Diverse Response Pattern to Anoxia in Three Freshwater Turtle Species
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Anoxia Stress and Recovery Experiments
2.3. Cerebral Antioxidant Enzyme Genes’ mRNA Levels
2.4. Biochemical Assays
2.5. Statistical Analysis
3. Results
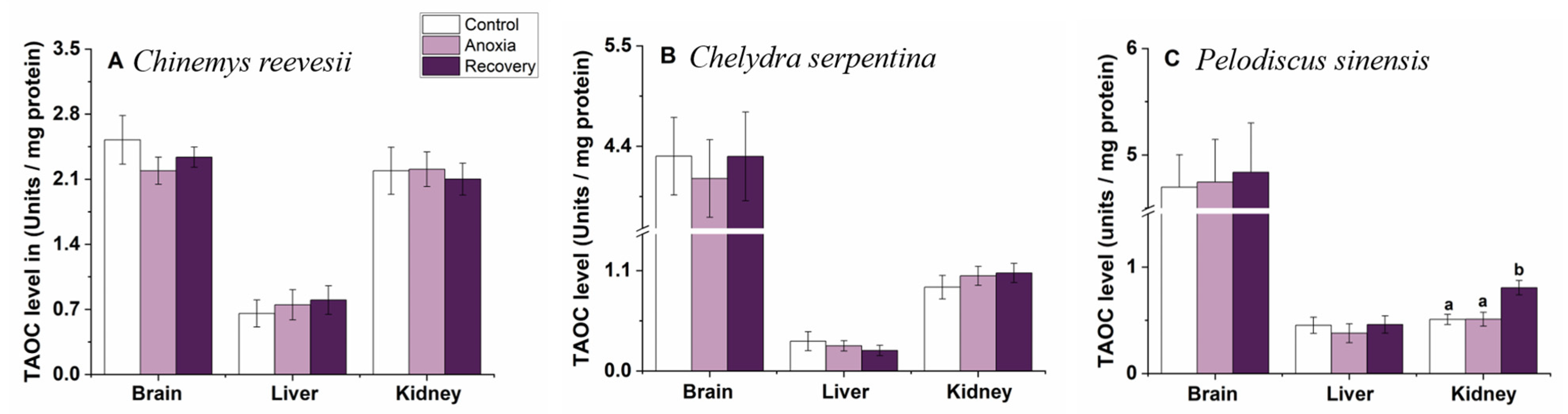
3.1. TAOC Level
3.2. MDA Concentration
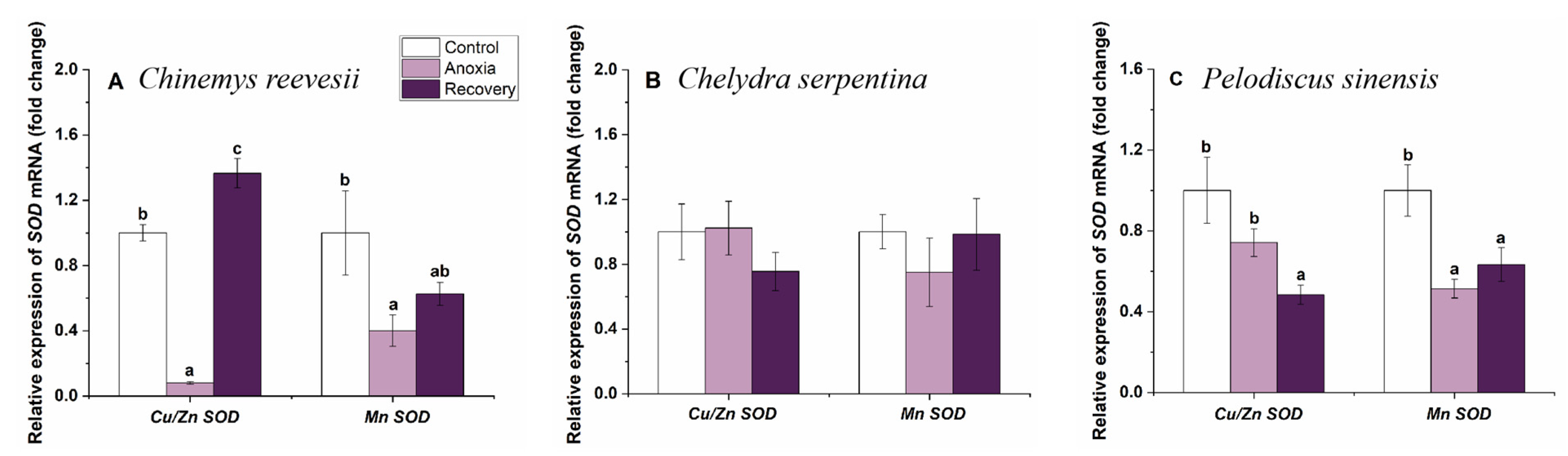
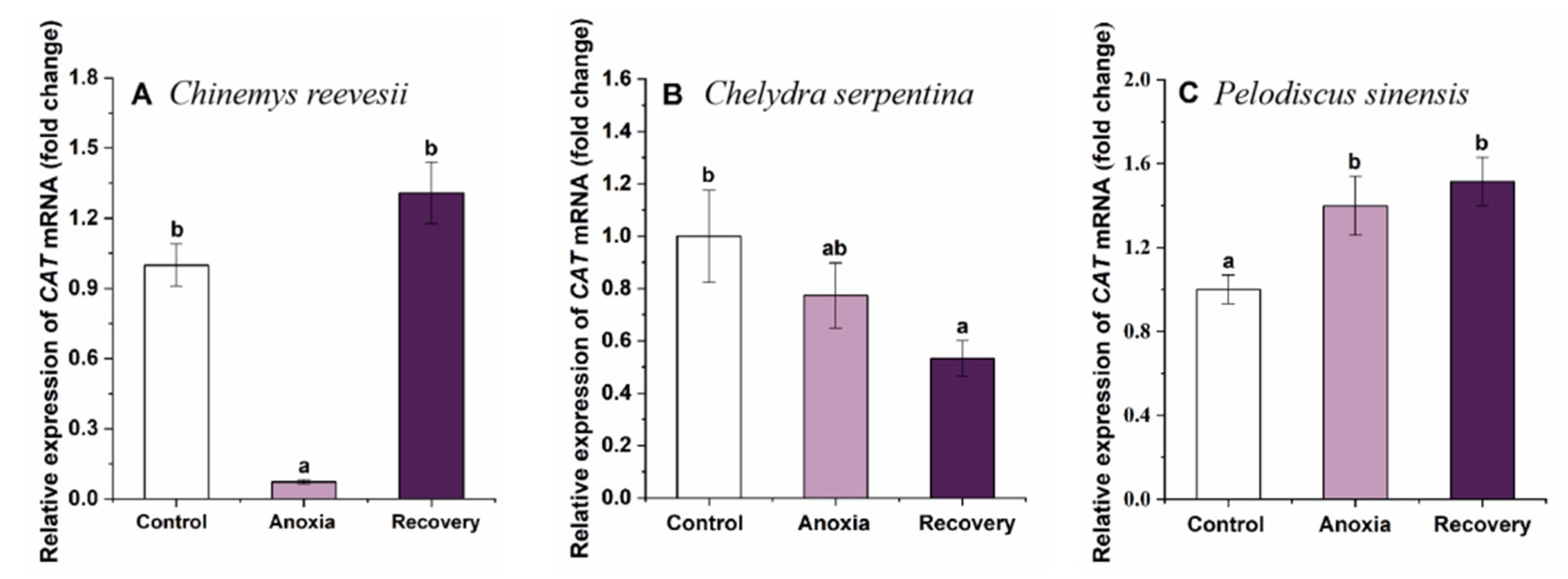
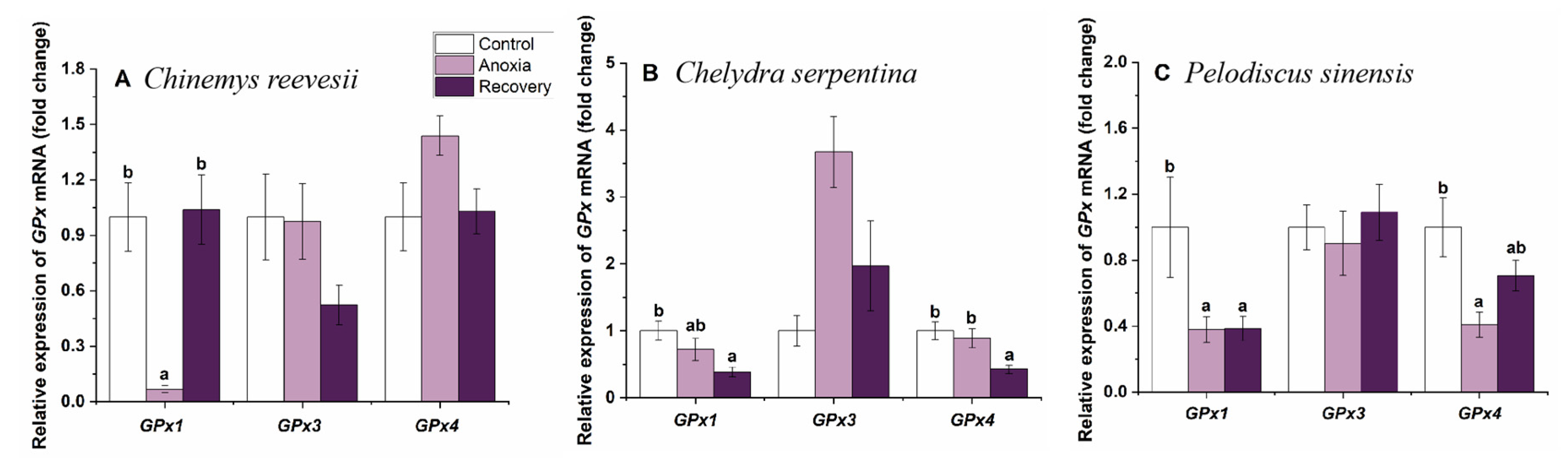
3.3. Gene mRNA Expression of Cerebral Antioxidant Enzymes
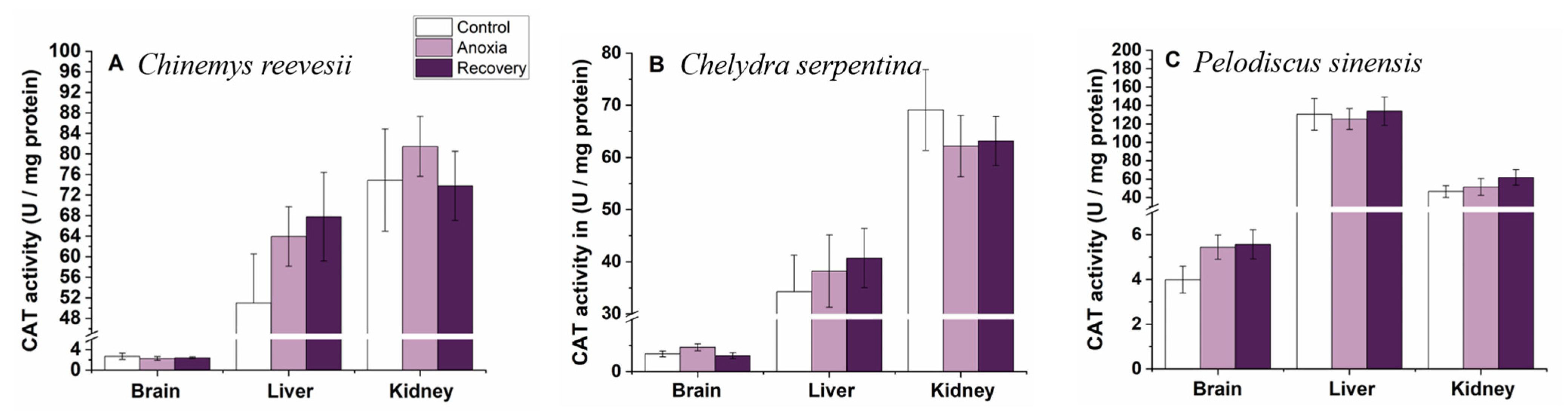
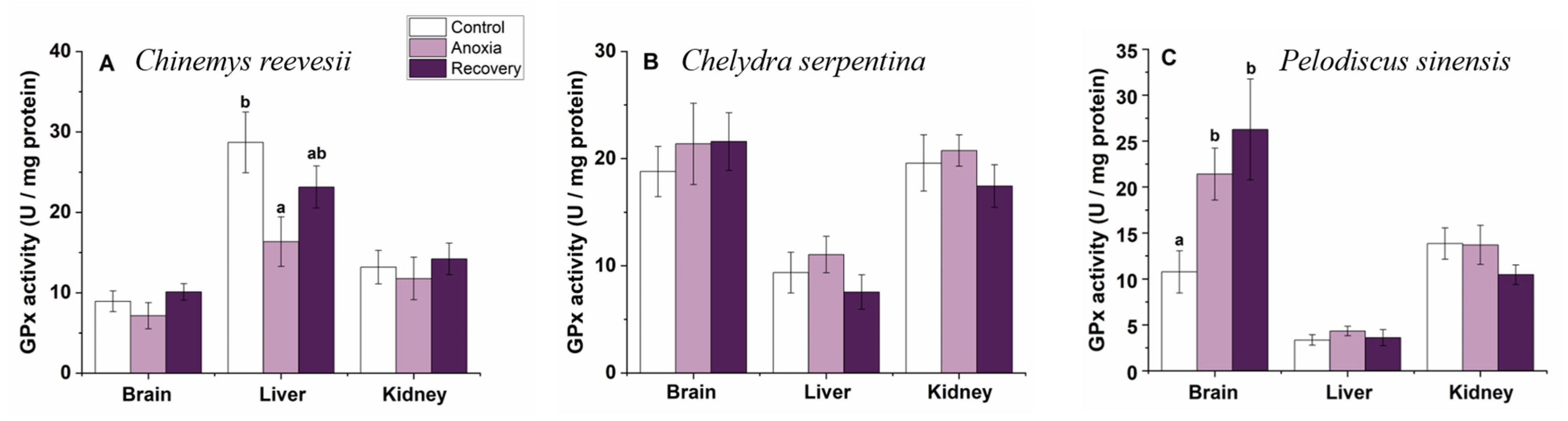
3.4. Antioxidant Enzymes
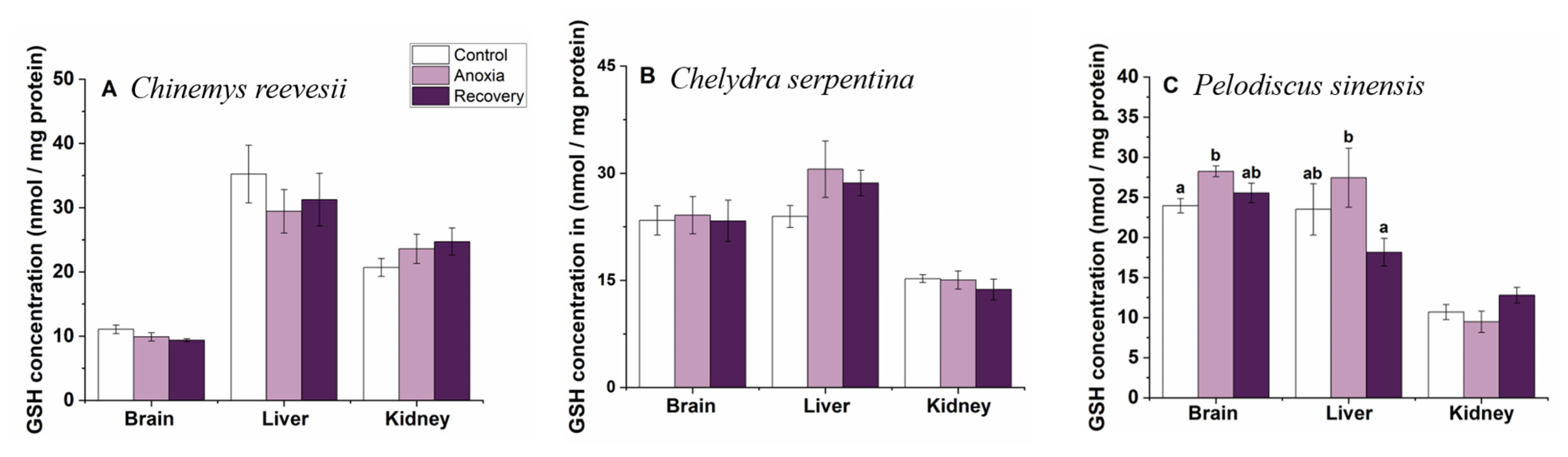
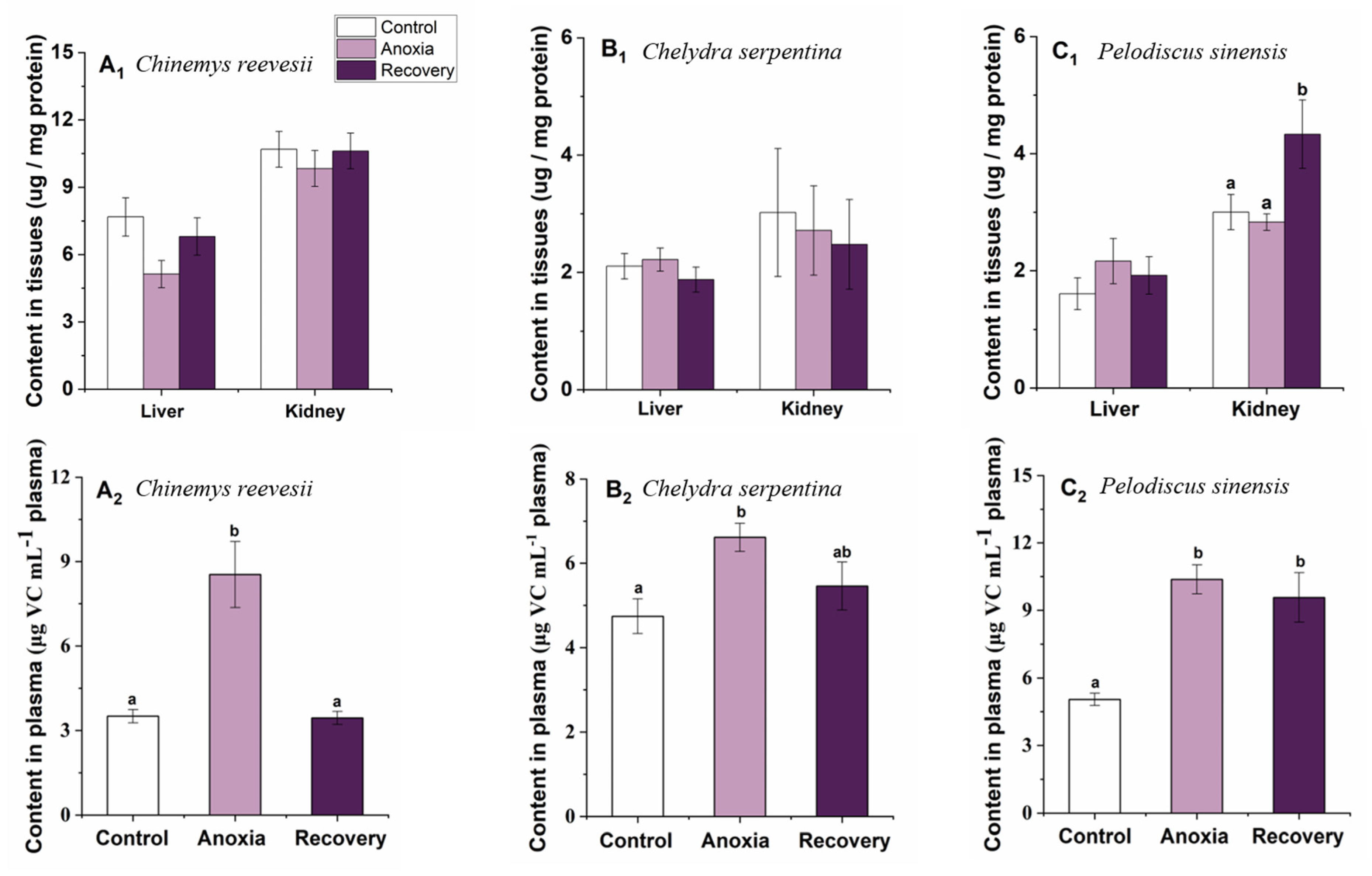
3.5. Small-Molecule Antioxidants
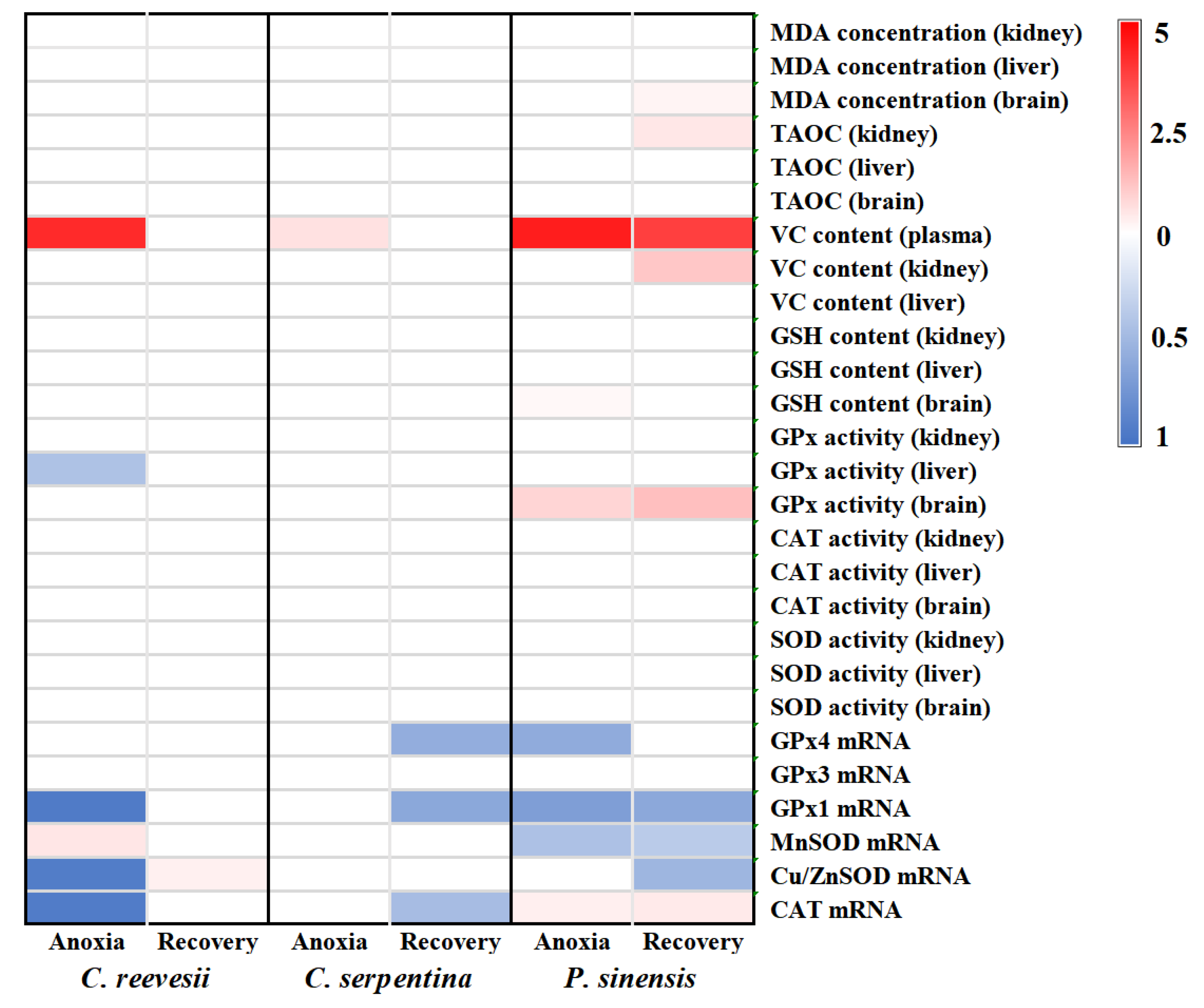
3.6. Comparison of the Response Pattern to Anoxia Stress among the Three Turtle Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jane, S.F.; Hansen, G.J.A.; Kraemer, B.M.; Leavitt, P.R.; Mincer, J.L.; North, R.L.; Pilla, R.M.; Stetler, J.T.; Williamson, C.E.; Woolway, R.I.; et al. Widespread deoxygenation of temperate lakes. Nature 2021, 594, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. 1995, 33, 245–303. [Google Scholar]
