Extracellular Compounds from Pathogenic Bacterium Pseudoalteromonas piscicida X-8 Cause Bleaching Disease, Triggering Active Defense Responses in Commercially Farmed Saccharina japonica
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Different Components of PpX-8
2.2. Sample Collection
2.3. Inoculation Assay with Different Components of PpX-8
2.4. Statistical Proportions of Bleached Tissue Pieces Following Inoculation with Different Components of PpX-8
2.5. Morphological Observations of the S. japonica Tissue Pieces Inoculated with PpX-8 Extracellular Compounds
2.6. Ultrastructural Changes in the S. japonica Tissues Inoculated with PpX-8 Extracellular Compounds Observed under a Transmission Electron Microscope (TEM)
2.7. TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assay
2.8. Detection of the Caspase-3-like Enzymatic Activity of S. japonica Tissues
3. Results
3.1. Inoculation Assay of S. japonica Tissues with Different Compounds of PpX-8
3.2. Analysis of the Proportions of the Bleached Tissue Pieces
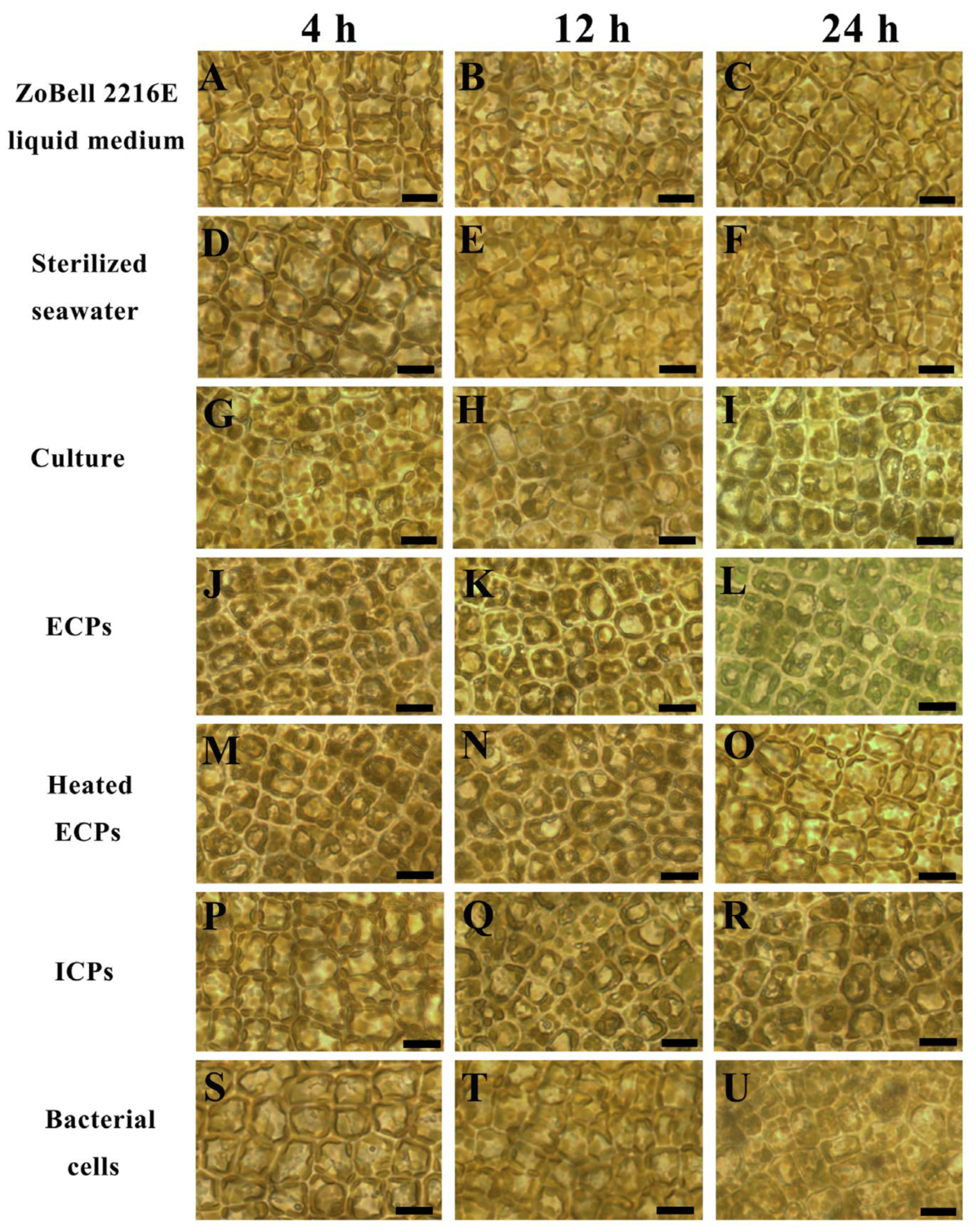
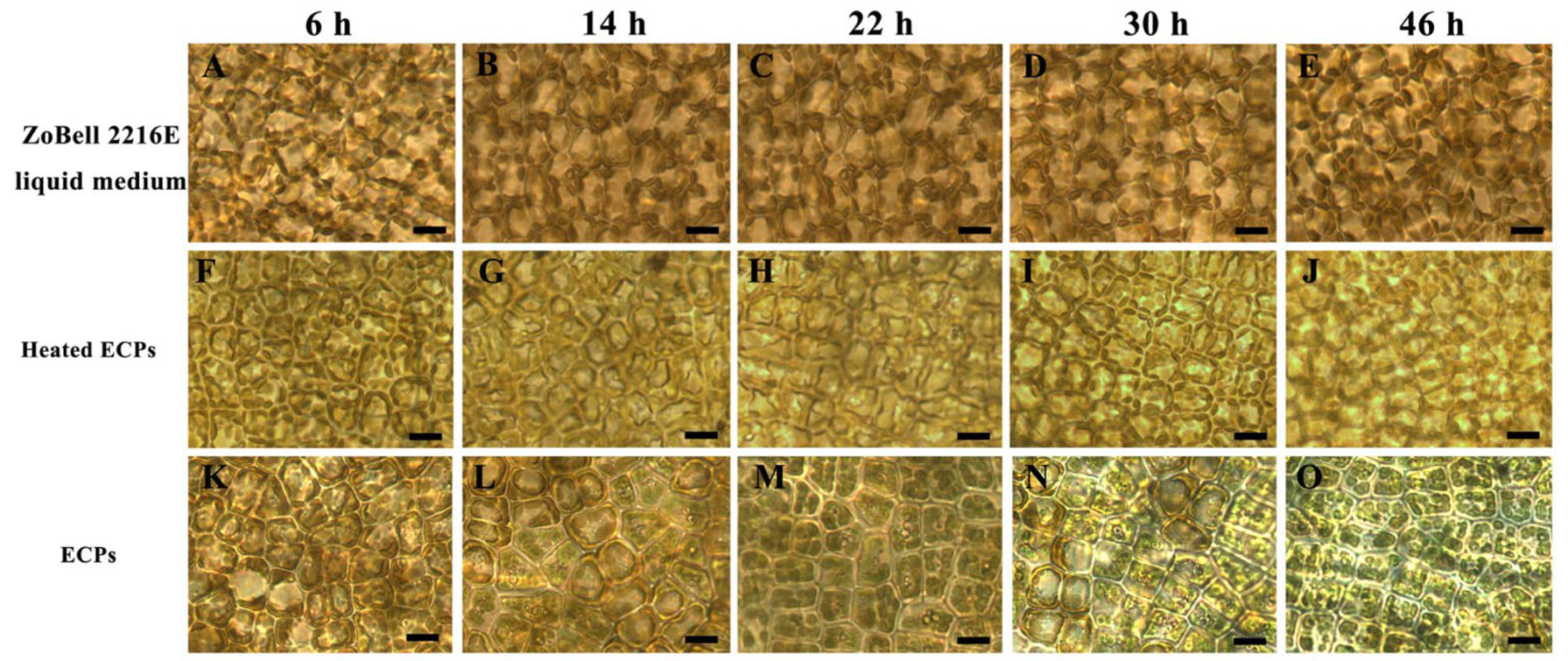
3.3. Morphological Observations of S. japonica Tissue Pieces Inoculated with PpX-8 Extracellular Compounds
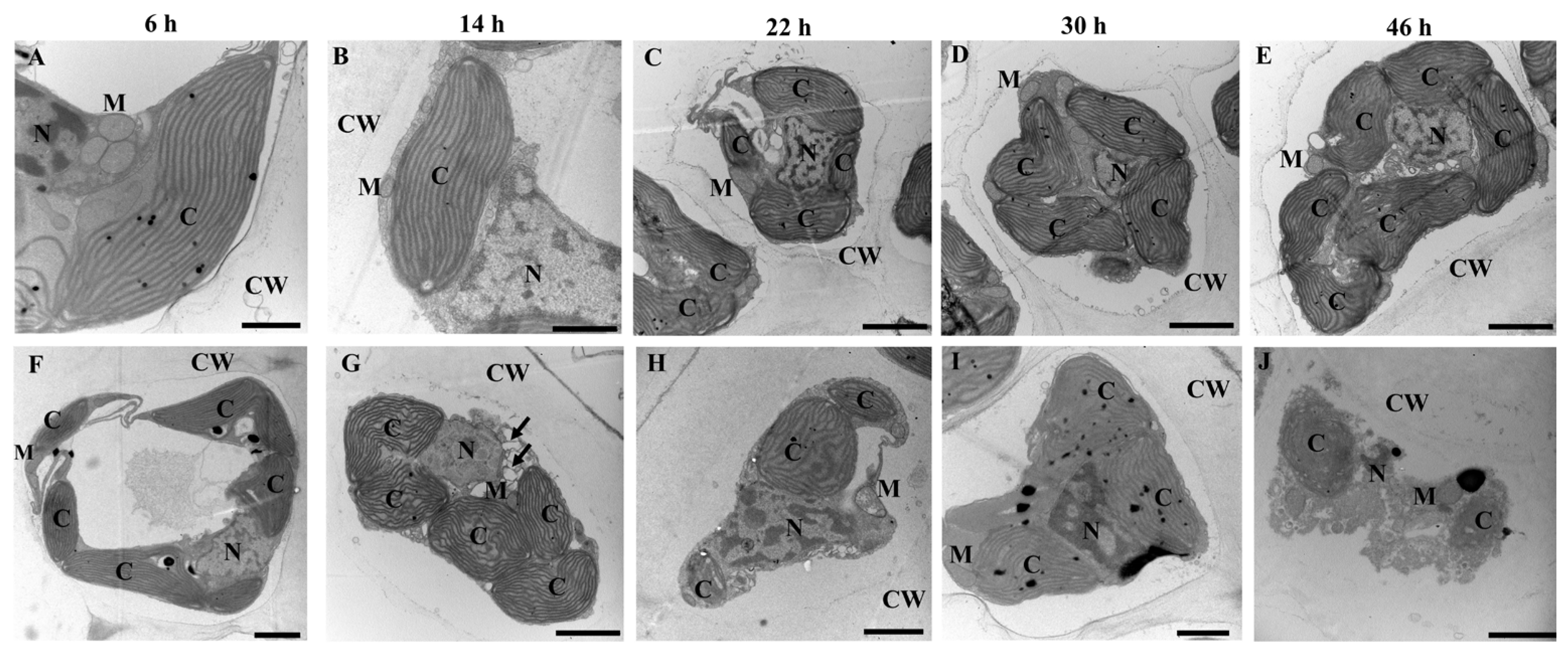
3.4. Ultrastructural Changes Observed under a Transmission Electron Microscope (TEM) in S. japonica Tissue Pieces Inoculated with Extracellular Compounds
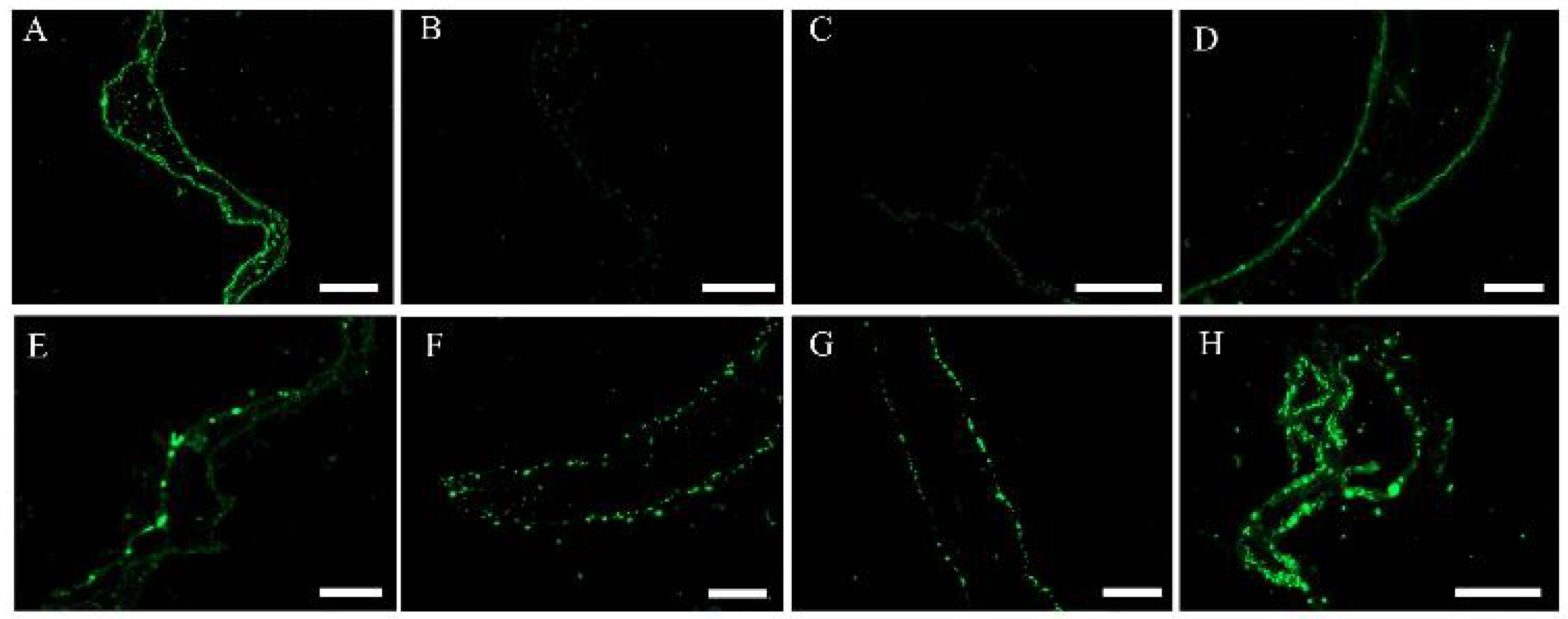
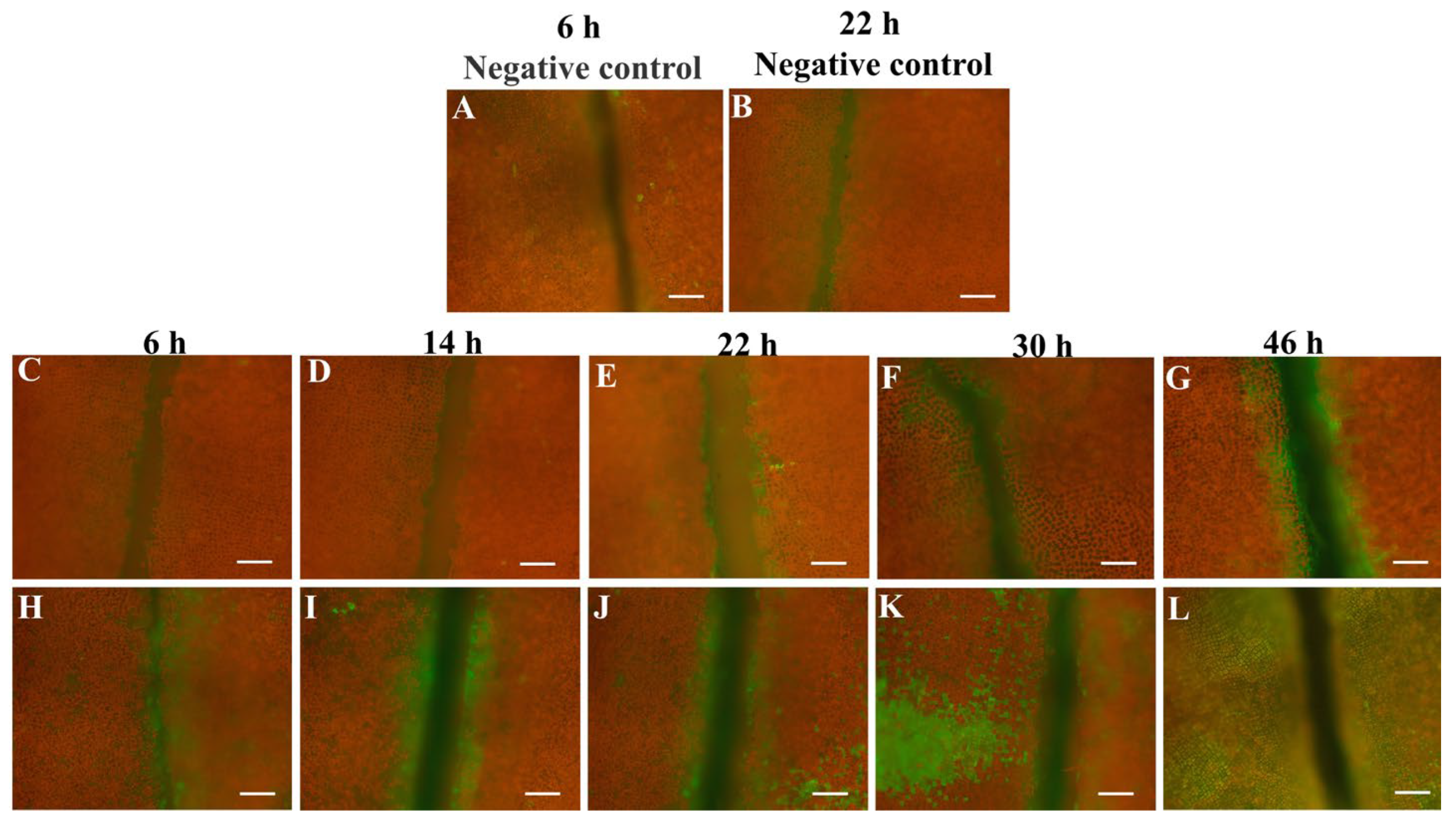
3.5. TUNEL Assay of S. japonica Tissue Pieces Inoculated with PpX-8 Extracellular Compounds
3.6. Caspase-3-like Enzymatic Activity in S. japonica Tissue Pieces Inoculated with PpX-8 Extracellular Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heath, M.C. Hypersensitive response-related death. Plant Mol. Biol. 2000, 44, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Programmed cell death in development and defense. Plant Physiol. 