Effects on Respiratory Pressures, Spirometry Biomarkers, and Sports Performance after Inspiratory Muscle Training in a Physically Active Population by Powerbreath®: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Quality Assessment
2.4. Quantitative Assessment
2.5. Risk of Bias Assessment
2.6. Meta-Analysis Data Analysis
2.7. Data Extraction
3. Results
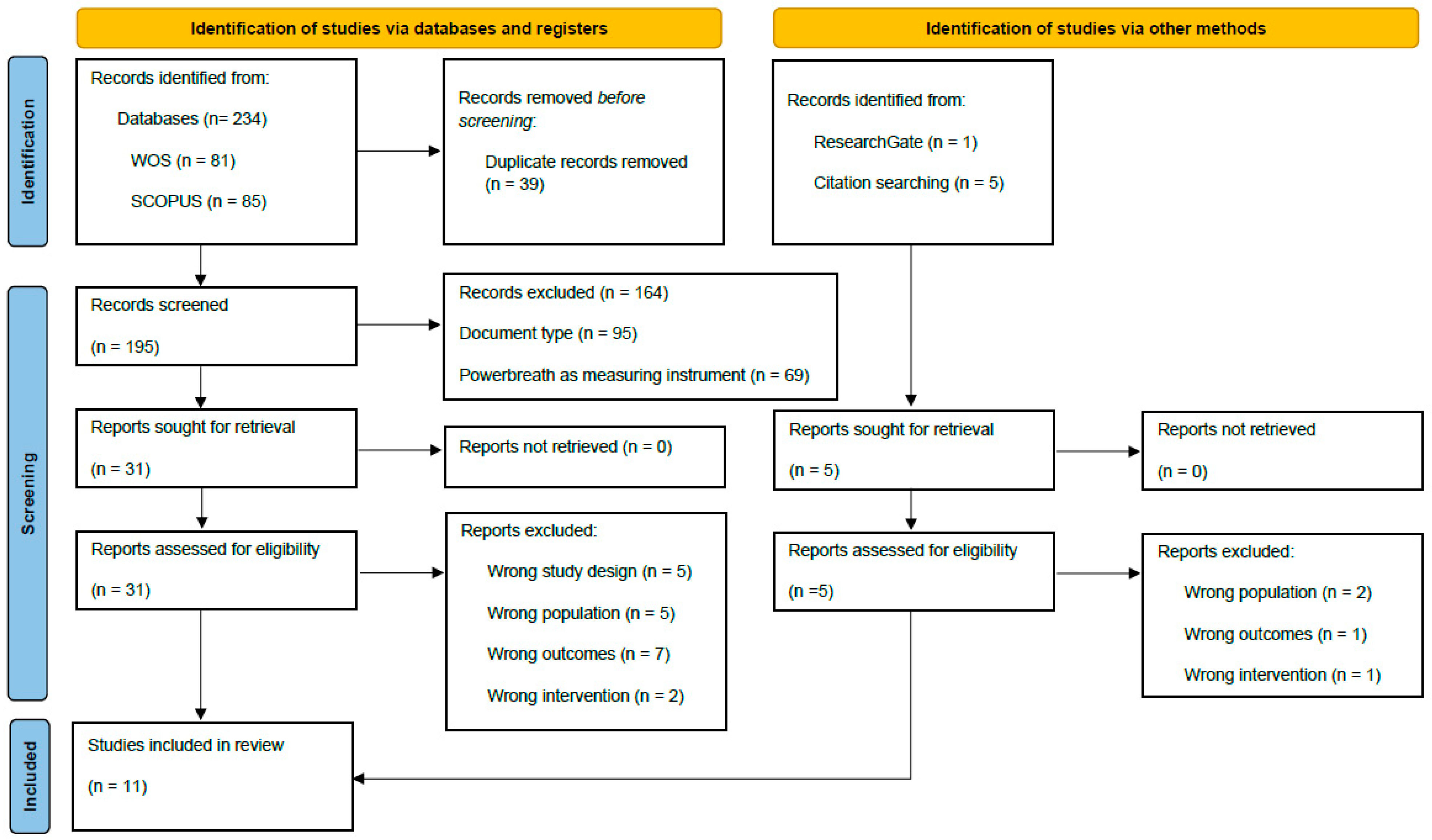
3.1. Study Selection
3.2. Characteristics of Participants
3.3. Outcome Evaluation
3.3.1. Intervention
3.3.2. Respiratory Pressures
3.3.3. Pulmonary Function
3.3.4. Sports Performance
3.4. Risk of Bias Assessment
3.5. Quality Assessment
3.5.1. PEDro Scale
3.5.2. Cochrane’s Assessment of Quality
3.6. Methodological Variables Assessment
