctopunctata
As the common region of HvEcRA and HvEcRB1 sequences was long, and no effective target fragment was predicted in the HvEcRA isoform, we designed a dsRNA to silence the HvEcRB1 (dsHvEcRB1) sequence.
Three days after dsHvEcRB1 injection, the levels of HvEcRB1 and the total level of two isoforms (HvEcR) were significantly reduced (Figure 5A,B). In contrast, the mRNA level of HvEcRA was not changed (Figure 5C). The expression of six 20E signaling genes (HvE74, HvE75, HvE93, HvHR3, HvHR4 and HvFTZ-F1) was also measured. Knockdown of HvEcRB1 did not decrease the mRNA levels of these genes (Figure S1).

Figure 5.
RNAi of HvEcRB1 delays vitellogenesis in Henosepilachna vigintioctopunctata female adults. The newly emerged female adults were treated with 0.1 μL dsegfp and dsEcRB1 (400 ng) by injection. The treated beetles were fed on fresh potato foliage. Three (A–C) or ten days (D,E) after treatment, transcript levels of HvEcR (two isoforms), HvEcRB1 and HvEcRA in whole bodies, HvVg in fat body and HvVgR in ovaries were determined. The relative transcripts refers to the ratios of relative copy numbers of the treated individuals to dsegfp-treated controls, which are set to 1. The number of eggs was recorded in 10-day-old females (F). Different instars indicate significant difference at p < 0.01 (**), 0.001 (***), or 0.0001 (****) using t-test. These columns use vertical lines to represent averages and indicating SD. NS, no significance. The 10-day-old ovaries are shown (G,H).
The transcript levels of HvVg in the fat body and HvVgR in the ovary were detected. Depletion of HvEcRB1 isoform negatively affected the expression of the two genes (Figure 5D,E). Consistently, the HvEcRB1 RNAi ovary contained less mature eggs (Figure 5H vs. Figure 5G); the 10-day-old HvEcRB1 RNAi females laid fewer eggs (Figure 5F).
3.6. Silence of Hvusp Disrupts Vitellogenesis in H. vigintioctopunctata
Injection of dsHvusp significantly reduced Hvusp mRNA level when measured three days after treatment (Figure 6A). In the 10-day-old normal ovaries, a matured oocyte was seen in each ovariole. Conversely, the primary oocytes were not matured in the 10-day-old Hvusp RNAi ovarioles (Figure 6B vs. Figure 6C). In the 30-day-old Hvusp RNAi ovarioles, misshapen eggs were accumulated; the colored matters were irregularly dispersed (Figure 6D).
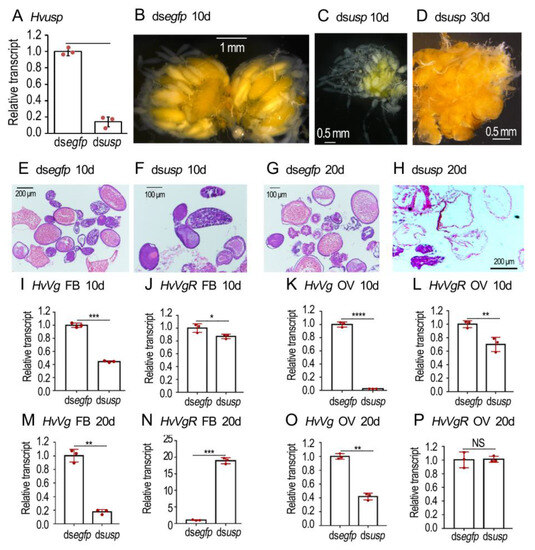
Figure 6.
RNAi of Hvusp represses vitellogenesis in Henosepilachna vigintioctopunctata. The newly emerged female adults were treated with 0.1 μL dsegfp and dsHvusp (400 ng) by injection. The treated beetles were fed on fresh potato foliage. Three, ten and twenty days (3d, 10d and 20d) after treatment, transcript levels of HvEcR (A), HvVg (I,K,M,O) and HvVgR (J,L,N,P) were determined. The relative transcripts refers to the ratios of relative copy numbers of the treated individuals to dsegfp-treated controls, which are set to 1. Different instars indicate significant difference at p < 0.05 (*), 0.01 (**), 0.001 (***), or 0.0001 (****) using t-test. These columns use vertical lines to represent averages and indicate SD. NS, no significance. The ovaries of 10- and 20-day-old females were dissected and imaged (B–D). The ovaries from 10- and 20-day-old adults were sectioned, stained with hematoxylin-eosin staining (HE), and imaged (E–H).