- Diaz, R.J.; Breitburg, D.L. The Hypoxic Environment. Fish. Physiol. 2009, 27, 1–23. [Google Scholar] [CrossRef]
- Storey, K.B. Oxidative stress: Animal adaptations in nature. Braz. J. Med. Biol. Res. 1996, 29, 1715–1733. [Google Scholar]
- Herbert, C.V.; Jackson, D.C. Temperature effects on the responses to prolonged submergence in the turtle Chrysemys picta bellii. II. metabolic rate, blood acid-base and ionic changes, and cardiovascular function in aerated and anoxic water. Physiol. Zool. 1985, 58, 670–681. [Google Scholar] [CrossRef]
- Ultsch, G.R. The viability of nearctic freshwater turtles submerged in anoxia and normoxia at 3 and 10 degrees C. Comp. Biochem. Phys. A 1985, 81, 607–611. [Google Scholar] [CrossRef]
- Ultsch, G.R. Ecology and physiology of hibernation and overwintering among freshwater fishes, turtles and snakes. Biol. Rev. 1989, 64, 435–516. [Google Scholar] [CrossRef]
- Reese, S.A.; Crocker, C.E.; Carwile, M.E.; Jackson, D.C.; Ultsch, G.R. The physiology of hibernation in common map turtles (Graptemys geographica). Comp. Biochem. Phys. A 2001, 130, 331–340. [Google Scholar] [CrossRef]
- Reese, S.A.; Jackson, D.C.; Ultsch, G.R. The physiology of overwintering in a turtle that occupies multiple habitats, the common snapping turtle (Chelydra serpentina). Physiol. Biochem. Zool. 2002, 75, 432–438. [Google Scholar] [CrossRef]