2001, 125, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Kato, N.; Lawton, M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature 2001, 411, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Segovia, M.; Haramaty, L.; Berges, J.A.; Falkowski, P.G. Cell death in the unicellular chlorophyte Dunaliella tertiolecta. A hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiol. 2003, 132, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hatsugai, N.; Iwasaki, S.; Tamura, K.; Kondo, M.; Fuji, K.; Ogasawara, K.; Nishimura, M.; Hara-Nishimura, I. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev. 2009, 23, 2496–2506. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Mysore, K.S. Nonhost resistance against bacterial pathogens: Retrospectives and prospects. Annu. Rev. Phytopathol. 2013, 51, 407–427. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Curtis, C.; Fu, Z.Q. Go in for the kill: How plants deploy effector-triggered immunity to combat pathogens. Virulence 2014, 5, 710–721. [Google Scholar] [CrossRef]
- Sawabe, T.; Tanaka, R.; Lqbal, M.; Tajima, K.; Ezura, Y.; Lvanova, E.; Christen, R. Assignment of Alteromonas elyakovii KMM 162T and five strains isolated from spotwounded fronds of Laminaria japonica to Pseudoalteromonas elyakovii comb. nov. and the extended description of the species. Int. J. Syst. Evol. Microbiol. 2000, 50, 265–271. [Google Scholar] [CrossRef]
- McCabe, P.F.; Levine, A.; Meijer, P.-J.; Tapon, N.A.; Pennell, R.I. A programmed cell death pathway activated in carrot cells cultured at low cell density. Plant J. 1997, 12, 267–280. [Google Scholar] [CrossRef]
- Del Pozo, O.; Lam, E. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr. Biol. 1998, 8, 1129–1132. [Google Scholar] [CrossRef]
- Woltering, E.J.; van der Bent, A.; Hoeberichts, F.A. Do plant caspases exist? Plant Physiol. 2002, 130, 1764–1769. [Google Scholar] [CrossRef]
- Danon, A.; Rotari, V.I.; Gordon, A.; Mailhac, N.; Gallois, P. Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and Defender against Apoptotic Death. J. Biol. Chem. 2004, 279, 779–787. [Google Scholar] [CrossRef]
