Simple Summary
Although global change is expected to modify the threat posed by plant pathogens, not much is known about how a changing climate will affect the epidemiology of generalist vector-borne diseases. In the present study, we developed a high-throughput screening method to test for the presence of a deadly plant pathogen, Xylella fastidiosa, in its insect vectors. Based on a four-year survey in climatically distinct areas of the island of Corsica (France), we found a significant positive correlation between the frequency of insect vectors positive for X. fastidiosa and temperature. We observed that a higher prevalence in insects corresponded with milder winters. We used future climate projections up to the year 2100, and found that the risk for X. fastidiosa outbreak will increase in the future. While the proportion of vectors that carry the pathogen should increase, the climate conditions will remain suitable for the bacterium and its main vector, with possible shifts towards higher elevations. Besides calling for research efforts to limit the incidence of plant diseases in temperate zones, this works reveals that recent molecular technologies could and should be used for massive screening of pathogens in vectors in order to scale-up surveillance and management efforts.
Abstract
Global change is expected to modify the threat posed by pathogens to plants. However, little is known regarding how a changing climate will influence the epidemiology of generalist vector-borne diseases. We developed a high-throughput screening method to test for the presence of a deadly plant pathogen, Xylella fastidiosa, in its insect vectors. Then, using data from a four-year survey in climatically distinct areas of Corsica (France), we demonstrated a positive correlation between the proportion of vectors positive to X. fastidiosa and temperature. Notably, a higher prevalence corresponded with milder winters. Our projections up to 2100 indicate an increased risk of outbreaks. While the proportion of vectors that carry the pathogen should increase, the climate conditions will remain suitable for the bacterium and its main vector, with possible range shifts towards a higher elevation. Besides calling for research efforts to limit the incidence of plant diseases in the temperate zone, this work reveals that recent molecular technologies could and should be used for massive screening of pathogens in vectors to scale-up surveillance and management efforts.
1. Introduction
Increased globalization of food production and climate change are facilitating the movement and local establishment of pests and pathogens [1]. The spread of plant pathogens is jeopardizing food security [2] and biodiversity [3]. Identifying how plants, pathogens, and their vectors will respond to a changing climate is challenging [4], even more so for unmanaged ecosystems [4,5,6]. However, improvements in molecular detection and climate modelling allow us to model realistic scenarios more accurately. Bacterial vector-borne plant diseases represent a major cost to producers worldwide [7], because they decrease the overall yield and require expensive control measures. Bacteria are mainly transmitted to plants by Hemipteran insects (e.g., psyllids, leafhoppers, and spittlebugs) that feed on the mesophyll, phloem, or xylem sap [8]. Climate change is expected to affect the distribution of vectors and, hence, the geographical range over which diseases are transmitted. Ambient temperature is also an important factor for determining the efficiency with which vectors transmit pathogens, how well plants can defend themselves, and possibly the multiplication rate of pathogens within hosts [9]. Despite generalist bacteria posing a severe and costly threat to both managed and unmanaged systems, it is largely unknown how they will respond to climate change.
With about 600 host species in over 85 families of wild and cultivated plants, the vector-borne bacterium Xylella fastidiosa Wells et al. (Xf) (Xanthomonadaceae) is a worldwide threat to agriculture, horticulture, forestry, and unmanaged habitats [10]. Biofilm-like colonies are formed; pectin gels and tyloses are produced by the plant, which reduce the hydraulic conductivity within the xylem and can lead to plant death [11]. Xf is transmitted to plants by xylem-feeding hemipterans (mainly sharpshooters and spittlebugs) [12,13]. There is no vertical transmission to offspring and infectivity is lost during molting, although adults that acquire Xf remain infective for life [13]. Sharpshooters can inoculate Xf with no latent period [14] and most vectors are polyphagous [12,13], which increases chances of transmission within and between semi-natural and cultivated habitats. At its center of origin in the New World, the bacterium has caused more than 100 million USD worth of losses each year to the Brazilian citrus industry and to the US grape industry [15,16]. Since the dramatic 2013 outbreak in the olive groves of Southern Italy [17], the presence of Xf has been confirmed in different Mediterranean regions of Europe (https://gd.eppo.int/taxon/XYLEFA/distribution, accessed on 13 July 2022). The economic impact on the European olive industry over 50 years could reach 5.2 billion EUR (5.6 billions USD) depending on cultivar resistance and effective control of the disease [18]. It is reasonable to expect that climate change will play a role in the worldwide epidemiology of Xf [19,20,21,22,23], but too few experimental studies have been conducted so far with which to generate robust predictions [19,20].
We conducted a four-year survey (2016–2019) across a range of climates in Corsica (France; Figure 1 and Figure 2), where Xf was detected for the first time in the summer of 2015. We explored relationships between climate variables (temperature and precipitation) and the prevalence of Xf in vectors (defined as the proportion of insects that carry Xf in sampled populations). We also generated hypotheses under a range of global change scenarios for the future climate suitability of Corsica for Xf and its main vector and for the prevalence of Xf in vectors. Our results are discussed with consideration regarding what we do, and do not know, about the pathosystem (Xf, its vectors, and their host feeding plants).
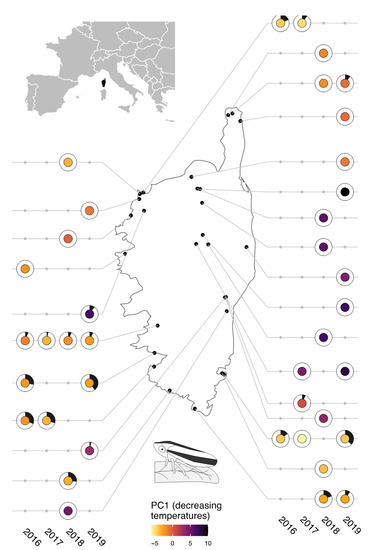
Figure 1.
Prevalence of X. fastidiosa in populations of insect vectors. The black portion of each donut represents the proportion of specimens from which Xf was sequenced. The color of the pies shows the score of the sampling sites on PC1 of the PCA performed on climate variables (the warmest colors correspond to the highest temperatures).