3.7. Effect Size Assessment
3.8. Evaluation of the Results of the Studies Included in the Synthesis and Meta-Analysis (n = 9 Included Studies)
3.8.1. Maximal Inspiratory Pressure (n = 8 Included Studies)
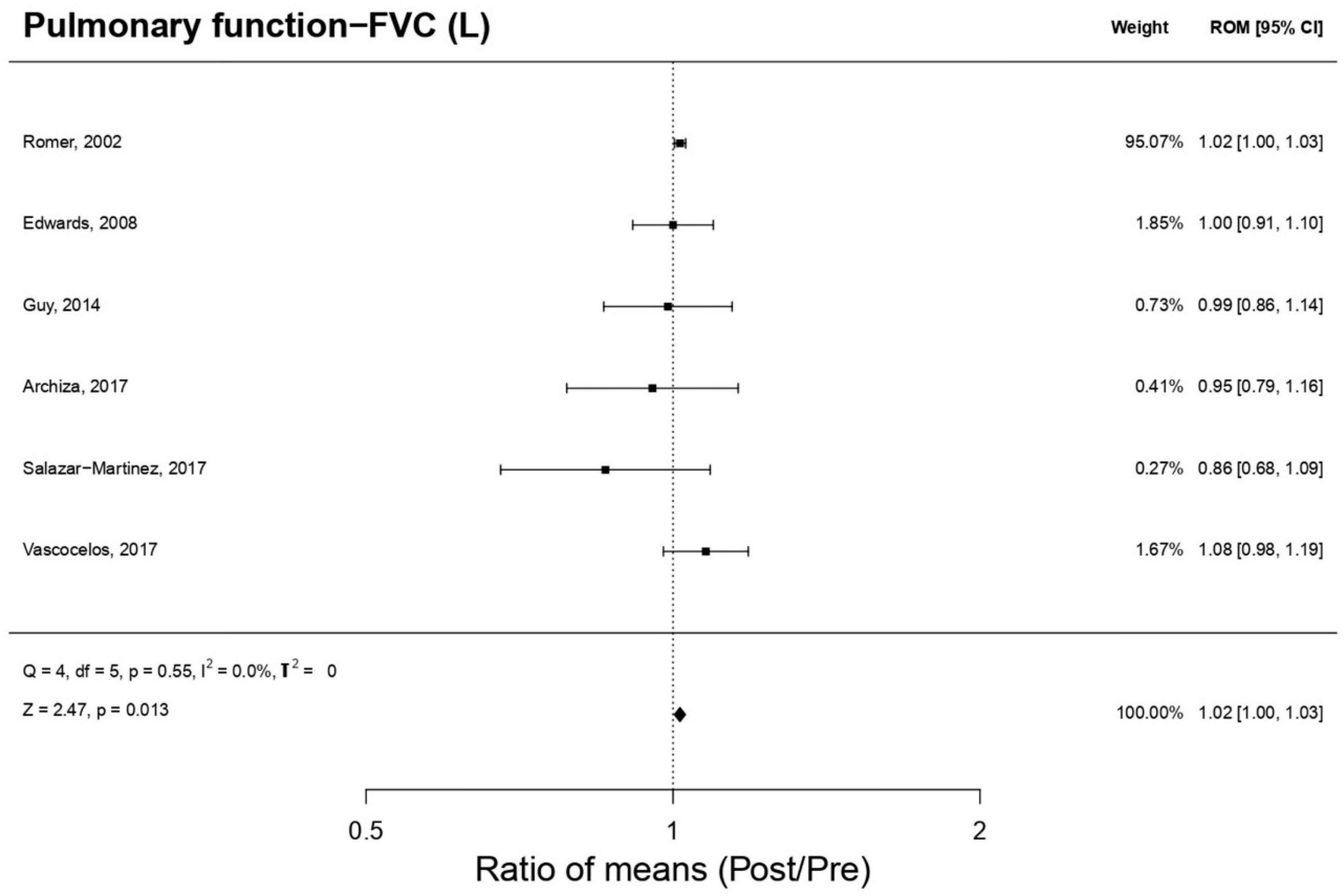
3.8.2. Forced Vital Capacity (n = 6 Included Studies)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D. Ergogenic Strategies for Optimizing Performance and Health in Regular Physical Activity Participants: Evaluation of the Efficacy of Compressive Cryotherapy, Exposure to Intermittent Hypoxia at Rest and Sectorized Training of the Inspiratory Muscles. Ph.D. Thesis, University of León, León, Spain, 2020. Available online: https://dialnet.unirioja.es/servlet/dctes?codigo=286163 (accessed on 5 September 2022).
