Using Macro- and Microscale Preservation in Vertebrate Fossils as Predictors for Molecular Preservation in Fluvial Environments
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Maturation Experiments
2.3. Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS)
2.4. Lipid Analyses
2.5. Fourier-Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS)
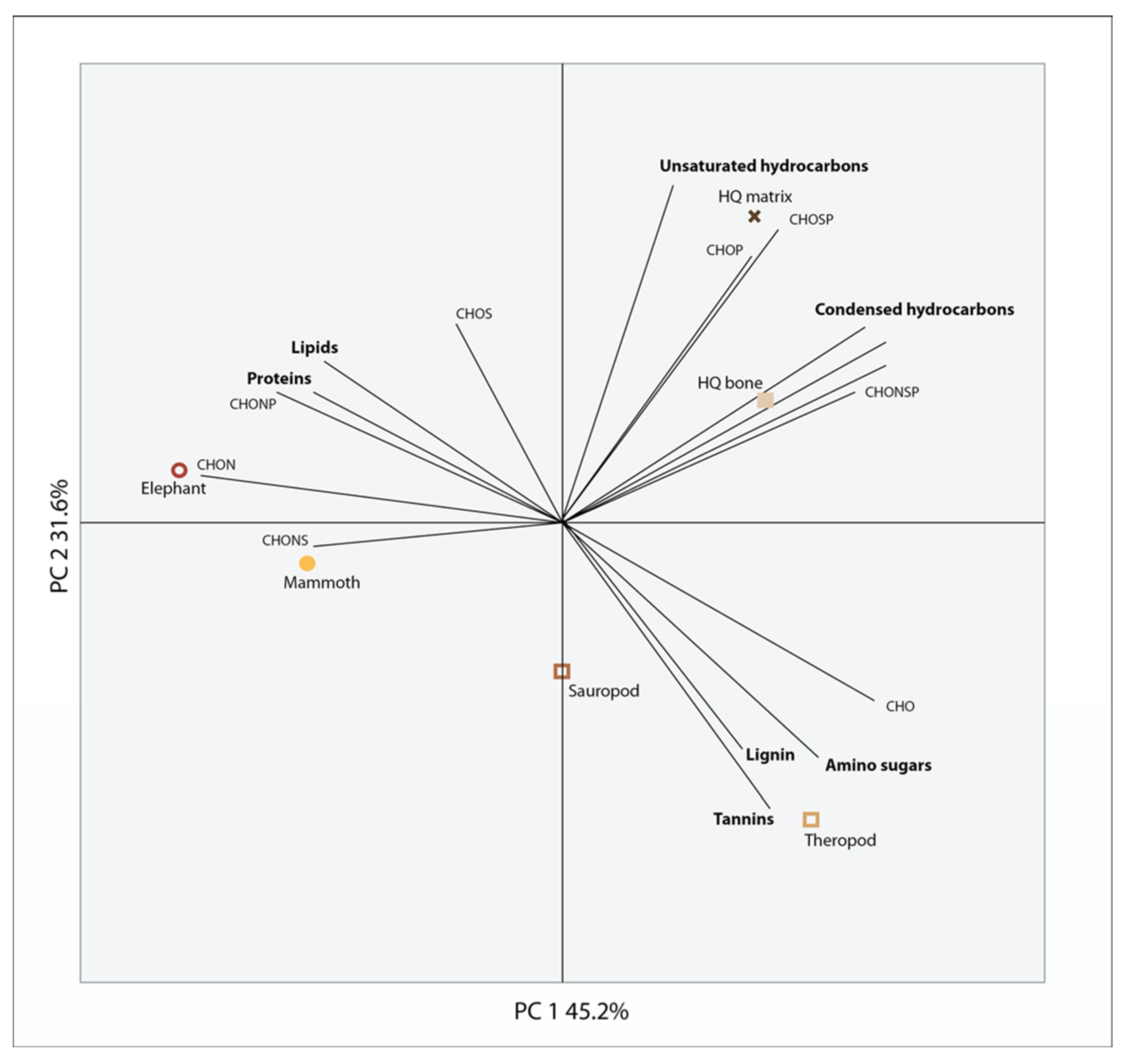
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briggs, D.E.; Summons, R.E. Ancient biomolecules: Their origins, fossilization, and role in revealing the history of life. Bioessays 2014, 36, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Allison, P.A.; Briggs, D.E. Exceptional fossil record: Distribution of soft-tissue preservation through the Phanerozoic. Geology 1993, 21, 527–530. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K. Taphonomic and ecologic information from bone weathering. Paleobiology 1978, 4, 150–162. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K.; Kidwell, S.M.; Gastaldo, R.A. Taphonomy and paleobiology. Paleobiology 2000, 26, 103–147. [Google Scholar] [CrossRef]
- Bertazzo, S.; Maidment, S.C.R.; Kallepitis, C.; Fearn, S.; Stevens, M.M.; Xie, H. Fibres and cellular structures preserved in 75-million–year-old dinosaur specimens. Nat. Commun. 2015, 6, 7352. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef]
- Surmik, D.; Boczarowski, A.; Balin, K.; Dulski, M.; Szade, J.; Kremer, B.; Pawlicki, R. Spectroscopic studies on organic matter from Triassic reptile bones, Upper Silesia, Poland. PLoS ONE 2016, 11, e0151143. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Zheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef]
- Wiemann, J.; Fabbri, M.; Yang, T.-R.; Stein, K.; Sander, P.M.; Norell, M.A.; Briggs, D.E.G. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Fabbri, M.; Wiemann, J.; Manucci, F.; Briggs, D.E. Three-dimensional soft tissue preservation revealed in the skin of a non-avian dinosaur. Palaeontology 2020, 63, 185–193. [Google Scholar] [CrossRef]
- Wiemann, J.; Menéndez, I.; Crawford, J.M.; Fabbri, M.; Gauthier, J.A.; Hull, P.M.; Norell, M.A.; Briggs, D.E.G. Fossil biomolecules reveal an avian metabolism in the ancestral dinosaur. Nature 2022, 606, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Ulmann, P.V.; Pandya, S.H.; Nellermoe, R. Patterns of soft tissue and cellular preservation in relation to fossil bone tissue structure and overburden depth at the Standing Rock Hadrosaur Site, Maastrichtian Hell Creek Formation, South Dakota, USA. Cretac. Res. 2019, 99, 1–13. [Google Scholar] [CrossRef]
- White, P.D.; Fastovsky, D.E.; Sheehan, P.M. Taphonomy and suggested structure of the dinosaurian assemblage of the Hell Creek Formation (Maastrichtian), eastern Montana and western North Dakota. Palaios 1998, 13, 41–51. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, C.-C.; Huang, P.-Y.; Chung, C.-Y.; Huang, T.D.; Wang, C.-C.; Chen, C.-I.; Chang, R.-S.; Liao, C.-H.; Reisz, R.R. Evidence of preserved collagen in an Early Jurassic sauropodomorph dinosaur revealed by synchrotron FTIR microspectroscopy. Nat. Commun. 2017, 8, 14220. [Google Scholar] [CrossRef]