- Ultsch, G.R.; Cochran, B.M. Physiology of northern and southern musk turtles (Sternotherus odoratus) during simulated hibernation. Physiol. Zool. 1994, 67, 263–281. [Google Scholar] [CrossRef]
- Ruhr, I.M.; Mccourty, H.; Bajjig, A.; Dane, I.I.; Shiels, H.A.; Galli, G.L.J. Developmental plasticity of cardiac anoxia-tolerance in juvenile common snapping turtles (Chelydra serpentina). Proc. R. Soc. B Biol. Sci. 2019, 286, 20191072. [Google Scholar] [CrossRef]
- Pell, S.M. Notes on the Habits of the Common Snapping Turtle, Chelydra serpentina (Linn.) in Central New York. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1941. [Google Scholar]
- Alibardi, L.; Toni, M. Skin structure and cornification proteins in the soft-shelled turtle Trionyx spiniferus. Zoology 2006, 109, 182–195. [Google Scholar] [CrossRef]
- Zhan, Q.; Gao, J.; Li, Y.; Hu, H.H.; Zhu, Z.R.; Yin, D.Z. Anoxic tolerance, blood physiological and biochemical advantages of the turtles and red-eaeed sliders during anoxic submengence. J. Nat. Sci. Hunan Norm. Univ. 2010, 33, 107–114. [Google Scholar] [CrossRef]