- Fernández-Lázaro, D.; Gallego-Gallego, D.; Corchete, L.A.; Fernández Zoppino, D.; González-Bernal, J.J.; García Gómez, B.; Mielgo-Ayuso, J. Inspiratory muscle training program using the powerbreath®: Does it have ergogenic potential for respiratory and/or athletic performance? A systematic review with meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 6703. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Augusto, V.M.; Eduardo, D.S.; Silveira, B.M.F.; Lemos, M.D.; Parreira, V.F. Inspiratory muscle training reduces dyspnea during activities of daily living and improves inspiratory muscle function and quality of life in patients with advanced lung disease. Physiother. Theory Pract. 2021, 37, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Santamaría, G.; Sánchez-Serrano, N.; Lantarón-Caeiro, E.; Seco-Calvo, J. Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome. Viruses 2022, 14, 2797. [Google Scholar] [CrossRef]
- Gething, A.D.; Williams, M.; Davies, B. Inspiratory resistive loading improves cycling capacity: A placebo controlled trial. Br. J. Sports Med. 2004, 38, 730–736. [Google Scholar] [CrossRef]
- Hartz, C.S.; Sindorf, M.A.G.; Lopes, C.R.; Batista, J.; Moreno, M.A. Effect of Inspiratory Muscle Training on Performance of Handball Athletes. J. Hum. Kinet. 2018, 63, 43–51. [Google Scholar] [CrossRef]
- Dickinson, J.; Whyte, G.; McConnell, A. Inspiratory muscle training: A simple cost-effective treatment for inspiratory stridor. Br. J. Sports Med. 2007, 41, 694–695. [Google Scholar] [CrossRef]
- Arya, S.; Kaji, A.H.; Boermeester, M.A. PRISMA Reporting Guidelines for Meta-analyses and Systematic Reviews. JAMA Surg. 2021, 156, 789–790. [Google Scholar] [CrossRef]
- van Tulder, M.; Furlan, A.; Bombardier, C.; Bouter, L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine 2003, 28, 1290–1299. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Rosenthal, R.; Rubin, D.B. Meta-Analytic Procedures for Combining Studies With Multiple Effect Sizes. Psychol. Bull. 1986, 99, 400–406. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Friedrich, J.O.; Adhikari, N.K.J.; Beyene, J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: A simulation study. BMC Med. Res. Methodol. 2008, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Archiza, B.; Andaku, D.K.; Caruso, F.C.R.; Bonjorno, J.C., Jr.; Oliveira, C.R.; de Ricci, P.A.; do Amaral, A.C.; Mattiello, S.M.; Libardi, C.A.; Phillips, S.A.; et al. Effects of inspiratory muscle training in professional women football players: A randomized sham-controlled trial. J. Sports Sci. 2018, 36, 771–780. [Google Scholar] [CrossRef]
- Edwards, A.M.; Wells, C.; Butterly, R. Concurrent inspiratory muscle and cardiovascular training differentially improves both perceptions of effort and 5000 m running performance compared with cardiovascular training alone. Br. J. Sports Med. 2008, 42, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.H.; Edwards, A.M.; Deakin, G.B. Inspiratory muscle training improves exercise tolerance in recreational soccer players without concomitant gain in soccer-specific fitness. J. Strength Cond. Res. 2014, 28, 483–491. [Google Scholar] [CrossRef]
- Hart, N.; Sylvester, K.; Ward, S.; Cramer, D.; Moxham, J.; Polkey, M.I. Evaluation of an inspiratory muscle trainer in healthy humans. Respir. Med. 2001, 95, 526–531. [Google Scholar] [CrossRef]
- Kilding, A.E.; Brown, S.; McConnell, A.K. Inspiratory muscle training improves 100 and 200 m swimming performance. Eur. J. Appl. Physiol. 2010, 108, 505–511. [Google Scholar] [CrossRef]
- Romer, L.M.; McConnell, A.K.; Jones, D.A. Effects of inspiratory muscle training upon recovery time during high intensity, repetitive sprint activity. Int. J. Sports Med. 2002, 23, 353–360. [Google Scholar] [CrossRef]
