Transcriptomic Analysis on the Effects of Altered Water Temperature Regime on the Fish Ovarian Development of Coreius guichenoti under the Impact of River Damming
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Process Control
2.2. Histological Examination
2.3. Transcriptomic Analysis
2.3.1. Library Construction and PacBio Sequencing
2.3.2. Library Construction, Illumina Sequencing, and Transcriptome Assembly
2.3.3. PacBio Transcriptome Quality Control and Analysis
2.3.4. Illumina Transcriptome Quality Control and Analysis
3. Results
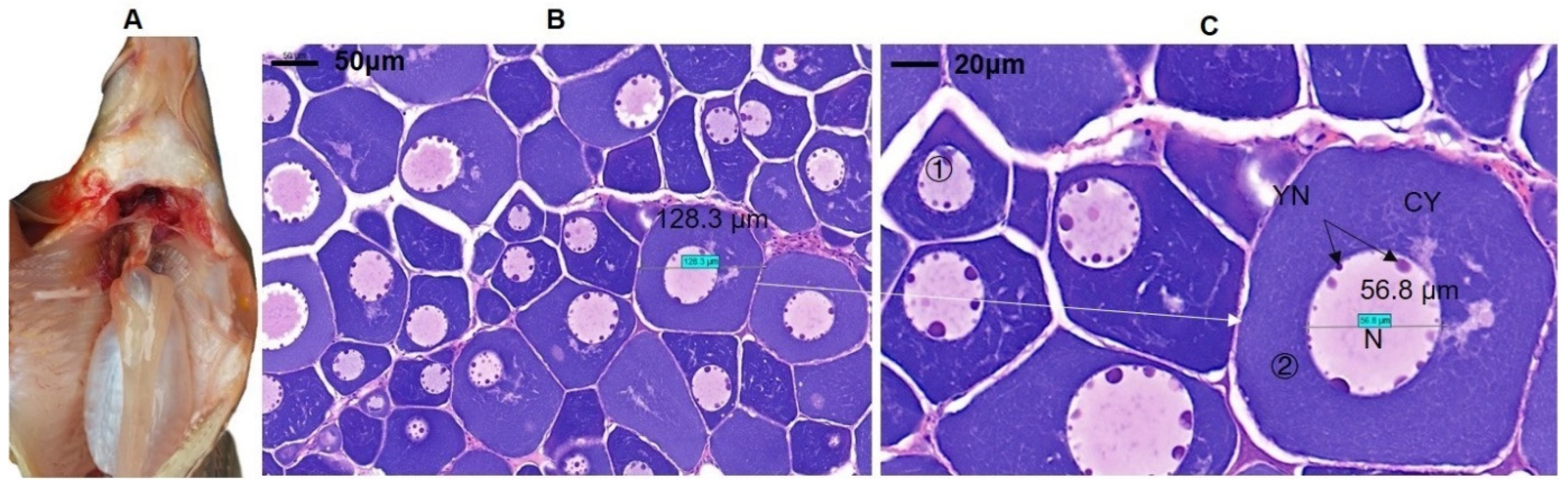
3.1. Morphological Characteristics of Ovarian Tissues
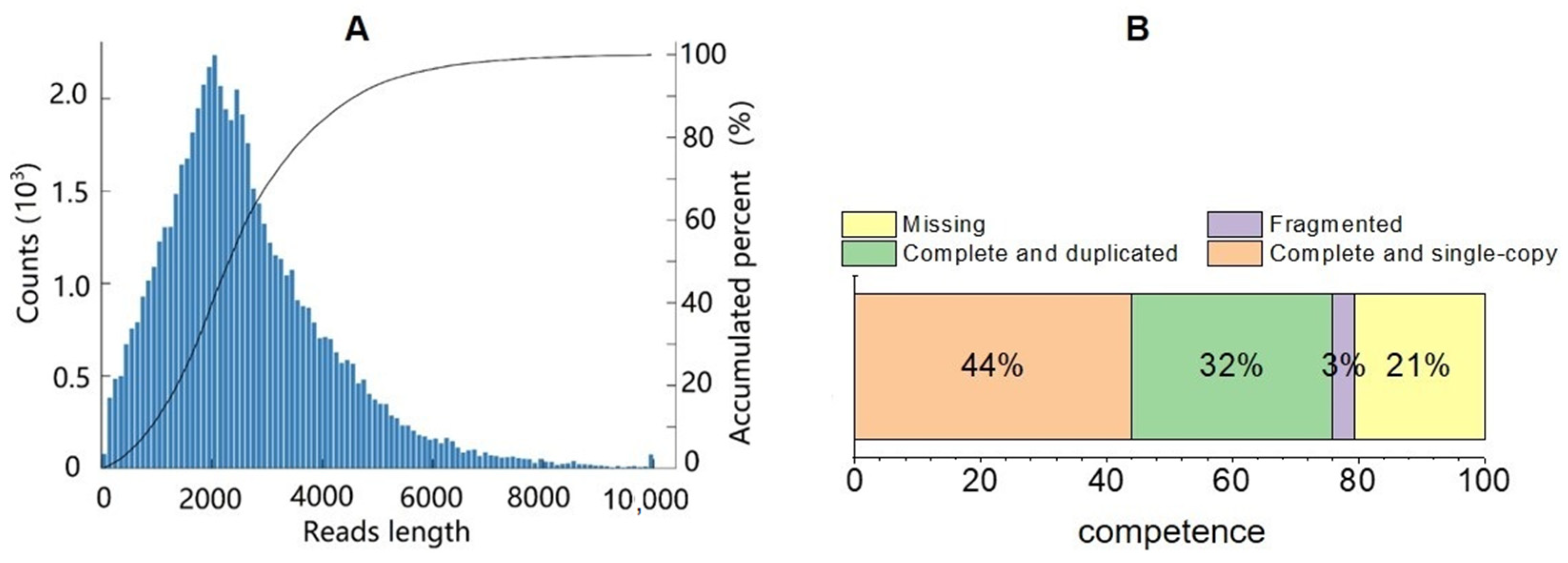
3.2. Transcriptome Data and Their Quality
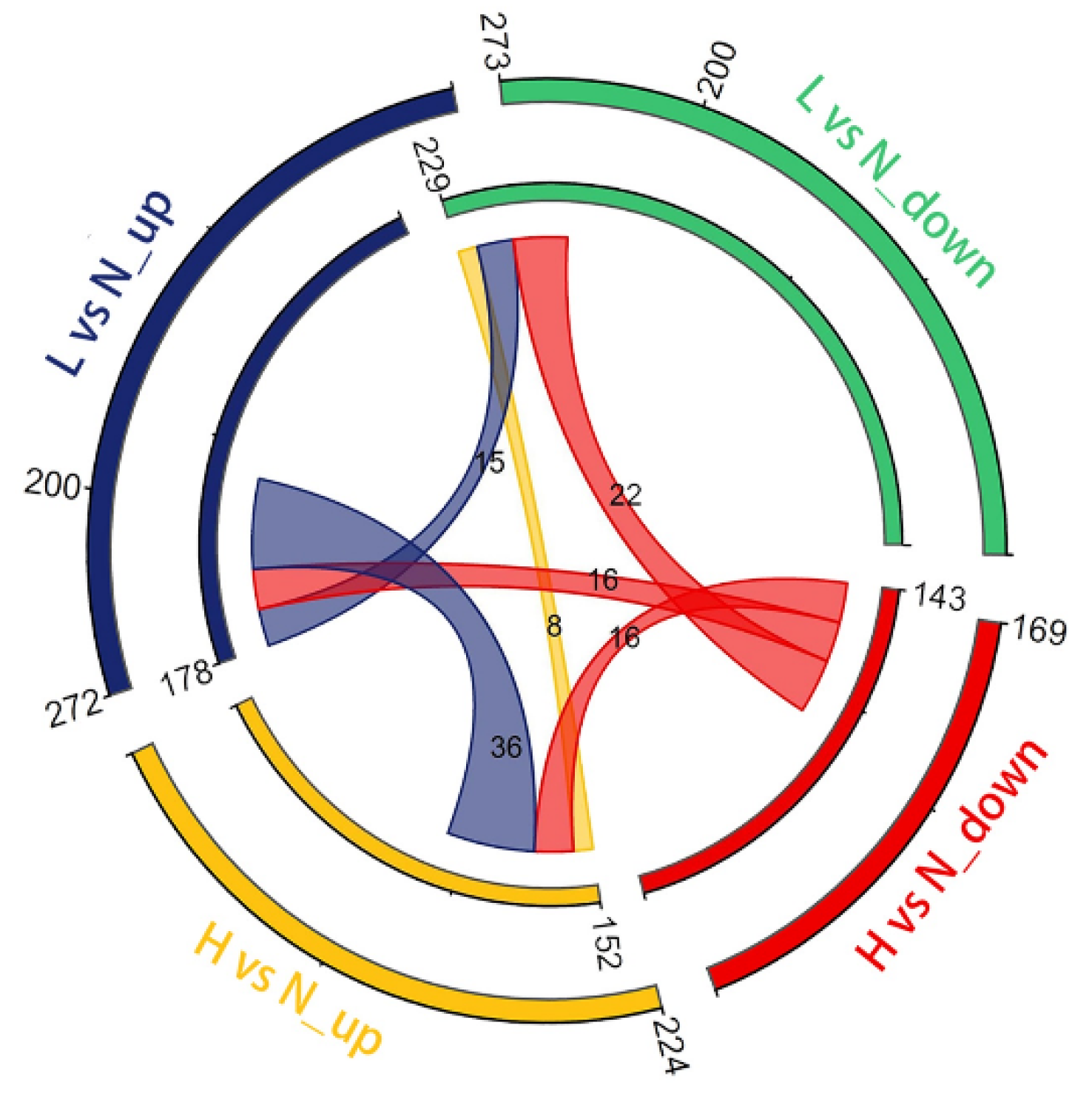
3.3. DETs and Their Functional Enrichment
3.4. Key DETs Involved in Oocyte Development Are Affected by Temperature
4. Discussion
4.1. Water Temperature Is a Key Factor Affecting Fish Spawning
4.2. Effects of Water Temperature on the Differentiation in DFO
4.3. Effects of Water Temperature on Vitellogenin Accumulation
4.4. Potential Impact of Variations in Water Temperature on Fish Reproduction in Dammed River
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Fish Spawning and Water Temperature Data Collection
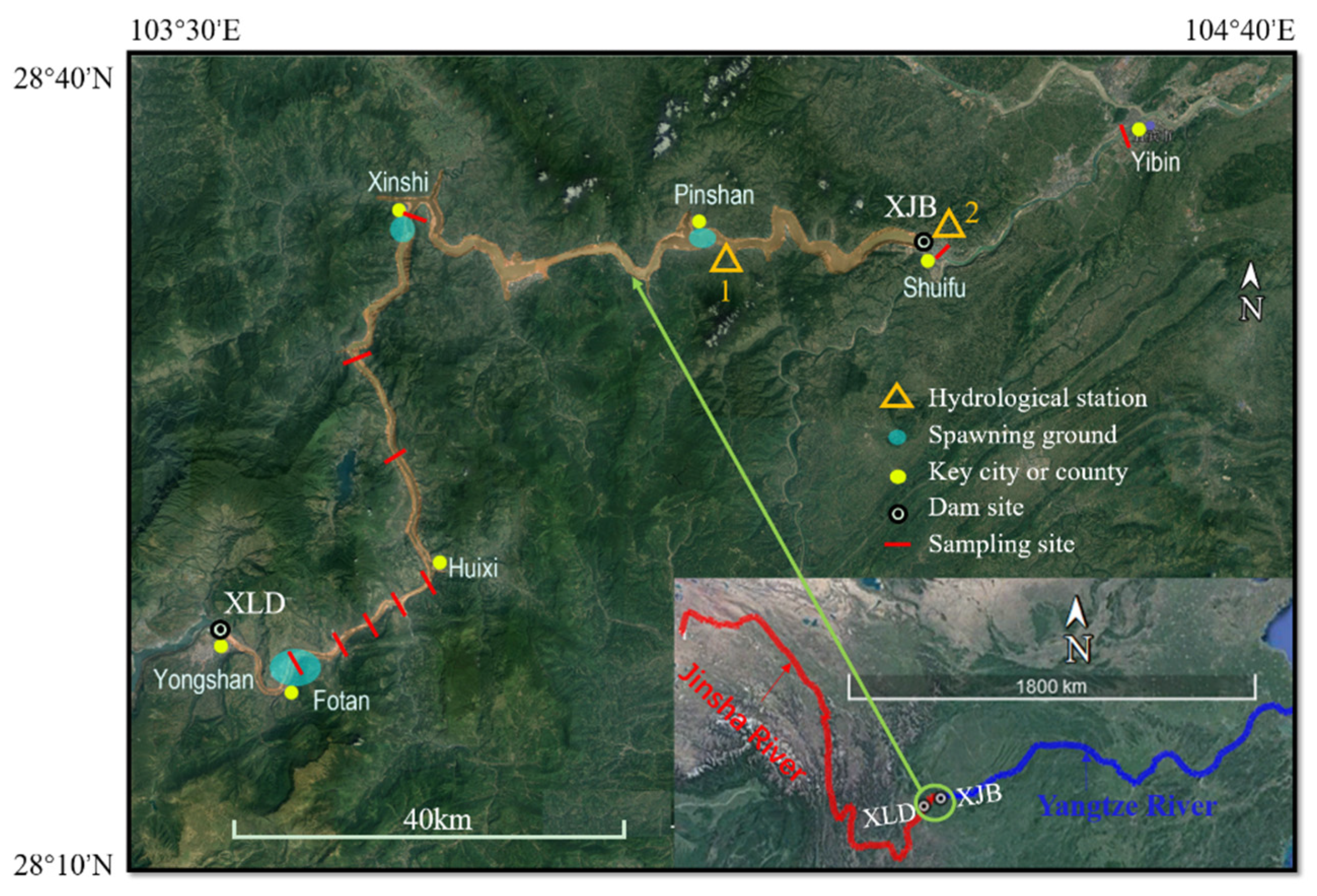
| Year | Sampling Site | Beginning and Ending Time | Water Temperature for Initial Spawning | Source |
|---|---|---|---|---|
| 2008 | Shuifu | 5/24–7/10 | 22.3 | Documentary recorded [88] |
| 2010 | Yibin | 4/21–7/12 | 20.4 | |
| 2011 | Yibin | 5/23–7/5 | 23.5 | |
| 2012 | Yibin | 5/21–7/15 | 22.2 | |
| 2013 | Yibin | 5/30–7/9 | – | |
| 2018 | Fotan–Xinshi | 5/6–7/4 | – | Field investigation |
Appendix A.2. Water Temperature Alteration and Spawning Abundance

References
- Vanderkelen, I.; Van Lipzig, N.P.M.; Sacks, W.J.; Lawrence, D.M.; Clark, M.; Mizukami, N. The impact of global reservoir expansion on the present-day climate. In Proceedings of the 23rd EGU General Assembly, Online, 19–30 April 2021; Available online: https://ui.adsabs.harvard.edu/abs/2021EGUGA..23..723V/abstract (accessed on 19 April 2021).
- Zhang, P.; Qiao, Y.; Grenouillet, G.; Lek, S.; Chang, J. Responses of spawning thermal suitability to climate change and hydropower operation for typical fishes below the three gorges dam. Ecol. Indic. 2021, 121, 107186. [Google Scholar] [CrossRef]
- Preece, R.M.; Jones, H.A. The effect of Keepit Dam on the temperature regime of the Namoi River, Australia. River Res. Appl. 2002, 184, 397–414. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Rhoads, B.; Wang, D.; Wu, J. Quantifying the impacts of the Three Gorges Reservoir on water temperature in the middle reach of the Yangtze River. J. Hydrol. 2019, 582, 124476. [Google Scholar] [CrossRef]