- Chen, B.J.; Niu, C.J.; Yuan, L. Ascorbic acid regulation in stress responses during acute cold exposure and following recovery in juvenile Chinese soft-shelled turtle (Pelodiscus sinensis). Comp. Biochem. Physiol. A 2015, 184, 20–26. [Google Scholar] [CrossRef]
- Storey, K.B. Reptile freeze tolerance: Metabolism and gene expression. Cryobiology 2006, 52, 1–16. [Google Scholar] [CrossRef]
- Chen, X.; Li, M.; Niu, C. Diverse defense responses to ammonia stress in three freshwater turtles. Aquaculture 2022, 546, 737302. [Google Scholar] [CrossRef]
- Randall, D.J.; Wilson, J.M.; Peng, K.W.; Kok, T.W.K.; Kuah, S.S.L.; Chew, S.F.; Lam, T.J.; Ip, Y.K. The mudskipper, Periophthalmodon schlosseri, actively transports NH4+ against a concentration gradient. Am. J. Phys. 1999, 277, 1562–1567. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Orbea, A.; Ortiz-Zarragoitia, M.; Sole, M.; Porte, C.; Cajaraville, M.P. Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish from the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat. Toxicol. 2002, 58, 75–98. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schunkert, H.; Ingelfinger, J.R.; Hirsch, A.T.; Pinto, Y.; Remme, W.J.; Jacob, H.; Dzau, V.J. Feedback regulation of angiotensin converting enzyme activity and mRNA levels by angiotensin II. Circ. Res. 1993, 72, 312. [Google Scholar] [CrossRef] [PubMed]