- Salazar-Martínez, E.; Gatterer, H.; Burtscher, M.; Orellana, J.N.; Santalla, A. Influence of Inspiratory Muscle Training on Ventilatory Efficiency and Cycling Performance in Normoxia and Hypoxia. Front. Physiol. 2017, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.K.; Fu, F.H.; Chung, P.K.; Eston, R.; Lu, K.; Quach, B.; Nie, J.; So, R. The effect of inspiratory muscle training on high-intensity, intermittent running performance to exhaustion. Appl. Physiol. Nutr. Metab. 2008, 33, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.K.; Fu, F.H.; Eston, R.; Chung, P.K.; Quach, B.; Lu, K. Chronic and acute inspiratory muscle loading augment the effect of a 6-week interval program on tolerance of high-intensity intermittent bouts of running. J. Strength Cond. Res. 2010, 24, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Tranchita, E.; Minganti, C.; Musumeci, L.; Squeo, M.R.; Parisi, A. Inspiratory muscles training in young basketball players: Preliminary evaluation. Med. Dello Sport 2014, 67, 411–422. [Google Scholar]
- Vasconcelos, T.; Hall, A.; Viana, R. The influence of inspiratory muscle training on lung function in female basketball players—A randomized controlled trial. Porto Biomed. J. 2017, 2, 86–89. [Google Scholar] [CrossRef]
- Forster, H.V.; Haouzi, P.; Dempsey, J.A. Control of breathing during exercise. Compr. Physiol. 2012, 2, 743–777. [Google Scholar]
- Harms, C.A.; Babcock, M.A.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Dempsey, J.A. Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol. 1997, 82, 1573–1583. [Google Scholar] [CrossRef]
- Harms, C.A.; Wetter, T.J.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Hanson, P.; Dempsey, J.A. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J. Appl. Physiol. 1998, 5, 609–618. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Romer, L.; Rodman, J.; Miller, J.; Smith, C. Consequences of exercise-induced respiratory muscle work. Respir. Physiol. Neurobiol. 2006, 151, 242–250. [Google Scholar] [CrossRef]
- Ianuzzo, C.D.; Hamilton, N.; O’Brien, P.J.; Desrosiers, C.; Chiu, R. Biochemical transformation of canine skeletal muscle for use in cardiac-assist devices. J. Appl. Physiol. 1990, 68, 1481–1485. [Google Scholar] [CrossRef]
- Romer, L.M.; Polkey, M.I. Exercise-induced respiratory muscle fatigue: Implications for performance. J. Appl. Physiol. 2008, 104, 879–888. [Google Scholar] [CrossRef]
- Martin, B.J.; Stager, J.M. Ventilatory endurance in athletes and non-athletes. Med. Sci. Sport Exerc. 1981, 13, 21–26. [Google Scholar] [CrossRef]
- McConnell, A.K.; Lomax, M. The influence of inspiratory muscle work history and specific inspiratory muscle training upon human limb muscle fatigue. J. Physiol. 2006, 77, 445–457. [Google Scholar] [CrossRef] [PubMed]
- González-Montesinos, J.L.; Pardal, C.V.; Santos, J.R.F.; Muñoz, A.A.; Sepúlveda, J.L.C.; de los Monteros, R.G.E. Efectos del entrenamiento de la musculatura respiratoria sobre el rendimiento. Revisión bibliográfica. Rev. Andal. Med. Del. Deport. 2012, 5, 163–170. [Google Scholar] [CrossRef]
- Bailey, S.J.; Romer, L.M.; Kelly, J.; Wilkerson, D.P.; DiMenna, F.J.; Jones, A.M. Inspiratory muscle training enhances pulmonary O2 uptake kinetics and high-intensity exercise tolerance in humans. J. Appl. Physiol. 2010, 109, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.A.; McConnell, A.K. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur. J. Appl. Physiol. 2007, 99, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Nicks, C.R.; Morgan, D.W.; Fuller, D.K.; Caputo, J.L. The influence of respiratory muscle training upon intermittent exercise performance. Int. J. Sports Med. 2009, 30, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Volianitis, S.; McConnell, A.K.; Jones, D.A. Assessment of maximum inspiratory pressure. Respiration 2001, 68, 22–27. [Google Scholar] [CrossRef]
- Durmic, T.; Lazovic, B.; Djelic, M.; Lazic, J.S.; Zikic, D.; Zugic, V.; Dekleva, M.; Mazic, S. Sport-specific influences on respiratory patterns in elite athletes. J. Bras. Pneumol 2015, 41, 516–522. [Google Scholar] [CrossRef]
- Beaumont, M.; Forget, P.; Couturaud, F.; Reychler, G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin. Respir. J. 2018, 12, 2178–2188. [Google Scholar] [CrossRef]