- Rojo, E.; Martín, R.; Carter, C.; Zouhar, J.; Pan, S.; Plotnikova, J.; Jin, H.; Paneque, M.; Sánchez-Serrano, J.J.; Baker, B.; et al. VPEgamma Exhibits a Caspase-like Activity that Contributes to Defense against Pathogens. Curr. Biol. 2004, 14, 1897–1906. [Google Scholar] [CrossRef]
- Vartapetian, A.B.; Tuzhikov, A.I.; Chichkova, N.V.; Taliansky, M.; Wolpert, T.J. A plant alternative to animal caspases: Subtilisin-like proteases. Cell Death Differ. 2011, 18, 1289–1297. [Google Scholar] [CrossRef]
- Lord, C.E.; Gunawardena, A.H. Programmed cell death in C. elegans, mammals and plants. Eur. J. Cell Biol. 2012, 91, 603–613. [Google Scholar] [CrossRef]
- Fagundes, D.; Bohn, B.; Cabreira, C.; Leipelt, F.; Dias, N.; Bodanese-Zanettini, M.H.; Cagliari, A. Caspases in plants: Metacaspase gene family in plant stress responses. Funct. Integr. Genom. 2015, 15, 639–649. [Google Scholar] [CrossRef]
- Bidle, K.D.; Haramaty, L.; Barcelos, E.R.J.; Falkowski, P. Viral activation and recruitment of metacaspases in the unicellular coccolithophore, Emiliania huxleyi. Proc. Natl. Acad. Sci. USA 2007, 104, 6049–6054. [Google Scholar] [CrossRef]
- Vardi, A.; Van Mooy, B.A.; Fredricks, H.F.; Popendorf, K.J.; Ossolinski, J.E.; Haramaty, L.; Bidle, K.D. Viral Glycosphingolipids Gnduce Lytic Infection and Cell Death in Marine Phytoplankton. Science 2009, 326, 861–865. [Google Scholar] [CrossRef]
- Bramucci, A.R.; Case, R.J. Phaeobacter inhibens induces apoptosis-like programmed cell death in calcifying Emiliania huxleyi. Sci. Rep. 2019, 9, 5215. [Google Scholar] [CrossRef]
- Wang, G.G.; Lin, W.; Zhang, L.J.; Yan, X.J.; Duan, D.L. Programmed cell death in Laminaria japonica (Phaeophyta) tissues infected with alginic acid decomposing bacterium. Prog. Nat. Sci. 2004, 14, 1064–1068. [Google Scholar] [CrossRef]
- Wang, G.; Lin, W.; Yan, X.; Delin, D. Study on the enzymatic activity of Caspase-3 in response to alginic acid decomposing bacteria in Laminaria japonica Aresch. (Phaeophyta). High Technol. Lett. 2005, 11, 80–84. [Google Scholar]