- Krivoruchko, A. Turtle Anoxia-Biochemistry and Gene Regulation in An Anaerobic Extremist. Ph.D. Thesis, Carleton University, Ottawa, ON, Canada, 2010. [Google Scholar]
- Willmore, W.G.; Storey, K.B. Glutathione systems and anoxia tolerance in turtles. Am. J. Physiol. Integr. Comp. Physiol. 1997, 273, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Rivera-Ingraham, G.A.; Moreira, D.C.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernandez, J.M.; Dafre, A.; Niu, C.J.; Tremblay, N.; Paital, B.; et al. Twenty years of the ‘Preparation for Oxidative Stress’ (POS) theory: Ecophysiological advantages and molecular strategies. Comp. Biochem. Phys. A 2019, 234, 36–49. [Google Scholar] [CrossRef]
- Diaz, A.; Yepes, M. Urokinase-type plasminogen activator promotes synaptic repair in the ischemic brain. Neural Regen. Res. 2018, 13, 2. [Google Scholar]
- Zhang, M.D.; Ling, C.; Sha, H.; Chen, M.; Wang, D.; Luo, Z.X.; Liang, H.W. Effects of hypoxic stress on antioxidant enzyme activity and SODs gene expression of Hypophthalmichthys molitrix. Acta Hydrobiol. Sin. 2022, 46, 498–506. [Google Scholar] [CrossRef]
- Kniffin, C.D.; Burnett, L.E.; Burnett, K.G. Recovery from hypoxia and hypercapnic hypoxia: Impacts on the transcription of key antioxidants in the shrimp Litopenaeus vannamei. Comp. Biochem. Phys. B 2014, 170, 43–49. [Google Scholar] [CrossRef]
