Characterization of Thermotoga neapolitana Alcohol Dehydrogenases in the Ethanol Fermentation Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Plasmids
2.2. Gene Expression and Enzyme Purification
2.3. Enzyme Activity Assay
3. Results
3.1. Gene Expression and Enzyme Purification
3.2. Biochemical Properties of the Recombinant Enzymes
3.3. Cofactor Dependence for the Three Putative Adhs
3.4. Involvement in the Ethanol Metabolic Pathway in T. neapolitana
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzoli, R.; Olson, D.G. Clostridium thermocellum: A microbial platform for high-value chemical production from lignocellulose. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 113, pp. 111–161. [Google Scholar]
- Ruchala, J.; Sibirny, A.A. Pentose metabolism and conversion to biofuels and high-value chemicals in yeasts. FEMS Microbiol. Rev. 2021, 45, fuaa069. [Google Scholar] [CrossRef] [PubMed]
- Naghshbandi, M.P.; Tabatabaei, M.; Aghbashlo, M.; Gupta, V.K.; Sulaiman, A.; Karimi, K.; Moghimi, H.; Maleki, M. Progress toward improving ethanol production through decreased glycerol generation in Saccharomyces cerevisiae by metabolic and genetic engineering approaches. Renew. Sustain. Energy Rev. 2019, 115, 109353. [Google Scholar] [CrossRef]
- Chang, D.D.; Ul Islam, Z.; Yang, Z.G.; Thompson, I.P.; Yu, Z.S. Conversion efficiency of bioethanol from levoglucosan was improved by the newly engineered Escherichia coli. Environ. Prog. Sustain. Energy 2021, 40, e13687. [Google Scholar] [CrossRef]
- Yia, X.; Gao, Q.; Bao, J. Expressing an oxidative dehydrogenase gene in ethanologenic strain Zymomonas mobilis promotes the cellulosic ethanol fermentability. J. Biotechnol. 2019, 303, 1–7. [Google Scholar] [CrossRef]
- Thomas, K.C.; Hynes, S.H.; Jones, A.M.; Ingledew, W.M. Production of fuel alcohol from wheat by VHG technology. Appl. Biochem. Biotechnol. 1993, 43, 211–226. [Google Scholar] [CrossRef]
- Jessen, J.E.; Orlygsson, J. Production of ethanol from sugars and lignocellulosic biomass by Thermoanaerobacter J1 isolated from a hot spring in Iceland. J. Biomed. Biotechnol. 2012, 2012, 186982. [Google Scholar] [CrossRef]
- Kim, B.; Du, J.; Eriksen, D.T.; Zhao, H. Combinatorial Design of a Highly Efficient Xylose-Utilizing Pathway in Saccharomyces cerevisiae for the Production of Cellulosic Biofuels. Appl. Environ. Microbiol. 2013, 79, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Boorla, V.S.; Dash, S.; Gopalakrishnan, S.; Jacobson, T.B.; Olson, D.G.; Amador-Noguez, D.; Lynd, L.R.; Maranas, C.D. Assessing the impact of substrate-level enzyme regulations limiting ethanol titer in Clostridium thermocellum using a core kinetic model. Metab. Eng. 2022, 69, 286–301. [Google Scholar] [CrossRef]