- Feiner, Z.S.; Wang, H.-Y.; Einhouse, D.W.; Jackson, J.R.; Rutherford, E.S.; Andergoot, C.S.V. Thermal environment and maternal effects shape egg size in a freshwater fish. Ecosphere 2016, 7, e01304. [Google Scholar] [CrossRef]
- Rogers, L.A.; Dougherty, A.B. Effects of climate and demography on reproductive phenology of a harvested marine fish population. Glob. Chang. Biol. 2019, 252, 708–720. [Google Scholar] [CrossRef]
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020, 97, 26. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 629, 1015. [Google Scholar] [CrossRef]
- Huang, Z.L.; Wang, L. Yangtze dams increasingly threaten the survival of the Chinese sturgeon. Curr. Biol. 2018, 28, 3640–3647. [Google Scholar] [CrossRef]
- Li, T.; Mo, K.; Wang, J.; Chen, Q.; Zhang, J.; Zeng, C. Mismatch between critical and accumulated temperature following river damming impacts fish spawning. Sci. Total Environ. 2021, 756, 144052. [Google Scholar] [CrossRef]
- Selman, K.; Wallace, R.A.; Sarka, A.; Qi, X. Stages of oocyte development in the Zebrafish, Brachydanio rerio. J. Morphol. 1993, 218, 203–224. [Google Scholar] [CrossRef]
- Sullivan, C.V.; Yilmaz, O. Vitellogenesis and yolk proteins, fish. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018; pp. 266–277. [Google Scholar]
- Ju, J.-C.; Jiang, S.; Tseng, J.-K.; Parks, J.E.; Yang, X. Heat shock reduces developmental competence and alters spindle configuration of bovine oocytes. Theriogenology 2005, 64, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Lubzens, E.; Young, G.; Bobe, J.; Cerda, J. Oogenesis in teleosts: How eggs are formed. Gen. Comp. Endocr. 2010, 1653, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, E.; Tranberg, H.K.; Sollid, J.; Nilsson, G.E.; Nikinmaa, M. Temperature regulates hypoxia-inducible factor-1 HIF-1 in a poikilothermic vertebrate, crucian carp Carassius carassius. J. Exp. Biol. 2006, 209, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Stensløkken, K.-O.; Ellefsen, S.; Larsen, H.K.; Vaage, J.; Nilsson, G.E. Expression of heat shock proteins in anoxic crucian carp Carassius carassius: Support for cold as a preparatory cue for anoxia. Am. J. Physiol.-Reg. I 2010, 298, R1499–R1508. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.X.; Teng, J.; Zhao, Y.; Li, N.; Wang, H.; Ji, X.S. Gonad transcriptome analysis of high-temperature-treated females and high-temperature-induced sex-reversed neomales in Nile Tilapia. Int. J. Mol. Sci. 2018, 19, 689. [Google Scholar] [CrossRef]
- Moura, M.T.; Paula-Lopes, F.F. Thermoprotective molecules to improve oocyte competence under elevated temperature. Theriogenology 2020, 156, 262–271. [Google Scholar] [CrossRef]