HE staining revealed that the normal eggs contained large amount of yolk granules when examined 10 and 20 days after eclosion (Figure 6E,G). Conversely, little yolk was accumulated in the 10- and 20-day-old Hvusp RNAi eggs (Figure 6F,H).
The expression levels of HvVg and HvVgR in the fat body and ovary were detected at the age of 10 (Figure 6I–L) and 20 (Figure 6M–P) days. RNAi of Hvusp significantly decreased the expression levels of HvVg in both the fat body and ovary (Figure 6I,K,M,O). Similarly, the HvVgR levels were significantly diminished in 10-day-old fat bodies and ovaries (Figure 6J,L), but were greatly increased in 20-day-old fat bodies (Figure 6N) in the Hvusp knockdown samples, compared with the dsegfp-treated group.
4. Discussion
Vitellogenesis is characterized by the synthesis of Vg in the fat body and secretion into the hemolymph, which is transported to the oocyte through the intercellular channels (patency) in the follicular epithelium and is absorbed and stored by the maturing oocyte [28]. The regulation of Vg synthesis, patency formation and Vg uptake are three key steps to affect Vg deposition in oocytes. In the current article, we examined the requirements of 20E signaling for vitellogenesis. Our functional analysis shows that 20E signaling plays a key role in the regulation of Vg synthesis and uptake in two representative Coleoptera species.
4.1. 20E Signaling Regulates Vg Synthesis in Fat Body, in a JH-Independent Pattern
In the current paper, we discovered that in vitro culture of L. decemlineata 1-day-old fat bodies with 20E significantly stimulated the expression of both Vg genes but not Kr-h1 and InR1 genes, in a cycloheximide-dependent pattern (Figure 1). Our findings indicate that 20E signaling activates Vg biosynthesis in a JH and ILP signals-independent manner. In line with our result, cumulative studies have confirmed that vitellogenesis is activated by the rise of ecdysteroid titer in some Hymenoptera, Lepidoptera, Diptera and Coleoptera insect species [29,30,31]. In Drosophila melanogaster, for instance, the presence of 20E contributes to the high Vg synthesis rate in the fat body [32]. Similarly, in Aedes aegypti, 20E stimulates Vg expression in the fat body and oocyte maturation in the ovary after a blood meal [33]. In T. castaneum, knockdown of 20E signaling pathway genes blocks Vg synthesis [4].
Repression of 20E-stimulated Vg expression by cycloheximide addition (Figure 1) demonstrates that protein synthesis is involved. It implies that active 20E cascade is critical for the stimulation of Vg biosynthesis in the fat body in L. decemlineata. Therefore, we knocked down either EcR or usp in the two Coleoptera species. Our results uncovered that the knockdown dramatically suppressed the transcription of Vg in adult fat bodies of the two beetle species (Figure 2, Figure 3, Figure 4 and Figure 6). Reduced Vg mRNA levels may lower the corresponding protein contents. As a result, oocyte maturation would be delayed in both beetle species. Thus, we observed that RNAi of EcR or usp in the two Coleoptera species delayed yolk accumulation and oocyte growth (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). Application of JH into the EcR or usp RNAi L. decemlineata females only partially rescued the decreased Vg levels (Figure 2 and Figure 3). The in vivo RNAi results provided another piece of evidence that 20E signaling regulates Vg synthesis in the fat body, in a JH-independent pattern.
Consistently, in some Lepidopterans such as Bombyx mori, Hyalophora cecropia, and Spodoptera frugiperda, 20E has a primary role in vitellogenesis [29,34]. Repression of 20E synthesis remarkably reduces the expression levels of Vg and VgR, resulting in egg development defects of Plutella xylostella [29]. A similar stimulatory role of 20E signaling in Vg synthesis has also been documented in D. melanogaster [13].
4.2. Interaction of 20E and JH Signaling Enhances Vg Synthesis in Fat Body
Partial rescuing of Vg expression by JH in the EcR or usp RNAi females in L. decemlineata (Figure 2 and Figure 3) also suggests interaction of 20E and JH signaling enhances Vg synthesis in fat body.