- Holwerda, E.K.; Olson, D.G.; Ruppertsberger, N.M.; Stevenson, D.M.; Murphy, S.J.L.; Maloney, M.I.; Lanahan, A.A.; Amador-Noguez, D.; Lynd, L.R. Metabolic and evolutionary responses of Clostridium thermocellum to genetic interventions aimed at improving ethanol production. Biotechnol. Biofuels 2020, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Olson, D.G.; Murphy, S.J.; Shao, X.; Tian, L.; Lynd, L.R. Both adhE and a Separate NADPH-Dependent Alcohol Dehydrogenase Gene, adhA, Are Necessary for High Ethanol Production in Thermoanaerobacterium saccharolyticum. J. Bacteriol. 2017, 199, e00542-16. [Google Scholar] [CrossRef]
- Zhou, J.; Olson, D.G.; Lanahan, A.A.; Tian, L.; Murphy, S.J.-L.; Lo, J.; Lynd, L.R. Physiological roles of pyruvate ferredoxin oxidoreductase and pyruvate formate-lyase in Thermoanaerobacterium saccharolyticum JW/SL-YS485. Biotechnol. Biofuels Bioprod. 2015, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Hon, S.; Olson, D.G.; Holwerda, E.K.; Lanahan, A.A.; Murphy, S.J.L.; Maloney, M.I.; Zheng, T.; Papanek, B.; Guss, A.M.; Lynd, L.R. The ethanol pathway from Thermoanaerobacterium saccharolyticum improves ethanol production in Clostridium thermocellum. Metab. Eng. 2017, 42, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.X.; Luo, S.; Dai, K.Q.; Qu, C.Y.; Wang, J.F. Engineering Thermoanaerobacterium aotearoense SCUT27/Δldh with pyruvate formate lyase-activating protein (PflA) knockout for enhanced ethanol tolerance and production. Process Biochem. 2021, 106, 184–190. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Recent advances in second generation ethanol production by thermophilic bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xiao, Y.; Xia, X.-X.; Zhao, X.-Q.; Peng, L.; Srinophakun, P.; Bai, F.-W. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xin, F.; Lu, J.; Dong, W.; Zhang, W.; Zhang, M.; Wu, H.; Ma, J.; Jiang, M. State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour. Technol. 2017, 245, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Sun, H.; Li, C.; Zhao, Z.; Liu, G. Advances in mechanisms and modifications for rendering yeast thermotolerance. J. Biosci. Bioeng. 2016, 121, 599–606. [Google Scholar] [CrossRef]
- Zuliani, L.; Serpico, A.; De Simone, M.; Frison, N.; Fusco, S. Biorefinery Gets Hot: Thermophilic Enzymes and Microorganisms for Second-Generation Bioethanol Production. Processes 2021, 9, 1583. [Google Scholar] [CrossRef]