- Tseng, C.K. Anthology of Tseng Cheng-Kui; Ocean Press: Beijing, China, 1994; pp. 871–873. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2016 (SOFIA): Contributing to Food Security and Nutrition for All, 4. State of World Fisheries & Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; pp. 40–41. [Google Scholar]
- Bennett, S.; Wernberg, T.; Connell, S.D.; Hobday, A.J.; Johnson, C.R.; Poloczanska, E.S. The ‘Great Southern Reef’: Social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshw. Res. 2016, 67, 47–56. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C. An introduction to farming and biomass utilisation of marine macroalgae. Phycologia 2019, 58, 443. [Google Scholar] [CrossRef]
- Hu, Z.M.; Shan, T.F.; Zhang, J.; Zhang, Q.S.; Critchley, A.T.; Choi, H.G.; Yotsukura, N.; Liu, F.L.; Duan, D.L. Kelp aquaculture in China: A retrospective and future prospects. Rev. Aquac. 2021, 13, 1324–1351. [Google Scholar] [CrossRef]
- Tseng, C.K. Manual of Cultivation of Haidai (Laminaria Japonica); Science Press: Beijing, China, 1962; pp. 146–154. (In Chinese) [Google Scholar]
- Tseng, C.K. Manual of Cultivation of Seaweeds; Shanghai Science and Technics Press: Shanghai, China, 1985; pp. 82–96. (In Chinese) [Google Scholar]
- Chen, D.; Lin, G.; Shen, S. Studies on alginic acid decomposing bacteria I. Action of alginic acid decomposing bacteria and alginase on Laminaria japonica. Oceanol. Limnol. Sin. 1979, 10, 329–333. [Google Scholar] [CrossRef]
- Chen, D.; Lin, G.; Shen, S. Studies on alginic acid decomposing bacteria II. Rot disease of Laminaria summer sporelings caused by alginic acid decomposing bacteria. Oceanol. Limnol. Sin. 1981, 12, 133–137. [Google Scholar]
- Wu, C.Y. Cultivation of temperate seaweeds in the Asia Pacific region. In Proceedings of the Regional Workshop on the Culture and Utilization of Seaweeds, Cebu City, Philippines, 27–31 August 1990; pp. 27–31. [Google Scholar]
- Xiang, J. Disease Occurrence and Control Strategies of Mariculture Organisms; Ocean Press: Beijing, China, 2001; pp. 78–83. [Google Scholar]