- de Oliveira, U.O.; Araujo, A.S.D.; Bello-Klein, A.; da Silva, R.S.M.; Kucharski, L.C. Effects of environmental anoxia and different periods of reoxygenation on oxidative balance in gills of the estuarine crab Chasmagnathus granulata. Comp. Biochem. Phys. B 2005, 140, 51–57. [Google Scholar] [CrossRef]
- Freire, C.A.; Welker, A.F.; Storey, J.; Storey, K.B.; Hermes-Lima, M. Oxidative Stress in Estuarine and Intertidal Environments (Temperate and Tropical). In Oxidative Stress in Aquatic Ecosystems; Wiley: Hoboken, NJ, USA, 2012; pp. 41–57. [Google Scholar] [CrossRef]
- Welker, A.F.; Moreira, D.C.; Campos, E.G.; Hermes-Lima, M. Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Phys. A 2013, 165, 384–404. [Google Scholar] [CrossRef]
- Ultsch, G.R. The ecology of overwintering among turtles: Where turtles overwinter and its consequences. Biol. Rev. 2006, 81, 339–367. [Google Scholar] [CrossRef]
- Willmore, W.G.; Storey, K.B. Antioxidant systems and anoxia tolerance in a freshwater turtle Trachemys scripta elegans. Mol. Cell. Biochem. 1997, 170, 177–185. [Google Scholar] [CrossRef]