- Nguyen, T.A.D.; Pyo Kim, J.; Sun Kim, M.; Kwan Oh, Y.; Sim, S.J. Optimization of hydrogen production by hyperthermophilic eubacteria, Thermotoga maritima and Thermotoga neapolitana in batch fermentation. Int. J. Hydrogen Energy 2008, 33, 1483–1488. [Google Scholar] [CrossRef]
- Bryant Frank, O.; Wiegel, J.; Ljungdahl Lars, G. Purification and Properties of Primary and Secondary Alcohol Dehydrogenases from Thermoanaerobacter ethanolicus. Appl. Environ. Microbiol. 1988, 54, 460–465. [Google Scholar] [CrossRef]
- Pei, J.; Zhou, Q.; Jiang, Y.; Le, Y.; Li, H.; Shao, W.; Wiegel, J. Thermoanaerobacter spp. control ethanol pathway via transcriptional regulation and versatility of key enzymes. Metab. Eng. 2010, 12, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Basen, M.; Schut, G.J.; Nguyen, D.M.; Lipscomb, G.L.; Benn, R.A.; Prybol, C.J.; Vaccaro, B.J.; Poole, F.L., II; Kelly, R.M.; Adams, M.W.W. Single gene insertion drives bioalcohol production by a thermophilic archaeon. Proc. Natl. Acad. Sci. USA 2014, 111, 17618–17623. [Google Scholar] [CrossRef] [PubMed]
- Eram, M.S.; Wong, A.; Oduaran, E.; Ma, K. Molecular and biochemical characterization of bifunctional pyruvate decarboxylases and pyruvate ferredoxin oxidoreductases from Thermotoga maritima and Thermotoga hypogea. J. Biochem. 2015, 158, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Eram, M.S.; Oduaran, E.; Ma, K. The bifunctional pyruvate decarboxylase/pyruvate ferredoxin oxidoreductase from Thermococcus guaymasensis. Archaea 2014, 2014, 349379. [Google Scholar] [CrossRef]
- Eram, M.S.; Ma, K. Pyruvate decarboxylase activity of the acetohydroxyacid synthase of Thermotoga maritima. Biochem. Biophys. Rep. 2016, 7, 394–399. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Sha, C.; Wang, H.; Ma, K.; Wiegle, J.; Abomohra, A.E.-F.; Shao, W. A novel bifunctional aldehyde/alcohol dehydrogenase catalyzing reduction of acetyl-CoA to ethanol at temperatures up to 95 °C. Sci. Rep. 2021, 11, 1050. [Google Scholar] [CrossRef]
- Larson, S.B.; Jones, J.A.; McPherson, A. The structure of an iron-containing alcohol dehydrogenase from a hyperthermophilic archaeon in two chemical states. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2019, 75, 217–226. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Ying, X.; Wang, Y.; Badiei, H.R.; Karanassios, V.; Ma, K. Purification and characterization of an iron-containing alcohol dehydrogenase in extremely thermophilic bacterium Thermotoga hypogea. Arch. Microbiol. 2007, 187, 499–510. [Google Scholar] [CrossRef]
- Nissen, L.S.; Basen, M. The emerging role of aldehyde:ferredoxin oxidoreductases in microbially-catalyzed alcohol production. J. Biotechnol. 2019, 306, 105–117. [Google Scholar] [CrossRef]