- Basso-Vanelli, R.P.; Di Lorenzo, V.A.; Labadessa, I.G.; Regueiro, E.M.; Jamami, M.; Gomes, E.L.; Costa, D. Effects of Inspiratory Muscle Training and Calisthenics-and-Breathing Exercises in COPD With and Without Respiratory Muscle Weakness. Respir. Care 2016, 61, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Mackała, K.; Kurzaj, M.; Okrzymowska, P.; Stodółka, J.; Coh, M.; Rożek-Piechura, K. The Effect of Respiratory Muscle Training on the Pulmonary Function, Lung Ventilation, and Endurance Performance of Young Soccer Players. Int. J. Environ Res. Public Health 2020, 17, 234. [Google Scholar] [CrossRef]
- Luzak, A.; Karrasch, S.; Thorand, B.; Nowak, D.; Holle, R.; Peters, A.; Schulz, H. Association of physical activity with lung function in lung-healthy German adults: Results from the KORA FF4 study. BMC Pulm. Med. 2017, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rubio, H.; Becerro-De-bengoa-vallejo, R.; Rodríguez-Sanz, D.; Calvo-Lobo, C.; Vicente-Campos, D.; Chicharro, J.L. Unraveling the Role of Respiratory Muscle Metaboloreceptors under Inspiratory Training in Patients with Heart Failure. Int. J. Environ. Res. Public Health 2021, 18, 1697. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Brown, A.M.; Frontera, W.R. Principles of Exercise Physiology: Responses to Acute Exercise and Long-term Adaptations to Training. PMR 2012, 4, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Santamaría, G.; Gutiérrez-Abejón, E.; Domínguez-Ortega, C.; García-Lázaro, S.M.; Seco-Calvo, J. Adequacy of an Altitude Fitness Program (Living and Training) plus Intermittent Exposure to Hypoxia for Improving Hematological Biomarkers and Sports Performance of Elite Athletes: A Single-Blind Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 9095. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Adams, D.P.; González-Bernal, J.J.; Fernández Araque, A.; Cano García, A.C.; Fernandez-Lazaro, C.I. Electromyography: A Simple and Accessible Tool to Assess Physical Performance and Health during Hypoxia Training. A Systematic Review. Sustainability 2020, 12, 9137. [Google Scholar] [CrossRef]
- Chung, Y.; Huang, T.Y.; Liao, Y.H.; Kuo, Y.C. 12-Week Inspiratory Muscle Training Improves Respiratory Muscle Strength in Adult Patients with Stable Asthma: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3267. [Google Scholar] [CrossRef]
- Brown, P.I.; McConnell, A.K. Respiratory-Related Limitations in Physically Demanding Occupations. Aviat. Space Environ. Med. 2012, 83, 424–430. [Google Scholar] [CrossRef]
- Taylor, N.A.S.; Peoples, G.E.; Petersen, S.R. Load carriage, human performance, and employment standards. Appl. Physiol. Nutr. Metab. 2016, 41, S131–S147. [Google Scholar] [CrossRef]



| First Author and Year of Publication | n (IG) | n (CG) | Age (Years) | Height (cm) | Sport Activity | Weekly Training Volume of Athletes |
|---|---|---|---|---|---|---|
| Archiza et al., 2017 [16] | Npre = 10 Npost = 10 | Npre = 8 Npost = 8 | IG: 22.0 (3.9) CG: 20.1 (2.0) | 160.0 (0.1) 160.0 (0.0) | Soccer (p) | 20 h × wk−1; 60% physical and 40% technical and tactical training |
| Edwards et al., 2008 [17] | Npre = 8 Npost = 8 | Npre = 8 Npost = 8 | NA | 180.1 (4.5) 181.3 (5.0) | Running (r) | NA |
| Guy et al., 2014 [18] | Npre = 24 Npost = 21 | Npre = 12 Npost = 10 | IG1: 26.6 (8.2) IG2: 23.9 (6.7) CG: 21.3 (4.9) | 182.0 (0.1) 175.0 (0.1) 175.0 (0.1) | Soccer (r) | Twice-weekly sessions for pre-season training |
| Hart et al., 2001 [19] | Npre = 6 Npost = 6 | Npre = 6 Npost = 6 | 32.0 (4.8) | NA | NA | NA |
| Kilding et al., 2016 [20] | Npre = 8 Npost = 8 | Npre = 8 Npost = 8 | IG: 19.1 (2.6) CG: 19.0 (2.1) | 176.5 (4.0) 180.5 (6.5) | Swimming (p) | NA |
| Romer et al., 2001 [21] | Npre = 12 Npost = 12 | Npre = 12 Npost = 12 | IG: 21.3 (1.1) CG: 20.2 (0.7) | 174.0 (0.1) 177.0 (0.1) | Soccer, rugby, field hockey, and basketball (p and/or a) | NA |