- Hermes-Lima, M.; Zenteno-Savin, T. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Phys. C 2002, 133, 537–556. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, L.P.; Mota, A.A.; Hermes-Lima, M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am. J. Physiol. Integr. Comp. Physiol. 2001, 280, 100–107. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Lushchak, O.V.; Storey, J.M.; Storey, K.B. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int. J. Biochem. Cell B 2005, 37, 1319–1330. [Google Scholar] [CrossRef]
- Kenneth, M.C. The winter environment of painted turtles, Chrysemys picta: Temperature, dissolved oxygen, and potential cues for emergence. Can. J. Zool. 1991, 69, 2493–2498. [Google Scholar]
- Ishida, T.; Li, W.; Liu, Z.; Kiwada, H. Stimulatory effect of polyethylene glycol (PEG) on gene expression in mouse liver following hydrodynamics-based transfection. J. Gene Med. 2006, 8, 324–334. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, C.J.; Xu, W.J. Effect of Dietary Vitamin C on the Antioxidant Defense System of Hibernating Juvenile Three-keeled Pond Turtles (Chinemys reevesii). Asian Herpetol. Res. 2012, 3, 151–156. [Google Scholar] [CrossRef]



| Gene Name | Accession No. | Forward Primer | Reverse Primer | Amplicon Size (bp) |
|---|---|---|---|---|