- Karaoglan, M.; Erden-Karaoglan, F.; Yilmaz, S.; Inan, M. Identification of major ADH genes in ethanol metabolism of Pichia pastoris. Yeast 2020, 37, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.; Zeikus, J.G. Purification of acetaldehyde dehydrogenase and alcohol dehydrogenases from Thermoanaerobacter ethanolicus 39E and characterization of the secondary-alcohol dehydrogenase (2° Adh) as a bifunctional alcohol dehydrogenase-acetyl-CoA reductive thioesterase. Biochem. J. 1994, 302, 163–170. [Google Scholar] [CrossRef]
- Peng, H.; Wu, G.; Shao, W. The aldehyde/alcohol dehydrogenase (AdhE) in relation to the ethanol formation in Thermoanaerobacter ethanolicus JW200. Anaerobe 2008, 14, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Olson, D.G.; Lynd, L.R. Characterization of the Clostridium thermocellum AdhE, NfnAB, ferredoxin and Pfor proteins for their ability to support high titer ethanol production in Thermoanaerobacterium saccharolyticum. Metab. Eng. 2019, 51, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Schut, G.J.; Adams, M.W.W. The Iron-Hydrogenase of Thermotoga maritima Utilizes Ferredoxin and NADH Synergistically: A New Perspective on Anaerobic Hydrogen Production. J. Bacteriol. 2009, 191, 4451–4457. [Google Scholar] [CrossRef]
- Esercizio, N.; Lanzilli, M.; Vastano, M.; Xu, Z.; Landi, S.; Caso, L.; Gallo, C.; Nuzzo, G.; Manzo, E.; Fontana, A.; et al. Improvement of CO2 and Acetate Coupling into Lactic Acid by Genetic Manipulation of the Hyperthermophilic Bacterium Thermotoga neapolitana. Microorganisms 2021, 9, 1688. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, X.; Liang, L.; Fang, J.; Chen, Y.; He, W.; Xue, T. Using CRISPR/Cas9 for multiplex genome engineering to optimize the ethanol metabolic pathway in Saccharomyces cerevisiae. Biochem. Eng. J. 2019, 145, 120–126. [Google Scholar] [CrossRef]
- Biswas, R.; Prabhu, S.; Lynd, L.R.; Guss, A.M. Increase in Ethanol Yield via Elimination of Lactate Production in an Ethanol-Tolerant Mutant of Clostridium thermocellum. PLoS ONE 2014, 9, e86389. [Google Scholar] [CrossRef]
- Biswas, R.; Zheng, T.; Olson, D.G.; Lynd, L.R.; Guss, A.M. Elimination of hydrogenase active site assembly blocks H-2 production and increases ethanol yield in Clostridium thermocellum. Biotechnol. Biofuels 2015, 8, 20. [Google Scholar] [CrossRef]
- Maeno, S.; Kajikaw, A.; Dicks, L.; Endo, A. Introduction of bifunctional alcohol/acetaldehyde dehydrogenase gene (adhE) in Fructobacillus fructosus settled its fructophilic characteristics. Res. Microbiol. 2019, 170, 35–42. [Google Scholar] [CrossRef]