- Wang, G.; Lu, B.; Shuai, L.; Li, D.; Zhang, R. Microbial diseases of nursery and field-cultivated Saccharina japonica (Phaeophyta) in China. Algol. Stud. 2014, 145/146, 39–51. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Saha, M.; Zhuang, Y.; Chang, L.; Xiao, L.; Wang, G. Pseudoalteromonas piscicida X-8 causes bleaching disease in farmed Saccharina japonica. Aquaculture 2022, 546, 737354. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Tang, K.; Shi, X.; Wang, S.; Zhu, W.M.; Zhang, X.H. Purification and characterization of antibacterial compounds of Pseudoalteromonas flavipulchra JG1. Microbiology 2012, 158, 835–842. [Google Scholar] [CrossRef]
- Hettwer, U.; Gross, M.; Rudolph, K. Purification and characterization of an extracellular levansucrase from Pseudomonas syringae pv. phaseolicola. J. Bacteriol. 1995, 177, 2834–2839. [Google Scholar] [CrossRef]
- Shindo, T.; Kaschani, F.; Yang, F.; Kovács, J.; Tian, F.; Kourelis, J.; Hong, T.N.; Colby, T.; Shabab, M.; Chawla, R.; et al. Screen of Non-annotated Small Secreted Proteins of Pseudomonas syringae Reveals a Virulence Factor That Inhibits Tomato Immune Proteases. PLoS Pathog. 2016, 12, e1005874. [Google Scholar] [CrossRef]
- Bodey, G.P.; Bolivar, R.; Fainstein, V.; Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 1983, 5, 279–313. [Google Scholar] [CrossRef]
- Yorgey, P.; Rahme, L.G.; Tan, M.W.; Ausubel, F.M. The roles of mucD and alginate in the virulence of Pseudomonas aeruginosa in plants, nematodes and mice. Mol. Microbiol. 2001, 41, 1063–1076. [Google Scholar] [CrossRef]
- Barbieri, J.; Sun, J. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 2004, 152, 79–92. [Google Scholar] [CrossRef]
- Lanotte, P.; Watt, S.; Mereghetti, L.; Dartiguelongue, N.; Rastegar-Lari, A.; Goudeau, A.; Quentin, R. Genetic features of Pseudomonas aeruginosa isolates from cystic fibrosis patients compared with those of isolates from other origins. J. Med. Microbiol. 2004, 53, 73–81. [Google Scholar] [CrossRef]