| GAPDH | NM_001286927.1 | TTCATGGCACTGTCAAGGCT | GGTTGACGCCCATCACAAAC | 243 |
| Cu/Zn SOD | JX470524 | TGCAGGTGCTCACTTCAATCC | CAACATGCCTCTCTTGATCTTGTG | 68 |
| Mn SOD | JX470525 | GCCATCAAGCGTGATTTCG | CTGATACTGCTGTCAGCTTCTCCTT | 61 |
| CAT | JX452102 | GCAGCGCTTCAATAGTGCAA | GTTCATCTTCTTTCAGCACTTTGG | 80 |
| GPx1 | KC357250 | GGAGCCCTTCAAACGCTACAG | TGAGGAGGCGCTGGATGT | 71 |
| GPx3 | JX470527 | AACCAGTTTGGCAAGCAAGAG | CGGGCCGGACGTATTTC | 70 |
| GPx4 | JX470528 | AGTAAGATAGAGGTCAACGGGAACA | TTCCTTTGGGCTGATCTTTCA | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Niu, C.; Chen, Y. Diverse Response Pattern to Anoxia in Three Freshwater Turtle Species. Biology 2023, 12, 50. https://doi.org/10.3390/biology12010050
Li M, Niu C, Chen Y. Diverse Response Pattern to Anoxia in Three Freshwater Turtle Species. Biology. 2023; 12(1):50. https://doi.org/10.3390/biology12010050
Chicago/Turabian StyleLi, Min, Cuijuan Niu, and Yixuan Chen. 2023. "Diverse Response Pattern to Anoxia in Three Freshwater Turtle Species" Biology 12, no. 1: 50. https://doi.org/10.3390/biology12010050
APA StyleLi, M., Niu, C., & Chen, Y. (2023). Diverse Response Pattern to Anoxia in Three Freshwater Turtle Species. Biology, 12(1), 50. https://doi.org/10.3390/biology12010050