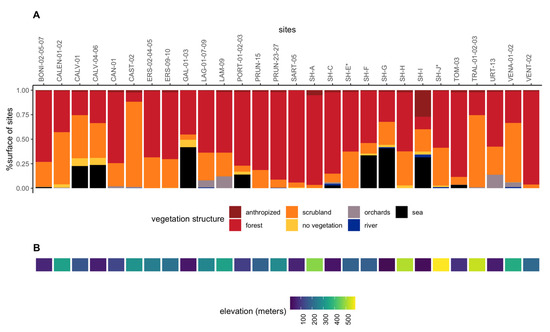
Figure 2.
Vegetation structure and elevation of the sampling sites. (A) Proportion of each vegetation structure in all sites sampled (B) Elevation of the sample sites (in the same order as in A). The vegetation structure is given within a radius of 1 km around the sampling sites. See also the additional map.
2. Materials and Methods
Sampling sites and collection of specimens—The sampling sites (Figure 1, Table S1, supplementary map) were selected with the primary aim of maximizing the range of climatic conditions (climate data retrieved from SAFRAN models (see next section)). Sampling sites were located in various types of vegetation (Figure 2A; vegetation data retrieved from the OCS GE database (© IGN—2022, https://geoservices.ign.fr/ocsge, accessed on 12 July 2022)) and at varying altitudes (Figure 2B). The sampling sites were not selected based on official detections of Xf in plants. Indeed, false positive or negative results may exist and surveillance does not cover all areas because it is almost exclusively directed towards symptomatic plants. We considered repeated measures for some sites to obtain an idea of the steadiness of Xf prevalence through time. However, as much as possible and because our aim was not to analyze time series, we selected insect populations originating from different sites to minimize data inter-dependence in the data set. Indeed, as advocated by Colegrave and Ruxton [24], even if data inter-dependence was now correctly accounted for by random effects in the generalized linear mixed model (GLMM) [25], it remains more powerful to collect independent data. A minimum of 30 adults were collected by sweep netting the vegetation at each sampling site (Table S1a). Insects were killed on site with ethyl acetate, and were quickly transferred to 8 mL vials filled with 70% EtOH and stored in 96% EtOH about 10 days later. The vials were stored at 4 °C until DNA extraction. The adults were collected in late October so that they had time to feed on infected plants and Xf could multiply in their foregut [26].
Prevalence data—Quantitative and conventional PCR are currently established as the gold standard when testing for the presence of Xf in plants, but drawbacks regarding sensitivity and versatility have been highlighted for vectors [27]. Therefore, we developed a high-throughput method to amplify and sequence leuA, one of the housekeeping genes of Xf [28], in insects. Philaenus spumarius (L.) (Aphrophoridae), the most significant vector of Xf in Europe [12], was used as a sentinel to track Xf in the environment. DNA was extracted from single specimens following Cruaud et al. [27]. A two-step PCR approach targeting leuA was set up following Cruaud et al. [29]. Details on the protocol are provided in the Supplementary Material, in Tables S4 and S5. A unique combination of 9-nt indexes was assigned to each specimen to track index hopping, and four PCR replicates were performed per insect to reduce false negatives (the same combination of indexes for all replicates). Sequencing was performed on a MiSeq system (2 × 250 bp). Analysis of the raw data was adapted from Cruaud et al. [29]. Adapter trimming and the selection of high quality paired reads was performed with Trimmomatic [30]; paired reads were merged with FLASH [31]; clustering of sequences was performed with SWARM [32]. Consensuses were aligned to the set of reference sequences available in pubMLST (http://pubmlst.org/xfastidiosa, accessed on 12 July 2022) and alignment was visually inspected in Geneious R11.1.4 (https://www.geneious.com, accessed on 12 July 2022) to discard non-target amplifications. The complete analytical workflow with examples is available from https://github.com/acruaud/prevalenceXfinsectclimate_2022 (accessed on 12 July 2022) and details are provided in Supplementary Material. The two step PCR approach was used for specimens collected in 2017–2019. Prevalence data for 2016 were retrieved from a previous study (nested PCR, Sanger sequencing [27]). We note that prevalence data for 2016 were not statistically different from those of the following years (Figure S5).
Climate variables—Twenty-five temperature- and five precipitation-related variables were computed to describe the climate profile of the sampling sites. Variables were chosen considering the phenology of P. spumarius in Corsica; literature on the multiplication of Xf in plants and P. spumarius and, in the absence of knowledge on the epidemiological dynamics of Xf multiplex in Europe, annual fluctuations of Pierce’s disease incidence in California. Five time slices were defined (as for growing and dormant seasons for plant/insects: March–November, December–February, and regarding P. spumarius phenology: March–June, July–August, and September–October). For each time slice, we computed the daily mean temperature. For each time slice but for the dormant season, we computed the maximum temperature of the time slice and the average daily maximum temperature over the time slice. For the dormant season, we computed the minimum temperature of the time slice and the average daily minimum temperature over the time slice. Finally, for the growing season, we computed the number of days with a daily maximal temperature strictly greater than 16 °C, 18 °C, 20 °C, 22 °C, 24 °C, and 30 °C, while the number of days with a daily minimum temperature strictly lower than 0 °C, 2 °C, 4 °C, and 6 °C was computed for the dormant season. For each time slice but for the dormant season, we computed the sum of the daily precipitation. Finally, we computed the sum of the daily precipitation for the growing season of the year Y-1 (see Supplementary Material and Table S1a for details). Raw data to compute climate variables were retrieved from Météo France (SAFRAN model), which interpolates temperature/precipitation measures made several times a day by a network of over 1000 meteorological stations spread over the French territory. SAFRAN provides the daily temperature (2 m above ground) and precipitation data simulated at a resolution of 8 km on an extended Lambert-II projection that were used to compute the studied climate variables. To estimate future and past climate conditions under different global circulation models (GCMs) and shared socioeconomic pathways (SSPs) (Supplementary Material and Table S1b,c), we relied on bioclimatic variables from the CHELSA v2.1 database (https://chelsa-climate.org, accessed on 12 July 2022).
Statistical analyses—GLMMs were built to analyze the effect of climate variables on prevalence (binomial distribution). Independent climate variables were built with two methods. First, a principal component analysis (PCA) was performed on the 30 climate variables and the scores of the sampling sites on PC1 and PC2 were used as the input for a first GLMM (GLMM1). Second, a partial least square regression analysis (PLSR) was conducted to rank climate variables in decreasing importance (using the Variable Importance on Projection, VIP [33]) regarding the correlation with Xf prevalence. Climate variables were selected step by step, in decreasing VIP order, with two conditions: VIP > 1 and Spearman correlation coefficient with variables selected in the previous steps lower than 0.7 [34]. The climate data table for sampling sites reduced to the selected variables was used as the input for a second GLMM (GLMM2). To account for repeated measures, we added the random effect of the sample site identifier. The year was included as an experimental design fixed effect owing to the number of factor levels being below 5. GLMM validity (correct distribution, dispersion, frequency of outliers, and homoscedasticity) was ascertained and we tested the significance of the fixed variables in models with type II analyses of deviance (two-sided type II Wald chi-square tests) (see Supplementary methods for all details).