| Salazar-Martínez et al., 2017 [22] | Npre = 8 Npost = 8 | Npre = 8 Npost = 8 | IG: 23.4 (2.7) CG: 25.4 (3.2) | 180.2 (3.5) 168.8 (5.1) | Cycling (a) | NA |
| Tong et al., 2008 [23] | Npre = 20 Npost = 20 | Npre = 10 Npost = 10 | IG1: 21.3 (0.9) IG2: 21.5 (2.1) CG: 22.0 (2.9) | 175.0 (5.4) 174.7 (6.8) 175.0 (5.4) | Soccer & rugby (a) | NA |
| Tong et al., 2010 [24] | Npre = 9 Npost = 9 | Npre = 9 Npost = 9 | IG: 21.1 (1.1) CG: 22.3 (1.0) | 172.9 (3.8) 175.6 (4.0) | Soccer & rugby (a) | 2–3 h × day−1 for 4–5 days × wk−1 |
| Tranchita et al., 2018 [25] | Npre = 15 Npost = 15 | Npre = 14 Npost = 14 | IG: 21.06 (2.5) CG: 19.0 (2.1) | 181.4 (9.9) 181.1 (9.6) | Basketball (a) | 8 h × wk−1 |
| Vasconcelos et al., 2017 [26] | Npre = 12 Npost = 11 | Npre = 11 Npost = 10 | IG: 22.0 (5.0) CG: 18.5 (5.8) | NA | Basketball (p) | NA |
| Study | T | Interventions | Instrument | Outcomes (Units) | Results | |
|---|---|---|---|---|---|---|
| IG: Changes from Baseline | IG vs. CG | |||||
| Archiza et al., 2017 [16] | 6 | IMT (2 × 30) With PwB device | Mouth pressure meter, spirometer | Respiratory pressures MIP (cmH2O) Pulmonary function FVC (L) Sports performance RSAmean (s) | ↑* MIP ↓ FVC ↑* RSAmean | ND |
| Edwards et al., 2008 [17] | 4 | IMT (1 × 30) + Cardiovascular training (CV1: 5 × 1000 m; CV2: 3 × 1600 m; SP: 20 min) With PwB device | Mouth pressure meter, portable ergospirometer | Respiratory pressures MIP (cmH2O) Pulmonary function FVC (L) Sports performance ET (s) 1000 m | ↑* MIP ↔ FVC ↑ ET | ↑* MIP ↔ FVC ↑* ET |
| Guy et al., 2014 [18] | 6 | IMT (2 × 30) + pre-season soccer training (2 days per week) With PwB device | Spirometer, chronometer, heart rate monitor, lactate analyzer | Respiratory pressures MIP (cmH2O) Pulmonary function-FVC (L) Sports performance-MSFT (m) | ↑* MIP ↓FVC ↑* MSFT | ND |
| Hart et al., 2001 [19] | 6 | IMT (2 × 30) With PwB device | Mouth pressure meter, spirometer, chronometer | Respiratory pressures MIP (cmH2O) Pulmonary function MVV (L/min) Sports performance ET (s) | ↑ MIP ↑ MVV ↑ ET | ↑ MIP ↑ MVV ↑ ET |
| Kilding et al., 2016 [20] | 6 | IMT (2 × 30) With PwB device | Mouth pressure meter, spirometer, lactate analyzer | Respiratory pressures MIP (cmH2O) Pulmonary function FVC (L) Sports performance TT 200 m (strokes/min) | ↑* MIP ↑ FVC ↑* TT | ↑* MIP ↑ FVC ↑ TT |
| Romer et al., 2001 [21] | 6 | IMT (2 × 30) With PwB device | Pneumotachograph spirometer, hand-held mouth pressure meter, lactate analyzer. | Respiratory pressures MIP (cmH2O) Pulmonary function FVC (L) Sports performance RSP (s) | ↑* MIP ↑ FVC ↑* RSP | ND |
| Salazar-Martínez et al., 2017 [22] | 6 | IMT (2 × 30) With PwB device | Spirometer, cycloergometer, portable gas analyzer | Respiratory pressures MIP (cmH2O) Pulmonary function FVC (L) Sport performance WTTmean (W) | ↑* MIP ↑ FVC ↑ WTT | ND |
| Tong et al., 2008 [23] | 6 | Warm-up + IMT (2 × 30) With PwB device | Bidirectional gas flow meter, RPE and RPB scales | Respiratory pressures MIP (cmH2O) Sports performance (number of repetitions) | ↑* MIP ↑* number of repetitions | ↑ MIP ↑ number of repetitions |
| Tong et al., 2010 [24] | 6 | Warm-up + IMT (2 × 30) + Interval training With PwB device | Differential pressure transducer, portable ergospirometer | Respiratory pressures MIP (cmH2O) Sports performance (number of repetitions) | ↑* MIP ↑* number of repetitions | ↑ MIP ↑ number of repetitions |
| Tranchita et al., 2018 [25] | 4 | IMT (2 × 30) With PwB device | Spirometer, Astrand-Rhyming Cycle Ergometer Test | Respiratory pressures MIP (cmH2O) Pulmonary function PIF (L/min) Pulmonary function MVV (L/min) Sports performance Max Power (W) | ↑* MIP ↑* PIF ↑*MVV ↑ Max Power | ND |
| Vasconcelos et al., 2017 [26] | 4 | IMT (1 × 30) With PwB device | Spirometer | Pulmonary function FVC (L) Pulmonary function PEF (L/s) | ↑* FVC ↑*PEF | ND |