- Morgan, C.H.; Zhang, H.; Bomblies, K. Are the effects of elevated temperature on meiotic recombination and thermotolerance linked via the axis and synaptonemal complex? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160470. [Google Scholar] [CrossRef]
- Zenzes, M.; Bielecki, R.; Casper, R.; Leibo, S. Effects of chilling to 0°C on the morphology of meiotic spindles in human metaphase II oocytes. Fertil. Steril. 2001, 754, 769–777. [Google Scholar] [CrossRef]
- Tseng, J.K.; Chen, C.H.; Chou, P.C.; Yeh, S.P.; Ju, J.C. Influences of follicular size on parthenogenetic activation and in vitro heat shock on the cytoskeleton in cattle oocytes. Reprod. Domest. Anim. 2010, 393, 146–153. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Ispada, J.; Risolia, P.; Rodrigues, M.T.; Lima, R.S.; Assumpcao, M. Thermoprotective effect of insulin-like growth factor 1 on invitro matured bovine oocyte exposed to heat shock. Theriogenology 2016, 868, 2028–2039. [Google Scholar] [CrossRef]
- Prosee, R.F.; Wenda, J.M.; Steiner, F.A. Adaptations for centromere function in meiosis. Essays Biochem. 2020, 642, 193–203. [Google Scholar]
- Laband, K.; Le Borgne, R.; Edwards, F.; Stefanutti, M.; Canman, J.C.; Verbavatz, J.M.; Dumont, J. Chromosome segregation occurs by microtubule pushing in oocytes. Nat. Commun. 2017, 81, 1499. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, N.W.; King, H.R. Temperature and salmonid reproduction: Implications for aquaculture. J. Fish Biol. 2010, 76, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Munoz-Cueto, J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocr. 2020, 291, 113439. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Lafont, A.G.; Maugars, G.; Baloche, S.; Pérez, L. Identification and stable expression of vitellogenin receptor through vitellogenesis in the European eel. Animal 2020, 146, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Wong, N.K.; Zhang, X.; Zhu, C.; Chen, T. Vitellogenin receptor VgR mediates oocyte maturation and ovarian development in the Pacific White Shrimp Litopenaeus vannamei. Front. Physiol. 2020, 11, 485. [Google Scholar]
- Hong, W.; Takshak, A.; Osunbayo, O.; Kunwar, A.; Vershinin, M. The effect of temperature on microtubule-based transport by cytoplasmic dynein and kinesin-1 motors. Biophys. J. 2016, 1116, 1287–1294. [Google Scholar] [CrossRef]
- Yadav, S.; Kunwar, A. Temperature-dependent activity of motor proteins: Energetics and their implications for collective behavior. Front. Cell Dev. Biol. 2021, 9, 610899. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Ishiwata, S.I. Temperature dependence of force, velocity, and processivity of single kinesin molecules. Biochem. Biophys. Res. Commun. 2020, 272, 895–899. [Google Scholar] [CrossRef]