- Javanmardi, F.; Emami, A.; Pirbonyeh, N.; Keshavarzi, A.; Rajaee, M. A systematic review and meta-analysis on Exo-toxins prevalence in hospital acquired Pseudomonas aeruginosa isolates. Infect. Genet. Evol. 2019, 75, 104037. [Google Scholar] [CrossRef]
- Georgescu, M.; Gheorghe, I.; Curutiu, C.; Lazar, V.; Bleotu, C.; Chifiriuc, M.-C. Virulence and resistance features of Pseudomonas aeruginosa strains isolated from chronic leg ulcers. BMC Infect. Dis. 2016, 16, 92. [Google Scholar] [CrossRef]
- Ben-Haim, Y.; Banim, E.; Kushmaro, A.; Loya, Y.; Rosenberg, E. Inhibition of photosynthesis and bleaching of zooxanthellae by the coral pathogen Vibrio shiloi. Environ. Microbiol. 1999, 1, 223–229. [Google Scholar] [CrossRef]
- Banin, E.; Khare, S.K.; Naider, F.; Rosenberg, E. Proline-rich peptide from the coral pathogen Vibrio shiloi that inhibits photosynthesis of Zooxanthellae. Appl. Environ. Microbiol. 2001, 67, 1536–1541. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Q.; He, Y.; Tao, Z.; Xu, M.; Luo, Q.; Chen, J.; Chen, H. Isolation and identification of Vibrio mediterranei 117-T6 as a pathogen associated with yellow spot disease of Pyropia (Bangiales, Rhodophyta). Aquaculture 2020, 526, 735372. [Google Scholar] [CrossRef]
- Danon, A.; Delorme, V.; Mailhac, N.; Gallois, P. Plant programmed cell death: A common way to die. Plant Physiol. Biochem. 2000, 38, 647–655. [Google Scholar] [CrossRef]
- Greenberg, J.T.; Yao, N. The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol. 2004, 6, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Reape, T.J.; Molony, E.M.; McCabe, P.F. Programmed cell death in plants: Distinguishing between different modes. J. Exp. Bot. 2008, 59, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Pennell, R.I.; Alvarez, M.E.; Palmer, R.; Lamb, C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 1996, 6, 427–437. [Google Scholar] [CrossRef]