Species distribution modelling—Species distribution models (SDMs) were built from worldwide occurrences of Xf ssp. multiplex and P. spumarius using 12 bioclimatic variables (Table S3; CHELSA database) and the Maxent algorithm [35] with 10,000 background points to define the available environmental conditions. The SDMs were used to predict the species future potential distribution using the same combinations of GCMs and SSPs as those used to model the future climate profiles of the sampling sites (see Supplementary methods for all details).
3. Results
Of the 1200 insects tested for Xf (39 populations of P. spumarius across Corsica; Supplementary Table S1a), 8% were recovered as positive for Xf ssp. multiplex. Prevalence in insect populations ranged from 0 to 40% (Figure 1, Table S1a). The first (PC1) and second (PC2) axes of the PCA performed on the 30 climate variables respectively supported 56.7% and 12.9% of the climate variability. PC1 opposed plots with high temperatures to plots with low temperatures. PC2 opposed plots with high maximal temperatures and high precipitations during the previous year to plots with high precipitations. In both GLMMs, variables that were significantly correlated with Xf prevalence were strongly linked to temperature descriptors (Table S2 and Figure 3). GLMM1 (built from scores of the sampling sites on PC1 and PC2 of the PCA; Figure S1 and Table S1a) and GLMM2 (built from uncorrelated most explanatory variables according to PLSR) provided similar results, showing that Xf prevalence in vectors was positively correlated to temperature. In GLMM1, prevalence decreased with the sampling site score on PC1, meaning that prevalence was higher in sites with higher temperatures (Figure 3A). GLMM2 identified the number of days from December to February, with a minimal temperature <6 °C (d6C_dec_fev) as the most explanatory variable. A higher prevalence was predicted under milder winter (Figure 3B).
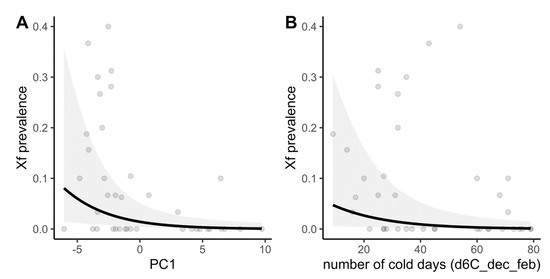
Figure 3.
Correlation between X. fastidiosa prevalence in vectors and climate. (A) GLMM1: Prevalence and sampling site scores on PC1 of the PCA performed on climate variables. (B) GLMM2: Prevalence and number of days in December to February (d6C_dec_feb) with minimal daily temperature <6 °C. Points are raw data, lines are regression curves from GLMMs, and grey ribbons are 95% confidence envelopes.
To explore the past and future climate profiles of the sampling sites, we computed the mean temperature of the coldest quarter (bio11), which is the bioclimatic variable that is the closest to d6C_dec_fev (Figure S2; Spearman’s rank correlation coefficient between bio11 and d6C_dec_feb for current climate = −0.87)) and for which the past and future values are publicly available. For all combinations of GCMs and SSPs, a general increase in winter temperatures (Figure 4 and Table S1b) was predicted. This, together with GLMM predictions, suggests that milder winters in the future will favour an increase in the prevalence of Xf in vectors.
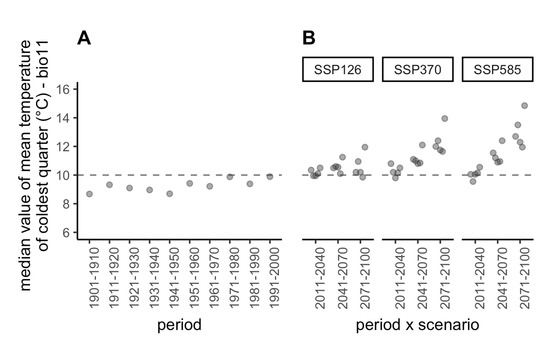
Figure 4.
Past and future projections of temperature for the coldest quarter. (A) Median value of bio11 (mean temperature of coldest quarter) over all sampling sites projected in the past. (B) Median value of bio11 over all sampling sites projected in the future under three shared socio-economic pathways (SSPs, three facets) and five global circulation models (GCMs, five points per facet jittered along abscissa) that reflect uncertainties regarding the response of humans to climate change and the evolution of physical processes in the atmosphere, oceans, cryosphere, and land surface, respectively (see Table S1c for details). The dashed line shows the current median value (for the period 2000–2018).
Only uncorrelated bioclimatic variables (variance inflation factor < 10) were retained for SDMs (bio5, bio8, bio9, bio10, bio11, bio13, bio17, and bio19 for Xf; bio5, bio6, bio8, bio9, bio13, bio14, and bio18 for P. spumarius). The MIAmaxent stepwise model fitting procedure led to a significant model (p = 6.83 × 10−5) based on four explanatory climate variables for P. spumarius, namely: bio5 (variable contribution = 53.7%), bio6 (36.6%), bio8 (4.9%), and bio13 (4.9%). The model was also significant for Xf (p = 1.11 × 10−3) and five variables were retained, namely: bio11 (60.0%), bio5 (24.1%), bio19 (13.0%), bio9 (1.5%), and bio8 (1.5%). Both models exhibited good evaluation metrics. The area under the curve (AUC) values and continuous Boyce indexes (CBI) were 0.822 and 0.992, respectively, for P. spumarius and 0.993 and 0.976, respectively, for Xf. Figure 5 shows the climate suitability of Corsica in the form of a consensus model based on the median of the predicted climate suitability using the model of each species and the five GCMs. Consensus models were computed for the periods covering 2011–2040 and 2041–2070 for both SSP370 and SSP585 using the medium to high end of plausible future pathways of greenhouse gas emissions (radiative forcing reaching respectively 7.0 W/m2 or 8.5 W/m2 in 2100; Table S1c). Other results (time periods and SSPs) are available in supplementary Figures S3 and S4. All predictions suggest that both Xf and its main vector will keep encountering suitable climate conditions in Corsica in the future. In the most extreme scenarios, climate change may lead to lower suitability on the coastal areas of Corsica for both partners, but suitable conditions could be found in areas that were previously unsuitable or less suitable, especially in higher elevation areas. The surface corresponding to suitable conditions for both species, i.e., for which there is an overlap between the vector and the pathogen decreases for the most extreme situations (Table S6, 2071–2100, SSP370 and SSP585), but remains high in all of the scenarios explored.