- Abraham, Z.; Hawley, E.; Hayosh, D.; Webster-Wood, V.A.; Akkus, O. Kinesin and dynein mechanics: Measurement methods and research applications. J. Biomech. Eng. 2018, 140, 020805. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tang, L.; Wang, L.; Wang, J.; Mo, K.L.; Chen, Q.W. Distribution characteristics and ecological types changes in fish communities under hydropower development from Xiluodu to Xiangjiaba reach. Acta Ecol. Sin. 2020, 404, 1–13. (In Chinese) [Google Scholar]
- Conesa, A.; Madrigal, P.; Tarazona, S. A survey of best practices for RNA-seq data analysis. Genome Boil. 2016, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Mukesh, J.; Liu, Z. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 72, e30619. [Google Scholar] [CrossRef] [PubMed]
- Salmela, L.; Rivals, E. LoRDEC: Accurate and efficient long read error correction. Bioinformatics 2014, 30, 3506–3514. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Panagiotis, I.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2005, 3119, 3210–3212. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 1–16. [Google Scholar] [CrossRef]
- Anders, S. Analyzing RNA-Seq data with the DESeq Package. Mol. Biol. 2010, 43, 1–17. [Google Scholar]
- Chen, Q.; Zhang, J.; Chen, Y.; Mo, K.; Zhang, Y. Inducing flow velocities to manage fish reproduction in regulated rivers. Engineering 2020, 7, 178–186. [Google Scholar] [CrossRef]
- Dahlke, F.T.; Wohlrab, S.; Butzin, M.; Prtner, H.O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 2020, 369, 65–70. [Google Scholar] [CrossRef]
- Glotzbecker, G.J.; Ward, J.L.; Walters, D.M.; Blum, M.J. Turbidity alters pre-mating social interactions between native and invasive stream fishes. Freshw. Biol. 2015, 60, 1784–1793. [Google Scholar] [CrossRef]
- Wang, N.; Teletchea, F.; Kestemont, P.; Milla, S.; Fontaine, P. Photothermal control of the reproductive cycle in temperate fishes. Rev. Aquacul. 2001, 2, 209–222. [Google Scholar] [CrossRef]
- Hu, Y.F.; Wu, W.M.; Chen, Z.H. Reservoir sediment computation of Xiangjiaba hydropower plant. Yangtze River 2008, 34, 36–38. (In Chinese) [Google Scholar]
- Food and Agriculture Organization of the United Nations. Farm Structures in Tropical Climates: Animal Environmental Requirements; FAO: Rome, Italy, 1986. [Google Scholar]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Henry, B.; Charmley, E.; Eckard, R.; Gaughan, J.B.; Hegarty, R. Livestock production in a changing climate: Adaptation and mitigation research in Australia. Crop Pasture Sci. 2012, 633, 191–202. [Google Scholar] [CrossRef]
- Fangue, N.A. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J. Exp. Biol. 2006, 20915, 2859–2872. [Google Scholar] [CrossRef]
- Mjiab, C.; Mjs, B.; Ak, A. What metabolic, osmotic and molecular stress responses tell us about extreme ambient heatwave impacts in fish at low salinities: The case of European seabass, Dicentrarchus labrax. Sci. Total Environ. 2020, 749, 141458. [Google Scholar]