| Items | Archiza et al., 2017 [16] | Edwards et al., 2008 [17] | Guy et al., 2014 [18] | Hart et al., 2001 [19] | Kilding et al., 2016 [20] | Romer et al., 2001 [21] | Salazar-Martínez et al., 2017 [22] | Tong et al., 2008 [23] | Tong et al., 2010 [24] | Tranchita et al., 2018 [25] | Vascocelos et al., 2017 [26] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES |
| 2 | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES |
| 3 | NO | NO | YES | NO | YES | YES | YES | YES | NO | NO | NO |
| 4 | YES | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO |
| 5 | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 6 | YES | NO | NO | YES | YES | YES | YES | YES | NO | YES | YES |
| 7 | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 8 | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| Total | 2 | 5 | 4 | 4 | 3 | 2 | 3 | 3 | 5 | 4 | 2 |
| Items | Archiza et al., 2017 [16] | Edwards et al., 2008 [17] | Guy et al., 2014 [18] | Hart et al., 2001 [19] | Kilding et al., 2016 [20] | Romer et al., 2001 [21] | Salazar-Martínez et al., 2017 [22] | Tong et al., 2008 [23] | Tong et al., 2010 [24] | Tranchita et al., 2018 [25] | Vascocelos et al., 2017 [26] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 8 | 1 | - | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| 9 | 1 | - | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| Total | 8 | 3 | 5 | 5 | 6 | 5 | 5 | 6 | 4 | 4 | 5 |
| Quality | G | P | R | R | G | R | R | G | R | R | R |
| Items | Archiza et al., 2017 [16] | Edwards et al., 2008 [17] | Guy et al., 2014 [18] | Hart et al., 2001 19] | Kilding et al., 2016 [20] | Romer et al., 2001 [21] | Salazar-Martínez et al., 2017 [22] | Tong et al., 2008 [23] | Tong et al., 2010 [24] | Tranchita et al., 2018 [25] | Vascocelos et al., 2017 [26] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES |
| 2 | YES | NO | NO | NO | NO | NO | NO | NO | NO | NO | YES |
| 3 | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 4 | YES | NO | YES | NO | YES | YES | YES | YES | NO | NO | NO |
| 5 | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO | NO |
| 6 | NO | NO | NO | NO | NO | YES | NO | NO | NO | NO | NO |
| 7 | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 8 | YES | - | YES | YES | YES | - | - | YES | - | - | YES |
| 9 | YES | - | YES | YES | NO | - | - | YES | - | - | YES |
| 10 | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| 11 | YES | - | NO | YES | NO | - | - | YES | - | - | NO |
| Total | 8 | 3 | 6 | 6 | 5 | 5 | 4 | 7 | 3 | 3 | 7 |
| Quality | G | P | G | G | R | R | R | G | P | P | G |
| Moderators’ Variables | k | Min. | Max. | Mean | SD |
|---|---|---|---|---|---|
| Intervention variables | |||||
| Duration (weeks) | 11 | 4 | 6 | 5.45 | 0.93 |
| Intensity (hours/week) | 0 | NA | NA | NA | NA |
| Magnitude (hours/intervention) | 0 | NA | NA | NA | NA |
| Subjects’ variables | |||||
| Age (years) | 10 | 19.05 | 32 | 22.47 | 3.73 |
| Methodology’s variables | |||||
| SS of the experimental group, at pretreatment | 11 | 6 | 24 | 12 | 5.6 |
| SS of the experimental group, at post-treatment | 11 | 6 | 21 | 11.63 | 5 |
| SS of the experimental group, at follow-up | 0 | - | - | - | - |
| SS of the control group, at pretreatment | 11 | 6 | 15 | 9.72 | 2.57 |
| SS of the control group, at post-treatment | 11 | 6 | 15 | 9.45 | 2.42 |
| SS of the control group, at follow-up | 0 | - | - | - | - |
| Mortality at post-treatment evaluation (%) | 11 | 0 | 13.88 | 2.05 | 4.71 |
| Mortality at follow-up evaluation (%) | 0 | - | - | - | - |
| Study | Outcomes (Units) | M (DT) pre | M (DT) Post | p-Value | ES |
|---|---|---|---|---|---|
| Archiza et al., 2017 [16] | Respiratory pressures MIP (cmH2O) | 137 (15.3) | 166.5 (17.1) | <0.05 | 1.51 |
| Pulmonary function FVC (L) | 4.4 (1.0) | 4.2 (0.9) | >0.05 | 0.27 | |
| Sports performance RSAmean (s) | 7.9 (0.2) | 7.6 (0.2) | <0.05 | 1.19 | |
| Edwards et al., 2008 [17] | Respiratory pressures MIP (cmH2O) | 148.1 (13.7) | 169.5 (9.1) | <0.01 | 1.56 |
| Pulmonary function FVC (L) | 5.5 (0.4) | 5.5 (0.6) | >0.05 | 0.00 | |
| Sports performance-ET (s) 1000 m | 210 (52.2) | 205 (53.8) | 0.09 | 0.09 | |