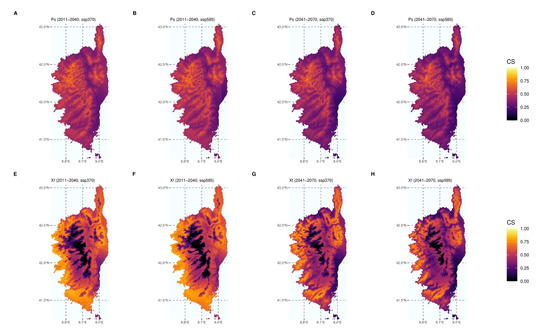
Figure 5.
Species distribution modelling. Consensus models showing climate suitability (CS) for P. spumarius (Ps, (A–D)) and Xf ssp. multiplex (Xf, (E–H)) according to two time periods (2011–2040 and 2041–2070) and two shared socio-economic pathways (SSP370 and SSP585). Climate suitability is estimated using the Maxent algorithm and climate reference data corresponding to the period of 1981–2010.
4. Discussion
This study has two major outcomes. First, it highlights the power of insect vectors to track Xf in ecosystems. Currently, the surveillance of Xf is almost exclusively directed towards symptomatic plants. However, infected vectors have now been recorded twice in areas supposedly free of Xf based on plant survey (e.g., northwestern and northeastern Corsica; this study and [27]). These findings clearly indicate the need for alternative early warning and long-term monitoring systems. Indeed, the surveillance of Xf in plants is actually more challenging than in insects. First, Xf is heterogeneously distributed within the plant tissue [36], which can lead to false negatives. This source of false negatives does not exist with insects for which the whole body is analyzed. In addition, the diversity of PCR inhibitors associated with numerous Xf host plant matrices adds technical challenges to the effective detection of the bacterium [37]. Finally, because many species are asymptomatic to low bacterial loads in natural conditions [23], targeting only symptomatic plants is not effective. A nuanced understanding of the factors leading to pathogenicity in this endophytic bacterium requires an explicit inclusion of insect vectors. Insects need to be surveyed at large scale using cutting-edge molecular tools.
The second major outcome is that the prevalence in vectors is highly likely to increase with ambient temperature. It has been established that Xf spread is directly linked to the abundance of infectious vectors [22], while plant mortality relates to the number of inoculation events [38]. A corollary of these factors is that areas experiencing milder winters and warmer springs and falls are at greater risk of new outbreaks. A mechanistic understanding requires a summary of what is known concerning the effect of temperature on Xf and its main vector.
Regarding the bacterium, the optimal temperature range may vary among strains of Xf, but temperatures below 10 °C and above 32 °C affect the survival of the bacterium according to in vitro and potted plant experiments [39]. Within this range, increasing temperatures favor higher multiplication rates [39], with plants achieving an infectious status faster [20]. Overwinter recovery of plants is observed in natural conditions and experiments show that exposure to freezing temperatures can lead to temporary or complete remission of symptoms [40]. Plants (vines) inoculated later in the growing season have better chances of recovery than those inoculated earlier [41]. However, the mechanisms leading to recovery are still not fully understood. Regarding the development of P. spumarius, data on the influence of temperature are inconsistent [42]. The minimum temperature for egg hatching and nymphal development ranges from 2.8 °C to 10 °C, and development is still observed at 27 °C, while the nymphal period becomes shorter with increasing temperatures [42]. Summer droughts can negatively impact P. spumarius populations and shifts from dry to less water-stressed plants or migration toward cooler climates have been documented [43,44]. In Corsica, field observations suggest that low numbers of adults survive until mid-February [45], while the first nymphs hatch in early February. Adults are virtually impossible to find in the summer, including in the riparian vegetation [45]. As such, the effects of temperature on the probing behavior or feeding rate of P. spumarius are unknown. The same applies for Xf multiplication within the insect foregut or transmission efficiency. In the US, a positive temperature-dependent transmission efficiency has been highlighted for some vectors [21] and a higher temperature also favors flight activity, feeding, and overwinter survival [22].
Our current understanding of vector and pathogen ecology allows us to propose the following. Milder winters likely increase the overwinter survival of Xf in plants, and the bacterial load is consequently higher in spring. The multiplication of Xf is favored by warmer weather during the growing season. A high cell density (which promotes biofilm formation [46]) is achieved earlier and, consequently, acquisition by insects happens sooner. A higher temperature in the growing season may also increase vector activity. Vectors may fly more frequently, disperse further, and take longer meals, which could favor the acquisition [47] and transmission of Xf. The probability of encounters between vectors and Xf is thus steadily increasing. A better understanding of the whole process would require lab and, above all, given the expected discrepancies, field experiments. These field experiments should primarily be designed to follow bacterial load in insects and plants throughout the year, but will also help to document unknown aspects of the ecology of P. spumarius. Of particular interest would be studies on overwinter survival of P. spumarius, because survival may steadily increase with milder winters. The population size may also increase and lead to a higher transmission efficiency. All of these factors could affect the epidemiology of Xf [48]. Obviously, an increased understanding of the environmental factors on other components of this complex pathosystem is also crucial [49,50].