- Metchat, A.; Akerfelt, M.; Bierkamp, C.; Delsinne, V.; Sistonen, L.; Alexandre, H. Mammalian heat shock factor 1 is essential for oocyte meiosis and directly regulates hsp90α expression. J. Biol. Chem. 2009, 28414, 9521. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, L.; Zhou, F.; Huang, J.; Guo, Y.; Yang, K. Molecular cloning and expression analysis of a heat shock protein Hsp90 gene from black tiger shrimp Penaeus monodon. Mol. Biol. Rep. 2009, 361, 127. [Google Scholar] [CrossRef]
- Dhamad, A.E.; Zhou, Z.; Zhou, J.; Du, Y.; Didier, P. Systematic proteomic Identification of the heat shock proteins Hsp that Interact with estrogen receptor alpha ERα and biochemical characterization of the ERα-Hsp70 Interaction. PLoS ONE 2016, 11, e0160312. [Google Scholar] [CrossRef]
- Seibert, J.T.; Adur, M.K.; Schultz, R.B.; Thomas, P.Q.; Ross, J.W. Differentiating between the effects of heat stress and lipopolysaccharide on the porcine ovarian heat shock protein response. J. Anim. Sci. 2019, 97, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Roll-Mecak, A. The tubulin code in microtubule dynamics and information encoding. Dev. Cell. 2020, 54, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Moore, J.K. Microtubule dynamics at low temperature: Evidence that tubulin recycling limits assembly. Mol. Biol. Cell. 2020, 3111, 1154–1166. [Google Scholar] [CrossRef]
- Reinemann, D.N.; Sturgill, E.G.; Das, D.K.; Degen, M.S.; Vörös, Z.; Hwang, W.; Ohi, R.; Lang, M.J. Collective force regulation in anti-parallel microtubule gliding by dimeric Kif15 kinesin motors. Curr. Biol. 2017, 27, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Körner, O.; Kohno, S.; Schönenberger, R.; Suter, J.F.; Knauer, K.; Guillette, L.J.; Burkhardt-Holm, P. Water temperature and concomitant waterborne ethinylestradiol exposure affects the vitellogenin expression in juvenile brown trout (Salmo trutta). Aquat. Toxicol. 2008, 903, 188–196. [Google Scholar] [CrossRef]
- Sato, N.; Kawazoe, I.; Suzuki, Y.; Aida, K. Effects of temperature on vitellogenesis in Japanese eel Anguilla japonica. Fish. Sci. 2006, 725, 961–966. [Google Scholar] [CrossRef]
- King, H.R.; Pankhurst, N.W.; Watts, M.; Pankhurst, P.M. Effect of elevated summer temperatures on gonadal steroid production, vitellogenesis and egg quality in female Atlantic salmon. J. Fish Biol. 2013, 631, 153–167. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Todo, T.; Sullivan, C.V.; Schilling, J.; Reading, B.J.; Matsubara, T. Ovarian yolk formation in fishes: Molecular mechanisms underlying formation of lipid droplets and vitellogenin-derived yolk proteins. Gen. Comp. Endocr. 2015, 221, 9–15. [Google Scholar] [CrossRef]