In agreement with our result, the interactions of 20E, JH and ILP cascades during stimulation of Vg biosynthesis have been documented in two beetle species, T. castaneum and C. bowringi [9,11,17], and non-Coleoptera insect species [2,35]. Firstly, 20E signaling, through the downstream component ETH, stimulates JH biosynthesis. In Periplaneta americana, injection of 20E inhibits the transcription of juvenile hormone acidmethyltransferase (jhamt), Vg and VgR [36]. In D. melanogaster, ETH plays a crucial role in the maintenance of JHAMT [37]. In Bactrocera dorsalis, injection of dsRNA targeting ETH or ETH receptor (ETHR) into female adults reduces the expression of jhamt, Vg2, and a few JH signal genes; 20E or methoprene can restore egg production to normal levels [35]. Secondly, JH signaling enhances the 20E cascade. For example, in D. melanogaster, JH acts on the phosphorylation of USP through the receptor tyrosine kinase-phospholipase C-protein kinase C (RTK-PLC-PKC) cascade, thus enhancing the role of 20E in activating Vg production [38]. In A. aegypti, JH promotes the ability of fat body for Vg synthesis, while 20E stimulates Vg expression in the fat body and oocyte maturation in the ovary after a blood meal [33]. Thirdly, 20E signaling can interact with the ILP pathway. For example, in A. aegypti, ETH mobilizes calcium ions from the endoplasmic reticulum through IP3 receptors to regulate the activities of JHAMT and JH [13]. Meanwhile, insulin and bombyxin (an insulin-like hormone) are directly stimulated in the prothoracic glands in B. mori. Both insulin and prothoracicotropic hormone (PTTH) stimulate the ecdysteroid secretion by increasing the phosphorylation of Akt [39].
How 20E signaling interacts with JH cascade during vitellogenesis in the two Coleoptera species in this deserves further investigation.
4.3. Isoform Specific Role of EcR in the Regulation of Vg Accumulation
In L. decemlineata, knockdown of either isoform of EcR suppressed Vg accumulation, indicated by the decreased Vg levels and the transparent ovarioles in the resultant females (Figure 3). In H. vigintioctopunctata, RNAi of HvEcRB1 greatly reduced the expression of HvVg. Moreover, the HvEcRB1 RNAi ovary contained less mature eggs and the 10-day-old HvEcRB1 RNAi females laid fewer eggs (Figure 5).
Consistently, EcR isoform-dependent regulation of oogenesis has been documented in other insects. In D. melanogaster, 20E regulates follicle rupture and ovulation by activating EcRB2. The deletion of EcR reduces egg laying of females, which can be reversed by ectopic expression of EcRB2 [40]. Moreover, the eggshell gene VM32E can produce components of the vitelline membrane and endochorion layers, and transcription of the gene is activated by EcRB1 and USP [41].
In this survey, we found that knockdown of HvEcRB1 did not decrease the mRNA levels of six 20E signaling genes (HvE74, HvE75, HvE93, HvHR3, HvHR4 and HvFTZ-F1) (Figure S1). It appears that the EcRB1/USP complex may directly trigger the expression of HvVg and HvVgR during vitellogenesis in H. vigintioctopunctata. In line with our data, the Vg 5′ regulatory region contains several EcR response elements (EcREs) in A. aegypti, providing evidence of direct control of this gene by EcR-USP [42,43].
Comparison of the negative effect on vitellogenesis in the HvEcRB1 and HvEcRA + HvEcRB1 RNAi ovaries indicates that the latter exhibits a severe defective phenotype in H. vigintioctopunctata (Figure 5 vs. Figure 4). It appears that the 20E-EcRA/USP complex may also be associated with the stimulation of vitellogenesis by an unknown signaling cascade. One possible way is a positive signal from the normal ovaries. It is known that a normal ovary releases an ovarian factor at a specific stage of development that stimulates the corpora allata of Diploptera punctata to synthesis JH [44]. We accordingly propose that failure to initiate Vg transcription in the fat bodies of EcR or usp RNAi beetles (this study) and T. castaneum [4] partially comes from an indirect effect caused by ovarian maturation retardation. Another possible way is negative feedback from accumulated Vg in hemolymph. In the present paper, we found that VgR was also expressed in the fat body (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6), and that Vg may serve as an autocrine molecule binding to VgR to regulate the expression of its gene in the fat body. In terms of the signaling role of Vg, some results have been reported in worker bees, where Vg plays a role in sensing fat body sugars and in gustatory perception [31,44].