We show that the prevalence might continue to increase, but how do these factors align across the pathosystem? Are vector populations, prevalence in vectors, and plant symptoms all favored by the same climatic conditions? As shown by our SDMs, the effect of climate on vector populations will probably be context-dependent: conditions becoming hotter and drier will favor P. spumarius at the cool-moist end of our climatic gradient (i.e., on the most elevated sites), while they will have an adverse effect on P. spumarius at the hot-dry end of our gradient (e.g., on sites already experiencing heat waves of up to 37.8 °C in summer, Table S1a). This is in line with the preference of P. spumarius for moist environments in Mediterranean climates [42,51] and the preference of vectors for fully irrigated plants [47]. The link between climate and Xf prevalence was clear for temperature, but inconclusive for precipitation, as none of the precipitation-related variables were retained in our models. The link between temperature and Xf symptoms is understudied, but Pierce’s disease severity is expected to be strongest in the warmest places of the Mediterranean basin, especially in those experiencing mild winters [52]. Finally, a negative feedback loop is likely to operate between vector populations and symptoms, because vectors strongly prefer to feed on healthy or asymptomatic plants in controlled conditions [20]. As a result, it is unlikely that rising temperatures will contribute to an exponential increase in the number of outbreaks. However, we can anticipate (i) increasing Xf prevalence and symptoms in the warmest places of Corsica, together with a decrease in P. spumarius abundance, and (ii) a progression of the Xf pathosystem towards more elevated sites, with the build-up of large vector populations. It is worth noting that adaptation to climate change for both partners (e.g., possibly longer aestivation for the vectors) is unknown and may influence our projections [53]. A final argument against runaway resides in the biology of Xf itself, which, for most plants, is a commensal exhibiting self-limiting behavior through quorum sensing [46].
Mitigating the effect of global warming requires knowledge on how the climate may affect different aspects of plant pathosystems [20]. Here, we provide a first assessment of how increasing temperatures may affect the prevalence of Xf in vectors. In addition, increased market globalization is also of concern as it may favor the introduction to Europe of other efficient vectors (e.g., Homalodisca vitripennis and Graphocephala atropunctata) or bacterial strains with which hybridizing is possible with unpredictable outcomes [54].
As illustrated here for X. fastidiosa, recent works have suggested that climate change will result in increasing the burden of plant pathogens at a high latitude in the Northern Hemisphere, particularly in Europe, China, and the central to eastern US [2,5]. Impact may vary depending on the ability of natural ecosystems and production systems to adapt [2,5,6]. Preventive and, when possible, curative plant protection have been underlined as key components to maintain and preserve current and future food security [5]. However, managed and unmanaged ecosystem should not be considered as separate compartments [6] with surveillance mainly targeting symptomatic cultivated plants. Indeed, especially for generalist vector-borne plant diseases, genetically diverse wild plants should be seen as potential reservoirs of pathogens for crops [6]. Conversely, infected cultivated plants that are introduced at the vicinity of natural ecosystems could become a source of biodiversity loss. In a rapidly changing world, early warning and long-term monitoring systems are crucial. This study is an example of how and why new sequencing technologies targeting pathogens in vectors are essential for scaling up surveillance efforts and protecting plant health. A blind and massive screening of vectors could also reveal undocumented vector–pathogen associations that may influence disease dynamics.
5. Conclusions
This study highlights how the surveillance of Xylella fastidiosa could be improved by using insects in addition to symptomatic plants. Indeed, we show that infected vectors are found in localities where symptomatic plants have never been recorded. This survey is an example of how and why new sequencing technologies targeting pathogens in vectors are essential for scaling up surveillance efforts and long-term monitoring.
Our results clearly indicate that Xf prevalence in its insect vectors is very likely to increase with ambient temperature. Areas experiencing milder winters and warmer springs and falls are expected to be at greater risk of outbreaks. Projections of the future potential distribution of both P. spumarius and X. fastidiosa indicate that although the climate may alter their current distribution, both species will find suitable climate conditions in more elevated areas of Corsica.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biology11091299/s1: Supplementary Methods; Table S1: Raw data collected and analyzed in this study; Table S2: Results of the GLMMs; Table S3: Climate descriptors used in species distribution modeling; Table S4: Primers, mix and cycling parameters used in the first PCR; Table S5: Primers, mix and cycling parameters used in the second PCR; Table S6: Spatial overlap between P. spumarius (Ps) and X. fastidiosa (Xf) in Corsica; Figure S1: Principal component analysis of the 30 climate variables used to describe the climate of the sampling sites during the 4 years of the survey; Figure S2: Spearman correlation matrix of climate variables calculated from SAFRAN data; Figure S3: Climate suitability for P. spumarius (Ps) and X. fastidiosa (Xf) according to reference climate condition (1981-2010) and for the periods 2011–2040, 2041–2070 and 2071–2100 for the shared socio-economic pathway SSP126; Figure S4: Consensus models showing the of climate suitability for P. spumarius (Ps) and X. fastidiosa (Xf) for 2071-2100 for the shared socio-economic pathways SSP370 and SSP585; Figure S5: Scatterplots of raw data and GLMM predictions for pairwise associations between prevalence of Xylella fastidiosa in populations of vector and sampling year; Supplementary Map: Distribution of sampling sites and landscape vegetation structure within 1000 m radius buffers zones around sites. [19,20,27,28,29,30,31,32,33,34,35,39,41,45,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100].
Author Contributions
Conceived and designed the study: J.-P.R., J.-Y.R. and A.C.; collected the data: P.F., M.C., X.M., M.L., J.-P.R., J.-Y.R. and A.C.; performed the analysis: P.F., M.C., X.M., J.-P.R., J.-Y.R. and A.C.; wrote the paper: P.F., M.C., X.M., J.-P.R., J.-Y.R. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
P.F. was funded by a French Ministry of Agriculture doctoral fellowship. A.C., J.-Y.R. and J.-P.R. were funded by the plant health and environment department of the INRAE. This study was supported by the Collectivité Territoriale de Corse and the European Union Horizon 2020 research and innovation program under grant agreement no. 727987 XF-ACTORS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw sequence reads generated as part of this study are available from the NCBI Sequence Read Archive under BioProject accession PRJNA873902. The code for the analysis of the sequence data is available from github: https://github.com/acruaud/prevalenceXfinsectclimate_2022 (accessed on 12 July 2022).