| Guy et al., 2014 [18] | Respiratory pressures MIP (cmH2O) | 134 (24.0) | 152 (21.0) | 0.002 | 0.75 |
| Pulmonary function FVC (L) | 5.25 (0.99) | 5.19 (0.9) | >0.05 | 0.06 | |
| Sports performance MSFT (m) | 1491 (410) | 1666 (460) | 0.02 | 0.42 | |
| Hart et al., 2001 [19] | Respiratory pressures MIP (cmH2O) | 127.8(ND) | 143.4 (NA) | 0.02 | - |
| Pulmonary function MVV (L/min) | 174 (ND) | 186 (NA) | 0.65 | ||
| Sports performance ET (s) | 848 (ND) | 887 (NA) | 0.22 | ||
| Kilding et al., 2016 [20] | Respiratory pressures MIP (cmH2O) | 115 (26.0) | NA | <0.01 | 0.41 |
| Pulmonary function FVC (L) | 5.2 (0.7) | 0.60 | −0.07 | ||
| Sports performance TT 200 m (strokes/min) | 43.7 (5.1) | 0.02 | −0.25 | ||
| Romer et al., 2001 [21] | Respiratory pressures MIP (cmH2O) | 130.3 (3.7) | 173.8 (6.0) | <0.01 | 1.29 |
| Pulmonary function FVC (L) | 5.63 (0.09) | 5.72 (0.09) | >0.05 | 1.00 | |
| Sports performance RSP (s) | 243.9 (9.2) | 227.2 (9.0) | <0.01 | 1.81 | |
| Salazar-Martínez et al., 2017 [22] | Respiratory pressures MIP (cmH2O) | 119.6 (37.36) | 166.91 (42.65) | <0.05 | 1.26 |
| Pulmonary function FVC (L) | 5.44 (1.14) | 4.67 (1.38) | >0.05 | 0.67 | |
| Sport performance WTTmean (W) | 217.25 (49.07) | 241.87 (56.01) | 0.02 | 0.50 | |
| Tong et al., 2008 [23] | Respiratory pressures MIP (cmH2O) | 145.1 (19.6) | 191.3 (22.2) | <0.05 | 1.35 |
| Sports performance (number of repetitions) | 37.6 (5.9) | 43.7 (6.6) | <0.05 | 1.03 | |
| Tong et al., 2010 [24] | Respiratory pressures MIP (cmH2O) | 163 (29.8) | 195.9 (23.5) | <0.01 | 1.10 |
| Sports performance (number of repetitions) | 40.3 (5.0) | 52.7 (6.4) | <0.05 | 0.89 | |
| Tranchita et al., 2018 [25] | Respiratory pressures MIP (cmH2O) | 97.75 (23.85) | 127.25 (22.12) | <0.001 | 0.95 |
| Pulmonary function PIF (L/min) | 66.67(23.60) | 87.58 (29.88) | 0.005 | 0.77 | |
| Pulmonary function MVV (L/min) | 125.50(20.37) | 133.83 (25.0) | 0.013 | 0.42 | |
| Sports performance Max Power (W) | 158 (34.48) | 161 (34.50) | >0.05 | 0.08 | |
| Vasconcelos et al., 2017 [26] | Pulmonary function FVC (L) | 4.03 (0.45) | 4.34 (0.51) | <0.05 | 0.68 |
| Pulmonary function PEF (L/s) | 6.73 (1.51) | 7.17 (1.58) | <0.05 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Corchete, L.A.; García, J.F.; Jerves Donoso, D.; Lantarón-Caeiro, E.; Cobreros Mielgo, R.; Mielgo-Ayuso, J.; Gallego-Gallego, D.; Seco-Calvo, J. Effects on Respiratory Pressures, Spirometry Biomarkers, and Sports Performance after Inspiratory Muscle Training in a Physically Active Population by Powerbreath®: A Systematic Review and Meta-Analysis. Biology 2023, 12, 56. https://doi.org/10.3390/biology12010056
Fernández-Lázaro D, Corchete LA, García JF, Jerves Donoso D, Lantarón-Caeiro E, Cobreros Mielgo R, Mielgo-Ayuso J, Gallego-Gallego D, Seco-Calvo J. Effects on Respiratory Pressures, Spirometry Biomarkers, and Sports Performance after Inspiratory Muscle Training in a Physically Active Population by Powerbreath®: A Systematic Review and Meta-Analysis. Biology. 2023; 12(1):56. https://doi.org/10.3390/biology12010056
Chicago/Turabian StyleFernández-Lázaro, Diego, Luis A. Corchete, Juan F. García, David Jerves Donoso, Eva Lantarón-Caeiro, Raúl Cobreros Mielgo, Juan Mielgo-Ayuso, David Gallego-Gallego, and Jesús Seco-Calvo. 2023. "Effects on Respiratory Pressures, Spirometry Biomarkers, and Sports Performance after Inspiratory Muscle Training in a Physically Active Population by Powerbreath®: A Systematic Review and Meta-Analysis" Biology 12, no. 1: 56. https://doi.org/10.3390/biology12010056
APA StyleFernández-Lázaro, D., Corchete, L. A., García, J. F., Jerves Donoso, D., Lantarón-Caeiro, E., Cobreros Mielgo, R., Mielgo-Ayuso, J., Gallego-Gallego, D., & Seco-Calvo, J. (2023). Effects on Respiratory Pressures, Spirometry Biomarkers, and Sports Performance after Inspiratory Muscle Training in a Physically Active Population by Powerbreath®: A Systematic Review and Meta-Analysis. Biology, 12(1), 56. https://doi.org/10.3390/biology12010056