- Schroeter, E.R.; DeHart, C.J.; Cleland, T.P.; Zheng, W.; Thomas, P.M.; Kelleher, N.L.; Bern, M.; Schweitzer, M.H. Expansion for the Brachylophosaurus canadensis collagen I sequence and additional evidence of the preservation of Cretaceous protein. J. Proteome Res. 2017, 16, 920–932. [Google Scholar] [CrossRef]
- Allison, P.A. Konservat-Lagerstätten: Cause and classification. Paleobiology 1988, 14, 331–344. [Google Scholar] [CrossRef]
- Eglinton, G.; Logan, G.A. Molecular preservation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991, 333, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Irmis, R.B.; Nesbitt, S.J.; Padian, K.; Smith, N.D.; Turner, A.H.; Woody, D.; Downs, A. A Late Triassic dinosauromorph assemblage from New Mexico and the rise of dinosaurs. Science 2007, 317, 358–361. [Google Scholar] [CrossRef]
- Irmis, R.B.; Mundil, R.; Martz, J.W.; Parker, W.G. High-resolution U–Pb ages from the Upper Triassic Chinle Formation (New Mexico, USA) support a diachronous rise of dinosaurs. Earth Planet. Sci. Lett. 2011, 309, 258–267. [Google Scholar] [CrossRef]
- Whiteside, J.H.; Lindström, S.; Irmis, R.B.; Glasspool, I.J.; Schaller, M.F.; Dunlavey, M.; Nesbitt, S.J.; Smith, N.D.; Turner, A.H. Extreme ecosystem instability suppressed tropical dinosaur dominance for 30 million years. Proc. Natl. Acad. Sci. USA 2015, 112, 7909–7913. [Google Scholar] [CrossRef] [Green Version]
- Werning, S.A. Evolution of Bone Histological Characters in Amniotes, and the Implications for the Evolution of Growth and Metabolism. Ph.D. Dissertation, University of California, Berkeley, CA, USA, 2013. [Google Scholar]
- Behrensmeyer, A.K.; Kidwell, S.M. Taphonomy’s contributions to paleobiology. Paleobiology 1985, 11, 105–119. [Google Scholar] [CrossRef]
- Nesbitt, S.J.; Smith, N.D.; Irmis, R.B.; Turner, A.H.; Downs, A.; Norell, M.A. A complete skeleton of a Late Triassic saurischian and the early evolution of dinosaurs. Science 2009, 326, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Dodson, P.; Behrensmeyer, A.K.; Bakker, R.T.; McIntosh, J.S. Taphonomy and paleoecology of the dinosaur beds of the Jurassic Morrison Formation. Paleobiology 1980, 6, 208–232. [Google Scholar] [CrossRef]
- Colleary, C.; Lamadrid, H.M.; O’Reilly, S.S.; Dolocan, A.; Nesbitt, S.J. Molecular preservation in mammoth bone and variation based on burial environment. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Colleary, C.; Dolocan, A.; Gardner, J.; Singh, S.; Wuttke, M.; Rabenstein, R.; Habersetzer, J.; Schaal, S.; Feseha, M.; Clemens, M.; et al. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proc. Natl. Acad. Sci. USA 2015, 112, 12592–12597. [Google Scholar] [CrossRef]
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I.; et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef]
- O’Reilly, S.S.; Mariotti, G.; Winter, A.R.; Newman, S.A.; Matys, E.D.; McDermott, F.; Pruss, S.B.; Bosak, T.; Summons, R.E.; Klepac-Ceraj, V. Molecular biosignatures reveal common benthic microbial sources of organic matter in ooids and grapestones from Pigeon Cay, The Bahamas. Geobiology 2016, 15, 112–130. [Google Scholar] [CrossRef]