- Manow, R.; Wang, C.; Garza, E.; Zhao, X.; Wang, J.; Grayburn, S.; Zhou, S. Expression of acetaldehyde dehydrogenase (aldB) improved ethanol production from xylose by the ethanologenic Escherichia coli RM10. World J. Microbiol. Biotechnol. 2020, 36, 59. [Google Scholar] [CrossRef]
- Keller, M.W.; Lipscomb, G.L.; Nguyen, D.M.; Crowley, A.T.; Schut, G.J.; Scott, I.; Kelly, R.M.; Adams, M.W.W. Ethanol production by the hyperthermophilic archaeon Pyrococcus furiosus by expression of bacterial bifunctional alcohol dehydrogenases. Microb. Biotechnol. 2017, 10, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Straub, C.T.; Schut, G.; Otten, J.K.; Keller, L.M.; Adams, M.W.W.; Kelly, R.M. Modification of the glycolytic pathway in Pyrococcus furiosus and the implications for metabolic engineering. Extremophiles 2020, 24, 511–518. [Google Scholar] [CrossRef] [PubMed]

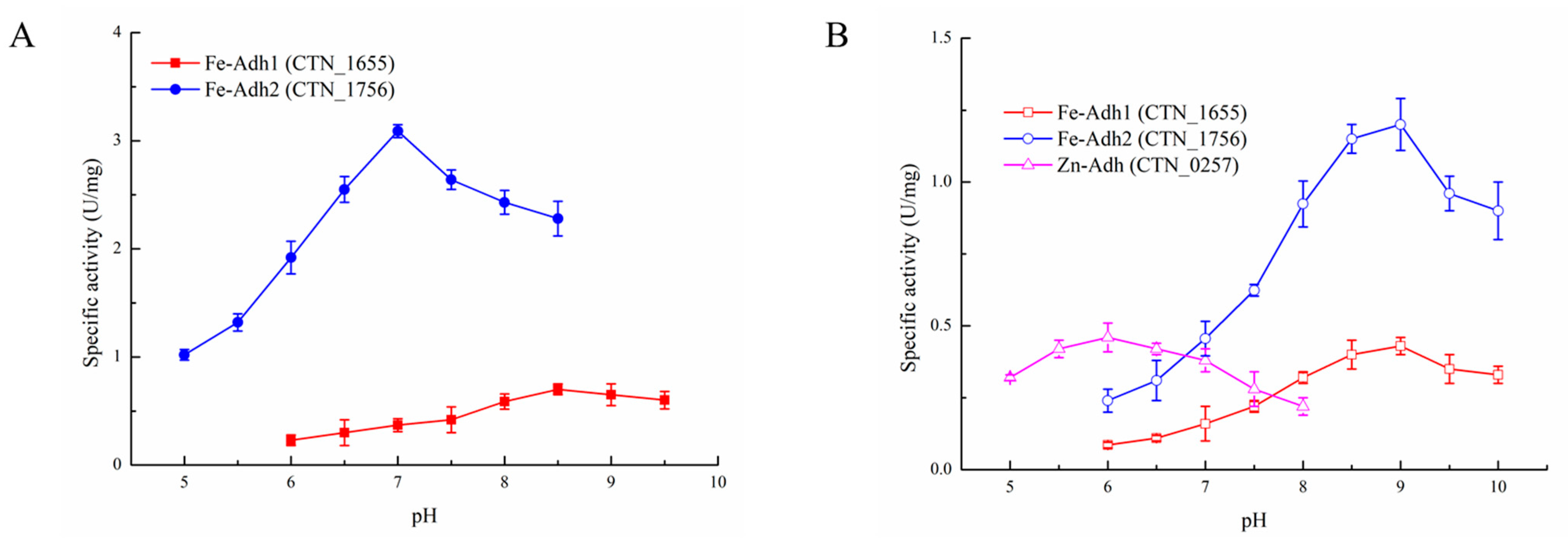
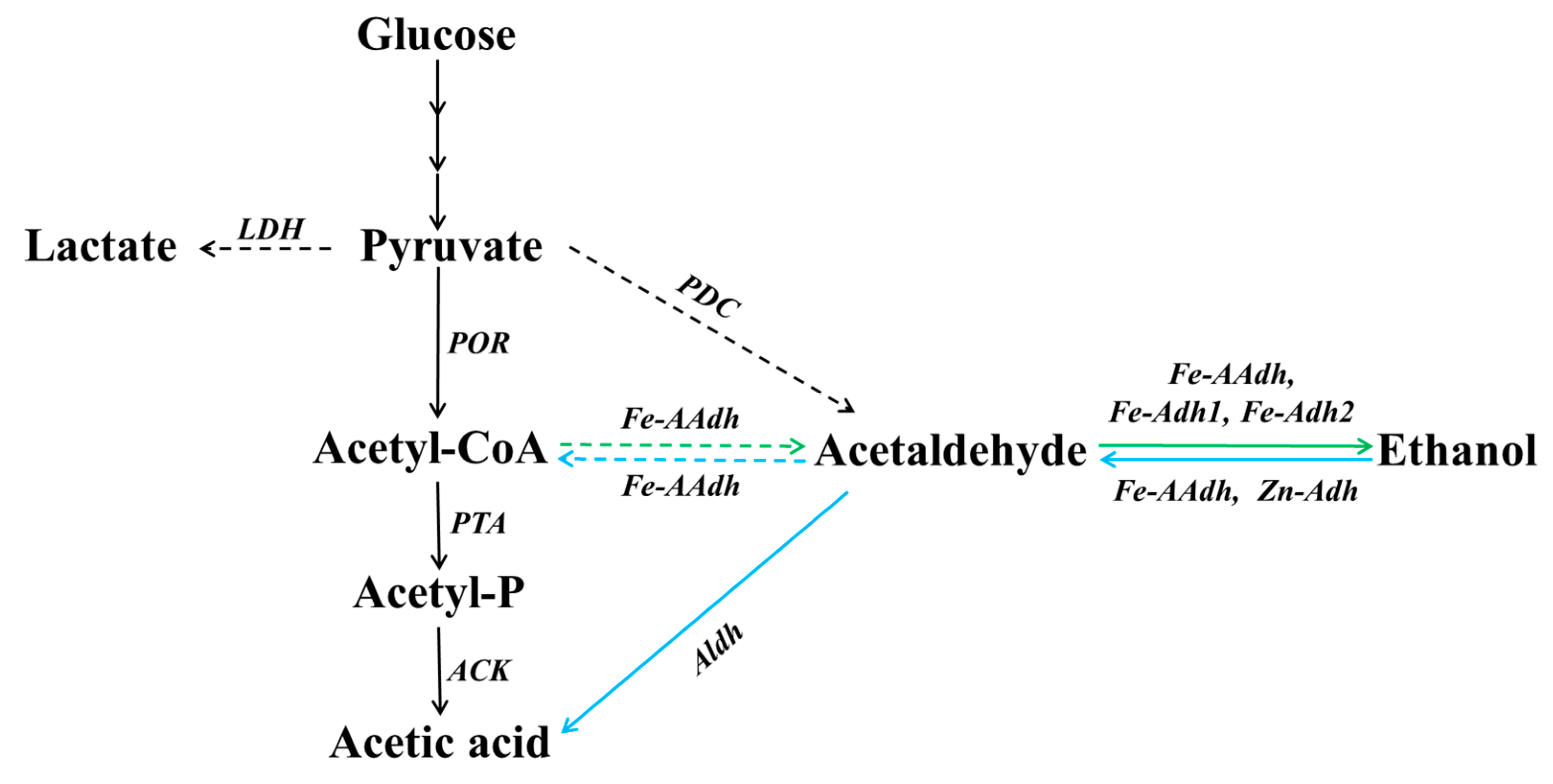
| Enzyme (Gene) | Fe-Adh1 (CTN_1655) | Fe-Adh2 (CTN_1756) | Zn-Adh (CTN_0257) | |||
|---|---|---|---|---|---|---|
| Reaction | Ac-ald→Eth | Ac-ald←Eth | Ac-ald→Eth | Ac-ald←Eth | Ac-ald→Eth | Ald←Eth |
| pHopt | 8.5 | 9.0 | 7.0 | 9.0 | ND | 6.0 |
| Topt | 95 °C | 95 °C | 85 °C | 100 °C | ND | 95 °C |
| Activity (U/mg) | ||||||
| NADPH | 0.70 ± 0.05 | 3.09 ± 0.06 | ND | |||
| NADH | ND | ND | ND | |||
| NADP | 0.43 ± 0.05 | 1.20 ± 0.06 | 0.46 ± 0.03 | |||
| NAD | 0.22 ± 0.04 | 0.25 ± 0.04 | 0.30 ± 0.02 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, C.; Wang, Q.; Wang, H.; Duan, Y.; Xu, C.; Wu, L.; Ma, K.; Shao, W.; Jiang, Y. Characterization of Thermotoga neapolitana Alcohol Dehydrogenases in the Ethanol Fermentation Pathway. Biology 2022, 11, 1318. https://doi.org/10.3390/biology11091318
Sha C, Wang Q, Wang H, Duan Y, Xu C, Wu L, Ma K, Shao W, Jiang Y. Characterization of Thermotoga neapolitana Alcohol Dehydrogenases in the Ethanol Fermentation Pathway. Biology. 2022; 11(9):1318. https://doi.org/10.3390/biology11091318
Chicago/Turabian StyleSha, Chong, Qiang Wang, Hongcheng Wang, Yilan Duan, Chongmao Xu, Lian Wu, Kesen Ma, Weilan Shao, and Yu Jiang. 2022. "Characterization of Thermotoga neapolitana Alcohol Dehydrogenases in the Ethanol Fermentation Pathway" Biology 11, no. 9: 1318. https://doi.org/10.3390/biology11091318
APA StyleSha, C., Wang, Q., Wang, H., Duan, Y., Xu, C., Wu, L., Ma, K., Shao, W., & Jiang, Y. (2022). Characterization of Thermotoga neapolitana Alcohol Dehydrogenases in the Ethanol Fermentation Pathway. Biology, 11(9), 1318. https://doi.org/10.3390/biology11091318