- Robert-Paganin, J.; Pylypenko, O.; Kikuti, C.; Sweeney, H.L.; Houdusse, A. Force generation by myosin motors: A structural perspective. Chem. Rev. 2019, 1201, 5–35. [Google Scholar] [CrossRef]
- Plagens, R.N.; Mossiah, I.; Guisbert, K.K.; Guisbert, E. Chronic temperature stress inhibits reproduction and disrupts endocytosis via chaperone titration in Caenorhabditis elegans. BMC Biol. 2021, 191, 75. [Google Scholar] [CrossRef]
- Scotland, P.B.; Heath, J.L.; Conway, A.E.; Porter, N.B.; Armstrong, M.B.; Walker, J.A.; Klebig, M.L.; Lavau, C.P.; Wechsler, D.S. The PICALM protein plays a key role in iron homeostasis and cell proliferation. PLoS ONE 2012, 78, e44252. [Google Scholar] [CrossRef] [PubMed]
- Papalazarou, V.; Machesky, L.M. The cell pushes back: The Arp2/3 complex is a key orchestrator of cellular responses to environmental forces. Curr. Opin. Cell Biol. 2021, 68, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, K.F.; Wu, Y.L.; Liu, Q.Y.; Zhao, P.X.; Li, Y. Analysis and restoration of an ecological flow regime during the Coreius guichenoti spawning period. Ecol. Eng. 2018, 123, 74–85. [Google Scholar] [CrossRef]
- Zhang, P.; Qiao, Y.; Schineider, M.; Chang, J.; Mutzner, R.; Sanmartín, J.F. Using a hierarchical model framework to assess climate change and hydropower operation impacts on the habitat of an imperiled fish in the Jinsha River, China. Sci. Total Environ. 2019, 646, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ma, C.; Zhang, J.; Lian, J.; Zhao, W. Multi-objective optimal operation of a large deep reservoir during storage period considering the outflow-temperature demand based on NSGA-II. J. Hydrol. 2020, 586, 124919. [Google Scholar] [CrossRef]
- Kjesbu, O.S.; Righton, D.; Krüger-Johnsen, M.; Thorsen, A.; Witthames, P. Thermal dynamics of ovarian maturation in Atlantic cod Gadus morhua. Can. J. Fish Aquat. Sci. 2010, 67, 605–625. [Google Scholar] [CrossRef]
- Firkus, T.; Rahel, F.J.; Bergman, H.L.; Cherrington, B.D. Warmed winter water temperatures alter reproduction in two fish species. Environ. Manag. 2018, 612, 291–303. [Google Scholar] [CrossRef]
- Farmer, T.M.; Marschall, E.A.; Dabrowski, K.; Ludsin, S.A. Short winters threaten temperate fish populations. Nat. Commun. 2015, 6, 7724. [Google Scholar] [CrossRef]
- Wright, P.J.; Orpwood, J.E.; Boulcott, P. Warming delays ovarian development in a capital breeder. Mar. Biol. 2017, 164, 1–9. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2020, 1217, 106976. [Google Scholar] [CrossRef]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell. Sci. 2014, 127, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.H.; Wang, J.W.; Lu, K.; Zhou, J.L.; Zhou, Q.; Zhang, G.R. The first vitellogenin receptor from a Lepidopteran insect: Molecular characterization, expression patterns and RNA interference analysis. Insect Mol. Biol. 2010, 20, 61–73. [Google Scholar] [CrossRef]