4.4. Hormonal Signals Control VgR Expression on Oocytes
During reproductive development, the expression of VgR is regulated by hormones. However, the regulation of VgR expression is complex. JH, 20E, ILP/TOR signaling pathways and micro RNAs have been documented to be involved [3].
In this paper, RNAi of either Ldusp or LdEcR significantly inhibited the expression of LdVgR in the ovaries in L. decemlineata. Meanwhile, application of JH into the Ldusp or LdEcR RNAi L. decemlineata females over-compensated LdVgR expression levels (Figure 2 and Figure 3). In another Coleoptera, C. bowringi, JH enhances VgR expression by the Met-Kr-h1 pathway [45]. Similarly, the RNAi of BmKr-h1 in B. mori reduces the deposition of vitellin and leads to partially transparent chorion [46]. Kr-h1 seems to trigger the expression of VgR in the ovaries. In adults, Kr-h1 is induced by 20E and is a key participant in the JH signaling pathway [47]. On one hand, the Met–Taiman complex binds the JH response element in the Kr-h1 promoter and directly regulates its transcription [1,48]. On the other hand, in vivo analysis displays that Kr-h1 is significantly up-regulated by 20E signaling in D. melanogaster [49,50] and B. mori [47,51]; in vitro culture shows that Kr-h1 is significantly up-regulated by 20E in T. castaneum TcA cells [52] and in Helicoverpa armigera larval epidermis [53]. Moreover, 20E significantly synergizes the BmKr-h1 induction by JH analog on cultured B. mori larval and pupal epidermis and the NIAS-Bm-aff3 cell line [54]. We can accordingly propose that Kr-h1 may also govern the expression of LdVgR in the L. decemlineata ovary. Given that LdKr-h1 may be independently activated by both 20E and JH cascades, suppression of 20E signaling in the LdEcR and Ldsup RNAi adults down-regulates LdKr-h1 expression and thus reduces the LdVgR level, while application of JH into LdEcR and Ldsup RNAi adults up-regulates LdKr-h1 expression and thus over-compensates the LdVgR level.
Consistent with our proposal, knockdown of either HvEcR or Hvusp caused a complicated influence on the expression of HvVgR in H. vigintioctopunctata ovaries (Figure 4 and Figure 6). Therefore, the transcription of VgR may also be regulated by both 20E and JH signaling through Kr-h1. Actually, the complex regulation of VgR expression has widely been documented in other insect species. For instance, in P. americana, the expression of VgR is suppressed by additional 20E treatment [36]. Inhibition of 20E synthesis significantly decreased the expression levels of VgR, resulting in the development of defective eggs in P. xylostella [29]. In Solenopsis invicta, JH significantly increased the expression of VgR in cultured ovaries [51]. Analogously, JH treatment induced the expression of VgR in Nilaparvata lugens adult females [55]. Further research will shed light on the hormonal control of VgR expression in both L. decemlineata and H. vigintioctopunctata ovaries.
Our data here also displayed that even though application of JH partially recovered the decreased LdVg expression and over-compensated LdVgR expression levels in the LdEcR or Ldusp RNAi females in L. decemlineata, it does not change the transparency of the ovaries (Figure 2 and Figure 3). These findings suggest that 20E signaling directly hinders oocyte maturation in L. decemlineata, similar to the results in another Coleoptera, T. castaneum [5]. Obviously, hindrance of oocyte maturation inhibits uptake of Vg proteins in the LdEcR or Ldusp RNAi females in L. decemlineata. This issue deserves further investigation.
5. Conclusions
The data in the current paper suggest that 20E signaling, through the EcR/USP complex, induces vitellogenesis in two Coleoptera species, in an isoform-dependent way. Our research provides a solid foundation for future studies to understand the molecular mechanisms of reproduction regulation in beetles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12101284/s1, Figure S1: Knocking down HvECRB1 did not reduce the expression of 20E signaling genes in female adults; Table S1: A list of primers used for RT-PCR, dsRNA synthesis and qRT-PCR. Supplementary materials are cited in the main text.
Author Contributions
Conceptualization, X.Z.; resources, original draft preparation, L.J.; data curation, X.Z.; writing—review and editing, G.L.; supervision, L.J.; project administration, G.L.; funding acquisition, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFC2601000), and China Agriculture Research System of MOF and MARA (CARS-09-P22) to Guo-Qing Li.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated in association with this study are available in the Supplementary Materials published online with this article.