- Tfaily, M.M.; Chu, R.K.; Tolić, N.; Roscioli, K.M.; Anderton, C.R.; Paša-Tolić, L.; Robinson, E.W.; Hess, N.J. Advanced solvent based methods for molecular characterization of soil organic matter by high-resolution mass spectrometry. Anal. Chem. 2015, 87, 5206–5215. [Google Scholar] [CrossRef]
- Tfaily, M.M.; Chu, R.K.; Toyoda, J.; Tolić, N.; Robinson, E.W.; Paša-Tolić, L.; Hess, N.J. Sequential extraction protocol for organic matter from soils and sediments using high resolution mass spectrometry. Anal. Chim. Acta. 2017, 972, 54–61. [Google Scholar] [CrossRef]
- Dittmar, T.; Koch, B.; Hertkorn, N.; Kattner, G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr. Methods. 2008, 6, 230–235. [Google Scholar] [CrossRef]
- Tolić, N.; Liu, Y.; Liyu, A.; Shen, Y.; Tfaily, M.M.; Kujawinski, E.B.; Longnecker, K.; Kuo, L.; Robinson, E.W.; Paša-Tolić, L.; et al. Formularity: Software for automated formula assignment of natural and other organic matter from ultrahigh-resolution mass spectra. Anal. Chem. 2017, 89, 12659–12665. [Google Scholar] [CrossRef] [PubMed]
- Minor, E.C.; Steinbring, C.J.; Longnecker, K.; Kujawinski, E.B. Characterization of dissolved organic matter in Lake Superior and its watershed using ultrahigh resolution mass spectrometry. Org. Geochem. 2012, 43, 1–11. [Google Scholar] [CrossRef]
- Kujawinski, E.B.; Behn, M.D. Automated analysis of electrospray ionization Fourier transform ion cyclotron resonance mass spectra of natural organic matter. Anal. Chem. 2006, 78, 4363–4373. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- AminiTabrizi, R.; Wilson, R.M.; Fudyma, J.D.; Hodgkons, S.B.; Heyman, H.M.; Rich, V.I.; Saleaska, S.R.; Chanton, J.P.; Tfaily, M.M. Controls on soil organic matter degradation and subsequent greenhouse gas emissions across a permafrost thaw gradient in Northern Sweden. Front. Earth Sci. 2020, 8, 557961. [Google Scholar] [CrossRef]
- Collins, M.J.; Nielsen–Marsh, C.M.; Hiller, J.; Smith, C.I.; Roberts, J.P.; Prigodich, R.V.; Wess, T.J.; Csapò, J.; Millard, A.R.; Turner–Walker, G. The survival of organic matter in bone: A review. Archaeometry 2002, 44, 383–394. [Google Scholar] [CrossRef]
- Keenan, S.W. From bone to fossil: A review of the diagenesis of bioapatite. Am. Mineral. 2016, 101, 1943–1951. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K. Vertebrate preservation in fluvial channels. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1988, 63, 183–199. [Google Scholar] [CrossRef]
- Cleland, T.P.; Schroeter, E.R.; Feranec, R.S.; Vashishth, D. Peptide sequences from the first Castoroides ohioensis skull and the utility of old museum collections for palaeoproteomics. Proc. R. Soc. B. 2016, 283, 20160593. [Google Scholar] [CrossRef]
- Wiemann, J.; Briggs, D.E. Validation of biosignatures confirms the informative nature of fossil organic Raman spectra. bioRxiv 2021. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Schroeter, E.R.; Cleland, T.P.; Zheng, W. Paleoproteomics of Mesozoic dinosaurs and other Mesozoic fossils. Proteomics 2019, 19, 1800251. [Google Scholar] [CrossRef] [PubMed]