- Mittler, R.; Lam, E. Sacrifice in the face of foes: Pathogen-induced programmed cell death in plants. Trends Microbiol. 1996, 4, 10–15. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Bostock, R.M.; Gilchrist, D.G. Apoptosis: A Functional Paradigm for Programmed Plant Cell Death Induced by a Host-Selective Phytotoxin and Invoked during Development. Plant Cell 1996, 8, 375–391. [Google Scholar] [CrossRef]
- Mittler, R.; Simon, L.; Lam, E. Pathogen-induced programmed cell death in tobacco. J. Cell Sci. 1997, 110, 1333–1344. [Google Scholar] [CrossRef]
- Orzáez, D.; Granell, A. DNA fragmentation is regulated by ethylene during carpel senescence in Pisum sativum. Plant J. 1997, 11, 137–144. [Google Scholar] [CrossRef]
- Yamada, T.; Ichimura, K.; van Doorn, W.G. DNA degradation and nuclear degeneration during programmed cell death in petals of Antirrhinum, Argyranthemum, and Petunia. J. Exp. Bot. 2006, 57, 3543–3552. [Google Scholar] [CrossRef]
- Yamada, T.; Takatsu, Y.; Kasumi, M.; Ichimura, K.; van Doorn, W.G. Nuclear fragmentation and DNA degradation during programmed cell death in petals of morning glory (Ipomoea nil). Planta 2006, 224, 1279–1290. [Google Scholar] [CrossRef]
- Domínguez, F.; Cejudo, F.J. A comparison between nuclear dismantling during plant and animal programmed cell death. Plant Sci. 2012, 197, 114–121. [Google Scholar] [CrossRef]
- Uzelac, B.; Janošević, D.; Budimir, S. In Situ Detection of Programmed Cell Death in Senescing Nicotiana tabacum Leaves Using TUNEL Assay. Methods Mol. Biol. 2018, 1744, 267–282. [Google Scholar] [CrossRef]
- Hatsugai, N.; Kuroyanagi, M.; Yamada, K.; Meshi, T.; Tsuda, S.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 2004, 305, 855–858. [Google Scholar] [CrossRef]
- Tran, V.; Weier, D.; Radchuk, R.; Thiel, J.; Radchuk, V. Caspase-Like Activities Accompany Programmed Cell Death Events in Developing Barley Grains. PLoS ONE 2014, 9, e109426. [Google Scholar] [CrossRef]
- Gopalan, S.; Wei, W.; He, S.Y. hrp gene-dependent induction of hin1: A plant gene activated rapidly by both harpins and the avrPto gene-mediated signal. Plant J. 1996, 10, 591–600. [Google Scholar] [CrossRef]