Acknowledgments
We acknowledge the general support for the molecular work from the CBGP-BM staff, thank Sabine Nidelet and Guénaëlle Genson for assistance in the laboratory methods and Audrey Weber for sequencing of the library. We are grateful to François Casabianca (INRAE) and Laetitia Hugot (CNBC) for their help in the setup of a research project in Corsica and to Simon Segar (Harper Adams University) for his helpful comments on a first version of this manuscript. We thank the two anonymous reviewers for their feedbacks on this manuscript. Analyses utilized the computing resources of the Genotoul bioinformatics platform Toulouse Midi-Pyrenees.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Chaloner, T.M.; Gurr, S.J.; Bebber, D.P. Plant pathogen infection risk tracks global crop yields under climate change. Nat. Clim. Chang. 2021, 11, 710–715. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Juroszek, P.; Racca, P.; Link, S.; Farhumand, J.; Kleinhenz, B. Overview on the review articles published during the past 30 years relating to the potential climate change effects on plant pathogens and crop disease risks. Plant Pathol. 2019, 69, 179–193. [Google Scholar] [CrossRef]
- IPPC Secretariat. Scientific Review of the Impact of Climate Change on Plant Pests—A Global Challenge to Prevent and Mitigate Plant Pest Risks in Agriculture, Forestry and Ecosystems; IPPC Secretariat: Rome, Italy, 2021. [Google Scholar]
- Jeger, M.J. The impact of climate change on disease in wild plant populations and communities. Plant Pathol. 2021, 71, 111–130. [Google Scholar] [CrossRef]
- Huang, W.; Reyes-Caldas, P.; Mann, M.; Seifbarghi, S.; Kahn, A.; Almeida, R.P.; Béven, L.; Heck, M.; Hogenhout, S.A.; Coaker, G. Bacterial Vector-Borne Plant Diseases: Unanswered Questions and Future Directions. Mol. Plant 2020, 13, 1379–1393. [Google Scholar] [CrossRef]
- Perilla-Henao, L.M.; Casteel, C.L. Vector-Borne Bacterial Plant Pathogens: Interactions with Hemipteran Insects and Plants. Front. Plant Sci. 2016, 7, 1163. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Delbianco, A.; Gibin, D.; Pasinato, L.; Morelli, M. Update of the Xylella spp. host plant database—Systematic literature search up to 30 June 2021. EFSA J. 2022, 20, 7039. [Google Scholar]
- Chatterjee, S.; Almeida, R.P.P.; Lindow, S. Living in two Worlds: The Plant and Insect Lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008, 46, 243–271. [Google Scholar] [CrossRef]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.-W.; Fereres, A.; Redak, R.A.; Perring, T.M.; Lopes, J.R.S. An overview on the worldwide vectors of Xylella fastidiosa. Èntomol. Gen. 2019, 39, 157–181. [Google Scholar] [CrossRef]
- Krugner, R.; Sisterson, M.S.; Backus, E.A.; Burbank, L.P.; Redak, R.A. Sharpshooters: A review of what moves Xylella fastidiosa. Austral Èntomol. 2019, 58, 248–267. [Google Scholar] [CrossRef]
- Purcell, A.H.; Finlay, A. Evidence of noncirculative transmission of Pierce’s Disease bacterium by sharpshooter leafhoppers. Phytopathology 1979, 69, 393–395. [Google Scholar] [CrossRef]
- Bové, J.M.; Ayres, A.J. Etiology of three recent diseases of citrus in São Paulo State: Sudden death, variegated chlorosis and huanglongbing. IUBMB Life 2007, 59, 346–354. [Google Scholar] [CrossRef]
- Tumber, K.P.; Alston, J.M.; Fuller, K.B. Pierce’s disease costs California $104 million per year. Calif. Agric. 2014, 68, 20–29. [Google Scholar] [CrossRef]
- Saponari, M.; Giampetruzzi, A.; Loconsole, G.; Boscia, D.; Saldarelli, P. Xylella fastidiosa in Olive in Apulia: Where We Stand. Phytopathology 2019, 109, 175–186. [Google Scholar] [CrossRef]
- Schneider, K.; van der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Lansink, A.O. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef]
- Daugherty, M.P.; Cooper, M.; Smith, R.; Varela, L.; Almeida, R. Has Climate Contributed to a Pierce’s Disease Resurgence in North Coast Vineyards? PWV: San Rafael, CA, USA, 2019. [Google Scholar]
- Daugherty, M.P.; Zeilinger, A.R.; Almeida, R.P.P. Conflicting Effects of Climate and Vector Behavior on the Spread of a Plant Pathogen. Phytobiomes J. 2017, 1, 46–53. [Google Scholar] [CrossRef]
- Daugherty, M.; Bosco, D.; Almeida, R. Temperature mediates vector transmission efficiency: Inoculum supply and plant infection dynamics. Ann. Appl. Biol. 2009, 155, 361–369. [Google Scholar] [CrossRef]
- Gruber, B.R.; Daugherty, M.P. Predicting the effects of seasonality on the risk of pathogen spread in vineyards: Vector pressure, natural infectivity, and host recovery. Plant Pathol. 2013, 62, 194–204. [Google Scholar] [CrossRef]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P. Xylella fastidiosa: Insights into an Emerging Plant Pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef] [Green Version]
- Colegrave, N.; Ruxton, G.D. Using Biological Insight and Pragmatism When Thinking about Pseudoreplication. Trends Ecol. Evol. 2018, 33, 28–35. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Non-Parametric Regression Models, 2nd ed.; Chapman & Hall: London, UK; CRC Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Bodino, N.; Cavalieri, V.; Pegoraro, M.; Altamura, G.; Canuto, F.; Zicca, S.; Fumarola, G.; Almeida, R.P.P.; Saponari, M.; Dongiovanni, C.; et al. Temporal dynamics of the transmission of Xylella fastidiosa subsp. pauca by Philaenus spumarius to olive plants. Èntomol. Gen. 2021, 41, 463–480. [Google Scholar] [CrossRef]
- Cruaud, A.; Gonzalez, A.-A.; Godefroid, M.; Nidelet, S.; Streito, J.-C.; Thuillier, J.-M.; Rossi, J.-P.; Santoni, S.; Rasplus, J.-Y. Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: A case study in Corsica. Sci. Rep. 2018, 8, 15628. [Google Scholar] [CrossRef]
- Yuan, X.; Morano, L.; Bromley, R.; Spring-Pearson, S.; Stouthamer, R.; Nunney, L. Multilocus Sequence Typing ofXylella fastidiosaCausing Pierce’s Disease and Oleander Leaf Scorch in the United States. Phytopathology 2010, 100, 601–611. [Google Scholar] [CrossRef]