Acknowledgments
This research was supported by the National Key Research and Development Program of China (2022YFC2601000), and China Agriculture Research System of MOF and MARA (CARS-09-P22).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Church, S.H.; de Medeiros, B.A.S.; Donoughe, S.; Márquez Reyes, N.L.; Extavour, C.G. Repeated loss of variation in insect ovary morphology highlights the role of development in life-history evolution. Proc. Biol. Sci. 2021, 288, 20210150. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Z.; Ahmad, S.; Ngegba, P.M.; Zhong, G. Role of endocrine system in the regulation of female insect reproduction. Biology 2021, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, L.; He, Q.; Zhou, S. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 2021, 8, 593613. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Sheng, Z.; Sun, Z.; Palli, S.R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 429–439. [Google Scholar] [CrossRef]
- Jowett, T.; Postlethwait, J.H. The regulation of yolk polypeptide synthesis in Drosophila ovaries and fat body by 20-hydroxyecdysone and a juvenile hormone analog. Dev. Biol. 1980, 80, 225–234. [Google Scholar] [CrossRef]
- Richard, D.S.; Watkins, N.L.; Serafin, R.B.; Richard, D.S. Ecdysteroids regulate yolk protein uptake by Drosophila melanogaster oocytes. J. Insect Physiol. 1998, 44, 637–644. [Google Scholar] [CrossRef]
- Lou, Y.H.; Pan, P.L.; Ye, Y.X.; Cheng, C.; Xu, H.J.; Zhang, C.X. Identification and functional analysis of a novel chorion protein essential for egg maturation in the brown planthopper. Insect Mol. Biol. 2018, 27, 393–403. [Google Scholar] [CrossRef]
- Lou, Y.H.; Shen, Y.; Li, D.T.; Huang, H.J.; Lu, J.B.; Zhang, C.X. A mucin-like protein is essential for oviposition in Nilaparvata lugens. Front. Physiol. 2019, 10, 551. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Telfer, W.H. The mechanism and control of yolk formation. Annu. Rev. Entomol. 1965, 10, 161–184. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Sun, Z.; Bai, H.; Palli, S.R. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010, 40, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.J. Age-dependent changes in corpus activity in vitro in the adult Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 1978, 24, 461–464. [Google Scholar] [CrossRef]
- Areiza, M.; Nouzova, M.; Rivera-Perez, C.; Noriega, F.G. Ecdysis triggering hormone ensures proper timing of juvenile hormone biosynthesis in pharate adult mosquitoes. Insect Biochem. Mol. Biol. 2014, 54, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Abrisqueta, M.; Süren-Castillo, S.; Maestro, J.L. Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2014, 49, 14–23. [Google Scholar] [CrossRef]
- Sheng, Z.; Xu, J.; Bai, H.; Zhu, F.; Palli, S.R. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 2011, 286, 41924–41936. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Bi, J.; Feng, Q.; Beerntsen, B.T.; Song, Q. Neuropeptide bursicon influences reproductive physiology in Tribolium Castaneum. Front. Physiol. 2021, 12, 717437. [Google Scholar] [CrossRef]
- Guo, S.; Tian, Z.; Wu, Q.W.; King-Jones, K.; Liu, W.; Zhu, F.; Wang, X.P. Steroid hormone ecdysone deficiency stimulates preparation for photoperiodic reproductive diapause. PLoS Genet. 2021, 17, e1009352. [Google Scholar] [CrossRef]
- Eid, D.M.; Chereddy, S.C.R.R.; Palli, S.R. The effect of E93 knockdown on female reproduction in the red flour beetle, Tribolium castaneum. Arch. Insect Biochem. Physiol. 2020, 104, e21688. [Google Scholar] [CrossRef]
- Xu, J.; Tan, A.; Palli, S.R. The function of nuclear receptors in regulation of female reproduction and embryogenesis in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 2010, 56, 1471–1480. [Google Scholar] [CrossRef]