- Reading, B.J.; Hiramatsu, N.; Schilling, J.; Molloy, K.T.; Glassbrook, N.; Mizuta, H.; Luo, W.; Baltzegar, D.A.; Williams, V.N.; Todo, T.; et al. Lrp13 is a novel vertebrate lipoprotein receptor that binds vitellogenins in teleostfishes. J. Lipid. Res. 2014, 55, 2287–2295. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Qin, L.T.; Liang, S.W.; Chen, P.; Chen, J.B. The expression and potential role of tubulin alpha 1b in Wilms’ tumor. BioMed Res. Int. 2020, 2020, 9809347. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, Z.K.; Zhu, D.S.; Guo, T.; Cai, Q.L.; Wang, Y. KIF20B promotes the progression of clear cell renal cell carcinoma by stimulating cell proliferation. J. Cell Physiol. 2019, 234, 16517–16525. [Google Scholar] [CrossRef]
- Cross, R.A.; Mcainsh, A. Prime movers: The mechanochemistry of mitotic kinesins. Nat. Rev. Mol. Cell Biol. 2014, 15, 257–271. [Google Scholar] [CrossRef]
- Campbell, P.D.; Marlow, F.L. Temporal and tissue specific gene expression patterns of the zebrafish kinesin-1 heavy chain family, kif5s, during development. Gene. Expression Patterns Gep. 2013, 13, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Yong, D.L.; Kim, B.; Jung, S.; Kim, H.; Kim, H. The dynactin subunit DCTN1 controls osteoclastogenesis via the Cdc42/PAK2 pathway. Exper. Mol. Med. 2020, 52, 514–528. [Google Scholar]
- Ansar, M.; Ullah, F.; Paracha, S.A.; Adams, D.J.; Antonarakis, S.E. Article bi-allelic variants in DYNC1I2 cause syndromic microcephaly with intellectual disability, cerebral malformations, and dysmorphic facial features. Am. J. Hum. Gen. 2019, 104, 1073–1087. [Google Scholar] [CrossRef]
- Piekny, A.J.; Maddox, A.S. The myriad roles of Anillin during cytokinesis. Semin. Cell Dev. Biol. 2010, 21, 881–891. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Shen, N.; Pi, W.; Jiang, W.; Huang, J.; Hu, Y.; Li, X.; Sun, L. Knockdown of ANLN by lentivirus inhibits cell growth and migration in human breast cancer. Mol. Cell Biochem. 2015, 398, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhou, X.; Zhu, L.; Shi, S.; Lv, J. Polymorphisms in the nonmuscle myosin heavy chain 9 gene (MYH9) are associated with the progression of IgA nephropathy in Chinese. Nephrol. Dial. Transpl. 2011, 26, 2544–2549. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.; Loos, B.; Moolman-Smook, J.C.; Kinnear, C.J. Ascribing novel functions to the sarcomeric protein, myosin binding protein H (MyBPH) in cardiac sarcomere contraction. Exp. Cell Res. 2015, 331, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, C.; Qu, X.; Xiong, F.; Paukert, C.P.; Chen, Y. Fish diversity, endemism, threats, and conservation in the jinsha river basin (upper yangtze river), China. N. Am. J. Fish. Manag. 2021, 41, 967–984. [Google Scholar] [CrossRef]