- Cruaud, P.; Rasplus, J.-Y.; Rodriguez, L.J.; Cruaud, A. High-throughput sequencing of multiple amplicons for barcoding and integrative taxonomy. Sci. Rep. 2017, 7, 41948. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Mahé, F.; Rognes, T.; Quince, C.; de Vargas, C.; Dunthorn, M. Swarm v2: Highly-scalable and high-resolution amplicon clustering. PeerJ 2015, 3, e1420. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A Review of Variable Selection Methods in Partial Least Squares Regression; Chemometr. Intell. Lab.: Amsterdam, The Netherlands, 2012; Volume 118, pp. 62–69. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Holland, R.M.; Christiano, R.S.C.; Gamliel-Atinsky, E.; Scherm, H. Distribution of Xylella fastidiosa in Blueberry Stem and Root Sections in Relation to Disease Severity in the Field. Plant Dis. 2014, 98, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Dupas, E.; Legendre, B.; Olivier, V.; Poliakoff, F.; Manceau, C.; Cunty, A. Comparison of real-time PCR and droplet digital PCR for the detection of Xylella fastidiosa in plants. J. Microbiol. Methods 2019, 162, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.S.; Blua, M.S.; Bethke, J.A.; Redak, R.A. Transmission of Xylella fastidiosa to Oleander by the Glassywinged Sharp-shooter, Homalodisca coagulata. HortScience 2000, 35, 1265–1267. [Google Scholar] [CrossRef]
- Feil, H.; Purcell, A.H. Temperature-Dependent Growth and Survival of Xylella fastidiosa in Vitro and in Potted Grapevines. Plant Dis. 2001, 85, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.H. Cold therapy of Pierce’s disease of grapevines. Plant Dis. Rep. 1977, 61, 514–518. [Google Scholar]
- Feil, H.; Feil, W.S.; Purcell, A.H. Effects of Date of Inoculation on the Within-Plant Movement of Xylella fastidiosa and Persistence of Pierce’s Disease Within Field Grapevines. Phytopathology 2003, 93, 244–251. [Google Scholar] [CrossRef]
- Cornara, D.; Bosco, D.; Fereres, A. Philaenus spumarius: When an old acquaintance becomes a new threat to European agri-culture. J. Pest Sci. 2018, 91, 957–972. [Google Scholar] [CrossRef]
- Cornara, D.; Panzarino, O.; Santoiemma, G.; Bodino, N.; Loverre, P.; Mastronardi, M.G.; Mattia, C.; De Lillo, E.; Addante, R. Natural areas as reservoir of candidate vectors of Xylella fastidiosa. B. Insectol. 2021, 74, 173–180. [Google Scholar]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Simonetto, A.; Fumarola, G.; Di Carolo, M.; Porcelli, F.; et al. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 17725. [Google Scholar] [CrossRef] [PubMed]
- Albre, J.; Carrasco, J.M.G.; Gibernau, M. Ecology of the meadow spittlebug Philaenus spumarius in the Ajaccio region (Corsica)—I: Spring. Bull. Èntomol. Res. 2020, 111, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Roper, C.; Castro, C.; Ingel, B. Xylella fastidiosa: Bacterial parasitism with hallmarks of commensalism. Curr. Opin. Plant Biol. 2019, 50, 140–147. [Google Scholar] [CrossRef]
- Krugner, R.; Backus, E.A. Plant Water Stress Effects on Stylet Probing Behaviors of Homalodisca vitripennis (Hemiptera: Cicadellidae) Associated with Acquisition and Inoculation of the Bacterium Xylella fastidiosa. J. Econ. Èntomol. 2014, 107, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.P.; Almeida, R.P.P. Understanding How an Invasive Vector Drives Pierce’s Disease Epidemics: Seasonality and Vine-to-Vine Spread. Phytopathology 2019, 109, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.P.; Blua, M.J.; Lopes, J.R.; Purcell, A.H. Vector Transmission of Xylella fastidiosa: Applying Fundamental Knowledge to Generate Disease Management Strategies. Ann. Èntomol. Soc. Am. 2005, 98, 775–786. [Google Scholar] [CrossRef]
- Mesmin, X.; Chartois, M.; Borgomano, S.; Rasplus, J.Y.; Rossi, J.P.; Cruaud, A. Interaction networks between spittlebugs and plants in and around olive and clementine groves of Corsica; implications for the management of Xylella fastidiosa. Agr. Ecosyst. Environ. 2022, 334, 107979. [Google Scholar] [CrossRef]
- Godefroid, M.; Morente, M.; Schartel, T.; Cornara, D.; Purcell, A.; Gallego, D.; Moreno, A.; Pereira, J.A.; Fereres, A. Climate tolerances of Philaenus spumarius should be considered in risk assessment of disease outbreaks related to Xylella fastidiosa. J. Pest. Sci. 2021, 95, 855–868. [Google Scholar] [CrossRef]
- Godefroid, M.; Cruaud, A.; Streito, J.C.; Rasplus, J.Y.; Rossi, J.P. Climate change and the potential distribution of Xylella fas-tidiosa in Europe. bioRxiv 2018. [Google Scholar] [CrossRef]
- Hällfors, M.H.; Liao, J.; Dzurisin, J.; Grundel, R.; Hyvärinen, M.; Towle, K.; Wu, G.C.; Hellmann, J.J. Addressing potential local adaptation in species distribution models: Implications for conservation under climate change. Ecol. Appl. 2016, 26, 1154–1169. [Google Scholar] [CrossRef]
- Vanhove, M.; Retchless, A.C.; Sicard, A.; Rieux, A.; Coletta-Filho, H.; De La Fuente, L.; Stenger, D.C.; Almeida, R.P.P. Genomic Diversity and Recombination among Xylella fastidiosa Subspecies. Appl. Environ. Microbiol. 2019, 85, e02972-18. [Google Scholar] [CrossRef] [PubMed]
- EPPO PM 7/24 (4) Xylella Fastidiosa. EPPO Bull. 2019, 49, 175–227. [CrossRef]
- Marshall, O.J. PerlPrimer: Cross-Platform, Graphical Primer Design for Standard, Bisulphite and Real-Time PCR. Bioinformatics 2004, 20, 2471–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcelletti, S.; Scortichini, M. Genome-Wide Comparison and Taxonomic Relatedness of Multiple Xylella Fastidiosa Strains Reveal the Occurrence of Three Subspecies and a New Xylella Species. Arch. Microbiol. 2016, 198, 803–812. [Google Scholar] [CrossRef]
- Martin, J.-F. Creating Error-Proof Indexes for High Throughput Sequencing 2019. Methods Mol. Biol. 2019, 840, 197–228. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Wistrom, C.; Purcell, A.H. The Fate of Xylella Fastidiosa in Vineyard Weeds and Other Alternate Hosts in California. Plant Dis. 2005, 89, 994–999. [Google Scholar] [CrossRef]
- Lieth, J.H.; Meyer, M.M.; Yeo, K.-H.; Kirkpatrick, B.C. Modeling Cold Curing of Pierce’s Disease in Vitis Vinifera ‘Pinot Noir’ and ‘Cabernet Sauvignon’ Grapevines in California. PhytopathologyTM 2011, 101, 1492–1500. [Google Scholar] [CrossRef]
- Purcell, A. Paradigms: Examples from the Bacterium Xylella Fastidiosa. Annu. Rev. Phytopathol. 2013, 51, 339–356. [Google Scholar] [CrossRef]
- Hewitt, W.B.; Frazier, N.W.; Freitag, J.H.; Winkler, A.J. Pierce’s Disease Investigations. Hilgardia 1949, 19, 207–264. [Google Scholar]
- R Core Team. R Version 3.5.1 (Feather Spray): A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: Https://Www.R-Project.Org/ (accessed on 12 July 2022).