- Jiang, H.; Meng, X.; Zhang, N.; Ge, H.; Wei, J.; Qian, K.; Zheng, Y.; Park, Y.; Reddy Palli, S.; Wang, J. The pleiotropic AMPK-CncC signaling pathway regulates the trade-off between detoxification and reproduction. Proc. Natl. Acad. Sci. USA 2023, 120, e2214038120. [Google Scholar] [CrossRef]
- Huang, Z.; Tian, Z.; Zhao, Y.; Zhu, F.; Liu, W.; Wang, X. MAPK signaling pathway is essential for female reproductive regulation in the cabbage beetle, Colaphellus bowringi. Cells 2022, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Guo, S.; Zhu, F.; Liu, W.; Wang, X.P. Targeting coat protein II complex genes via RNA interference inhibits female adult feeding and reproductive development in the cabbage beetle Colaphellus bowringi. Pest. Manag. Sci. 2022, 78, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Wang, F.; Guo, J.; Deng, X.Y.; Chen, J.Y.; Lin, L.B. Characterization of ladybird Henosepilachna vigintioctopunctata transcriptomes across various life stages. Sci. Data 2018, 5, 180093. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-Y.; Deng, P.; Zhang, Q.; Li, A.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. Ecdysone receptor isoforms play distinct roles in larval-pupal-adult transition in Leptinotarsa decemlineata. Insect Sci. 2020, 27, 487–499. [Google Scholar] [CrossRef]
- Ze, L.-J.; Xu, P.; Kang, W.-N.; Wu, J.-J.; Jin, L.; Anjum, A.A.; Li, G.-Q. Disruption of kynurenine pathway reveals physiological importance of tryptophan catabolism in Henosepilachna vigintioctopunctata. Amino Acids 2021, 53, 1091–1104. [Google Scholar] [CrossRef]
- Lü, J.; Chen, S.; Guo, M.; Ye, C.; Qiu, B.; Wu, J.; Yang, C.; Pan, H. Selection and validation of reference genes for RT-qPCR analysis of the ladybird beetle Henosepilachna vigintioctomaculata. Front. Physiol. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Shi, X.Q.; Guo, W.C.; Wan, P.J.; Zhou, L.T.; Ren, X.L.; Ahmat, T.; Fu, K.Y.; Li, G.Q. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Res Notes 2013, 13, 6–93. [Google Scholar] [CrossRef]
- Seago, A.E.; Brady, P.; Vigneron, J.P.; Schultz, T.D. Gold bugs and beyond: A review of iridescence and structural colour mechanisms in beetles (Coleoptera). J. R. Soc. Interface 2009, 6, S165–S184. [Google Scholar] [CrossRef]
- Peng, L.; Wang, L.; Zou, M.M.; Vasseur, L.; Chu, L.N.; Qin, Y.D.; Zhai, Y.-L.; You, M.-S. Identification of halloween genes and RNA interference-mediated functional characterization of a halloween gene shadow in Plutella xylostella. Front. Physiol. 2019, 10, 1120. [Google Scholar] [CrossRef]
- Telfer, W.H. Egg formation in lepidoptera. J. Insect Sci. 2009, 9, 50. [Google Scholar] [CrossRef]
- Wang, Y.; Brent, C.S.; Fennern, E.; Amdam, G.V. Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet. 2012, 8, e1002779. [Google Scholar] [CrossRef]
- Berger, E.M.; Dubrovsky, E.B. Juvenile hormone molecular actions and interactions during development of Drosophila melanogaster. Vitam. Horm. 2005, 73, 175–215. [Google Scholar] [CrossRef]
- Shin, S.W.; Zou, Z.; Saha, T.T.; Raikhel, A.S. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc. Natl. Acad. Sci. USA 2012, 109, 16576–16581. [Google Scholar] [CrossRef]
- Milas, A.; Telley, I.A. Polarity events in the Drosophila melanogaster oocyte. Front. Cell Dev. Biol. 2022, 10, 895876. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, T.-Y.; Jiang, H.-B.; Liu, X.-Q.; Dou, W.; Park, Y.; Smagghe, G.; Wang, J.-J. The ecdysis triggering hormone system, via ETH/ETHR-B, is essential for successful reproduction of a major pest insect, Bactrocera dorsalis (Hendel). Front. Physiol. 2019, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, A.; Mikani, A.; Mohamed, A.A.; Elgendy, A.M.; Takeda, M. Crosstalk among indoleamines, neuropeptides and JH/20E in regulation of reproduction in the American cockroach, Periplaneta americana. Insects 2020, 11, 155. [Google Scholar] [CrossRef]