- Gao, S.B.; Tang, H.Y.; Chen, S.; Yang, Z.; Dong, F.Y. Effects of the first phase of Jinsha River hydropower project on fish recruitment: Early life history stages of Coreius guichenoti in the Upper Yangtze River. J. Hydroecol. 2015, 36, 6–10. (In Chinese) [Google Scholar]
- Kawakami, T.; Aoyama, J.; Tsukamoto, K. Morphology of pelagic fish eggs identified using mitochondrial DNA and their distribution in waters west of the mariana islands. Environ. Biol. Fish. 2010, 7, 221–235. [Google Scholar] [CrossRef]
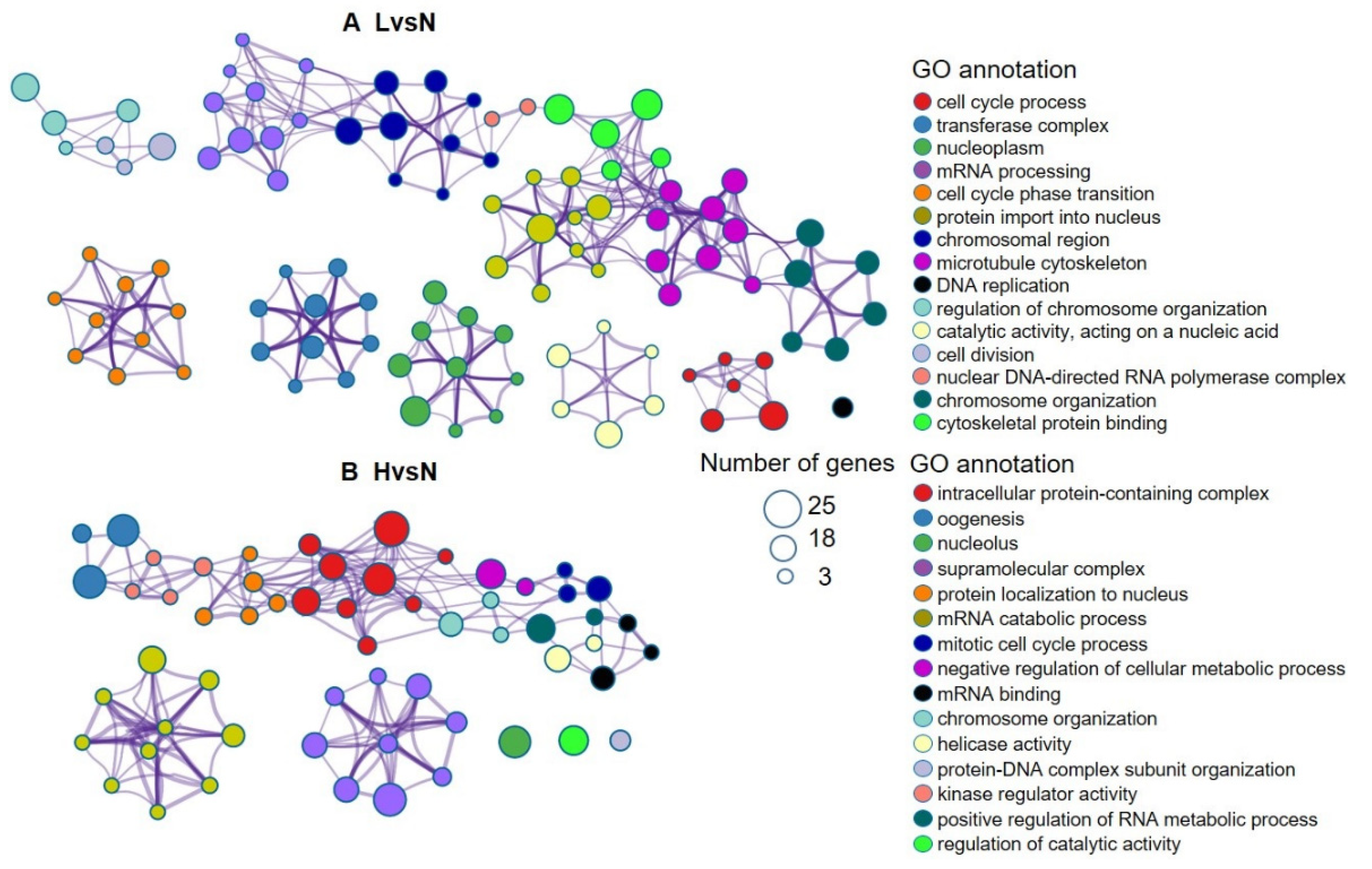
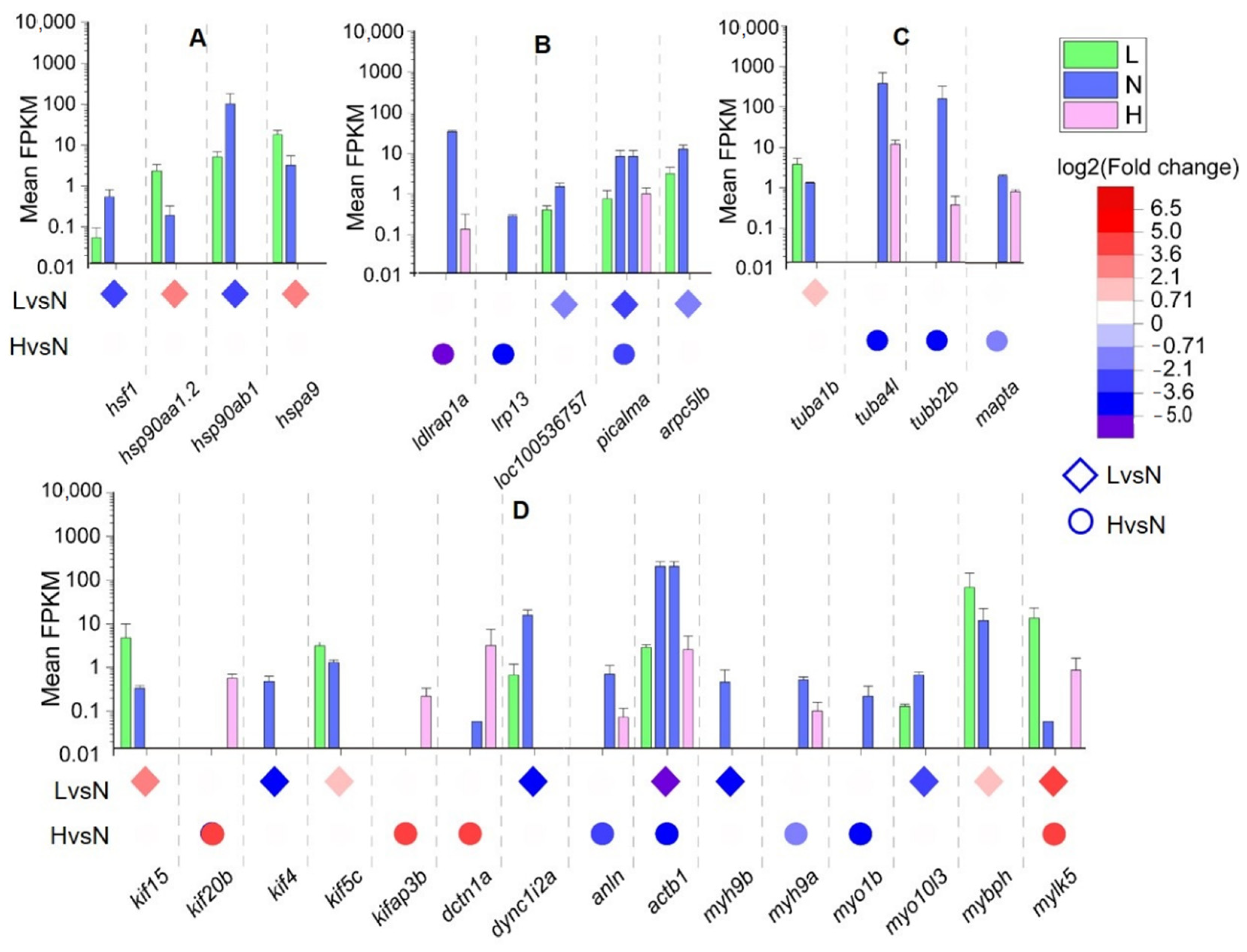
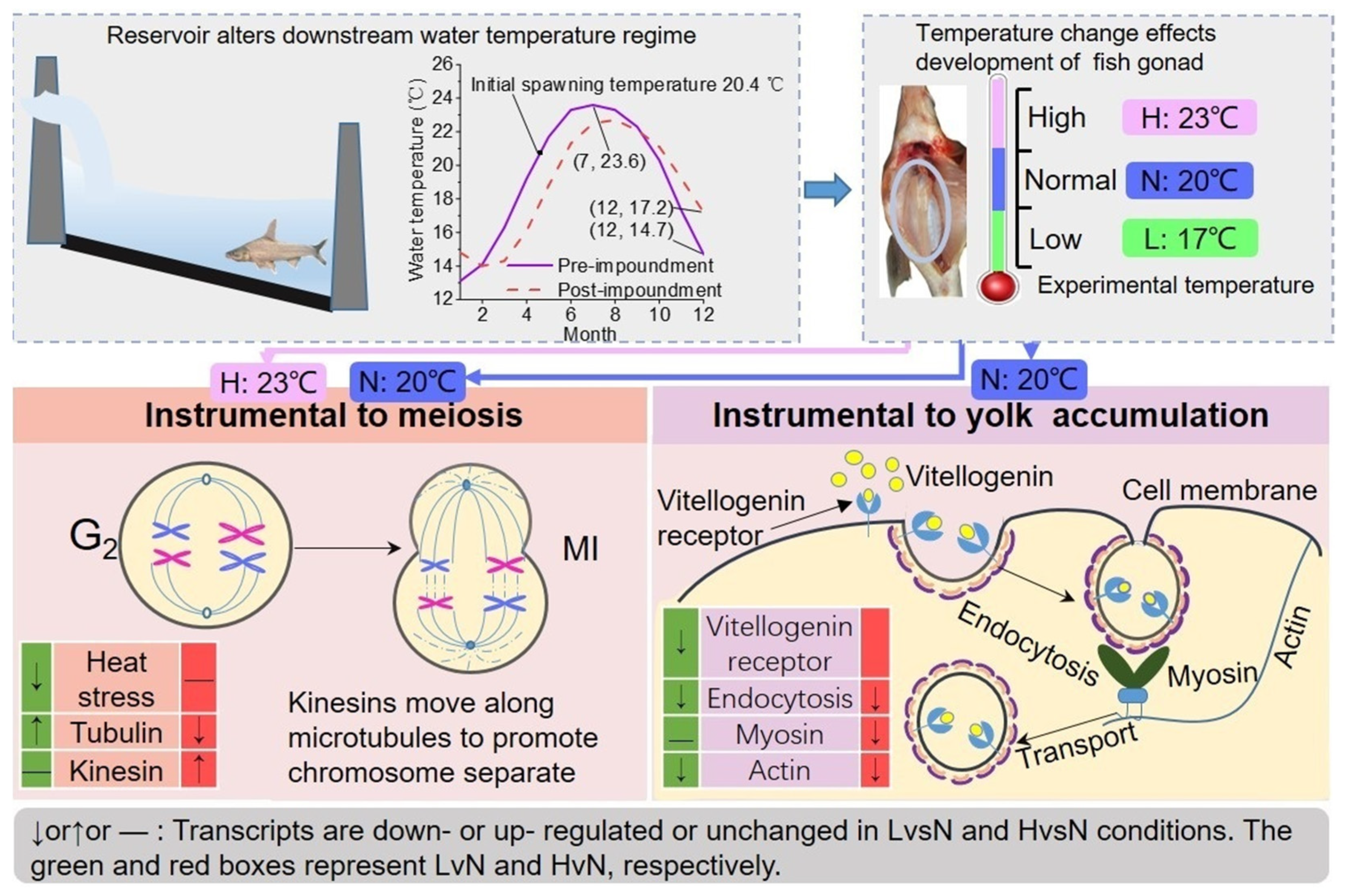
| Samples | Total Clean Reads Number | GC Content (%) | ≥Q30 (%) | Uniquely Mapped Reads | Multiple Mapped Reads | Unmapped Reads |
|---|---|---|---|---|---|---|
| N1 | 2.70 × 107 | 48.70 | 95.04 | 39.11% | 43.67% | 16.71% |
| N2 | 2.24 × 107 | 48.39 | 95.01 | 38.29% | 42.48% | 16.57% |
| N3 | 2.38 × 107 | 48.80 | 94.92 | 38.94% | 43.24% | 16.80% |
| L1 | 2.39 × 107 | 48.72 | 95.45 | 40.44% | 45.41% | 16.10% |
| L2 | 2.13 × 107 | 48.89 | 95.07 | 41.22% | 46.96% | 16.95% |
| L3 | 2.06 × 107 | 48.97 | 95.44 | 38.82% | 43.64% | 16.61% |
| H1 | 2.60 × 107 | 48.90 | 94.94 | 39.51% | 44.42% | 16.10% |
| H2 | 2.42 × 107 | 48.90 | 95.06 | 38.76% | 43.48% | 16.50% |
| H3 | 2.76 × 107 | 48.87 | 94.96 | 38.09% | 43.15% | 16.65% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Chen, Q.; Zhang, Q.; Feng, T.; Zhang, J.; Lin, Y.; Yang, P.; He, S.; Zhang, H. Transcriptomic Analysis on the Effects of Altered Water Temperature Regime on the Fish Ovarian Development of Coreius guichenoti under the Impact of River Damming. Biology 2022, 11, 1829. https://doi.org/10.3390/biology11121829
Li T, Chen Q, Zhang Q, Feng T, Zhang J, Lin Y, Yang P, He S, Zhang H. Transcriptomic Analysis on the Effects of Altered Water Temperature Regime on the Fish Ovarian Development of Coreius guichenoti under the Impact of River Damming. Biology. 2022; 11(12):1829. https://doi.org/10.3390/biology11121829
Chicago/Turabian StyleLi, Ting, Qiuwen Chen, Qi Zhang, Tao Feng, Jianyun Zhang, Yuqing Lin, Peisi Yang, Shufeng He, and Hui Zhang. 2022. "Transcriptomic Analysis on the Effects of Altered Water Temperature Regime on the Fish Ovarian Development of Coreius guichenoti under the Impact of River Damming" Biology 11, no. 12: 1829. https://doi.org/10.3390/biology11121829
APA StyleLi, T., Chen, Q., Zhang, Q., Feng, T., Zhang, J., Lin, Y., Yang, P., He, S., & Zhang, H. (2022). Transcriptomic Analysis on the Effects of Altered Water Temperature Regime on the Fish Ovarian Development of Coreius guichenoti under the Impact of River Damming. Biology, 11(12), 1829. https://doi.org/10.3390/biology11121829