- Saitta, E.T.; Liang, R.; Lau, M.C.Y.; Brown, C.M.; Longrich, N.R.; Kaye, T.G.; Novak, B.J.; Salzberg, S.L.; Norell, M.A.; Abbott, G.D.; et al. Cretaceous dinosaur bone contains recent organic material and provides an environment conducive to microbial communities. eLife 2018, 8, e46205. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.A.; O’Reilly, S.S.; Luo, G.; Briggs, D.E.G.; Summons, R.E. Prospects for sterane preservation in sponge fossils from museum collections and the utility of sponge biomarkers for molecular clocks. Bull. Peabody Mus. Nat. Hist. 2016, 57, 181–189. [Google Scholar] [CrossRef]
- Liebenau, K.; Kiel, S.; Vardeh, D.; Treude, T.; Thiel, V. A quantitative study of the degradation of whale bone lipids: Implications for the preservation of fatty acids in marine sediments. Org. Geochem. 2015, 89, 23–30. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Shen, X.; Wang, J.; Yuan, Q. Recent advances in microbial production of phenolic compounds. Chin. J. Chem. Eng. 2021, 30, 54–61. [Google Scholar] [CrossRef]
- Tuross, N.; Behrensmeyer, A.K.; Eanes, E.D. Strontium increases and crystallinity changes in taphonomic and archaeological bone. J. Archaeol. Sci. 1989, 16, 661–672. [Google Scholar] [CrossRef]



| Specimen Number | Specimen | Locality | Time Period | Analyses |
|---|---|---|---|---|
| GR1063 | Coprolite | Hayden Quarry (H2) | Triassic | TOF-SIMS |
| GR1064 | Phytosaur tooth | Hayden Quarry (H2) | Triassic | TOF-SIMS |
| GR1065 | Tawa hallae femur | Hayden Quarry (H2) | Triassic | TOF-SIMS |
| GR1066 | Rib fragment | Hayden Quarry (H4) | Triassic | TOF-SIMS, GC-MS, FT-ICR MS |
| GR1067 | Rib fragment | Hayden Quarry (H2) | Triassic | GC-MS, FT-ICR MS |
| Matrix sample | Hayden Quarry (H4) | Triassic | TOF-SIMS, GC-MS | |
| LACM 154089 | Sauropod rib | Morrison | Jurassic | TOF-SIMS, FT-ICR MS |
| LACM 23844 | Theropod rib | Hell Creek | Cretaceous | TOF-SIMS, FT-ICR MS |
| Mammoth rib | FT-ICR MS | |||
| TMMM-12613 | Alligator rib | Modern | TOF-SIMS | |
| MS-E01 | Elephant rib | Modern | TOF-SIMS, FTIR-MS | |
| MS-E01 | Elephant rib (experimentally matured) | Modern | TOF-SIMS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colleary, C.; O’Reilly, S.; Dolocan, A.; Toyoda, J.G.; Chu, R.K.; Tfaily, M.M.; Hochella, M.F., Jr.; Nesbitt, S.J. Using Macro- and Microscale Preservation in Vertebrate Fossils as Predictors for Molecular Preservation in Fluvial Environments. Biology 2022, 11, 1304. https://doi.org/10.3390/biology11091304
Colleary C, O’Reilly S, Dolocan A, Toyoda JG, Chu RK, Tfaily MM, Hochella MF Jr., Nesbitt SJ. Using Macro- and Microscale Preservation in Vertebrate Fossils as Predictors for Molecular Preservation in Fluvial Environments. Biology. 2022; 11(9):1304. https://doi.org/10.3390/biology11091304
Chicago/Turabian StyleColleary, Caitlin, Shane O’Reilly, Andrei Dolocan, Jason G. Toyoda, Rosalie K. Chu, Malak M. Tfaily, Michael F. Hochella, Jr., and Sterling J. Nesbitt. 2022. "Using Macro- and Microscale Preservation in Vertebrate Fossils as Predictors for Molecular Preservation in Fluvial Environments" Biology 11, no. 9: 1304. https://doi.org/10.3390/biology11091304
APA StyleColleary, C., O’Reilly, S., Dolocan, A., Toyoda, J. G., Chu, R. K., Tfaily, M. M., Hochella, M. F., Jr., & Nesbitt, S. J. (2022). Using Macro- and Microscale Preservation in Vertebrate Fossils as Predictors for Molecular Preservation in Fluvial Environments. Biology, 11(9), 1304. https://doi.org/10.3390/biology11091304