- Meiselman, M.; Lee, S.S.; Tran, R.-T.; Dai, H.; Ding, Y.; Rivera-Perez, C.; Wijesekera, T.P.; Dauwalder, B.; Noriega, F.G.; Adams, M.E. Endocrine network essential for reproductive success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E3849–E3858. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, S.; Jia, Q.; Wu, L.; Yuan, D.; Li, E.Y. Juvenile hormone membrane signaling phosphorylates USP and thus potentiates 20-hydroxyecdysone action in Drosophila. Sci. Bull. 2022, 67, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-H.; Lin, J.-L.; Lin, P.-L.; Chen, C.-H. Insulin stimulates ecdysteroidogenesis by prothoracic glands in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2009, 39, 171–179. [Google Scholar] [CrossRef]
- Knapp, E.; Sun, J. Steroid signaling in mature follicles is important for Drosophila ovulation. Proc. Natl. Acad. Sci. USA 2017, 114, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.; Romani, P.; Tzertzinis, G.; Gargiulo, G.; Cavaliere, V. EcR-B1 and Usp nuclear hormone receptors regulate expression of the VM32E eggshell gene during Drosophila oogenesis. Dev. Biol. 2009, 328, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Kokoza, V.A.; Martin, D.; Mienaltowski, M.J.; Ahmed, A.; Morton, C.M.; Raikhel, A.S. Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene 2001, 274, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, L.; Sun, G.; Raikhel, A.S. The competence factor βFtz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol. Cell Biol. 2006, 26, 9402–9412. [Google Scholar] [CrossRef] [PubMed]
- Amdam, G.V.; Norberg, K.; Page, R.E.J.; Erber, J.; Scheiner, R. Downregulation of vitellogenin gene activity increases the gustatory responsiveness of honey bee workers (Apis mellifera). Behav. Brain Res. 2006, 169, 201–205. [Google Scholar] [CrossRef]
- Liu, W.; Guo, S.; Sun, D.; Zhu, L.; Zhu, F.; Lei, C.L.; Sheng, L.; Phelps, B.; Wang, X.-P. Molecular characterization and juvenile hormone-regulated transcription of the vitellogenin receptor in the cabbage beetle Colaphellus bowringi. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 229, 69–75. [Google Scholar] [CrossRef]
- Zhu, Z.D.; Hu, Q.H.; Tong, C.M.; Yang, H.G.; Zheng, S.C.; Feng, Q.L.; Deng, H.M. Transcriptomic analysis reveals the regulation network of BmKrüppel homolog 1 in the oocyte development of Bombyx mori. Insect Sci. 2021, 28, 47–62. [Google Scholar] [CrossRef]
- Zhu, Z.; Tong, C.; Qiu, B.; Yang, H.; Xu, J.; Zheng, S.; Song, Q.; Feng, Q.; Deng, H. 20E-mediated regulation of BmKr-h1 by BmKRP promotes oocyte maturation. BMC Biol. 2021, 19, 39. [Google Scholar] [CrossRef]
- Berrigan, D. The allometry of egg size and number in insects. Oikos 1991, 60, 313–321. [Google Scholar] [CrossRef]
- Büning, J. The trophic tissue of telotrophic ovarioles in polyphage Coleoptera. Zoomorphologie 1979, 93, 33–50. [Google Scholar] [CrossRef]
- Richter, P.; Baker, C. Ovariole number in Scarabaeoidea (Coleoptera: Lucanidae, Passalidae, Scarabaeidae). Proc. Entomol. Soc. Wash. 1974, 76, 480–498. Available online: https://biostor.org/reference/59572 (accessed on 12 May 2023).
- Chen, M.E.; Lewis, D.K.; Keeley, L.L.; Pietrantonio, P.V. cDNA cloning and transcriptional regulation of the vitellogenin receptor from the imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Insect Mol. Biol. 2004, 13, 195–204. [Google Scholar] [CrossRef]
- Mirth, C.K.; Alves, A.N.; Piper, M.D. Turning food into eggs: Insights from nutritional biology and developmental physiology of Drosophila. Curr. Opin. Insect Sci. 2019, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tanwar, P.S.; Raftery, L.A. Drosophila follicle cells: Morphogenesis in an eggshell. Semin. Cell Dev. Biol. 2008, 19, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Lu, K.; Shu, Y.; Zhou, J.; Zhang, X.; Zhang, X.; Chen, M.; Yao, Q.; Zhou, Q.; Zhang, W. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stål). J. Insect Physiol. 2015, 73, 20–29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).