- Quintana-Seguí, P.; Le Moigne, P.; Durand, Y.; Martin, E.; Habets, F.; Baillon, M.; Canellas, C.; Franchisteguy, L.; Morel, S. Analysis of Near-Surface Atmospheric Variables: Validation of the SAFRAN Analysis over France. J. Appl. Meteorol. Climatol. 2008, 47, 92–107. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Chessel, D.; Dufour, A.-B.; Thioulouse, J. The Ade4 Package—I: One-Table Methods. R News 2004, 4, 5–10. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; Chapman and Hall/CRC: Boca Raton, FL, 2006. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized Linear Mixed Models: A Practical Guide for Ecology and Evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2020. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 12 July 2022).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Social Sciences Mcmaster: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2021. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 12 July 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Xu, T.; Hutchinson, M. ANUCLIM Version 6.1 User Guide; Australia National University: Canberra, Australia, 2011. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. Available online: https://cran.r-project.org/web/packages/dismo/dismo.pdf (accessed on 12 July 2022).
- Karger, D.N.; Schmatz, D.R.; Dettling, G.; Zimmermann, N.E. High-Resolution Monthly Precipitation and Temperature Time Series from 2006 to 2100. Sci. Data 2020, 7, 248. [Google Scholar] [CrossRef]
- Buisson, L.; Thuiller, W.; Casajus, N.; Lek, S.; Grenouillet, G. Uncertainty in Ensemble Forecasting of Species Distribution. Glob. Change Biol. 2010, 16, 1145–1157. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; van Vuuren, D.P. A New Scenario Framework for Climate Change Research: The Concept of Shared Socioeconomic Pathways. Clim. Change 2014, 122, 387–400. [Google Scholar] [CrossRef]
- Godefroid, M.; Cruaud, A.; Streito, J.-C.; Rasplus, J.-Y.; Rossi, J.-P. Xylella Fastidiosa: Climate Suitability of European Continent. Sci. Rep. 2019, 9, 8844. [Google Scholar] [CrossRef] [PubMed]
- Falsini, S.; Tani, C.; Sambuco, G.; Papini, A.; Faraoni, P.; Campigli, S.; Ghelardini, L.; Bleve, G.; Rizzo, D.; Ricciolini, M.; et al. Anatomical and Biochemical Studies of Spartium Junceum Infected by Xylella fastidiosa Subsp. Multiplex ST 87. Protoplasma 2021, 259, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample Selection Bias and Presence-Only Distribution Models: Implications for Background and Pseudo-Absence Data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Varela, S.; Anderson, R.P.; García-Valdés, R.; Fernández-González, F. Environmental Filters Reduce the Effects of Sampling Bias and Improve Predictions of Ecological Niche Models. Ecography 2014, 2014, 1084–1091. [Google Scholar] [CrossRef]
- VanDerWal, J.; Shoo, L.P.; Graham, C.; Williams, S.E. Selecting Pseudo-Absence Data for Presence-Only Distribution Modeling: How Far Should You Stray from What You Know? Ecol. Model. 2009, 220, 589–594. [Google Scholar] [CrossRef]
- Seabra, S.G.; Rodrigues, A.S.B.; Silva, S.E.; Neto, A.C.; Pina-Martins, F.; Marabuto, E.; Thompson, V.; Wilson, M.R.; Yurtsever, S.; Halkka, A.; et al. Population Structure, Adaptation and Divergence of the Meadow Spittlebug, Philaenus Spumarius (Hemiptera, Aphrophoridae), Revealed by Genomic and Morphological Data. PeerJ 2021, 9, e11425. [Google Scholar] [CrossRef]
- Vollering, J.; Halvorsen, R.; Mazzoni, S. The MIAmaxent R Package: Variable Transformation and Model Selection for Species Distribution Models. Ecol. Evol. 2019, 9, 12051–12068. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, S.; Halvorsen, R.; Bakkestuen, V. MIAT: Modular R-Wrappers for Flexible Implementation of MaxEnt Distribution Modelling. Ecol. Inform. 2015, 30, 215–221. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the Ability of Habitat Suitability Models to Predict Species Presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Yackulic, C.B.; Chandler, R.; Zipkin, E.F.; Royle, J.A.; Nichols, J.D.; Campbell Grant, E.H.; Veran, S. Presence-Only Modelling Using MAXENT: When Can We Trust the Inferences? Methods Ecol. Evol. 2013, 4, 236–243. [Google Scholar] [CrossRef]
- Streito, J.-C.; Chartois, M.; Pierre, É.; Dusoulier, F.; Armand, J.-M.; Gaudin, J.; Rossi, J.-P. Citizen Science and Niche Modeling to Track and Forecast the Expansion of the Brown Marmorated Stinkbug Halyomorpha Halys (Stål, 1855). Sci. Rep. 2021, 11, 11421. [Google Scholar] [CrossRef]
- Broennimann, O.; Cola, V.D.; Guisan, A. Ecospat: Spatial Ecology Miscellaneous Methods; 2020. Available online: https://cran.r-project.org/web/packages/ecospat/index.html (accessed on 12 July 2022).
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An Improved Dual-Indexing Approach for Multiplexed 16S RRNA Gene Sequencing on the Illumina MiSeq Platform. Microbiome 2014, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2017. Available online: https://cran.r-project.org/web/packages/factoextra/readme/README.html (accessed on 12 July 2022).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. 2017. Available online: https://scirp.org/reference/referencespapers.aspx?referenceid=3067218 (accessed on 12 July 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).