- Ryerson, D.E.; Heath, M.C. Cleavage of Nuclear DNA into Oligonucleosomal Fragments during Cell Death Induced by Fungal Infection or by Abiotic Treatments. Plant Cell 1996, 8, 393–402. [Google Scholar] [CrossRef]
- Pontier, D.; Tronchet, M.; Rogowsky, P.; Lam, E.; Roby, D. Activation of hsr203, a Plant Gene Expressed During Incompatible Plant-Pathogen Interactions, Is Correlated with Programmed Cell Death. Mol. Plant-Microbe Interact. 1998, 11, 544–554. [Google Scholar] [CrossRef]
- Del Pozo, O.; Lam, E. Expression of the baculovirus p35 protein in tobacco affects cell death progression and compromises N gene-mediated disease resistance response to Tobacco mosaic virus. Mol. Plant-Microbe Interact. 2003, 16, 485–494. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, X.; Ma, M.; Zhuang, Y.; Chang, L.; Xiao, L.; Wang, G. Extracellular Compounds from Pathogenic Bacterium Pseudoalteromonas piscicida X-8 Cause Bleaching Disease, Triggering Active Defense Responses in Commercially Farmed Saccharina japonica. Biology 2023, 12, 47. https://doi.org/10.3390/biology12010047
Chen Y, Zhang X, Ma M, Zhuang Y, Chang L, Xiao L, Wang G. Extracellular Compounds from Pathogenic Bacterium Pseudoalteromonas piscicida X-8 Cause Bleaching Disease, Triggering Active Defense Responses in Commercially Farmed Saccharina japonica. Biology. 2023; 12(1):47. https://doi.org/10.3390/biology12010047
Chicago/Turabian StyleChen, Yao, Xiaoyang Zhang, Mingyu Ma, Yingrui Zhuang, Lirong Chang, Luyang Xiao, and Gaoge Wang. 2023. "Extracellular Compounds from Pathogenic Bacterium Pseudoalteromonas piscicida X-8 Cause Bleaching Disease, Triggering Active Defense Responses in Commercially Farmed Saccharina japonica" Biology 12, no. 1: 47. https://doi.org/10.3390/biology12010047
APA StyleChen, Y., Zhang, X., Ma, M., Zhuang, Y., Chang, L., Xiao, L., & Wang, G. (2023). Extracellular Compounds from Pathogenic Bacterium Pseudoalteromonas piscicida X-8 Cause Bleaching Disease, Triggering Active Defense Responses in Commercially Farmed Saccharina japonica. Biology, 12(1), 47. https://doi.org/10.3390/biology12010047





